Abstract
Saccade adaptation is a mechanism that adjusts saccade landing positions if they systematically fail to reach their intended target. In the laboratory, saccades can be shortened or lengthened if the saccade target is displaced during execution of the saccade. In this study, saccades were performed from different positions to an adapted saccade target to dissociate adaptation to a spatiotopic position in external space from a combined retinotopic and spatiotopic coding. The presentation duration of the saccade target before saccade execution was systematically varied, during adaptation and during test trials, with a delayed saccade paradigm. Spatiotopic shifts in landing positions depended on a certain preview duration of the target before saccade execution. When saccades were performed immediately to a suddenly appearing target, no spatiotopic adaptation was observed. These results suggest that a spatiotopic representation of the visual target signal builds up as a function of the duration the saccade target is visible before saccade execution. Different coordinate frames might also explain the separate adaptability of reactive and voluntary saccades. Spatiotopic effects were found only in outward adaptation but not in inward adaptation, which is consistent with the idea that outward adaptation takes place at the level of the visual target representation, whereas inward adaptation is achieved at a purely motor level.
Keywords: reference frame, saccade adaptation, spatiotopic
saccade adaptation is a convenient method to experimentally modify the size of saccade eye movements where the saccade target is systematically displaced during the execution of the saccade (Hopp and Fuchs 2004; McLaughlin 1967; Pelisson et al. 2010). Since the eye will not reach the intended saccade goal position, the oculomotor system starts adjusting the saccade size, and usually within 100 trials a good calibration is achieved. Depending on the direction of the target displacement, the saccade gain can be increased or decreased: inward displacements will lead to shorter saccade amplitudes, whereas outward displacements will lead to longer saccade amplitudes. Many studies suggest that inward and outward adaptation rely on different neuronal mechanisms (Cecala and Freedman 2009; Ethier et al. 2008; Hernandez et al. 2008; Miller et al. 1981; Mueller et al. 2012; Panouilleres et al. 2009; Schnier and Lappe 2011, 2012; Semmlow et al. 1989; Straube and Deubel 1995; Straube et al. 1997; Zimmermann and Lappe 2010). Whereas inward adaptation is most probably established at a purely motor stage (cf. Pelisson et al. 2010 for review), transfer of adaptation to visual perception (Garaas and Pomplun 2011; Schnier et al. 2010; Zimmermann and Lappe 2010) and to hand-pointing movements (Hernandez et al. 2008) suggest that saccade outward adaptation occurs at a target localization stage, changing the visual registration of the saccade target.
Remarkable differences in saccade adaptation have also been repeatedly reported between different types of saccades: reactive saccades, which are driven by a sudden onset of the saccade target, are independently adaptable from voluntary saccades, in which the saccade targets are presented continuously and saccades are performed by the subject in a self-paced manner (Collins and Dore-Mazars 2006; Cotti et al. 2007; Deubel 1995; Erkelens and Hulleman 1993; Fujita et al. 2002; Hopp and Fuchs 2004). If saccades of one type are adaptively changed, the adaptation transfers only partly to the other type, suggesting the involvement of different neural mechanisms (Alahyane et al. 2007, 2008; Cotti et al. 2009; Schnier and Lappe 2012). After adaptation of reactive saccades, mislocalization of targets flashed briefly before saccade execution (Awater et al. 2005; Collins et al. 2007) and mislocalization of the saccade target (Bahcall and Kowler 1999; Collins et al. 2009; Klingenhoefer and Bremmer 2011) have been observed. However, after adaptation of scanning saccades, stationary visual objects that have been inspected for a long time also are perceived shifted in the direction of adaptation (Zimmermann and Lappe 2009). Reactive and voluntary saccade adaptation thus might take place at different levels of the oculomotor transform, coded in separate coordinate frames. Indeed, a recent brain imaging study found differential activation for reactive (cerebellum, middle temporal, temporoparietal, and frontal areas) and voluntary (cerebellar, frontal, and parietal areas) saccade adaptation (Gerardin et al. 2012).
The main difference between reactive and voluntary saccades is the duration the saccade targets are visible before the saccades are initiated. The preview duration of the saccade target before saccade execution can be manipulated with the delayed saccade paradigm. By varying the temporal delay between saccade target appearance and fixation point offset, saccades from the reactive to voluntary range can be triggered. If there is no temporal delay, reactive saccades will be triggered; if, however, the temporal delay is larger (e.g., 500 ms), voluntary saccades are triggered. Variations in the delay duration confirmed a systematic relationship between the presentation duration of the visual saccade target signal and the amount of transfer from one saccade type to the other (Deubel 1995; Schnier and Lappe 2011). Adaptation of reactive saccades transferred strongly to saccades performed in trials with low delay durations. Adaptation transfer was very poor, however, when the delay duration in the test trials had been increased. This finding suggests that the separate adaptability of reactive and voluntary saccades is not an all-or-none phenomenon of two separate saccade classes but a process that gradually builds up over time. Effects of eye position, which modulates saccade adaptation (Alahyane and Pelisson 2004; Havermann et al. 2011; Wulff et al. 2012), also suggested the involvement of different coordinate systems in reactive and voluntary adaptation: strong eye position effects were found in voluntary, but not in reactive, outward adaptation (Zimmermann and Lappe 2011). A following investigation on the reference frame of voluntary saccade outward adaptation dissociated retinal from spatiotopic coordinates and found that saccade outward adaptation was linked to a position in external space, rather than to a retinal or a cranial position (Zimmermann et al. 2011). When the target was presented in the same spatial position as during adaptation, saccade targeting was strongly influenced by adaptation. However, when the target was presented in the same retinal or the same cranial position, saccade targeting was barely affected.
The present study sought to investigate the reference frames in saccade outward and inward adaptation for reactive and voluntary saccades. By varying the saccade target presentation duration, saccades from the reactive and the voluntary range were tested. To dissociate between a retinal and a spatiotopic coding of the saccade target, the following method was used (Zimmermann et al. 2011): rightward saccades from the left part of the screen were adapted with either an outward or an inward target displacement. After adaptation, two saccade directions were tested. First, rightward saccades were tested. These saccades had the same retinal vector and went to the same spatial position as the adapted saccade. They were applied to estimate the transfer of adaptation to the test trials. Second, to dissociate the retinal vector from the spatial position, leftward saccades starting from the right part of the screen had to be performed to the adapted target location. If adaptation is coded in spatiotopic coordinates, these leftward saccades should then be shortened. If the spatiotopic effect rises with increasing delay duration of the saccade target, this would suggest that reactive and voluntary saccade outward adaptation mainly differ in the coordinate systems they are coded in.
METHODS
Participants.
Four subjects (3 naive subjects and the author; mean age 29 yr) participated in all of the experiments. All subjects had normal or corrected-to-normal vision, and all subjects gave informed consent. The study was approved by the ethics committee of the German Society of Psychology. The experiments were carried out in accordance with the principles laid down in the Declaration of Helsinki.
Procedure.
Subjects were seated 57 cm from a 21-inch CRT color monitor (Barco Calibrator) with the head stabilized by a chin- and headrest. The visible screen diagonal was 20 in., resulting in a visual field of 40° × 30°. Stimuli were presented on the monitor with a vertical frequency of 120 Hz at a resolution of 800 × 600 pixels. Eye movements were monitored by the EyeLink 1000 system (SR Research), which samples gaze positions with a frequency of 2,000 Hz. Viewing was binocular, but only the dominant eye was recorded. The system detected start and end of a saccade when eye velocity exceeded or fell below 22°/s and acceleration was above or below 4,000°/s2. All fixation points and saccade targets were 1° in diameter and were presented on a homogeneously gray background.
