Abstract
Puberty in primates is timed by 2 hypothalamic events: during late infancy a decline in pulsatile GnRH release occurs, leading to a hypogonadotropic state that maintains quiescence of the prepubertal gonad; and in late juvenile development, pulsatile GnRH release is reactivated and puberty initiated, a phase of development that is dependent on kisspeptin signaling. In the present study, we determined whether the arrest of GnRH pulsatility in infancy was associated with a change in kisspeptin expression in the mediobasal hypothalamus (MBH). Kisspeptin was determined using immunohistochemistry in coronal hypothalamic sections from agonadal male rhesus monkeys during early infancy when GnRH release as reflected by circulating LH concentrations was robust and compared with that in juveniles in which GnRH pulsatility was arrested. The distribution of immunopositive kisspeptin neurons in the arcuate nucleus of the MBH of infants was similar to that previously reported for adults. Kisspeptin cell body number was greater in infants compared with juveniles, and at the middle to posterior level of the arcuate nucleus, this developmental difference was statistically significant. Neurokinin B in the MBH exhibited a similar distribution to that of kisspeptin and was colocalized with kisspeptin in approximately 60% of kisspeptin perikarya at both developmental stages. Intensity of GnRH fiber staining in the median eminence was robust at both stages. These findings indicate that the switch that shuts off pulsatile GnRH release during infancy and that guarantees the subsequent quiescence of the prepubertal gonad involves a reduction in a stimulatory kisspeptin tone to the GnRH neuronal network.
In higher primates, the timing of puberty is regulated by 2 major hypothalamic events that govern the postnatal pattern of pulsatile GnRH release in these species (1, 2). The first occurs during late infancy and results in a decline in GnRH release that leads, in turn, to the low levels of gonadotropin secretion that are characteristic of the remainder of prepubertal development in primates and that thereby guarantee the relative quiescence of the ovary and testis in children (human) and juveniles (1, 2). The second occurs at the termination of the juvenile phase of development and results in reactivation of a robust pattern of pulsatile GnRH release that drives the pubertal rise in LH and FSH secretion, which leads, in turn, to initiation of menstrual cyclicity and spermatogenesis (1, 2). The characteristic on-off-on pattern of GnRH pulsatility from birth to puberty in higher primates is independent of the gonads (1, 2).
The mechanisms underlying the hypothalamic switch that leads to the reactivation of robust GnRH pulsatility at the end of juvenile development and that therefore triggers the onset of primate puberty, have been relatively extensively investigated (1, 2). Compelling evidence has emerged over the last decade from both primate and nonprimate species to indicate that kisspeptin expressing neurons in the arcuate nucleus of the mediobasal hypothalamus (MBH) play an important role in the onset of puberty and in maintaining the activity of the neuroendocrine axis regulating gonadal function postpubertally (3). This breakthrough in our understanding of the importance of kisspeptin signaling in the hypothalamic control of the pituitary-gonadal axis had its origin in 2003 when 2 landmark publications appeared demonstrating that loss-of-function mutations of the kisspeptin receptor (KISS1R) in man were associated with hypogonadotropic hypogonadism and an absence or delay in puberty (4, 5). Since then, studies by many laboratories of several species have revealed the essential neurobiological bases for the clinical phenotype associated with loss-of-function mutations of KISS1R (3). In the monkey, KISS1, the gene that encodes for kisspeptin, is expressed in the arcuate nucleus of male and females and KISS1 mRNA content in the MBH increases in association with the pubertal increase in gonadotropin secretion (6). In the female, the release of kisspeptin in the stalk-median eminence region of the MBH is pulsatile and increases with the progression of pubertal development (7), and in prepubertal females local application of kisspeptin to the stalk-median eminence elicits premature GnRH release (7, 8), whereas administration of a KISS1R antagonist to the median eminence of pubertal females results in a decrease in hypothalamic GnRH release as assessed by microdialysis (9, 10). Moreover, in the agonadal juvenile male, in which spontaneous GnRH and LH release is very low, chronic intermittent release of endogenous GnRH induced by a pulsatile iv infusion of kisspeptin elicits a sustained train of LH discharges that is reminiscent of the spontaneous pattern of release of the gonadotropin in pubertal and adult animals (11)
In contrast to the switch that reactivates robust GnRH pulsatility at the end of the juvenile phase of primate development and triggers initiation of puberty, the nature of the switch that suppresses GnRH pulsatility in the infant primate and thereby guarantees the long delay to puberty in these species has received little attention. The purpose of the present study was therefore to begin to address this hiatus in our knowledge by examining the hypothesis that loss of robust GnRH pulsatility in the infant/juvenile transition in the male monkey is related to a reduction in expression of kisspeptin in the arcuate nucleus, ie, the inverse of what takes place during the initiation of puberty.
In both nonprimate and primate species, many kisspeptin neurons in the arcuate nucleus coexpress neurokinin B (NKB) and dynorphin and have therefore been termed kisspeptin/neurokinin B/dynorphin (KNDy) neurons (12, 13). In man, loss-of-function mutations in NKB or its receptor (NK3R) are associated, at the expected time of puberty, with a phenotype similar to that reported earlier for KISS1R (14–16). Moreover, NKB has been shown to stimulate GnRH release in the juvenile monkey, and the site of this action of NKB appears to be upstream to that of kisspeptin (17, 18). For these reasons, we used dual-label immunohistochemistry to compare the expression of these 2 hypothalamic neuropeptides in infant monkeys, in which pulsatile GnRH release was robust, with that in juvenile monkeys in which GnRH pulse generation had been arrested. Lastly, we took the opportunity to compare the distribution of GnRH immunopositive fibers in the region of the median eminence of infantile and juvenile animals.
