Abstract
Locomotor adaptation in humans is not well understood. To provide insight into the neural reorganization that occurs following a significant disruption to one's learned neuromuscular map relating a given motor command to its resulting muscular action, we tied the mechanical action of a robotic exoskeleton to the electromyography (EMG) profile of the soleus muscle during walking. The powered exoskeleton produced an ankle dorsiflexion torque proportional to soleus muscle recruitment thus limiting the soleus' plantar flexion torque capability. We hypothesized that neurologically intact subjects would alter muscle activation patterns in response to the antagonistic exoskeleton by decreasing soleus recruitment. Subjects practiced walking with the exoskeleton for two 30-min sessions. The initial response to the perturbation was to “fight” the resistive exoskeleton by increasing soleus activation. By the end of training, subjects had significantly reduced soleus recruitment resulting in a gait pattern with almost no ankle push-off. In addition, there was a trend for subjects to reduce gastrocnemius recruitment in proportion to the soleus even though only the soleus EMG was used to control the exoskeleton. The results from this study demonstrate the ability of the nervous system to recalibrate locomotor output in response to substantial changes in the mechanical output of the soleus muscle and associated sensory feedback. This study provides further evidence that the human locomotor system of intact individuals is highly flexible and able to adapt to achieve effective locomotion in response to a broad range of neuromuscular perturbations.
Keywords: biomechanics, motor adaptation, motor learning, gait, exoskeleton, orthosis
how does the human locomotor control system adapt to perturbations to its neuromuscular map? Acute changes in either the gain or connections relating a given motor command to a desired muscular action can challenge one's ability to effectively walk. Humans are adept at compensating for common gain perturbations such as muscular fatigue during gait (Nessler et al. 2011). In instances of significant gain change, including muscle impairment, upper limb research suggests that the nervous system does not optimally modulate muscle activation patterns (de Rugy et al. 2012). On rare occasions, the mechanical action of a specific muscle is altered as occurs with tendon transfer. Currently, there is no consensus on whether humans can effectively adapt their locomotor patterns to acute changes in the connections of the neuromuscular map. Comparisons of locomotor muscle activation patterns pre- and post-muscle transplantation surgery has found both relatively unchanged activations in children with cerebral palsy (Perry and Hoffer 1977; Waters et al. 1982) and substantial modifications in patients with poliomyelitis (Close and Todd 1959; Sutherland et al. 1960). Evidence from controlled animal studies found the original locomotor electromyography (EMG) patterns persisted following nerve crossing or muscle transpositions surgeries (Forssberg and Svartengren 1983; Sperry 1945). Cumulatively, this research suggests that there may be limitations of the motor system to adapt to either significant gain or connection changes in the neuromuscular map.
We have previously employed robotic exoskeletons to investigate human adaptation to acute disruptions of the neuromuscular map of two plantar flexor muscles: soleus and gastrocnemius (Cain et al. 2007; Gordon and Ferris 2007; Kao et al. 2010a; Kinnaird and Ferris 2009). These two muscles typically display similar activation profiles during gait (Arsenault et al. 1986). However, physiological differences between these muscles result in divergent biomechanical contributions to gait. The gastrocnemius is biarticular and contributes primarily to body weight support and leg swing initiation. The soleus is uniarticular and contributes to body weight support and serves as a primary contributor to forward propulsion (McGowan et al. 2008, 2009; Neptune et al. 2001). Individuals modulate the recruitment of each muscle independently in response to the specific mechanical demands imposed during walking (Courtine et al. 2006; Gordon and Ferris 2007; Gottschall and Kram 2003; McGowan et al. 2008). This last property makes these muscle groups desirable for studying locomotor adaptation.
We found that humans significantly reduced soleus recruitment when walking with a powered exoskeleton that acutely increased the gain between either soleus (Gordon and Ferris 2007; Kao et al. 2010a) or gastrocnemius (Kinnaird and Ferris 2009) activation and the resulting plantar flexor torque. This resulted in total (human + exoskeleton) ankle moments that were comparable to unassisted walking (Kao et al. 2010a). Subjects made both rapid adaptations to maintain ankle joint moments (Kao et al. 2010b) and slower adaptations to minimize negative work produced by the exoskeleton (Gordon and Ferris 2007; Kinnaird and Ferris 2009). This research provides evidence that the human nervous system is proficient at making meaningful and independent adjustments to soleus and gastrocnemius activation profiles during gait in response to perturbations that disrupt the gain between a given efferent command and the resulting muscular output.
To further study the adaptability of the human locomotor system, it would be interesting to use the powered exoskeleton to impose a connection change on the neuromuscular map by modifying the function of the soleus from a plantar flexor to a dorsiflexor. As the exoskeleton can only create torques that supplement the actual mechanical output of the subjects' existing muscles, this is not entirely possible. However, the exoskeleton can create an antagonistic torque proportional to activation of the soleus that will effectively negate the ability of the soleus to produce a plantar flexion torque. Such a coactivation setup would simulate an acute mechanical impairment of the soleus muscle.
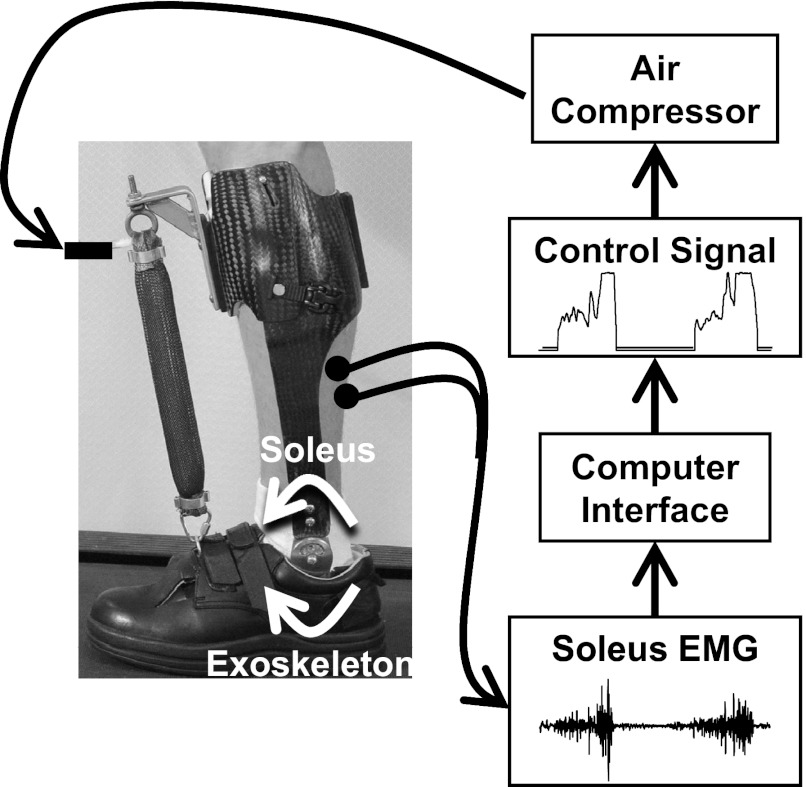
In the current experiment, we investigated the ability of humans to reorganize locomotor patterns when faced with a mechanical impairment of a single plantar flexor muscle. The impairment was imposed using a myoelectrically controlled exoskeleton that resisted the mechanical action of the soleus (Fig. 1). The exoskeleton provided a real-time dorsiflexion torque proportional to the user's soleus EMG amplitude. This setup created an antagonistic coactivation about the ankle joint (human soleus plantar flexor torque vs. exoskeleton dorsiflexor torque) any time the user recruited the soleus muscle. This setup exposed subjects to a novel and substantial perturbation to their neuromuscular map (activation of the soleus muscle independently would no longer produce a net plantar flexion torque). We first hypothesized that subjects would decrease soleus recruitment to minimize the energetically costly effects of “fighting” the antagonistic exoskeleton configuration. In past experiments, subjects decreased soleus recruitment when the exoskeleton effectively increased plantar flexor torque of the soleus. In the current experiment, we believe that subjects will decrease soleus recruit as well. In this case, we theorize that decreases in soleus recruitment will be to minimize energetic costs rather than to maintain plantar flexor torque. Second, we hypothesize that subjects will increase gastrocnemius recruitment in an effort to offset decrements in plantar flexor torque resulting from the selective soleus impairment. Although the gastrocnemius may not be able to fully offset the biomechanical impact of a soleus impairment, both muscles do contribute to body weight support during gait. We theorize that individuals will increase gastrocnemius recruitment with the goal of maintaining normal plantar flexor moments about the ankle joint.