Adaptation trials.
Saccade adaptation was induced with a delay saccade paradigm, which is sketched in Fig. 1. Subjects fixated on a fixation point 9° to the left of the screen center. After 1,000 ms, the saccade target was shown at the screen center. The subject was instructed to perform a saccade to the saccade target as soon as the fixation point disappeared. In separate sessions the fixation point was either switched off simultaneously with the onset of the saccade target or 250 or 500 ms later, thus varying the overlap duration of saccade target and fixation point (indicated by the gray vertical bar in Fig. 1B). Before adaptation began, 20 baseline trials were presented in which the saccade target remained in its initial position. The aim of these trials was to establish the baseline saccade landing position to a stationary target. In the subsequent adaptation trials the saccade target was displaced 4° to the right in outward adaptation sessions or 4° to the left in inward adaptation sessions. The target displacement was applied when the eye had moved more than 3° to the right of the fixation point. Adaptation trials lasted between 2,000 and 2,500 ms depending on the delay duration.
Fig. 1.
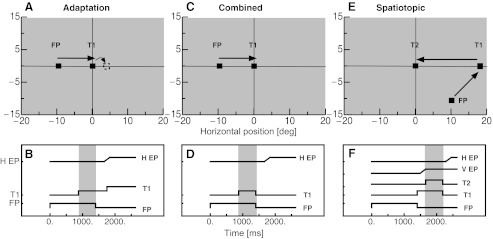
A: positions of the fixation point (FP) and the saccade target (T1) for the adaptation procedure. During execution of the saccade, the saccade target jumped in the outward direction. B: time course of events for the adaptation procedure. While subjects kept their gaze on the fixation point, saccade target T1 appeared. When the fixation point disappeared, subjects had to perform a saccade to T1. With this method, 3 different overlap durations (indicated by the gray vertical bar) of fixation point and saccade target (0, 250, 500 ms) could be tested. When the eye tracker detected the change in the horizontal eye position (H EP), the saccade target was displaced. C: positions of the fixation point and the saccade target (T1) in the “combined condition” were the same as in the adaptation procedure. D: time course of events in the combined condition. Subjects performed a saccade to the position of the saccade target when the fixation point disappeared. The saccade target was shown for either 50 or 500 ms together with the fixation point. E: positions of the fixation point and the saccade targets (T1 and T2) in the “spatiotopic condition.” Subjects fixated on the fixation point until saccade target T1 appeared and then performed a saccade to T1. From T1 they had to initiate a saccade to T2. F: time course of events in the spatiotopic condition. The gray vertical bar indicates the overlap period of saccade targets T1 and T2 (which could be either 50 or 500 ms). When T1 disappeared, subjects had to saccade to the position of T2. V EP, vertical eye position.
Pre- and postadaptation trials.
Test trials were applied before and after adaptation. Two conditions were presented in the test trials. The “combined condition” tested the combined coding of the retinotopic and the spatiotopic target representation. It mimicked the adaptation trials except that the saccade target was not displaced during the saccade but switched off when the fixation point disappeared. The aim of these trials was to test the transfer of adaptation to trials where the saccade target disappeared. Figure 1C shows the spatial arrangement of the targets and Fig. 1D their time courses. The saccade target overlapped in time with the fixation point for either 50 or 500 ms (the delay period is indicated by the gray vertical bar in Fig. 1D). Fixation point and saccade target were then simultaneously switched off. Subjects were instructed to perform a saccade to the memorized saccade target position when the fixation point disappeared. If the eye tracker detected a saccade before the target had disappeared, the target was switched off immediately. The positions of the fixation point and the saccade target were the same as in adaptation trials except that across trials the position of the saccade target was jittered across three positions (−1.5°, 0°, and 1.5°) to prevent stereotyped saccading to a fixed position.
The presentation of the “spatiotopic condition” followed the procedure described in Zimmermann et al. (2011). In these trials (shown in Fig. 1, E and F) a sequence of two saccades had to be performed. The first saccade was necessary to bring gaze into the right part of the screen. To avoid de-adaptation, the path of the first saccade was in the diagonal direction, rather than horizontal as during adaptation. The second saccade of this sequence was directed to the adapted saccade target position but with the opposite saccade vector from that during adaptation. The fixation point was shown 9° to the right and 10° below the screen center for a randomly chosen duration between 1,300 and 1,500 ms. The first saccade target then appeared 18° to the right of the screen center and at the vertical midline. This target position was chosen because spatiotopic effects were found to be strongest for saccades starting from this position (Zimmermann et al. 2011). After a randomly chosen duration between 800 and 1,000 ms, the second saccade target appeared at the adapted target position (position T1 in Fig. 1A), for either 50 or 500 ms. The second saccade target was jittered across three positions (−1.5°, 0°, and 1.5°). The aim of the jittering of the saccade target position was to check that saccades were not initiated in a stereotyped manner. The subject was instructed to wait until the first saccade target turned off. The first saccade target was switched off together with the second, and the subject had to perform a saccade to the memorized position of the second saccade target. The screen then remained blank for the rest of the trial.
The fixation point changed color to inform the subject about the condition. A green fixation point warned the subject to keep fixation until the fixation point disappeared. The fixation point was red when the delay duration was 0 ms and the subject had to saccade directly to the targets. Test trials lasted 2,050 or 2,500 ms depending on condition.
Structure of a session.
A session started with 60 preadaptation-trials in which each of the 2 × 2 (50 ms/500 ms target presentation duration and combined/spatiotopic) conditions was presented for 15 trials. After the preadaptation trials, 20 baseline trials were applied in which the saccade target remained in its initial position after saccade execution, to estimate the unadapted saccade gain. In the next 150 trials the saccade target was displaced intrasaccadically either 4° in outward direction to induce outward adaptation or in separate sessions in inward direction to induce inward adaptation. Inward and outward adaptation sessions were identical in all respects except for the direction of the target displacement. After adaptation, 60 postadaptation trials were applied. The postadaptation trials were intermingled with 165 reinforcement adaptation trials whose aim was to keep adaptation at a steady level. Before and after adaptation, the presentation of the trials was completely randomized.
RESULTS
Adaptation magnitude.
