Abstract
Epiretinal implants for the blind are designed to stimulate surviving retinal neurons, thus bypassing the diseased photoreceptor layer. Single-unit or multielectrode recordings from isolated animal retina are commonly used to inform the design of these implants. However, such electrical recordings provide limited information about the spatial patterns of retinal activation. Calcium imaging overcomes this limitation, as imaging enables high spatial resolution mapping of retinal ganglion cell (RGC) activity as well as simultaneous recording from hundreds of RGCs. Prior experiments in amphibian retina have demonstrated proof of principle, yet experiments in mammalian retina have been hindered by the inability to load calcium indicators into mature mammalian RGCs. Here, we report a method for labeling the majority of ganglion cells in adult rat retina with genetically encoded calcium indicators, specifically GCaMP3 and GCaMP5G. Intravitreal injection of an adeno-associated viral vector targets ∼85% of ganglion cells with high specificity. Because of the large fluorescence signals provided by the GCaMP sensors, we can now for the first time visualize the response of the retina to electrical stimulation in real-time. Imaging transduced retinas mounted on multielectrode arrays reveals how stimulus pulse shape can dramatically affect the spatial extent of RGC activation, which has clear implications in prosthetic applications. Our method can be easily adapted to work with other fluorescent indicator proteins in both wild-type and transgenic mammals.
Keywords: calcium imaging, electrophysiology, viral vectors, retinal prosthesis
epiretinal prostheses have restored partial vision to the blind via a multielectrode array (MEA) that electrically stimulates surviving retinal neurons (Humayun et al. 2012). To optimize the design of such prostheses, several groups are using in vitro retina preparations to measure population activity of retinal ganglion cells (RGCs) during electrical stimulation. Typically, MEA electrodes record extracellular spikes from dozens of RGCs simultaneously (Ahuja et al. 2008; Grumet et al. 2000; Sekirnjak et al. 2008; Stett et al. 2000). This approach is well-suited for measuring temporal response properties of RGCs, but spatial resolution is limited by the area covered by the electrodes, the spacing between each electrode, and the proximity of RGCs to electrodes. State-of-the-art high-density MEAs (30-μm electrode spacing) can record from nearly all ganglion cells in a small patch of retina, yet the number of electrodes is limited to ∼500 (Field et al. 2010; Litke et al. 2004; Segev et al. 2004). This number should increase as high-resolution CMOS-based MEAs become available (Eversmann et al. 2003; Ferrea et al. 2012; Hutzler et al. 2006); however, MEA recordings have other limitations. Complex spike sorting algorithms (Segev et al. 2004) and stimulus artifact subtraction techniques (Sekirnjak et al. 2006) are needed to assign spikes to individual RGCs. These techniques are biased toward identifying primarily larger neurons that are closer to the recording electrodes (Briggman and Euler 2011; Segev et al. 2004). To overcome these limitations, our group developed an optical method to record from populations of RGCs in isolated retina. Calcium imaging reports RGC activity during stimulation with one or more MEA electrodes (Behrend et al. 2009). We recently demonstrated the power of this technique for identifying the spatial properties of RGC activation during electrical stimulation (Behrend et al. 2011).
Our method relies on being able to label large populations of RGC somata selectively with fluorescent calcium indicator. In the past, we accomplished this by retrogradely loading dextran-conjugated calcium dye via the cut optic nerve (Behrend et al. 2009). This approach labeled nearly all ganglion cells in amphibian retina, yet the same approach did not succeed in adult mammalian retina due to dye extrusion. Others have also reported difficulty achieving dense, widespread labeling of adult mammalian RGCs with synthetic calcium indicators, using techniques such as membrane-permeant dye loading (Morgan et al. 2005), biolistic delivery (Kettunen et al. 2002; Roizenblatt et al. 2006), and electroporation (Yu et al. 2009). This led us to investigate genetically encoded calcium indicators (GECIs; Mank and Griesbeck 2008), which can target specific mammalian cell populations through viral transduction (Kay et al. 2001). GECIs are chimeric proteins consisting of a calcium-binding domain fused to one or two fluorescent molecules. The GCaMP family of GECIs, for example, contains a circularly permuted green fluorescent protein (cpGFP) linked to calmodulin (CaM) and the CaM-binding M13 fragment of myosin light chain kinase (Nakai et al. 2001; Tian et al. 2009). On binding to calcium, CaM wraps around the M13 peptide and blocks solvent access to the cpGFP chromophore, causing it to become brighter (Akerboom et al. 2009).
Until recently, GECIs have produced signals inferior to those of synthetic calcium dyes (Hendel et al. 2008; Palmer and Tsien 2006). However, recent advances in GECI biotechnology have led to sensors with signal-to-noise ratios (SNRs) that rival those of synthetic probes (Akerboom et al. 2012; Tian et al. 2009; Zhao et al. 2011). GECIs are also more photostable than their synthetic counterparts (Borghuis et al. 2011). They can be delivered to cells via electroporation, viral vectors, or generation of transgenic animals (Zariwala et al. 2012). Of these three methods, viral vectors have shown the greatest cellular specificity in the retina (Borghuis et al. 2011; Hellström et al. 2008).
Recombinant adeno-associated viral (AAV) vectors are most commonly used for retinal gene transfer due to their lack of pathogenicity and toxicity as well as their superior ability to target specific retinal cell types (Grimm and Kay 2003). AAV tropism is dictated by the viral capsid, which differs among serotypes (Hellström et al. 2008). By using various combinations of serotypes and promoters, Borghuis et al. (2011) delivered the GECI GCaMP3 (Tian et al. 2009) to the five major neuron classes in mouse retina. Intravitreal injection of AAV2/1-SYN1-GCaMP3 targeted RGCs with high specificity, although some horizontal cells were also labeled. In general, labeling was described as patchy, with >70% of RGCs in a patch showing GCaMP3 expression (Borghuis et al. 2011).
Of the many AAV serotypes that have been identified (Schmidt et al. 2008), AAV2 has been reported to be best for labeling RGCs (Auricchio et al. 2001; Hellström et al. 2008). AAV2 also transduces the greatest number of cells following intravitreal injection (Hellström et al. 2008). When coupled with the CAG promoter, AAV2 vectors can transduce ∼85% of RGCs in adult rat retina (Martin et al. 2002).
Based on these findings, we designed AAV2-CAG vectors incorporating the GECIs GCaMP3 (Tian et al. 2009) and GCaMP5G (Akerboom et al. 2012). We found these vectors to label the majority of RGCs in adult rat retina with high specificity. Subsequent electrical stimulation with MEAs evoked large increases in fluorescence intensity, much larger than we observed in amphibian retina with synthetic calcium dyes (Behrend et al. 2009). For the first time, we are able to visualize spatial patterns of RGC electrical activation in real-time. We demonstrate how our technique can be used to test and optimize stimulation strategies for epiretinal prostheses.
MATERIALS AND METHODS
Overview.
Adult rats received an intravitreal injection of an AAV vector encoding a GECI. Retinas were dissected out 2–4 wk later, placed ganglion cell-side-down on a transparent MEA, and maintained in heated, oxygenated saline. Stimuli were delivered through one or more electrodes while imaging evoked calcium transients with an inverted fluorescence microscope. All procedures were approved by the Institutional Animal Care and Use Committee and the Institutional Biosafety Committee at the University of Southern California (USC).
Generation of recombinant AAV vectors.
The pGFP plasmid (Klein et al. 2002; Wu et al. 2003) was used as a backbone for constructing two GECI-containing vectors. The GFP was replaced with the GECIs GCaMP3 (Tian et al. 2009) and GCaMP5G (Akerboom et al. 2012) to create pAAV-CAG-GCaMP3 and pAAV-CAG-GCaMP5G, respectively (Fig. 1). The resulting constructs contained the strong and ubiquitous CAG promoter (Niwa et al. 1991) followed by GCaMP3 or GCaMP5G cDNA. Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) was placed downstream of the transgene to enhance protein translation (Loeb et al. 1999). The entire cassette was flanked by AAV2 inverted terminal repeats.
