Abstract
QX-314 (N-ethyl-lidocaine) is a cationic lidocaine derivative that blocks voltage-dependent sodium channels when applied internally to axons or neuronal cell bodies. Coapplication of external QX-314 with the transient receptor potential vanilloid 1 protein (TRPV1) agonist capsaicin produces long-lasting sodium channel inhibition in TRPV1-expressing neurons, suggestive of QX-314 entry into the neurons. We asked whether QX-314 entry occurs directly through TRPV1 channels or through a different pathway (e.g., pannexin channels) activated downstream of TRPV1 and whether QX-314 entry requires the phenomenon of “pore dilation” previously reported for TRPV1. With external solutions containing 10 or 20 mM QX-314 as the only cation, inward currents were activated by stimulation of both heterologously expressed and native TRPV1 channels in rat dorsal root ganglion neurons. QX-314-mediated inward current did not require pore dilation, as it activated within several seconds and in parallel with Cs-mediated outward current, with a reversal potential consistent with PQX-314/PCs = 0.12. QX-314-mediated current was no different when TRPV1 channels were expressed in C6 glioma cells, which lack expression of pannexin channels. Rapid addition of QX-314 to physiological external solutions produced instant partial inhibition of inward currents carried by sodium ions, suggesting that QX-314 is a permeant blocker. Maintained coapplication of QX-314 with capsaicin produced slowly developing reduction of outward currents carried by internal Cs, consistent with intracellular accumulation of QX-314 to concentrations of 50–100 μM. We conclude that QX-314 is directly permeant in the “standard” pore formed by TRPV1 channels and does not require either pore dilation or activation of additional downstream channels for entry.
Keywords: dorsal root ganglion, sodium channel, capsaicin
noxious stimulus-sensing primary sensory neurons (nociceptors) express a variety of receptors and channels that have little or no expression in other types of neurons (reviewed by Julius and Basbaum 2001; Woolf and Ma 2007). Prominent among these is the transient receptor potential vanilloid 1 protein (TRPV1), a cation channel that is activated by a variety of painful stimuli, including noxious heat, low pH, and vanilloid ligands such as capsaicin (Caterina et al. 1997; Caterina and Julius 2001). TRPV1 molecules form ion channels that are permeable to many cations, including calcium, and have little selectivity among Na, K, and Cs ions (Caterina et al. 1997). Compared with most other nonselective cation channels such as nicotinic acetylcholine receptors and NMDA receptors, TRPV1 channels are unusual in allowing permeation of very large cations. Current through TRPV1 channels can be carried by cations as large as tetraethylammonium (TEA; 130-Da mol mass), N-methyl-d-glucamine (NMDG; 195-Da mol mass), and spermine (202-Da mol mass; Ahern et al. 2006; Hellwig et al. 2004). There is also less direct evidence for entry of even larger molecules like the dyes YO-PRO (375-Da mol mass) and FM1-43 (452-Da mol mass; Banke et al. 2010; Chung et al. 2008; Meyers et al. 2003), based on measuring accumulation of dye (usually on a time scale of minutes) rather than electrical current through the channels.
We previously hypothesized that the pore of TRPV1 channels might be large enough to pass QX-314 (263-Da mol mass), a cationic derivative of lidocaine that blocks voltage-dependent sodium channels when applied inside axons (Frazier et al. 1970; Strichartz 1973). Application of QX-314 together with capsaicin produced long-lasting inhibition of voltage-dependent sodium channels in TRPV1-expressing neurons and selectively blocked function of specific populations of sensory neurons in vivo (Binshtok et al. 2007; Kim et al. 2010), suggestive of QX-314 entry through TRPV1 channels followed by block of sodium channels. However, another possibility is that QX-314 does not permeate directly through TRPV1 channels but instead enters cells through some pathway activated secondarily by TRPV1 stimulation, which produces many downstream signaling events (e.g., Mohapatra and Nau 2005; Wu et al. 2005; Zhang et al. 2011). In the case of P2X7 purinergic receptors, originally proposed to form pores capable of passing molecules as large as YO-PRO (Virginio et al. 1999), it was later suggested that such molecules may instead enter through large-pore pannexin channels activated indirectly by P2X7 stimulation (Pelegrin et al. 2006; cf. Donnelly-Roberts et al. 2004). The same possibility exists for TRPV1 channels. Both for P2X7 channels and TRPV1 channels, some stimuli produce a delayed enhancement in the permeability of large cations, a process that can be alternatively interpreted as “pore dilation” of the primary channel (Chung et al. 2008; Yan et al. 2010) or a time-dependent recruitment of downstream pathways such as pannexin channels.
Here, we tested the hypothesis that QX-314 can enter neurons by direct permeation through TRPV1 channels. We find that QX-314 permeates TRPV1 channels rapidly enough to carry macroscopic electrical current immediately on channel activation and that QX-314 entry requires neither pannexin expression nor time-dependent pore dilation.
METHODS
Preparation of DRG neurons.
Dissociated neurons were prepared from dorsal root ganglia (DRG) dissected from Sprague-Dawley rats (postnatal days 14–28) using a protocol approved by the Institutional Animal Care and Use Committee of Harvard Medical School. Rats were deeply anesthetized with isoflurane and decapitated. DRG were dissected out, cut in half, and treated for 20 min at 34–35°C with 20 U/ml papain (Worthington Biochemical, Lakewood, NJ) and 5 mM dl-cysteine in a Ca2+- and Mg2+-free (CMF) Hank's solution containing (in mM): 136.9 NaCl, 5.4 KCl, 0.34 Na2HPO4, 0.44 KH2PO4, 5.55 glucose, 5 HEPES, 0.005% phenol red, pH 7.4 with NaOH. Ganglia were then treated for 20 min at 37°C with 3 mg/ml collagenase (Type I; Sigma-Aldrich, St. Louis, MO) and 4 mg/ml dispase II (Boehringer Mannheim, Indianapolis, IN) in CMF Hank's solution and dispersed by trituration with a fire-polished glass Pasteur pipette in a solution of Leibovitz's L-15 medium (Invitrogen, San Diego, CA) supplemented with 10% fetal calf serum, 5 mM HEPES, 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM l-glutamine, and 100 ng/ml NGF (Invitrogen). Cells were plated on glass coverslips treated with 200 μg/ml poly-d-lysine and 20 μg/ml laminin and incubated in the supplemented L-15 solution at 37°C (in 5% CO2) for 2–3 h, after which they were stored at 4°C in Neurobasal medium (Gibco) and used over the next 2–3 days.
Small DRG neurons (cell diameters 18–30 μm) were chosen for recording. Cells were first tested for capsaicin-activated current, and only cells responding to capsaicin (∼80% of those tested), corresponding to a subset of nociceptors (Cardenas et al. 1995; Petruska et al. 2000), were used for further experiments.
TRPV1 expression in N1E-115 and C6 cells.
The mouse neuroblastoma cell line N1E-115 and the rat glioma cell line C6 were both obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were grown in Dulbecco's modified Eagle's medium (30-2002; ATCC) supplemented with 10% fetal bovine serum and 100 U/ml penicillin-streptomycin and cotransfected with rat TRPV1, enhanced green fluorescent protein (eGFP) plasmids, and PolyJet DNA in vitro transfection reagent (SignaGen, Ijamsville, MD) according to the manufacturer's instructions. Cells were used the day after transfection.