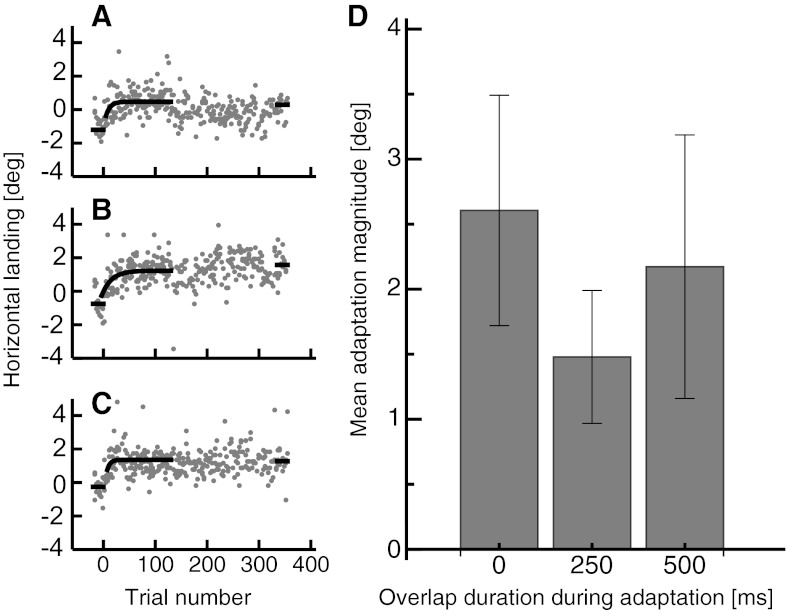
Figure 2, A–C, shows saccade landing positions in the baseline trials and the adaptation trials from a representative subject for the three different delay durations. The median landing position from the 20 baseline trials and the last 20 adaptation trials is indicated by the black horizontal bars. The saccade target was presented at 0°. In the 20 baseline trials, where the delay was 0 ms (shown in Fig. 2A), the saccade undershot the target by 1.2° on average. The average undershoot size diminished as a function of saccade target delay duration (for 250-ms delay, 0.75°; for 500-ms delay, 0.25°). This finding is consistent with earlier studies reporting reduced undershoot for saccades performed to stationary saccade targets (Lemij and Collewijn 1989). The amount of adaptation was estimated by the difference between the average saccade landing in the 20 baseline trials, where the target was not displaced, and the last 20 adaptation trials.
Fig. 2.
A: adaptation curve from a representative subject for an adaptation with 0-ms overlap duration between fixation point and saccade target. Black points represent horizontal saccade landing positions in preadaptation trials, and gray points represent horizontal saccade landing positions in adaptation trials. An exponential fit function is fitted to the first 150 adaptation trials (shown in black). Black lines indicate the median landing position in the pre- and the postadaptation trials. The postadaptation trials shown contain the reinforcement adaptation trials, which should keep adaptation at the steady level. B: adaptation curve from a representative subject for an adaptation with 250-ms overlap duration between fixation point and saccade target. Same conventions as in A. C: adaptation curve from a representative subject for an adaptation with 500-ms overlap duration between fixation point and saccade target. Same conventions as in A. D: mean adaptation magnitude from all 3 overlap duration sessions averaged across subjects. Error bars represent SE.
Figure 2D shows the adaptation amount averaged across all subjects for the three different delay durations. The average adaptation amount was statistically indistinguishable between delay durations [repeated-measures ANOVA, degrees of freedom (df) = 2, F = 0.66, P = 0.602].
Since the presaccadic gain varied between adaptation sessions, adaptation magnitude was also calculated as a percentage of the unadapted saccade gain [(landing adapted − landing unadapted)/landing unadapted × 100]. In adaptation sessions with 0-ms delay the percentage was 29.9% (SE 9.4), in adaptation sessions with 250-ms delay it was 16% (SE 4.9), and in adaptation sessions with 500-ms delay it was 24.7% (SE 11.37). Similarly, when calculated as a percentage, adaptation magnitude did not differ significantly between sessions (repeated-measures ANOVA, df = 2, F = 0.735, P = 0.576). For each adaptation session an exponential fit function [a + b × E(−t/c)] was fitted to the first 150 adaptation trials to estimate the number of trials after which the adaptation steady state was reached. On average, in adaptation sessions with 0-ms delay the adaptation steady state was reached after 63 (SE 38) trials, in sessions with 250-ms delay after 45 (SE 29) trials, and in sessions with 500-ms delay after 47 (SE 27) trials. The adaptation speed did not differ significantly between sessions (repeated-measures ANOVA, df = 2, F = 3.980, P = 0.201).
Average saccade landing positions.
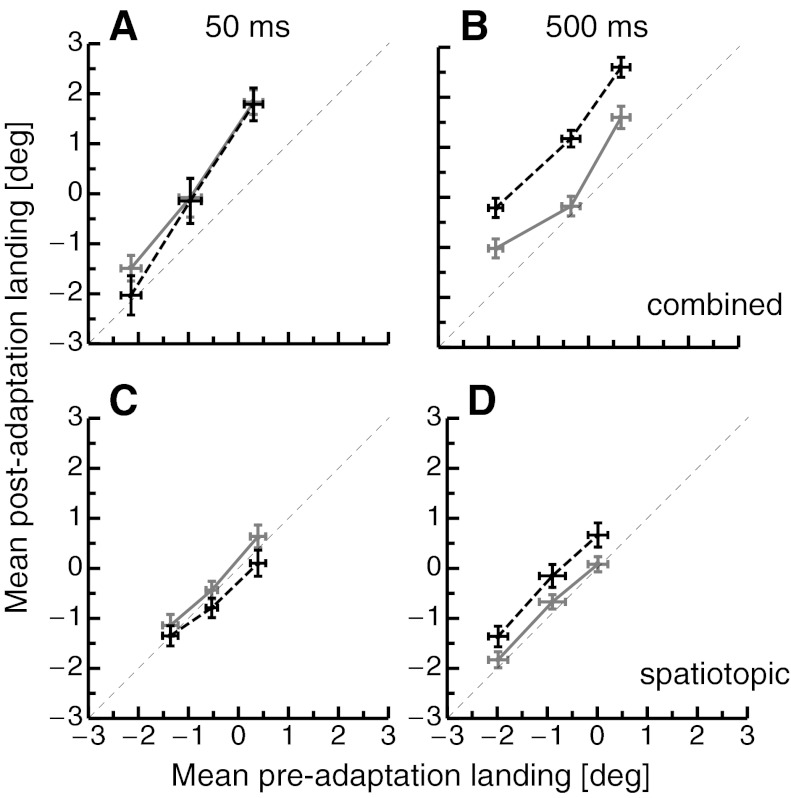
Figure 3 shows mean saccade landing positions from the test trials (pre- vs. posttrials), exemplary for outward adaptation with 0- and 500-ms delay. The data show that the three jitter positions were clearly reflected in saccade landings, thus indicating that the saccades were guided by the visual target signal and not performed in a stereotyped manner. If saccades were unchanged by adaptation, all data points should be found on the identity line. When saccade targets were presented for 50 ms in the combined condition, a shift of saccade landing positions in outward direction was seen. The gray solid line in Fig. 3 represents data from adaptation sessions with 0-ms delay, and the black dashed line represents data from adaptation sessions with 500-ms delay. The shift is very similar for both adaptation sessions (Fig. 3A). When saccade targets were presented for 500 ms, however, a strong shift was observed only after adaptation with 500-ms delay but not after adaptation with 0-ms delay (Fig. 3B). This is consistent with the often reported finding that voluntary saccade adaptation but not reactive saccade adaptation transfers to voluntary test saccades. In the spatiotopic condition with saccade targets presented for 50 ms (Fig. 3C), the 0- and 500-ms saccade target delay durations during adaptation did not produce considerable differences in the saccade landing. Only the jittering of the saccade target position was clearly reflected in the saccade landing. When saccades executed to saccade targets with a presentation duration of 500 ms (Fig. 3D) were tested in the spatiotopic condition, the delay duration during adaptation clearly shifted the saccade landing positions. With 0-ms delay duration, saccade landing positions (gray points, solid line) are centered on the identity line, indicating that they are almost identical between pre- and postadaptation trials. With 500-ms delay duration during adaptation (black points, black dashed line), saccade landing in the postadaptation trials is shifted in the rightward direction, which is the direction the saccade target was displaced during adaptation. Thus saccades in the spatiotopic condition, which were leftward saccades, were shortened by the rightward target displacement in the adaptation trials. This result is in accordance with a mechanism that adapts the representation of external spatial positions rather than vector-specific saccade gain.
