Abstract
After reproductive senescence or gonadectomy, changes occur in neural gene expression, ultimately altering brain function. The endocrine mechanisms underlying these changes in gene expression beyond immediate hormone loss are poorly understood. To investigate this, we measured changes in gene expression the dorsal striatum, where 17β-estradiol modulates catecholamine signaling. In human caudate, quantitative PCR determined a significant elevation in β1-adrenergic receptor (β1AR) expression in menopausal females when compared with similarly aged males. No differences were detected in β2-adrenergic and D1- and D2-dopamine receptor expression. Consistent with humans, adult ovariectomized female rats exhibited a similar increase in β1AR expression when compared with gonadectomized males. No sex difference in β1AR expression was detected between intact adults, prepubertal juveniles, or adults gonadectomized before puberty, indicating the necessity of pubertal development and adult ovariectomy. Additionally, increased β1AR expression in adult ovariectomized females was not observed if animals were masculinized/defeminized with testosterone injections as neonates. To generate a model system for assessing functional impact, increased β1AR expression was induced in female-derived cultured striatal neurons via exposure to and then removal of hormone-containing serum. Increased β1AR action on cAMP formation, cAMP response element-binding protein phosphorylation and gene expression was observed. This up-regulation of β1AR action was eliminated with 17β-estradiol addition to the media, directly implicating this hormone as a regulator of β1AR expression. Beyond having implications for the known sex differences in striatal function and pathologies, these data collectively demonstrate that critical periods early in life and at puberty program adult gene responsiveness to hormone loss after gonadectomy and potentially reproductive senescence.
Reproductive senescence or gonadectomy fundamentally changes adult brain function (1, 2), creating differences between pre- and postmenopause and also between age-matched females and males. Indeed, complex changes in gene expression (3, 4) and catecholamine action (5–7) have been widely documented. The endocrine mechanisms causal to these changes beyond the immediate loss of estrogens and other hormones are not well understood, especially given the multiple hormone-sensitive periods throughout life that underlie sexual differentiation (2, 8–11). Here we investigate how early-life hormone-sensitive periods program adult responsiveness to hormone loss, employing catecholamine receptor expression in dorsal striatum as a model system.
The dorsal striatum, comprised of the caudate and putamen, receives projections from many brain regions and mediates multiple functions. These include but are not limited to sensorimotor control and aspects of cognition. This brain region is also particularly impacted by pathologies such as Parkinson's disease and drugs of abuse. All of these normal functions and pathologies are modulated by estrogens and show phenotypic sex differences (11–19). Organizational and activational hormone and sex-linked genomic effects have been heavily implicated as underlying mechanisms, modulating dopamine and norepinephrine release as well as dopaminergic and adrenergic receptor binding characteristics and/or expression (20–25).
The catecholamine receptors, including β1- and β2-adrenergic and D1- and D2-dopamine receptors, are all highly expressed in human and rodent caudate (26–30). Indeed, proper functioning of catecholamine systems is necessary for striatal function, and catecholamine systems are highly sensitive to steroid sex hormone action. Previous studies have shown increases in β1-adrenergic receptor density after estrogen loss in the cardiovascular system (31–36) and in the brain, including the dorsal striatum (37–43). We thus focused upon β1-adrenergic receptor expression in the dorsal striatum as a model system for studying how early-life endocrine events program how a relevant gene in the nervous system responds to adult steroid sex hormone loss. To that end, here we use quantitative PCR (qPCR) to measure expression of β1-adrenergic and other catecholamine receptors in postmenopausal females and age-matched males, male and female rats (an experimentally tractable model system), and male and female primary striatal cell cultures. Broadly, these results provide an example of how early neonatal and pubertal life endocrine events program how the adult nervous system will respond to the effects of menopause and gonadectomy.
Materials and Methods
Human caudate tissue
All protocols were approved by the Internal Review Board at the University of Minnesota. Postmortem samples from human caudate were obtained from the National Disease Research Interchange (http://ndriresource.org; Philadelphia, Pennsylvania). After dissection, tissue was rapidly frozen using liquid nitrogen and placed in RNAlater (QIAGEN, Valencia, California). Tissue was shipped frozen to the University of Minnesota and stored at −20°C until mRNA extraction. Seven samples were acquired (n = 3 males and 4 females). A priori subject exclusion criteria included known compromising conditions at the time of death: Parkinson's disease, Alzheimer's disease, psychostimulant use, intracranial hemorrhage, anoxia, or thyroidectomy. None of the females were known to receive hormone replacement therapy. The age of menopause onset is not known. There were no significant differences between males and females in all attributes monitored (P > .05), including time after death to tissue recovery (males, 6.9 ± 0.7 hours; females, 7.6 ± 0.4 hours), tissue recovery time (males, 436.7 ± 177.6 seconds; females, 933.8 ± 157.3 seconds), age (males, 67.7 ± 5.5 years; females, 70.0 ± 3.4 years), and RNA integrity number (males, 5.1 ± 0.6; females, 4.0 ± 0.7). RNA integrity numbers were calculated using the Agilent 2100 Bioanalyzer system and fall within the normal range of human postmortem tissue (44).
Animals
All protocols were approved by the Animal Care and Use Committee at the University of Minnesota, the University of Michigan, or Harlan Laboratories (Madison, Wisconsin). For experiments carried out in the Mermelstein laboratory, adult male and female intact or gonadectomized Sprague-Dawley rats were purchased from Harlan Laboratories. Animals were bilaterally gonadectomized on postnatal day 60 (P60) at Harlan Laboratories, transported to the University of Minnesota, and then housed in a room maintained at 20°C to 21°C, with a 12-hour light, 12-hour dark cycle and water available ad libitum. Absence of gonads was verified postmortem. Rats were anesthetized with isoflurane and killed on P70. This date was chosen based upon earlier work (38). The brain was rapidly removed and blocked 3 and 5 mm caudal from the base of the olfactory bulb. The dorsal striatum was microdissected from the anterior brain slice, and the hippocampus was removed from the caudal portion of the brain. All dissections were made in ice-cold modified Hank's balanced salt solution (HBSS) containing (in mM) 4.2 NaHCO3 and 1 HEPES (pH 7.35, 300 mOsm), as previously described (45, 46). Tissue was immediately placed into RNAlater. Tissue was stored at 4°C overnight and then at −20°C until mRNA extraction. Neonatal rats were born in the Mermelstein laboratory colony and group housed with their dam. These animals were killed at P8, and the brain was rapidly removed. Brains from P8 animals were processed the same as adult brains, except they were blocked 2 and 4 mm anterior from the intersection of the midsagittal and transverse sinuses.
