Abstract
Many critically ill patients in intensive care units suffer from an infection-induced whole body inflammatory state known as sepsis, which causes severe weakness in patients who survive. The mechanisms by which sepsis triggers intensive care unit-acquired weakness (ICUAW) remain unclear. Currently, research into ICUAW is focused on dysfunction of the peripheral nervous system. During electromyographic studies of patients with ICUAW, we noticed that recruitment was limited to few motor units, which fired at low rates. The reduction in motor unit rate modulation suggested that functional impairment within the central nervous system contributes to ICUAW. To understand better the mechanism underlying reduced firing motor unit firing rates, we moved to the rat cecal ligation and puncture model of sepsis. In isoflurane-anesthetized rats, we studied the response of spinal motoneurons to injected current to determine their capacity for initiating and firing action potentials repetitively. Properties of single action potentials and passive membrane properties of motoneurons from septic rats were normal, suggesting excitability was normal. However, motoneurons exhibited striking dysfunction during repetitive firing. The sustained firing that underlies normal motor unit activity and smooth force generation was slower, more erratic, and often intermittent in septic rats. Our data are the first to suggest that reduced excitability of neurons within the central nervous system may contribute to ICUAW.
Keywords: action potential, critical illness myopathy, critical illness neuropathy, motor neuron
weakness following critical illness is a common problem that greatly complicates weaning from ventilators and rehabilitation (for review, see Bolton 2005; Khan et al. 2008; Latronico and Bolton 2011; Stevens et al. 2009). The syndrome of profound weakness following critical illness is currently termed intensive care unit (ICU)-acquired weakness (ICUAW). It is widely thought that the primary cause of ICUAW is dysfunction of the peripheral nervous system. The primary mechanisms underlying dysfunction of the peripheral nervous system have been identified as critical illness neuropathy and critical illness myopathy (Bolton 2005; Khan et al. 2008; Latronico and Bolton 2011; Stevens et al. 2009). Despite the focus on the peripheral nervous system as the locus of ICUAW, there is also evidence of central nervous system (CNS) dysfunction in critical illness. Many patients with ICUAW have septic encephalopathy. The etiology of septic encephalopathy is poorly understood (Flierl et al. 2010; Pytel and Alexander 2009), but it provides clear evidence of CNS dysfunction. No studies have been performed to determine whether CNS dysfunction contributes to ICUAW.
During a prospective electromyographic (EMG) study of ICUAW (Khan et al. 2006), we identified myopathy and neuropathy, but in many patients these factors seemed insufficient to explain the virtual paralysis exhibited by conscious patients attempting to produce maximal voluntary contraction. With further study, we identified an additional factor in recordings of motor unit activity obtained using concentric needle EMG. In these recordings, recruitment was limited to very few motor units, which fired at low rates. Thus the two primary mechanisms by which the CNS regulates muscle force, recruitment of motor units and rate modulation of motor unit firing, were both reduced. This suggested that dysfunction of the CNS contributes to ICUAW. To explore potential mechanisms underlying poor recruitment and rate modulation of motor units, we moved to a rat model of sepsis to assess motoneuron excitability. Motoneurons from septic rats fired at slower rates and demonstrated abnormal pauses in firing during current injection. These data raise the possibility that deficits in motoneuron excitability occurring within the CNS contribute to ICUAW.
MATERIALS AND METHODS
Ethical approval.
All procedures performed on patients were approved by the Institutional Review Board of Emory University, and each patient or legally authorized representative gave informed written consent. All procedures involving animals were approved by the Wright State University Laboratory Animal Care and Use Committee (LACUC).
Nerve conduction/EMG in patients.
Subjects with weakness following a diagnosis of severe sepsis were enrolled from the medical intensive care units at two participating institutions, Emory University Hospital and Grady Health System, using criteria for the diagnosis of sepsis that we used previously (Khan et al. 2006). Subjects less than 18 years of age and those with known preexisting neuromuscular disorders were excluded.
Demographic and clinical information regarding patient's sex, age, race, medical comorbidities, and source of infection if known were recorded. None of the patients received neuromuscular blocking agents or glucocorticoids. All aspects of patient care, including nutritional support, ventilator management, and general ICU supportive care were left to the discretion of the primary physician caring for the patient.