Fig. 3.
A: mean preadaptation landing vs. mean postadaptation landing positions averaged separately for all 3 jitter positions in the combined condition where saccade targets were shown for 50 ms. The gray solid line indicates data from adaptation sessions with 0-ms overlap, and the black dashed line represents data from adaptation sessions with 500-ms overlap. Error bars represent SE. B: landing positions in the combined condition where saccade targets were shown for 500 ms. Same conventions as in A. C: landing positions in the spatiotopic condition where saccade targets were shown for 50 ms. Same conventions as in A. D: landing positions in the spatiotopic condition where saccade targets were shown for 500 ms. Same conventions as in A.
Average outward adaptation in the combined condition.
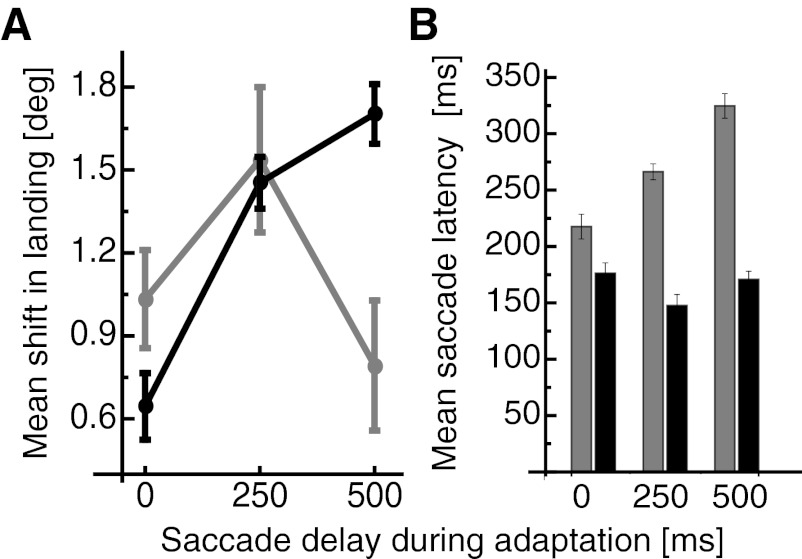
Before and after adaptation, two conditions were tested in random order: the combined condition, so called because retinotopic and spatiotopic coding of the saccade target position were not dissociated, and the spatiotopic condition, in which the pure spatiotopic coding was tested. In each of these two conditions the final saccade target was presented for either 50 or 500 ms. To calculate the average amount of adaptation transfer to the test trials, saccade landing was averaged for each of the three jitter positions separately in the pre- and the posttrials. The adaptation transfer was given by the difference between pre- and posttest landing positions. Landing positions from the posttrials from each jitter position were subtracted by the average landing positions in the pretrials from the respective jitter position. These data were then collapsed within each condition and averaged. Average changes in saccade landing in the combined condition are shown in Fig. 4A. Strong adaptive shifts for saccades performed to targets that were presented for 50 ms (shown in gray) were found after adaptation with delay durations of 0 ms [1° (SE 0.2°)] and 250 ms [1.5° (SE 0.3°)]. A weaker adaptive shift was found after adaptation with 500-ms delay duration [0.8° (SE 0.2°)]. Saccades executed to targets shown for 50 ms belong to the category of reactive saccades. The weaker adaptation transfer for the 500-ms delay duration during adaptation is consistent with the previously reported independent adaptation of reactive saccades and saccades with longer delay durations (Deubel 1995; Schnier and Lappe 2011). Landing positions of saccades that were performed to targets presented for 500 ms are shown in black. The shift size of these saccades is rather weak after adaptation with 0-ms delay duration [0.6° (SE 0.1°)]. However, the magnitude of the shift increases with longer delay durations and reaches a shift size of 1.7° (SE 0.1°) after a delay duration of 500 ms. The different shift sizes for the varying delay durations are again consistent with the finding of adaptation selectivity for variations in the delay durations. A two-way repeated-measures ANOVA confirmed a significant main effect for the two target durations (df = 1, F = 6.665, P < 0.012) and for the three delay durations (df = 2, F = 13.509, P < 0.001). A significant interaction effect showed that the influence of delay duration during adaptation on shift size was stronger for saccades performed to targets presented for 500 ms (df = 2, F = 5.481, P < 0.006), which is in agreement with the weak transfer between reactive and voluntary saccades: adaptation with 500-ms delay leads to stronger adaptation for test saccades performed to targets presented for 500 ms. Conversely, adaptation with 0-ms delay leads to stronger adaptation for test saccades performed to targets presented for 50 ms.
Fig. 4.
A: mean shift in saccade landing position for the 3 overlap durations during adaptation in the combined condition. Shifts of saccades performed to targets presented for 50 ms are shown in gray, and shifts of saccades performed to targets presented for 500 ms are shown in black. Error bars represent SE. B: mean saccade latencies in the combined condition. Latencies of saccades performed to targets presented for 50 ms are shown in gray, and latencies of saccades performed to targets presented for 500 ms are shown in black. Error bars represent SE.
Since the adaptation magnitude varied between conditions (see Fig. 2D), the adaptation transfer to the test saccades must also be related to the according adaptation strength. When calculated as a percentage, [(postspatiotopic trials − prespatiotopic trials)/prespatiotopic trials]/%adaptation × 100, the shift in saccade landing for targets presented for 500 ms is stronger when the saccade target delay is longer than 0 ms [0-ms delay, 12.3% (SE 2.3%); 250-ms delay, 51.7% (SE 3.3%); 500-ms delay, 39.4% (SE 2.5%)], whereas the transfer was different when targets were presented for 50 ms [0-ms delay, 20.2% (SE 3.5%); 250-ms delay, 55.9% (SE 9.6%); 500-ms delay, 18.8% (SE 5.6%)]. A repeated-measures ANOVA calculated with the percentage data showed a significant main effect for target duration (df = 1, F = 5.51, P = 0.022) and for delay duration (df = 2, F = 35.43, P < 0.001), as well as a significant interaction effect (df = 2, F = 5.97, P = 0.004), which showed that the delay duration during adaptation affected the shift size more strongly when saccade targets were presented for 500 ms.
Figure 4B shows saccade latencies in the combined condition. Subjects were required to initiate their saccades when the saccade target disappeared. The duration between saccade target onset and saccade initiation thus depends on the delay duration and the saccade latency. Saccade latencies were defined as the duration between offset of the fixation point and start of the saccade. Saccade latencies were significantly higher when targets were presented for 50 ms (df = 1, F = 330.229, P < 0.001). Saccade latencies also varied significantly for the three delay durations during adaptation (df = 2, F = 58.661, P < 0.001). A significant interaction effect (df = 2, F = 11.717, P < 0.001) showed that the latency increase with overlap duration was stronger for 50-ms target durations than for 500-ms target durations. No significant correlation between saccade latency and the adaptive shift size was found [r(6) = −0.3].
Average outward adaptation in the spatiotopic condition.