Fig. 1.
Electrodes placed on the skin of the subject's leg recorded electromyographic (EMG) signals from the soleus muscle, a plantar flexor. A computer processed the soleus EMG to control air pressure sent to an artificial pneumatic muscle so that there was a proportional relationship between EMG amplitude and air pressure. As air pressure increased, it caused the artificial muscle to develop tension by shortening in length. The exoskeleton effectively created a cocontraction about the ankle joint proportional to soleus muscle activation. White arrows about the ankle joint show the direction of torque created by the exoskeleton and user's soleus muscle. Thus, when the soleus muscle was activated, the exoskeleton produced a dorsiflexor torque limiting the ability of the soleus to produce plantar flexor torque.
METHODS
Ten able-bodies subjects (5 male, 5 female; means ± SD: age 27 ± 2.5 years, body mass 69.2 ± 10.3 kg, and height 1.70 ± 0.11 m) gave written informed consent and participated in the study. The University of Michigan Medical School Institutional Review Board approved the protocol. All subjects were considered to be nonpathological ambulators in good health. With the exception of the antagonistic setup, the testing procedures used during this experiment were identical to those described in an earlier study (Gordon and Ferris 2007).
We constructed a custom fit exoskeleton for each subject's left lower limb (Fig. 1). Design specifications and mechanical performance of earlier versions of the exoskeleton have been described in detail (Ferris et al. 2005, 2006; Gordon and Ferris 2007; Gordon et al. 2006). The exoskeleton consisted of a carbon fiber shank section and a polypropylene foot section. A metal hinge joint between the shank and foot sections permitted free sagittal plane rotation about the ankle. An artificial pneumatic muscle fastened between two steel brackets affixed to the dorsum of the foot and on the uppermost anterior surface of the exoskeleton produced a dorsiflexor torque when pressurized. The average weight of the exoskeletons was 1.07 ± 0.09 (means ± SD) kg. The artificial pneumatic muscle had an average moment arm length of 10.8 ± 0.9 cm (means ± SD) when the ankle joint was placed in the neutral position. Moment arm lengths were assumed to be constant during walking. These small differences in exoskeleton weight and moment arm length were a result of anthropometric differences between subjects. Four parallel proportional pressure regulators (MAC Valves, Wixom, MI) transmitted compressed air to the artificial pneumatic muscle via nylon tubing (0–6.2 bar). An analog-controlled, solenoid valve (MAC Valves) connected in parallel with the air supply tubing facilitated exhaust.
We used a desktop computer and real-time control board (dSPACE, Northville, MI) to implement proportional myoelectric control of the artificial pneumatic muscle. We wrote custom software in Simulink (The Mathworks, Natick, MA) and converted it to ControlDesk (dSPACE). The software regulated a 0- to 10-V analog signal that was sent to four proportional pressure regulators and solenoid valves working in parallel to control activation and deactivation of the artificial pneumatic muscles. The air pressure sent to the artificial pneumatic muscle was proportional to processed soleus EMG amplitude. For use as a real-time control signal, soleus EMG signals were high-pass filtered with a second-order Butterworth filter (cutoff frequency 20 Hz) to remove movement artifact, full-wave rectified, and low-pass filtered with a second-order Butterworth filter (cutoff frequency 10 Hz) to smooth the signal. These filters were selected for the real-time controller with the goal of limiting the number of computations performed that could potentially increase processing time when working with noisy signals. We applied threshold cutoffs to eliminate background noise and adjustable gains to scale the control signals. Gains and thresholds were typically identical between testing sessions for a given subject.
Gains and thresholds were set each testing day from the processed real-time soleus EMG control signals generated during the baseline walking condition. An initial multiplier was set such that peak soleus EMG amplitude (calculated from 10 consecutive strides) would send a 10-V (maximal activation) signal to the pressure regulators. The value of the multiplier was doubled, and this value was used as the gain for the remainder of the testing period. This resulted in EMG amplitudes greater than 50% of the peak soleus EMG amplitude during the baseline to send a 10-V signal to the pressure regulators. EMG amplitudes less than this value sent a proportional voltage between 0 and 10 V to the regulator. This method of setting the gain was instituted based on pilot studies to create a substantial mechanical perturbation and has been used in our previous research (Gordon and Ferris 2007). Thresholds were selected to remove baseline noise considered to be the period during swing between soleus activations.
The force produced by an artificial pneumatic muscle is attributed to multiple factors. In isometric conditions, increasing activation (air pressure sent to the artificial pneumatic muscle) will increase its force production (Chou and Hannaford 1996). During dynamic conditions, such as walking, force produced by the artificial pneumatic muscle will be attributed to three primary factors: activation, length, and bandwidth (Gordon et al. 2006). As a result, there is no simple linear gain relating real-time EMG amplitude to artificial muscle force or exoskeleton torque. In this experiment, the amplitude of the processed real-time soleus EMG control signals was used to directly control activation (air pressure sent to the artificial muscle) and as such the recruitment of the user's soleus directly influenced the resulting force produced by the artificial pneumatic muscle.
Subjects completed two identical days of treadmill training with the exoskeleton. During each training day, subjects initially walked for 10 min with the exoskeleton passive (baseline). Then the exoskeleton was powered using the antagonistic exoskeleton setup (human soleus activation vs. exoskeleton dorsiflexor torque), and subjects walked for another 30 min (resistance). Transitions between the two conditions were performed as the subject walked without stopping. For safety reasons, the subjects received a verbal warning immediately prior to the device being powered. The second training session was completed 72 h after the first session. A 72-h rest period was selected to allow the subjects to recover from potential muscle soreness and to allow sufficient time for motor consolidation to occur (Krakauer and Shadmehr 2006). Subjects had no prior experience wearing the exoskeleton before participating in the study. Subjects were instructed to walk in the manner that they felt most comfortable and to let their arms swing freely without holding onto the treadmill for support. We did not explicitly explain to the subjects how the exoskeleton would be controlled. We limited the amount of instructions subjects received with the objective of not biasing subjects' natural, self-selected gait parameters. All treadmill walking was performed at 1.25 m/s which is close to the preferred walking speed for healthy adults (Ralston 1958).