Recording solutions were identical to experiments with DRG neurons.
Electrophysiology and solution exchange.
Whole cell recordings were made using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Patch pipettes were pulled from borosilicate glass (100-μl microcapillaries; VWR, South Plainfield, NJ) using a Sutter P-97 puller (Sutter Instrument, Novato, CA). The resistances of the pipettes were 1.8–2.5 MΩ when filled with the standard internal CsCl-based internal solution. The shanks of the patch pipettes were wrapped with Parafilm to reduce pipette capacitance. After the whole cell configuration was established, the cell was lifted up and placed in front of an array of quartz fiber flow pipes (250-μm internal diameter, 350-μm external diameter) containing the test solutions. Solutions were changed (in ∼1 s) by moving the cell from one pipe to another. In experiments testing rapid application of external QX-314 after activation of TRPV1 current, more rapid and precisely timed solution changes were made using a system (RSC-200; Bio-Logic SAS, Claix, France) with a stepper motor under computer control to move perfusion tubes (540-μm diameter quartz tubing) arranged in a semicircular array. The time course of solution exchange with this system was measured by applying capsaicin in normal external solution and then switching to an external solution in which half of the sodium is replaced by NMDG (with capsaicin still present). The relaxation of current to the new lower value occurred with a time constant of 5–10 ms in different cells.
All experiments were done at room temperature (22 ± 2°C).
Solutions.
The standard internal solution was (in mM): 112 CsCl, 13 CsF, 9 NaCl, 1.8 MgCl2, 9 EGTA, 9 HEPES, 14 Tris-creatine PO4, 4 Mg-ATP, and 0.3 Na-GTP, pH adjusted to 7.2 with CsOH. For recording QX-314-mediated inward currents, some experiments (e.g., Figs. 1C, 2B, and 3B) used an NMDG-based internal solution (135 mM NMDG, 5 mM EGTA, 10 mM HEPES, pH adjusted to 7.2 with HCl) that allowed measurement of QX-314-mediated currents at less negative voltages than Cs-based solutions. The standard external solution was a modified Tyrode's solution containing (in mM): 151 NaCl, 2 BaCl2, 0.03 CdCl2, 10 HEPES, 13 glucose, pH 7.4 with NaOH.
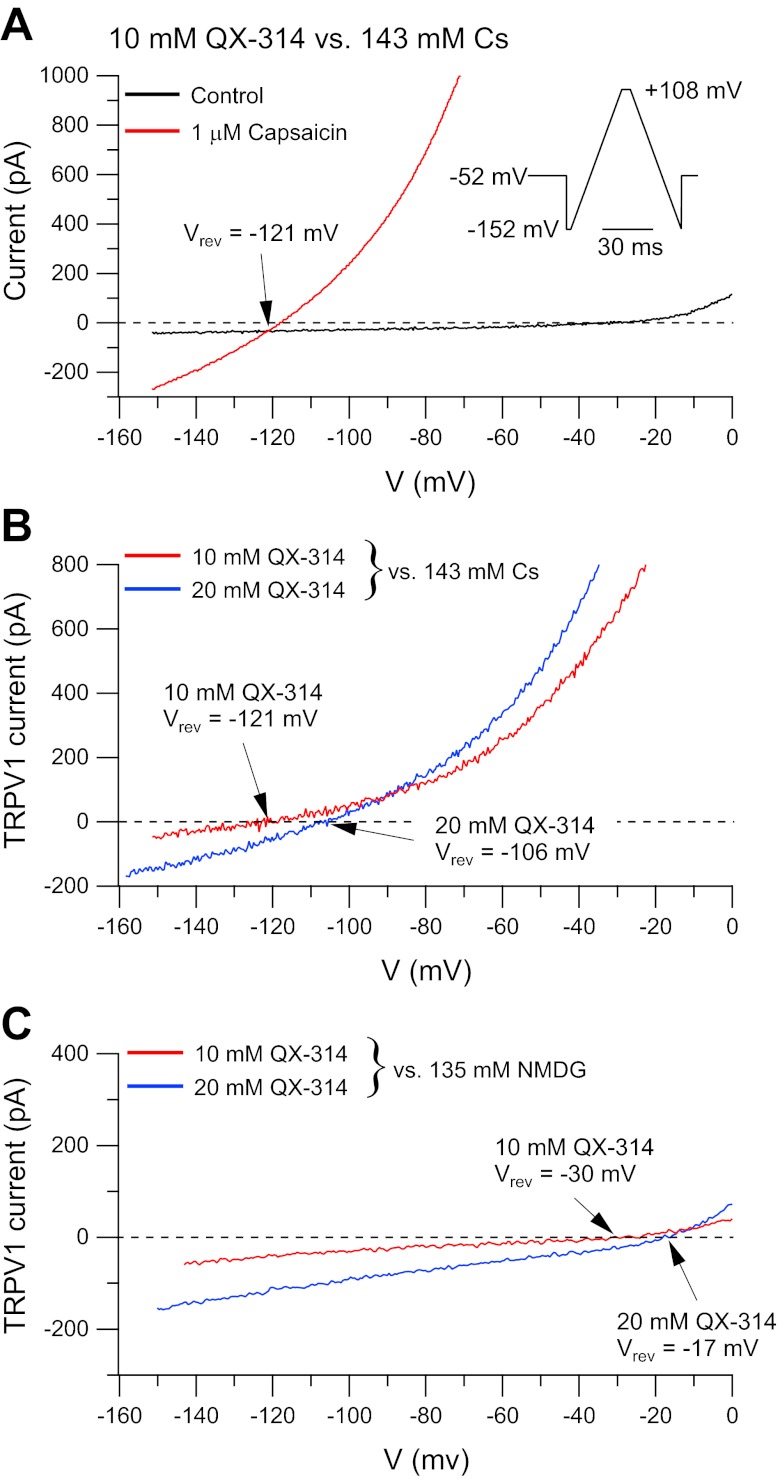
Fig. 1.
Current carried by QX-314 in transient receptor potential vanilloid 1 protein (TRPV1) channels expressed in N1E-115 cells. A: currents evoked by voltage ramps (inset) before and after application of 1 μM capsaicin in an external solution with QX-314 as the sole cation (10 mM QX-314-hydroxide, 277 mM sucrose, 5 mM HEPES, pH adjusted to 7.4 with HCl). Internal solution was 135 mM CsCl, 5 mM EGTA, 10 mM HEPES, pH adjusted to 7.2 with ∼8 mM CsOH. Currents from the downward-going ramp were averaged from 10 sweeps each, before and after application of capsaicin, and plotted as current vs. voltage. Vrev, reversal potential. B: TRPV1 currents with external solutions containing either 10 or 20 mM QX-314 vs. a Cs-based internal solution (different cell than A). Solutions as in A, with the 20 mM QX-314 solution consisting of 20 mM QX-314-hydroxide, 250 mM sucrose, 5 mM HEPES, pH adjusted to 7.4 with HCl. Capsaicin-activated current is plotted (subtraction of currents before and after application of 1 μM capsaicin with 5 sweeps signal-averaged in each condition). C: same as B but with an N-methyl-d-glucamine (NMDG)-based internal solution (135 mM NMDG, 5 mM EGTA, 10 mM HEPES, pH adjusted to 7.2 with HCl).
