Abstract
During the first 2 wk of mouse postnatal development, transient retinal circuits give rise to the spontaneous initiation and lateral propagation of depolarizations across the ganglion cell layer (GCL). Glutamatergic retinal waves occur during the second postnatal week, when GCL depolarizations are mediated by ionotropic glutamate receptors. Bipolar cells are the primary source of glutamate in the inner retina, indicating that the propagation of waves depends on their activation. Using the fluorescence resonance energy transfer-based optical sensor of glutamate FLII81E-1μ, we found that retinal waves are accompanied by a large transient increase in extrasynaptic glutamate throughout the inner plexiform layer. Using two-photon Ca2+ imaging to record spontaneous Ca2+ transients in large populations of cells, we found that despite this spatially diffuse source of depolarization, only a subset of neurons in the GCL and inner nuclear layer (INL) are robustly depolarized during retinal waves. Application of the glutamate transporter blocker dl-threo-β-benzyloxyaspartate (25 μM) led to a significant increase in cell participation in both layers, indicating that the concentration of extrasynaptic glutamate affects cell participation in both the INL and GCL. In contrast, blocking inhibitory transmission with the GABAA receptor antagonist gabazine and the glycine receptor antagonist strychnine increased cell participation in the GCL without significantly affecting the INL. These data indicate that during development, glutamate spillover provides a spatially diffuse source of depolarization, but that inhibitory circuits dictate which neurons within the GCL participate in retinal waves.
Keywords: retina, development, retinal waves, glutamate spillover, crossover inhibition
before the maturation of the light response, the murine retina is spontaneously active, exhibiting laterally propagating depolarizations termed retinal waves. This spontaneous activity is necessary for refinement of retinal ganglion cell (RGC) axons within target regions of the brain (for reviews, see Guido 2008 and Huberman et al. 2008). In addition, there is growing evidence that retinal waves may play a role of the stratification of dendrites of a subset of RGCs (Xu et al. 2010) as well as in the synaptic maturation of particular retinal circuits (Kerschensteiner et al. 2009; Soto et al. 2012).
Retinal waves are detected for an extended period perinatally, from 1 wk before to 2 wk after birth in mice. During this time, the retina undergoes a substantial amount of development, and, as a result, the circuits that mediate waves change with age (Blankenship and Feller 2010). During the first postnatal week, waves are mediated by a transient cholinergic circuit composed of starburst amacrine cells (Zhou 2001; Ford and Feller 2011). Volume release of acetylcholine provides a diffuse source of depolarization that drives the participation of all nearby starburst amacrine cells and RGCs (Syed et al. 2004; Ford et al. 2012).
Just before eye opening, from postnatal day (P)10–P14, retinal waves are dependent upon the activation of ionotropic glutamate receptors (iGluRs) (Bansal et al. 2000; Wong et al. 2000; Zhou and Zhao 2000) and are modulated by inhibition. Similar to cholinergic waves, glutamatergic waves are accompanied by increases in extrasynaptic glutamate (Blankenship et al. 2009) providing a diffuse source of depolarization. In both ferrets and mice, GABAA transmission modulates the percentage of retinal waves that recruit On and Off RGCs (Fischer et al. 1998; Kerschensteiner and Wong 2008). However, the role of inhibitory transmitters in determining the percentage of cells that are recruited into waves in the inner nuclear layer (INL) and ganglion cell layer (GCL) is unknown. Here, we used an optical indicator of extrasynaptic glutamate and two-photon Ca2+ imaging to explore how the balance of diffuse excitation and inhibition influences the participation of retinal neurons in glutamatergic waves.
METHODS
Mice.
All experiments were performed on acutely isolated mouse retinas. Male and female C57BL/6 mice obtained from Harlan were used for all wild-type recordings. GLAST-tdTomato mice were generated by crossing mice that express Cre recombinase under control of the GLAST promoter [Tg(Slc1a3-cre/ERT)1Nat/J] to mice that ubiquitously express tdTomato preceeded by a loxP-flanked stop cassette [B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, both from The Jackson Laboratory]. Intraperitoneal injection of 0.5 mg of 4-hydroxytamoxifen (50:50 E and Z isomers, Sigma) at P5 reliably induced the expression of tdTomato in Müller glial cells by P10. In Grm6-EFGP mice, enhanced green fluorescent protein (EGFP) is expressed under control of the metabotropic glutamate receptor (mGluR)6 promoter, labeling On bipolar cells (Morgan et al. 2006). All procedures were approved by the Institutional Animal Care and Use Committees of the University of California (Berkeley, CA) and conformed with guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Public Health Service Policy, and the Society for Neuroscience Policy on the Use of Animals in Neuroscience Research.
Retinal preparation.
P10–P12 C57BL/6 mice of either sex were deeply anesthetized with isoflurane and decapitated. Eyes were removed, and retinas were isolated in oxygenated artificial cerebrospinal fluid [aCSF; containing (in mM) 119.0 NaCl, 26.2 NaHCO3, 11 glucose, 2.5 KCl, 1.0 K2HPO4, 2.5 CaCl2, and 1.3 MgCl2]. For Ca2+ and fluorescence resonance energy transfer (FRET) imaging, retinas were mounted GCL-up on filter paper (Millipore) and perfused continuously with oxygenated aCSF.
Ca2+ imaging.