Fig. 1.
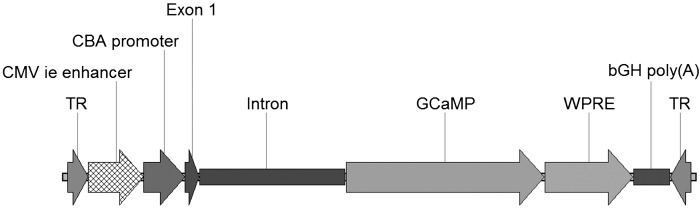
Map of pAAV2-CAG-GCaMP. The CMV enhancer, chicken β-actin promoter (CBA promoter), exon, and intron collectively form the CAG promoter. Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) is placed downstream of the GCaMP3/GCaMP5G transgene to increase protein translation. The cassette is flanked by adeno-associated virus type 2 (AAV2) inverted terminal repeats (TR). CMV ie enhancer, cytomegalovirus immediate-early enhancer; bGH poly(A), bovine growth hormone polyadenylation signal.
Recombinant AAV vectors were produced by the two-plasmid cotransfection method (Zolotukhin et al. 1999). Briefly, one CellSTACK (Corning, Corning, NY) with ∼1 × 109 HEK-293 cells was cultured in Dulbecco's modified Eagle's medium (HyClone Laboratories, Logan, UT) supplemented with 5% fetal bovine serum and antibiotics (cDMEM). A calcium phosphate precipitation transfection was set up by mixing a 1:1 molar ratio of vector plasmid DNA and a serotype-2-specific rep-cap helper plasmid, pDG. This precipitate was added to 1,100 ml of cDMEM, and the mixture was applied to the cell monolayer. The transfection was allowed to incubate at 37°C, 7% CO2, for 60 h. The cells were then harvested and lysed by three freeze/thaw cycles. The crude lysate was clarified by centrifugation, and the resulting vector-containing supernatant was divided among four discontinuous iodixanol step gradients. The gradients were centrifuged at 350,000 g for 1 h. Five milliliters of the AAV containing 40–60% interface was removed from each gradient and combined. This combined iodixanol fraction was further purified and concentrated by column chromatography on a 5-ml HiTrap Q Sepharose (anion exchange) column using an ÄKTA FPLC system (Pharmacia, Piscataway, NJ). The vector was eluted from the column using 215 mM NaCl, pH 8.0, and the AAV peak was collected. The AAV-containing fraction was then concentrated and buffer exchanged in Alcon Balanced Salt Solution (BSS) with 0.014% Polysorbate 20 using a Biomax 100K concentrator (Millipore, Billerica, MA). The AAV was titered for DNase-resistant vector genomes by real-time PCR relative to a standard. Purity of the final vectors was assayed by polyacrylamide gel electrophoresis to determine the fraction of total protein that was AAV viral capsids VP1, VP2, and VP3 (>95%). Final concentrations of AAV2-CAG-GCaMP3 and AAV2-CAG-GCaMP5G were 4.0 × 1012 and 3.2 × 1012 vector genomes per milliliter, respectively. AAV2/1-SYN1-GCaMP3 (University of Pennsylvania Vector Core, Philadelphia, PA), which was used in some experiments, had a concentration of 1.7 × 1013 vector genomes per milliliter. Viral stock was diluted in BSS before injection.
Intravitreal injections.
Adult female Long-Evans (Harlan Laboratories, Indianapolis, IN) rats, aged postnatal day 60-256, were anesthetized via intraperitoneal injection of ketamine (60 mg/kg) and xylazine (8 mg/kg). Phenylephrine (2.5%) and tropicamide (1%) were dropped into one eye to induce pupil dilation. Tetracaine (0.5%) was applied to the same eye as a local anesthetic. A 30-gauge needle was used to make a pilot hole through the sclera, choroid, and retina, 1–2 mm posterior to the corneal limbus. A microliter syringe (Hamilton, Reno, NV) attached to a blunt 32-gauge needle was used to inject 4–5 μl of virus into the vitreous. Care was taken to avoid injury of the lens. Injections were given slowly over a period of 30 s to allow diffusion of the virus. The needle was left in place for 30 s after the injection and withdrawn slowly to prevent leakage. The injection site was visualized with an ophthalmic microscope to confirm absence of cataract, intraocular bleeding, and air bubbles. Antibiotic eye ointment (neomycin and polymyxin B sulfates and bacitracin zinc) was applied to prevent infection.
Fluorescent dye loading.
Rats were deeply anesthetized with ketamine/xylazine and rapidly decapitated. The treated eye was enucleated and hemisected with dissection scissors. Vitreous was removed with a custom extractor (Sekirnjak et al. 2006) to allow for a tight interface between the retina and MEA. The red tracer dye Alexa Fluor 594 hydrazide, sodium salt (30 mM, 1.5 μl; Life Technologies, Grand Island, NY) was retrogradely loaded into RGCs via the cut optic nerve, as described previously (Behrend et al. 2009). This enabled us to determine whether GECI-expressing cells were RGCs or other cell types. Dye loading was performed at 30°C for 1 h while superfusing the eyecup at 4–5 ml/min.
Bicarbonate-buffered Ames' Medium (Sigma-Aldrich, St. Louis, MO) was used as superfusate in all experiments. Media was supplemented with penicillin-streptomycin to prevent bacterial growth, equilibrated with 5% CO2-95% O2 gas, and adjusted to pH 7.4 and 280 mOsm. Ames' Medium used before dye loading contained 2.5 mM MgCl2 and 5 mM EGTA to chelate calcium (Baldridge 1996) and prevent Ca2+-dependent resealing of the optic nerve (Yawo and Kuno 1985).
In experiments separate from those involving GECIs, we attempted retrograde loading of synthetic calcium dyes (Life Technologies) via the optic nerve. These experiments were performed in adult Long-Evans rats (Harlan Laboratories) and C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME). All dyes were dextran-conjugated potassium salts: 10-kDa fluo-4-dextran (20 mM), 10-kDa fura-dextran (20 mM), and 70-kDa Calcium Green-1-dextran (5 mM). An equimolar mixture (10 mM) of 10-kDa Oregon Green 488 BAPTA-1 (OGB-1)-dextran and 10-kDa Alexa Fluor 594-dextran was also applied in some experiments. Required loading times were predicted by a finite element model based off of the diffusion equation (Behrend et al. 2009). All 10-kDa dyes were loaded for 4–14 h. Calcium Green-1-dextran (70 kDa) was loaded for 15 h. Probenecid (2.5 mM) and sulfinpyrazone (250 μM) were added to the superfusate to inhibit organic anion transporter activity (Di Virgilio et al. 1988).
Imaging and electrophysiology.
After dye loading, the retina was removed from the eyecup and mounted on a porous membrane (cat. no. JVWP01300; Millipore). The retina was placed ganglion cell-side-down on a transparent MEA and imaged with an inverted epifluorescence microscope. Fluorescence excitation was provided by a super bright white light-emitting diode (LED) except for fura-dextran, which was excited by an ultraviolet LED. Excitation and emission light were filtered through Semrock (Rochester, NY) filter sets: cat. no. GFP-4050A for GCaMP3/GCaMP5G, cat. no. TXRED-4040B for Alexa Fluor 594, and cat. no. FITC-3540B for the remaining calcium dyes. Images were viewed through a Nikon (Tokyo, Japan) Plan Apo 0.75-numerical aperture (NA) ×20 or 0.45-NA ×10 objective and captured by an Andor Technology (Belfast, Northern Ireland) iXon electron-multiplied charge-coupled device (CCD) camera. The retina was continuously superfused during the course of each experiment at a flow rate of 4–5 ml/min and a temperature of 33°C.