Nerve conduction studies were performed in the ICU using standard techniques with surface stimulation and recording. Responses were categorized as normal or abnormal based on standard normal values used at the EMG laboratories at Grady Memorial Hospital and Emory University Hospital. When possible, sural sensory, peroneal motor, tibial motor, median sensory, radial sensory, and median motor studies were obtained. Amplitudes were measured from baseline to negative peak for motor and sensory responses. Concentric needle EMG was performed on both proximal and distal arm and leg muscles in each patient. Muscles studied included the deltoid, biceps, and triceps, first dorsal interosseous, tibialis anterior, gastrocnemius, and vastus lateralis.
Studies of motoneuron excitability in the septic rat.
We used the cecal ligation and puncture procedure to induce sepsis in rats, which we used previously (Novak et al. 2009). Briefly, rats were anesthetized with inhaled isoflurane (1–3% mixed in 100% O2), and the anterior abdomen was shaved, cleaned, and incised. The cecum was ligated halfway between its tip and the ileum and punctured with an 18-gauge needle. For continuous relief of pain, an ALZET 2-ml osmotic pump (DURECT, Cupertino, CA) that delivered 30 μg·kg−1·h−1 of oxymorphine was inserted into the abdomen before closing the incision. At the end of surgery, rats were given a single dose of buprenorphine (0.12 mg/kg) subcutaneously for pain relief until the oxymorphine took effect.
Following 1 day of sepsis, data were collected in vivo from motoneurons in rats anesthetized by inhalation of isoflurane (1.2–1.5% mixed in 100% O2) and fixed in a rigid recording frame. Properties of medial gastrocnemius motoneurons were measured by intracellular recording with glass micropipettes (K-acetate, 5–10 MΩ) advanced through the dorsal spinal cord, exposed by laminectomy as detailed in earlier reports from this laboratory (Bullinger et al. 2011). All recordings were performed using an Axoprobe 1A (Sunnyvale, CA) amplifier in bridge mode. Action potentials were initiated in motoneurons by injecting suprathreshold depolarizing current (square pulses lasting ≥100 ms) directly into a motoneuron soma within the spinal cord, bypassing synaptic activation of the motor unit. In normal rats, motoneurons fire one action potential at rheobase current and fire repetitively throughout longer pulses as current is increased. Repetitive firing during 5-s pulses was assessed at several different levels of current. Both rheobase current and repetitive firing behaviors reflect the translation of current into firing rates by motoneurons and are standard indices of the intrinsic excitability of motoneurons (Powers and Binder 2001).
Statistical analysis of data.
The effect of sepsis in rats was identified by statistical comparison of treated vs. control groups. Data sampled from rats in each group were pooled and tested for group differences using nested ANOVA, which identified the effect of sepsis as well as its dependence on individual rats.
RESULTS
Reduced recruitment of motor units contributes to ICUAW in patients.
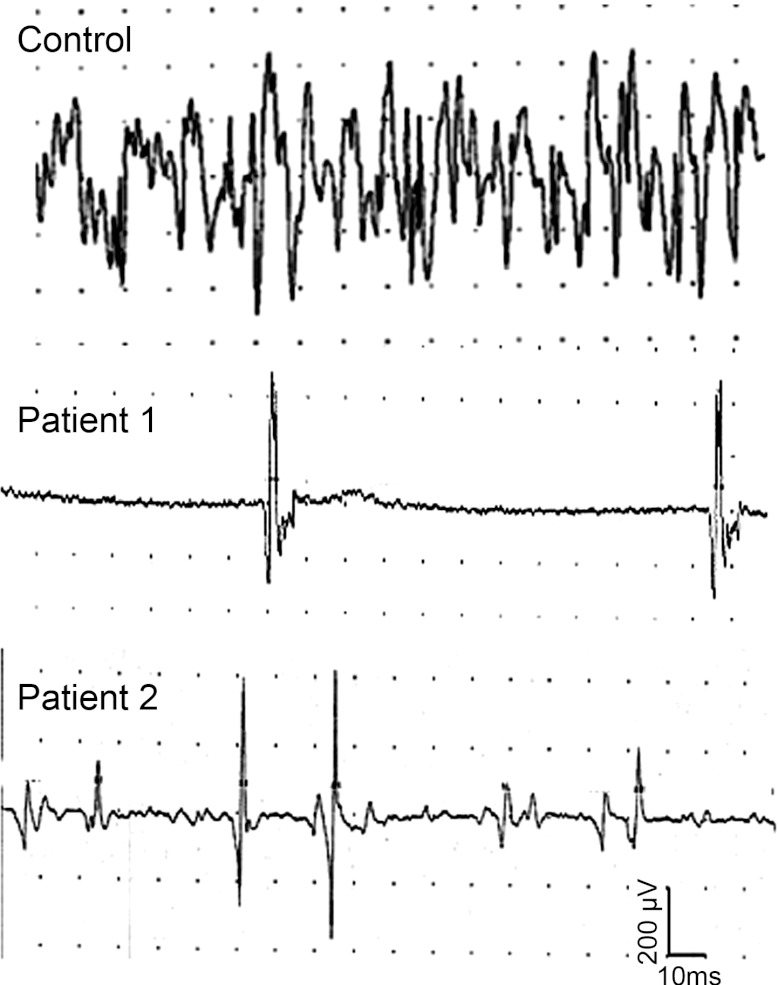
We performed nerve conduction/EMG studies in 4 patients in the early stage (2–3 wk after onset) of recovery from sepsis. Clinical and neurophysiological characteristics of the patients are included in Table 1. EMG was performed in both proximal and distal muscles in an arm and leg of each patient. At the time of EMG study, all patients were awake and able to cooperate but had severe weakness with an average Medical Research Council (MRC) scale of or less in both proximal and distal muscles. Despite apparent cooperation, EMG in all 4 patients revealed an inability to recruit more than a few motor units during maximal voluntary contraction of both distal and proximal limb muscles (bottom traces in Fig. 1).