Retinas were loaded with Oregon green 488 BAPTA-1 AM (OGB) using the multicell bolus loading technique (Stosiek et al. 2003; Blankenship et al. 2009). Two-photon Ca2+ imaging of neurons in the INL and GCL was performed using a custom-modified two-photon microscope (Fluoview 300, Olympus America). XYZ scans were used to localize neurons in the GCL and INL. Time series images were acquired at 1 Hz using a ×60 objective (Olympus LUMPlanFl/IR ×60/0.90W) with the excitation laser tuned to 790 nm. Images were corrected for motion artifacts using the Turboreg ImageJ plugin. Ten × ten-pixel regions (12 × 12 µm) of interest were manually selected within all cells in the field of view. Fluorescence signals were averaged within these regions over time. Cell events were identified when change in fluorescence exceeded 15% of the cell's baseline fluorescence within 1 s. Cells were categorized as participating in a retinal wave if cell events correlated with neighboring cells.
FRET imaging.
The FLII81E-1μ glutamate sensor was purified as previously described (Dulla et al., 2008). Whole mount retinas were bath loaded with ∼50 μg/ml of the sensor diluted in aCSF for 20 min at room temperature. Live imaging was performed on an upright Zeiss Axioskop 2 using a ×20 objective (Olympus UMPlanFl N/×20/0.50W). Retinas loaded with the FLII81E-1μ indicator were transferred from the loading solution directly into the microscope perfusion. After retinas had been loaded, the sensor diffused out of the tissue in roughly 5–8 min, limiting the duration of imaging runs. dl-Threo-β-benzyloxyaspartate (TBOA; 25 μM), gabazine (Gbz; 5 μM), and strychnine (Stry; 4 μM) were applied by immersing the loaded retina for ∼5 min before imaging. The imaging plane was located within the inner plexiform layer (IPL).
Individual FRET channel detection was accomplished by using a Dual-View image splitter (DV-FC, Optical Insights) with appropriate yellow (535 nm/30) and cyan (480 nm/40) emission filters. Images were captured on a Photometrics CoolSnap HQ charge-coupled device camera and analyzed in ImageJ. Images were acquired at 2 Hz (200-ms exposures). Background fluorescence (F) was subtracted from both channels, and enhance cyan fluorescent protein (eCFP) bleedthrough into the Venus channel was corrected (FVenus = FFRET − 0.51 × FeCFP) (Gordon et al. 1998; van Rheenen et al. 2004). FRET ratios (R) were calculated as follows: R = (FVenus/FeCFP). FRET ratio data were then analyzed with custom MATLAB (Mathworks) scripts to define the spatiotemporal properties of spillover, similar to the process used to define Ca2+ waves as described by Blankenship et al. (2009).
Immunohistochemistry.
Retinas were fixed in 4% paraformaldehyde and then cut into 120-μm sections with a vibratome. Sections were stained using sheep anti-Chx10 primary antibody (1:200, Exalpha Biologicals) and Alexa fluor 647 donkey anti-sheep secondary antibody (1:200, Invitrogen), collected on slides, and mounted in medium containing 4′,6-diamidino-2-phenylindole before being imaged on a Zeiss LSM 780 confocal microscope.
RESULTS
Glutamate waves are detected using a FRET-based sensor.
We have previously demonstrated that glutamatergic waves are accompanied by increases in extrasynaptic glutamate by probing the IPL with an outside-out patch of RGC membrane (Blankenship et al. 2009). To visualize the spatial extent of glutamate spillover during retinal waves, we developed a new strategy using the FRET-based optical sensor of glutamate FLII81E-1μ. FLII81E-1μ is a diffusible extracellular sensor that has been previously optimized to detect glutamate spillover in brain slices (Dulla et al. 2008). It contains the Esherichia coli glutamate binding protein YbeJ with internally fused eCFP and COOH-terminally fused Venus, a variant of yellow fluorescent protein (Deuschle et al. 2005). Upon binding of glutamate, there is a decrease in FRET between the fluorophores, allowing for a ratiometric analysis of glutamate transients by fluorescence.