Materials and Methods
Animals
Nine male rhesus monkeys, born at the Primate Core of the Specialized Cooperative Centers Program in Infertility and Reproduction Research at the University of Pittsburgh, were used. Seven of these monkeys were bilaterally orchidectomized at 3-9 days of age and remained with their mothers in individual cages until the termination of the experiments. The remaining 2 animals were castrated between 33 and 42 weeks of age. All monkeys were maintained under controlled photoperiod (lights on between 7:00 am and 7:00 pm) and temperature (approximately 21°C). A high-protein monkey diet (LabDiet; PMI Nutrition International, Richmond, Indiana) was fed at approximately 11:00 am, and this was supplemented with fruit in the afternoon. Water was available ad libitum. The study was approved by the University of Pittsburgh Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental protocol
Experimental design
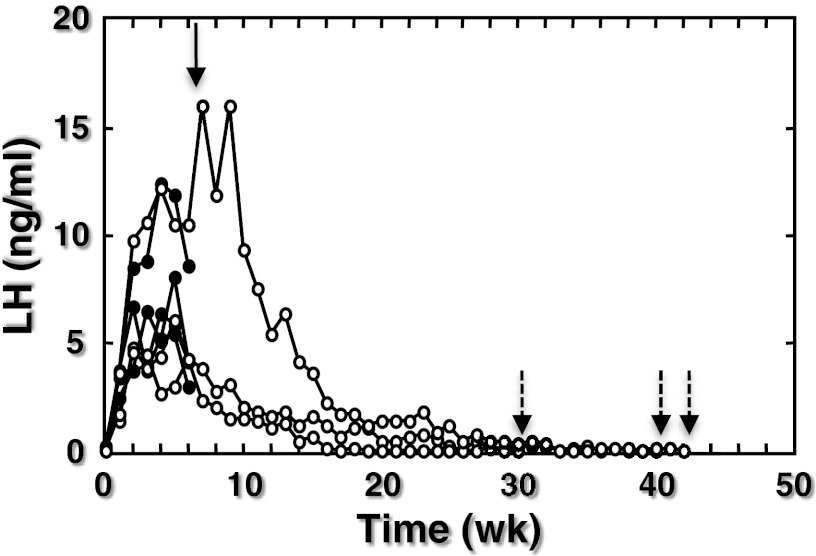
In the 7 monkeys castrated shortly after birth, pulsatile GnRH release was tracked indirectly by measuring plasma LH concentrations in morning blood samples collected at weekly intervals. Four of these monkeys were killed as infants at 7 weeks of age, a time of robust GnRH pulse generator activity as reflected by elevated LH levels (Figure 1). The remaining 3 animals were killed as juveniles at 32–43 weeks of age after arrest of pulsatile GnRH release was confirmed by the decline in circulating LH concentrations to undetectable values (Figure 1). Two procedures were used to kill the animals and to subsequently process the brain. In the first procedure, the brain was fixed by transcardial perfusion of 4% paraformaldehyde as previously described (19), while the animals were deeply anesthetized with sodium pentobarbital (Nembutal sodium solution, ∼30 mg/kg; Abbott Laboratories, North Chicago, Illinois). Brains were removed from the cranium and the hypothalamus isolated as a single block comprised of preoptic area and MBH. Tissue blocks were transferred to 30% sucrose in PBS and stored at 4°C for 48–72 hours prior to sectioning. In the second procedure, animals were killed with an overdose of sodium pentobarbital (∼40 mg/kg), and the brains were rapidly removed postmortem. The hypothalamus was then isolated from the rest of the brain as described (19) and the hypothalamus bisected. For the present study, 1 hemihypothalamus was immersion fixed in 4% paraformaldehyde at 4°C for 48–72 hours at which time tissue was transferred to 30% sucrose in PBS at 4°C for 72–108 hours before sectioning.
Figure 1.
Circulating LH concentrations in 7 male rhesus monkeys that were castrated during the first week of postnatal life. In 4 animals, the brain was collected at 7 weeks of age (infants, closed data points and solid arrow). The brain from the remaining 3 animals was taken after LH had declined to undetectable levels when the monkeys were between 32 and 43 weeks of age (juveniles, open data points and broken arrows).
Table 1 provides the identity of the infant (INF) and juvenile (JUV) monkeys that were paired to provide a total of 6 hypothalamic INF-JUV comparisons (pairs 1–6). The table also shows how the hypothalamic pairs were fixed and the specific experiment for which they were used. It may be seen that 1 infant monkey was paired with 2 juvenile monkeys (pairs 3 and 4) and 1 juvenile was paired with 2 infants (pairs 3 and 5). It is important to note, however, that pairs generated from the same monkey were used for independent experiments. It is also important to note that animals in any given pair were fixed in the same manner. The ages at perfusion of the juveniles that were castrated as juveniles (numbers 3285 and 3288) were 61 and 71 weeks of age, respectively. The duration of the juvenile phase of development in the rhesus monkey when the GnRH release is diminished extends from approximately 24 to 120 weeks of age (20).
Table 1.