Table 1.
Patient data
| Patient no. | Medical Conditions | Time of EMG Study Relative to Onset of Sepsis | Mean Motor Amplitude as a % of the Lower Limit of Normal at the Time of EMG | Mean Sensory Amplitude as a % of the Lower Limit of Normal at the Time of EMG | Summary of EMG Findings |
|---|---|---|---|---|---|
| 1 | Pneumonia | 20 Days | 58% | 0% | + Fibrillations, normal motor-unit amplitudes, reduced motor-unit activation, and firing rate in proximal and distal muscles |
| Sepsis | n = 6 | n = 4 | |||
| ARDS | |||||
| Hypertension | |||||
| CHF | |||||
| CRD | |||||
| 2 | Pneumonia | 21 Days | 76% | 65% | + Fibrillations, reduced motor-unit amplitudes, reduced motor-unit activation, and firing rate in proximal and distal muscles |
| Sepsis | n = 5 | n = 4 | |||
| ARDS | |||||
| ATN | |||||
| Sinusitis | |||||
| Hypertension | |||||
| 3 | Sepsis | 17 Days | 42% | 10% | No spontaneous activity, reduced motor-unit amplitudes, reduced motor-unit activation, and firing rate in proximal and distal muscles |
| Leukemia | n = 5 | n = 4 | |||
| Bone marrow transplant | |||||
| GI bleed | |||||
| 4 | Pneumonia | 14 Days | 32% | 38% | + Fibrillations, no motor-unit activation in proximal and distal muscles |
| Sepsis | n = 1 | n = 4 | |||
| Alcohol Abuse | |||||
| Gout |
For motor and sensory studies, n = the number of nerves studied. ARDS, adult respiratory distress syndrome; ATN, acute tubular necrosis; CHF, congestive heart failure; CRD, chronic renal disease; GI, gastrointestinal; EMG, electromyography.
Fig. 1.
Reduced motor-unit recruitment during maximal voluntary contraction in patients recovering from sepsis. Shown are needle electromyogram traces from the biceps muscle during maximal voluntary muscle contraction in a control and patients 1 and 2 from Table 1.
A potential explanation for poor recruitment of motor units in patients with ICUAW is neuropathy. In ICU-acquired neuropathy, limb weakness is worse distally (Zochodne et al. 1987). This pattern of weakness is due to more severe loss of motor units in distal muscles with relative sparing of proximal muscles. All patients studied had neuropathy as evidenced by reduction of nerve-evoked sensory and motor responses (Table 1). However, there was a disproportionate lack of motor unit recruitment. On average, motor amplitudes were reduced in distal muscles by ∼50% (Table 1). This should have been reflected in a 50% reduction in motor unit recruitment in distal muscles with milder reduction in motor unit recruitment in proximal muscles. Instead, proximal muscles also showed severe reduction in motor unit recruitment instead of the milder reduction expected. Our findings suggest that neuropathy is insufficient to account for the reduction in recruitment of motor units in patients with severe ICUAW.
In addition to the reduction in number of motor units recruited, we noticed a defect in the rate of motor unit firing. Rates of motor unit firing in these patients were uncommonly low (5–10 Hz) during maximum voluntary contraction and did not achieve the normal maximal values of 15 Hz and greater (De Luca and Contessa 2012). These subnormal values contrast starkly with those typically observed in patients with neuropathy wherein the firing rates of surviving motor units are high.