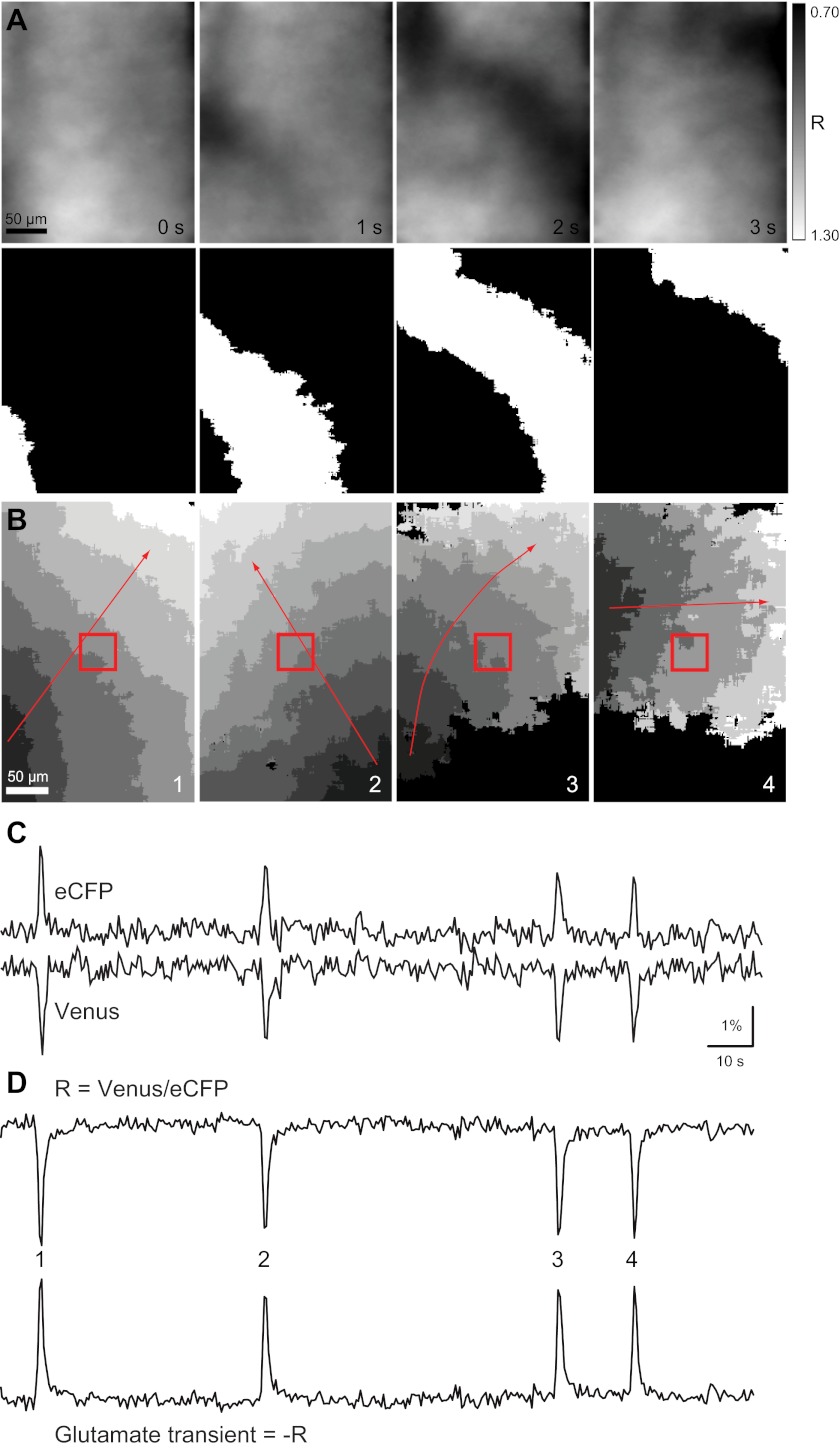
FLII81E-1μ was bath loaded into P10–P12 whole mount retinas. FRET imaging was then used to detect glutamate in the IPL for a period of 5 min. Periodic increases of glutamate could be visualized as a spatially diffuse band of FRET change that propagated through the IPL (Fig. 1). The band of FRET changes, which we refer to as glutamate waves, had clearly defined front and back edges (Fig. 1A) and passed through the field of view at a median frequency of 1.8 waves/min (wave interval median, lower quartile/upper quartile: 33 s, 22/78 s, N = 47 waves; Fig. 1, B and C). Increases in extrasynaptic glutamate were transient, lasting 2–4 s in control conditions. The addition of 20 μM 6,7-dinitroquinoxaline-2,3-dione and 50 μM d-2-amino-5 phosphonopentanoic acid, iGluR antagonists that have previously been shown to block retinal waves (Blankenship et al. 2009), completely abolished glutamate waves (data not shown, n = 4 retinas). These data indicate that glutamatergic retinal waves are accompanied by large increases in extrasynaptic glutamate that propagate throughout the IPL.
Fig. 1.
The fluorescence resonance energy transfer (FRET)-based glutamate sensor FLII81E-1μ detects coherent wave fronts of glutamate propagating through the inner plexiform layer (IPL) in postnatal day (P)10–P12 retinas. A: example of a single glutamate wave detected by FRET imaging of FLII81E-1μ in a P11 whole mount retina. The raw ratio data (top) and binarized version used for analysis (bottom) are shown. B: each image represents the spatial extent and duration of one glutamate transient. Within an image, each grayscale value represents the active area in one frame, with an imaging rate of 2 Hz. Dark gray is the first frame in which glutamate was detected, whereas white is the last. Black is baseline. Red arrows indicate the propagation direction. The red box is the area where ΔR/R was averaged to determine whether an increase in glutamate occurred in that region. C: example trace of raw fluorescence changes for enhance cyan fluorescent protein (eCFP) and Venus. D, top: resulting trace when the ratio of Venus to eCFP was taken. The downward peaks indicate the decrease in FRET when glutamate binds. Bottom, reflection of the top trace. The upward peaks indicate increases in glutamate. Numbered peaks correspond to the wave events shown in B.
Neurons in the INL participate in retinal waves.
To investigate whether neuronal populations in the INL participate in retinal waves, we used two-photon Ca2+ imaging to record from large numbers of neurons simultaneously. Retinas were acutely isolated from P10–P12 mice and bolus loaded with the Ca2+ indicator OGB as previously described (Stosiek et al. 2003; Blankenship et al. 2009; Ford et al. 2012). Two-photon imaging enabled us to record Ca2+ transients in different neuronal populations with single cell resolution by varying the depth of the imaging plane (Fig. 2A) without contamination from out-of-focus fluorescence.
Fig. 2.
Two-photon Ca2+ imaging reveals that neurons in the inner nuclear layer (INL) participate in glutamatergic retinal waves. A: example of wave propagation in the INL (top) and ganglion cell layer (GCL; bottom) observed with two-photon Ca2+ imaging at a frame rate of 1 Hz. Leftmost images are the retinal sample loaded with Oregon green BAPTA-1 AM (OGB). Circles are identified cells; black indicates cells with ΔF/F of >15% for the first time in that frame, and gray indicates cells with persisting ΔF/F above threshold. B: sample ΔF/F traces from averaged regions within cells. Each trace represents a different cell. C: raster plots of neuronal Ca2+ transients of >15% ΔF/F for all cells in the field of view. The total imaging duration was 5 min. D: histogram of interwave intervals for glutamate and Ca2+ imaging from both the GCL and INL (Ca2+ imaging: N = 150 wave intervals and FRET: N = 47 wave intervals).
