Abstract
In the mammalian retina, some ganglion cells express the photopigment melanopsin and function as photoreceptors. Five morphological types of these intrinsically photosensitive retinal ganglion cells (ipRGCs), M1–M5, have been identified in mice. Whereas M1 specializes in non-image-forming visual functions and drives such behaviors as the pupillary light reflex and circadian photoentrainment, the other types appear to contribute to image-forming as well as non-image-forming vision. Recent work has begun to reveal physiological diversity among some of the ipRGC types, including differences in photosensitivity, firing rate, and membrane resistance. To gain further insights into these neurons' functional differences, we conducted a comprehensive survey of the electrophysiological properties of all five morphological types. Compared with the other types, M1 had the highest membrane resistance, longest membrane time constant, lowest spike frequencies, widest action potentials, most positive spike thresholds, smallest hyperpolarization-activated inwardly-rectifying current-induced “sagging” responses to hyperpolarizing currents, and the largest effects of voltage-gated K+ currents on membrane potentials. M4 and M5 were at the other end of the spectrum for most of these measures, while M2 and M3 tended to be in the middle of this spectrum. Additionally, M1 and M2 cells generated more diverse voltage-gated Ca2+ currents than M3–M5. In conclusion, M1 cells are significantly different from all other ipRGCs in most respects, possibly reflecting the unique physiological requirements of non-image-forming vision. Furthermore, the non-M1 ipRGCs are electrophysiologically heterogeneous, implicating these cells' diverse functional roles in both non-image-forming vision and pattern vision.
Keywords: melanopsin, retina, non-image-forming vision, action potentials, voltage-gated channels
image-forming visual analysis begins in the retina. Following phototransduction by rod and cone photoreceptors, different attributes at every point in the visual scene are analyzed in parallel, first by about 40 types of interneurons, and then by as many as 20 types of ganglion cells (Masland 2011). These retinal ganglion cells (RGCs) subsequently signal to higher brain centers for further analysis of the visual scene. The various types of RGCs have different dendritic structures, presynaptic networks, and intrinsic electrophysiological properties, which presumably contribute to these retinal output neurons' diverse photoresponses and physiological functions (Berson 2008; Masland 2011).
About a decade ago, a new ganglion cell type was discovered that specializes in non-image-forming visual functions, such as the pupillary light reflex, photoentrainment of circadian rhythms, and photic regulation of hormone secretion. These novel RGCs express the photopigment melanopsin and function as photoreceptors and thus are called ganglion-cell photoreceptors or intrinsically photosensitive RGCs (ipRGCs) (Berson et al. 2002; Hattar et al. 2002; Provencio et al. 1998). In their seminal study of ipRGCs, Berson et al. (2002) injected fluorescent dyes into the suprachiasmatic nucleus (SCN) of rats to retrogradely label these cells and found that all retrolabeled ipRGCs had similar morphologies, suggesting that a single type of ganglion-cell photoreceptor innervates the circadian pacemaker. Subsequent studies revealed four additional morphological types of melanopsin-expressing RGCs in mice. The morphological type originally described by Berson et al. (2002) is now called M1, while the four new types have been named M2 through M5. The five cell types have different dendritic stratification levels, dendritic morphologies, and soma sizes (Berson et al. 2010; Ecker et al. 2010; Estevez et al. 2012; Schmidt and Kofuji 2011; Schmidt et al. 2008; Viney et al. 2007). Besides innervating non-image-forming visual nuclei, these new types project prominently to the dorsal lateral geniculate nucleus and the superior colliculus and hence could contribute to image-forming vision (Brown et al. 2010; Ecker et al. 2010; Estevez et al. 2012).
In addition to anatomical differences, some of the five ipRGC types have been shown to have different melanopsin expression levels, photoresponse characteristics, and intrinsic electrophysiological properties, suggesting they likely perform diverse physiological functions (Ecker et al. 2010; Sand et al. 2012). In terms of intrinsic electrophysiology, it has been reported that M2 and M3 have similar membrane resistances (Rm) and firing rates, whereas M1 cells have significantly higher Rm and lower spike frequencies (Schmidt and Kofuji 2009, 2011). However, virtually nothing is known about the intrinsic physiological properties of M4 and M5 cells. Moreover, while several studies have demonstrated the presence of voltage-gated currents in rat ganglion-cell photoreceptors (Hartwick et al. 2007; Van Hook and Berson 2010; Warren et al. 2003), potential differences in the expression of these currents among mouse ipRGC types have not been explored. Here, we filled this knowledge gap by carrying out a comprehensive characterization of the electrophysiology of all five types of mouse melanopsin RGCs. Specifically, we examined their Rm, membrane time constants (τm), membrane capacitances (Cm), spiking behaviors, and voltage-gated currents, and found that the five cell types are significantly different in most of these parameters. These results have reinforced the notion that the various ipRGC types subserve different functions.
MATERIALS AND METHODS
Animals
All experimental procedures were approved by the University Committee on Use and Care of Animals at the University of Michigan. To enable identification of ipRGCs for whole cell recording, we used mice in which all five types of melanopsin-expressing ganglion cells were selectively labeled with enhanced green fluorescent protein (EGFP). These mice were generated by crossing a line in which both melanopsin promoters drive Cre recombinase, with a commercially available line in which EGFP expression is induced selectively in cells containing Cre (Ecker et al. 2010). The animals ranged from 6 wk to 4 mo of age, and both sexes were used. All animals were housed in a 12:12-hr light-dark environment, and all experiments were performed during the light phase.
Whole Cell Recording
The isolation of mouse retinas.
Prior to each experiment, an animal was kept in a ventilated light-proof box overnight. Euthanasia and tissue preparation were performed under either red light or infrared illumination. Following euthanasia with carbon dioxide, both eyes were harvested and put in Ames' medium (Sigma, St. Louis, MO) gassed with 95% O2/5% CO2. The retinas were isolated from the retinal pigment epithelium, and forceps were used to remove most vitreous from the retinas. Each retina was cut into three to four pieces, which were incubated in Ames' medium at room temperature and kept in a dimly lit environment (<11 log quanta·cm−2·s−1) for up to 7 h prior to recording.
Electrophysiological recording.
A piece of retina was flattened on the glass bottom of a superfusion chamber with the ganglion cell side up and was held down by a weighted net. The superfusion chamber was positioned on a fixed-stage upright microscope (Eclipse FN1; Nikon Instruments, Melville, NY). The bathing solution (see Chemicals and solutions below) was maintained at 32°C with a temperature controller (Warner Instruments, Hamden, CT) and fed into the recording chamber by a peristaltic pump at 2–3 ml/min. The same pump was used to remove the bathing solution from the recording chamber. After the retina had been exposed to epifluorescence excitation (450–490 nm with an intensity of 16.3 log quanta·cm−2·s−1) for 3–10 s to locate EGFP-expressing RGCs, it was maintained in a dimly lit environment (<11 log quanta·cm−2·s−1) throughout the experiment. The ganglion cell layer was visualized through infrared transillumination using NIS Elements D imaging software (Nikon Instruments), and whole cell recordings were obtained from EGFP-labeled RGCs using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Glass micropipettes with tip resistances 6–8 MΩ were pulled from thick-walled borosilicate tubings on a Narishige PC-10 puller (East Meadow, NY). PCLAMP 9 software (Molecular Devices) was used for data acquisition. Signals were low-pass filtered at 2.4 kHz and sampled at 10 kHz. Series resistances were typically between 20 and 40 MΩ and were compensated by 40–70%.
Chemicals and solutions.
Two kinds of intracellular solution were used. The “K+-based intracellular solution” contained (in mM): 120 K-gluconate; 5 NaCl; 4 KCl; 10 HEPES; 2 EGTA; 4 Mg-ATP; 0.3 Na-GTP; 7 Tris-phosphocreatine; 0.1% Lucifer Yellow; and pH was adjusted to 7.3 with KOH. The “Cs+-based intracellular solution” contained (in mM): 120 Cs-methanesulfonate; 5 NaCl; 4 tetraethylammonium chloride; 10 HEPES; 2 EGTA; 4 Mg-ATP; 0.3 Na-GTP; 7 Tris-phosphocreatine; 0.1% Lucifer Yellow; and pH was adjusted to 7.3 with CsOH. The K+-based intracellular solution was used for all current-clamp recordings and for the voltage-clamp measurement of K+ currents (IK), whereas the Cs+-based intracellular solution was used in all other voltage-clamp experiments. Liquid junction potentials were calculated using CLAMPEX software (Molecular Devices) and were ∼13 mV for the K+ intracellular solution and ∼10 mV for the Cs+ intracellular solution; they were taken into account in all measurements.
Three kinds of bathing solution were used. Unless stated otherwise, the bathing solution was artificial cerebrospinal fluid (ACSF) that contained (in mM): 120 NaCl; 3.6 KCl; 1.15 CaCl2; 1.24 MgCl2; 22.6 NaHCO3; 16 d-glucose; 0.5 l-glutamine; this solution was gassed continuously with 95% oxygen/5% carbon dioxide. In the voltage-clamp experiment that measured voltage-gated Ca2+ currents (ICa), we used a “5 mM-Ca2+ Ringer solution” that contained (in mM): 105.4 NaCl; 20 tetraethylammonium chloride; 10 CsCl; 5 CaCl2; 1.24 MgCl2; 10 HEPES; 16 d-glucose; 0.5 l-glutamine; 0.0003 tetrodotoxin (TTX); and pH was adjusted to 7.4 using NaOH. This 5 mM-Ca2+ Ringer made ICa larger and hence easier to measure. In the voltage-clamp experiment that blocked IK (see Fig. 8, A and B), the “K+-blocking external solution” contained (in mM): 60.3 NaCl; 57.6 tetraethylammonium chloride; 10 CsCl; 10 BaCl2; 1.24 MgCl2; 10 HEPES; 16 d-glucose; 0.5 l-glutamine; and NaOH for adjusting the pH to 7.4. A manifold was used to switch the superfusion between different bathing solutions. TTX, 3-{[(3,4-dichlorophenyl)methyl]amino}propyl diethoxymethyl phosphinic acid (CGP 52432, a GABAB receptor antagonist), and (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA, a GABAC receptor antagonist) were purchased from Tocris (Minneapolis, MN). All other chemicals were purchased from Sigma.