Reductions in maximal firing rate of motor units cannot be accounted for by either neuropathy or myopathy and strongly suggest that functional impairment within the CNS contributes to ICUAW. There are two possible explanations for reduced motor unit recruitment and firing rates in patients with ICUAW. One is a reduction in excitatory drive of motoneurons due to either poor effort secondary to encephalopathy or weakening of synaptic inputs onto motoneurons. A second explanation is reduction in motoneuron excitability so that motoneurons are less responsive to depolarizing current. The possibility that motoneurons have reduced excitability was particularly interesting to us in light of our earlier demonstrations of reduced excitability of muscle and sensory nerve axons early in the course of critical illness (Novak et al. 2009; Rich et al. 1996, 1997). To go beyond our qualitative studies in patients and quantify motoneuron excitability, it was necessary to move to an animal model of sepsis to perform intracellular recordings from motoneurons in vivo.
Selective defects in central excitability of motoneurons in septic rats.
Reduced excitability of the peripheral nervous system in rats was well-explained by sodium channelopathy (Filatov and Rich 2004; Novak et al. 2009; Rich et al. 1998; Rich and Pinter 2001, 2003), and it was intriguing that such problems might extend to the CNS where they would alter how neurons integrate and respond to synaptic current. To this end, we studied the capacity of motoneurons to respond to current injected directly into their soma within the spinal cord. Data from untreated rats were compared against those from rats made septic by cecal ligation and puncture 24 h earlier.
We examined central excitability of motoneurons using injection of current to elicit single action potentials. In rat models of critical illness neuropathy and critical illness myopathy, reduced peripheral excitability manifested as lower action potential amplitude and reduced rate of action potential rise (dV/dt; Novak et al. 2009; Rich and Pinter 2001). By contrast, there was no change in single action potentials generated by brief current pulses injected centrally into the soma of motoneurons in septic rats: both action potential amplitude and rate of rise of were normal (Table 2). Other measures of factors that affect baseline excitability were also normal. There was no difference between control and septic motoneurons in amplitude and decay time of the afterhyperpolarization of the action potential (Table 2). Resting potential was normal, consistent with our earlier finding that electrolyte concentrations are normal at early time points in sepsis in rats (Novak et al. 2009). Neither input resistance nor rheobase were significantly different between control and septic motoneurons (Table 2). Thus, with regard to generation of a single action potential, excitability was normal.
Table 2.
Action potential and passive properties of control and septic rat motoneurons
| Control | Septic | |
|---|---|---|
| Resting potential, mV | −68.9 ± 3.0 | −68.6 ± 2.0 |
| Input resistance, MΩ | 1.9 ± 0.3 | 1.8 ± 0.1 |
| Rheobase current, nA | 12.6 ± 2.3 | 12.9 ± 1.4 |
| AP amplitude, mV | 78.2 ± 4.7 | 76.7 ± 2.0 |
| Rate of AP rise, mV/ms | 405 ± 9 | 396 ± 6 |
| AHP amplitude, mV | 1.3 ± 0.4 | 1.5 ± 0.5 |
| AHP half-decay time, ms | 11.3 ± 1.5 | 11.6 ± 1.0 |
Values are means ± SE. Control, n = 6 motoneurons from 5 rats. Septic, n = 8 motoneurons from 4 rats. No differences were statistically significant. AP, action potential; AHP, afterhyperpolarization.
Normal operation of motoneurons is based on repetitive firing, and that is where we found evidence of reduced excitability. When repetitive firing of motoneurons was triggered using prolonged stimulation, there were striking abnormalities. Motoneurons from control rats respond to increasing current by increasing their rate of firing (Fig. 2). This is the mechanism underlying modulation of firing rate, which is essential for maximal generation of force. In motoneurons from septic rats, the increase in firing rate triggered by increased current injection was blunted such that firing rates were reduced at all levels of current injection (Fig. 2). The slope of the frequency-current (F–I) relation was reduced from 4.6 ± 1.3 pulses per second per nanoampere in the control to 3.6 ± 0.8 pulses per second per nanoampere in septic rats. In part because of the reduced firing rate, the number of spikes generated by septic motoneurons during 5 s of stimulation averaged only ⅓ that of control motoneurons injected with the same current (Fig. 2; P < 0.01). Reduction in firing rate was not the only difference between septic and control motoneurons. In control motoneurons, at each level of current injection, the rate of firing was relatively stable throughout the duration of that current injection. In motoneurons from septic rats, the firing rate was often normal initially but then fell, producing highly variable firing rates (Fig. 3). Further evidence of an activity-dependent reduction in excitability came from the pattern of firing of septic motoneurons. In septic motoneurons, firing intermittently stopped completely for 1–2 s (Fig. 4). This “stuttering” pattern of firing has not been observed in any of ∼50 motoneurons examined by us in control rats for other studies. These data strongly suggested that although the threshold properties of septic motoneurons are normal, their ability to sustain prolonged repetitive firing was severely impaired.