Neurons in the INL and GCL exhibited propagating waves of Ca2+ transients (Fig. 2, A–C). The rapid rise time and short time course of the Ca2+ transients are consistent with membrane depolarization rather than Ca2+ release from internal stores. The frequency of waves detected with Ca2+ imaging was similar in the INL and GCL, with a median frequency of ∼1.6 waves/min (wave interval median, lower quartile/upper quartile: 36 s, 20/82 s, GCL: 89 waves, 10 retinas and INL: 76 waves, 6 retinas; Fig. 2D), and waves were blocked in both retinal layers by bath application of iGluR antagonists (n = 6 retinas; data not shown). Consistent with a previous study (Blankenship et al. 2009), we found that retinal waves often occur in episodic clusters during which two to five waves occur in rapid succession followed by a much longer interval of inactivity. This pattern was observed in both the INL and GCL (Fig. 2C). Note that FLII81E-1μ was unable to detect a rapid succession of glutamate waves (Fig. 2D). We believe this is a limitation in the temporal resolution of the glutamate imaging, because increases in extrasynaptic glutamate were previously shown to occur in clusters by outside-out patch (Blankenship et al. 2009).
There are four general classes of cell types whose somas are roughly stratified within the INL by P10. From the inner to outer plexiform layer, there are amacrine cells, Müller glial cells, bipolar cells, and horizontal cells. We used an antibody against Chx10, a transcription factor expressed in all bipolar cells, to identify bipolar cell somas and determine their location relative to the somas of other cell types in the P10–P13 INL (Fig. 3A) (Katoh et al. 2010). Bolus loading of OGB labeled both neurons and glial cells indiscriminately throughout the INL and GCL (Fig. 3B) (Sullivan et al., 2005). Müller glial cells have been reported to have both spontaneous and neurally evoked Ca2+ transients (Newman 2001, 2005). To see if glial Ca2+ transients correlated with retinal waves, we performed Ca2+ imaging in P11–P13 retinas from GLAST-tdTomato mice, which express tdTomato in Müller glial cells. After bolus loading with OGB, tdTomato was used to identify Müller somas within the INL (Fig. 3B). We chose a single XY plane that contained both Müller and neuronal somas for 1-Hz two-photon imaging (Fig. 3C). We found that spontaneous Ca2+ transients in Müller somas were infrequent and uncorrelated with wave events seen in OGB-loaded neurons (n = 6 retinas; Fig. 3, D and E). Hence, the correlated Ca2+ transients we observed in the INL originate from neurons and not from glial cells.
Fig. 3.
Müller glial somas are not depolarized during retinal waves. A: P11 GLAST-tdTomato retina stained for anti-Chx10 showing the location of Müller glial and bipolar somas within the INL. The confocal image is of a vibratome section. Green is anti-Chx10, magenta is tdTomato, and blue is 4′,6-diamidino-2-phenylindole (DAPI). B: orthogonal projections of two-photon Z-stacks. The dashed line indicates the XY plane chosen for 1-Hz imaging. Left, GLAST-tdTomato labeling of Müller glial cells; right: bolus loading of OGB labeling both neurons and glial cells. C: XY plane from B, left. G and N indicate examples of Müller glial or neuronal somas, respectively. D: sample ΔF/F traces from averaged regions within identified neuronal or glial somas. E: raster plot of neuronal and glial Ca2+ transients of >15% ΔF/F.
Pharmacological manipulation of glutamatergic waves.
To further strengthen our conclusion that glutamate waves and correlated Ca2+ transients in the INL and the GCL all originate from the same wave events, we used pharmacology to alter wave frequency. First, we blocked glutamate uptake through transporters by the bath application of 25 μM TBOA, an approach we have previously shown to increase extrasynaptic glutamate levels and the frequency of retinal waves (Blankenship et al. 2009). TBOA led to a prolonged duration of glutamate waves, consistent with glutamate lingering in the extracellular space longer, whereas block of inhibitory GABA and glycine receptors with Gbz/Stry did not (Fig. 4A). As measured by both FLII81E-1μ and Ca2+ imaging, TBOA increased the frequency of glutamate waves in the IPL and the frequency of correlated Ca2+ transients in neurons of the INL and GCL (Fig. 4, A–C). Previously, we have shown that inhibition shapes cholinergic waves by decreasing wave frequency via increased shunting inhibition to RGCs through potentiated amacrine cells (Wang et al. 2007). Similarly, we found that Gbz/Stry led to a dramatic increase in event frequency, as assessed by both FLII81E-1μ and Ca2+ imaging (Fig. 4, A–C). Consistent with a previous report (Blankenship et al. 2009), we found that inhibition had the most marked effect on wave initiation rate in glutamatergic waves, by shifting the wave interval distribution to lower values, indicating that inhibition is important for the timing of triggering a wave (Fig. 4C).
Fig. 4.

dl-Threo-β-benzyloxyaspartate (TBOA) and gabazine (Gbz)/strychnine (Stry) increase wave frequency as detected by FLII81E-1μ and two-photon Ca2+ imaging of neurons in the INL and GCL. A: representative traces of glutamate transients for control (top), Gbz and Stry (middle) and TBOA (bottom). B: raster plots of neuronal Ca2+ transients detected by two-photon Ca2+ imaging as in Fig. 2C. C: cumulative distributions of interwave intervals for control (black), TBOA (gray), and Gbz/Str (blue) for FRET imaging in the IPL and Ca2+ imaging in the INL and GCL. Binning = 10 s. FRET glutamate transients: control, 47 wave intervals, 8 retinas; Gbz/Stry, 135 intervals, 5 retinas; and TBOA, 42 intervals, 5 retinas; Ca2+ INL: control, 84 intervals, 16 retinas; Gbz/Stry, 116 intervals, 8 retinas; and TBOA, 127 intervals, 8 retinas; and Ca2+ GCL: control, 156 intervals, 15 retinas; Gbz/Stry, 122 waves, 9 retinas; and TBOA, 42 intervals, 6 retinas. D: each image represents the spatial extent and duration of one glutamate transient in control (left), Gbz/Stry (middle left), and TBOA (middle right and right). Gray values correspond to the time during which that region of the retina was active, as in Fig. 1B. Red arrows indicate the propagation direction.