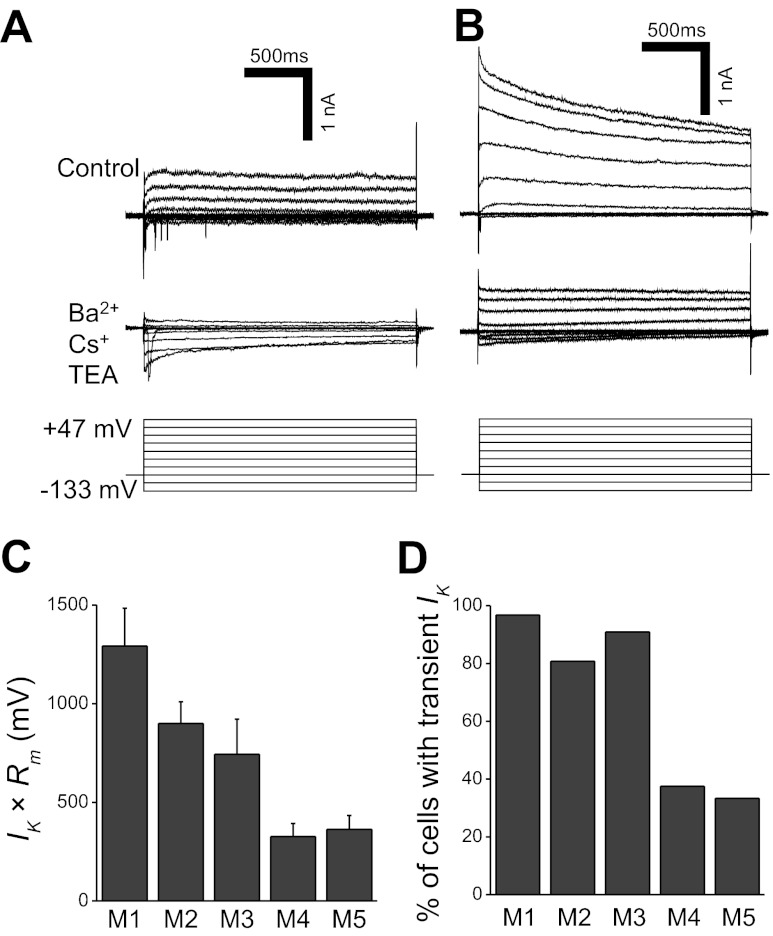
Fig. 8.
Voltage-dependent K+ currents (IK) in ipRGCs. A and B: in response to a series of hyperpolarizing and depolarizing voltage steps in 20-mV increments, some ipRGCs generated sustained outward IK (A, top traces), whereas others generated transient outward IK (B, top traces). Upon the application of the K+ channel blockers Ba2+, Cs+, and TEA (tetraethylammonium), both sustained and transient currents were significantly attenuated (bottom recording traces). Both cells were M2, and all recordings had been leak-subtracted. The holding potential was −93 mV, and the command potentials ranged from −133 mV to +47 mV. The inward currents that emerged in the presence of the K+ blockers were due to the enhancement of Ca2+ currents by Ba2+. C: the products of IK amplitude (measured at +47 mV) and Rm. For M1, n = 22 cells; M2, n = 48; M3, n = 10; M4, n = 15; M5, n = 3. D: percentages of the five morphological types that generated transient IK in response to the +47-mV voltage step. The total numbers of cells tested, including the ones without transient IK, were M1, n = 31 cells; M2, n = 52; M3, n = 11; M4, n = 16; M5, n = 3.
Experimental protocols and data analysis.
We made quantitative measurements of 10 parameters of ipRGC physiology: Rm, τm, Cm, peak spike frequency, average spike frequency, spike width, spike threshold, sag amplitude, voltage-gated ICa amplitudes, and voltage-dependent IK amplitude. An explanation of the experimental and analytical methods utilized in these measurements follows.
1) Rm.
See Fig. 2B. Under current clamp, each neuron was maintained at −83 ± 5 mV with an appropriate holding current to suppress spontaneous spiking activity. A 1-s hyperpolarizing current step with an amplitude (ΔI) of 25–50 pA was presented, and the peak voltage change (ΔV) was measured as illustrated in Fig. 2A. Rm was calculated using Ohm's law, Rm = ΔV/ΔI. Because the ACSF used in this experiment permitted synaptic input, Rm reflected synaptically activated as well as nonsynaptic conductances.
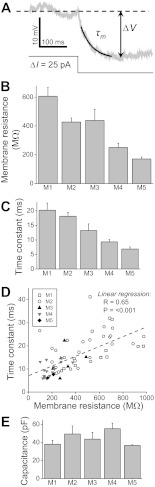
Fig. 2.
Membrane time constants (τm), resistances (Rm), and capacitances (Cm) of ipRGCs. A: methods for measuring τm and Rm. For both measurements, a hyperpolarizing current step with an amplitude of 25–50 pA was injected into the neuron. To determine τm, the voltage response was fitted with a single-exponential decay curve (black curve) between ∼10 and ∼100 ms after the onset of the step, and the time constant of this fit was used as τm. To calculate Rm, Ohm's law (Rm = ΔV/ΔI) was used, with ΔV measured as the difference between the preinjection membrane potential (dashed line) and the peak of the voltage response (ΔI). The cell illustrated here was an M1 cell. B: mean Rm of the five ipRGC types. For M1, n = 24 cells; M2, n = 56; M3, n = 12; M4, n = 36; M5, n = 4. C: mean τm of the five ipRGC types. The n values are M1, 15; M2, 29; M3, 6; M4, 16; M5, 2. D: a positive correlation was found between Rm and τm. The linear regression (dashed gray line) has an R value of 0.65 and a P value of <0.001. E: mean Cm of the five ipRGC types. The n values are the same as those for C.
2) τm.
See Fig. 2C. Cells were held at −83 ± 5 mV under current clamp, and a 25- to 50-pA hyperpolarizing current step was applied to each neuron. Using Clampfit software (Molecular Devices), the voltage response was fitted with a single-exponential decay curve between ∼10 and ∼100 ms after the onset of the current step (see Fig. 2A), and the time constant of this exponential fit was used as τm. To assess the quality of the fits, their correlation coefficients were calculated using Clampfit software. These coefficients ranged from 0.963 to 0.998, and only the cells yielding at least 0.990 were used in this analysis. Because ACSF allowed synaptic input, our τm measurements were influenced by synaptic as well as nonsynaptic conductances.
3) Cm.
See Fig. 2E. For the ipRGCs with high-quality τm fits (see the preceding paragraph), the Cm of each cell was calculated using the equation Cm = τm/Rm.
4) PEAK SPIKE FREQUENCY.
See Fig. 3B. Under current clamp, each cell was held at −83 ± 5 mV with an appropriate holding current to suppress spontaneous spiking. At 5-s intervals, 1-s depolarizing current steps of increasing amplitudes were applied in 25- to 50-pA increments, up to an amplitude of at least 425 pA. As shown in Fig. 3A, large-amplitude current steps caused ipRGCs to enter into depolarization block, resulting in very small spikelike events. Thus spikes <10 mV in amplitude were disregarded in this analysis. To determine the peak firing rate for each 1-s current step, we used the interval between the first two spikes evoked by the current step to calculate the instantaneous spike frequency (Schmidt and Kofuji 2009). In Fig. 3C, each column represents the mean of each ipRGC type's maximum peak firing rates induced by current steps up to 425 pA in amplitude. To calculate this mean, each neuron's highest peak firing rate induced by current steps up to 425 pA was identified, and the values from all of the cells within each ipRGC type were averaged.
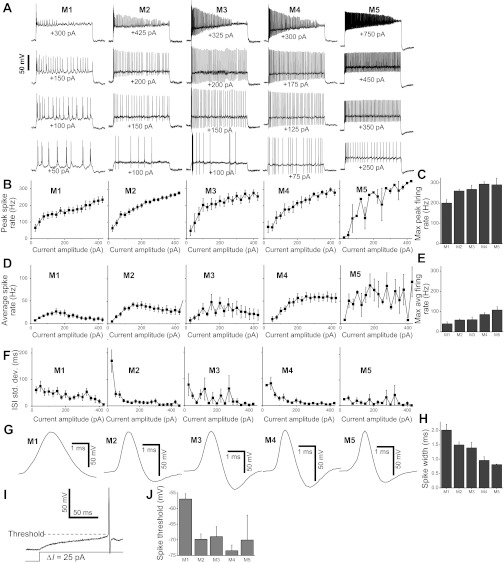
Fig. 3.
Spiking behaviors of ipRGCs. A: representative spiking responses of five ipRGCs to 1-s depolarizing current steps of various amplitudes. For each cell, an appropriate holding current was injected to maintain the baseline membrane potential at −83 ± 5 mV. The number under each response trace indicates the amplitude of the depolarizing current step. B: peak firing rates, calculated from the interval between the first and second spikes in the response to each current step. For individual data points in the M1 plot, n ranged from 8 cells to 17 cells; M2, n = 22 to 27; M3, n = 5 to 6; M4, n = 9 to 13; M5, n = 1 to 3. C: the highest peak firing rates of the five ipRGC types that were induced by currents up to 425 pA in amplitude. N values: M1, 17; M2, 27; M3, 6; M4, 13; M5, 3. D: average spike frequencies, measured by counting the number of spikes induced by each 1-s depolarizing current step. The n values are M1, 11 to 17; M2, 15 to 24; M3, 5 to 6; M4, 12 to 13; M5, 2 to 4. E: the highest average firing rates of the five cell types that were induced by currents up to 425 pA in amplitude. N values: M1, 17; M2, 24; M3, 6; M4, 13; M5, 4. F: spike timing regularity, quantified as the standard deviation of the interspike intervals (ISI). N values are the same as for D. G: representative spike waveforms of the five ipRGC types. H: the half-height spike widths of the five ipRGC types. For M1, n = 25 cells; M2, n = 53; M3, n = 9; M4, n = 22; M5, n = 2. I: measurement of the spike threshold. J: mean spike thresholds. For M1, n = 22 cells; M2, n = 52; M3, n = 10; M4, n = 33; M5, n = 3.