Fig. 2.
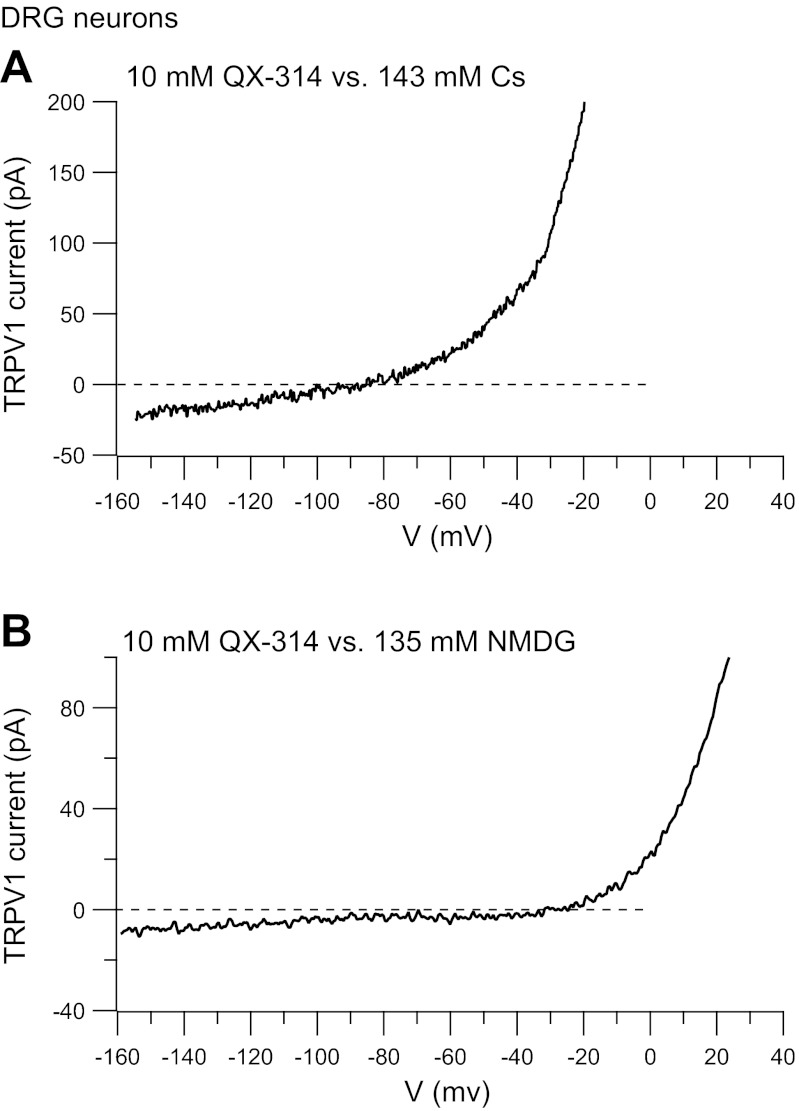
QX-314-mediated current in native TRPV1 channels in dorsal root ganglion (DRG) neurons. A: capsaicin-activated current in a DRG neuron with 10 mM external QX-314 vs. 143 mM internal Cs+. Solutions as in Fig. 1A. B: capsaicin-activated current in a DRG neuron with 10 mM external QX-314 vs. 135 mM internal NMDG+. Solutions as in Fig. 1C. Current-voltage currents were generated using ramp commands as in Fig. 1, subtracting current before and after application of 5 μM capsaicin.
Fig. 3.
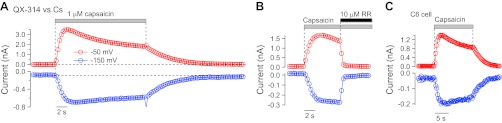
QX-314-mediated current activates rapidly and does not require pannexins. A: time course of activation of inward current carried by external 10 mM QX-314 (at −150 mV; blue symbols) compared with outward current carried by internal Cs (at −50 mV; red symbols) when capsaicin is rapidly applied to TRPV1 channels expressed in an N1E-115 cell. A triangular waveform like that in Fig. 1A was applied at 3 Hz, and current at −150 and −50 mV was measured in each sweep (i.e., every 333 ms). B: QX-314-mediated inward current is blocked rapidly by 10 μM ruthenium red (RR). TRPV1 in N1E-115 cell using external 10 mM QX-314 vs. NMDG-based internal solution as in Fig. 1C. Inward current carried by external 10 mM QX-314 (at −64 mV; blue symbols) compared with outward current carried by internal NMDG (at +10 mV; red symbols). A triangular waveform from −64 to +116 mV was applied at 3 Hz. C: QX-314-mediated current carried by TRPV1 channels expressed in C6 glioma cells lacking pannexin expression. Solutions and voltage protocol as in A.
The solutions used for testing for currents carried by QX-314 contained QX-314 as the only external cation (other than 10−7 M H+). The composition of the 10 mM QX-314 solution was 10 mM QX-314 hydroxide, 277 mM sucrose, and 5 mM HEPES, with pH adjusted to 7.4 with HCl. Solutions with 20 mM QX-314 were identical except with a base of 250 mM sucrose. It was necessary to use the hydroxide salt of QX-314 (custom synthesized for this purpose by Ascent Scientific, Bristol, United Kingdom) because solutions made using QX-314 bromide or chloride plus HEPES were acidic, and any base used to adjust the pH (e.g., Tris or NMDG hydroxide) contributes a cation that itself is likely to be permeant in TRPV1.
Voltage protocols.
TRPV1 current was determined by subtraction of currents before and after application of 1 μM capsaicin. The current-voltage relationship for TRPV1 current was determined using 2 mV/ms voltage ramps. Ramps were usually delivered in the same sweep both from −160 to +100 mV and from +100 to −160 mV (Fig. 1A, inset), with the “down ramp” preceded by 25–200 ms at either 0 or +100 mV. With this procedure, currents carried by voltage-dependent sodium channels were largely inactivated before the down ramp, which allowed more precise determination of TRPV1 currents near the reversal potential by eliminating overlapping sodium channel currents. However, in the experiments in Figs. 5 and 6, the rectification ratio for currents at +80 mV and −80 mV was measured using the “up ramp”; the rectification ratio using the down ramp was more variable, most likely reflecting variable enhancement of the inward current occurring because of time-dependent and voltage-dependent gating processes (Ahern and Premkumar 2002; Matta and Ahern 2007; Nilius et al. 2005; Voets et al. 2004) engaged by maintained depolarization.
Fig. 5.