During each session, we recorded kinematic, kinetic, and EMG data during the first 10 s of every minute that subjects walked on the treadmill. We placed 29 reflective markers on the subjects' pelvis and lower limbs and used an eight-camera video system (120 Hz; Motion Analysis, Santa Rosa, CA) to record three-dimensional kinematics. We collected foot-ground contact data using footswitches (1,200 Hz; B&L Engineering, Tustin, CA) placed in each shoe. We recorded surface EMG (1,200 Hz; Konigsberg Instruments, Pasadena, CA) from the left soleus, tibialis anterior, medial gastrocnemius, lateral gastrocnemius, vastus lateralis, vastus medialis, rectus femoris, and medial hamstring muscles using bipolar surface electrodes. The EMG amplifier had a bandwidth of 1,000 Hz. To minimize crosstalk, we visually inspected EMG signals during manual muscle tests prior to walking and repositioned electrodes as necessary. We marked the location of the electrodes on the subjects' skin using permanent marker to ensure constant electrode placement between training sessions. Artificial pneumatic muscle force was recorded using a tension compression force transducer (1,200 Hz; Omega Engineering, Stamford, CT) placed in series with the artificial muscle.
Subjects returned for a third testing session during which we recorded data during normal overground walking at 1.25 m/s without the subject wearing the exoskeleton. The purpose of this third session was to examine subjects' normal gait kinetics to make an estimate of the relative resistance the powered exoskeleton created during the treadmill walking sessions. Overground walking was performed because an instrumented force treadmill was not available for this study. During overground walking, we collected ground reaction force data from two force plates in addition to the kinematic data described earlier. A stopwatch connected to two light triggers was used to determine overground walking speed. For each subject, we recorded 10 trials in which overground walking speed had a less than 5% difference from the target 1.25 m/s treadmill walking speed.
For the treadmill walking conditions, we calculated average step cycle profiles for EMG, kinematic, and kinetic variables for each subject using data from each minute of walking. Average step cycle profiles were computed from all complete step cycles occurring during the 10-s recording period for each minute of walking. To examine changes in EMG amplitude, peak EMG values occurring during every gait cycle were detected for each subject for each minute of walking. High-pass filtered (high-pass, 4th-order Butterworth filter, cutoff frequency 20 Hz) and rectified EMG data were used to detect peak EMG values. In addition, we created step cycle profiles for ankle, knee, and hip joint angles (low-pass, 4th-order Butterworth filter, cutoff frequency 6 Hz). To examine changes in kinematics across time, we found the peak ankle plantar flexion angle occurring between 50 and 75% of the gait cycle, a period when the ankle actively plantar flexes (Perry 1992). We also found the peak knee and hip extension occurring between 12 and 62% of the gait cycle, a period when ankle plantar flexion moments assist in creating knee extension (Perry 1992).
Finally, to examine changes in exoskeleton kinetics, we calculated exoskeleton dorsiflexor torque and created average step cycle exoskeleton mechanical power profiles. From exoskeleton power, we calculated average exoskeleton positive, negative, and total absolute mechanical work (| positive work | + | negative work |) during the step cycle.
From the overground walking data, we calculated net torque about the ankle joint using commercial software (Visual3D; C-Motion, Rockville, MD) combining kinematic marker and force platform data. Lower limb inertial properties were estimated based on anthropometric measurements of the subjects (Zatsiorsky 2002). While there are differences in gait kinetics during treadmill and overground walking, there do not appear to be differences in ankle joint powers over the full gait cycle or in the ankle moments from 30–50% of the gait cycle (Lee and Hidler 2008), which were the variables of interest in this study. In addition, the overground data was not used for any statistical purposes; it was used solely to get a relative measure of the magnitude of the dorsiflexor moments produced by the exoskeleton during treadmill walking.
We tested for differences in exoskeleton-produced kinetics, lower limb gait kinematics, and EMG activity. We used a two-tailed, paired Student's t-test to look for differences in the magnitude of the exoskeleton dorsiflexor torque and total absolute exoskeleton work between subjects' initial exposure to the powered exoskeleton, day 1, resistance minute 1 (initial resistance), and subjects' final minute of powered walking, day 2, resistance minute 30 (final resistance). As the exoskeleton was not powered during baseline, comparisons of the exoskeleton kinetics were only compared between initial and final resistance. We used one-way repeated measures ANOVAs to compare peak ankle plantar flexion, knee extension, and hip extension angles and peak EMG amplitudes between baseline, initial resistance, and final resistance time points. For all testing, we set the significance level at P < 0.05 and used Tukey Honestly Significant Difference (THSD) post hoc tests to make individual comparisons when necessary. Baseline data were taken from the final, 10th minute of baseline walking on day 1 as opposed to day 2. Paired Student's t-tests confirmed that there was no difference in either kinematic or EMG data between days (P < 0.05) for any of the baseline variables examined. Finally, we calculated cross-correlations on raw unrectified EMG during every step cycle recorded during baseline between soleus-medial gastrocnemius and soleus-lateral gastrocnemius to ensure that the soleus EMG signals used for controlling the exoskeleton were free from crosstalk (Wakeling 2009). We also performed cross-correlations relating the change in peak EMG values from baseline to final resistance for soleus-medial gastrocnemius and soleus-lateral gastrocnemius to assess the relationship in the direction that subjects modulated the recruitment of these two muscles.
RESULTS
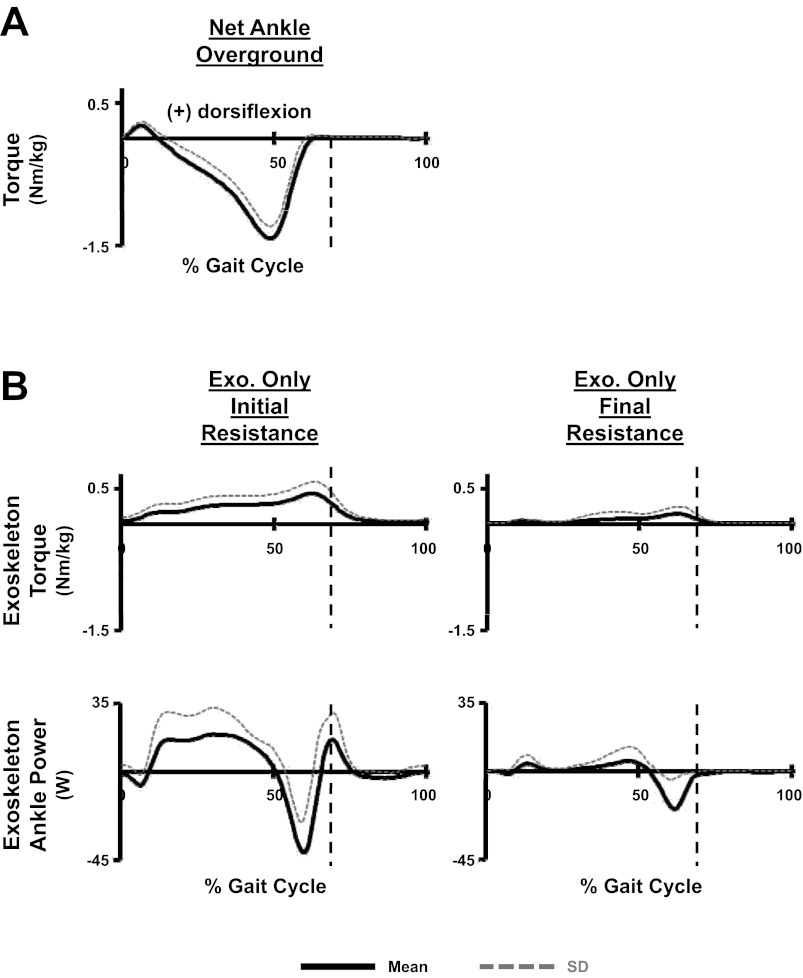
As designed, when powered, the exoskeleton produced a dorsiflexor torque about the ankle joint. During initial resistance, the exoskeleton produced a peak stance-phase dorsiflexor torque normalized to subjects' bodyweight of 0.45 ± 0.17 Nm/kg (Fig. 2B). The magnitude of this dorsiflexor torque was ∼32% of the peak stance-phase plantar flexor torque (1.42 ± 0.16 Nm/kg) subjects' produced during overground walking with no exoskeleton (Fig. 2A). At final resistance, the magnitude of the peak exoskeleton dorsiflexor torque had been significantly reduced, 0.16 ± 0.11 Nm/kg (t-test: P = 0.001073; Fig. 2B). Accompanying these reductions in dorsiflexor torque, the subjects also reduced both positive and negative power produced by the exoskeleton (Fig. 2B). The total absolute work, calculated from the exoskeleton power profiles, produced during initial resistance, was 13.2 ± 6.4 J/kg. At final resistance, the total absolute work produced had been significantly reduced (t-test: P = 0.0023) by ∼73% to 3.6 ± 3.0 J/kg.