We designed and fabricated MEAs at the W. M. Keck Photonics Laboratory at USC, a class 100 cleanroom. Arrays were patterned from indium tin oxide (ITO) on no. 1 cover glass substrates (Vaculayer, Mississauga, Ontario, Canada). A dual-insulation layer, consisting of silicon nitride and SU-8 epoxy photoresist (Gholmieh et al. 2006), was deposited atop the ITO. Vias were opened over the electrodes and contact pads on the perimeter of the substrate. Some electrodes were electroplated with platinum/iridium by cyclic voltammetry to increase charge injection limits.
Electrical stimuli consisted of a train of charge-balanced, biphasic current pulses or sinusoids. Voltage stimuli were output from a computer-controlled stimulus generator (Multi Channel Systems, Reutlingen, Germany) and fed through a custom voltage-to-current converter. Signals were relayed to MEA electrodes through a custom circuit board that interfaced with the electrode array. A platinum wire encircling the top of the recording chamber served as the return electrode.
All stimuli were repetitive to evoke a burst of spikes and generate a detectable calcium transient (Akerboom et al. 2012; Behrend et al. 2009; Borghuis et al. 2011; Tian et al. 2009). Rectangular pulse trains (167 or 333 Hz, 120- or 360-ms duration, cathodic-first) and sine waves (20 Hz, 200-ms duration, anodic-first) were applied in different experiments. Rectangular pulses consisted of a 60- or 400-μs cathodic phase followed by an anodic phase of twice the duration and half of the amplitude. (All stated amplitude and pulse width values are for the cathodic phase. For sinusoidal stimulation, current is specified in zero-to-peak amplitude.) Images were acquired at 5 or 10 Hz and synchronized with the onset of each stimulus. Kynurenic acid (1 mM), a broad-spectrum glutamate receptor antagonist, was applied in one experiment to isolate RGCs from bipolar cell input pharmacologically (Massey and Miller 1988).
Electrical thresholds of RGCs were measured as described previously (Behrend et al. 2009, 2011), with slight modifications. Stimuli were delivered 14 times on 3.6-s intervals and repeated over 10 monotonically increasing amplitudes. Electrically evoked responses were detected by convolving the fluorescence intensity of each ganglion cell body with a difference filter and identifying rapid changes in fluorescence temporally correlated with the stimuli. A dose-response curve was generated for every RGC by plotting the fraction of the 14 stimuli that elicited a response at each amplitude. A sigmoidal function was fit to this curve, and threshold was defined as the stimulation amplitude that yielded a 50% response. Threshold maps (Fig. 7) were generated by binning cells in a grid according to their spatial location relative to the stimulating electrode. Maps from separate retinas were rotated and shifted into the same reference frame (relative to the optic disc position). Thresholds of cells in each grid bin were averaged. Data processing was performed in ImageJ 1.43u (National Institutes of Health, Bethesda, MD) and MATLAB R2009b (The MathWorks, Natick, MA). Except for the identification of ganglion cell bodies, all processing steps were automated and identical for each data set. Statistical significance was determined with unpaired t-tests using a significance level of 5%.
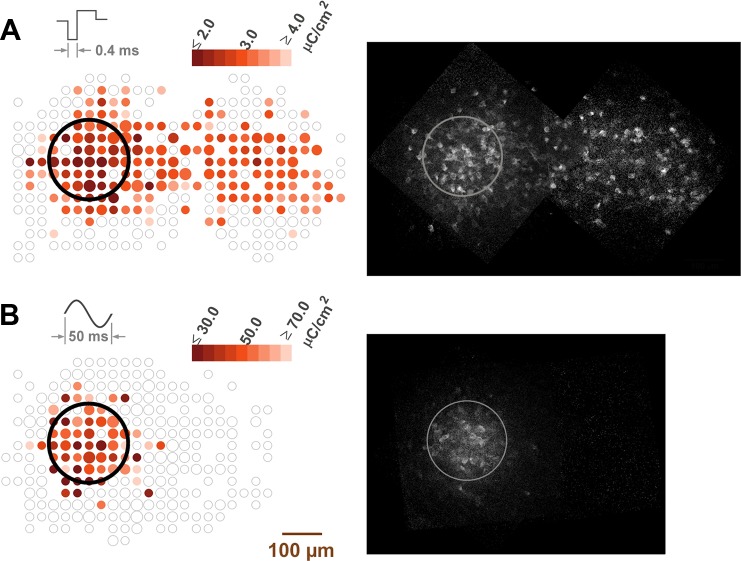
Fig. 7.
Left: threshold maps of RGCs in response to 167-Hz pulsatile stimulation with 400-μs pulses (A) and 20-Hz sinusoidal stimulation (B). Stimuli were delivered by a transparent 200-μm-diameter electrode (black circle). RGCs are drawn as colored circles according to their stimulus thresholds. Small, medium, and large circles indicate 1–2, 3–4, and 5+ cells, respectively. Data in A were generated from 2 retinas (682 somata), and data in B were generated from 3 retinas (686 somata). Maps from each retina were rotated and shifted into the same reference frame such that the optic disc lies to the left of each image. Cells were binned in a grid according to spatial location relative to the electrode, and thresholds in each bin were averaged. Right: background-subtracted fluorescence responses to the highest stimulus amplitude. Images from each retina were transformed to the same reference frame and averaged. Brightness and contrast were adjusted to accentuate the responses. The electrode perimeter is outlined in gray.
Histology.
Eyes treated with AAV were enucleated, and their anterior segments were carefully removed. Posterior eyecups were fixed in 4% paraformaldehyde in 1× PBS for 1 h. Following fixation, eyecups were washed three times in 1× PBS for 10 min each. Samples were transferred to 30% sucrose in 1× PBS and kept overnight at 4°C. Eyecups were then embedded in O.C.T. compound (Tissue-Tek; Sakura Finetek, Torrance, CA) and sectioned to a thickness of 8 μm. Sections were collected on slides and stored at −80°C until imaging. Before imaging, slides were thawed at room temperature and immersed in 1× PBS for 10 min. Nuclei were stained with ProLong Gold Antifade Reagent with 4′,6′-diamidino-2-phenylindole (DAPI; Life Technologies). Imaging was performed at the Doheny Eye Institute Specialized Microscopy Core on a Zeiss (Thornwood, NY) LSM 510 confocal laser scanning microscope equipped with a Plan-Neofluar 1.3-NA ×40 objective. GCaMP fluorescence was excited with a 488-nm argon laser and collected through a 505- to 530-nm band-pass filter. DAPI was excited at 800 nm by a Ti:sapphire laser and collected through a 390- to 465-nm band-pass filter.
RESULTS
Following intravitreal injection of AAV2-CAG vectors encoding for GECIs, ganglion cells began to show green fluorescence after 1 wk. Expression levels became strong after 2 wk, at which point retinas were harvested for experiments. Transduced retinas were dissected and mounted on a transparent MEA for imaging and electrophysiology.
GCaMP expression profiles.
Retinal whole mounts showed widespread GCaMP expression with anywhere from one-quarter to the entire whole mount exhibiting green fluorescence (Fig. 2). Ganglion cell bodies were generally brighter than their axons, which permitted imaging of somata through the superficial nerve fiber layer (Fig. 3A). When experiments were performed <4 wk postinjection, fluorescence remained predominantly localized to RGC cytoplasms (Fig. 3C, inset). As time progressed, baseline fluorescence increased and became apparent in ganglion cell nuclei, indicating GCaMP overexpression and cytomorbidity (Akerboom et al. 2012; Borghuis et al. 2011; Tian et al. 2009). Indeed, we found that very bright cells and/or ones with filled nuclei did not fire spikes or exhibit GCaMP fluorescence transients during electrical stimulation. To limit overexpression, viral stock was diluted in BSS before administration. Optimal dose was roughly 1–5 × 109 vector genomes per injection.
Fig. 2.