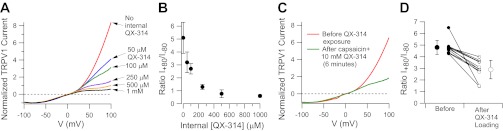
Dose-dependent reduction of outward current by intracellular QX-314 in DRG neurons. A: the current-voltage relationship for current activated by 1 μM capsaicin was determined in DRG neurons after dialysis with various concentrations of QX-314 in the intracellular solution. Currents were normalized to the current at −80 mV. B: collected results for reduction in capsaicin-activated outward current by intracellular QX-314, plotted as the ratio of current at +80 mV to current at −80 mV (I+80/I−80). C: reduction of outward current following loading of QX-314 by exposure to external QX-314 (10 mM) and capsaicin (1 μM) for 6 min. Current-voltage curve was determined after washout of external QX-314. D: collected results in 11 DRG neurons for change in rectification produced by exposure to 10 mM QX-314 and 1 μM capsaicin for times ranging from 100 to 350 s.
Fig. 6.
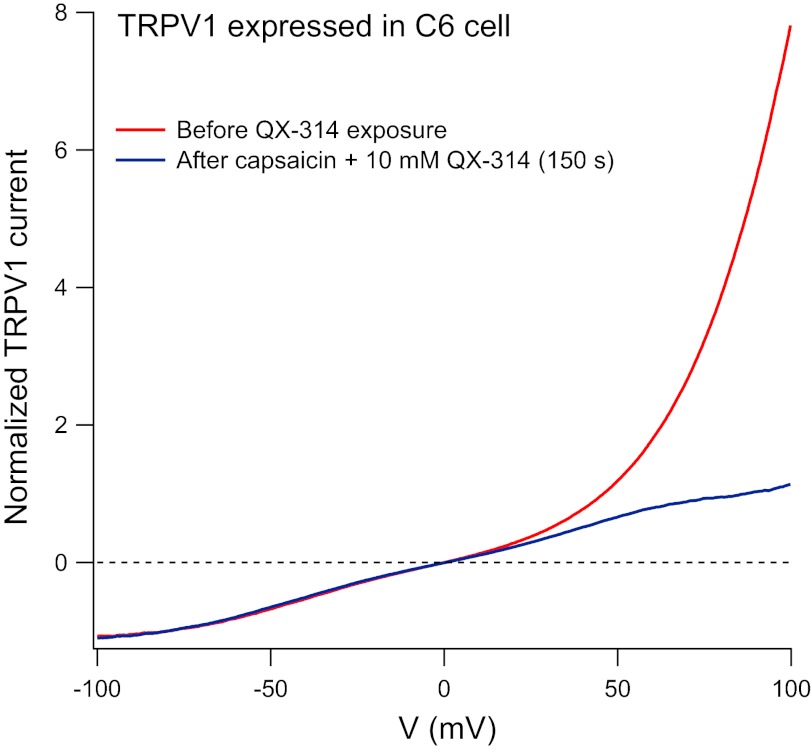
QX-314 loading in a TRPV1-expressing C6 cell. Solutions and protocols were the same as in Fig. 5, C and D, but using a TRPV1-expressing C6 cell. The voltage-dependence of capsaicin-activated current was determined (using voltage ramps) before (red) or after (blue) exposure to external QX-314 (10 mM) and capsaicin (1 μM) for 150 s. Current-voltage curve after loading was determined after washout of external QX-314. Currents were normalized relative to the current at −80 mV.
Data acquisition and analysis.
Currents and voltages were controlled and sampled using a Digidata 1321A interface and pCLAMP 9 software (Axon Instruments). Current or voltage signals were filtered at 10 kHz (−3 dB, 4-pole Bessel) and digitized at 50 kHz. Analysis was performed using pCLAMP 9 and IGOR Pro (version 4.06; WaveMetrics, Lake Oswego, OR) using DataAccess (Bruxton, Seattle, WA) to import pCLAMP files into IGOR.
In the experiments determining reversal potentials using QX-314-based external solutions, reported voltages are corrected for junction potentials, which were measured using a flowing 3 M KCl reference electrode according to the procedure described by Neher (1992). There were two relevant junction potentials: first, the junction potential between the internal solution in the patch pipette and the Tyrode's solution in the bath in which the pipette current was zeroed before the experiment and, second, the junction potential between the QX-314-sucrose solution (flowing from a flow pipe) and the Tyrode's solution in the chamber. The first junction potential was −4 mV (pipette relative to bath) for the CsCl-based solution and +4 mV for the NMDG-based solution. The second junction potential was −12 mV for the 10 mM QX-314-sucrose solution relative to the bath and −5 mV for the 20 mM QX-314-sucrose solution relative to the bath. Thus a net correction of +8 mV was applied to raw voltages for CsCl vs. 10 mM QX-314, +1 mV for CsCl vs. 20 mM QX-314, +16 mV for NMDG Cl vs. 10 mM QX-314, and +9 mV for NMDG Cl vs. 20 mM QX-314.
Data are reported as means ± SD.
RESULTS
QX-314 carries macroscopic current.
The most direct evidence for QX-314 permeation through TRPV1 channels would be measurement of macroscopic ionic currents carried by QX-314 cations. We therefore attempted to measure directly QX-314-mediated ionic currents when using external solutions in which QX-314 is the sole cation added (other than protons, present at 10−7 M). Using the hydroxide salt of QX-314, we made an external solution containing 10 mM QX-314 on a background of 277 mM sucrose (using 5 mM HEPES and hydrochloric acid to buffer the pH to 7.4). Figure 1A shows the current activated by capsaicin in heterologously expressed rat TRPV1 channels using this external solution and a Cs-based internal solution. With these solutions, there was a clear capsaicin-activated inward current at voltages below −130 mV. In collected results from 15 cells, the capsaicin-activated current reversed at −123 ± 5 mV. Using the Goldman-Hodgkin-Katz equation to estimate relative permeabilities (Hille 2001), this reversal potential corresponds to a relative permeability PQX-314/PCs = 0.12 ± 0.02.
The inward current seen with the QX-314-sucrose external solution is most simply interpreted as QX-314 cations entering the cell. Another possibility is that the inward current instead results from chloride efflux, if there were a small but nonzero anion permeability of the TRPV1 channel revealed at strong hyperpolarizations. To test this, we did experiments in which we compared currents with external solutions containing either 10 or 20 mM QX-314. If the inward current is from influx of QX-314, the reversal potential should shift in the depolarizing direction with higher QX-314. However, if the inward current is from efflux of chloride, the reversal potential should, if anything, shift in the hyperpolarizing direction with the 20 mM QX-314 solution because the external chloride concentration is higher in this solution (as a result of more chloride being added in the form of HCl to buffer the extra hydroxide from the QX-314-hydroxide used to make the solution), thus requiring stronger hyperpolarization for a given chloride efflux. In fact, when the concentration of QX-314 in the external solution was increased to 20 mM (Fig. 1B), the reversal potential shifted in the depolarizing direction, with a reversal potential of −104 ± 4 mV (n = 10). Assuming permeability only to QX-314 and Cs, this corresponds to a relative permeability PQX-314/PCs = 0.13 ± 0.02, essentially identical to the permeability ratio obtained with 10 mM QX-314. The close match of the predicted and measured shift in reversal potential with the increase in external QX-314 from 10 to 20 mM argues strongly that the inward current is indeed carried by QX-314. As well as ruling out chloride permeability, the match of predicted and measured reversal potentials also argues that there is no significant contribution of current carried by external protons (present at 0.1 μM) or by contaminating calcium in the external solution (at perhaps ∼1 μM). Although both calcium (Caterina et al. 2001) and protons (Hellwig et al. 2004) are likely more intrinsically permeant than QX-314, the fact that they are present at concentrations 104–105-fold lower than QX-314 is obviously consistent with a negligible contribution under the conditions of the experiment.