5) AVERAGE SPIKE FREQUENCY.
See Fig. 3D. Action potentials were evoked as described above for the peak firing frequency measurement. To determine the average spike frequency of the response to each 1-s current step, the total number of spikes induced by the current step was counted. In Fig. 3E, each column depicts the mean of each ipRGC type's maximum average firing rates. To calculate this mean, each neuron's highest average spike frequency induced by current steps up to 425 pA in amplitude was identified, and the values from all of the cells within each ipRGC type were averaged.
6) SPIKE WIDTH.
See Fig. 3H. Action potentials were evoked as described above for the peak spike frequency analysis. To determine each cell's spike width, the current-step response with the highest average spike frequency was used. The half-height widths of all of the spikes in this response were measured and averaged.
7) SPIKE THRESHOLD.
See Fig. 3J. Depolarizing current steps of various amplitudes were applied to each ipRGC as described above for the peak spike frequency analysis. The lowest-amplitude current step that caused the cell to spike was identified, and the spike threshold was measured as the membrane potential at which the upstroke of the first action potential started (see Fig. 3I).
8) SAG AMPLITUDE.
See Fig. 4B. Under current clamp, each cell was held at −83 ± 5 mV using an appropriate holding current. At 5-s intervals, 1-s hyperpolarizing current steps of decreasing amplitudes were presented in 25- to 50-pA decrements, inducing hyperpolarizing responses that gradually decayed or “sagged” upward (see Fig. 4A). The trial that induced a peak hyperpolarization of −103 ± 10 mV was selected, and the sag amplitude was measured as the voltage difference between the peak hyperpolarization and the voltage at the end of the 1-s current step.
Fig. 4.
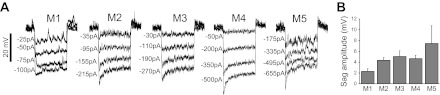
Responses of ipRGCs to hyperpolarizing current steps. A: representative voltage responses to 1-s hyperpolarizing current steps of various amplitudes. Note that these responses gradually “sagged” upward during the current steps. B: average “sag” amplitudes of the five ipRGC types' responses to hyperpolarizing current steps. M1, n = 25; M2, n = 55; M3, n = 10; M4, n = 33; M5, n = 3.
9) VOLTAGE-GATED ICa AMPLITUDES.
See Fig. 6B. Cells were recorded under voltage clamp using the Cs+-based intracellular solution and superfused with the 5 mM Ca2+ Ringer solution. The various types of voltage-gated Ca2+ channels show varying degrees of inactivation upon membrane depolarization (Hille 2001). To gain insight into whether the five ipRGC types might express different varieties of Ca2+ channels, we first held each cell at −80 mV and presented 500-ms voltage steps from −90 mV to +10 mV in 10-mV increments at 2-s intervals. The cell was then held at −40 mV to inactivate some of the voltage-gated Ca2+ channels, and the same series of 500-ms voltage steps was applied again. The responses to both sets of voltage steps were leak-subtracted using Clampfit software. To determine the amplitudes of the ICa inactivated by the −40-mV holding potential (ICa,inact), the responses induced from this holding potential were subtracted from those induced from the −80-mV holding potential (see Fig. 6A), and the amplitude of each response was measured from the prestep baseline to the response peak. To determine the amplitudes of the ICa that remained non-inactivated at the −40-mV holding potential (ICa,non-inact), the voltage-step responses induced from the holding potential of −40 mV were used, and the amplitude of each response was measured from the prestep baseline to the response peak. In all of the histograms shown in Fig. 6, C–E, each cell's largest ICa,inact and ICa,non-inact amplitudes detected in this experiment were used.
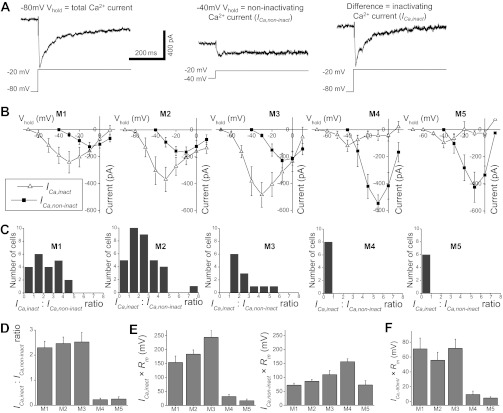
Fig. 6.
Voltage-gated Ca2+ currents in ipRGCs. A: the vast majority of ipRGCs generated both Ca2+ currents that were inactivated by the holding potential of −40 mV (ICa,inact) and those that remained non-inactivated at −40 mV (ICa,non-inact). These recordings from an M2 cell illustrate how these two kinds of currents were elicited. Left: each cell was first held at −80 mV and presented with a series of depolarizing voltage steps. Shown here is the leak-subtracted response to the −20-mV step, which comprised both ICa,inact and ICa,non-inact. Middle: the cell was then held at −40 mV, and the same series of voltage steps presented. This holding potential selectively eliminated ICa,inact so that the voltage steps induced only ICa,non-inact. Shown here is the leak-subtracted response to the −20-mV step. Right: to isolate ICa,inact, the responses induced from the −40-mV holding potential were subtracted from those induced from −80 mV. B: current-voltage relationships of the ICa,inact and ICa,non-inact of all of the ipRGCs examined. For M1, n = 21; M2, n = 34; M3, n = 12; M4, n = 8; M5, n = 6. C: distributions of the ICa,inact-to-ICa,non-inact amplitude ratios. D: the mean ICa,inact-to-ICa,non-inact amplitude ratios. E, left: to estimate the effectiveness of ICa,inact in changing membrane potentials, the peak ICa,inact amplitude of each ipRGC was multiplied by the corresponding cell type's mean Rm (Fig. 2B), and the mean values of this product are shown in this plot. Right: the products of peak ICa,non-inact amplitude and mean Rm. F: to estimate the impact of putative T-type Ca2+ currents on membrane potentials, the amplitude of these currents (ICa,−50 mV) was determined as described in the text and multiplied by each ipRGC type's mean Rm (Fig. 2B). For C–F, n values are the same as those for B.
10) VOLTAGE-DEPENDENT IK AMPLITUDE.
See Fig. 8C. Cells were recorded under voltage clamp using the K+-based intracellular solution. A 2-s voltage step was applied to depolarize the cell from −93 mV to +47 mV to activate IK (i.e., the largest depolarizing steps shown in Fig. 8, A and B). After leak subtraction using Clampfit software, the amplitude of the IK response was measured from the prestep baseline to the peak of the response. In the plot shown in Fig. 8C, each ipRGC's IK amplitude was multiplied by the cell's Rm measured as described above in the Rm section.
To test whether each of the above physiological parameters was related to morphological type, the data sets from the five cell types were first subjected to the Kruskal-Wallis statistical test. If a significant relationship was observed for a given physiological parameter, a post hoc test was then applied to systematically compare all 10 possible pairs of cell types. All statistical analyses were performed using SPSS software (IBM, Armonk, NY).
Light-evoked responses.
Light-evoked responses were recorded from 13 ipRGCs to assess the quality of the voltage clamp. Each cell was superfused using ACSF supplemented with 200 μM picrotoxin, 10 μM CGP52432, 30 μM TPMPA, 5 μM strychnine, and 300 nM TTX; these drugs were used to block amacrine-cell input to ipRGCs (Wong et al. 2007). The light stimuli were full-field 490-nm light generated by filtering the microscope's tungsten-halogen lamp with a narrowband filter. All light stimuli were introduced from below the superfusion chamber's glass bottom and had an intensity of 15.5 log quanta·cm−2·s−1 at the retina. A logic-controlled electromechanical shutter regulated stimulus timing. The amplitude of each light response was measured from the prestimulus baseline to the peak of the response.
Morphological Analysis
Immediately after electrophysiological recording from each cell, intracellular Lucifer Yellow staining was visualized by through-focus microscopy and saved on the computer as a movie in AVI format using the imaging software. These movies were analyzed offline to determine the recorded neurons' morphological types, according to published criteria. Specifically, RGCs with sparse dendrites terminating in a single layer deep beneath the retinal surface were categorized as M1 cells, whose dendrites stratify exclusively in the OFF sublamina of the inner plexiform layer (Berson et al. 2002; 2010). Cells with relatively sparse dendrites stratifying only near the retinal surface were classified as M2 neurons (Berson et al. 2010; Ecker et al. 2010; Estevez et al. 2012; Schmidt and Kofuji 2009; Schmidt et al. 2008). Bistratified cells were classified as M3 cells (Berson et al. 2010; Schmidt and Kofuji 2011; Viney et al. 2007). RGCs with relatively dense and thick dendrites that branched in a radiate pattern and stratified only near the retinal surface were identified as M4. An additional characteristic of M4 cells is their relatively large cell bodies (Ecker et al. 2010; Estevez et al. 2012). However, we found that some ipRGCs' cell bodies changed in shape and/or size during recording, making soma size a somewhat unreliable metric. Thus we identified M4 cells mainly based on their dendritic characteristics and used soma size only as a secondary criterion. Cells with bushy dendrites that covered relatively small dendritic fields and that stratified only near the retinal surface were categorized as M5 (Ecker et al. 2010). Recorded cells with equivocal morphologies were discarded.