For experiments involving neonatal/peripubertal hormone exposure or ovariectomy, male and female Sprague-Dawley rats were born in the Becker laboratory colony and housed 3 to 4 per cage. The animals were housed in a room maintained at 20°C to 21°C, with a 14-hour light, 10-hour dark cycle and free access to phytoestrogen-free rodent chow (2014 Teklad Global, 14% protein rodent maintenance diet, Harlan rat chow; Harlan Teklad, Madison, Wisconsin) and water available ad libitum. On P0 and P1, following a well-established protocol known to masculinize/defeminize rat brain (47), female pups received a sc injection of 100 μg testosterone propionate in 0.1 mL peanut oil or peanut oil alone. Male pups received peanut oil. Injection sites were sealed with 3M tissue adhesive. On P21, animals were gonadectomized or received a sham surgery using techniques described previously (48). On P22 to P37, some animals received a daily injection of 0.1 mL peanut oil or 50 μg/kg 17β-estradiol benzoate. On P60, animals were bilaterally gonadectomized or received a sham surgery. All animals were killed on P70. Caudate was microdissected and placed in RNAlater at 4°C overnight and then stored at −20°C until transport to the University of Minnesota. Upon arrival, tissue was stored at −20°C until mRNA extraction.
Cell culture
Striatal or hippocampal neurons were cultured from P2 to P3 Sprague-Dawley rat pups as previously described (46, 49). Male and female cultures were prepared in parallel from pups obtained from the same litter as previously described (50). Chemicals were purchased from Sigma (St Louis, Missouri) unless stated otherwise. After decapitation, the striatum or hippocampus was isolated in ice-cold modified HBSS containing (in mM) 4.2 NaHCO3 and 1 HEPES (pH 7.35, 300 mOsm). The tissue was then washed and digested for 5 minutes in a trypsin solution (type XI; 10 mg/mL) containing (in mM) 137 NaCl, 5 KCl, 7 Na2HPO4, 25 HEPES, and 1500 U deoxyribonuclease (pH 7.2, 300 mOsm). After additional washes with HBSS and HBSS containing 20% fetal bovine serum (FBS) (lots B1090 and L11059; Atlanta Biologicals, Lawrenceville, Georgia), tissue was dissociated and pelleted twice by centrifugation (180g for 10 minutes) to remove contaminants. Cells were then plated onto 10-mm coverslips (treated with Matrigel to promote adherence; BD Biosciences, San Jose, California) and incubated for 20 minutes at 24°C. In normal cultured neuron experiments, neurons were grown in 2 mL per coverslip of MEM (Invitrogen, Grand Island, New York) containing (in mM) 28 glucose, 2.4 NaHCO3, 0.0013 transferrin (Calbiochem, La Jolla, California), 2 glutamine, and 0.0042 insulin with 1% B-27 supplement (Invitrogen) and 10% FBS (pH 7.35, 300 mOsm). Culture media did not contain phenol red, and FBS was not charcoal stripped. To inhibit glial growth, 1 mL medium was replaced with a solution containing 4μM cytosine 1-β-d-arabinofuranoside and 5% FBS 48 hours after plating. At 96 hours later, 1 mL medium was replaced with a modified MEM solution containing 5% FBS. Gentamicin (2 μg/mL; Invitrogen) was added to all media solutions to eliminate bacterial growth. In serum-free cultured neuron experiments, FBS was not added to media.
Quantitative PCR
The qPCR was performed using standard protocols (46, 51). mRNA was extracted and reverse transcribed from cultured neurons or brain tissue using standard kits (RNAeasy Mini or Midi Kit and QuantiTect Reverse Transcription Kit from QIAGEN and Transcriptor First-Strand cDNA Synthesis Kit from Roche Diagnostics, Indianapolis, Indiana). Some qPCR amplification was performed using RT2 Real-Time SYBR Green PCR master mix PA-010 (SABiosciences, Frederick, Maryland) or QuantiFast SYBR Green PCR master mix (QIAGEN) on an Opticon 2 PCR machine (MJ Research, Schaumburg, Illinois). No differences in experimental results were found between SYBRs. The critical cycle threshold was set at 25 SDs above baseline and standardized to the ribosome-related gene rpl13a. The thermal cycling program used for the RT2 Real-Time SYBR was an initial denaturing step at 95°C for 11 minutes, followed by at least 30 cycles consisting of a 15-second denaturing step at 95°C, annealing step for 35 seconds at 55°C, an extension step for 30 seconds at 72°C, and a measurement of fluorescent intensity. The thermal cycling program used with QuantiFast SYBR was an initial denaturing step at 95°C for 6 minutes, followed by at least 30 cycles consisting of a 10-second denaturing step at 95°C, annealing/extension step for 30 seconds at 60°C, and a measurement of fluorescent intensity. Other qPCR amplifications were performed using LightCycler 480 SYBR Green I Master Mix (Roche) on a LightCycler 480 II PCR machine (Roche). Threshold values were calculated using the second derivative max method and standardized to the ribosome-related gene rpl13a (LightCycler 480 software version 1.5; Roche). The thermal cycling program used was a preincubation step at 95°C for 5 minutes, followed by at least 45 cycles consisting of a 10-second denaturing step at 95°C, annealing step for 10 seconds at 60°C, an extension step for 10 seconds at 72°C, and a measurement of fluorescent intensity. At the end of each cycling program, a melting curve was run. PCR products were sequenced for verification of product identity. PCR for individual cDNA samples was performed in triplicate, and overall experiments were repeated at least twice. No differences in experimental results were found between PCR machines or SYBR.
The upper and lower primer sequences used for humans were as follows: rpl13a (GenBank accession number NM_012423.2), 5′-GCCCTCAAGGTCGTGCGTCTG-3′ and 5′-CCAGCTTCCTATGTCCCAGGGCTGC-3′; adrb1 (GenBank accession number NM_000684.2), 5′-ACCCCAAGTGCTGCGATTTCGT-3′ and 5′-GCTCGCAGCTGTCGATCTTCTT-3′; adrb2 (GenBank accession number NM_000024.5), 5′-GTGATCGCAGTGGATCGCTA-3′ and 5′-AGTTGATGGCTTCCTGGTGG-3′; drd1a (GenBank accession number NM_000794.3), 5′-TGGGTGGGCGAATTCTTCCCTGAA-3′ and 5′-GCCCCATTGTTGTTAATGCTCACCG-3′; and drd2 (GenBank accession number NM_016574.3 and NM_000795.3, a shared sequence across both human drd2 isoforms), 5′-ATGTGTGTGATGAAGAAGGGCAGCC-3′ and 5′-ATGCCCAATGGCAAAACCCGGA-3′.