Fig. 2.
A: mean and SD of step cycle torque profiles calculated from all 10 subjects. Data for net ankle overground is the net ankle torque subjects created during overground walking without the exoskeleton. B: data for the two powered exoskeleton (Exo.) conditions including the dorsiflexion torque and power produced solely by the exoskeleton during treadmill walking. The exoskeleton is only able to actively produce dorsiflexion torques. When the power created by the exoskeleton is positive, it indicates the exoskeleton is generating energy occurring during periods when the artificial pneumatic muscle is both shortening (during ankle joint dorsiflexion) and producing torque. Negative values indicate the exoskeleton is absorbing energy, which occur when the artificial pneumatic muscle is both lengthening (during ankle joint plantar flexion) and producing torque. Vertical dashed lines indicate stance to swing transitions.
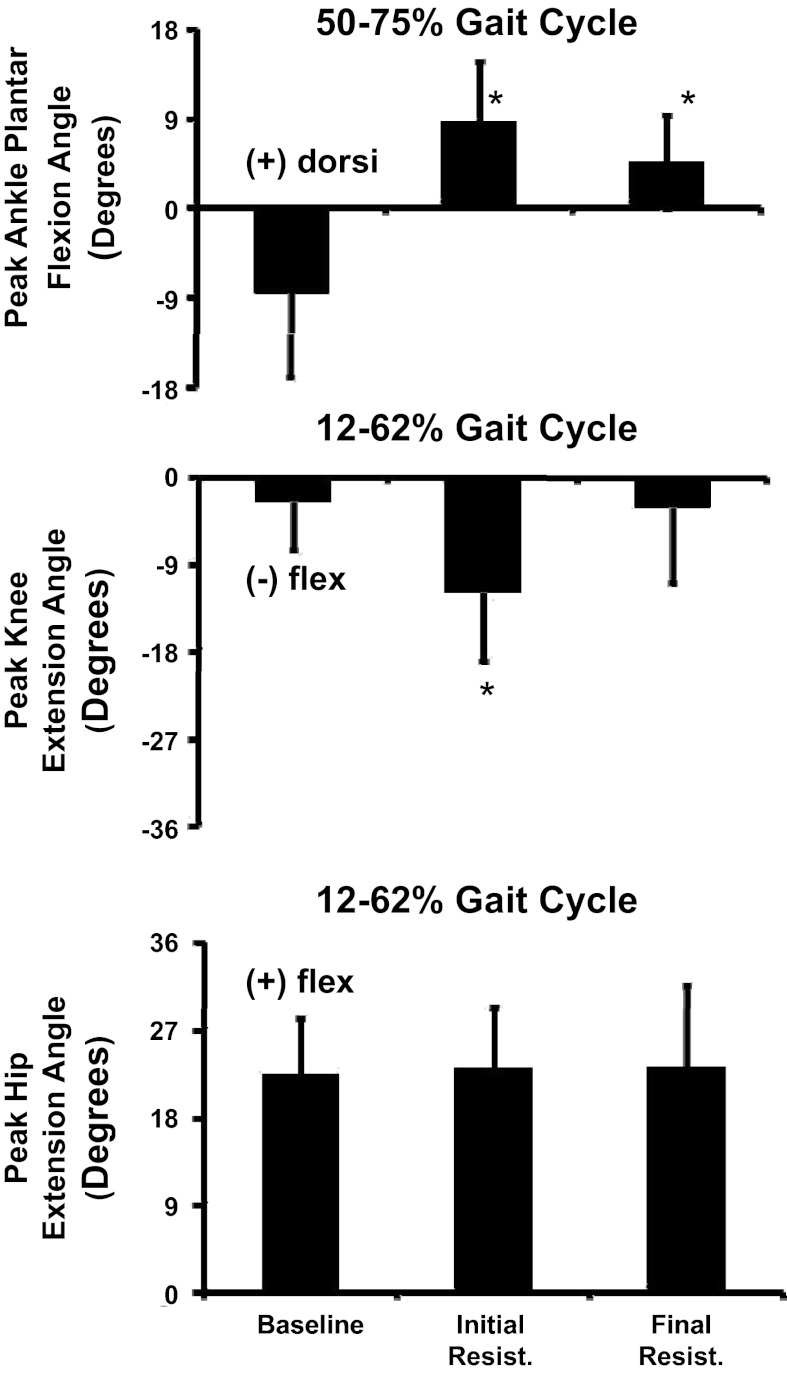
During baseline walking subjects displayed typical sagittal plane joint angle kinematic patterns with two periods of peak plantar flexion occurring during early stance phase and again during the period of pre-swing thru initial-swing, and peak knee and hip extension occurring during late stance (Fig. 3). Subjects' initial kinematic response to the robotic-imposed resistance to soleus activation was both substantial and uniform. Subjects visually presented a limp on the resisted limb that remained through final resistance (Fig. 3). At the ankle joint, subjects significantly reduced their peak plantar flexion angle occurring during pre-swing thru initial-swing (ANOVA: P < 0.0001) when comparing baseline to both initial resistance and final resistance periods (THSD, P < 0.05) (Fig. 4). At the knee joint there were significant differences in the maximum knee extension angle subjects achieved (ANOVA: P = 0.0004) across the testing period. Post hoc tests revealed that during initial resistance, subjects significantly increased knee flexion compared with baseline (THSD, P < 0.05) (Fig. 4). During final resistance, there were no significant differences in peak knee extension compared with baseline (THSD, P > 0.05). Statistical testing found difference in peak hip extension (ANOVA: P = 0.0002). However, effects test found differences only between subjects (P < 0.0001) and not between time periods (P = 0.8770) (Fig. 4).
Fig. 3.