Fig. 2.
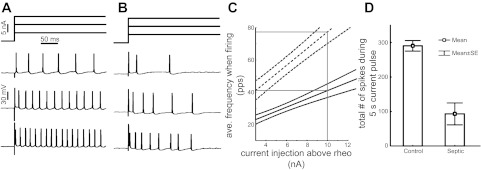
Motoneurons fire more slowly in septic rats. Shown at the top of A and B is the pulse paradigm for current injection into motoneurons from a control (A) and a septic (B) rat. Shown below the pulse protocols are the firing pattern triggered by each of the 3 current injections. With increasing current injection into the control motoneuron (A), it fires more rapidly (bottom traces). B: the motoneuron from the septic rat fires more slowly than the control at each level of current injection. C: plotted are the average (ave.) and 95% confidence intervals of firing frequency vs. current injection above rheobase for control (dashed line) and septic (solid line) motoneurons. Both the initial firing rate and the slope of the relationship are reduced in motoneurons from septic rats (P < 0.01 for both comparisons). D: plotted is the mean number of spikes in response to a 5-s current injection at 10 nA above rheobase for motoneurons from both control and septic rats. Motoneurons from septic rats fire approximately ⅓ as many spikes as motoneurons from control (P < 0.01). n = 6 control and 8 septic motoneurons.pps, Pulses per second; rheo, rheobase current, the minimal current sufficient to trigger an action potential.
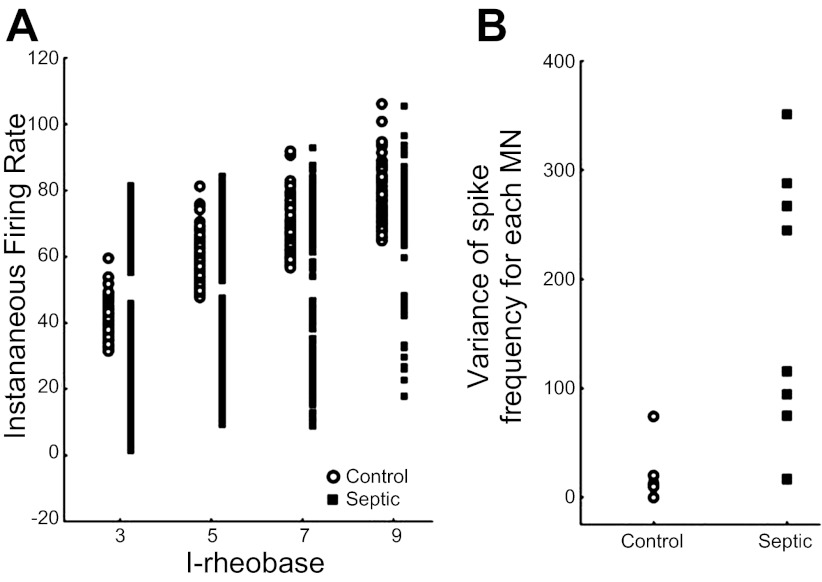
Fig. 3.
Motoneurons fire irregularly after induction of sepsis. A: shown is a scatter plot of the instantaneous firing frequency throughout a 5-s current injection for a control motoneuron and a motoneuron from a septic rat plotted vs. current injection above rheobase current (I-rheobase). The instantaneous firing frequency varies over a much wider range at each level of current injection in the motoneuron from the septic rat. B: shown is a scatter plot of the average variance of spike frequency in 6 control and 8 septic motoneurons (MN). Average variance of spike frequency is higher in the motoneurons from septic rats. Several of the control points are superimposed.
Fig. 4.