Summary of Monkey Identities Used to Generate the 6 INF-JUV Pairs, the Methods of Fixation, and the Experiments for Which the Pairs Were Used
| Pair | Monkey INF-JUV | Fixation (4% PFA) | KP Cell in ARC (Immunofluor) | KP-NKB Colocalization (Immunofluor) | KP-GnRH Fibers in ME (Immunofluor) | KP Cell Size (Peroxidase) |
|---|---|---|---|---|---|---|
| 1 | 3342–3317 | Immersion | — | — | — | |
| 2 | 3336–3288a | Perfusion | — | — | — | |
| 3 | 3313–3341 | Perfusion | — | — | — | |
| 4 | 3313–3285a | Perfusion | — | |||
| 5 | 3336–3341 | Perfusion | — | |||
| 6 | 3295–3291 | Immersion | — |
Abbreviations: ARC, arcuate nucleus; KP, kisspeptin; ME, median eminence; PFA, paraformaldehyde.
, castrated when juvenile.
Pairs 1–3 were used to determine kisspeptin neuron number (immunoflourescence), kisspeptin/NKB colocalization (immunoflourescence) and kisspeptin cell body size (immunoperoxidase). Pairs 4–6 were used to qualitatively examine the GnRH fiber network in the median eminence. It is important to note that for any given immunohistochemical procedure the infant and juvenile comprising a particular pair were always processed together.
Antibodies
The GnRH antibody (LR1; 1:100K), raised in rabbit against [D-Lys (6)] GnRH and kindly provided by Robert Benoit (Montréal General Hospital, Montréal, Canada), kisspeptin antibody (GQ2; 1:120K), raised in sheep against synthetic human kisspeptin-54 and kindly provided by Stephen R. Bloom (Imperial Collage London, Hammersmith Hospital, London, United Kingdom) and NKB antibody (IS681; 1:6K), raised in rabbit against human prepro-NKB and kindly provided by Philippe Ciofi (Institut Francois Magendie and University of Bordeaux, Bordeau Cedex, France) have been validated previously for fluorescence immunohistochemistry on monkey hypothalamus (17, 19). For the detection of GnRH and NKB immunoactivity, Cy3-conjugated AffiniPure donkey antirabbit IgG (Jackson ImmunoResearch Laboratories, Inc, West Grove, Pennsylvania) was used as secondary antibody. For detection of kisspeptin immunofluorescence, Alexa Fluor 488 donkey antisheep IgG (Invitrogen Corp, Carlsbad, California) was used (17, 19). Dual-fluorescence immunohistochemistry was performed for localizing kisspeptin in combination with either NKB (pairs 1–3) or GnRH (pairs 4–6).
Kisspeptin immunopositive neurons were also localized using the 3, 3-diaminobenzidine (DAB) method. To this end, the primary antibody for kisspeptin (GQ2) was used at 1:120K and the secondary antibody (biotinylated rabbit antisheep antibody; Vector Laboratories, Inc, Burlingame, California) was used at 1:100. The staining was visualized by incubation in Vectastain ABC kit followed by ImmPACT DAB precipitation (both from Vector Laboratories). Pairs 1–3 were used for this purpose.
Immunohistochemistry
Hypothalamic or hemihypothalamic blocks were sectioned at 25 μm in the coronal plane using a freezing microtome (Leica SM 2000R; Leica Instruments GMBH, Germany), and corresponding sections were stored in cryoprotectant (21) in a series of 10 wells, each well containing sections collected at 250-μm intervals throughout the block. The sections were stored in cryoprotectant at −20°C until used for dual-label fluorescence immunohistochemistry as described previously (17, 19) or immunoperoxidase (see below).
For the detection of kisspeptin neurons by the DAB method, hypothalamic sections (750 μm apart) representing anterior to posterior MBH were mounted on Superfrost Plus slides (Fisherbrand; Fisher Scientific, Pittsburgh, Pennsylvania), allowed to dry at room temperature for 30 minutes and at 45°C for 2 hours, and stored at −80°C until use. At that time sections were thawed at room temperature for 30 minutes, washed twice for 15 minutes each in potassium PBS (KPBS; pH 7.3), incubated for 30 minutes in 3% hydrogen peroxide, and washed 6 times for 5 minutes each in KPBS. They were then incubated for 1 hour at 4°C on a shaker in the blocking serum buffer (5% normal horse serum; Vector Laboratories) followed by 24 hours incubation in the primary (kisspeptin) antibody at 4°C on a shaker. The next day, the slides were washed 4 times for 5 minutes each in KPBS followed by incubation in the second antibody for 1 hour at 4°C on a shaker and washed again 4 times for 5 minutes each in KPBS. They were then incubated in ABC Vector kit solution for 1 hour at 4°C on a shaker, washed 4 times for 5 minutes each in KPBS, and, finally, in the DAB solution for approximately 2 minutes for the development of chromagen precipitation. The slides were briefly washed in distilled water, air dried, and coverslipped using Permount (Fisher Scientific, Fair Lawn, New Jersey).
Confocal microscopy
Confocal imaging of dual-immunofluorescence staining of kisspeptin-NKB or kisspeptin-GnRH was conducted as previously described (17, 19).
Cell counting and size determination
For enumerating the number of kisspeptin immunopositive perikarya in the arcuate nucleus, a complete series of sections (ie, 1 section every 250 μm apart) of the MBH from pairs 1–3 (Table 1) was processed for fluorescence immunohistochemistry. The first retrochiasmatic section was identified for each animal, and 4 sections, typically 750 μm apart, were then selected for cell counting (hemisections counted), using a Leica fluorescence microscope (LEITZS DMRB; Leica, Wetzlar, Germany) fitted with a motorized stage control (MAC 5000; Ludl Electronic Products Ltd, Hawthorne, New York) in combination with Bioquant Nova Prime software (Bioquant Image Analysis Corp, Nashville, Tennessee).