The images shown in Fig. 1A were created from the AVI movies of the dye fills. For each cell, six frames acquired at six different focal planes were exported from the movie into separate JPEG files. All of the out-of-focus cellular structures in each JPEG image were masked manually, and a Z projection of the six processed JPEG files were then generated using ImageJ software (National Institutes of Health, Bethesda, MD).
Fig. 1.
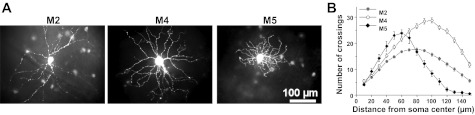
Morphologies of the three types of ON-stratifying intrinsically photosensitive retinal ganglion cells (ipRGCs). A: Lucifer Yellow fills of an M2 cell, an M4 cell, and an M5 cell. Due to the large dendritic fields of M2 and M4 cells, the dendritic arbors of the vast majority of these cells extended beyond the field of view of our imaging system. The bright spots around the dye-filled cells are green fluorescent protein signals from the somas of surrounding ipRGCs. B: Sholl's analysis of 94 M2 cells, 37 M4 cells, and 9 M5 cells. The error bars represent SEM.
The branching patterns of some of the recorded cells were analyzed using Sholl's method (Sholl 1953). Fifteen concentric circles with 10-μm spacing were laid around the center of each cell's soma. The number of dendrites crossing each circle was plotted vs. the distance from the soma center (Fig. 1B). Due to the limited field of view of our imaging system, this analysis could be performed only up to 150 μm from the soma center.
RESULTS
Identification of M2, M4, and M5 Cells
Among the five morphological types of ipRGCs, M1 and M3 cells can be identified unequivocally based on their unique dendritic stratification patterns: M1 cells are the only ipRGCs that monostratify in the OFF sublayer of the innerplexiform layer, whereas M3 cells are the only ipRGCs that stratify in both ON and OFF sublayers. M5 cells' uniquely small dendritic fields also enable them to be identified with ease (Ecker et al. 2010) (Fig. 1A, right). By contrast, it is harder to distinguish M2 and M4 because both types have large dendritic fields (Fig. 1A, left and middle) and monostratify in the ON sublamina. Thus, after identifying M2 and M4 cells qualitatively as described in materials and methods, we performed Sholl's analysis to compare their dendritic patterns quantitatively. Similar to a previous study (Estevez et al. 2012), we found that M4 cells had nearly twice as many dendrites as M2 cells at most distances from the soma (Fig. 1B), thereby confirming the accuracy of the qualitative categorization. We also performed Sholl's analysis on M5 cells because the dendritic morphology of these cells had not been characterized in detail. On average, the number of dendrites peaked at ∼24 at a distance of ∼60 μm from the soma. This number dropped to nearly zero at ∼140 μm, reflecting the small dendritic field size of this cell type (Fig. 1B).
Rm, τm, Cm
In the experiments measuring these parameters, retinas were superfused in normal ACSF, which permitted synaptic transmission. Because bipolar and amacrine cells spontaneously release neurotransmitters that activate ionotropic receptors on ipRGCs (Perez-Leon et al. 2006; Wong et al. 2007), the Rm measured in these experiments reflected both synaptically activated and nonsynaptic conductances. The Rm of the five morphological types ranged from ∼170 MΩ for M5 to just over 600 MΩ for M1 and are summarized in Fig. 2B and Table 1. The dependence of Rm on morphological type was statistically significant, with a Kruskal-Wallis H value of 38.0 and P value of <0.001. A post hoc analysis revealed statistically significant differences between 7 of the 10 possible pairs of cell types (Table 2), with the most significant differences between M1 vs. M4 (P value < 0.001) and between M2 vs. M4 (P value < 0.001).
Table 1.
Summary of intrinsic physiological properties of mouse ganglion-cell photoreceptors
| Cell Type | Rm, MΩ | τm, ms | Cm, pF | Max Peak Firing, Hz | Max Avg Firing, Hz | Spike Width, ms | Spike Threshold, mV | Sag, mV | ICa,inact/ ICa,non-inact | ICa,inact × Rm, mV | ICa,non-inact × Rm, mV | ICa,−50 mV × Rm, mV | IK × Rm, mV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 607 ± 59 | 20.2 ± 2.5 | 38.0 ± 4.3 | 201 ± 16 | 40 ± 9 | 2.01 ± 0.21 | −57.0 ± 1.7 | 2.31 ± 0.50 | 2.30 ± 0.26 | 154 ± 23 | 72 ± 7 | 71 ± 14 | 1292 ± 192 |
| M2 | 427 ± 29 | 18.1 ± 1.3 | 49.3 ± 9.0 | 260 ± 9 | 58 ± 5 | 1.50 ± 0.10 | −69.8 ± 1.7 | 4.35 ± 0.51 | 2.47 ± 0.26 | 184 ± 15 | 86 ± 6 | 56 ± 11 | 900 ± 110 |
| M3 | 439 ± 77 | 13.2 ± 2.3 | 43.7 ± 7.6 | 268 ± 20 | 60 ± 13 | 1.40 ± 0.19 | −68.9 ± 3.2 | 5.06 ± 1.04 | 2.53 ± 0.37 | 244 ± 26 | 110 ± 15 | 72 ± 12 | 744 ± 178 |
| M4 | 251 ± 30 | 9.3 ± 0.8 | 55.2 ± 6.2 | 294 ± 12 | 86 ± 8 | 0.96 ± 0.13 | −73.5 ± 1.8 | 4.67 ± 0.59 | 0.23 ± 0.06 | 32 ± 8 | 156 ± 10 | 10 ± 5 | 327 ± 66 |
| M5 | 171 ± 14 | 6.9 ± 0.7 | 36.7 ± 0.8 | 291 ± 33 | 108 ± 17 | 0.82 ± 0.02 | −70.1 ± 8.0 | 7.48 ± 3.28 | 0.25 ± 0.09 | 17 ± 6 | 73 ± 15 | 5 ± 2 | 364 ± 69 |
Values are means ± SE. Rm, membrane resistance; τm, membrane time constant; Cm, membrane capacitance; ICa,inact, amplitude of the Ca2+ currents that were inactivated when intrinsically photosensitive retinal ganglion cells were held at −40 mV; ICa,non-inact, amplitude of the Ca2+ currents that could be induced when cells were held at −40 mV; ICa,−50 mV, amplitude of ICa,inact evoked by the −50-mV voltage step which probably activated mainly T-type channels; IK, voltage-dependent K+ current amplitude.
Table 2.
Pairwise comparisons of physiological properties that showed statistically significant differences
| M1 | M2 | M3 | M4 | |
| M2 | Rm, fpeak, favg, W, t, S, K | |||
| M3 | fpeak, W, t, S, I, N | I | ||
| M4 | Rm, τm, fpeak, favg, W, t, S, IN, I, N, T, K | Rm, τm, fpeak, favg, W, IN, I, N, T, K | Rm, W, IN, I, N, T, K | |
| M5 | Rm, τm, favg, W, S, IN, I, T, K | Rm, τm, favg, IN, I, T | Rm, IN, I, T | N |
Each letter represents a physiological parameter: Rm, τm, maximum peak firing frequency (fpeak), maximum average firing frequency (favg), spike width (W), spike threshold (t), sag amplitude (S), ICa,inact-to-ICa,non-inact amplitude ratio (IN), ICa,inact amplitude × Rm (I), ICa,non-inact amplitude × Rm (N), ICa,−50 mV amplitude × Rm (T), and voltage-dependent K+ current amplitude × Rm (K).
Similarly, the τm of the five ipRGC types spanned a large range, from ∼7 ms for M5 to ∼20 ms for M1 (Fig. 2C and Table 1), and morphological type was found to have significant influence on this physiological property (Kruskal-Wallis H value = 25.8, P value < 0.001). Four of the 10 possible cell type pairs were significantly different (Table 2), with the most significant differences between M1 and M4 (P value < 0.001) and between M2 and M4 (P value < 0.001). Since τm equals Rm times Cm and our Rm measurements included both synaptic and nonsynaptic conductances, our τm measurements were influenced by both kinds of conductances. A positive correlation was observed between τm and Rm, with an R value of 0.65 and a P value of <0.001 (Fig. 2D).
By contrast, the five cell types' Cm spanned a relatively small range, from ∼37 pF for M5 to ∼55 pF for M4 (Fig. 2E and Table 1) and were not significantly influenced by morphological type (Kruskal-Wallis H value = 8.6, P value = 0.071).
Spike Rates
All five ipRGC types generated action potentials in response to sufficiently large depolarizing current injection. Representative spiking responses of five different cells are shown in Fig. 3A. For each neuron illustrated in this panel, the bottom response was evoked by the lowest amplitude current that induced at least five spikes, while the top response was elicited by the lowest amplitude current that resulted in spike block. The two traces in between show these cells' responses to intermediate-amplitude current steps. At all current amplitudes that did not induce spike block, all five cell types spiked continuously throughout the 1-s injection. Fig. 3, B and D, plot the five morphological types' peak spike rates and average spike rates as a function of current amplitude. For all cell types, average spike rates were lower than peak firing rates at all current amplitudes, reflecting frequency adaptation to sustained stimulation. A positive correlation existed between current amplitude and peak firing rate over the entire range of current amplitudes presented (Fig. 3B). By contrast, due to spike block caused by high current amplitudes, most cells responded to increasing current amplitudes with increasing average spike rates only up to ∼150–250 pA, beyond which average spike rates either plateaued or declined (Fig. 3D). On average, spike block was induced when ipRGCs were depolarized above −28.9 ± 1.9 mV (n = 105). Low-amplitude spiking responses tended to have irregular patterns, and bursts of two or more action potentials were occasionally observed in these near-threshold responses, e.g., the M1 cell's bottom response trace in Fig. 3A. However, spiking patterns became more regular as spike rates increased, resulting in smaller standard deviations of interspike intervals (Fig. 3F).