As measured by FLII81E-1μ, the spatial properties of glutamate waves in the IPL also showed differential effects of TBOA versus Gbz/Stry (Fig. 4D). In contrast to the spatially coherent wavefronts of glutamate waves in control conditions, glutamate waves in the presence of Gbz/Stry exhibited a greater range of rotational propagation trajectories. In the presence of TBOA, glutamate waves propagated in multiple directions simultaneously. In addition, there were regions of sustained decreases in FRET, indicative of stationary pools of glutamate. These observations indicate that the excitability of the circuit, set by extrasynaptic glutamate and inhibition, is not only a critical determinant of wave initiation (Blankenship et al. 2009) but also the trajectory of glutamate wave propagation.
Inhibition limits ganglion cell participation in retinal waves.
The single cell resolution of two-photon Ca2+ imaging revealed that not all cells participate in waves (Fig. 2A). This is in sharp contrast to cholinergic retinal waves, where the vast majority of RGCs and displaced starburst amacrine cells are depolarized in each event (Ford et al. 2012). In the INL, we found that, on average, roughly half the identified cells in the field of view participated in at least one wave over the entire recording duration of 8 min (mean: 54 ± 16%, n = 6 retinas; Fig. 5A control). The GCL had slightly higher percentages of cells that participated in at least one wave (mean: 68 ± 30%, n = 6 retinas; Fig. 5A control). Cell participation per wave was even lower. In the INL, on average, 25 ± 16% of cells participated per wave (76 waves). The GCL had nearly identical levels of cell participation per wave (25 ± 18% of cells, 89 waves). Of the cells that did not participate in waves, some had detectable Ca2+ events between waves (10 ± 1.0% of nonwaving cells in the INL and 15 ± 4.2% of nonwaving cells in the GCL, n = 4 retinas). This indicates that a cell's participation in waves is linked to its circuitry and is not an artifact of dye loading.
Fig. 5.
TBOA increases cell participation in the INL and GCL, whereas Gbz/Stry only increases GCL participation. A: cell participation in retinal waves. The INL (top) and GCL (bottom) in a whole mount retina loaded with OGB are shown. Magenta indicates cells that participated in at least one wave during the duration of the recording; open circles indicate cells that did not participate. The same field of view from an example retina was compared in control (left), Gbz/Stry (middle), and TBOA (right). B: changes in the proportion of waving cells. The change in the proportion of cells participating in at least one wave in the control versus drug condition were quantified for each retina, and these values were then averaged across all retinas (INL: TBOA, N = 8; Gbz/Stry, N = 8 and GCL: TBOA, N = 6; Gbz/Stry, N = 9). C: summary of effects of TBOA and Gbz/Stry on the average proportion of cells that participated per wave. Lines connect values of average cell participation per wave for one retina in control versus TBOA or Gbz/Stry in the INL and GCL. Open circles are group means and SD. See Table 1 for details.
The sparse participation of cells in both the INL and GCL suggest that the diffuse excitation provided by glutamate spillover is balanced by specific inhibitory circuits, influencing the strength of depolarization of cells during retinal waves. To test this hypothesis, we assayed the effects of increasing glutamate spillover and blocking inhibitory signaling on the percentage of cells that participate in waves.
Using two-photon Ca2+ imaging, the proportion of cells participating in at least one wave was tracked across different drug conditions in both the INL and GCL (Fig. 5, A and B). This method of quantification revealed changes in the population of cells depolarized by waves. In the INL, TBOA increased cell participation (Table 1). In contrast, Gbz/Stry did not significantly affect an INL neuron's likelihood of participating in a wave. In the GCL, TBOA increased cell participation and Gbz/Stry dramatically increased the proportion of cells participating in waves. Few to no neurons within the GCL failed to participate in any waves when inhibitory neurotransmission was blocked. Interestingly, blockade of inhibition had a significantly greater effect on neuron participation in the GCL compared with the INL (F(3,27) = 3.35, P < 0.05).
Table 1.
Quantification of cell participation in waves
| n | Control | Drug | P Value | |
|---|---|---|---|---|
| Percentage of cells that participated in a wave per retina | ||||
| GCL | ||||
| Gbz/Stry | 6 | 58 ± 18 | 95 ± 6 | <0.001 |
| TBOA | 6 | 69 ± 13 | 94 ± 2 | <0.001 |
| INL | ||||
| Gbz/Stry | 8 | 33 ± 15 | 43 ± 16 | 0.22 |
| TBOA | 8 | 41 ± 22 | 71 ± 18 | 0.01 |
| Average percentage of cells participating per wave | ||||
| GCL | ||||
| Gbz/Stry | 9 | 33 ± 13 | 53 ± 15 | 0.0092 |
| TBOA | 6 | 40 ± 18 | 65 ± 9 | 0.013 |
| INL | ||||
| Gbz/Stry | 8 | 15 ± 7 | 12 ± 8 | 0.43 |
| TBOA | 8 | 24 ± 18 | 33 ± 19 | 0.35 |
Values are means ± SD; n, number of retinas. The percentages of cells that participated in any wave that propagated through an imaged region of the retina as well as the percentages of cells that participated per wave are shown.GCL, ganglion cell layer; Gbz, gabazine; Stry, strychnine; TBOA, dl-threo-β-benzyloxyaspartate; INL, inner nuclear layer.