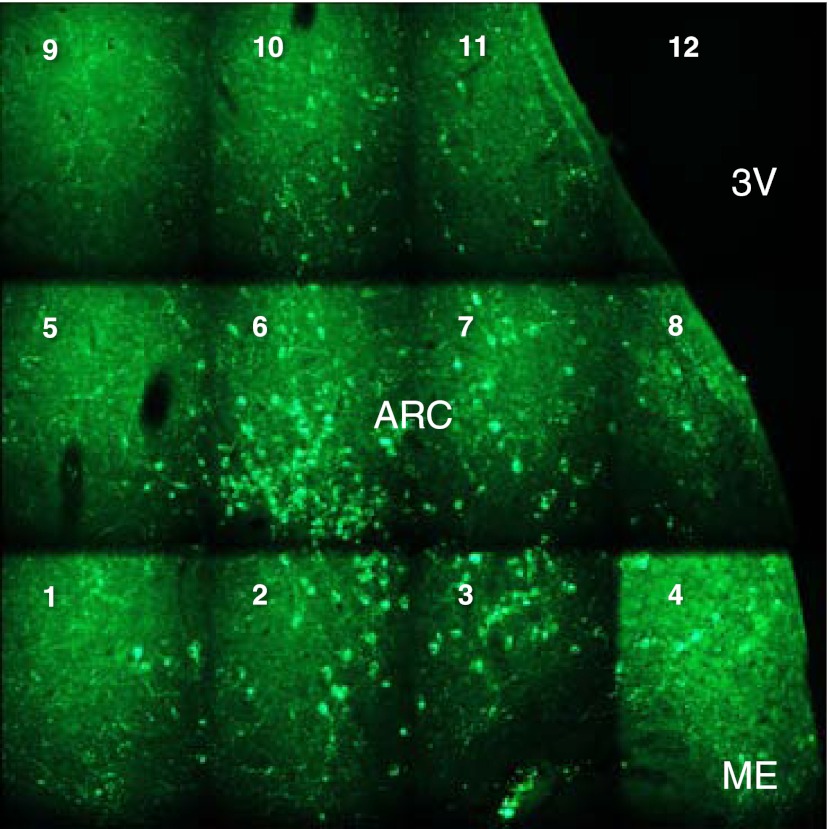
Using the sequential imaging option of the Bioquant software, the maximum number of frames required to overlay all kisspeptin neurons at the anteroposterior level of the arcuate nucleus in which the cross-sectional area occupied by kisspeptin-immunopositive cells was greatest was first determined at low magnification (×10) (Figure 2). Then, by controlling the motorized stage, the preset grid of frames was moved systematically over the area of immunopositive cells in any given hemisection as shown in Figure 2. The number of kisspeptin perikarya in each frame was counted under high power (×40) and recorded on a spreadsheet. Consistent differences in kisspeptin immunostaining of perfusion vs immersion-fixed sections were not noticed, and because the 2 animals in any given pair were fixed in the same manner, this issue was not pursued further.
Figure 2.
A montage of the frames of immunopositive kisspeptin neurons in the arcuate nucleus produced by the sequential imaging option of the Bioquant software that was used to count kisspeptin neurons in coronal hemisections of this nucleus. The motorized stage of the microscope was moved sequentially from frame 1 (bottom left) to frame 12 (top right), and the number of kisspeptin perikarya in each frame was counted under high power (×40) and recorded on a spread sheet. ARC, arcuate nucleus; ME, median eminence; 3V, third ventricle.
The percentage of kisspeptin cells immunopositive for NKB were determined in 2 hemisections from each animal comprising pairs 1–3 (Table 1) using a Leica fluorescent microscope. Typically these sections were taken in the midtuberal region and contained between 15 and 109 (mean 60) kisspeptin neurons. All neurons in each section were scored for colocalization. The effect of fixation on double labeling was not examined.
The size of 10 kisspeptin-positive cell bodies in the arcuate nucleus was determined from a hemisection taken at the middle to posterior level of the nucleus and stained by the DAB protocol by calculating perikaryal area using MetaMorph software (Molecular Devices, LLC, Sunnyvale, California).
Statistics
The significance of differences in the number of kisspeptin neurons between infant and juvenile groups was determined using the Student's t test and that of perikaryal area of kisspeptin positive neurons using the Mann-Whitney U test. Significance was accepted at P < .05.