Maximum peak firing rates ranged from ∼200 Hz for M1 cells to just under 300 Hz for M4 (Fig. 3C and Table 1) and were significantly influenced by ipRGC type (Kruskal-Wallis H value = 17.3, P value = 0.002). The cell type pairs with significantly different maximum peak firing rates are listed in Table 2, with the most significant differences observed between M1 and M2 (P value = 0.004), and between M1 and M4 (P value < 0.001). Maximum average spike rates ranged from 40 Hz for M1 to nearly 110 Hz for M5 (Fig. 3E and Table 1) and showed strong dependence on cell type (Kruskal-Wallis H value = 23.2, P value < 0.001). Table 2 lists the cell pairs with significant differences in this measure, and the most significant differences were M1 vs. M2 (P value = 0.001) and M1 vs. M4 (P value < 0.001).
Spike Width
Representative examples of the spike waveforms of the five ipRGC types are shown in Fig. 3G. Spike widths ranged from ∼0.8 ms for M5 cells to ∼2.0 ms for M1 cells (Fig. 3H and Table 1) and were significantly dependent on cell type (Kruskal-Wallis H value = 29.5, P value < 0.001). Of the 10 possible pairwise comparisons, 6 pairs showed significantly different spike widths (Table 2), with the most significant differences between M1 and M4 (P value < 0.001) and between M2 and M4 (P value < 0.001).
Spike Threshold
Spike threshold was statistically related to ipRGC type (Kruskal-Wallis H value = 33.5, P value < 0.001). Whereas the spike thresholds for M2–M5 cells spanned a relatively small range (about −74 mV to −69 mV) and were statistically indistinguishable, those for M1 cells were more positive, at about −57 mV on average (Fig. 3J and Table 1). M1 cells were significantly different from M2–M4 cells in this measure, with P values ranging from <0.001 to 0.003.
“Sagging” Responses To Hyperpolarizing Currents
It was previously shown that rat M1 cells' responses to sustained hyperpolarizing currents decayed or “sagged” toward the resting potential, due to the recruitment of the hyperpolarization-activated inwardly-rectifying current (Ih) (Van Hook and Berson 2010). We found that the vast majority of mouse ganglion-cell photoreceptors (n = 114 out of 130) responded to hyperpolarizing currents in a similar manner, and representative examples are shown in Fig. 4A. Under our experimental conditions (see materials and methods), the amplitude of this sag ranged from ∼2.3 mV for M1 to ∼7.5 mV for M5 (Fig. 4B and Table 1). The amplitude of this sag was correlated with morphological type, with a Kruskal-Wallis H value of 14.4 and P value of 0.006. Of the 10 possible pairwise comparisons, 4 showed statistically significant differences in this parameter, all of them between M1 and the other types (Table 2). The most significant differences were between M1 and M2 (P value = 0.003), and between M1 and M4 (P value = 0.002).
Assessing Voltage-clamp Quality
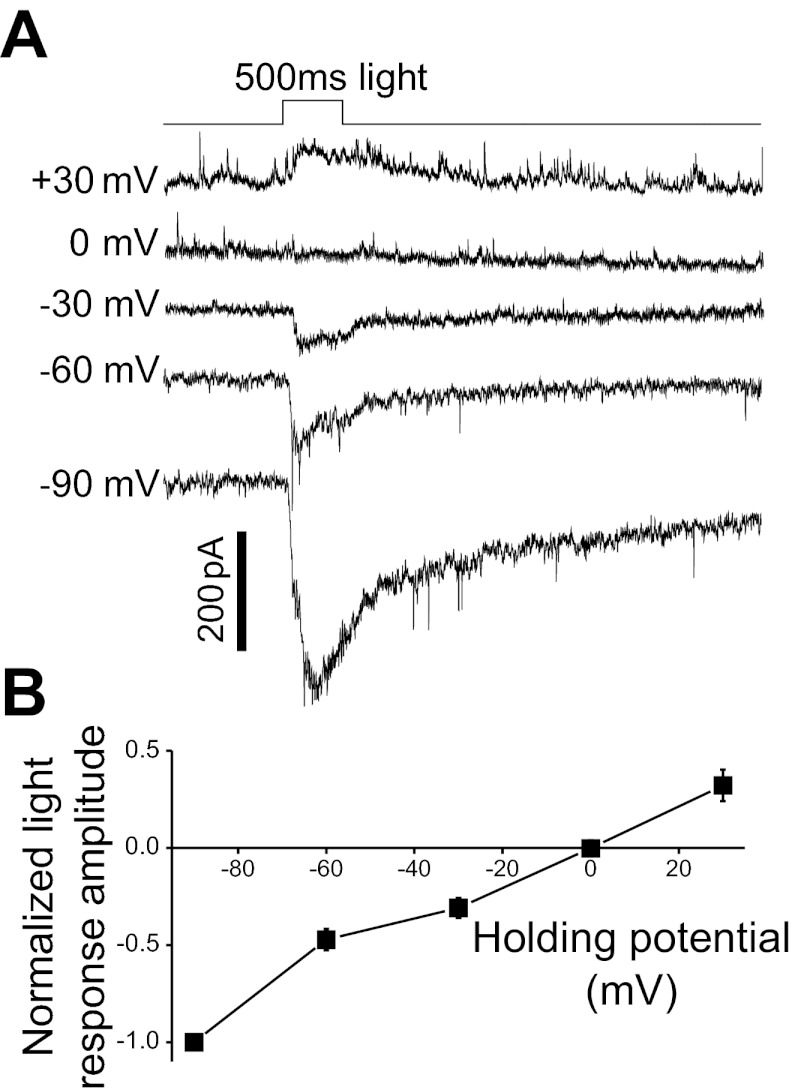
In the studies to be described below, we made voltage-clamp recordings from ipRGCs to analyze voltage-gated currents. Because M1–M4 cells have long dendrites, it was important to verify that these dendrites could be adequately clamped. We took advantage of the fact that both bipolar cell-driven and melanopsin-based photoresponses are mediated by increases in cationic conductances, and that both responses occur throughout the entire dendritic field of an ipRGC (Berson et al. 2002; Warren et al. 2003; Wong et al. 2007; Xue et al. 2011). M1–M4 cells were recorded using the Cs+-based intracellular solution and superfused with ACSF containing drugs that blocked amacrine-cell signaling (see Light-evoked responses in materials and methods). Each cell was voltage-clamped at five different holding potentials, and, at each potential, a full-field light stimulus was presented. We found that the light-evoked responses of 13 cells reversed at 2.6 ± 2.7 mV, as expected for cationic conductances (Fig. 5). Based on this result, we believe that our voltage-clamp recordings obtained using the Cs+-based intracellular solution were of sufficiently high quality.
Fig. 5.
Evidence for accurate voltage clamp of ipRGC dendrites. In this experiment, amacrine cell signaling was blocked using GABA and glycine antagonists (200 μM picrotoxin, 10 μM CGP52432, 30 μM TPMPA, and 5 μM strychnine) so that all ipRGC photoresponses were mediated by melanopsin- and bipolar cell-driven increases in cationic conductances. All light stimuli were full-field and thus illuminated each cell's entire dendritic field. A: voltage-clamp recordings from an M1 cell. B: average of all ipRGCs tested: M1, n = 5; M2, n = 6; M3, n = 1; M4, n = 1. Each cell's largest photoresponse amplitude was normalized to 1. These light responses reversed at 2.6 ± 2.7 mV, as expected for cationic currents.
Voltage-gated ICa
To facilitate measurement of voltage-gated ICa, all ipRGCs were superfused with the 5 mM Ca2+ Ringer solution; see Chemicals and solutions in materials and methods. Under our experimental conditions, all ipRGCs tested (n = 92) generated ICa when held at −40 mV, indicating the presence of Ca2+ channels that remained non-inactivated at this holding potential (ICa,non-inact), and all but two M4 and two M5 cells also generated ICa that were inactivated at −40 mV (ICa,inact). ICa,inact was relatively transient, whereas ICa,non-inact was more sustained (Fig. 6A, middle and right). The current-voltage relationships of the five ipRGC types are plotted in Fig. 6B. For some cells, ICa,inact was larger than ICa,non-inact, whereas for others, ICa,non-inact dominated. M1 and M2 consisted of both ICa,inact-dominant and ICa,non-inact-dominant cells, whereas all M3 cells were ICa,inact-dominant, and all M4 and M5 cells were ICa,non-inact-dominant (Fig. 6C). As a result, the ratio of ICa,inact to ICa,non-inact amplitudes was highly dependent on ipRGC type (Fig. 6D), with a Kruskal-Wallis H value of 32.6 and P value of <0.001. Six of the 10 possible cell type pairs were found to be significantly different in this ratio (Table 2), all of them with a P value of <0.001.
To assess how ICa,inact and ICa,non-inact might differentially impact the five cell types' membrane potentials (ΔV), each ipRGC's peak ICa,inact and ICa,non-inact amplitudes (ΔI) were multiplied by the corresponding cell type's mean Rm (determined using cells recorded with the K+-based intracellular solution; see Fig. 2B) according to Ohm's law, ΔV = ΔI × Rm. Note that this calculation aimed merely to compare the relative rather than absolute impacts of ICa on the membrane potentials of the five ipRGC types. Under physiological conditions, these currents' effects would be much smaller, for two reasons. First, the physiological extracellular Ca2+ concentration ([Ca2+]o) is only 1.15 mM (Ames and Nesbett 1981), and we found that ICa were roughly 50% smaller in 1.15 mM than in 5 mM [Ca2+]o. For example, the peak ICa,inact amplitude of M2 cells was 430 ± 36 pA in 5 mM [Ca2+]o (n = 34), but only 223 ± 37 pA in 1.15 mM [Ca2+]o (n = 3). Moreover, the Rm used in this calculation was obtained from ipRGCs held at −83 mV, below the activation range for most voltage-gated ion channels, and Rm would become significantly lower when these channels are activated. As shown in Fig. 6E, ICa,inact probably exerts the greatest impact on the membrane potentials of M3 cells while affecting M5 cells the least, whereas ICa,non-inact probably affects M4 cells the most and M1/5 cells the least. A significant correlation was found between ICa,inact × Rm and cell type (Kruskal-Wallis H value = 37.5, P value < 0.001). Eight of the 10 possible cell type pairs were significantly different (Table 2), with 5 of these pairwise comparisons showing P values of <0.001, specifically M1 vs. M4, M1 vs. M5, M2 vs. M4, M2 vs. M5, and M3 vs. M4. Similarly, ICa,non-inact × Rm was significantly influenced by cell type (Kruskal-Wallis H value = 20.6, P value < 0.001). Five of the 10 possible cell pairs were significantly different in ICa,non-inact × Rm (Table 2), and the most pronounced differences were those between M1 and M4 (P value < 0.001) and between M2 and M4 (P value < 0.001).