Stuttering in firing is not due to inactivation of sodium channels. A: shown is the firing of a motoneuron from a septic rat during a 5-s current injection. The motoneuron stops firing for ∼2 s during the middle of the current injection. B: shown on an expanded time scale are the last action potential before the pause (large arrowhead in A, on left in B) and the 1st action potential after the pause in firing (small arrowhead in A, on right in B). The 1st potential after the pause has a threshold that is 5 mV higher, a slower rate of rise and a lower peak. These differences in action potential characteristics suggest firing resumes despite sodium channel inactivation that is greater than before the pause. C: the maximum rate of rise of action potentials is plotted vs. time during the 5-s current injection. The plot is aligned with the action potential plot shown in A. The rate of action potential rise gradually decreases to a steady-state value before the pause. The rate of rise of the action potential is lowest when the motoneuron resumes firing after the pause and then gradually increases to the steady-state value before the pause in firing.
In our previous studies of animal models of ICUAW, we found that increased inactivation of the sodium channels that generate the action potential was the mechanism of reduced excitability of peripheral nerve and skeletal muscle (Filatov and Rich 2004; Novak et al. 2009; Rich and Pinter 2001, 2003). We wished to determine whether a similar mechanism could account for failure to sustain firing of motoneurons in septic rats. If sodium channel inactivation is the sole mechanism underlying stuttering in firing, the degree of inactivation should be greatest before pauses in firing, and relief of inactivation should occur before resumption of firing. The rate of action potential rise in motoneurons is proportional to the number of sodium channels available to open (Brownstone et al. 2010; Miles et al. 2005). To estimate the relative degree of sodium channel inactivation, we measured the maximal rate of rise of action potentials (dV/dt) during repetitive firing in response to a 5-s suprathreshold current step (Fig. 4C). We compared the maximal dV/dt of the last action potential before each pause (Fig. 4B, left) with that of the first action potential following resumption of firing (Fig. 4B, right). The first action potential after a pause had a significant reduction in the rate of rise relative to the final action potential before the pause (210 ± 14 vs. 243 ± 14 mV/ms; P < 0.01, paired t-test) as well as reduction in amplitude (48.9 ± 2.9 vs. 55.1 ± 2.5 mV; P < 0.01, paired t-test). These data demonstrate resumption of firing following a pause occurs when sodium channel availability is relatively low. This suggests that resumption of firing is not solely due to relief of sodium channel inactivation.
DISCUSSION
During EMG studies of patients recovering from sepsis, we found that neither neuropathy nor myopathy appeared sufficient to account for profound weakness. In looking for an explanation for weakness, we identified reduced firing rates of motor units as a potential contributor. Using a rat model of sepsis, we identified a novel form of reduced motoneuron excitability that is only expressed during repetitive firing. Our findings suggest there is a defect in mechanisms specific to central portions of motoneurons that encode repetitive firing. Our findings suggest that reduced motoneuron excitability may contribute to ICUAW.
Comparison between studies of septic patients and rats.
We used an in vivo cecal ligation and puncture model of sepsis in rats to determine whether reduced motoneuron excitability was a potential contributor to reduced motor unit recruitment in sepsis. The rat model of sepsis we used closely mimics the clinical setting in patients who are septic; however, EMG studies of patients were performed several weeks after the onset of sepsis, when patients were recovering, whereas rats were studied within 24 h of the onset of sepsis. In patients, we could not examine motor unit recruitment during the acute phase of critical illness because patients were too encephalopathic to cooperate with voluntary motor unit examination. This might not be coincidental if septic encephalopathy and reduced motoneuron excitability are due to the same underlying mechanism. Although the time point at which they were studied differed, the parallels in motoneuron behavior raise the possibility that reduced motoneuron excitability identified in rats also underlies reduced motor unit firing rates identified in patients. It also suggests the possibility that a mechanism in the CNS, which can be observed within 24 h of the onset of sepsis, might contribute to the early phase of ICUAW.
Mechanisms underlying reduced excitability of rat motoneurons.
In the central portion of motoneurons in septic rats, properties of single action potentials as well as resting potential and membrane conductance were all normal. Defects in the central excitability of motoneurons only emerged during repetitive firing. In previous studies where we identified reduced excitability of peripheral nerve and muscle, we found abnormalities of single action potentials fired from rest (Novak et al. 2009; Rich et al. 1998). This difference suggests the mechanism underlying reduced motoneuron excitability is distinct from the hyperpolarized shift in sodium channel inactivation that we previously identified as the mechanism underlying reduced excitability of skeletal muscle and peripheral nerve (Filatov and Rich 2004; Novak et al. 2009; Rich and Pinter 2001, 2003). However, two recent computational studies suggest a hyperpolarized (left) shift in activation and inactivation of sodium channels, when coupled with reduced efficacy of the Na-K-ATPase, can lead to stuttering in firing of neurons with pauses that last for seconds (Boucher et al. 2012; Yu et al. 2012). These studies raise the possibility that reduced excitability in motoneurons involves multiple mechanisms but shares the shift in sodium channel inaction seen in peripheral nerve and muscle.