Results
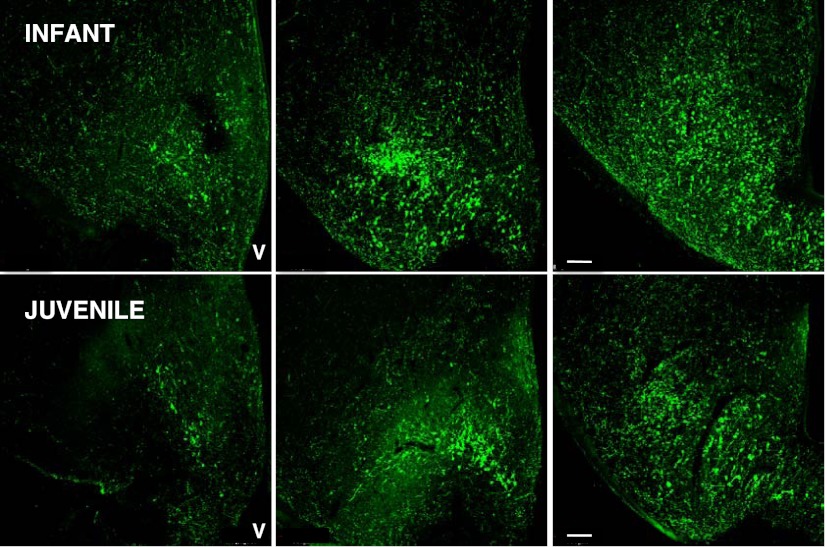
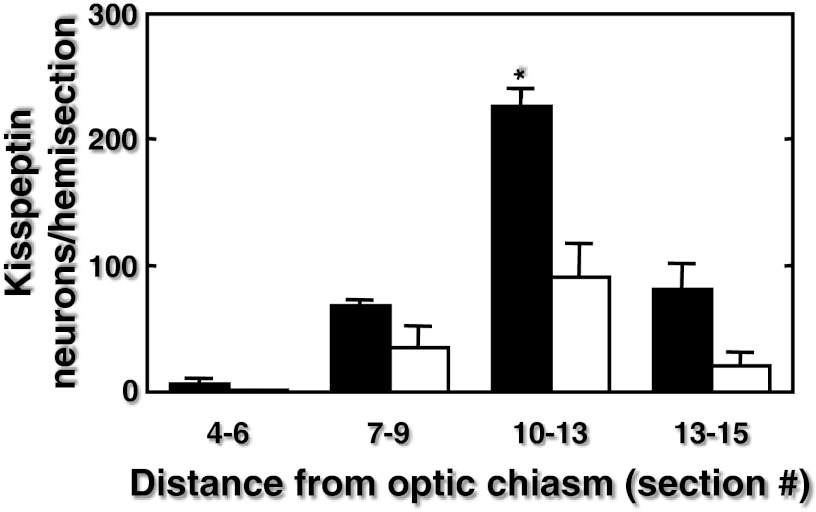
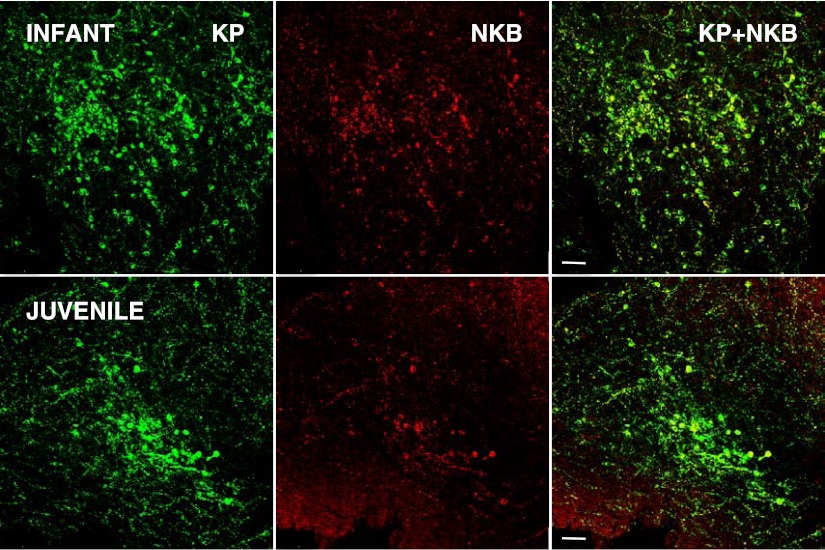
Immunopositive kisspeptin neurons were located in the arcuate nucleus of the MBH of both infant and juvenile monkeys (Figure 3). Typically, at both stages of development, kisspeptin perikarya in the anteroposterior plane were first observed in sections taken 750–1000 μm posterior to the optic chiasm. The number of kisspeptin neurons per hemisection progressively increased in the posterior direction with greatest numbers observed in the posterior tuberal/premammillary region of the arcuate nucleus (Figure 4). Immunopositive NKB was colocalized in 59% and 61% of the arcuate kisspeptin perikarya in the infant and juvenile MBH, respectively, and NKB-immunopositive-only neurons were seldom observed (Figure 5). At all levels of the arcuate nucleus, the number of kisspeptin cell bodies per section were greater in infants compared with juveniles, and in sections taken 2250–3250 μm posterior to the optic chiasm this developmental differences was statistically significant (Figure 4). The area of kisspeptin-immunopositive cell bodies in the arcuate nucleus of the infants was 102.5 ± 19.3 μm2 (mean ± SEM), and this compared with a value of 129.0 ± 19.5 μm2 for the juveniles (n = 3, P > .05)
Figure 3.
Confocal projections (×10, 1 μm optical sections) illustrating the distribution of kisspeptin neurons (visualized with green Alexa Fluor 488) in coronal hemisections of the MBH at 3 anteroposterior levels (left to right, respectively) from an infant (top panels) and juvenile (bottom panels) agonadal male rhesus monkey. Each projection is orientated with midline to the right. V, third ventricle. Scale bar, 100 μm.
Figure 4.
Distribution of kisspeptin neurons/hemicoronal section (mean ± SEM) throughout the arcuate nucleus of infant (closed bars) and juvenile (open bars) agonadal rhesus monkeys (pairs 1–3, n = 3/group). Section number indicates distance posterior from the optic chiasm with section 1 the first retrochiasmatic section. The distance between 2 sequentially numbered sections was 250 μm. *P < .05 infant vs juvenile.
Figure 5.