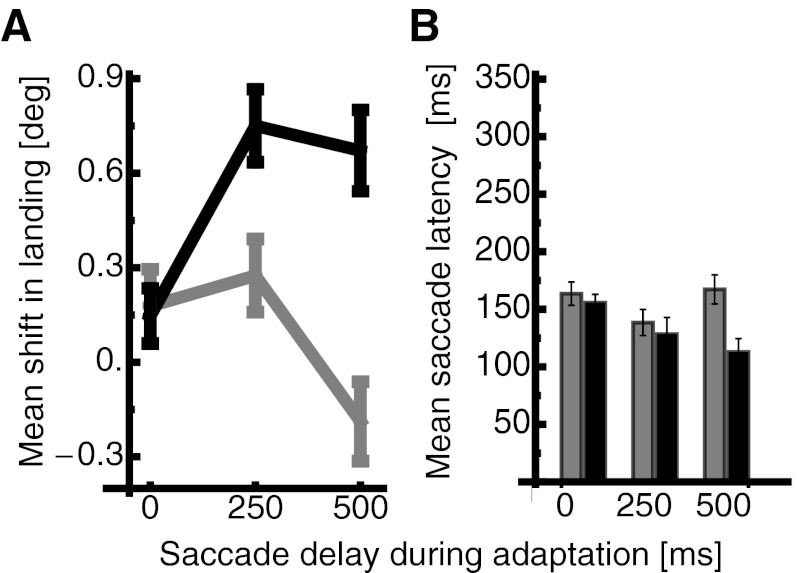
Average changes in saccade landing against the three delay durations during adaptation in the spatiotopic condition are shown in Fig. 5A. The average change in saccade landing is calculated as the difference between the landing in the pre- and the postadaptation trials. Mean shifts in landing are shown for saccades that were performed to targets presented for 50 ms (shown in gray) and to targets presented for 500 ms (shown in black). Mean shifts in saccade landing for targets that were visible for only 50 ms were generally weak for all three delay durations. However, when targets were presented for 500 ms, the size of the shifts increased as a function of delay duration. For 0-ms delay duration the average shift was 0.1° (SE 0.1°); with a delay duration of 250 ms, the shift increased to 0.8° (SE 0.1°) and remained at that level with 500-ms delay duration [0.7° (SE 0.1°)]. Thus saccades in the spatiotopic condition, which were leftward saccades, were shortened by the rightward target displacement in the adaptation trials. This result is in accordance with a mechanism that adapts the representation of external spatial positions, rather than vector-specific saccade gain. A two-way repeated-measures ANOVA confirmed a significant difference in shift size between targets presented for 50 ms and targets presented for 500 ms (df = 1, F = 21.168, P < 0.001). A significant interaction effect between the factors saccade target presentation duration and delay duration during adaptation (df = 2, F = 5.447, P = 0.007) confirmed that the increase of the spatiotopic adaptation occurred only for targets presented for 500 ms.
Fig. 5.
A: mean shift in saccade landing position for the 3 overlap durations during adaptation in the spatiotopic condition. Same conventions as in Fig. 4A. Error bars represent SE. B: mean saccade latencies in the spatiotopic condition. Same conventions as in Fig. 4B. Error bars represent SE.
Calculated as a percentage of adaptation magnitude, the spatiotopic effect for targets presented for 500 ms still rose with increasing delay duration in the adaptation sessions [0-ms delay, 6.1% (SE 3.6%); 250-ms delay, 57.9% (SE 8.9%); 500-ms delay, 33.7% (SE 6.5%)], whereas the effect remained low or absent when targets were presented for 50 ms [0-ms delay, 7% (SE 4.6%); 250-ms delay, 20.1% (SE 8.5%); 500-ms delay, −8.9% (SE 5.9%)]. A repeated-measures ANOVA, calculated with the percentage data, confirmed significantly stronger adaptive shifts when targets were presented for 500 ms (df = 1, F = 31.58, P < 0.001) and significantly stronger adaptation for longer delay duration during adaptation (df = 2, F = 6.86, P = 0.02). A significant interaction effect (df = 2, F = 5.93, P = 0.04) showed that the influence of the delay duration during adaptation was stronger when targets were shown for 500 ms.
Saccade latencies for the two target presentation durations are shown in Fig. 5B. Latencies were significantly higher when targets were presented for 50 ms (2-way repeated-measures ANOVA, df = 1, F = 43.825, P < 0.001). Saccade latencies for the three different delay durations during adaptation differed significantly also (df = 2, F = 18.415, P < 0.001). No significant interaction effect was found (df = 2, F = 2.721, P = 0.073). A strong significant negative correlation between saccade latency and the adaptive spatiotopic shift was found [r(6) = 0.9, P < 0.0001], indicating that lower latencies yielded larger spatiotopic shifts. This result shows that in the latency period, where the visual target signal no longer is present, the spatiotopic representation of the target deteriorates in memory. A spatiotopic reference frame thus depends on a constantly present visual target signal to build up. A 2 × 2 × 3 repeated-measures ANOVA was calculated with the factors condition (combined/spatiotopic), target duration (50 ms/500 ms), and delay duration during adaptation (0 ms/250 ms/500 ms). Saccade latencies were significantly longer in the combined condition (df = 1, F = 17.861, P < 0.001) and significantly longer when saccade targets were presented for 50 ms (df = 1, F = 256.156, P < 0.001). The influence of saccade target duration and delay duration during adaptation on saccade latencies was significantly stronger in the combined condition, as indicated by a significant interaction effect between condition and target duration (df = 1, F = 34.133, P < 0.001), a significant interaction effect between condition and delay duration during adaptation (df = 2, F = 86.338, P < 0.001), and a significant interaction effect between condition, target duration, and delay duration during adaptation (df = 2, F = 8.760, P < 0.001).
Average inward adaptation in the combined and the spatiotopic conditions.
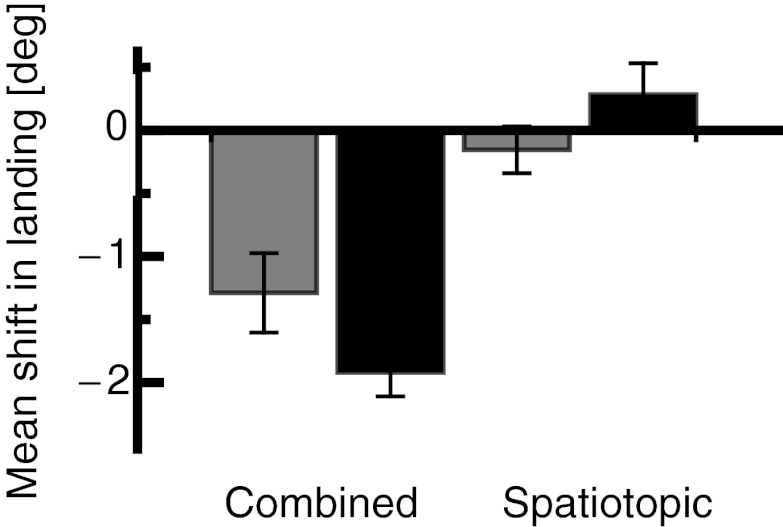
The combined and the spatiotopic conditions were also measured for inward adaptation with saccade target presentation durations of 50 and 500 ms. For inward adaptation, saccade landing was measured after adaptation with a delay duration of 500 ms, since spatiotopic effects after outward adaptation were strongest for that duration (see Fig. 3A). Figure 6 shows the average amount of shift in saccade landing after adaptation in the inward direction for the combined and the spatiotopic conditions (gray colors refer to a target duration of 50 ms and black color to a target duration of 500 ms). The shift in the spatiotopic condition was almost zero for targets presented for 50 ms [0.2° (SE 0.2°)] and for targets presented for 500 ms [−0.4° (SE 0.3°)]. In the combined condition, however, there was a clear shift in landing for saccades executed to targets presented for 50 ms [1.3° (SE 0.3°)] and for saccades executed to targets presented for 500 ms [1.8° (SE 0.2°)]. A two-way repeated-measures ANOVA revealed significantly different adaptation magnitudes between the combined and the spatiotopic conditions (df = 1, F = 43.326, P < 0.001). No significant interaction effect was found. The saccade target presentation durations, however, did not influence saccade landing significantly.