We also tested external QX-314 vs. an internal solution containing 135 mM NMDG, reasoning that inward current carried by QX-314 should be evident at less-hyperpolarized voltages if tested against a larger intracellular cation with less permeability than Cs. As expected, inward current attributable to QX-314 was present over a wider voltage range and reversed at more depolarized voltages with internal NMDG compared with internal Cs (Fig. 1C). The reversal potential was −39 ± 14 mV with 10 mM QX-314 (n = 11), corresponding to a relative permeability PQX-314/PNMDG = 3.3 ± 1.9. With external solutions containing 20 mM QX-314, the reversal potential shifted to −20 ± 12 mV (n = 6), corresponding to a relative permeability PQX-314/PNMDG = 3.5 ± 1.7. Thus QX-314 actually has a substantially greater permeability than NMDG, at least as determined from reversal potentials, despite being a considerably larger molecule.
We next tested for permeability of QX-314 in native TRPV1 channels expressed in rat DRG neurons. With 10 mM QX-314 in the external solution and 143 mM Cs in the internal solution, 5 μM capsaicin activated an inward current that reversed at −114 ± 15 mV (n = 9; Fig. 2A), corresponding to a relative permeability PQX-314/PCs = 0.20 ± 0.13. In experiments done with an NMDG-based internal solution, the capsaicin-activated inward current reversed at −41 ± 6 mV (n = 8; Fig. 2B), corresponding to a relative permeability PQX-314/PNMDG = 2.8 ± 0.7. Thus native TRPV1 channels behave very similarly to heterologously expressed channels and also carry QX-314-mediated current.
QX-314-carried current activates and deactivates in parallel with Cs-carried current.
With rapid application of capsaicin, the inward currents carried by external QX-314 activated within seconds, in parallel with the outward current carried by internal Cs (Fig. 3A), with no evidence of a lag that might be expected if QX-314 entered through a downstream pathway or if QX-314 permeation required pore dilation. On removal of capsaicin, the QX-314-carried inward current also deactivated immediately and in parallel with Cs-mediated outward current. In addition, QX-314-mediated inward current and Cs-mediated outward current were blocked immediately and simultaneously by 10 μM ruthenium red, a pore blocker of TRP channels (n = 7; Fig. 3B). The rapid activation and deactivation and immediate block by ruthenium red are consistent with the QX-314-mediated inward current being carried directly through TRPV1 channels and not though a second messenger-activated pathway such as pannexin channels. The rapid activation of QX-314-mediated inward current in parallel with Cs-mediated outward current also suggests that permeation occurs through the standard pore structure and does not require the time-dependent pore dilation process previously documented for TRPV1 channels (Banke et al. 2010; Chung et al. 2008).
Pannexin channels are not required for QX-314-mediated current.
To test further for possible involvement of pannexin channels in the QX-314-mediated current, we expressed TRPV1 channels in C6 glioma cells, a cell line that lacks expression of these channels (Lai et al. 2007). Just as for TRPV1 expressed in N1E-115 cells or in native DRG channels (Fig. 3C), solutions with QX-314 as the only cation carried inward current when capsaicin activated TRPV1 expressed in C6 cells and the QX-314-mediated inward current activated and deactivated immediately on application or removal of capsaicin, in parallel with the Cs-mediated outward current. There was no difference compared with QX-314-mediated current for TRPV1 expressed in N1E-115 cells or from native TRPV1 channels in DRG neurons.
External QX-314 is a permeant blocker in the pore of TRPV1 channels.
We next examined the effect of external QX-314 added to solutions in which sodium is the main cation. In this series of experiments, the external solution was a modified Tyrode's solution with 155 mM Na as the main cation, with a Cs-based internal solution. As expected, 1 μM capsaicin activated a current reversing near 0 mV. The current-voltage relationship showed the strongly outwardly rectifying shape typical of TRPV1 current (Caterina and Julius 1997). Rapid substitution of an external solution that was identical to the capsaicin-containing external solution but with the addition of 10 mM QX-314 produced an instantaneous reduction in the inward limb of the current-voltage relationship (Fig. 4A). This suggests that although QX-314 is permeant through the TRPV1 channels, its permeation impedes the passage of Na ions, i.e., it is a permeant blocker. Instantaneously, QX-314 had little effect on the outward limb of the current-voltage relationship, as if external QX-314 has little or no effect on the efflux of Cs ions. However, with maintained application of 10 mM QX-314 in the presence of capsaicin, there was a gradual reduction over seconds in the outward current carried by Cs. We hypothesized that this effect is caused by intracellular QX-314 accumulating inside the cell as it enters through TRPV1 channels and then acting as a permeant blocker from the inner face of the TRPV1 pore to reduce outward-going Cs current. Consistent with this, when QX-314 was abruptly removed from the external solution, the effect on the inward limb of the current-voltage curve reversed immediately, but the effect on the outward limb remained (Fig. 4B).
Fig. 4.
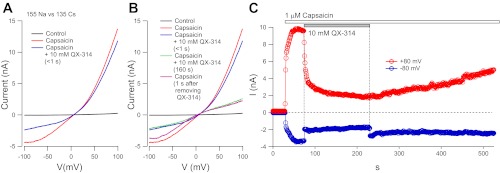
Partial block of Na- and Cs-mediated currents by QX-314. A: currents in a rat DRG neuron in control (black), after applying 1 μM capsaicin (red), and immediately (∼100 ms) after switching to a solution with 10 mM QX-314 added to the same 1 μM capsaicin-containing external solution (blue). Current-voltage relationships were determined from a 100-ms downward-going ramp from +100 to −100 mV (following 200 ms at +100 mV to inactivate voltage-dependent sodium currents maximally). Voltage protocols were delivered every second from a steady holding potential of −80 mV. B: continuation of the experiment in A showing currents 160 s after adding 10 mM QX-314 to the external solution (green) and immediately after changing to a QX-314-free solution (violet). C: time course of block and recovery from block by externally applied QX-314 for the experiment in A and B.
Figure 4C shows the time course with which outward current was inhibited and then recovers from coapplication of QX-314 and capsaicin. Outward current was reduced to about half in the first 3 s followed by a slower decline over the next 5 min. On washout of external QX-314, there was slow and partial recovery of the outward current that was still incomplete after 5 min of exposure to capsaicin alone. We interpret the recovery as reflecting a combination of efflux of QX-314 through the activated TRPV1 channels along with diffusion of QX-314 from the cell body into the much larger volume of QX-314-free internal solution in the recording pipette.