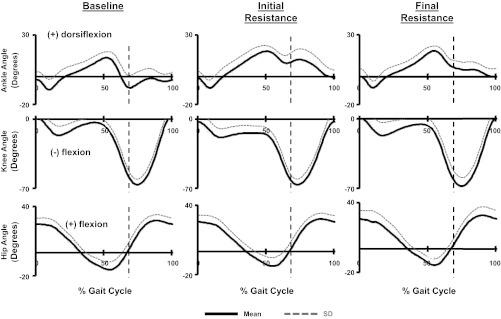
Mean and SD of step cycle kinematic profiles for the ankle, knee, and hip joint angles calculated from all 10 subjects during baseline, initial resistance, and final resistance time periods. Vertical dashed lines indicate stance to swing transitions.
Fig. 4.
Mean and SD of maximum ankle plantar flexion, maximum knee extension, and maximum hip extension occurring at specific phases of the gait cycle during the baseline, initial resistance, and final resistance time periods. Data were calculated from all 10 subjects. *Significantly different from baseline.
Results from the cross-correlations performed on raw EMG signals during baseline found minimal correlation between soleus and medial gastrocnemius, R2 = 0.014 ± 0.018 (means ± SD), with the maximum value for all subjects being R2 = 0.062. Cross-correlations between soleus and lateral gastrocnemius similarly found minimal correlation, R2 = 0.03 ± 0.04, with the maximum value for all subjects being R2 = 0.12. These results suggest that there was minimal common signal recorded from the distinct muscles and that different populations of motor units were recorded (Wakeling 2009).
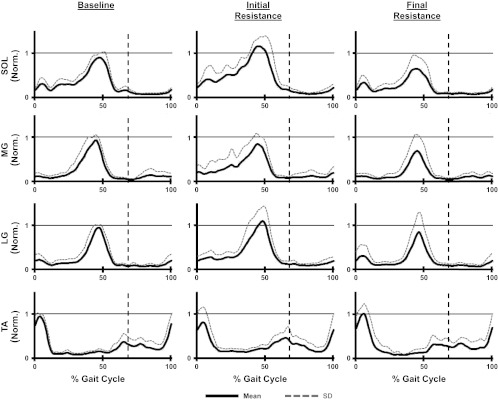
During baseline walking subjects displayed typical EMG patterns in the lower limb with soleus, medial gastrocnemius, and lateral gastrocnemius all demonstrating primary activation during late stance and the tibialis anterior being active primarily during early stance and late swing (Fig. 5). ANOVAs examining peak EMG amplitude found significant differences in lower limb muscle activity across the training period for both the soleus and lateral gastrocnemius (Table 1). The soleus significantly increased peak EMG values during initial resistance compared with baseline (THSD, P < 0.05). At final resistance, the soleus had significantly decreased peak EMG values (THSD, P < 0.05) compared with baseline. There were significant differences in peak lateral gastrocnemius values between initial resistance and final resistance (THSD, P < 0.05). However, neither of these values was different from baseline (THSD, P > 0.05).
Fig. 5.
Mean and SD of step cycle EMG profiles calculated from all 10 subjects for the soleus (SOL), medial gastrocnemius (MG), lateral gastrocnemius (LG), and tibialis anterior (TA) during the baseline, initial resistance, and final resistance time periods. EMG values were normalized to the peak value occurring during baseline. Vertical dashed lines indicate stance to swing transitions.
Table 1.
Peak EMG values (normalized to baseline)
| SOL | TA | MG | LG | VM | VL | RF | MH | |
|---|---|---|---|---|---|---|---|---|
| Initial resistance | ||||||||
| Means | 1.46* | 1.01 | 1.10 | 1.29 | 1.29 | 1.69 | 1.56 | 1.32 |
| SD | 0.27 | 0.32 | 0.26 | 0.33 | 0.43 | 1.55 | 0.60 | 0.30 |
| Final resistance | ||||||||
| Means | 0.71* | 1.19 | 0.79 | 0.88 | 0.97 | 0.88 | 1.23 | 0.84 |
| SD | 0.25 | 0.55 | 0.42 | 0.49 | 0.49 | 0.40 | 0.52 | 0.25 |
| ANOVA | 0.0001 | 0.0662 | 0.1773 | 0.0139 | 0.1257 | 0.5243 | 0.1149 | 0.0654 |
Means and SD of peak electromyography (EMG) values normalized to baseline.
Post hoc testing found the values are significantly different from baseline. Note ANOVAs compare EMG values that have not been normalized between all 3 time points (baseline, initial resistance, and final resistance).
For the upper leg, subjects also displayed typical EMG patterns during baseline walking with the rectus femoris, vastus medialis, and vastus lateralis all displaying primary bursts of activity during early stance phase. Rectus femoris also had a second burst of activity occurring during early swing. The medial hamstrings were active during baseline primarily during late swing and early stance (Fig. 6). ANOVAs examining the peak EMG amplitude of the upper leg muscles across time found no significant differences in any of the muscles examined (Table 1).
Fig. 6.
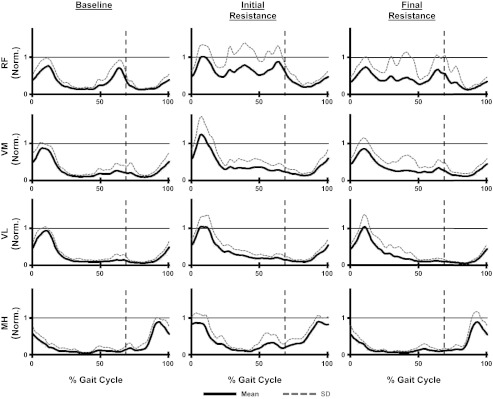
Mean and SD of step cycle EMG profiles calculated from all 10 subjects for the rectus femoris (RF), vastus medialis (VM), vastus lateralis (VL), and medial hamstring (MH) during the baseline, initial resistance, and final resistance time periods. EMG values were normalized to the peak value occuring during baseline. Vertical dashed lines indicate stance to swing transitions.