Another possibility is that the mechanism underlying reduced motoneuron excitability is distinct from the one underlying reduced excitability of peripheral nerve and muscle. Striatal neurons normally fire in a stuttering pattern that is very similar to the pattern of firing of motoneurons from septic rats (Sciamanna and Wilson 2011). Stuttering of striatal neurons is dependent on the balance between persistent inward currents (PICs) and outward currents mediated by subthreshold voltage-activated K channels (Sciamanna and Wilson 2011). Increasing the ratio of PICs to subthreshold K currents eliminated stuttering of striatal neurons. PICs are present in motoneurons from rats (Button et al. 2006; Dai and Jordan 2011; Hamm et al. 2010) and are under neuromodulatory control by monoamines, which play an important role in regulation of motoneuron excitability (Heckman et al. 2003, 2008; Powers and Binder 2001; Rekling et al. 2000). Changes in neuromodulatory drive could reduce PICs and diminish the ability of motoneurons to sustain firing. Further experiments will be necessary to distinguish between these and other mechanisms that could underlie reduced excitability of motoneurons in septic rats.
Implications of reduced motoneuron excitability.
Motoneurons are the final common pathway through which the CNS encodes muscle force and its gradation. Motoneurons execute their role in modulating force output by converting the synaptic current delivered to the soma into a firing rate that determines muscle force output (Kernell 2006; Powers and Binder 2001). Our data suggest that this process is selectively disrupted by the reduction in motoneuron excitability triggered by sepsis. The fact that this change is restricted to a specific motoneuron function encourages the possibility of a selective treatment that normalizes firing. It is encouraging that the motoneuron is able to fire at least one normal action potential.
Motoneuron central excitability was significantly reduced within 24 h of induction of sepsis. If there is a similar rapid reduction in motoneuron excitability in patients, it might have implications for efforts to prevent ICUAW. The rapid loss of motoneuron excitability could trigger weakness before interventions have time to work. Reduced excitability of motoneurons might affect efforts to prevent later complications of critical illness such as muscle atrophy.
We have not determined whether reduced excitability of motoneurons is fully reversed on recovery from sepsis. If reduced excitability persists, it might be a contributing factor to the complaints of fatigue in patients who have recovered from sepsis (Cheung et al. 2006; Fletcher et al. 2003; Herridge et al. 2003). In rats, motoneurons were able to fire at normal frequencies for brief periods but unable to sustain firing rates. If this sort of defect in firing occurs in patients, it would manifest as the inability to sustain force and might be perceived as fatigue.
Septic encephalopathy is one of the most common and troubling complications of critical illness (Pytel and Alexander 2009). Despite a number of studies, the mechanism underlying septic encephalopathy remains a mystery (Pytel and Alexander 2009). Our findings raise the possibility that reduced excitability of neurons within the CNS might underlie septic encephalopathy. If this is the case, a single therapy to improve neuronal excitability might treat both ICUAW and septic encephalopathy.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant P01-NS-057228 (M. M. Rich and T. C. Cope).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.N. and J.K. performed experiments; P.N., J.K., R.K.P., T.C.C., and M.M.R. analyzed data; P.N., J.K., R.K.P., T.C.C., and M.M.R. interpreted results of experiments; P.N., J.K., R.K.P., T.C.C., and M.M.R. prepared figures; P.N., J.K., R.K.P., T.C.C., and M.M.R. approved final version of manuscript; R.K.P., T.C.C., and M.M.R. conception and design of research; R.K.P., T.C.C., and M.M.R. drafted manuscript; R.K.P., T.C.C., and M.M.R. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Lori Goss for technical assistance.