Fig. 6.
Mean shift in saccade landing in the spatiotopic and combined conditions for 50-ms (black) and 500-ms (gray) saccade target presentations after inward adaptation with 500-ms overlap duration. Error bars represent SE.
Calculated as a percentage of adaptation magnitude, the transfer of adaptation in the combined condition remained strong [50-ms target duration, −52.9% (SE 12.9%); 500-ms target duration, −59.12% (SE 5.8%)], whereas the spatiotopic effect after inward adaptation still remained absent [50-ms target duration, −6.35% (SE 7.6%); 500-ms target duration, 9.22% (SE 8%)]. A two-way repeated-measures ANOVA confirmed a significant main effect for the combined vs. the spatiotopic condition (df = 1, F = 38.816, P < 0.001) but not for the durations of the saccade target (df = 1, F = 0.004, P = 0.948). No significant interaction effect was found.
That spatiotopic effects were absent for inward adaptation confirms the assumption of a general gain reduction mechanism for inward saccade adaptation (Albano 1996; Deubel 1995; Frens and van Opstal 1994; Noto et al. 1999; Semmlow et al. 1989).
DISCUSSION
The present series of experiments investigated the reference frame in saccade adaptation. Saccade landing positions were studied in a paradigm following a previous study (Zimmermann et al. 2011): saccades were performed to an adapted saccade target in the rightward direction from the left part of the screen as during adaptation, to test for combined effects of retinal vector and spatial position coding. To test for a spatiotopic coding of the saccade target position, leftward saccades starting from the right part of the screen were executed to the adapted target. If saccade outward adaptation induces a shift in the saccade target representation in spatiotopic coordinates, then rightward saccades performed in the same direction as during adaptation should be lengthened and leftward saccades from the opposite direction should be shortened. A spatiotopic representation of the saccade target position depended on the delay duration of the saccade target in the test trials: the target had to be presented a certain duration before saccade execution to shorten saccades performed from the opposite direction of that during adaptation to the adapted saccade target. A spatiotopic representation of the saccade target position thus needs a certain amount of time to build up. This spatiotopic adaptation is consistent with earlier findings showing that the motor and visual changes in saccade outward adaptation are strongly modulated by eye position when saccades to stationary rather than to suddenly appearing targets are adapted (Zimmermann and Lappe 2011). The availability of the visual saccade target thus might play an important role in the construction of a spatiotopic reference frame (Niemeier and Karnath 2003).
The required size of saccades that had to be performed in the spatiotopic posttrials were larger than those in the adaptation trials. It might therefore be that a range effect (Kapoula and Robinson 1986) or fatigue could explain the shortening of the saccades in the spatiotopic trials. However, the finding that the spatiotopic effect rises with increasing delay adaptation and also varies between inward and outward adaptation rules out explanations for the effect which are not related to adaptation. Saccade adaptation is selective for the type of saccade tested: when reactive saccades performed to suddenly appearing targets are adapted, transfer to saccades performed to stationary targets is only partial (Alahyane et al. 2007; Collins and Dore-Mazars 2006; Cotti et al. 2007; Deubel 1995; Erkelens and Hulleman 1993; Fujita et al. 2002; Hopp and Fuchs 2004; Zimmermann and Lappe 2009). Although there is general agreement that the reactive saccade type is independent from the group of voluntary saccade types, it is not clear what exactly is the functional difference between these two types of saccades. The crucial experimental variable that dissociates between the two types is the duration the saccade target has been presented before saccade execution. The percentage of transfer from reactive saccade adaptation to delayed saccades, a type of voluntary saccades, decreases as a function of delay duration of the saccade targets (Deubel 1995; Schnier and Lappe 2011).
The data in the combined condition confirm the independence of reactive and voluntary saccades: if saccades were adapted with 0-ms delay duration (i.e., reactive saccades), stronger adaptation transfer was found for saccades performed to targets presented for 50 ms than for saccades performed to targets presented for 500 ms. Conversely, if saccades were adapted with 500-ms delay duration (i.e., voluntary saccades), stronger transfer was found for saccades performed to targets presented for 500 ms than for saccades performed to targets presented for 50 ms. The data thus suggest that the reactive-voluntary difference in saccade outward adaptation is not really dependent on the “intentionality” of the saccade but on the type of reference frame in which the saccades are coded.
Regarding the definition of reactive saccades as externally triggered and voluntary saccades as internally triggered, all saccades in the present study should be classified as externally triggered, since the saccade go signal always was the offset of the fixation point. However, the delay duration during adaptation yielded significantly different shifts in test saccade landing positions for targets presented for 50 ms and for targets presented for 500 ms. Voluntary saccades with long saccade target presentations are usually associated with higher saccade latencies than reactive saccades, where the target suddenly appears (cf. Pelisson et al. 2010 for review). Saccade latencies in voluntary saccade conditions in this study (250- and 500-ms delay duration during adaptation) were generally quite low, which is a well-known phenomenon, occurring when saccades can be anticipated or preplanned. Kalesnykas and Hallett (1987) estimated that the latencies of anticipated saccades range between 100 and 120 ms. Especially in saccades performed to targets shown for 500 ms, the oculomotor system has ample time to preplan the eye movement and delay saccade initiation until offset of the fixation point. Consistently, latencies of saccades to targets with 500-ms presentation duration were shorter than those of saccades to targets with 50-ms presentation duration. In the combined condition, latencies of saccades to targets with 50-ms duration increased depending on the delay duration during adaptation. This might be a training effect from the adaptation period: it is likely that subjects here transferred to the test trials their habit to delay their saccades in the adaptation trials. This effect is saccade vector specific, since it does not occur in the spatiotopic condition. The construction of a spatiotopic representation of the saccade target position depends on the permanent presence of the visual saccade target. In the saccade latency period, no visual saccade target was shown. A strong negative correlation between saccade latency and spatiotopic shift size thus showed that in the absence of a visual signal, the spatiotopic representation decays in memory.