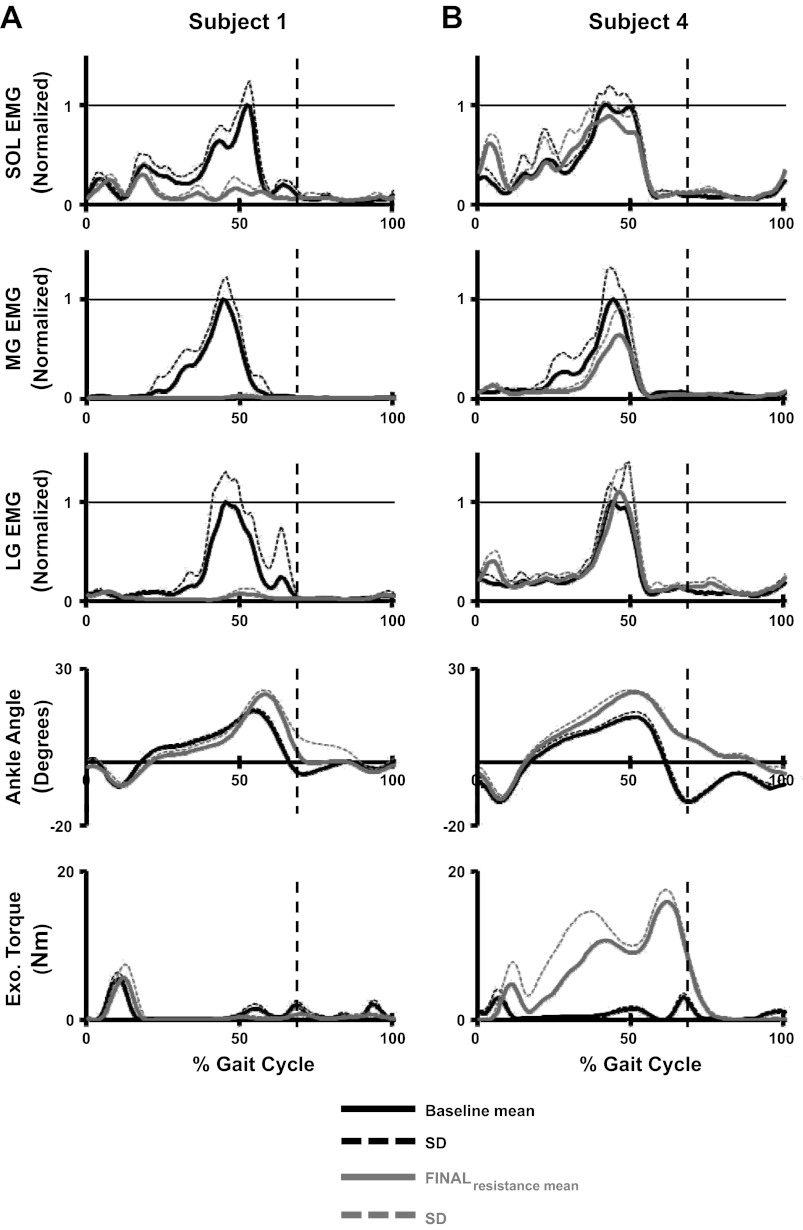
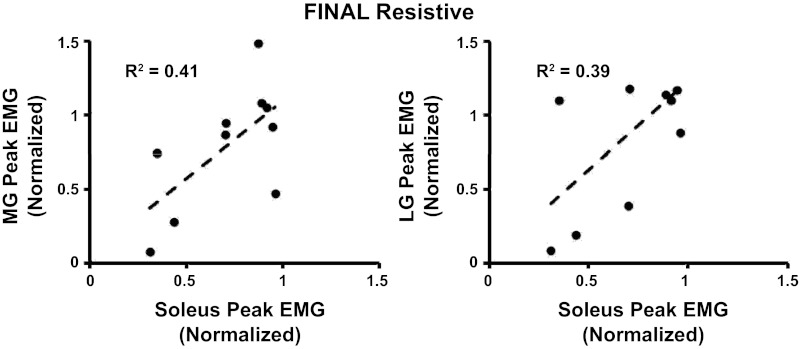
A closer examination of individual muscle activation patterns over the 60 min of resisted walking found, between subjects, variations in how the subjects modulated soleus and gastrocnemius recruitment. At the two extremes, some subjects adapted to the antagonistic robotic perturbation by minimizing all plantar flexor muscle activity (Fig. 7A), whereas other subjects selected to maintain their baseline muscle activation patterns (Fig. 7B). As observed in the individual data (Fig. 7, A and B), there was a trend for subjects to modulate both the soleus and the gastrocnemius muscles in a similar direction during the testing period, meaning that if subjects decreased soleus activation, they likely decreased gastrocnemius activity as well. Figure 8 shows linear regressions of the magnitude of change from baseline to final resistance in the peak EMG values for the soleus and medial/lateral gastrocnemius. The changes in soleus-medial gastrocnemius and soleus-lateral gastrocnemius magnitude had correlation coefficients of r = 0.64 and r = 0.63, respectively. This relationship indicates that the muscles were being modulated in the same direction.
Fig. 7.
Mean and SD of step cycle profiles from individual subjects showing data of the SOL, MG, LG, ankle angle, and exoskeleton dorsiflexor torque at the baseline and final resistance time periods. Subject 1 selected to minimize plantar flexor activity in response to the exoskeleton resistance (A) and Subject 4 selected to maintain muscle activity at levels similar to baseline in response to the exoskeleton resistance (B). Vertical dashed lines indicate stance to swing transitions.
Fig. 8.
Linear regressions showing the relationship between the changes in peak EMG values calculated from soleus-medial gastrocnemius and soleus-lateral gastrocnemius during the final resistive time period. EMG values have been normalized to baseline.
DISCUSSION
In response to perturbations, people adapt their motor control strategies to achieve purposeful locomotion. In the current study, subjects were challenged with a very difficult task: to walk while wearing a powered exoskeleton that directly counteracted the mechanical action of their soleus. In response to this perturbation, subjects adapted their locomotor control patterns by making significant reductions in soleus recruitment. Although kinematic patterns also changed significantly in response to the exoskeleton resistance, subjects were able to maintain forward progression at the target speed. Also, over the course of the training period, subjects were able to significantly reduce the antagonistic effects of the exoskeleton. The results from this study provide insight into how the central nervous system regulates control of the major plantar flexor muscle groups during human walking.
The powered exoskeleton in this experiment was intended to create a substantial perturbation to the user's ability to control locomotion by producing a resistive torque offsetting the mechanical action of the soleus. Because the resistive dorsiflexor torque created by the exoskeleton was considerable and was tied directly to the user's soleus recruitment, this setup resulted in a substantial reduction in the metabolic efficiency (mechanical power output/metabolic power) of the soleus muscle. Given the strong evidence that humans will select gait patterns that minimize metabolic costs (Alexander 2002; Bertram and Ruina 2001; Cavagna and Franzetti 1986; Donelan et al. 2001, 2002; Minetti et al. 2003), we hypothesized that individuals would significantly reduce soleus recruitment. Within this experimental setup, maintaining normal soleus activation profiles would be both metabolically costly and mechanically ineffective. As predicted, by the final resistance period, subjects made significant reductions in peak soleus activation. It is worth noting that while the average reduction in peak soleus activation was ∼29%, the amount of reduction was distributed over a wide range from no change up to a 65% reduction. There was no clear indication from the data we collected to explain why we saw this spread. We speculated that some subjects were more willing to explore alternative walking strategies than others, but further examinations of stride-to-stride EMG and joint kinematic variability provided no support for this theory.
Decreasing soleus amplitude directly reduced the antagonistic effects (dorsiflexor torque and total absolute work) produced by the exoskeleton during walking. However, the reduction in soleus recruitment also resulted in a significant reduction in the peak ankle plantar flexion angle during late stance. It has been proposed that a strong, well-timed ankle push-off occurring just prior to initial contact of the contralateral limb, can significantly reduce the energy required for step-to-step transition costs (Kuo and Donelan 2010). Loss of this push-off can be compensated for by performing work at other joints, but doing so may be up to four times more energetically costly (Kuo 2002). Thus, by choosing to decrease the recruitment of their soleus, which reduced the metabolic cost associated with producing force in that muscle, subjects accepted a locomotor strategy that was likely less metabolically efficient than normal walking because they would have to compensate for the loss of ankle push-off by performing more work at other joints. This strategy was implemented over the course of the training period as subjects' initial response to the antagonistic exoskeleton was to increase soleus recruitment and attempt presumably (although unsuccessfully) to maintain normal ankle joint moments as has been indicated in previous studies (Kao et al. 2010a). These results suggest that the human locomotor control system may tune both individual muscle activation profiles and gross locomotor patterns (joint torque and kinematics) to minimize metabolic energy costs.