REFERENCES
- Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve 32: 140–163, 2005 [DOI] [PubMed] [Google Scholar]
- Boucher PA, Joos B, Morris CE. Coupled left-shift of Nav channels: modeling the Na(+)-loading and dysfunctional excitability of damaged axons. J Comput Neurosci 33: 301–319, 2012 [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Krawitz S, Jordan LM. Reversal of the late phase of spike frequency adaptation in cat spinal motoneurons during fictive locomotion. J Neurophysiol 105: 1045–1050, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons. J Neurophysiol 106: 2471–2485, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button DC, Gardiner K, Marqueste T, Gardiner PF. Frequency-current relationships of rat hindlimb alpha-motoneurones. J Physiol 573: 663–677, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matte A, Barr A, Mehta S, Mazer CD, Guest CB, Stewart TE, Al-Saidi F, Cooper AB, Cook D, Slutsky AS, Herridge MS. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 174: 538–544, 2006 [DOI] [PubMed] [Google Scholar]
- Dai Y, Jordan LM. Tetrodotoxin-, dihydropyridine-, and riluzole-resistant persistent inward current: novel sodium channels in rodent spinal neurons. J Neurophysiol 106: 1322–1340, 2011 [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Contessa P. Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107: 178–195, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov GN, Rich MM. Hyperpolarized shifts in the voltage dependence of fast inactivation of Nav1.4 and Nav1.5 in a rat model of critical illness myopathy. J Physiol 559: 813–820, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher SN, Kennedy DD, Ghosh IR, Misra VP, Kiff K, Coakley JH, Hinds CJ. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med 31: 1012–1016, 2003 [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Huber-Lang MS, Stahel PF. Pathophysiology of septic encephalopathy–an unsolved puzzle. Crit Care 14: 165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm TM, Turkin VV, Bandekar NK, O'Neill D, Jung R. Persistent currents and discharge patterns in rat hindlimb motoneurons. J Neurophysiol 104: 1566–1577, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Hyngstrom AS, Johnson MD. Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol 586: 1225–1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci 26: 688–695, 2003 [DOI] [PubMed] [Google Scholar]
- Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348: 683–693, 2003 [DOI] [PubMed] [Google Scholar]
- Kernell D. The Motoneurone and its Muscle Fibres. Oxford, UK: Oxford Univ. Press, 2006 [Google Scholar]
- Khan J, Harrison TB, Rich MM. Mechanisms of neuromuscular dysfunction in critical illness. Crit Care Clin 24: 165–177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan J, Harrison TB, Rich MM, Moss M. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology 67: 1421–1425, 2006 [DOI] [PubMed] [Google Scholar]
- Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 10: 931–941, 2011 [DOI] [PubMed] [Google Scholar]
- Miles GB, Dai Y, Brownstone RM. Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurones. J Physiol 566: 519–532, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak KR, Nardelli P, Cope TC, Filatov G, Glass JD, Khan J, Rich MM. Inactivation of sodium channels underlies reversible neuropathy during critical illness in rats. J Clin Invest 119: 1150–1158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143: 137–263, 2001 [DOI] [PubMed] [Google Scholar]
- Pytel P, Alexander JJ. Pathogenesis of septic encephalopathy. Curr Opin Neurol 22: 283–287, 2009 [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MM, Bird SJ, Raps EC, McCluskey LF, Teener JW. Direct muscle stimulation in acute quadriplegic myopathy. Muscle Nerve 20: 665–673, 1997 [DOI] [PubMed] [Google Scholar]
- Rich MM, Pinter MJ. Crucial role of sodium channel fast inactivation in muscle fibre inexcitability in a rat model of critical illness myopathy. J Physiol 547: 555–566, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MM, Pinter MJ. Sodium channel inactivation in an animal model of acute quadriplegic myopathy. Ann Neurol 50: 26–33, 2001 [DOI] [PubMed] [Google Scholar]
- Rich MM, Pinter MJ, Kraner SD, Barchi RL. Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Ann Neurol 43: 171–179, 1998 [DOI] [PubMed] [Google Scholar]
- Rich MM, Teener JW, Raps EC, Schotland DL, Bird SJ. Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology 46: 731–736, 1996 [DOI] [PubMed] [Google Scholar]
- Sciamanna G, Wilson CJ. The ionic mechanism of gamma resonance in rat striatal fast-spiking neurons. J Neurophysiol 106: 2936–2949, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, de Jonghe B, Ali NA, Sharshar T. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med 37: S299–S308, 2009 [DOI] [PubMed] [Google Scholar]
- Yu N, Morris CE, Joós B, Longtin A. Spontaneous excitation patterns computed for axons with injury-like impairments of sodium channels and Na/K pumps. PLoS Comput Biol 8: e1002664, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne DW, Bolton CF, Wells GA, Gilbert JJ, Hahn AF, Brown JD, Sibbald WA. Critical illness polyneuropathy. A complication of sepsis and multiple organ failure. Brain 110: 819–841, 1987 [DOI] [PubMed] [Google Scholar]