We also tracked the proportion of cells participating per wave (Fig. 5C and Table 1). This is a measure of the density of cells participating in waves and how that changes in different drug conditions. Interestingly, the proportion of GCL neurons that exhibited an increase in intracellular Ca2+ per wave significantly increased after blockade of inhibition with Gbz/Stry. These results indicate that inhibitory neurons in the INL, and glutamate levels in the IPL, may be responsible for selecting which GCL cells participate in waves. In contrast, cell participation in the INL was primarily reliant on glutamate levels.
A subset of On-bipolar cells participates in retinal waves.
To further determine which inhibitory circuits are responsible for determining cell participation, we manipulated the On-bipolar cell circuit that inhibits Off cells through crossover inhibition (Werblin 2010; Demb and Singer 2012). To directly measure whether bipolar cells participate in retinal waves, we performed Ca2+ imaging in retinas from Grm6-eGFP mice, which express eGFP in On-cone bipolar cells and rod bipolar cells (Fig. 6A, i) (Morgan et al. 2006). We found that GFP-positive cell somas directly adjacent to the outer plexiform layer, where rod bipolar cell somas are located (Morgan et al. 2006; Morrow et al. 2008), did not exhibit detectable Ca2+ transients. However, a subset of GFP-positive cells that were deeper in the INL, closer to the IPL, exhibited correlated spontaneous Ca2+ transients that were blocked with 5 μM l-amino-4-phosphonobutyric acid (l-AP4), a group III mGluR agonist that keeps On-bipolar cells in a more hyperpolarized state (Fig. 6A,ii) (Slaughter and Miller 1981). Hence, we concluded that a subset of On-bipolar cells are recruited during retinal waves. We then tracked how INL and GCL cell participation during waves was affected by application of l-AP4. Interestingly, l-AP4 did not significantly affect wave interval but did alter the population of cells that participated in waves (Fig. 6B). In the presence of l-AP4, some cells that were depolarized during waves in control became inactive and some cells that were inactive during waves in control began to participate in waves. l-AP4 did not affect overall levels of cell participation in the INL but significantly increased levels of cell participation in the GCL (mean increase: 30 ± 10%, n = 4 retinas; Fig. 6C). These findings suggest that On-bipolar cells play a role in selecting which cells in the GCL participate in waves.
Fig. 6.

The On-bipolar cell (BPC) blocker l-amino-4-phosphonobutyric acid (l-AP4) affects cell participation in glutamatergic waves. A,i: Grm6:eGFP-labeled On-BPCs (left) and bolus-loaded INL and GCL neurons (right). Top, orthogonal projections of two-photon Z-stacks. The dashed line indicates the XY plane chosen for 1-Hz imaging. Bottom, XY plane. Yellow arrows indicate On-BPCs. A,ii, top: sample ΔF/F traces from averaged regions within identified On-BPC somas. Bottom, raster plot of neuronal Ca2+ transients of >15% ΔF/F. Regions of interests 1–30 are identified On-BPCs; the remainder are unidentified neurons within the same imaging plane. On-BPCs stopped participating after the onset of l-AP4 washin (n = 5 retinas). aCSF, artificial cerebrospinal fluid. B: cell participation in retinal waves. The INL (top) and GCL (bottom) in a whole mount retina loaded with OGB are shown. Magenta indicates cells that participated in at least one wave during the duration of the recording; open circles indicate cells that did not participate. The same field of view from an example retina was compared in control (left) and l-AP4 (right). Red indicates increased activity compared with control; blue indicates decreased activity; magenta cells were unaffected. C: summary of effects of l-AP4 on the average proportion of cells that participated per wave. Lines connect values of average cell participation per wave for one retina in control versus l-AP4 in the INL and GCL. Open circles are group means and SD. See Table 1 for details.
DISCUSSION
We have presented two-photon Ca2+ imaging data demonstrating that neurons within the INL, including a subset of On-bipolar cells, participate in retinal waves. In addition, using an optical sensor for glutamate, we found that glutamatergic retinal waves are accompanied by significant transient increases in extrasynaptic glutamate throughout the IPL. Prolonging the duration of glutamate waves with TBOA indiscriminately increased the percentage of both INL and GCL neurons that participated in retinal waves, whereas block of inhibition with Gbz/Stry selectively affected the GCL, where it drastically increased the percentage of cells that participated in retinal waves. Taken together, this evidence suggests that glutamatergic waves may activate a specific neural circuit that selects for subsets of neurons within the GCL rather than a gross wavefront of glutamate-based depolarization that activates all cells. This is in contrast to cholinergic waves, where every cell in the GCL is recruited during waves, suggesting that there is a maturation of inhibitory inputs during glutamatergic waves. Additionally, use of l-AP4 to prevent On-bipolar cells from depolarizing changes the pattern of cell activation in the INL and GCL and increases participation in the GCL, indicating that crossover inhibition mediated by specific amacrine cell subtypes may be responsible for activating distinct circuits during development (Kerschensteiner et al. 2008).