The upper and lower primer sequences used for rats were as follows: rpl13a (GenBank accession number NM_173340), 5′-TGCTGCCGCACAAGACCAAA-3′ and 5′-AACTTTCTGGTAGGCTTCAGCCGC-3′ (46); adrb1 (GenBank accession number NM_012701), 5′-ACCCCAAGTGCTGCGATTTCGT-3′ and 5′-GCTCGCAGCTGTCGATCTTCTT-3′ (46); adrb2 (GenBank accession number NM_012492.2), 5′-TTCTGTGCCTTCGCCGGTCTTCTT-3′ and 5′-ATGCCAGGGGCTTCCTCACAAA-3′; drd1a (GenBank accession number NM_012546.2), 5′-TGGGTGGGCGAATTCTTCCCTGAA-3′ and 5′-GCCCCATTGTTGTTAATGCTCACCG-3′; drd2 (GenBank accession number NM_012547.1), 5′-ATGCCCAATGGCAAAACCCGGA-3′ and 5′-ATGTGCGTGATGAAGAAGGGCAGCCA-3′; and ngf (GenBank accession number XM_227525.4), 5′-GCAGTGCCCCTGCTGAACCA-3′ and 5′-TCCGAAGAGGTGGGTGGAGGC-3′.
For select qPCR experiments, before mRNA extraction, cultured striatal neurons were incubated in Tyrode's solution containing tetrodotoxin (1μM) and AP-5 (25μM) at 24°C for 3 hours and then stimulated with isoproterenol (10μM) or vehicle for 2 hours.
cAMP assay
Using previously described protocols (46, 52), we measured cAMP concentrations in cultured striatal neurons (8–9 days in vitro) using a Parameter cAMP kit (R&D Systems, Minneapolis, Minnesota). Cells were incubated in a Tyrode's solution containing tetrodotoxin (1μM; Sigma) and AP-5 (25μM; Sigma) at 24°C for 3.0 hours. Cell stimulations were performed as follows: vehicle for 10 minutes and isoproterenol for 10 minutes (10μM; Sigma). Each experiment was performed at least 3 times to verify results.
Immunocytochemistry
Immunocytochemistry protocols followed those described previously (46, 53). Briefly, cultured striatal or hippocampal neurons at 8 to 9 days in vitro were incubated in a Tyrode's solution containing tetrodotoxin (1μM) and AP-5 (25μM) at 24°C for 3.0 hours. Some neurons were exposed to 17β-estradiol (1nM; Tocris, Ellisville, Missouri) in MEM or MEM alone (Invitrogen) for 24 hours before incubation in 17β-estradiol in Tyrode's solution or Tyrode's solution alone. Cell stimulations were performed as follows: vehicle for 10 minutes, isoproterenol for 10 minutes (10μM; Sigma), 6-chloro-PB for 10 minutes (500nM; Sigma), and 20mM K+ for 5 minutes. After fixation, permeabilization, and blocking, cells were incubated at 37°C for 1 hour in blocking solution containing a monoclonal antibody directed against serine 133 phosphorylated cAMP response element-binding protein (CREB) (1:1000, 05-667; Upstate Biotechnology, Lake Placid, New York), and to identify individual cell morphology, a polyclonal antibody targeting microtubule-associated protein 2 (1:1000, AB5622; Calbiochem). Cells were exposed to Alexa Fluor 488-conjugated antirabbit and 635-conjugated antimouse (Invitrogen, Carlsbad, California) secondary antibodies for visualization of microtubule-associated protein 2 and phosphorylated CREB, respectively. After mounting, nuclear fluorescent intensities for phosphorylated CREB were blindly acquired using a Leica DM5500Q confocal system and quantified using Leica LAS AF (version 1.9.0; Leica, Deerfield, Illinois). Intensities of randomly selected neurons (n ≥ 30 per group) were measured blind following established protocols described in detail elsewhere (46). Each experiment was performed at least 3 times to verify results.
Statistics
Experiments were analyzed using 2-tailed Student's t tests, or 1- or 2-way ANOVA and Tukey's multiple-comparison post hoc tests (Prism version 4.03 from GraphPad Software, La Jolla, California, and SigmaStat version 3.00 from Systat, Chicago, Illinois). Statistical differences between treatment groups are depicted within each figure as different alphabetical characters. Probability values < .05 were considered a priori as significant. Data are presented as mean ± SEM.
Results
Postmenopausal, aged women show increased β1-adrenergic receptor expression in the caudate compared with age-matched males
Given the important role of catecholamines in caudate function, and the known sex differences in these systems, we used qPCR to assess β1- and β2-adrenergic and D1- and and D2-dopamine receptor expression in human caudate from postmenopausal females and age-matched males. β1-Adrenergic receptor expression was elevated in female caudate compared with age-matched males (Figure 1A, P = .0076). No other sex differences were detected (Figure 1, B–D, P > .05). This sex difference could be generated via a direct genomic effect, generated by a direct result of loss of hormones after menopause or by other aging processes that differ between males and females. Acquiring tissue from young humans (both premenopausal and those with their ovaries removed) or adult females undergoing hormone replacement therapy without confounding conditions was not feasible for this study. Thus, we continue with studies in ovariectomized rats. Ovariectomized rats have been previously demonstrated to model various aspects of hormone loss associated with menopause (1), with known limitations including abrupt onset, and lack of perimenopause and associated hormonal cycling.
Figure 1.
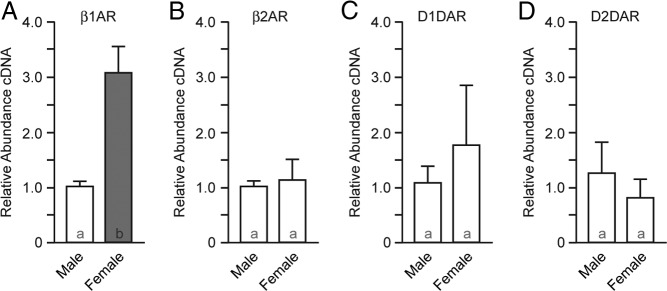
β1-Adrenergic receptor expression is increased in menopausal human female caudate compared with age-matched males. A, qPCR analysis of β1-adrenergic receptor (β1AR) expression. B, β2-Adrenergic receptor (β2AR). C, D1-dopamine receptor (D1DAR). D, D2-dopamine receptor (D2DAR). Bar color and letters indicate statistically significantly different groups.