The soleus is a primary contributor to forward trunk propulsion and body weight support during gait (McGowan et al. 2008, 2009; Neptune et al. 2001). To maintain locomotor function, individuals in the current study had to compensate for the reduced biomechanical contributions of the soleus. As such, we hypothesized that subjects would increase gastrocnemius recruitment based on a computer modeling study suggesting that isolated decreases in soleus strength could be offset primarily by increases in gastrocnemius work combined with secondary increases in work performed by the vasti, rectus femoris, and gluteus maximus (Goldberg and Neptune 2007). Subjects did not increase gastrocnemius activity in response to the soleus-controlled antagonistic exoskeleton. In fact, there was a moderate trend for subjects to decrease both gastrocnemius and soleus. Gait analysis of individuals with stroke has found that reduced forward propulsion created by deficits of soleus and other muscles on the paretic limb is compensated for by the non-paretic limb (Hall et al. 2011). As we did not observe significant changes in any muscle other than the soleus on the resisted limb, it is quite possible that subjects also compensated for the mechanically reduced soleus output by making locomotor changes to the contralateral limb. Future research examining the contributions of the contralateral limb may be helpful for understanding how individuals may compensate for the exoskeleton perturbations.
There are two primary explanations why individuals would not increase gastrocnemius activity to offset decreases in soleus mechanical output. First, it is possible that the two plantar flexor muscles perform a diverse set of biomechanical functions during gait, meaning that the gastrocnemius may not be able to directly compensate for soleus impairments. The gastrocnemius has been suggested to contribute to body weight support and the initiation of leg swing (McGowan et al. 2008, 2009; Neptune et al. 2001), which do not overlap the role of the soleus as the primary contributor to forward propulsion. In addition, simulations have suggested that modifying soleus output is the primary mechanism by which the mechanical output of the leg is regulated in response to changes in demand for body weight support and forward propulsion (McGowan et al. 2009). Thus, the gastrocnemius may not be able to replace the biomechanical actions of the soleus during gait. Recent simulations finding a limited ability of the leg to compensate for individual muscle loss lend further support to this perspective (Kutch and Valero-Cuevas 2011).
Second, the nervous system may simplify the complex task of controlling walking by forming combinations of activation patterns that cross multiple muscles known as synergies (Lacquaniti et al. 2012; Ting and McKay 2007; Tresch and Jarc 2009). It has been shown that the EMG of the leg and trunk during human walking can be represented by the linear combination of four or five of these basic activation patterns (Clark et al. 2010; Ivanenko et al. 2004). The soleus and gastrocnemius have been identified as belonging to the same muscle synergy during human walking (Clark et al. 2010; Lacquaniti et al. 2012; McGowan et al. 2010). If the activation of the soleus and gastrocnemius are indeed controlled as a unit, the nervous system may not have the flexibility to both reduce the drive to the soleus while at the same time increase activation of the gastrocnemius. Our observation that there was a trend to reduce the activation of both the soleus and gastrocnemius, even though only the soleus was perturbed, supports the idea that the nervous system controls groups of muscles rather than individual muscles. McGowan et al. (2010) found that flexible weighting of gastrocnemius and soleus activity was required to respond to substantial changes in imposed mechanical demands during walking. The flexible plantar flexor module they propose allowing the relative activations of muscles within the module to change could account for the data we observed.
One of the challenges subjects may have had in proficiently adapting to the exoskeleton resistance was that the sensory feedback received by the subjects in response to activation of the soleus was likely inconsistent with their forward model. The forward model predicts the sensory feedback that will be received based on the state of the limb and the current motor command (Wolpert et al. 1995). Typically during walking, activation of the soleus will result in an angular acceleration of the ankle joint into plantar flexion. In predictable environments, the movement-related parameters created by activation of the soleus will result in corresponding sensory feedback that, compared with the individuals' forward model, allows the central nervous system to rapidly detect movement errors and to appropriately respond to these errors using an established inverse model. During human walking, external perturbations that increase or decrease the anticipated load on the triceps surae muscles result in rapid within-step modifications to EMG amplitude (Gordon et al. 2009, 2010; Sinkjaer et al. 2000).
In the current experiment, when the soleus was activated the resulting angular acceleration of the ankle joint was less than the individual would predict during normal gait. One might theorize that the initial response to this feedback would be to increase activation of the soleus to correct the perceived underperformance. In the current study, we observed exactly this, subjects made an initial significant increase in soleus activation when walking with the antagonistic exoskeleton. However, the problem with this response is that it is based on an incorrect internal model (composed of both incorrect forward and inverse models). Increasing soleus activation in this situation does not reduce the subject's movement errors. Franklin et al. (2008) has proposed a simple learning algorithm suggesting that, in response to unpredictable environments, individuals will create a general initial cocontraction about their joint. The drive to both agonist and antagonist groups will then be gradually decreased over multiple iterations of a movement, provided movement error remains small without the requirement that the nervous system has first developed an appropriate inverse dynamic model. We have applied this model to explain the locomotor adaptations observed when individuals walk with an exoskeleton configured to increase soleus mechanical output (Reinkensmeyer et al. 2009). This model could also be used to explain the generalized locomotor adaptation response (initial increases in muscle activation that decay over time with practice) observed in the current experiment.
Conclusion.
Performing tractable studies on human locomotor motor adaptation in response to internal perturbations that disrupt one's neuromuscular mapping is challenging. Myoelectrically controlled, powered exoskeletons have the potential to serve as a tool to investigate this motor adaptation process. In the current study, subjects significantly reduced soleus recruitment in response to a powered exoskeleton that counteracted the mechanical output of the muscle. The reduction of soleus activation likely decreased the metabolic energy cost of walking with the antagonistic exoskeleton but resulted in significant kinematic changes. The results from this study demonstrate the ability of the nervous system to recalibrate locomotor output in response to substantial changes in the mechanical output of the soleus muscle and associated sensory feedback. This study provides further evidence that the human locomotor system of intact individuals is highly flexible and able to adapt to achieve effective, although kinematically altered, locomotion in response significant perturbations of the neuromuscular map.
GRANTS
This project was funded by National Institute of Neurological Disorders and Stroke Grant R01-NS-045486.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.E.G. and D.P.F. conception and design of research; K.E.G. and C.R.K. performed experiments; K.E.G. and C.R.K. analyzed data; K.E.G. and D.P.F. interpreted results of experiments; K.E.G. prepared figures; K.E.G. drafted manuscript; K.E.G., C.R.K., and D.P.F. edited and revised manuscript; K.E.G., C.R.K., and D.P.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ammanath Peethambaran and the staff of the University of Michigan Orthotics and Prosthetics Center for help with designing and fabricating parts of the exoskeleton. We also thank members of the Human Neuromechanics Laboratory for assistance in collecting and analyzing data.