In terms of inactivation properties, voltage-gated Ca2+ channels can be divided into three groups. Specifically, in response to sufficiently depolarized holding potentials, T-type channels inactivate completely, whereas the P/Q, N, and R types exhibit partial inactivation, and the L type inactivates minimally and very slowly (Hille 2001). Thus the ICa,inact we recorded in this experiment was probably generated mainly by T-, P/Q-, N-, and/or R-type channels, whereas ICa,non-inact likely arose primarily from L-, P/Q-, N-, and/or R-type channels. Because T-type channels are activated at voltages as negative as −70 mV, whereas the activation thresholds for all the other types are positive to around −30 mV (Hille 2001), we compared the T-type currents in ipRGCs by analyzing the amplitudes of their ICa,inact responses to the −50-mV voltage step (ICa,−50 mV), which was probably not sufficiently depolarized to activate the non-T-type Ca2+ channels. By multiplying ICa,−50 mV amplitudes by Rm, we found that these currents probably affect the membrane potentials of M1 and M3 cells the most, and those of M4 and M5 the least (Fig. 6F). The product of ICa,−50 mV amplitude and Rm was correlated with morphological type (Kruskal-Wallis H value = 19.7, P value = 0.001). Six of the 10 pairwise comparisons were significantly different (Table 2), and the differences were most significant between M1 and M4 (P value = 0.001), and between M3 and M4 (P value = 0.001).
Voltage-gated Na+ Currents
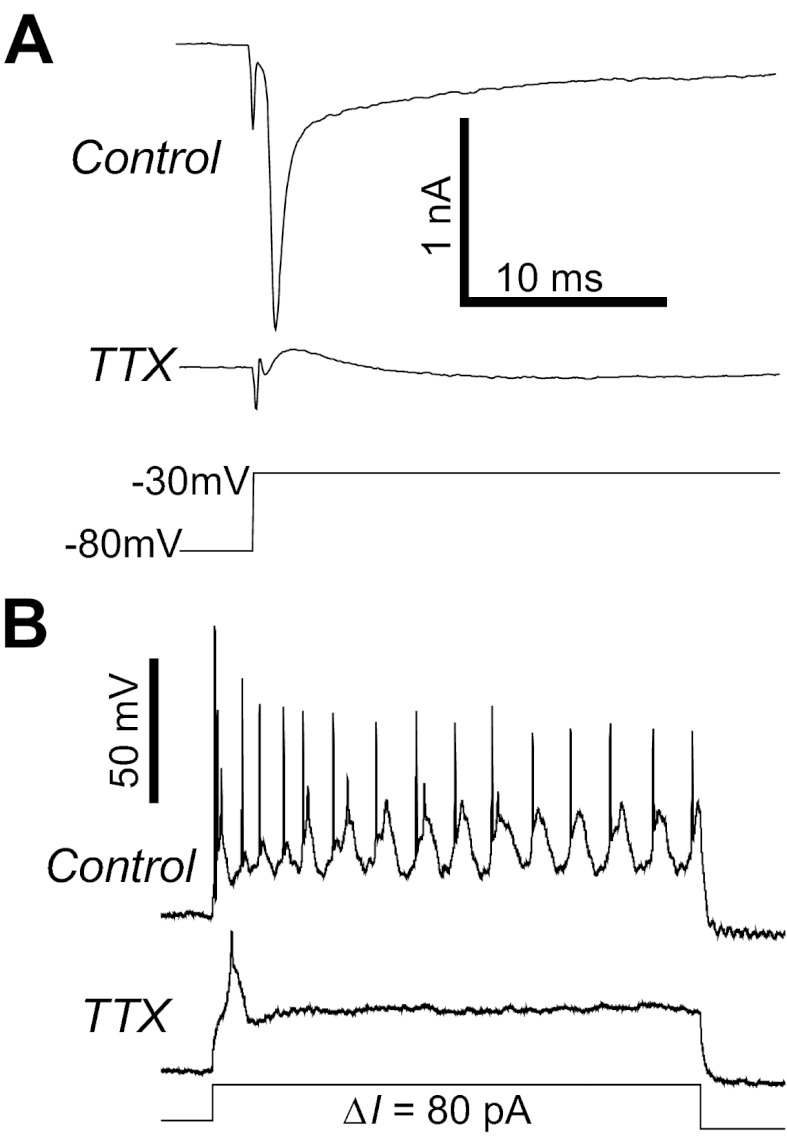
To study voltage-gated Na+ (NaV) currents, voltage-clamped ipRGCs were held at −80 mV, and depolarizing voltage steps of various amplitudes were presented at 5-s intervals. In response to the voltage steps that significantly exceeded the spike thresholds (Fig. 3J), all ipRGCs generated short-latency, rapidly inactivating inward currents (Fig. 7A, top recording) that were far shorter in duration than the above-mentioned voltage-gated ICa (Fig. 6A). These short-duration currents were abolished by 300 nM TTX (n = 48; Fig. 7A, bottom recording), confirming that they were NaV currents. 300 nM TTX also completely blocked all action potentials induced by depolarizing current steps (n = 34; Fig. 7B). In the presence of this drug, a few ipRGCs generated broad, transient depolarizations at the onset of some current steps (Fig. 7B, bottom recording). These TTX-resistant events were likely to be Ca2+ spikes.
Fig. 7.
Voltage-gated Na+ (NaV) currents in ipRGCs. A: for all ipRGCs, sufficiently large depolarizing voltage steps induced transient inward currents that were abolished by 300 nM tetrodotoxin (TTX), indicating the presence of NaV channels. These leak-subtracted recordings were obtained from an M2 cell. B: for all ipRGCs, 300 nM TTX eliminated Na+ action potentials induced by 1-s depolarizing current steps. In some cells, a broad, low-amplitude spike was induced at the beginning of some depolarizing current steps, which was likely a Ca2+ spike. These current-clamp recordings came from an M2 cell different from the one shown in A.
Voltage-dependent IK
In normal ACSF, all ipRGCs' current responses to voltage steps of various amplitudes showed outward rectification (Fig. 8, A and B, top recordings). These outwardly rectifying currents were suppressed in the presence of Ba2+, tetraethylammonium, and Cs+, indicating they were IK (Fig. 8, A and B, bottom recordings). These currents could be mediated by voltage-gated K+ channels, or Ca2+-activated K+ channels, or both; no attempt was made to distinguish these two classes of channels. To use Ohm's law to assess the potential impact of IK on the membrane potential, each ipRGC's peak IK amplitude in the response to the +47-mV voltage step was multiplied by the cell's Rm (Fig. 8C). The product of these parameters was statistically dependent on ipRGC type (Kruskal-Wallis H value = 20.3, P value < 0.001), and 5 of the 10 possible cell type pairs had significant differences (Table 2). The most significant differences were observed between M1 vs. M4 (P value < 0.001) and between M2 vs. M4 (P value = 0.001). Two varieties of IK responses were observed. For a few M1–M3 cells and the majority of M4 and M5 cells, IK was sustained throughout the 2-s voltage steps (Fig. 8A). By contrast, for most M1–M3 cells and a minority of M4 and M5 cells, IK slowly decayed toward steady-state levels during the 2-s voltage steps (Fig. 8, B and D). For these latter ipRGCs, the responses to the +47-mV step decayed with time constants ranging from 243 to 8,192 ms or 2,001 ± 235 ms on average.
DISCUSSION
Potential Technical Pitfalls
Significant differences among the five ipRGC types were observed for nearly all of the physiological parameters analyzed. However, for some parameters, variability within each cell type was rather high as evident from the error bars in some of the plots, which could conceivably be caused by variability in cell health, regional differences across the retina, or errors in cell type identification. Without such variability, physiological differences among the cell types could have been even more pronounced than suggested by our data. The small number of M5 cells in our sample could have further obscured across-type differences. On the other hand, some readers might be concerned that the variability in our data could have artificially caused at least some of the across-type differences reported here. We do not believe that these differences were artificial because, for the parameters that Schmidt and Kofuji (2009, 2011) reported previously for M1–M3, their measurements were usually in close agreement with ours. Furthermore, in general terms, our findings were largely congruent with those described in O'Brien and colleagues' large-scale studies of mammalian RGCs (O'Brien et al. 2002; Wong et al. 2012).
Another potential concern is the reliability of our voltage-clamp data. Most investigations of voltage-gated currents in RGCs have utilized dissociated cells because the removal of dendrites improves space clamp quality (Lilley and Robbins 2005; Lipton and Tauck 1987; Van Hook and Berson 2010). We chose to obtain voltage-clamp recordings in situ, because intact cells are required for morphological categorization and because we wanted to preserve dendritic as well as somatic ion channels (Henderson and Miller 2003). Whenever possible, we enhanced our ability to space-clamp the ipRGCs' long dendrites by using the Cs+-based intracellular solution to block most K+ conductances. Under these conditions, cation-based photoresponses generated in ipRGC dendrites reversed near the expected equilibrium potential of 0 mV. Further, ICa,non-inact peaked around −10 mV (Fig. 6B), agreeing with measurements made from dissociated RGCs (Karschin and Lipton 1989). In conclusion, our intact ipRGCs were probably adequately space-clamped when recorded using the Cs+-based intracellular solution. By contrast, our voltage-clamp studies of IK necessitated the preservation of K+ conductances and hence the use of the K+-based intracellular solution. Consequently, space clamp was likely relatively poor in those experiments, and our results concerning voltage-dependent IK should be interpreted with caution.