Increased β1-adrenergic receptor expression in adult females relies on neonatal feminization and puberty followed by the loss of ovarian function during adulthood
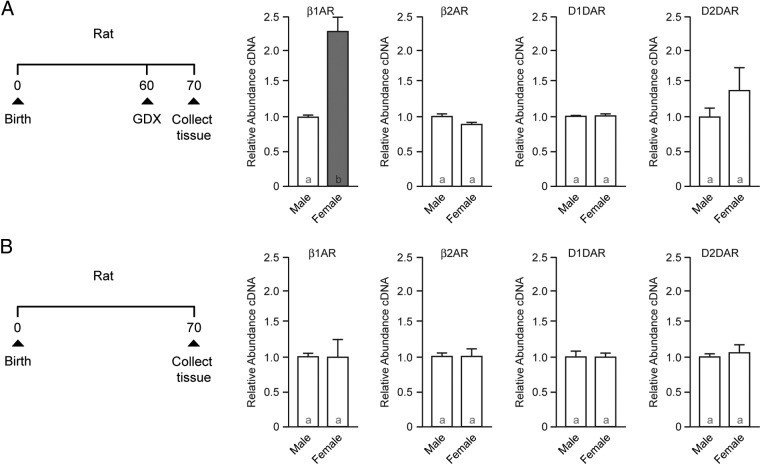
In the first set of experiments, male and female rats were gonadectomized on P60 (Figure 2A), whereas a second group was left intact (Figure 2B). The dorsal striatum (caudate/putamen) from all groups was collected on P70. Gene expression in the gonadectomized rats recapitulated that observed in human caudate, with β1-adrenergic receptor expression increased in females compared with males (Figure 2A; P = .036), with no other sex differences (P > .05). In contrast, intact animals exhibited no sex differences in any of the genes assayed (Figures 2B; P > .05). Hence, this sex difference in β1-adrenergic receptor expression is dependent on the loss of ovarian function.
Figure 2.
β1-Adrenergic receptor expression is increased in the dorsal striatum (caudate/putamen) of ovariectomized but not intact rat females compared with males. A, Experimental protocol (left): rats were gonadectomized (GDX) on P60; tissue was collected on P70; qPCR analysis of β1-adrenergic receptor (β1AR), β2-adrenergic receptor (β2AR), D1-dopamine receptor (D1DAR), and D2-dopamine receptor (D2DAR) expression in gonadectomized rat (right). B, Experimental protocol (left): tissue was collected from intact rats on P70; qPCR analysis of β1- and β2-adrenergic and D1- and D2-dopamine receptor expression in in intact rat (right). Bar color and letters indicate statistically significantly different groups.
To determine whether this sex difference is due to a simple lack of circulating ovarian hormones or whether developmental processes also play a role, we assessed receptor expression in P8 animals. At this early, prepubertal age, ovarian activity is quiescent. No sex differences in β1-adrenergic receptor expression were detected at P8 (Figure 3A; P > .05), indicating increased β1-adrenergic receptor is not simply due to a lack of ovarian hormones but that developmental processes must first occur. No sex differences were detected in D1- or D2-dopamine or β2-adrenergic receptor expression at P8 (P > .05 for all).
Figure 3.
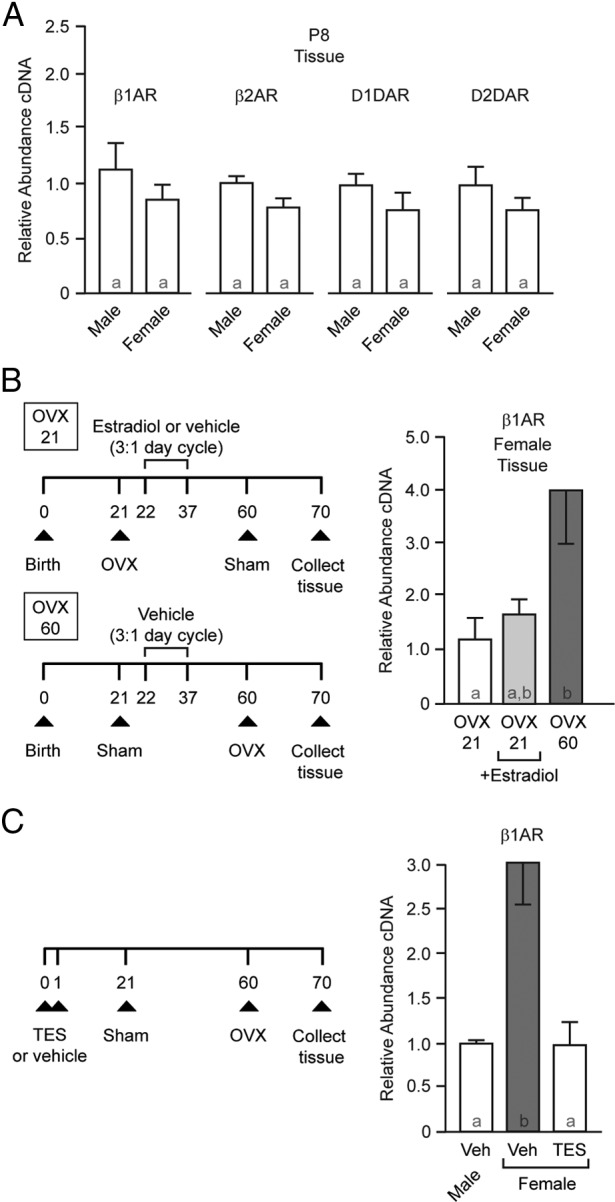
Natal and peripubertal hormone-sensitive periods program adult β1-adrenergic receptor gene expression sensitivity to ovariectomy. A, qPCR analysis of β1- and β2-adrenergic and D1- and D2-dopamine receptor expression in prepubertal rat (P8). No sex differences were detected. B, Prepubertal ovariectomy eliminates increased β1-adrenergic receptor expression in females, and cyclic peripubertal 17β-estradiol exposure is not sufficient to induce increased β1-adrenergic receptor expression. Experimental protocol is shown on the left. Females were ovariectomized or experienced sham surgery on P21 and P60. Between P22 and P37, animals were injected daily for 3 days with 17β-estradiol or vehicle, and then there was 1 day of no injection. This cycle was repeated 4 times, ending on P37. On the right is shown β1-adrenergic receptor expression in female rat caudate/putamen. Data are normalized to OVX 21 expression. C, Masculinization/defeminization induced by early hormone exposure blocks sex differences in β1-adrenergic receptor expression. Left: Experimental protocol. Right: Neonatal testosterone exposure blocks the ovariectomy-induced increase in β1-adrenergic receptor expression. Bar color and letters indicate statistically significantly different groups. Abbreviations: β1AR, β1-adrenergic receptor; β2AR, β2-adrenergic receptor; D1DAR, D1-dopamine receptor; D2DAR, D2-dopamine receptor; OVX, ovariectomized; TES, testosterone; Veh, vehicle.