REFERENCES
- Alexander RM. Energetics and optimization of human walking and running: the 2000 Raymond Pearl Memorial Lecture. Am J Hum Biol 14: 641–648, 2002 [DOI] [PubMed] [Google Scholar]
- Arsenault AB, Winter DA, Marteniuk RG. Is there a ‘normal’ profile of EMG activity in gait? Med Biol Eng Comput 24: 337–343, 1986 [DOI] [PubMed] [Google Scholar]
- Bertram JE, Ruina A. Multiple walking speed-frequency relations are predicted by constrained optimization. J Theor Biol 209: 445–453, 2001 [DOI] [PubMed] [Google Scholar]
- Cain SM, Gordon KE, Ferris DP. Locomotor adaptation to a powered ankle-foot orthosis depends on control method. J Neuroeng Rehabil 4: 48, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna GA, Franzetti P. The determinants of the step frequency in walking in humans. J Physiol 373: 235–242, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CP, Hannaford B. Measurement and modeling of McKibben pneumatic artificial muscles. IEEE Trans Robot Automat 12: 90–102, 1996 [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol 103: 844–857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close JR, Todd FN. The phasic activity of the muscles of the lower extremity and the effect of tendon transfer. J Bone Joint Surg Am 41: 189–235, 1959 [PubMed] [Google Scholar]
- Courtine G, Papaxanthis C, Schieppati M. Coordinated modulation of locomotor muscle synergies constructs straight-ahead and curvilinear walking in humans. Exp Brain Res 170: 320–335, 2006 [DOI] [PubMed] [Google Scholar]
- de Rugy A, Loeb GE, Carroll TJ. Muscle coordination is habitual rather than optimal. J Neurosci 32: 7384–7391, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. Mechanical and metabolic determinants of the preferred step width in human walking. Proc Biol Sci 268: 1985–1992, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J Exp Biol 205: 3717–3727, 2002 [DOI] [PubMed] [Google Scholar]
- Ferris DP, Czerniecki JM, Hannaford B. An ankle-foot orthosis powered by artificial pneumatic muscles. J Appl Biomech 21: 189–197, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris DP, Gordon KE, Sawicki GS, Peethambaran A. An improved powered ankle-foot orthosis using proportional myoelectric control. Gait Posture 23: 425–428, 2006 [DOI] [PubMed] [Google Scholar]
- Forssberg H, Svartengren G. Hardwired locomotor network in cat revealed by a retained motor pattern to gastrocnemius after muscle transposition. Neurosci Lett 41: 283–288, 1983 [DOI] [PubMed] [Google Scholar]
- Franklin DW, Burdet E, Tee KP, Osu R, Chew CM, Milner TE, Kawato M. CNS learns stable, accurate, and efficient movements using a simple algorithm. J Neurosci 28: 11165–11173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EJ, Neptune RR. Compensatory strategies during normal walking in response to muscle weakness and increased hip joint stiffness. Gait Posture 25: 360–367, 2007 [DOI] [PubMed] [Google Scholar]
- Gordon KE, Ferris DP. Learning to walk with a robotic ankle exoskeleton. J Biomech 40: 2636–2644, 2007 [DOI] [PubMed] [Google Scholar]
- Gordon KE, Sawicki GS, Ferris DP. Mechanical performance of artificial pneumatic muscles to power an ankle-foot orthosis. J Biomech 39: 1832–1841, 2006 [DOI] [PubMed] [Google Scholar]
- Gordon KE, Wu M, Kahn JH, Dhaher YY, Schmit BD. Ankle load modulates hip kinetics and EMG during human locomotion. J Neurophysiol 101: 2062–2076, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KE, Wu M, Kahn JH, Schmit BD. Feedback and feedforward locomotor adaptations to ankle-foot load in people with incomplete spinal cord injury. J Neurophysiol 104: 1325–1338, 2010 [DOI] [PubMed] [Google Scholar]
- Gottschall JS, Kram R. Energy cost and muscular activity required for propulsion during walking. J Appl Physiol 94: 1766–1772, 2003 [DOI] [PubMed] [Google Scholar]
- Hall AL, Peterson CL, Kautz SA, Neptune RR. Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis. Clin Biomech 26: 509–515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol 556: 267–282, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao PC, Lewis CL, Ferris DP. Invariant ankle moment patterns when walking with and without a robotic ankle exoskeleton. J Biomech 43: 203–209, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao PC, Lewis CL, Ferris DP. Joint kinetic response during unexpectedly reduced plantar flexor torque provided by a robotic ankle exoskeleton during walking. J Biomech 43: 1401–1407, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird CR, Ferris DP. Medial gastrocnemius myoelectric control of a robotic ankle exoskeleton. IEEE Trans Neural Syst Rehabil Eng 17: 31–37, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci 29: 58–64, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AD. Energetics of actively powered locomotion using the simplest walking model. J Biomech Eng 124: 113–120, 2002 [DOI] [PubMed] [Google Scholar]
- Kuo AD, Donelan JM. Dynamic principles of gait and their clinical implications. Phys Ther 90: 157–174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutch JJ, Valero-Cuevas FJ. Muscle redundancy does not imply robustness to muscle dysfunction. J Biomech 44: 1264–1270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacquaniti F, Ivanenko YP, Zago M. Patterned control of human locomotion. J Physiol 590: 2189–2199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Hidler J. Biomechanics of overground vs. treadmill walking in healthy individuals. J Appl Physiol 104: 747–755, 2008 [DOI] [PubMed] [Google Scholar]
- McGowan CP, Kram R, Neptune RR. Modulation of leg muscle function in response to altered demand for body support and forward propulsion during walking. J Biomech 42: 850–856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan CP, Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: adaptations to altered mechanical demands. J Biomech 43: 412–419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan CP, Neptune RR, Kram R. Independent effects of weight and mass on plantar flexor activity during walking: implications for their contributions to body support and forward propulsion. J Appl Physiol 105: 486–494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti AE, Boldrini L, Brusamolin L, Zamparo P, McKee T. A feedback-controlled treadmill (treadmill-on-demand) and the spontaneous speed of walking and running in humans. J Appl Physiol 95: 838–843, 2003 [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech 34: 1387–1398, 2001 [DOI] [PubMed] [Google Scholar]
- Nessler JA, Huynh H, McDougal M. A single bout of resistance exercise does not affect nonlinear dynamics of lower extremity kinematics during treadmill walking. Gait Posture 34: 285–287, 2011 [DOI] [PubMed] [Google Scholar]
- Perry J. Gait Analysis of Normal and Pathological Function. Thorofare, NJ: Slack Incorporated, 1992 [Google Scholar]
- Perry J, Hoffer MM. Preoperative and postoperative dynamic electromyography as an aid in planning tendon transfers in children with cerebral palsy. J Bone Joint Surg Am 59: 531–537, 1977 [PubMed] [Google Scholar]
- Ralston HJ. Energy-speed relation and optimal speed during level walking. Int Z Angew Physiol 17: 277–283, 1958 [DOI] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Akoner O, Ferris DP, Gordon KE. Slacking by the human motor system: computational models and implications for robotic orthoses. Conf Proc IEEE Eng Med Biol Soc 1: 2129–2132, 2009 [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523: 817–827, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry RW. The problem of central nervous reorganization after nerve regeneration and muscle transposition. Q Rev Biol 20: 311–369, 1945 [DOI] [PubMed] [Google Scholar]
- Sutherland DH, Bost FC, Schottstaedt ER. Electromyographic study of transplanted muscles about the knee in poliomyelitic patients. J Bone Joint Surg Am 42: 919–939, 1960 [Google Scholar]
- Ting LH, McKay JL. Neuromechanics of muscle synergies for posture and movement. Curr Opin Neurobiol 17: 622–628, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol 19: 601–607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling JM. The recruitment of different compartments within a muscle depends on the mechanics of the movement. Biol Lett 5: 30–34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RL, Frazier J, Garland DE, Jordan C, Perry J. Electromyographic gait analysis before and after operative treatment for hemiplegic equinus and equinovarus deformity. J Bone Joint Surg Am 64: 284–288, 1982 [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science 269: 1880–1882, 1995 [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM. Kinetics of human motion. Champaign, IL: Human Kinetics, 2002 [Google Scholar]