Rm and τm
Schmidt and Kofuji (2011) previously reported that mouse M2 and M3 cells had comparable Rm, whereas M1 had significantly higher Rm. We have confirmed this observation and further found that M4 had lower Rm than M1–M3, and that M5 had the lowest Rm among ipRGCs, although our experimental conditions did not allow us to address whether these differences arose from differences in synaptic conductances, or nonsynaptic conductances, or both. The low Rm of M4 cells was expected because these neurons have large cell bodies and thick dendrites and because morphologically they resemble the alpha-cells (Ecker et al. 2010), which have the lowest Rm among RGCs (O'Brien et al. 2002; Wong et al. 2012). On the other hand, M5 cells' even lower Rm was somewhat surprising, considering their narrow dendritic fields. However, their dendrites are dense (Ecker et al. 2010) and hence might contain many neurotransmitter receptors, which were probably tonically activated during our recordings. We noticed a positive correlation between τm and Rm, similar to that observed for cat and rat RGCs (O'Brien et al. 2002; Wong et al. 2012). Under our recording conditions, M1 was found to have longer τm than all other ipRGCs, which could help it integrate synaptic inputs and melanopsin photoresponses over longer durations than for the non-M1 ipRGCs. Such a long τm could also contribute to M1's relatively broad action potentials.
Spiking Behavior
We determined the peak firing rates of M1–M5 to be around 200, 260, 270, 295, and 290 Hz, respectively. Interestingly, we have also observed this rank order in firing rates during photic stimulation of mouse ipRGCs. For example, when presented with a 10-s light step that was about 12 log quanta·cm−2·s−1 in intensity, the spike rates of M1–M5 during the first second of stimulation are around 45, 60, 70, 125, and 115 Hz, respectively (X. Zhao and K. Y. Wong, unpublished observations). For comparison, in response to current step injection, the peak firing frequencies of cat and rat RGCs varied from ∼30 to 260 Hz (O'Brien et al. 2002; Wong et al. 2012). Thus ipRGCs' peak spike rates are near the upper limit for ganglion cells, possibly because ganglion-cell photoreceptors need a very large dynamic range to encode an enormous span of light intensities (Dacey et al. 2005). The alpha-like M4 cells' peak spike rate was higher than those of all the other ipRGCs. Likewise, O'Brien et al. reported the alpha-cells' peak firing rate to be the highest for cat and rat RGCs (O'Brien et al. 2002; Wong et al. 2012).
For all five ipRGC types, maximum average firing rates were elicited by moderate-intensity current injections (Fig. 3D) because all cells entered into spike block when high-amplitude currents depolarized the membrane beyond about −30 mV. In response to sufficiently intense light, ganglion-cell photoreceptors often depolarize above this potential and enter into spike block, although subsequent light adaptation allows spiking to resume (Wong et al. 2005). Thus spike block is a physiologically relevant phenomenon.
Similar to other RGCs (O'Brien et al. 2002; Wong et al. 2012), we found that spike width was inversely correlated with spike rate (Fig. 3, C, E, and H). It has been hypothesized that spike width differences may contribute to spike rate differences (O'Brien et al. 2002). According to this hypothesis, a broad action potential activates voltage-gated Ca2+ channels for longer than a narrower impulse, causing the influx of more Ca2+, which opens more Ca2+-activated K+ channels, thereby reducing spike frequency. In addition, we found that M1 had significantly higher spike thresholds than all other ipRGCs, which could further contribute to the relatively low firing rates of M1 cells. As discussed below, additional differences in IK across the ipRGC types could also underlie their spike rate differences. Moreover, because cell geometry can influence firing behavior (Fohlmeister and Miller 1997b), it is conceivable that the different morphologies of the five ipRGC types are partly responsible for their different firing rates.
Voltage-Gated Currents
Ih.
Ih responses are mediated by hyperpolarization-activated cyclic nucleotide-gated channels (Santoro et al. 1998). Pharmacologically blocking Ih was previously shown to have no effect on the resting potential or spiking responses of rat M1 cells, suggesting that this current may not be functionally relevant to these cells (Van Hook and Berson 2010). However, we found that, for mouse ipRGCs, M2–M5 generated significantly larger Ih-mediated “sags” than M1, and so it is possible that Ih plays a more prominent role in the non-M1 ipRGCs. O'Brien and colleagues detected Ih in most types of cat and rat RGCs (O'Brien et al. 2002; Wong et al. 2012) and observed that OFF-stratifying cell types had a tendency to exhibit smaller Ih than both ON-stratifying and bistratifying ones (Wong et al. 2012), matching what we found for ipRGCs.
ICa.
All ipRGCs exhibited ICa,non-inact and ∼95% also showed ICa,inact. M1 and M2 comprised both ICa,inact- and ICa,non-inact-dominant cells, whereas all M3 cells were ICa,inact dominant, while all M4 and M5 cells were ICa,non-inact dominant. Thus, in terms of ICa, M1 and M2 appear more diverse than the other ganglion-cell photoreceptors. It will be of interest to learn whether these two M1 subgroups correspond to the two subtypes of mouse M1 cells that differ in molecular phenotype and axonal projection, namely, the Brn3b-negative M1 cells that innervate the SCN, and the Brn3b-positive M1 cells that project to all other non-image-forming visual nuclei (Chen et al. 2011).
As mentioned earlier, the ICa,inact we recorded from ipRGCs was likely generated by T-, P/Q-, N-, and/or R-type channels, whereas ICa,non-inact probably included L-, P/Q-, N-, and/or R-type currents. ICa,non-inact almost certainly included L-type currents. This is because all adult RGCs studied to date exhibited this current type (Ishida 1995), and because, in a Ca2+ imaging study of rat ipRGCs, L-type Ca2+ channel blockers virtually eliminated these cells' light-evoked Ca2+ dye signals (Hartwick et al. 2007). This imaging result may appear surprising considering that most mouse ipRGCs generated robust ICa,inact in response to the −50-mV voltage step, which probably mainly activated T-type channels (Fig. 6F). However, T-type currents are transient (“T” = transient), whereas L-type currents are comparatively sustained (“L” = long-lasting) (Nowycky et al. 1985). Thus, during an ipRGC's response to prolonged light, such as the 20-s stimuli used by Hartwick et al. (2007), T-type Ca2+ channels may be activated only briefly after light onset, whereas L-type channels remain active for much longer. Another plausible explanation is that Hartwick et al. (2007) used dissociated ipRGCs that were largely devoid of dendrites. There is some evidence that, in RGCs, T-type ICa are primarily located in the dendrites (Henderson and Miller 2003, 2007). If this is the case for ipRGCs, then a substantial fraction of T-type Ca2+ channels would probably have been removed from the cells studied by Hartwick and colleagues (2007).
A number of potential functions have been postulated for voltage-gated Ca2+ channels in RGCs. First, dendritic T-type channels have been proposed to facilitate the propagation of excitatory synaptic currents toward the cell body (Henderson and Miller 2007; Miller et al. 2002). Dendritic T-type currents could serve a similar function in ipRGCs, because we found that putative T-type currents were most prominent in M1–M3 cells (Fig. 6F) whose long and thin dendrites probably have high impedance. Thus these dendrites might depend on T-type Ca2+ channels to propagate synaptic currents and melanopsin-based photoresponses toward the soma. By contrast, the M4 and M5 cells we tested appeared to exhibit either very small or no T-type currents. These cells' dendrites probably have lower impedance than those of M1–M3 because, even though the dendrites of M4 are long, they are very thick, and even though M5's dendrites are thin, they are shorter than those of M1–M3 cells. Therefore, M4 and M5 cells might be less dependent on dendritic T-type currents, and, indeed, a recent paper reported a lack of dendritic T-type currents in mouse ON alpha-cells (Margolis et al. 2010). Another proposed function of T-type channels is to increase the excitability of RGCs. However, because these channels are mostly inactivated at resting potentials, they are probably activated mainly after a prolonged hyperpolarization, triggering a burst of rebound spikes (Lee et al. 2003). Third, because ICa,non-inact does not inactivate during sustained depolarization, it is thought to promote repetitive spiking (Rothe et al. 1999). Thus ICa,non-inact may contribute to the ability of ipRGCs to signal light intensity continuously for many hours (Wong 2012). Finally, voltage-gated ICa can modulate spike frequency through the regulation of Ca2+-activated K+ channels (Fohlmeister et al. 1990; Fohlmeister and Miller 1997a). Although we did not probe specifically for Ca2+-activated IK (IK,Ca), they are likely present in at least some ganglion-cell photoreceptors (see below).
Na+ currents.
All ipRGCs generated NaV currents, and the NaV blocker TTX eliminated all cells' impulses. There is immunohistochemical evidence for the existence of TTX-resistant NaV channels in mouse retinal neurons, including RGCs with large somas, which have been proposed to include alpha-cells (O'Brien et al. 2008). Our observation that TTX blocked all action potentials in the alpha-like M4 cells indicates that these ipRGCs either lack such channels or express them at very low levels.
IK.