We thus next established whether a peripubertal critical period is necessary for the observed increase in β1-adrenergic receptor expression and whether peripubertal 17β-estradiol exposure alone is sufficient for increased adult β1-adrenergic receptor expression. There were 3 treatment groups. Animals were either ovariectomized or received a sham surgery before puberty on P21. Between P22 and P37 (during the peripubertal period), animals were injected daily for 3 days with either vehicle or 50 μg/kg 17β-estradiol benzoate in peanut oil and then 1 day of no injection. This cycle was repeated 4 times, ending on P37. On P60 (adulthood/postpuberty), animals received either ovariectomy or sham surgery. Caudate from all 3 groups was collected on P70. Females ovariectomized after puberty (P60) exhibited increased β1-adrenergic receptor expression compared with those ovariectomized before puberty (P21) and not receiving peripubertal 17β-estradiol (Figure 3B; overall ANOVA, P = .0438; post hoc, P < .05). This indicates that the presence of ovaries during a peripubertal period (and potentially during adulthood) was necessary for the increase in receptor expression after loss of ovarian function in the adult. β1-Adrenergic receptor expression in females ovariectomized before puberty (P21) exposed to peripubertal 17β-estradiol did not significantly differ from either of the other 2 groups (P > .05 for both), with absolute expression level being more comparable to that of females ovariectomized on P21. These data argue that peripubertal 17β-estradiol exposure alone may not be sufficient for inducing increased β1-adrenergic receptor expression. As a control, no difference was detected in D2-dopamine receptor expression among the 3 groups (P > .05).
Around the time of birth, the testes of male rats produce testosterone. This process masculinizes and defeminizes neural tissue and would have occurred in all experiments described thus far. Female testosterone levels at this age are low or nondetectable, although we note that they are exposed to some steroid sex hormones in utero. To test whether the lack of neonatal testosterone is essential for adult sex differences in β1-adrenergic receptor expression, we injected female neonate rat pups with either vehicle or testosterone on P0 and P1; males were injected with vehicle. All animals received a sham surgery on P21 and were gonadectomized as adults on P60. β1-Adrenergic receptor expression was measured on P70 (Figure 3C). As expected, there was a sex difference between vehicle-treated animals. In contrast, testosterone injections masculinized/defeminized β1-adrenergic receptor expression in female animals (Figure 3C; P = .0064). No significant differences were found between groups in D2-dopamine receptor expression (P > .05). This finding supports the hypothesis that early masculinizing/defeminizing testosterone exposure eliminates estrogenic regulation of β1-adrenergic receptor expression in the adult. This could occur by acting to directly masculinize/defeminize neural tissue or by possibly shutting down pubertal/adult ovary function (54, 55).
Increased β1-adrenergic receptor expression leads to functional sex differences in cellular physiology
Primary neuronal cultures were used to establish a model system in which to assess the functional significance of increased β1-adrenergic receptor expression. Striatal cultures were generated from pups aged 2 to 3 days, after the initial hormone surge occurs in males but not females (Figure 4A). These sex-specific cultures were placed in either serum-free (hormone-free) media or media containing serum. Nine days later, all cultures were placed in serum-free media for at least 3 hours to induce hormone loss before cell lysis and qPCR. Striatal neurons raised in serum-free media did not show a difference in β1-adrenergic receptor expression after exposure to additional serum-free media (Figure 4B; P > .05). In contrast, striatal neurons raised in hormone-containing media and then exposed to serum-free media showed increased β1-adrenergic receptor expression in female-derived cultures (Figure 4B; P = .0376). As an additional control, there were no observed sex differences in D1- or D2-dopamine receptor expression (Table 1; P > .05).
Figure 4.
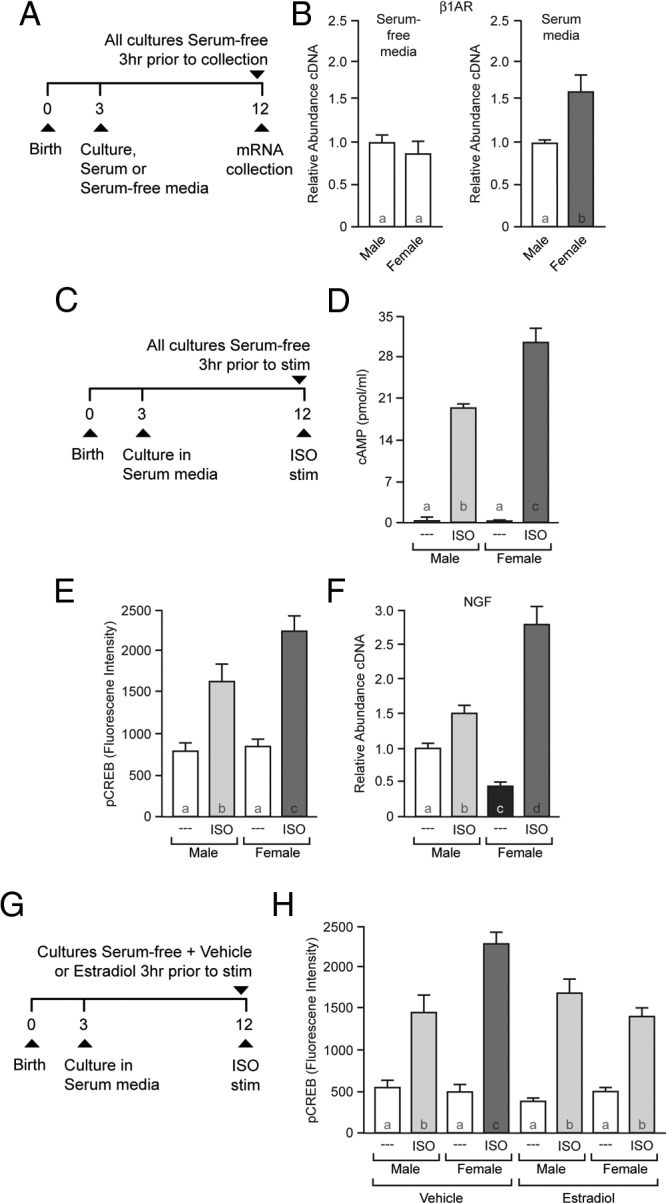
Increases in β1-adrenergic receptor expression impacts cellular physiology and is blocked by 17β-estradiol. A, Experimental protocol: increased β1-adrenergic receptor expression can be induced in vitro using primary cultured female striatal neurons. B, Neurons continuously grown in serum-free media do not exhibit sex differences in β1-adrenergic receptor (β1AR) expression (left); β1-adrenergic receptor expression shows sex differences in striatal-neuron cultures first exposed to hormone-containing serum and then placed in serum-free Tyrode's solution for at least 3 hours (right). C, Sex differences in β1-adrenergic receptor expression differentially impact male and female cellular physiology. D, The β-adrenergic receptor agonist ISO induces sex differences in cAMP formation. The effects of ISO in this paradigm have been previously shown to be β1-adrenergic receptor-specific (see Results). E, ISO-induced CREB phosphorylation shows sex differences. F, ISO exposure induces sex differences in NGF gene expression. G, Experimental protocol to test whether 17β-estradiol exposure is sufficient to block sex differences in β1-adrenergic receptor action after feminization. H, 17β-Estradiol exposure blocks increased β1-adrenergic receptor action in female cultures. Striatal neurons exposed to vehicle show sex differences in ISO-induced phosphorylation of CREB, whereas neurons exposed to 17β-estradiol do not. Bar color and letters indicate statistically significantly different groups.