The results in Fig. 4 were typical. In collected results, when 10 mM QX-314 was applied rapidly in the Na-based external solution during continuous activation of TRPV1 current by capsaicin, the inward current at −80 mV was instantaneously reduced by 66 ± 12%, whereas current at +80 mV was reduced by only 37 ± 10% (n = 18) when measured quickly (<1 s) after the application of 10 mM QX-314. This reduction of outward current is likely an overestimate of the instantaneous reduction of outward current because the time-dependent reduction interpreted as reflecting accumulation of intracellular QX-314 began to develop within seconds.
The reduction of capsaicin-activated inward current by external QX-314 fits well with previous observations for TRPV1 expressed in both HEK cells (Leffler at al. 2008) and Xenopus oocytes (Rivera-Acevedo et al. 2011) and shows that the effect of QX-314 on current is highly rectifying, consistent with the reduction of current resulting from permeation effects and not a reduction in the efficacy of channel activation. Rivera-Acevedo and colleagues (2011) found that QX-314 can actually act as an agonist for activating TRPV1 channels when applied at concentrations of 30–60 mM. In our experiments, 10 mM QX-314 applied alone never activated TRPV1 currents detectably (data not shown), in agreement with earlier results for TRPV1 in HEK cells (Leffler et al. 2008). Therefore, it seems clear that the effects seen with QX-314 coapplied with capsaicin represent effects on channel permeation and not regulation of channel activation.
Internal QX-314 is a potent inhibitor of outward current through TRPV1 channels.
To test the hypothesis that the time-dependent reduction of outward-going current seen with coapplication of QX-314 and capsaicin is caused by accumulation of intracellular QX-314, we tested directly the effect of intracellular QX-314 applied in the patch pipette using recordings of capsaicin-activated current in DRG neurons. We found that intracellular QX-314 produced a dose-dependent change in the shape of the current-voltage relationship for capsaicin-activated current (Fig. 5, A and B), suggesting a voltage-dependent inhibition of outward-going current. There was little apparent effect of intracellular QX-314 on the shape of the current-voltage relationship for inward current and no change in reversal potential, but outward current at +80 (normalized to inward current at −80 mV) was reduced by ∼50% by 100 μM intracellular QX-314 and by ∼90% by 1 mM QX-314. The data suggest that intracellular QX-314 produces powerful block of outward current.
The quantifiable and reproducible change in the rectification of capsaicin-activated current by intracellular QX-314 suggested that it should be possible to use this change to estimate the concentrations of intracellular QX-314 that develop when extracellular QX-314 is coapplied with capsaicin. Figure 5C shows an example of the current-voltage relationship for capsaicin-activated current before and after presumptive QX-314 loading by coapplication of 10 mM QX-314 with 1 μM capsaicin (with the current-voltage relationship after loading measured after removing external QX-314). The ratio of current at +80 mV to that at −80 mV changed dramatically, from 4.3 in control to 1.5 after QX-314 loading for ∼6 min. By comparison with the changes seen with known amounts of QX-314 applied intracellularly (Fig. 5B), this change suggests that in this cell intracellular QX-314 accumulated to a concentration of ∼200 μM. In collected results from 11 neurons with QX-314 loading by application of 10 mM QX-314 with 1 μM capsaicin for times ranging from 100 s to 6 min (average of 184 ± 88 s), the rectification ratio changed from 4.8 ± 0.6 before loading to 2.9 ± 0.8 after loading (Fig. 5D), suggesting an accumulation to typical concentrations of 50–100 μM.
The simplest interpretation of these results is that QX-314 accumulates intracellularly as a result of permeation through TRPV1 channels. In agreement with this interpretation, the same effects were seen in experiments using TRPV1 expressed in pannexin-lacking C6 cells. Figure 6 shows an example for a TRPV1-expressing C6 cell, in which the rectification ratio for current at +80 mV relative to that at −80 mV changed from 3.9 in control to 0.95 after coapplication of 10 mM QX-314 with 1 μM capsaicin for 2.5 min. By comparison with the effects of calibrated concentrations of intracellular QX-314, the change suggests an intracellular accumulation to near 500 μM QX-314. The results suggest that accumulation of intracellular QX-314 occurs equally effectively in cells that lack expression of pannexin channels as in native DRG neurons, consistent with entry through TRPV1 channels.
DISCUSSION
These experiments show that that QX-314 is rapidly permeant through the pore of TRPV1 channels and can accumulate within minutes to reach intracellular concentrations in excess of 50–100 μM when coapplied externally together with capsaicin to activate TRPV1 channels.
QX-314 permeability relative to other ions.
At 263 Da, QX-314 is the largest cation so far demonstrated to carry macroscopic currents through TRPV1 channels. Interestingly, the permeability of QX-314 as estimated from the Goldman-Hodgkin-Katz equation is actually considerably higher than NMDG, a smaller molecule (195 Da), with PQX-314/PNMDG = 3.5. This permeability ratio is based on reversal potentials, and in cases in which permeating ions bind transiently to binding sites within the channel, higher permeability as measured from reversal potentials can be associated with tighter binding within the channel (Adams et al. 1981; Hille 2001). Thus the higher permeability of QX-314 vs. NMDG probably reflects tighter transient binding within the pore. Such binding is consistent with QX-314 partially inhibiting current carried by Na or Cs (Leffler et al. 2008; Rivera-Acevedo et al. 2011).
The shift in reversal potential between solutions containing 10 and 20 mM external QX-314 on a sucrose background exactly matched the predicted shift assuming that the inward current corresponds only to QX-314 entry, with no contribution of chloride efflux. Thus, although permeable to surprisingly large cations, the TRPV1 channel apparently discriminates perfectly between cations and chloride. These results agree with similar experiments using NMDG (Chung et al. 2008) and extend the resolution for any possible component from chloride efflux to voltages negative to −120 mV. The high selectivity of TRPV1 channels for cations has also been shown by their ability to pass the cationic dye N-(ethoxycarbonylmethyl)-6-methoxyquinolinium (MQAE) but not a neutral derivative of similar size (Hellwig et al. 2004).
QX-314 as a permeant blocker.
Although QX-314 permeates through TRPV1 channels, it also partially inhibits current carried by Na or Cs ions, as seen previously using extracellular QX-314 (Leffler et al. 2008; Rivera-Acevedo et al. 2011). Thus QX-314 is a permeant blocker in TRPV1. We found that inhibition by QX-314 was strongly asymmetric, depending on whether it was applied externally or internally. In both cases, inhibition was strongly voltage-dependent but with opposite directionality. Externally applied QX-314 reduced inward-going current much more than outward current (at least instantaneously), whereas internally applied QX-314 appeared to affect only outward current. The asymmetry of QX-314 inhibition is also reflected in the stronger effects of internal QX-314 (1 mM reducing outward current by ∼90%) compared with external application (10 mM reducing inward current by ∼66%).
Rivera-Acevedo and colleagues (2011) found that external QX-314 inhibited inward TRPV1 currents with IC50 of 8 μM, implying a high-affinity binding site, and inhibition was incomplete even at high QX-314 concentrations, consistent with QX-314 being a permeant blocker. The parent compound of QX-314, lidocaine, also potently inhibits TRPV1 channels (Leffler et al. 2008; Rivera-Acevedo et al. 2012), but the neutral local anesthetic benzocaine is far weaker, suggesting that it is the charged (protonated) form of lidocaine, similar in structure to permanently charged QX-314, that blocks by binding within the ion permeation pathway (Rivera-Acevedo et al. 2012).