Depolarization-induced IK were detected in all ipRGCs. Most M4 and M5 cells and a few M1–M3 cells exhibited sustained IK, which probably arose from the non-inactivating delayed-rectifier channels (Lasater and Witkovsky 1990; Lipton and Tauck 1987). By contrast, the remaining ipRGCs displayed transient IK that decayed with time constants between ∼240 ms and ∼8.1 s, too slow for them to be IA whose inactivation time constant is only ∼10 ms (Lipton and Tauck 1987). Instead, they probably consisted of IB, IC, and/or IK,Ca, all of which inactivate slower than IA (Lasater and Witkovsky 1990; Lukasiewicz and Werblin 1988).
Through mathematical modeling, Fohlmeister and colleagues demonstrated that IA modulates spike waveform, IK,Ca regulates spike frequency, and IB, IC, and non-inactivating IK participate in both aspects of regulation (Fohlmeister et al. 1990; Fohlmeister and Miller 1997a). We found an inverse correlation between firing frequency and the effectiveness of IK in inducing membrane potential change (contrast Fig. 3, C and E, with Fig. 8C), raising the possibility that these currents contribute to spike rate regulation. The slow inactivation of the transient IK could also partly explain the slow induction of spike block in some ipRGCs (Fig. 3A, top traces): during a high-amplitude depolarizing current step, the slow inactivation of IK gradually shifts the membrane potential upward, causing significant inactivation of NaV ∼1–2 s after the onset of the current step (Lukasiewicz and Werblin 1988).
Summary
It has been well established that M1 cells project nearly exclusively to non-image-forming visual nuclei, including the SCN and the shell of the olivary pretectal nucleus (Hattar et al. 2006). By contrast, M2–M5 cells project primarily to other parts of the non-image-forming visual system, such as the olivary pretectal nucleus core and the ventral lateral geniculate nucleus (Baver et al. 2008; Ecker et al. 2010) and to the image-forming visual system (Ecker et al. 2010). Thus it has been hypothesized that M1 cells support different non-image-forming visual functions than M2–M5 cells, and that the latter cell types contribute to image-forming vision. Consistent with these notions, the melanopsin-based light responses of the five cell types have different intensity thresholds and amplitudes (Ecker et al. 2010; Estevez et al. 2012; Schmidt and Kofuji 2009, 2011). Moreover, M1 and M2 were previously reported to be differentially influenced by cone-driven synaptic inputs (Schmidt and Kofuji 2010), have different Rm, and spike at different frequencies (Schmidt and Kofuji 2009, 2011). In the present study, we included many physiological parameters that were not covered in Schmidt and Kofuji's earlier investigations. For most parameters, we found that M1 cells were significantly different from the other ipRGCs, reinforcing the possibility that M1 and the other cell types differentially regulate non-image-forming vision. Diversity was also observed among the four non-M1 types. However, except for one parameter, differences between M2 and M3 were insignificant (Table 2), suggesting that these two morphological types may serve similar functions. This observation is consistent with Berson and colleagues' (2010) proposal that M3 may not be a true cell type, but is “an anomalous transitional form” between M1 and M2, with greater similarity to M2. Finally, we learned that M4 and M5 were statistically indistinguishable for most of the parameters analyzed (Table 2). However, considering that M4 and M5 have vastly different dendritic field diameters (Fig. 1), they should at least have different receptive field sizes and thus perform different functions.
GRANTS
This work was funded by National Eye Institute (NEI) Grant R00 EY18863 to K. Y. Wong, a Research to Prevent Blindness Scientific Career Development Award to K. Y. Wong, National Science Foundation Graduate Student Research Fellowship DGE 1256260 to D. D. Hill, and NEI Grant P30 EY007003 to the Kellogg Eye Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.H. performed experiments; C.H., D.D.H., and K.Y.W. analyzed data; C.H. and K.Y.W. prepared figures; C.H., D.D.H., and K.Y.W. approved final version of manuscript; K.Y.W. conception and design of research; K.Y.W. interpreted results of experiments; K.Y.W. drafted manuscript; K.Y.W. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Samer Hattar for the melanopsin::cre mice, Austra Liepa for animal husbandry, and Christine Crilly for secretarial support.
REFERENCES
- Ames A, 3rd, Nesbett FB. In vitro retina as an experimental model of the central nervous system. J Neurochem 37: 867–877, 1981 [DOI] [PubMed] [Google Scholar]
- Baver SB, Pickard GE, Sollars PJ. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci 27: 1763–1770, 2008 [DOI] [PubMed] [Google Scholar]
- Berson DM. Retinal ganglion cell types and their central projections. In: The Senses: A Comprehensive Reference, Vol. 1, Vision I, edited by Basbaum AI, Kaneko A, Shepherd GM, Westheimer G. San Diego: Academic, 2008 [Google Scholar]
- Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp Neurol 518: 2405–2422, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295: 1070–1073, 2002 [DOI] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol 8: e1000558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 476: 92–95, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433: 749–754, 2005 [DOI] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 67: 49–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez ME, Fogerson PM, Ilardi MC, Borghuis BG, Chan E, Weng S, Auferkorte ON, Demb JB, Berson DM. Form and function of the m4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. J Neurosci 32: 13608–13620, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohlmeister JF, Coleman PA, Miller RF. Modeling the repetitive firing of retinal ganglion cells. Brain Res 510: 343–345, 1990 [DOI] [PubMed] [Google Scholar]
- Fohlmeister JF, Miller RF. Impulse encoding mechanisms of ganglion cells in the tiger salamander retina. J Neurophysiol 78: 1935–1947, 1997a [DOI] [PubMed] [Google Scholar]
- Fohlmeister JF, Miller RF. Mechanisms by which cell geometry controls repetitive impulse firing in retinal ganglion cells. J Neurophysiol 78: 1948–1964, 1997b [DOI] [PubMed] [Google Scholar]
- Hartwick AT, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ, Pickard GE. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci 27: 13468–13480, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol 497: 326–349, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295: 1065–1070, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D, Miller RF. Evidence for low-voltage-activated (LVA) calcium currents in the dendrites of tiger salamander retinal ganglion cells. Vis Neurosci 20: 141–152, 2003 [DOI] [PubMed] [Google Scholar]
- Henderson D, Miller RF. Low-voltage activated calcium currents in ganglion cells of the tiger salamander retina: experiment and simulation. Vis Neurosci 24: 37–51, 2007 [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates, 2001, p. 814 [Google Scholar]
- Ishida AT. Ion channel components of retinal ganglion cells. Prog Retin Eye Res 15: 261–280, 1995 [Google Scholar]
- Karschin A, Lipton SA. Calcium channels in solitary retinal ganglion cells from post-natal rat. J Physiol 418: 379–396, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM, Witkovsky P. Membrane currents of spiking cells isolated from turtle retina. J Comp Physiol A 167: 11–21, 1990 [DOI] [PubMed] [Google Scholar]
- Lee SC, Hayashida Y, Ishida AT. Availability of low-threshold Ca2+ current in retinal ganglion cells. J Neurophysiol 90: 3888–3901, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley S, Robbins J. The rat retinal ganglion cell in culture: an accessible CNS neurone. J Pharmacol Toxicol Methods 51: 209–220, 2005 [DOI] [PubMed] [Google Scholar]
- Lipton SA, Tauck DL. Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina. J Physiol 385: 361–391, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz P, Werblin F. A slowly inactivating potassium current truncates spike activity in ganglion cells of the tiger salamander retina. J Neurosci 8: 4470–4481, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Gartland AJ, Euler T, Detwiler PB. Dendritic calcium signaling in ON and OFF mouse retinal ganglion cells. J Neurosci 30: 7127–7138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. Cell populations of the retina: the Proctor lecture. Invest Ophthalmol Vis Sci 52: 4581–4591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RF, Stenback K, Henderson D, Sikora M. How voltage-gated ion channels alter the functional properties of ganglion and amacrine cell dendrites. Arch Ital Biol 140: 347–359, 2002 [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 316: 440–443, 1985 [DOI] [PubMed] [Google Scholar]
- O'Brien BJ, Caldwell JH, Ehring GR, Bumsted O'Brien KM, Luo S, Levinson SR. Tetrodotoxin-resistant voltage-gated sodium channels Na(v)1.8 and Na(v)1.9 are expressed in the retina. J Comp Neurol 508: 940–951, 2008 [DOI] [PubMed] [Google Scholar]
- O'Brien BJ, Isayama T, Richardson R, Berson DM. Intrinsic physiological properties of cat retinal ganglion cells. J Physiol 538: 787–802, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Brown RL. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur J Neurosci 24: 1117–1123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A 95: 340–345, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe T, Juttner R, Bahring R, Grantyn R. Ion conductances related to development of repetitive firing in mouse retinal ganglion neurons in situ. J Neurobiol 38: 191–206, 1999 [PubMed] [Google Scholar]
- Sand A, Schmidt TM, Kofuji P. Diverse types of ganglion cell photoreceptors in the mammalian retina. Prog Retin Eye Res 31: 287–302, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93: 717–729, 1998 [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J Neurosci 30: 16262–16271, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci 29: 476–482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J Comp Neurol 519: 1492–1504, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol 100: 371–384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87: 387–406, 1953 [PMC free article] [PubMed] [Google Scholar]
- Van Hook MJ, Berson DM. Hyperpolarization-activated current [I(h)] in ganglion-cell photoreceptors. PLos One 5: e15344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol 17: 981–988, 2007 [DOI] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci 17: 1727–1735, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY. A retinal ganglion cell that can signal irradiance continuously for 10 hours. J Neurosci 32: 11478–11485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Berson DM. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron 48: 1001–1010, 2005 [DOI] [PubMed] [Google Scholar]
- Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol 582: 279–296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RC, Cloherty SL, Ibbotson MR, O'Brien BJ. Intrinsic physiological properties of rat retinal ganglion cells with a comparative analysis. J Neurophysiol 108: 2008–2023, 2012 [DOI] [PubMed] [Google Scholar]
- Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P, Stolz S, Flockerzi V, Freichel M, Simon MI, Clapham DE, Yau KW. Melanopsin signalling in mammalian iris and retina. Nature 479: 67–73, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]