Table 1.
Catecholamine Receptor Expression and Function in Cultured Neuronsa
| Cultured Neuron Origin | Catecholamine Receptor | Receptor Expression | CREB Phosphorylation |
|---|---|---|---|
| Dorsal striatum (caudate/putamen) | D1DAR | M: 1.00 ± 0.02b | M, Veh: 593 ± 115b |
| F: 1.23 ± 0.15b | M, 6-C: 1704 ± 136c | ||
| F, Veh: 568 ± 83b | |||
| F, 6-C: 1454 ± 135c | |||
| Dorsal striatum (caudate/putamen) | D2DAR | M: 1.00 ± 0.08b | Not measured |
| F: 0.98 ± 0.13b | |||
| Hippocampus | β1AR | M: 1.00 ± 0.06b | M, Veh: 1192 ± 97b |
| F: 1.01 ± 0.04b | M, ISO: 2337 ± 173c | ||
| F, Veh: 1210 ± 48b | |||
| F, ISO: 2206 ± 178c | |||
| Hippocampus | β2AR | M: 1.04 ± 0.03b | Not measured |
| F: 1.20 ± 0.15b | |||
| Hippocampus | D2DAR | M: 1.00 ± 0.08b | Not measured |
| F: 0.97 ± 0.13b |
Abbreviations: β1AR, β1-adrenergic receptor; β2AR, β2-adrenergic receptor; 6-C, 6-chloro PB; D1DAR, D1-dopamine receptor; D2DAR, D2-dopamine receptor; F, female; M, male; Veh, vehicle.
Values are mean ± SEM. Catecholamine receptor expression values are relative gene expression normalized to males and are unitless. CREB phosphorylation values are fluorescence intensity. 6-Chloro PB is a D1-dopamine receptor agonist; ISO is a β-adrenergic receptor agonist.
Differences in significance are indicated by superscript letters; no sex differences were detected.
Sex differences in β1-adrenergic receptor expression in the striatal cultures afforded the ability to assess functional cellular relevance. Hence, we assessed the effects of β1-adrenergic receptor stimulation on downstream cAMP formation, CREB phosphorylation, and target gene expression (ie, nerve growth factor [NGF]). For experiments measuring cAMP and CREB phosphorylation, serum-containing cultures were treated as described above followed by 10 minutes stimulation with isoproterenol (ISO) (Figure 4C). Under these experimental conditions, ISO predominantly stimulates the β1-adrenergic receptor (46). ISO-exposed female striatal neurons exhibited increased cAMP formation compared with ISO-exposed male cultures (Figure 4D; effect of sex, P = .020; effect of drug, P < .001; interaction of sex and drug: P = .013; post hoc, female ISO compared with male ISO, P = .002). Similarly, ISO produced sex differences in CREB phosphorylation, with females showing increased responsiveness compared with males (Figure 4E; effect of sex, P = .036; effect of drug, P < .001; interaction of sex and drug, P = .040; post hoc, female ISO compared with male ISO, P = .004).
As a control, we stimulated D1-dopamine receptors with 6-chloro PB. CREB phosphorylation induced by the D1-dopamine receptor agonist 6-chloro PB did not differ by sex (Table 1; effect of sex, P > .05; effect of drug, P < .001; interaction of sex and drug, P > .05). Because the D1-dopamine and the β1-adrenergic receptor are coexpressed in medium spiny neurons (56) and activate similar signal transduction pathways, this controlled for a potential sex differences in downstream signal transduction molecules. We also tested the regional specificity of the sex difference in β1-adrenergic receptor expression by examining β1-adrenergic receptor expression in cultured hippocampal neurons. We found no sex differences in β1-adrenergic receptor expression in cultured hippocampal neurons (Table 1; P > .05). Similarly, ISO-induced CREB phosphorylation in hippocampal cultures did not differ by sex (Table 1; effect of sex, P > .05; effect of drug, P < .001; interaction of sex and drug, P > .05). These in vitro data are consistent with in vivo catecholamine receptor expression, because no sex differences in β1- or β2-adrenergic or D1- or D2-dopamine receptor expression were detected in adult hippocampus from gonadectomized or intact rats (Table 2; P > .05 for all comparisons).
Table 2.
Catecholamine Receptor Expression in Adult Rat Hippocampus a
| Catecholamine Receptor | Hippocampus |
|
|---|---|---|
| Gonadectomized | Intact | |
| β1-Adrenergic receptor | ||
| Male | 1.04 ± 0.15 | 1.00 ± 0.04 |
| Female | 1.28 ± 0.09 | 1.06 ± 0.10 |
| β2-Adrenergic receptor | ||
| Male | 1.04 ± 0.20 | 1.02 ± 0.08 |
| Female | 1.21 ± 0.14 | 0.85 ± 0.11 |
| D1-dopamine receptor | ||
| Male | 1.00 ± 0.04 | 1.01 ± 0.06 |
| Female | 0.87 ± 0.05 | 1.08 ± 0.14 |
| D2-dopamine receptor | ||
| Male | 1.10 ± 0.30 | 1.03 ± 0.11 |
| Female | 0.88 ± 0.47 | 1.29 ± 0.31 |
Gene expression was measured in animals aged P70. Gonadectomy was performed on P60. Values are mean ± SEM. Values are relative gene expression normalized to males and are unitless. No significant differences were detected between males and female (P > .05 for all comparisons).
These sex differences in cAMP formation and CREB phosphorylation could also lead to sex differences in a target gene regulated by this pathway. For this experiment, we examined changes in NGF expression, which is regulated by CREB and produced by the striatum (57) to locally affect striatal neurons (58). A 2-hour exposure to ISO produced increased expression of NGF in female striatal neurons compared with male (Figure 4F; effect of sex, P = .043; effect of drug, P < .001; interaction of sex and drug, P < .001; post hoc, female ISO compared with male ISO, P < .001). Of note, we additionally found that baseline NGF levels differed between males and females (P = .029), with female expression lower than male. Collectively, these data indicate that sex differences in β1-adrenergic receptor expression have functional impacts upon cellular physiology.