Confocal projections (×20, 1 μm optical sections) illustrating the relationship between kisspeptin (KP; green fluorescence, Alexa Fluor 488, left hand panels) and NKB (red fluorescence, Cy 3, center panels) in a coronal hemisection of the arcuate nucleus at a premamillary anteroposterior level of an infant (top panels) and juvenile (bottom panels) agonadal monkey. The right-hand panels show the merged projections and indicate colocalization of the 2 peptides in neurons of the arcuate nucleus. Each projection is orientated with midline to the right. V, third ventricle. Scale bar, 50 μm.
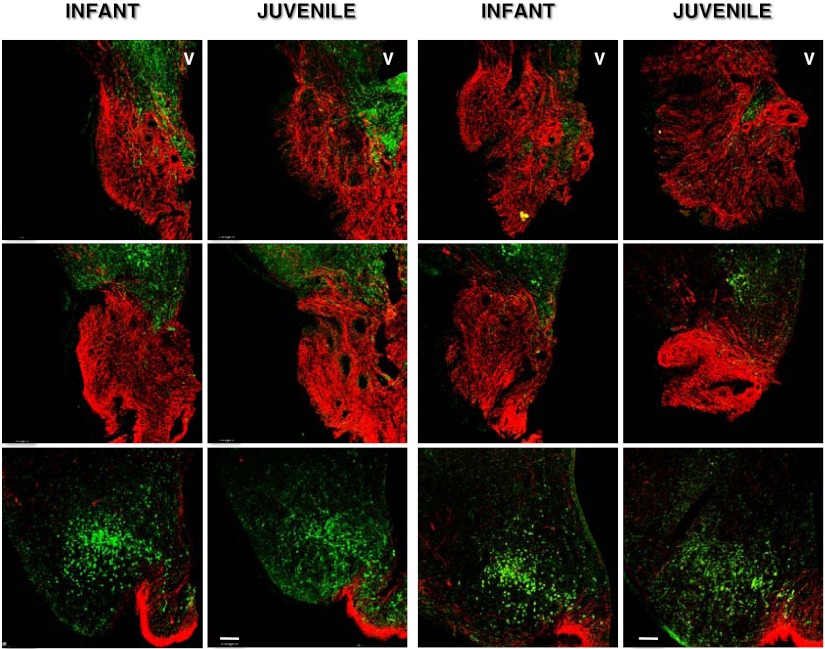
In contrast to the marked decrease in immunopositive kisspeptin cell body number during the infant-juvenile transition, the intensity of GnRH fiber staining in the median eminence as assessed qualitatively was robust at both stages of postnatal development (Figure 6).
Figure 6.
Confocal projections ×10, 1 μm optical sections) illustrating GnRH fiber projections (red fluorescence, Cy 3) to the anterior (top row of panels), midtuberal (middle row of panels) and posterior (bottom row of panels) level of the median eminence in coronal hemisections from 2 infant-juvenile pairs (left and right double panels, respectively) of agonadal male monkeys. The relationship to kisspeptin (green fluorescence, Alexa Fluor 488) may also be seen. At the posterior level of the median eminence, the arcuate nucleus with kisspeptin perikarya is also included in the projection. Each projection is orientated with midline to the right. V, third ventricle. Scale bar, 100 μm.
The mean number of sections encompassing the MBH (first retrochiasmatic section to the final premammillary section) was 11 in both infants and juveniles.
Discussion
The general distribution of immunopositive kisspeptin neurons in the MBH of both infant and juvenile agonadal male rhesus monkeys was similar to that previously reported for castrated adult males (17, 19). These neurons were absent from the anterior pole of the arcuate nucleus (22) but were found in progressively increasing numbers as more posterior sections were examined. The greatest numbers of kisspeptin neurons were in the posterior tuberal/premamillary region of the arcuate nucleus. As in the adult castrate male monkey, the number of immunopositive kisspeptin cell bodies was greater than those for NKB at all anteroposterior levels of the arcuate nucleus (17). This is in contrast to the situation in the normal human male in which the number of NKB-immunopositve perikarya in the arcuate nucleus was found to be 5-fold greater than that of kisspeptin (23). The reasons for this are unclear, but differences due to immunohistochemical procedures used and/or gonadal status cannot be excluded at the present time.
Typically and regardless of developmental stage of the monkeys, approximately 60% of kisspeptin neurons were also immunopositive for NKB: a proportion similar to that previously reported for the castrated adult male rhesus macaque (17). It should be noted that colocalization was not systematically assessed throughout the entire anterior-posterior extent of the arcuate nucleus, and therefore, the degree of coexpression seen for the 2 peptides may not be representative for all regions of this nucleus. Although most studies of other species indicate a similar or higher percentage of kisspeptin neurons that coexpress NKB (24–27), we consider there may be little comparative significance to these differences because the extent of double labeling will reflect, in part, the relative characteristics of the 2 primary antibodies used in any one study.
The number of kisspeptin neurons per coronal section along the anteroposterior plane of the arcuate nucleus was greater in the infant MBH than in the juvenile (Figure 4), and at the posterior tuberal/premamillary level of the arcuate nucleus, the mean number of kisspeptin cells per section in the infant was 2.5-fold greater than that in the juvenile. Although neurogenesis in the primate brain is completed prenatally (28), the possibility that the developmental reduction in the number of kisspeptin neurons per section in the coronal plane was due to postnatal growth resulting in an increase of the anteroposterior dimension of the arcuate nucleus over the 6- to 11-month period of maturation that separated the infant and juvenile stages is considered unlikely. This is because the anteroposterior length of the MBH (and presumably therefore of the arcuate nucleus), as indicated by the number of sequential coronal sections encompassing the entire MBH, was similar in both infant and juvenile monkeys. Thus, it is reasonable to propose that the decline in pulsatile GnRH release during the infant juvenile transition was associated with a decrease in the number of kisspeptin-immunopositive neurons of the arcuate nucleus during this developmental transition.