Previously, we demonstrated that waves led to an increase in extrasynaptic glutamate using outside-out patches (Blankenship et al. 2009). However, these experiments were limited in that they only assessed extrasynaptic glutamate at one point in space and the electrode may have caused tissue damage that led to increases in glutamate in local regions around the pipette. Here, we used an optical sensor, FLII81E-1μ, to demonstrate that retinal waves lead to a spatially diffuse glutamate wave that propagates throughout the IPL (Fig. 1). FLII81E-1μ is one of a family of FRET-based indicators that has been used to measure glutamate spillover in brain slices and in vivo (Okumoto et al. 2005; Namiki et al. 2007; Dulla et al. 2008; Matsuo et al. 2009; Okubo et al. 2010; Tani et al. 2010). This study further demonstrates the applicability of this sensor in measuring glutamate dynamics in response to neuronal activity in intact tissue.
Robust glutamate spillover has been observed in both developing and mature circuits. In the mature retina, glutamate spillover has been detected both at photoreceptor (Szmajda and Devries 2011) and bipolar cell terminals (Hasegawa et al. 2006; Veruki et al. 2006; Rowan et al. 2010), where it is thought to inhibit glutamate release by the activation of transporters or mGluRs, providing a source of negative feedback (Awatramani and Slaughter 2001; Higgs et al. 2002). Extrasynaptic glutamate is also a source of excitation for RGCs (Chen and Diamond 2002). During development, extrasynaptic glutamate has been implicated in regulating the early differentiation of neurons in the ventricular zone (Demarque et al. 2002) and might modulate neuronal migration (Manent et al. 2005). In addition, it is a source of depolarization that is critical for generating spontaneous activity in the cortex and hippocampus (Garaschuk et al. 2000; Allene et al. 2008). Similarly, volume release of ACh between starburst amacrine cells mediates an earlier stage of spontaneous activity in the retina (Ford et al. 2012).
We posit that glutamate spillover stimulates the propagation of glutamatergic retinal waves through the INL, activating both excitatory and inhibitory neurons. Block of glutamate uptake with TBOA enables an additional population of neurons in the INL to become depolarized during waves. In addition, TBOA leads to prolonged elevations of extrasynaptic glutamate in the IPL. We hypothesize that this, in turn, recruits additional neurons in the GCL to participate in waves by direct spillover from bipolar cells onto neighboring amacrine and retinal ganglion cells.
Depolarization of neurons in the GCL depends on the balance of excitatory and inhibitory inputs, acting as a readout of the circuits that are activated during retinal waves. Here, we show that blockade of inhibition selectively enabled more neurons in the GCL to participate in waves, with no significant effect on cell participation in the INL. These data suggest that inhibition shapes cell participation in the GCL based on targets from inhibitory INL neurons.
The time around eye opening represents a significant period in retinal development, where a large number of RGC subtypes are undergoing dendritic stratification (Coombs et al. 2007; Kim et al. 2010) and bipolar cell-amacrine and bipolar cell-RGC synapses are forming. Interestingly, recent studies have indicated that the development of some retinal microcircuits may be sensitive to activity, whereas other are not (Kerschensteiner et al. 2009; Elstrott and Feller 2010; Xu et al. 2010; Soto et al. 2012). These data suggest that perhaps as subsets of retinal microcircuits emerge, the maturation of inhibitory synapses exerts a more powerful influence on the structure of correlated activity.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant RO1-EY-013528 (to M. B. Feller), National Science Foundation Grant IOS-0818983 (to M. B. Feller), NIH Grant 5-T32-E-Y007043-33 (to G. S. Sack), NIH Grant T32-EB-005586-05 (to A. Firl), and a Dana Foundation Immuno-Imaging grant (to H. Tani).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.F., G.S.S., Z.L.N., H.T., and M.B.F. conception and design of research; A.F., G.S.S., and Z.L.N. performed experiments; A.F., G.S.S., and Z.L.N. analyzed data; A.F., G.S.S., Z.L.N., H.T., and M.B.F. interpreted results of experiments; A.F., G.S.S., Z.L.N., and M.B.F. prepared figures; A.F., G.S.S., Z.L.N., and M.B.F. drafted manuscript; A.F., G.S.S., Z.L.N., H.T., and M.B.F. edited and revised manuscript; A.F., G.S.S., Z.L.N., H.T., and M.B.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank John Hugenard and Richard Reimer (Stanford University) for the generous gift of the FLII81E-1μ glutamate sensor, John Flannery and Noga Vardi for the Grm6-GFP mice, Holly Aaron (Univ. of California, Berkeley Molecular Imaging Center) for technical assistance, and members of the Feller laboratory for commenting on this manuscript.