Retinal whole mount of an adult rat infected with AAV2-CAG-GCaMP3 (14 days postinjection). The retina was mounted on a multielectrode array and imaged with an inverted fluorescence microscope. GCaMP3 expression is visible throughout the ganglion cell layer (GCL). The mosaic was created by stitching together 88 ×10 images. The 2 black circles are 200-μm-diameter platinum/iridium electrodes.
Fig. 3.
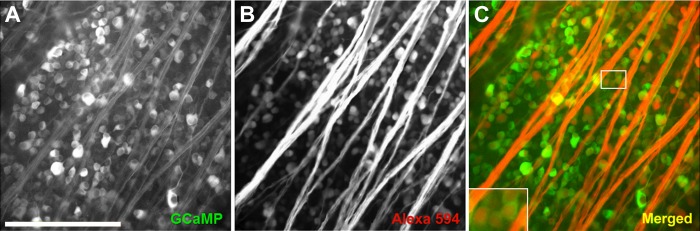
To investigate the specificity of the vector for transducing retinal ganglion cells (RGCs), ganglion cells were double-labeled with the red tracer dye Alexa Fluor 594. A: green fluorescence image of rat retina infected with AAV2-CAG-GCaMP3. Axons are weakly labeled. B: red fluorescence image of ganglion cells retrogradely loaded with Alexa Fluor 594 (same field of view as A). C: merging A and B reveals that the majority of RGCs are transduced with GCaMP3. The inset shows how GCaMP3 fluorescence is localized to the cytoplasm, a characteristic indicative of cells that have not been damaged by GCaMP overexpression (Akerboom et al. 2012; Borghuis et al. 2011; Tian et al. 2009). Scale bar is 200 μm.
Double-labeling RGCs with the red tracer dye Alexa Fluor 594 enabled us to determine whether transduced cells were RGCs or other cell types (Fig. 3). We compared expression profiles induced by our AAV2-CAG-GCaMP3 vector with those of AAV2/1-SYN1-GCaMP3, which labels mouse RGCs with high efficiency (Borghuis et al. 2011). Cell counting revealed that both vectors had a high specificity for RGCs. Although differences were not statistically significant, AAV2-CAG-GCaMP3 labeled more RGCs and less non-RGCs than AAV2/1-SYN1-GCaMP3 (Table 1). Roughly 83% of ganglion cells were transduced by AAV2-CAG-GCaMP3, which achieved a labeling density of 1,813 ± 343 cells/mm2. Similarly, Martin et al. (2002) found that AAV2-CAG-GFP targeted 84.5% of adult rat RGCs and labeled 1,828 ± 299 cells/mm2. Given that 85.3% of all AAV2-CAG-GCaMP3-labeled cells (i.e., ∼1,550 cells/mm2) were RGCs (Table 1), our counts are consistent with counts of approximately 1,500–1,600 RGCs per square millimeter obtained by FluoroGold backfilling through rat optic nerve and superior colliculus (Salinas-Navarro et al. 2009).
Table 1.
Percentage of RGCs and other retinal cells labeled with GCaMP3 following intravitreal injection
| AAV2-CAG | AAV2/1-SYN1 | P Value | |
|---|---|---|---|
| RGCs labeled, % | 82.9 ± 9.2 | 75.4 ± 7.5 | 0.14 |
| Labeled cells that were not RGCs, % | 14.7 ± 7.6 | 17.6 ± 3.9 | 0.39 |
| Labeled cell density, cells per square millimeter | 1,813 ± 343 | 1,553 ± 367 | 0.24 |
| No. counted cells | 2,714 | 1,492 |
Percentages indicate means ± SD. Cells were counted in regions with dense genetically encoded calcium indicator GCaMP3 labeling. We counted 8 400- × 400-μm regions from 2 retinas transduced with AAV2-CAG-GCaMP3 and 5 400- × 400-μm regions from 2 retinas transduced with AAV2/1-SYN1-GCaMP3. Retinal ganglion cells (RGCs) were identified after retrograde loading with Alexa Fluor 594. P values imply no significant differences between the labeling profile of each vector.
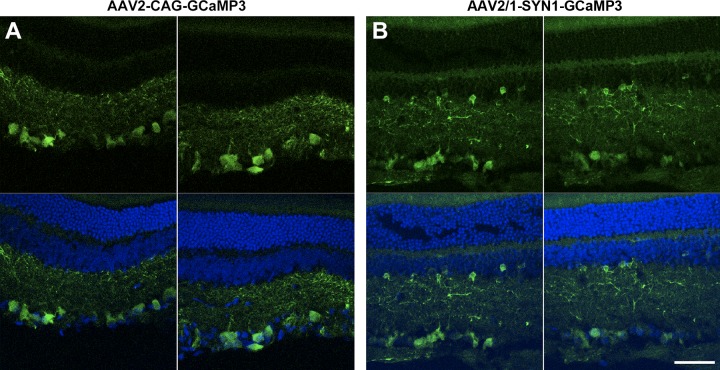
To determine which types of non-RGCs were targeted by AAV2-CAG-GCaMP3 and AAV2/1-SYN1-GCaMP3, we examined cross-sections of retina transduced by each vector. With rare exception, we found AAV2-CAG-GCaMP3 labeling to be confined to the ganglion cell layer (GCL), implying transduction of RGCs and displaced amacrine cells (Fig. 4A). In contrast, AAV2/1-SYN1-GCaMP3 expression often extended into the inner nuclear layer, where it labeled amacrine cells (Fig. 4B). Coupled with our double-labeling and cell counting results, these data indicate that the 14.7% of non-RGCs transduced by AAV2-CAG-GCaMP3 (Table 1) were displaced amacrine cells. Similarly, Harvey et al. (2002) found that 12–13% of all cells transduced by intravitreal injection of AAV2-CMV-GFP in adult rat were amacrine cells.
Fig. 4.
Representative sections of rat retina infected with AAV2-CAG-GCaMP3 (A) and AAV2/1-SYN1-GCaMP3 (B). AAV2-CAG-GCaMP3 labeling is limited to the GCL (RGCs and displaced amacrine cells), whereas AAV2/1-SYN1-GCaMP3 labeling extends into the inner nuclear layer (amacrine cells). Top images show GCaMP3 fluorescence; bottom images show GCaMP3 + 4′,6′-diamidino-2-phenylindole (DAPI) fluorescence. Scale bar is 50 μm.
GCaMP responses to electrical stimulation.
Electrical stimulation evoked large calcium transients that were clearly visible when viewed through the microscope. As shown in Fig. 5, burst stimulation with a 20-μm-diameter electrode elicited strong fluorescence responses in GCaMP3-expressing RGCs. A rapid train of 120 pulses (1.2 μA, 400-μs pulse width, 333 Hz) stimulated a bundle of passing axons, antidromically activating a streaklike pattern of RGC somata (see Supplemental Video S1 available in the data supplement online at the Journal of Neurophysiology web site). Normalized change in fluorescence (ΔF/F) for responding cells was 75.9 ± 21.5% (n = 34). Application of 1 mM CdCl2 to the superfusate abolished all fluorescence signals within minutes.
Fig. 5.
Electrical stimulation activates a pattern of RGCs as revealed through large changes in GCaMP3 fluorescence intensity (see Supplemental Video S1). A: before stimulation, cells are at baseline fluorescence. B: delivering a burst of 120 suprathreshold pulses from a 20-μm-diameter electrode (arrows) causes electrically activated cells to become visibly brighter. C: image subtraction of A from B highlights the pattern of responding cells. Stimulation with 400-μs pulses activates an axon bundle, causing a streaklike antidromic response that extends away from the optic disc. Average normalized change in fluorescence (ΔF/F) for responding RGCs was 75.9 ± 21.5% (n = 34). Scale bar is 100 μm.