Although peptide content is directly related to peptide synthesis on the one hand and inversely related to terminal release of peptide on the other, the decrease in kisspeptin-immunopositive neurons in the juvenile is most likely explained by a decrease in kisspeptin expression during the infant-juvenile transition. The notion that elevated expression of kisspeptin in KNDy neurons of the infant primate is a critical component of the mechanism responsible for robust pulsatile GnRH release at this stage of development is consistent with the finding from human genetics that an infant boy with a loss-of-function mutation of KISS1R was reported to be hypogonadotropic (29). In this regard, it is likely that the stimulatory role of kisspeptin signaling in regulating GnRH release develops prenatally in primates as indicated by the recent finding in the human fetus that kisspeptin perikarya are present in the MBH as early as 15 weeks of gestation (30).
Studies of postnatal development of kisspeptin neurons of nonprimate species are limited, and changes in number of immunopositive kisspeptin cell bodies during neonatal life have not been reported (31–33). KiSS1 mRNA has been detected in the arcuate nucleus of male rats as early as postnatal day 1, and expression of the gene increases progressively throughout the prepubertal period (34, 35). In the newborn male mouse, however, kisspeptin signaling does not appear to be driving gonadotropin secretion at this stage of postnatal development (36).
Interestingly, the size of the cell body of kisspeptin neurons of the arcuate nucleus was unrelated to the arrest in GnRH pulse generation during the infant-juvenile transition. Thus, the gonad-independent switch that turns off GnRH pulse generation during infancy in primates appears to inhibit kisspeptin neuronal activity by a mechanism that is distinct from that used by gonadal steroids to restrain kisspeptin neuron activity post pubertally. In the latter situation, the size of kisspeptin perikarya in the arcuate nucleus in gonadally intact females is markedly smaller than that observed in the hypergonadotropic state induced by ovariectomy or associated with menopause in the female (37, 38).
The neurobiology that is involved in relaying the decreased kisspeptin tone in KNDy neurons to the GnRH neuronal network to affect reduced GnRH pulsatility during the infant-juvenile transition in primates is unknown, but a reasonable hypothesis is that it uses the same pathway that underlies reactivation in pulsatile GnRH release several years later at the onset of puberty, although at this later stage of development, the kisspeptin signal to the GnRH neuronal network is increasing rather than decreasing. In this regard, mammalian GnRH neurons are considered to express KISS1R (39), and studies by Terasawa and her colleagues (7, 8) in the female rhesus monkey have shown using microdialysis that kisspeptin release in the region of the arcuate nucleus/median eminence is pulsatile and increases with the onset of puberty. The median eminence is the site of GnRH release into the primary plexus of the hypophysial portal circulation, and in the monkey beaded kisspeptin axons come into intimate association with GnRH fibers projecting to the portal vessels (19). Moreover, local administration of a kisspeptin receptor antagonist to the arcuate nucleus-median eminence region of the pubertal monkey hypothalamus results in a suppression of pulsatile GnRH release (9, 10). Thus, it seems reasonable to propose that the decrease in kisspeptin in KNDy neurons during the infant juvenile transition is accompanied by a decrease in intermittent release of kisspeptin in the median eminence near GnRH fibers/terminals responsive to this peptide, and this reduction in kisspeptinergic tone leads, in turn, to the hypogonadotropic state of juvenile development.
Because the degree of colocalization of kisspeptin with NKB was similar in infant and juvenile animals, the number of NKB-immunopositive neurons must have declined in association with those of kisspeptin during this developmental transition. NKB staining was generally much weaker than that of kisspeptin, and the NKB signal (Cy3) declined relatively rapidly under the sustained fluorescence required for counting, and therefore, NKB cell number has not been reported.
The foregoing considerations are consistent with emerging models of GnRH pulse generation that posit that this mode of GnRH release originates within the arcuate nucleus as a result of reciprocal stimulatory and inhibitory interactions between KNDy cell bodies that are mediated by NKB and dynorphin signaling pathways, respectively, and that the output of the pulse generator to the GnRH neuronal network is mediated by kisspeptin (13, 18, 25, 27, 40). During the infantile-juvenile transition when GnRH pulsatility is being restrained, kisspeptin expression in KNDy neurons and kisspeptin release in the median eminence is reduced, but during the juvenile-pubertal transition when GnRH pulsatility is being reaugmented, kisspeptin activity of KNDy neurons is again up-regulated. According to this schemata, kisspeptin plays no regulatory role in timing the onset of puberty but rather is simply the output of the GnRH pulse generator, the activity of which during postnatal development in primates is governed by an as-yet-unidentified puberty control system that lies upstream of the GnRH pulse generator (3).
Although we have previously reported that overall GnRH content in the MBH of agonadal infant and juvenile monkeys are identical, as are corresponding levels of GnRH mRNA (41, 42), the strikingly intense staining of GnRH fibers in the median eminence at both these stages of development is nevertheless intriguing. This is because the secretory output of the network of GnRH fibers in the median eminence is dramatically different at these 2 stages of development: in the infant a robust pulsatile discharge of GnRH is produced that drives pituitary gonadotropin secretion, whereas in the juvenile the network is quiescent, and LH secretion, even in the absence of the testis, is arrested (1, 20). Similarly, during the juvenile-pubertal transition when robust GnRH pulsatility is reactivated, the peptide content of the MBH remains unchanged (41). Thus, the network of GnRH fibers in the median eminence may be viewed as providing a reservoir of peptide, the developmental release of which is dictated by the strength of the intermittent kisspeptin output of the GnRH pulse generator in the arcuate nucleus.