No spatiotopic shifts in saccade landing were found for saccade inward adaptation, consistent with earlier claims that inward adaptation operates on a general gain reduction (Albano 1996; Deubel 1995; Frens and van Opstal 1994; Noto et al. 1999; Semmlow et al., 1989). Several studies have shown significant differences between inward and outward saccade adaptation in time course and strength of adaptation (Cecala and Freedman 2009; Miller et al. 1981; Schnier and Lappe 2011) as well as in modifications of motor parameters (Ethier et al. 2008; Straube and Deubel 1995; Straube et al. 1997; Zimmermann and Lappe 2010). Transfer of adaptation to antisaccades (Panouilleres et al. 2009), to hand-pointing movements (Hernandez et al. 2008), and to visual perception (Zimmermann and Lappe 2010) after saccade adaptation was found only for outward but not for inward adaptation. These differences suggest separate neural mechanisms for inward and outward adaptation (Pelisson et al. 2010). Computational modeling studies that took into account the saccade dynamics of adapted saccades suggested that inward adaptation results from changes to the internal monitoring in a forward model, whereas outward adaptation is achieved by changes in the motor command, i.e., a shift in the saccade target representation (Chen-Harris et al. 2008; Ethier et al. 2008; Xu-Wilson et al. 2009). Consistent with that proposal, Semmlow et al. (1989) observed the amount of shifts in saccade landing positions of adapted saccades over a wide range of several starting and landing positions and concluded that outward adaptation operated on a remapping of final position in world coordinates, whereas inward adaptation is achieved through an overall reduction of gain.
Electrophysiological studies in monkeys (Catz et al. 2008; Kojima et al. 2010; Prsa and Thier 2011; Soetedjo and Fuchs 2006; Soetedjo et al. 2008), lesion studies in humans (Golla et al. 2008; Xu-Wilson et al. 2009), and a brain imaging study (Desmurget et al. 1998) have found changes after saccade adaptation in the oculomotor vermis of the cerebellum. Disruptions of the lateral (Panouilleres et al. 2012) and the posterior (Jenkinson and Miall 2010) cerebellum with transcranial magnetic stimulation demonstrated reductions in saccade adaptation. Changes in other subcortical structures, such as the nucleus reticularis tegmenti pontis (Takeichi et al. 2005), have also been reported. To modify the saccade target registration, adaptation should occur at higher levels of the oculomotor system. An involvement of the superior colliculus has been controversially debated (Edelman and Goldberg 2002; Quessy et al. 2010; Takeichi et al. 2007). However, ascending projections from the cerebellum to the cortex are also involved in saccade adaptation: a lesion study in humans showed reduced saccade adaptation after lesions in the cerebellar thalamus (Gaymard et al. 2001). The idea that reactive and voluntary saccade adaptation are generated on separate levels in the oculomotor transform is supported by physiological evidence of recent brain imaging results (Blurton et al. 2012; Gerardin et al. 2012) and a study in human patients (Alahyane et al. 2007).
In conclusion, spatiotopic maps of visual space guiding saccade eye movements take time to construct. The transformation from a general gain adaptation mechanism to one that is selective for position in external space takes time to build up. That positional selectivity evolves as a function of time may explain why different saccade types are separately adaptable.
GRANTS
This research was supported by the European Union, Project STANIB Grant FP7-ERC, and the Italian Ministry of University and Research (MIUR-PRIN).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
E.Z. conception and design of research; E.Z. performed experiments; E.Z. analyzed data; E.Z. interpreted results of experiments; E.Z. prepared figures; E.Z. drafted manuscript; E.Z. edited and revised manuscript; E.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Prof. Markus Lappe and Dr. Marco Cicchini for helpful suggestions on the manuscript.
REFERENCES
- Alahyane N, Fonteille V, Urquizar C, Salemme R, Nighoghossian N, Pelisson D, Tilikete C. Separate neural substrates in the human cerebellum for sensory-motor adaptation of reactive and of scanning voluntary saccades. Cerebellum 7: 595–601, 2008 [DOI] [PubMed] [Google Scholar]
- Alahyane N, Pelisson D. Eye position specificity of saccadic adaptation. Invest Ophthalmol Vis Sci 45: 123–130, 2004 [DOI] [PubMed] [Google Scholar]
- Alahyane N, Salemme R, Urquizar C, Cotti J, Guillaume A, Vercher JL, Pélisson D. Oculomotor plasticity: are mechanisms of adaptation for reactive and voluntary saccades separate? Brain Res 1135: 107–121, 2007 [DOI] [PubMed] [Google Scholar]
- Albano JE. Adaptive changes in saccade amplitude: oculocentric or orbitocentric mapping? Vision Res 36: 2087–2098, 1996 [DOI] [PubMed] [Google Scholar]
- Awater H, Burr D, Lappe M, Morrone MC, Goldberg ME. Effect of saccadic adaptation on localization of visual targets. J Neurophysiol 93: 3605–3614, 2005 [DOI] [PubMed] [Google Scholar]
- Bahcall DO, Kowler E. Illusory shifts in visual direction accompany adaptation of saccadic eye movements. Nature 400: 864–866, 1999 [DOI] [PubMed] [Google Scholar]
- Blurton SP, Raabe M, Greenlee MW. Differential cortical activation during saccadic adaptation. J Neurophysiol 107: 1738–1747, 2012 [DOI] [PubMed] [Google Scholar]
- Catz N, Dicke PW, Thier P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proc Natl Acad Sci USA 105: 7309–7314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecala AL, Freedman EG. Head-unrestrained gaze adaptation in the rhesus macaque. J Neurophysiol 101: 164–183, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. J Neurosci 28: 2804–2813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Dore-Mazars K. Eye movement signals influence perception: evidence from the adaptation of reactive and volitional saccades. Vision Res 46: 3659–3673, 2006 [DOI] [PubMed] [Google Scholar]
- Collins T, Dore-Mazars K, Lappe M. Motor space structures perceptual space: evidence from human saccadic adaptation. Brain Res 1172: 32–39, 2007 [DOI] [PubMed] [Google Scholar]
- Collins T, Rolfs M, Deubel H, Cavanagh P. Post-saccadic location judgments reveal remapping of saccade targets to non-foveal locations. J Vis 9: 29.1–29.9, 2009 [DOI] [PubMed] [Google Scholar]
- Cotti J, Guillaume A, Alahyane N, Pelisson D, Vercher JL. Adaptation of voluntary saccades, but not of reactive saccades, transfers to hand pointing movements. J Neurophysiol 98: 602–612, 2007 [DOI] [PubMed] [Google Scholar]
- Cotti J, Panouilleres M, Munoz DP, Vercher JL, Pélisson D, Guillaume A. Adaptation of reactive and voluntary saccades: different patterns of adaptation revealed in the antisaccade task. J Physiol 587: 127–138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Pélisson D, Urquizar C, Prablanc C, Alexander GE, Grafton ST. Functional anatomy of saccadic adaptation in humans. Nat Neurosci 1: 524–528, 1998 [DOI] [PubMed] [Google Scholar]
- Deubel H. Is adaptation saccadic context-specific? In: Eye Movement Research: Mechanisms, Processes and Applications. Amsterdam: Elsevier Science, 1995, p. 177–178 [Google Scholar]
- Edelman JA, Goldberg ME. Effect of short-term saccadic adaptation on saccades evoked by electrical stimulation in the primate superior colliculus. J Neurophysiol 87: 1915–1923, 2002 [DOI] [PubMed] [Google Scholar]