We compared calcium transients from GCaMP3-, GCaMP5G-, and Oregon Green-labeled RGCs by delivering bursts of 40 suprathreshold pulses (60-μs pulse width, 333 Hz) through a 200-μm-diameter electrode (Fig. 6). GCaMP3 fluorescence responses in rat RGCs were similar in amplitude to Oregon Green responses in salamander RGCs (Behrend et al. 2009) but were generally less noisy. GCaMP5G signals in rat were roughly 3–4 times larger than those of GCaMP3. After rising to full magnitude, GCaMP3 and GCaMP5G fluorescence decayed with time constants of 0.91 s ± 0.10 SE (n = 24) and 0.50 s ± 0.03 SE (n = 66), respectively. Single stimulus pulses never elevated GCaMP5G fluorescence above the baseline noise level; a rapid burst of 5–10 suprathreshold pulses was needed. For RGCs expressing GCaMP3, 20–30 pulses were needed to generate a detectable calcium transient. Because of the short pulse width (60 μs), it is likely that each stimulus pulse elicited one or two action potentials per RGC (Ahuja et al. 2008; Freeman et al. 2011; Jensen et al. 2005; Sekirnjak et al. 2006).
Fig. 6.
ΔF/F plotted for RGCs in response to repeated stimuli (tick marks). Each stimulus was a rapid burst of 40 suprathreshold pulses. The bottom traces are from salamander RGCs (n = 34) labeled with 10-kDa Oregon Green 488 BAPTA-1 (OGB-1)-dextran (Behrend et al. 2009). The middle traces (n = 24) and top traces (n = 66) are from rat RGCs labeled with GCaMP3 and GCaMP5G, respectively. GCaMP3 and Oregon Green signals are similar in amplitude, whereas GCaMP5G provides a 3–4× improvement in ΔF/F.
We gathered statistics about the properties of electrically responsive cells across seven GCaMP5G-labeled retinas. Retinas were stimulated with a transparent 200-μm-diameter electrode, and only RGCs lying directly above the electrode were used for analysis (n = 418). In response to high-amplitude stimulation, 82.3 ± 10.5% of cells responded with detectable calcium transients (stimulus amplitude was 2–3 times greater than the mean threshold of responding RGCs). For responding cells, there was little to no correlation between baseline fluorescence, F0, and response magnitude, ΔF = Fstim − F0 (correlation coefficient = 0.19 ± 0.24). Within each retina, baseline fluorescence of RGC somata was highly uniform (mean coefficient of variation = 12.4 ± 4.9%).
To investigate whether GCaMP overexpression (i.e., very bright baseline fluorescence) was the cause of nonresponsiveness, we compared F0 of responding cells with that of nonresponding ones. Baseline fluorescence between these two populations varied by only 1.0 ± 5.9% (P = 0.67), indicating that cytomorbidity was not the primary cause. Indeed, cells that were bright (F0 > 1 SD from the mean) and not responsive made up only 3.1% of the total population.
Stimulus threshold mapping.
Previous studies have suggested that pulse shape affects the target of epiretinal electrical stimulation: short pulses excite RGCs directly, whereas longer pulses target bipolar cells (Freeman et al. 2010; Greenberg 1998; Jensen et al. 2005; Margalit and Thoreson 2006; Shah et al. 2006). By avoiding RGC axon bundles, long pulses can potentially confine the response area to the site of stimulation. This would have implications for epiretinal prostheses, which currently face the potential problem of stimulating RGC axons (Nanduri et al. 2011).
Because prior studies have relied on single-unit recordings, they were unable to quantify the spatial extent of RGC activation. To investigate this, we mapped RGC thresholds in response to stimulation with two markedly different pulse shapes: 400-μs rectangular pulses and 20-Hz sine waves. As shown in Fig. 7A, 400-μs pulses activated a streak of RGC somata, the path of which followed the trajectory of their axons. (Although not shown in the figure, this streak extended to the edge of the retina.) Mean RGC threshold directly under the electrode was 2.6 ± 0.5 μC/cm2. The shape of the response map resembles those of prior calcium imaging studies by our group in salamander retina (Behrend et al. 2009, 2011).
In contrast to short, rectangular pulses, 20-Hz sine waves produced focal activation of RGC somata (Fig. 7B). Responding cells were largely confined to the vicinity of the electrode. Mean threshold charge density was 49.3 ± 18.5 μC/cm2, much higher than for 400-μs pulses. Sine-wave thresholds increased by 32.7 ± 4.1% SE (n = 31; P < 0.001) in the presence of kynurenic acid, suggesting that responses were mediated in part by inner retinal stimulation. Comparatively, Freeman et al. (2010) found that low-frequency sinusoids (10–25 Hz) preferentially activated bipolar cells and avoided passing axons.
Effects of rat strain and temperature.
All data reported in this study were collected from Long-Evans rats, an outbred strain. Injecting AAV2-CAG-GCaMP into the vitreous of inbred Copenhagen rats also led to widespread RGC transduction. However, GCaMP-induced cytomorbidity (Borghuis et al. 2011; Tian et al. 2009) was much more pronounced in Copenhagen retina, especially at higher temperatures. At 23°C, Copenhagen RGCs showed very dim baseline fluorescence. Electrical stimulation evoked calcium transients in these cells, but fluorescence changes were much smaller than those of Long-Evans. When bath temperature was increased to 28–30°C, Copenhagen RGCs became very bright and stopped responding to stimulation. This was accompanied by cessation of spontaneous spiking and a gradual increase in nuclear fluorescence. Reducing GCaMP expression levels with lower viral titers did not eliminate cytomorbidity. For experiments in Long-Evans rats, temperature had little to no effect on calcium transients (although higher temperatures sometimes led to increased basal fluorescence, especially with higher viral doses).
Synthetic dye loading.
Before employing GECIs, we attempted to load synthetic calcium dyes down the cut optic nerve. Previous attempts with 10-kDa OGB-1-dextran in adult rat were unsuccessful, presumably owing to dye extrusion by organic anion transporters (Behrend et al. 2009). In this study, we tried loading other dextran-conjugated calcium dye variants in both adult rats and mice. Experiments were performed in the presence of the organic anion transporter blockers probenecid and sulfinpyrazone.
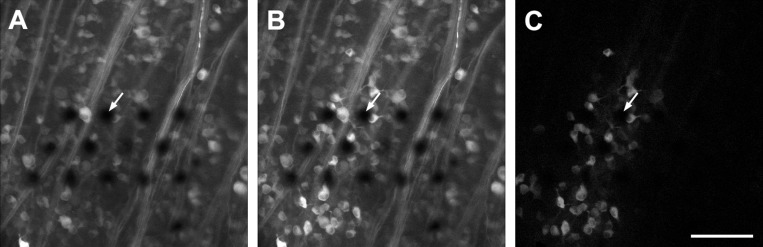
As a control experiment, we applied an equimolar mixture of 10-kDa Alexa Fluor 594-dextran and 10-kDa OGB-1-dextran to the cut optic nerve. The weakly charged Alexa tracer was retained by RGC somata (Fig. 8A), but the highly charged Oregon Green was not (Fig. 8B), further supporting the hypothesis that organic anion transporters are responsible for calcium dye extrusion. Attempts to load 10-kDa fluo-4-dextran (Fig. 8C) and 10-kDa fura-dextran (Fig. 8D) were also unsuccessful. Reasoning that a heavier dye might be more resistant to externalization, we tried loading 70-kDa Calcium Green-1-dextran; however, this dye was not retained (Fig. 8E).
Fig. 8.
Attempts to load dextran-conjugated calcium dyes via the cut optic nerve were unsuccessful in adult rat. Retrograde loading with an equimolar mixture of 10-kDa Alexa Fluor 594-dextran and 10-kDa OGB-1-dextran led to retention of the Alexa dye (A) but not Oregon Green (B). Very weak OGB-1 staining can be seen in some RGC somata, but we could not evoke calcium transients in these cells. Ten-kilodalton fluo-4-dextran (C), 10-kDa fura-dextran (D), and 70-kDa Calcium Green-1-dextran (E) were also not retained by adult rat RGCs even in the presence of probenecid and sulfinpyrazone. Results in adult mice were similar. Scale bars are 100 μm.