In summary, our finding that the number of immunopositive kisspeptin neurons in the arcuate nucleus of the male monkey decline during the transition from the infantile to the juvenile stage of development, indicates that the switch that shuts off pulsatile GnRH release during this critical period of primate development and that thereby guarantees the subsequent quiescence of the prepubertal gonad involves a reduction in a stimulatory kisspeptin output from this hypothalamic nucleus to the GnRH neuronal network.
Acknowledgments
We are most grateful for support from the staff of the Primate (Mr Michael Cicco and Ms Rachel Rosland) and Assay (Ms Carolyn Phalin) Cores of the Center for Research in Reproductive Physiology. Ms Roslund also assisted with the immunohistochemistry. We would also like to thank Drs Stephen Bloom, Philippe Ciofi, and Robert Benoit for gifts of the primary antibodies used.
This work was supported by National Institutes of Health Grants HD R01 13254 and HD U54 08610.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DAB
- 3, 3-diaminobenzidine
- INF
- infant
- JUV
- juvenile
- KISS1R
- kisspeptin receptor
- KNDy
- kisspeptin/neurokinin B/dynorphin
- KPBS
- potassium PBS
- MBH
- mediobasal hypothalamus
- NKB
- neurokinin B.
References
- 1. Plant TM, Witchel SF. Puberty in nonhuman primates and primates. In: Neill JD, ed. The Physiology of Reproduction. Vol 2, 3rd ed San Diego: Academic Press; 2006:2177–2230 [Google Scholar]
- 2. Terasawa E, Kurian JR. Neuroendocrine mechanism of puberty. In: Fink G, Pfaff DW, Levine JE, eds. Handbook of Neuroendocrinology. London: Academic Press; 2012:433–484 [Google Scholar]
- 3. Terasawa E, Guerriero KA, Plant TM. Kisspeptin and puberty in mammals. In: Kauffman AS, Smith JT, eds. Kisspeptin Signaling in Reproductive Biology. Springer Science. In Press 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 6. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guerriero KA, Keen KL, Terasawa E. Developmental increase in kisspeptin-54 in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta). Endocrinology. 2012;153:1887–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roseweir AK, Kauffman AS, Smith JT, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guerriero KA, Keen KL, Millar RP, Terasawa E. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology. 2012;153:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plant TM, Ramaswamy S, DiPietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013 [DOI] [PubMed] [Google Scholar]
- 12. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153:5105–5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TAC3R mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Young J, Bouligand J, Francoi B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95:2287–2295 [DOI] [PubMed] [Google Scholar]
- 16. Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal live followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramaswamy S, Seminara S, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology. 1985;116:1341–1350 [DOI] [PubMed] [Google Scholar]
- 21. Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159 [DOI] [PubMed] [Google Scholar]
- 22. Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic co-ordinates. San Diego: Academic Press; 2000 [Google Scholar]
- 23. Hrabovszky E, Sipos MT, Molnár CS, et al. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012;153:4978–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hrabovszky E, Ciofi P, Vida B, et al. The kisspeptin system of the human hypothalamus:sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–1998 [DOI] [PubMed] [Google Scholar]
- 25. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe also express dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 26. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterization of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neuroscience. 2010;30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rakic P. Neurogenesis in adult primates. Prog Brain Res. 2002;138:3–14 [DOI] [PubMed] [Google Scholar]
- 29. Semple RK, Achermann JC, Ellery J, et al. Novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–1855 [DOI] [PubMed] [Google Scholar]
- 30. Guimot F, Chevrier L, Dreux S, et al. Negative fetal FSH/LH regulation in late pregnancy is associated with declined kisspeptin/kiss1R expression in the tuberal hypothalamus. J Clin Endocrinol Metab. 2012;97(12):E2221–E2229 [DOI] [PubMed] [Google Scholar]
- 31. Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One. 2010;5:e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2006;147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710 [DOI] [PubMed] [Google Scholar]
- 34. Takumi K, Iijima N, Ozawa H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci. 2011;43:138–145 [DOI] [PubMed] [Google Scholar]
- 35. Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors a and b and kiss 1 in neonatal male and female rats. J Comp Neurol. 2011;519:2954–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology. 2012;153:782–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 38. Alcin E, Sahu A, Ramaswamy S, et al. Ovarian regulation of kisspeptin neurons in the arcuate nucleus of the rhesus monkey (Macaca mulatta) [published online January 17, 2013]. J Neuroendocrinol. doi: 10.1111/jne.12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Navarro VM, Gottsch ML, Wu M, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fraser MO, Pohl CR, Plant TM. The hypogonadotropic state of the prepubertal male rhesus monkey (Macaca mulatta) is not associated with a decrease in hypothalamic gondaotropin-releasing hormone content. Biol Reprod. 1989;40:972–980 [DOI] [PubMed] [Google Scholar]
- 42. El Majdoubi, Sahu A, Plant TM. Changes in hypothalamic gene expression associated with the arrest of pulsatile gonadotropin-releasing hormone release during infancy in the agonadal male rhesus monkey (Macaca mulatta). Endocrinology. 2000;141:3273–3277 [DOI] [PubMed] [Google Scholar]