17β-Estradiol down-regulates β1-adrenergic receptor action
Across multiple organs and in other brain regions, β1-adrenergic receptor expression in the adult animal is sensitive to 17β-estradiol. 17β-Estradiol, along with many other hormones and relevant molecules, is present in the serum used to induce increased β1-adrenergic receptor expression in the cultured striatal neurons. To test whether 17β-estradiol treatment alone is sufficient to block the up-regulation in β1-adrenergic receptor action after serum deprivation, male- and female-derived cultures were treated as described above, except that the serum-free media contained either vehicle or 1nM 17β-estradiol. Similar to Figure 4E, vehicle in the serum-free media did not block the sex difference in ISO-induced CREB phosphorylation between male and female cultures (Figure 4H). In contrast, adding 17β-estradiol to the serum-free media eliminated this sex difference (vehicle group: effect of sex, P = .010; effect of drug, P < .001; interaction of sex and drug, P = .004; post hoc, female ISO compared with male ISO, P < .001; 17β-estradiol group: effect of sex, P > .05; effect of ISO, P < .001; interaction of sex and drug, P > .05; post hoc, female ISO compared with male ISO, P > .05) This result is consistent with the model that 17β-estradiol is sufficient to prevent up-regulation of the β1-adrenergic receptor.
Discussion
Here we present data generated from humans to cultured neurons that collectively argue that critical hormone-sensitive organizational periods early in life directly sensitize adult gene responsiveness to loss of gonadal hormones after ovariectomy, and possibly menopause (Figure 5). In experiments with human postmortem caudate tissue, we found that β1-adrenergic receptor expression was increased in menopausal human females compared with age-matched males. Then, using ovariectomized rats as a standard model for loss of hormone effects after menopause, we found that β1-adrenergic receptor expression was increased in gonadectomized but not intact female rats compared with males. To elucidate the underlying endocrine mechanisms, we determined that this sex difference was eliminated by prepubertal ovariectomy or early masculinizing/defeminizing hormone exposure, consistent with data from other model systems indicating a peripubertal steroid sex hormone-sensitive period beyond the pre/neonatal critical period (8, 9). Sex differences in β1-adrenergic receptor expression were then induced in cultured striatal neurons, determining that differences in β1-adrenergic receptor expression directly impact cellular physiology. Broadly, these findings provide a specific example of how early life hormone-sensitive periods program how neural gene expression responds to hormone loss associated with menopausal/ovariectomy.
Figure 5.

Schematic illustrating possible roles of early sexual differentiation and peripubertal hormone-sensitive periods in programming changes in β1-adrenergic receptor expression after adult hormone loss. Abbreviations: β1AR, β1-adrenergic receptor; OVX, ovariectomized.
Increased striatal β1-adrenergic receptor expression induced by loss of ovary function joins a large literature indicating estrogenic influence on β-adrenergic receptor expression and function in and outside the nervous system. The first hint of this relationship dates to at least the 1960s, when it was discovered that the effects of norepinephrine on vascular reactivity was more potent in pregnant than in nonpregnant human females (59). In 1976, it was reported that estrogens reduced β-adrenergic receptor modulation of rat vascular reactivity (31). These initial reports in the vascular system foreshadowed later research in many systems, including but not limited to cardiac muscle (35), myometrium (60), and brown adipose cells (61), all of which report 17β-estradiol down-regulating β-adrenergic receptor action or density. Likewise, ovariectomy up-regulated β1-adrenergic receptors in rat heart (32). In rat brain, 17β-estradiol has direct actions on norepinephrine-producing neurons and receptors (39), and rat locus coeruleus cell number and volume increase in females during puberty (62). Measured using autoradiographic receptor binding, 17β-estradiol exposure decreases β-adrenergic receptor responses and/or density in rat cortex, olfactory bulb, striatum (42), and hypothalamus (39). As predicted, ovariectomy increased β-adrenergic receptor density in cortex and hypothalamus (38). Exposure to 17β-estradiol can also uncouple the associated signaling transduction pathway, at least in the hypothalamus (39). We conclude from these studies that loss of 17β-estradiol generally up-regulates β-adrenergic receptor expression and action.
This conclusion was supported by our studies using primary cultured striatal neurons, where 17β-estradiol exposure eliminated sex differences in β1-adrenergic receptor action. Increased β1-adrenergic receptor expression was induced by first harvesting striatal neurons from female animals after the first pre/neonatal masculinization/defeminization period, by providing hormone-containing serum during the neuron growth phase and then by removing serum. Providing 17β-estradiol during serum removal prevented increased β1-adrenergic receptor action. This protocol does not necessarily mimic in vivo processes per se but instead establishes that 17β-estradiol and potentially other hormones act relatively rapidly and directly on striatal neurons to program gene expression. This is consistent with the rapid signaling of membrane-associated α- and β-estrogen receptors expressed by both medium spiny neurons and cholinergic interneurons (20, 63–66) and the sex differences exhibited in some cellular properties (67–70).
The use of primary cultured striatal neurons enabled clear tests of whether sex differences in β1-adrenergic receptor expression led to functional consequences for cellular physiology. We assessed this by turning to the downstream action of the β1-adrenergic receptor on cellular physiology, given that we were unable to generate quality Western blots of the β1-adrenergic receptor to directly assess protein levels. To address this limitation, we reasoned that if a sex difference in functional, membrane-associated β1-adrenergic receptor protein exists, then receptor stimulation should likewise induce sex differences in downstream target molecules. Thus, we employed the cultured striatal neuron model and measured cAMP formation, CREB phosphorylation, and target gene expression (ie, NGF). Given the sex differences in these measurable endpoints after β1-adrenergic receptor stimulation, we conclude that functional sex differences exist in membrane β1-adrenergic receptor expression, consistent with classical studies measuring β1-adrenergic receptor density (42, 71).
Adrenergic receptor action in the caudate was understudied for many years, especially beginning in the late 1970s after dopamine (instead of norepinephrine) was established as the primary reward neuromodulator. This model, however, is being augmented by both older and more recent experiments that show abundant β1-adrenergic receptor expression in human caudate (29) and rat striatum on both D1- and D2-dopamine receptor-expressing medium spiny neurons (46, 56) and cholinergic interneurons (72). Likewise, norepinephrine is present in the striatum (although at lower levels than dopamine) (73), reflecting sparse noradrenergic projections from the locus coeruleus and brainstem nuclei (74). This projection may be sexually dimorphic, because norepinephrine levels are greater in females than in males and vary across the ovarian cycle (23) and rat locus coeruleus cell number and volume are increased in females compared with males (62). Like dopamine, extracellular concentrations of norepinephrine are increased by psychostimulant exposure (75) and reduced in Parkinson's disease (76), with the β-adrenergic receptor antagonist propranolol modulating locomotor responses in both contexts (77, 78). We note that there is also evidence that dopamine can activate β-adrenergic and especially α-adrenergic receptors (72, 79–81).