DISCUSSION
We have described an optical technique to record from populations of mammalian RGCs during electrical stimulation. By relying on calcium imaging, rather than traditional electrical recording approaches, we can identify the precise location of every activated neuron, including those distant from the stimulation site. Furthermore, patterns of electrical activity can be visualized in real-time due to the large fluorescence transients produced by GCaMP3 and GCaMP5G.
Comparison with other calcium indicator loading techniques.
A number of studies have focused on bulk-labeling RGCs with fluorescent calcium dyes. Early protocols utilized membrane-permeant dye loading or biolistic delivery (via gene gun), although these methods have significant drawbacks: membrane-permeant dyes do not load well in adult retina and lack cell-type specificity (Morgan et al. 2005). Biolistic delivery is also not cell-type specific and results in sparse labeling (Kettunen et al. 2002; Roizenblatt et al. 2006). In search of a new method, our group found that dextran-conjugated calcium dyes could be loaded into ganglion cell populations through optic nerve backfilling (Behrend et al. 2009). This technique labeled nearly all RGCs with complete specificity yet was ineffective in mature mammalian retina. We have since tested other calcium dye variants, including heavier dextran conjugates, in both adult rat and mouse (Fig. 8). None were retained to a level sufficient for detecting fluorescence transients.
To date, only one group has reported a method for bulk-loading mature mammalian RGCs with calcium dye (Briggman and Euler 2011). Their approach relies on in vitro electroporation and is able to stain the entire GCL. However, displaced amacrine cells, which constitute roughly 50–60% of cells in the rodent GCL (Jeon et al. 1998; Perry 1981), are labeled indiscriminately. Because we are interested in recording RGC responses to electrical stimulation (since RGC activity defines retinal output), we wanted a method for labeling populations of RGCs preferentially over other cell types. This led us to investigate GECIs, which have become an attractive alternative to synthetic dyes. Recent advancements in GECI engineering have led to novel proteins (Akerboom et al. 2012; Zhao et al. 2011) that exceed small molecule dyes in terms of SNR and photostability (Borghuis et al. 2011).
Various approaches have been developed for loading GECI genetic material into RGCs. In vivo (Dezawa et al. 2002; Mo et al. 2002) and in utero (Garcia-Frigola et al. 2007) electroporation can transfect RGC populations, but the extent of labeling is limited and is not RGC-specific. Transgenic mouse lines offer an alternative means to express GECIs in neuronal populations. The Pvalb-2A-Cre:Ai38 line, for example, expresses GCaMP3 in RGCs, horizontal cells, and Müller glia (Zariwala et al. 2012). Because expression levels remain stable without increases over time, GCaMP3-induced cytomorbidity does not occur in these mice, even after several months. However, the lack of a pan-ganglion-cell-specific promoter makes it difficult to generate a mouse line that expresses GCaMP exclusively in RGCs (Feng et al. 2000). Furthermore, establishing stable transgenic lines is costly and takes many months, making it impractical to incorporate new GECIs as they become available. Viral vectors overcome these limitations. They can be designed to target specific neuronal classes and produced within a matter of weeks. We chose to use AAV because it is easily administered (via intravitreal injection), is not pathogenic, and can be designed to target the overwhelming majority of RGCs in mammalian retina (Martin et al. 2002).
The AAV2-CAG-GCaMP vectors we generated in this study labeled ∼83% of adult rat RGCs while causing minimal expression in other cell types (Table 1). We found that AAV2-CAG-GCaMP3 transduced more RGCs and fewer non-RGCs than AAV2/1-SYN1-GCaMP3, which targets mouse RGCs with high specificity (Borghuis et al. 2011). This was not unexpected, given that prior studies have reported superior RGC labeling with AAV2 vs. AAV2/1 (Auricchio et al. 2001; Hellström et al. 2008). Although both vectors we tested contained different promoters, CAG and synapsin-1 are both strong promoters that produce similar amounts of transgene expression (Shevtsova et al. 2005). Furthermore, the extent of retinal transduction is limited by the ability of the AAV particles to penetrate the retina, which is determined by the capsid serotype, not the promoter (Dalkara et al. 2009; Petrs-Silva et al. 2010).
Effects of rat strain and temperature.
We were surprised to find that GCaMP expression led to different phenotypes in different rat strains. GCaMP-induced cytomorbidity was much more pronounced in Copenhagen RGCs than in Long-Evans. We believe this behavior may be attributed to differences in genetic background: Long-Evans is an outbred strain, whereas Copenhagens are inbred. Prior studies have reported problems with inbred lines that do not occur with outbreds, possibly due to deleterious homozygous recessive alleles that result from inbreeding. For example, Carlson et al. (1997) found that overexpressing Alzheimer amyloid precursor protein (Kang et al. 1987) was lethal to inbred mice but not to outbreds. Similarly, Kinney and Sidman (1986) found that a mutation causing spongiform encephalopathy killed inbred mice within 3 mo but was well-tolerated by outbred strains. Although the focus of these studies is unrelated, their findings demonstrate how phenotype can be affected by genetic background. We also observed that higher temperatures exacerbated the effects of cytomorbidity, especially in Copenhagen rats. This resembles a finding by Newman (2003) in which temperatures above 24°C altered calcium fluorescence in rat retina and caused deterioration of retinal function.
Limitations.
Despite the relative ease of AAV vector production and delivery to the eye, GCaMP-induced cytomorbidity limits the types of experiments that can be performed. In agreement with another study, we found that retinas harvested after ∼4 wk exhibited abnormal cellular physiology (Borghuis et al. 2011). This has been suggested to arise from GCaMP overexpression and interaction of the sensor CaM and/or M13 motifs with endogenous proteins (Hasan et al. 2004). It may be possible to limit overexpression by systemically administering hyperosmotic mannitol before AAV injection (Burger et al. 2005; Kuhn et al. 2012), although this might also cause GCaMP expression to extend beyond the GCL.
Because the degree of cytomorbidity varies with rat strain, investigators wishing to use a different mammalian species or rat strain (other than Long-Evans) should test for cytomorbidity before proceeding with experiments. Since GCaMP expression will eventually reach a level that is toxic to cells (after a few weeks), our method does not permit chronic in vivo imaging. Chronic experiments would require the use of transgenic GECI knockins, as expression levels in these animals remain stable over time (Zariwala et al. 2012).
Future applications.
Although we were unable to detect single action potentials in GECI-expressing RGCs, future generations of calcium indicators may overcome this limitation. GECIs still lag behind synthetic dyes in terms of detecting sparse neural activity (Akerboom et al. 2012). However, given the rate at which calcium indicator technology is progressing (Looger and Griesbeck 2011), measurement of single spikes may soon become possible. This can already be accomplished with genetically encoded voltage sensors (Kralj et al. 2011), although the fast signals of those sensors make it difficult to image simultaneous activity from neuronal populations.
In this study, we confirmed that changing the stimulus pulse shape can dramatically alter the pattern of electrically activated RGCs. Importantly, 20-Hz sine waves avoid axon bundles and stimulate cells only near the electrode. This may have implications for epiretinal prostheses, which currently face the problem of stimulating RGC axons (Nanduri et al. 2011). Since these prostheses operate on diseased retina, it would be helpful to understand how retinal degeneration affects the spatial patterns of RGC activation. This could be investigated in transgenic rodent models (Chader 2002; Marc et al. 2003) such as the S334ter-line-3 rat model of retinitis pigmentosa (Steinberg et al. 1996). Despite extensive retinal remodeling that occurs during degeneration (Jones and Marc 2005), AAV2 transduction profiles in S334ter-line-3 retina are similar to those of wild-type rats (Kolstad et al. 2010).
GRANTS
This work was supported in part by the National Science Foundation (Grant EEC-0310723 to M. S. Humayun), the National Eye Institute (Grant EY-03040 to the Doheny Eye Institute), the National Institute of General Medical Sciences (Grant GM-85791 to R. H. Chow), and the National Institute of Diabetes and Digestive and Kidney Diseases (Grant DK-091445 to R. H. Chow).