The permeation and block by QX-314 has similarities to TEA ion, which can also carry measurable macroscopic current when present at millimolar concentrations externally (Hellwig et al. 2004) and acts as a blocker when applied either internally or externally at submillimolar concentrations (Jara-Oseguera et al. 2008; Rivera-Acevedo et al. 2012). The entry and exit of TEA and related alkylammonium blockers are influenced by permeating sodium ions in a manner suggesting that the TRPV1 channel is a multi-ion pore (Jara-Oseguera et al. 2008; Oseguera et al. 2007). Similarly, interaction of QX-314 with permeating monovalent ions can explain the sidedness with which it acts, with inward current flow by monovalents effectively displacing internally applied QX-314 from its binding site, so that it blocks only outward current, and similarly for outward current flow displacing externally applied QX-314. The ability of QX-314 and large quaternary ammonium molecules to act as permeant blockers in TRPV1 is reminiscent of the behavior of large permeant blockers like the cationic antibiotic dihydrostreptomycin in the hair cell mechanoelectrical transducer (MET) channel, another large-pore channel (Farris et al. 2004; Fettiplace 2009).
External QX-314 acts as an open-channel blocker in skeletal muscle acetylcholine receptor channels (Neher and Steinbach 1978) but in this case does not permeate. Even at strongly negative voltages, externally applied QX-314 appears to exit the channel only by unbinding back into the extracellular solution (Neher and Steinbach 1978) and not by permeating. Also, in acetylcholine receptor channels, QX-314 cannot reach its binding site from the inside (Horn et al. 1980), consistent with the acetylcholine receptor channel having a considerably smaller pore (at the narrowest point) than the TRPV1 channel (Dwyer et al. 1980; Jara-Oseguera et al. 2008; Owsianik et al. 2006).
Pore dilation not required.
Our experiments suggest that QX-314 permeates effectively through the standard pore conformation of the TRPV1 channel and does not require pore dilation. In the experiments with QX-314 as the only external cation, the capsaicin-activated inward current carried by QX-314 activated rapidly, within seconds, and in parallel with the outward current carried by Cs. This contrasts with the behavior of the dilated conformation of the pore, which develops over minutes after an initial delay (Chung et al. 2008). With our recording conditions, capsaicin activation of current occurred rapidly with a smooth, single component, whether carried by Na or QX-314, and we did not observe the second delayed increase in current associated with the dilated pore conformation. Pore dilation is subject to modulatory influences (e.g., protein kinase C; Chung et al. 2008), which may depend on culture or recording conditions. In our experiments, cells were lifted away from the bottom of the chamber away from possible modulatory influences from neighboring cells. Whatever the reason the dilated form of the channel was not evident in our experiments, the results show that QX-314 permeation through the standard pore is effective enough to produce rapid entry and accumulation in cells. Entry through the dilated form of the pore would likely be even greater.
Accumulation in cytoplasm by entry of extracellular QX-314.
Based on the changes in rectification produced by internal QX-314, coapplication of 10 mM QX-314 with capsaicin for 2–6 min produced accumulation of intracellular QX-314 to typical concentrations of 50–100 μM (and up to 200–500 μM in some cells). This agrees well with previous estimates of QX-314 accumulation based on the reduction of voltage-dependent sodium current (Brenneis et al. 2013). In the present experiments, the accumulation of internal QX-314 evoked by capsaicin clearly did not require pannexin channels because it was equally prominent and rapid when TRPV1 was expressed in C6 cells lacking pannexin expression. This fits well with previous evidence that accumulation of the cationic dye YO-PRO in TRPV1-expressing cells is not prevented by a wide range of agents that inhibit pannexins and gap-junction hemichannels (Banke et al. 2010; Chung et al. 2008).
Our experiments examined accumulation of QX-314 into DRG cell bodies. QX-314 is likely to accumulate to higher concentrations in TRPV1-expressing axons or nerve terminals, which have a much higher surface-to-volume ratio than cell bodies. The accumulation of QX-314 in our experimental configuration is also reduced by the diffusion of the entering QX-314 from the cell into the large volume of the recording pipette. Therefore, the levels of 50–100 μM intracellular QX-314 reached in our experiments probably considerably underestimate the concentrations that would be reached in nerve endings or in cell bodies that are not being dialyzed.
Implications for in vivo and clinical use.
These experiments were motivated by the concept of using TRPV1 channels as portals for delivering charged drug molecules into primary pain-sensing neurons to inhibit pain signaling in vivo selectively (Binshtok et al. 2007; Kim et al. 2010; Liu et al. 2011; Roberson et al. 2011), an approach recently extended to using light-controlled QX-314 derivatives (Mourot et al. 2012) and to using TRPA1 channels as portals (Lennertz et al. 2012). Our finding that QX-314 can enter through the standard pore and does not require pore dilation is likely advantageous for in vivo and potential clinical use because pore dilation typically requires large concentrations of agonist (Banke et al. 2010; Chung et al. 2008), which may be difficult to deliver and maintain in vivo (e.g., using perineural infusion to produce nerve block). High agonist concentrations also tend to produce rapid TRPV1 desensitization. Submaximal stimulation of TRPV1 allowing slow but steady entry of drug may therefore best optimize entry of QX-314 for therapeutic benefit in vivo.
GRANTS
This study was supported by the National Institute of Neurological Disorders and Stroke Grants NS-064254 and NS-072040.
DISCLOSURES
Endo Pharmaceuticals has licensed technology from Harvard University, invented by Bruce Bean and Clifford Woolf, for using TRP-mediated entry of sodium channel blockers to produce analgesia. Drs. Bean and Woolf have received licensing fees and are eligible to receive royalties from potential clinical use of such technology.
AUTHOR CONTRIBUTIONS
M.P., A.M.B., S.B.O., C.J.W., and B.P.B. conception and design of research; M.P., G.-L.Y., and S.B.O. performed experiments; M.P. and B.P.B. analyzed data; M.P., A.M.B., S.B.O., and B.P.B. interpreted results of experiments; M.P. and B.P.B. prepared figures; M.P. and B.P.B. drafted manuscript; M.P., A.M.B., S.B.O., and B.P.B. edited and revised manuscript; M.P., A.M.B., G.-L.Y., S.B.O., C.J.W., and B.P.B. approved final version of manuscript.
ACKNOWLEDGMENTS
Thanks to Dr. Tony Priestley (Endo Pharmaceuticals) for helpful discussions.
Present address of M. Puopolo: Dept. of Anesthesiology, Stony Brook Univ. School of Medicine, Stony Brook, NY 11794-8480.
Present address of A. M. Binshtok: Dept. of Medical Neurobiology, Institute for Medical Research Israel-Canada, The Hebrew Univ. Medical School, Jerusalem 91120, Israel.
Present address of S. B. Oh: National Research Laboratory for Pain, Dental Research Institute and Dept. of Neurobiology and Physiology, School of Dentistry, Seoul National Univ., Seoul 110-749, Republic of Korea.