- Erkelens CJ, Hulleman J. Selective adaptation of internally triggered saccades made to visual targets. Exp Brain Res 93: 157–164, 1993 [DOI] [PubMed] [Google Scholar]
- Ethier V, Zee DS, Shadmehr R. Changes in control of saccades during gain adaptation. J Neurosci 28: 13929–13937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frens MA, van Opstal AJ. Transfer of short-term adaptation in human saccadic eye movements. Exp Brain Res 100: 293–306, 1994 [DOI] [PubMed] [Google Scholar]
- Fujita M, Amagai A, Minakawa F, Aoki M. Selective and delay adaptation of human saccades. Brain Res Cogn Brain Res 13: 41–52, 2002 [DOI] [PubMed] [Google Scholar]
- Garaas TW, Pomplun M. Distorted object perception following whole-field adaptation of saccadic eye movements. J Vis 11: 2, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard B, Rivaud-Pchoux S, Yelnik J, Pidoux B, Ploner CJ. Involvement of the cerebellar thalamus in human saccade adaptation. Eur J Neurosci 14: 554–560, 2001 [DOI] [PubMed] [Google Scholar]
- Gerardin P, Mique A, Urquizar C, Pélisson D. Functional activation of the cerebral cortex related to sensorimotor adaptation of reactive and voluntary saccades. Neuroimage 61: 1100–1112, 2012 [DOI] [PubMed] [Google Scholar]
- Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci 27: 132–144, 2008 [DOI] [PubMed] [Google Scholar]
- Havermann K, Zimmermann E, Lappe M. Eye position effects in saccadic adaptation. J Neurophysiol 106: 2536–2545, 2011 [DOI] [PubMed] [Google Scholar]
- Hernandez TD, Levitan CA, Banks MS, Schor CM. How does saccade adaptation affect visual perception? J Vis 8: 3.1–3.16, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog Neurobiol 72: 27–53, 2004 [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Miall RC. Disruption of saccadic adaptation with repetitive transcranial magnetic stimulation of the posterior cerebellum in humans. Cerebellum 9: 548–555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnykas RP, Hallett PE. The differentiation of visually guided and anticipatory saccades in gap and overlap paradigms. Exp Brain Res 68: 115–121, 1987 [DOI] [PubMed] [Google Scholar]
- Kapoula Z, Robinson DA. Saccadic undershoot is not inevitable: saccades can be accurate. Vision Res 26: 735–743, 1986 [DOI] [PubMed] [Google Scholar]
- Klingenhoefer S, Bremmer F. Saccadic suppression of displacement in face of saccade adaptation. Vision Res 51: 881–889, 2011 [DOI] [PubMed] [Google Scholar]
- Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci 30: 3715–3727, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemij HG, Collewijn H. Differences in accuracy of human saccades between stationary and jumping targets. Vision Res 29: 1737–1748, 1989 [DOI] [PubMed] [Google Scholar]
- McLaughlin SC. Parametric adjustment in saccadic eye movements. Percept Psychophys 2: 59–362, 1967 [Google Scholar]
- Miller JM, Anstis T, Templeton WB. Saccadic plasticity: parametric adaptive control by retinal feedback. J Exp Psychol Hum Percept Perform 7: 356–366, 1981 [DOI] [PubMed] [Google Scholar]
- Mueller AL, Davis AJ, Robinson FR. Long-term size-increasing adaptation of saccades in macaques. Neuroscience 8: 38–47, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeier M, Karnath HO. Stimulus-driven and voluntary saccades are coded in different coordinate systems. Curr Biol 13: 585–589, 2003 [DOI] [PubMed] [Google Scholar]
- Noto CT, Watanabe S, Fuchs AF. Characteristics of simian adaptation fields produced by behavioral changes in saccade size and direction. J Neurophysiol 81: 2798–2813, 1999 [DOI] [PubMed] [Google Scholar]
- Panouilleres M, Weiss T, Urquizar C, Salemme R, Munoz DP, Pélisson D. Behavioral evidence of separate adaptation mechanisms controlling saccade amplitude lengthening and shortening. J Neurophysiol 101: 1550–1559, 2009 [DOI] [PubMed] [Google Scholar]
- Panouilleres M, Neggers SF, Gutteling TP, Salemme R, van der Stigchel S, van der Geest JN, Frens MA, Pélisson D. Transcranial magnetic stimulation and motor plasticity in human lateral cerebellum: dual effect on saccadic adaptation. Hum Brain Mapp 33: 1512–1525, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelisson D, Alahyane N, Panouillres M, Tilikete C. Sensorimotor adaptation of saccadic eye movements. Neurosci Biobehav Rev 34: 1103–1120, 2010 [DOI] [PubMed] [Google Scholar]
- Prsa M, Thier P. The role of the cerebellum in saccadic adaptation as a window into neural mechanisms of motor learning. Eur J Neurosci 33: 2114–2128, 2011 [DOI] [PubMed] [Google Scholar]
- Quessy S, Quinet J, Freedman EG. The locus of motor activity in the superior colliculus of the rhesus monkey is unaltered during saccadic adaptation. J Neurosci 30: 14235–14244, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnier F, Lappe M. Differences in intersaccadic adaptation transfer between inward and outward adaptation. J Neurophysiol 106: 1399–1410, 2011 [DOI] [PubMed] [Google Scholar]
- Schnier F, Lappe M. Mislocalization of stationary and flashed bars after saccadic inward and outward adaptation of reactive saccades. J Neurophysiol 107: 3062–3070, 2012 [DOI] [PubMed] [Google Scholar]
- Schnier F, Zimmermann E, Lappe M. Adaptation and mislocalization fields for saccadic outward adaptation in humans. J Eye Mov Res 3: 1–18, 2010 [Google Scholar]
- Semmlow JL, Gauthier GM, Vercher JL. Mechanisms of short-term saccadic adaptation. J Exp Psychol Hum Percept Perform 15: 249–258, 1989 [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Fuchs AF. Complex spike activity of Purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J Neurosci 26: 7741–7755, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetedjo R, Kojima Y, Fuchs AF. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? J Neurophysiol 100: 1949–1966, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube A, Deubel H. Rapid gain adaptation affects the dynamics of saccadic eye movements in humans. Vision Res 35: 3451–3458, 1995 [DOI] [PubMed] [Google Scholar]
- Straube A, Fuchs AF, Usher S, Robinson FR. Characteristics of saccadic gain adaptation in rhesus macaques. J Neurophysiol 77: 874–895, 1997 [DOI] [PubMed] [Google Scholar]
- Takeichi N, Kaneko CR, Fuchs AF. Discharge of monkey nucleus reticularis tegmenti pontis neurons changes during saccade adaptation. J Neurophysiol 94: 1938–1951, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi N, Kaneko CRS, Fuchs AF. Activity changes in monkey superior colliculus during saccade adaptation. J Neurophysiol 97: 4096–4107, 2007 [DOI] [PubMed] [Google Scholar]
- Wulff S, Bosco A, Havermann K, Placenti G, Fattori P, Lappe M. Eye position effects in saccadic adaptation in macaques. J Neurophysiol 108: 2819–2826, 2012 [DOI] [PubMed] [Google Scholar]
- Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci 29: 12930–12939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann E, Burr D, Morrone MC. Spatiotopic visual maps revealed by saccadic adaptation in humans. Curr Biol 21: 1380–1384, 2011 [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Lappe M. Mislocalization of flashed and stationary visual stimuli after adaptation of reactive and scanning saccades. J Neurosci 29: 11055–11064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann E, Lappe M. Motor signals in visual localization. J Vis 6: 11, 2010 [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Lappe M. Eye position effects in oculomotor plasticity and visual localization. J Neurosci 31: 7341–7348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]