DISCLOSURES
M. S. Humayun has a financial interest in Second Sight Medical Products, Inc. Although no Second Sight products were used in the study, we did perform basic research on electrical stimulation of the retina.
W. W. Hauswirth and the University of Florida have a financial interest in the use of AAV therapies and own equity in a company (AGTC, Inc.) that might, in the future, commercialize some aspects of this work.
AUTHOR CONTRIBUTIONS
A.C.W. performed the experiments, analyzed the data, and wrote the manuscript; M.R.B. developed many of the experimental procedures; N.S.L. and R.L.K. aided with construction of the viral plasmid; V.A.C. and W.W.H. produced the AAV vectors; M.S.H., J.D.W., and R.H.C. oversaw the research and provided experimental guidance.
Supplementary Material
ACKNOWLEDGMENTS
GCaMP3 and GCaMP5G cDNA were gracious gifts from Loren L. Looger and Jasper Akerboom (Howard Hughes Medical Institute, Ashburn, VA). We thank Artin Petrossians for providing electroplating solution and Xiaopeng Wang for assistance with histology. We also thank A. P. Sampath, Eun-Jin (Grace) Lee, Bart Borghuis, Keith Martin, Ed Callaway, Chris Sekirnjak, and Clare Hulse for their helpful advice.
REFERENCES
- Ahuja AK, Behrend MR, Kuroda M, Humayun MS, Weiland JD. An in vitro model of a retinal prosthesis. IEEE Trans Biomed Eng 55: 1744–1753, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr R, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SS, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci 32: 13819–13840, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom J, Rivera JD, Guilbe MM, Malavé EC, Hernandez HH, Tian L, Hires SA, Marvin JS, Looger LL, Schreiter ER. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J Biol Chem 284: 6455–6464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auricchio A, Kobinger G, Anand V, Hildinger M, O'Connor E, Maguire AM, Wilson JM, Bennett J. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet 10: 3075–3081, 2001 [DOI] [PubMed] [Google Scholar]
- Baldridge WH. Optical recordings of the effects of cholinergic ligands on neurons in the ganglion cell layer of mammalian retina. J Neurosci 16: 5060–5072, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrend MR, Ahuja AK, Humayun MS, Chow RH, Weiland JD. Resolution of the epiretinal prosthesis is not limited by electrode size. IEEE Trans Neural Syst Rehabil Eng 19: 436–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrend MR, Ahuja AK, Humayun MS, Weiland JD, Chow RH. Selective labeling of retinal ganglion cells with calcium indicators by retrograde loading in vitro. J Neurosci Methods 179: 166–172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Tian L, Xu Y, Nikonov SS, Vardi N, Zemelman BV, Looger LL. Imaging light responses of targeted neuron populations in the rodent retina. J Neurosci 31: 2855–2867, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Euler T. Bulk electroporation and population calcium imaging in the adult mammalian retina. J Neurophysiol 105: 2601–2609, 2011 [DOI] [PubMed] [Google Scholar]
- Burger C, Nguyen FN, Deng J, Mandel RJ. Systemic mannitol-induced hyperosmolality amplifies rAAV2-mediated striatal transduction to a greater extent than local co-infusion. Mol Ther 11: 327–331, 2005 [DOI] [PubMed] [Google Scholar]
- Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG. Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet 6: 1951–1959, 1997 [DOI] [PubMed] [Google Scholar]
- Chader GJ. Animal models in research on retinal degenerations: past progress and future hope. Vision Res 42: 393–399, 2002 [DOI] [PubMed] [Google Scholar]
- Dalkara D, Kolstad KD, Caporale N, Visel M, Klimczak RR, Schaffer DV, Flannery JG. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther 17: 2096–2102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezawa M, Takano M, Negishi H, Mo X, Oshitari T, Sawada H. Gene transfer into retinal ganglion cells by in vivo electroporation: a new approach. Micron 33: 1–6, 2002 [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Fasolato C, Steinberg TH. Inhibitors of membrane transport system for organic anions block fura-2 excretion from PC12 and N2A cells. Biochem J 256: 959–963, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eversmann B, Jenkner M, Hofmann F, Paulus C, Brederlow R, Holzapfl B, Fromherz P, Merz M, Brenner M, Schreiter M. A 128 × 128 CMOS biosensor array for extracellular recording of neural activity. IEEE J Solid-State Circuits 38: 2306–2317, 2003 [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: 41–51, 2000 [DOI] [PubMed] [Google Scholar]
- Ferrea E, Maccione A, Medrihan L, Nieus T, Ghezzi D, Baldelli P, Benfenati F, Berdondini L. Large-scale, high-resolution electrophysiological imaging of field potentials in brain slices with microelectronic multielectrode arrays. Front Neural Circuits 6: 1–14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Gauthier JL, Sher A, Greschner M, Machado TA, Jepson LH, Shlens J, Gunning DE, Mathieson K, Dabrowski W. Functional connectivity in the retina at the resolution of photoreceptors. Nature 467: 673–677, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DK, Eddington DK, Rizzo JF, Fried SI. Selective activation of neuronal targets with sinusoidal electric stimulation. J Neurophysiol 104: 2778–2791, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DK, Rizzo JF, Fried SI. Encoding visual information in retinal ganglion cells with prosthetic stimulation. J Neural Eng 8: 035005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Frigola C, Carreres M, Vegar C, Herrera E. Gene delivery into mouse retinal ganglion cells by in utero electroporation. BMC Dev Biol 7: 103, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholmieh G, Soussou W, Han M, Ahuja A, Hsiao MC, Song D, Tanguay AR, Jr, Berger TW. Custom-designed high-density conformal planar multielectrode arrays for brain slice electrophysiology. J Neurosci Methods 152: 116–129, 2006 [DOI] [PubMed] [Google Scholar]
- Greenberg RJ. Analysis of electrical stimulation of the vertebrate retina: work towards a retinal prosthesis. In: The Wilmer Ophthalmological Institute. Baltimore, MD: The Johns Hopkins Univ., 1998 [Google Scholar]
- Grimm D, Kay MA. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther 3: 281–304, 2003 [DOI] [PubMed] [Google Scholar]
- Grumet AE, Wyatt JL, Rizzo JF. Multi-electrode stimulation and recording in the isolated retina. J Neurosci Methods 101: 31–42, 2000 [DOI] [PubMed] [Google Scholar]
- Harvey AR, Kamphuis W, Eggers R, Symons NA, Blits B, Niclou S, Boer GJ, Verhaagen J. Intravitreal injection of adeno-associated viral vectors results in the transduction of different types of retinal neurons in neonatal and adult rats: a comparison with lentiviral vectors. Mol Cell Neurosci 21: 141–157, 2002 [DOI] [PubMed] [Google Scholar]
- Hasan MT, Friedrich RW, Euler T, Larkum ME, Giese G, Both M, Duebel J, Waters J, Bujard H, Griesbeck O. Functional fluorescent Ca2+ indicator proteins in transgenic mice under TET control. PLoS Biol 2: 0763–0775, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström M, Ruitenberg MJ, Pollett MA, Ehlert EM, Twisk J, Verhaagen J, Harvey AR. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Ther 16: 521–532, 2008 [DOI] [PubMed] [Google Scholar]
- Hendel T, Mank M, Schnell B, Griesbeck O, Borst A, Reiff DF. Fluorescence changes of genetic calcium indicators and OGB-1 correlated with neural activity and calcium in vivo and in vitro. J Neurosci 28: 7399–7411, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayun MS, Dorn JD, da Cruz L, Dagnelie G, Sahel JA, Stanga PE, Cideciyan AV, Duncan JL, Eliott D, Filley E, Ho AC, Santos A, Safran AB, Arditi A, Del Priore LV, Greenberg RJ; Argus II Study Group Interim results from the international trial of Second Sight's visual prosthesis. Ophthalmology 119: 779–788, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler M, Lambacher A, Eversmann B, Jenkner M, Thewes R, Fromherz P. High-resolution multitransistor array recording of electrical field potentials in cultured brain slices. J Neurophysiol 96: 1638–1645, 2006 [DOI] [PubMed] [Google Scholar]