REFERENCES
- Adams DJ, Nonner W, Dwyer TM, Hille B. Block of endplate channels by permeant cations in frog skeletal muscle. J Gen Physiol 78: 593–615, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern GP, Premkumar LS. Voltage-dependent priming of rat vanilloid receptor: effects of agonist and protein kinase C activation. J Physiol 545: 441–451, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern GP, Wang X, Miyares RL. Polyamines are potent ligands for the capsaicin receptor TRPV1. J Biol Chem 281: 8991–8995, 2006 [DOI] [PubMed] [Google Scholar]
- Banke TG, Chaplan SR, Wickenden AD. Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am J Physiol Cell Physiol 298: C1457–C1468, 2010 [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449: 607–610, 2007 [DOI] [PubMed] [Google Scholar]
- Brenneis C, Kistner K, Puopolo M, Segal D, Roberson D, Sisignano M, Labocha S, Ferreirós N, Strominger A, Cobos EJ, Ghasemlou N, Geisslinger G, Reeh PW, Bean BP, Woolf CJ. Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing. J Neurosci 33: 315–326, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Scroggs RS. Variation in serotonergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J Neurophysiol 74: 1870–1879, 1995 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487–517, 2001 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- Chen J, Kim D, Bianchi BR, Cavanaugh EJ, Faltynek CR, Kym PR, Reilly RM. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Mol Pain 5: 3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Güler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci 11: 555–564, 2008 [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic MT, Faltynek CR, Jarvis MF. Mitogen-activated protein kinase and caspase signaling pathways are required for P2X7 receptor (P2X7R)-induced pore formation in human THP-1 cells. J Pharmacol Exp Ther 308: 1053–1061, 2004 [DOI] [PubMed] [Google Scholar]
- Dwyer TM, Adams DJ, Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol 75: 469–492, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria RX, Cascabulho CM, Reis RA, Alves LA. Large-conductance channel formation mediated by P2X7 receptor activation is regulated through distinct intracellular signaling pathways in peritoneal macrophages and 2BH4 cells. Naunyn Schmiedebergs Arch Pharmacol 382: 73–87, 2010 [DOI] [PubMed] [Google Scholar]
- Farris HE, LeBlanc CL, Goswami J, Ricci AJ. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J Physiol 558: 769–792, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R. Defining features of the hair cell mechanoelectrical transducer channel. Pflügers Arch 458: 1115–1123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier DT, Narahashi T, Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther 171: 45–51, 1970 [PubMed] [Google Scholar]
- Hellwig N, Plant TD, Janson W, Schafer M, Schultz G, Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J Biol Chem 279: 34553–34561, 2004 [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001 [Google Scholar]
- Horn R, Brodwick MS, Dickey WD. Asymmetry of the acetylcholine channel revealed by quaternary anesthetics. Science 210: 205–207, 1980 [DOI] [PubMed] [Google Scholar]
- Jara-Oseguera A, Llorente I, Rosenbaum T, Islas LD. Properties of the inner pore region of TRPV1 channels revealed by block with quaternary ammoniums. J Gen Physiol 132: 547–562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 413: 203–210, 2001 [DOI] [PubMed] [Google Scholar]
- Kim HY, Kim K, Li HY, Chung G, Park CK, Kim JS, Jung SJ, Lee MK, Ahn DK, Hwang SJ, Kang Y, Binshtok AM, Bean BP, Woolf CJ, Oh SB. Selectively targeting pain in the trigeminal system. Pain 150: 29–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res 67: 1545–1554, 2007 [DOI] [PubMed] [Google Scholar]
- Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, Gavva NR, Reeh PW, Nau C. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest 118: 763–776, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennertz RC, Kossyreva EA, Smith AK, Stucky CL. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS One 7: e43597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang HX, Hou HY, Lu XF, Wei JQ, Wang CG, Zhang LC, Zeng YM, Wu YP, Cao JL. Acid solution is a suitable medium for introducing QX-314 into nociceptors through TRPV1 channels to produce sensory-specific analgesic effects. PLoS One 6: e29395, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta JA, Ahern GP. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol 585: 469–482, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1–43 loading of sensory cells through nonselective ion channels. J Neurosci 23: 4054–4065, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem 280: 13424–13432, 2005 [DOI] [PubMed] [Google Scholar]
- Mourot A, Fehrentz T, Le Feuvre Y, Smith CM, Herold C, Dalkara D, Nagy F, Trauner D, Kramer RH. Rapid optical control of nociception with an ion-channel photoswitch. Nat Methods 9: 396–402, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207: 123–131, 1992 [DOI] [PubMed] [Google Scholar]
- Neher E, Steinbach JH. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol 277: 153–176, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: a voltage connection? J Physiol 567: 35–44, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseguera AJ, Islas LD, Garcia-Villegas R, Rosenbaum T. On the mechanism of TBA block of the TRPV1 channel. Biophys J 92: 3901–3914, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol 68: 685–717, 2006 [DOI] [PubMed] [Google Scholar]
- Pascual JM, Karlin A. Delimiting the binding site for quaternary ammonium lidocaine derivatives in the acetylcholine receptor channel. J Gen Physiol 112: 611–621, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25: 5071–5082, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol 84: 2365–2379, 2000 [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Agarwal S, Steffen D. Single-channel properties of native and cloned rat vanilloid receptors. J Physiol 545: 107–117, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Acevedo RE, Pless SA, Ahern CA, Schwarz SK. The quaternary lidocaine derivative, QX-314, exerts biphasic effects on transient receptor potential vanilloid subtype 1 channels in vitro. Anesthesiology 114: 1425–1434, 2011 [DOI] [PubMed] [Google Scholar]
- Rivera-Acevedo RE, Pless SA, Schwarz SK, Ahern CA. Extracellular quaternary ammonium blockade of transient receptor potential vanilloid subtype 1 channels expressed in Xenopus laevis oocytes. Mol Pharmacol 82: 1129–1135, 2012 [DOI] [PubMed] [Google Scholar]
- Roberson DP, Binshtok AM, Blasl F, Bean BP, Woolf CJ. Targeting of sodium channel blockers into nociceptors to produce long-duration analgesia: a systematic study and review. Br J Pharmacol 164: 48–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz GR. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol 62: 37–57, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543, 1998 [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci 2: 315–321, 1999 [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430: 748–754, 2004 [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors–noxious stimulus detectors. Neuron 55: 353–364, 2007 [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. Transient receptor potential vanilloid type 1 activation down-regulates voltage-gated calcium channels through calcium-dependent calcineurin in sensory neurons. J Biol Chem 280: 18142–18151, 2005 [DOI] [PubMed] [Google Scholar]
- Yan Z, Khadra A, Li S, Tomic M, Sherman A, Stojilkovic SS. Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci 30: 14213–14224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Daugherty SL, de Groat WC. Activation of CaMKII and ERK1/2 contributes to the time-dependent potentiation of Ca2+ response elicited by repeated application of capsaicin in rat DRG neurons. Am J Physiol Regul Integr Comp Physiol 300: R644–R654, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]