REFERENCES
- Allene C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y, Cossart R. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci 28: 12851–12863, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani G, Slaughter M. Intensity-dependent, rapid activation of presynaptic metabotropic glutamate receptors at a central synapse. J Neurosci 21: 741–749, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns, and reveal a limited role for retinal waves in forming O.N and OFF circuits in the inner retina. J Neurosci 20: 7672–7681, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci 11: 18–29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, Feller MB. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron 62: 230–241, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci 22: 2165–2173, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JL, Van Der List D, Chalupa LM. Morphological properties of mouse retinal ganglion cells during postnatal development. J Comp Neurol 503: 803–814, 2007 [DOI] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron 36: 1051–1061, 2002 [DOI] [PubMed] [Google Scholar]
- Demb JB, Singer JH. Intrinsic properties, and functional circuitry of the AII amacrine cell. Vis Neurosci 29: 51–60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle K, Okumoto S, Fehr M, Looger LL, Kozhukh L, Frommer WB. Construction, and optimization of a family of genetically encoded metabolite sensors by semirational protein engineering. Protein Sci 14: 2304–2314, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulla C, Tani H, Okumoto S, Frommer WB, Reimer RJ, Huguenard JR. Imaging of glutamate in brain slices using FRET sensors. J Neurosci Methods 168: 306–319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrott J, Feller MB. Direction-selective ganglion cells show symmetric participation in retinal waves during development. J Neurosci 30: 11197–11201, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KF, Lukasiewicz PD, Wong RO. Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci 18: 3767–3778, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KJ, Feller MB. Assembly and disassembly of a retinal cholinergic network. Vis Neurosci 29: 61–71, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KJ, Felix AL, Feller MB. Cellular mechanisms underlying spatiotemporal features of cholinergic retinal waves. J Neurosci 32: 850–863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci 3: 452–459, 2000 [DOI] [PubMed] [Google Scholar]
- Gordon GW, Berry G, Liang XH, Levine B, Herman B. Quantitative fluorescence resonance energy transfer measurements using fluorescence microscopy. Biophys J 74: 2702–2713, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W. Refinement of the retinogeniculate pathway. J Physiol 586: 4357–4362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa J, Obara T, Tanaka K, Tachibana M. High-density presynaptic transporters are required for glutamate removal from the first visual synapse. Neuron 50: 63–74, 2006 [DOI] [PubMed] [Google Scholar]
- Higgs MH, Romano C, Lukasiewicz PD. Presynaptic effects of group III metabotropic glutamate receptors on excitatory synaptic transmission in the retina. Neuroscience 115:163–172, 2002 [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci 31: 479–509, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Omori Y, Onishi A, Sato S, Kondo M, Furukawa T. Blimp1 suppresses Chx10 expression in differentiating retinal photoreceptor precursers to ensure proper photoreceptor development. J Neurosci 19: 6515–6526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Wong RO. A precisely timed asynchronous pattern of ON and OFF retinal ganglion cell activity during propagation of retinal waves. Neuron 58: 851–858, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature 460: 1016–1020, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci 30: 1452–1462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, Represa A. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci 25: 4755–4765, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo R, Kobayashi S, Watanabe S, Namiki S, Iinuma S, Sakamoto H, Hirose K, Ito E. Glutamatergic neurotransmission in the procerebrum (olfactory center) of a terrestrial mollusk. J Neurosci Res 87: 3011–3023, 2009 [DOI] [PubMed] [Google Scholar]
- Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nature neurosci 9: 85–92, 2006 [DOI] [PubMed] [Google Scholar]
- Morrow EM, Chen CM, Cepko CL. Temporal order of bipolar cell genesis in the neural retina. Neural Dev 3: 2, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki S, Sakamoto H, Iinuma S, Iino M, Hirose K. Optical glutamate sensor for spatiotemporal analysis of synaptic transmission. Eur J Neurosci 25: 2249–2259, 2007 [DOI] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci 21: 2215–2223, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci 25: 5502–5510, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, Sekiya H, Namiki S, Sakamoto H, Iinuma S, Yamasaki M, Watanabe M, Hirose K, Iino M. Imaging extrasynaptic glutamate dynamics in the brain. Proc Natl Acad Sci USA 107: 6526–6531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumoto S, Looger LL, Micheva KD, Reimer RJ, Smith SJ, Frommer WB. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc Natl Acad Sci USA 102:8740–8745, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan MJ, Ripps H, Shen W. Fast glutamate uptake via EAAT2 shapes the cone-mediated light offset response in bipolar cells. J Physiol 588:3943–3956, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science 211: 182–185, 1981 [DOI] [PubMed] [Google Scholar]
- Soto F, Ma X, Cecil JL, Vo BQ, Culican SM, Kerschensteiner D. Spontaneous activity promotes synapse formation in a cell-type-dependent manner in the developing retina. J Neurosci 32: 5426–5439, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA 100: 7319–7324, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MR, Nimmerjahn A, Sarkisov DV, Helmchen F, Wang SS. In vivo calcium imaging of circuit activity in cerebellar cortex. J Neurophysiol 94: 1636–1644, 2005 [DOI] [PubMed] [Google Scholar]
- Syed MM, Lee S, He S, Zhou ZJ. Spontaneous waves in the ventricular zone of developing mammalian retina. J Neurophysiol 91: 1999–2009, 2004 [DOI] [PubMed] [Google Scholar]
- Szmajda BA, Devries SH. Glutamate spillover between mammalian cone photoreceptors. J Neurosci 31: 13431–13441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Dulla CG, Huguenard JR, Reimer RJ. Glutamine is required for persistent epileptiform activity in the disinhibited neocortical brain slice. J Neurosci 30: 1288–1300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rheenen J, Langeslag M, Jalink K. Correcting confocal acquisition to optimize imaging of fluorescence resonance energy transfer by sensitized emission. Biophys J 86: 2517–2529, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Morkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci 9: 1388–1396, 2006 [DOI] [PubMed] [Google Scholar]
- Wang CT, Blankenship AG, Anishchenko A, Elstrott J, Fikhman M, Nakanishi S, Feller MB. GABAA receptor-mediated signaling alters the structure of spontaneous activity in the developing retina. J Neurosci 27: 9130–9140, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis Neurosci 27: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT, Myhr KL, Miller ED, Wong RO. Developmental changes in the neurotransmitter regulation of correlated spontaneous retinal activity. J Neurosci 20: 351–360, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Chen H, Ding Q, Xie ZH, Chen L, Diao L, Wang P, Gan L, Crair MC, Tian N. The immune protein CD3zeta is required for normal development of neural circuits in the retina. Neuron 65: 503–515, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ. The function of the cholinergic system in the developing mammalian retina. Prog Brain Res 131: 599–613, 2001 [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Zhao D. Coordinated transitions in neurotransmitter systems for the initiation, and propagation of spontaneous retinal waves. J Neurosci 20: 6570–6577, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]