- Jensen RJ, Ziv OR, Rizzo JF. Thresholds for activation of rabbit retinal ganglion cells with relatively large, extracellular microelectrodes. Invest Ophthalmol Vis Sci 46: 1486–1496, 2005 [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci 18: 8936–8946, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res 81: 123–137, 2005 [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736, 1987 [DOI] [PubMed] [Google Scholar]
- Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med 7: 33–40, 2001 [DOI] [PubMed] [Google Scholar]
- Kettunen P, Demas J, Lohmann C, Kasthuri N, Gong Y, Wong RO, Gan WB. Imaging calcium dynamics in the nervous system by means of ballistic delivery of indicators. J Neurosci Methods 119: 37–43, 2002 [DOI] [PubMed] [Google Scholar]
- Kinney HC, Sidman RL. Pathology of the spongiform encephalopathy in the gray tremor mutant mouse. J Neuropathol Exp Neurol 45: 108–126, 1986 [DOI] [PubMed] [Google Scholar]
- Klein RL, Hamby ME, Gong Y, Hirko AC, Wang S, Hughes JA, King MA, Meyer EM. Dose and promoter effects of adeno-associated viral vector for green fluorescent protein expression in the rat brain. Exp Neurol 176: 66–74, 2002 [DOI] [PubMed] [Google Scholar]
- Kolstad KD, Dalkara D, Guerin K, Visel M, Hoffmann N, Schaffer DV, Flannery JG. Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Hum Gene Ther 21: 571–578, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D, Cohen AE. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat Methods 9: 90–95, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B, Ozden I, Lampi Y, Hasan MT, Wang SS. An amplified promoter system for targeted expression of calcium indicator proteins in the cerebellar cortex. Front Neural Circuits 6: 1–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litke AM, Bezayiff N, Chichilnisky EJ, Cunningham W, Dabrowski W, Grillo AA, Grivich M, Grybos P, Hottowy P, Kachiguine S. What does the eye tell the brain?: development of a system for the large-scale recording of retinal output activity. IEEE Trans Nucl Sci 51: 1434–1440, 2004 [Google Scholar]
- Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ. Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther 10: 2295–2305, 1999 [DOI] [PubMed] [Google Scholar]
- Looger LL, Griesbeck O. Genetically encoded neural activity indicators. Curr Opin Neurobiol 22: 18–23, 2011 [DOI] [PubMed] [Google Scholar]
- Mank M, Griesbeck O. Genetically encoded calcium indicators. Chem Rev 108: 1550–1564, 2008 [DOI] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res 22: 607–655, 2003 [DOI] [PubMed] [Google Scholar]
- Margalit E, Thoreson WB. Inner retinal mechanisms engaged by retinal electrical stimulation. Invest Ophthalmol Vis Sci 47: 2606–2612, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods 28: 267–275, 2002 [DOI] [PubMed] [Google Scholar]
- Massey SC, Miller RF. Glutamate receptors of ganglion cells in the rabbit retina: evidence for glutamate as a bipolar cell transmitter. J Physiol 405: 635–655, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X, Yokoyama A, Oshitari T, Negishi H, Dezawa M, Mizota A, Adachi-Usami E. Rescue of axotomized retinal ganglion cells by BDNF gene electroporation in adult rats. Invest Ophthalmol Vis Sci 43: 2401–2405, 2002 [PubMed] [Google Scholar]
- Morgan J, Huckfeldt R, Wong RO. Imaging techniques in retinal research. Exp Eye Res 80: 297–306, 2005 [DOI] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol 19: 137–141, 2001 [DOI] [PubMed] [Google Scholar]
- Nanduri D, Fine I, Greenberg R, Horsager A, Boynton GM, Weiland J. Predicting the percepts of electrical stimulation in retinal prosthesis subjects (Abstract). In: Cosyne 2011. Salt Lake City, UT: 2011 [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci 23: 1659–1666, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Ken-ichi Y, Jun-ichi M. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108: 193–199, 1991 [DOI] [PubMed] [Google Scholar]
- Palmer AE, Tsien RY. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc 1: 1057–1065, 2006 [DOI] [PubMed] [Google Scholar]
- Perry VH. Evidence for an amacrine cell system in the ganglion cell layer of the rat retina. Neuroscience 6: 931–944, 1981 [DOI] [PubMed] [Google Scholar]
- Petrs-Silva H, Dinculescu A, Li Q, Deng WT, Pang J, Min SH, Chiodo V, Neeley AW, Govindasamy L, Bennett A. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther 19: 293–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizenblatt R, Weiland JD, Carcieri S, Qiu G, Behrend M, Humayun MS, Chow RH. Nanobiolistic delivery of indicators to the living mouse retina. J Neurosci Methods 153: 154–161, 2006 [DOI] [PubMed] [Google Scholar]
- Salinas-Navarro M, Mayor-Torroglosa S, Jimenez-Lopez M, Avilés-Trigueros M, Holmes TM, Lund RD, Villegas-Pérez MP, Vidal-Sanz M. A computerized analysis of the entire retinal ganglion cell population and its spatial distribution in adult rats. Vision Res 49: 115–126, 2009 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid-and heparan sulfate proteoglycan-independent transduction activity. J Virol 82: 1399–1406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev R, Goodhouse J, Puchalla J, Berry MJ. Recording spikes from a large fraction of the ganglion cells in a retinal patch. Nat Neurosci 7: 1155–1162, 2004 [DOI] [PubMed] [Google Scholar]
- Sekirnjak C, Hottowy P, Sher A, Dabrowski W, Litke A, Chichilnisky E. Electrical stimulation of mammalian retinal ganglion cells with multielectrode arrays. J Neurophysiol 95: 3311–3327, 2006 [DOI] [PubMed] [Google Scholar]
- Sekirnjak C, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. High-resolution electrical stimulation of primate retina for epiretinal implant design. J Neurosci 28: 4446–4456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah HA, Montezuma SR, Rizzo JF. In vivo electrical stimulation of rabbit retina: effect of stimulus duration and electrical field orientation. Exp Eye Res 83: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- Shevtsova Z, Malik JM, Michel U, Bähr M, Kügler S. Promoters and serotypes: targeting of adeno-associated virus vectors for gene transfer in the rat central nervous system in vitro and in vivo. Exp Physiol 90: 53–59, 2005 [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Flannery JG, Naash M, Oh P, Matthes MT, Yasumura D, Lau-Villacorta C, Chen J, LaVail MM. Transgenic rat models of inherited retinal degeneration caused by mutant opsin genes. Invest Ophthalmol Vis Sci 37: S698, 1996 [Google Scholar]
- Stett A, Barth W, Weiss S, Haemmerle H, Zrenner E. Electrical multisite stimulation of the isolated chicken retina. Vision Res 40: 1785–1795, 2000 [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 6: 875–881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Klein RL, Meyers CA, King MA, Hughes JA, Millard WJ, Meyer EM. Long-term neuronal effects and disposition of ectopic preproNGF gene transfer into the rat septum. Hum Gene Ther 14: 1463–1472, 2003 [DOI] [PubMed] [Google Scholar]
- Yawo H, Kuno M. Calcium dependence of membrane sealing at the cut end of the cockroach giant axon. J Neurosci 5: 1626–1632, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Daniels BA, Baldridge WH. Slow excitation of cultured rat retinal ganglion cells by activating group I metabotropic glutamate receptors. J Neurophysiol 102: 3728–3739, 2009 [DOI] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen TW. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci 32: 3131–3141, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T. An expanded palette of genetically encoded Ca2+ indicators. Science 333: 1888–1891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther 6: 973–985, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.