The clear effect of estrogens on β1-adrenergic receptor function is a direct contrast to estrogenic effects on dopamine neurotransmission and receptor expression. Although it is clear that 17β-estradiol increases dopamine release in the caudate, the effects of 17β-estradiol on dopamine receptors are more complex (22, 82). Consistent with the current study, most investigators find little evidence for sex differences or 17β-estradiol effects upon dopamine receptor mRNA expression (21, 83). Rather, 17β-estradiol effects on dopamine receptor density are usually detected using receptor-binding techniques (20, 22, 84–86). Indeed, one study that measured D2-dopamine receptors 14 days after ovariectomy in rats found differences using receptor binding but not PCR (86). This suggests that 17β-estradiol modulates dopamine receptor action after transcription, consistent with a rapid, nonclassical 17β-estradiol action on striatal dopamine receptors. The studies cited above were conducted in rodents. We are aware of only one human study, which detected no significant sex difference in D2-dopamine receptor density (87).
Given the caudate's integral role in sensorimotor function, it is tempting to speculate that increased β1-adrenergic receptor expression after adult hormone loss may play a role in the known pathologies associated with this brain region. For instance, one characteristic behavioral phenotype of Parkinson's disease, bradykinesia, is caused by loss of neurons in the substantia nigra that produce dopamine and neurons in the locus coeruleus that produce norepinephrine, thereby compromising striatal function. The most common treatment for this pathology is the dopamine/norepinephrine precursor l-DOPA (l-3,4-dihydroxyphenylalanine), which attenuates bradykinesia but can induce dyskinesia. l-DOPA-induced dyskinesia is more prevalent in females than in males (88), although women have an overall lower incidence of Parkinson's disease (18). Because more β1-adrenergic receptors are expressed in menopausal females than in males, this may provide more opportunity for norepinephrine and perhaps dopamine to influence neuronal function. Consistent with this speculation, the β-adrenergic receptor antagonist propranolol reduces or eliminates l-DOPA-induced dyskinesia in human Parkinson's disease patients and rat models (78, 89). Propranolol is also recommended as a first-line therapy for tardive dyskinesia (90), a pathology modulated by steroid sex hormones (22).
Acknowledgments
We thank Kyla Britson, Kelsey Pflepsen, Krista Tuomela, Dr Robert Meisel, Dr Laura Been, Dr Nancy Staffend, and Brittni Peterson for their support.
This work was supported by NIH T32 DA07234 and F32 DA030828 (to J.M.), R01 DA012677 (to J.B.B.), NSF IOS-1146015 (to P.G.M.), and core funding NIH NS062158.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CREB
- cAMP response element-binding protein
- FBS
- fetal bovine serum
- HBSS
- Hank's balanced salt solution
- ISO
- isoproterenol
- l-DOPA
- l-3,4-dihydroxyphenylalanine
- NGF
- nerve growth factor
- P60
- postnatal day 60
- qPCR
- quantitative PCR.
References
- 1. Diaz Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology. 2012;153:3571–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCarthy MM. What can development teach us about menopause? Brain Res. 2011;1379:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berchtold NC, Cribbs DH, Coleman PD, et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci U S A. 2008;105:15605–15610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sárvári M, Kalló I, Hrabovszky E, et al. Estradiol replacement alters expression of genes related to neurotransmission and immune surveillance in the frontal cortex of middle-aged, ovariectomized rats. Endocrinology. 2010;151:3847–3862 [DOI] [PubMed] [Google Scholar]
- 5. Bossé R, DiPaolo T. The modulation of brain dopamine and GABAA receptors by estradiol: a clue for CNS changes occurring at menopause. Cell Mol Neurobiol. 1996;16:199–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chisholm NC, Packard AR, Koss WA, Juraska JM. The effects of long-term treatment with estradiol and medroxyprogesterone acetate on tyrosine hydroxylase fibers and neuron number in the medial prefrontal cortex of aged female rats. Endocrinology. 2012;153:4874–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scarpace PJ, Abrass IB. α- and β-adrenergic receptor function in the brain during senescence. Neurobiol Aging. 1988;9:53–58 [DOI] [PubMed] [Google Scholar]
- 8. Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gibbs RB. Does short-term estrogen therapy produce lasting benefits in brain? Endocrinology. 2010;151:843–845 [DOI] [PubMed] [Google Scholar]
- 11. Sherwin BB. Estrogen and cognitive functioning in women: lessons we have learned. Behav Neurosci. 2012;126:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Becker JB. Hormonal influences on sensorimotor function. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, eds. Behavioral Endocrinology. Cambridge, MA: MIT Press; 2002:497–525 [Google Scholar]
- 13. Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav. 1987;27:53–59 [DOI] [PubMed] [Google Scholar]
- 14. Haaxma CA, Bloem BR, Borm GF, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990;15:97–111 [DOI] [PubMed] [Google Scholar]
- 16. Hampson E, Kimura D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav Neurosci. 1988;102:456–459 [DOI] [PubMed] [Google Scholar]
- 17. Mayeux R, Denaro J, Hemenegildo N, et al. A population-based investigation of Parkinson's disease with and without dementia. Relationship to age and gender. Arch Neurol. 1992;49:492–497 [DOI] [PubMed] [Google Scholar]
- 18. Pavon JM, Whitson HE, Okun MS. Parkinson's disease in women: a call for improved clinical studies and for comparative effectiveness research. Maturitas. 2010;65:352–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saunders-Pullman R, Gordon-Elliott J, Parides M, Fahn S, Saunders HR, Bressman S. The effect of estrogen replacement on early Parkinson's disease. Neurology. 1999;52:1417–1421 [DOI] [PubMed] [Google Scholar]
- 20. Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X, Grisham W, Arnold AP. X chromosome number causes sex differences in gene expression in adult mouse striatum. Eur J Neurosci. 2009;29:768–776 [DOI] [PubMed] [Google Scholar]
- 22. Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41 [DOI] [PubMed] [Google Scholar]
- 23. Jennes L, Jennes ME, Purvis C, Nees M. c-fos expression in noradrenergic A2 neurons of the rat during the estrous cycle and after steroid hormone treatments. Brain Res. 1992;586:171–175 [DOI] [PubMed] [Google Scholar]
- 24. Leret ML, González MI, Tranque P, Fraile A. Influence of sexual differentiation on striatal and limbic catecholamines. Comp Biochem Physiol C. 1987;86:299–303 [DOI] [PubMed] [Google Scholar]
- 25. Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546 [DOI] [PubMed] [Google Scholar]
- 26. Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL, Watson SJ. Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology. 1996;15:17–29 [DOI] [PubMed] [Google Scholar]
- 27. Biegon A, Israeli M. Regionally selective increases in β-adrenergic receptor density in the brains of suicide victims. Brain Res. 1988;442:199–203 [DOI] [PubMed] [Google Scholar]
- 28. Joyce JN, Lexow N, Kim SJ, et al. Distribution of β-adrenergic receptor subtypes in human post-mortem brain: alterations in limbic regions of schizophrenics. Synapse. 1992;10:228–246 [DOI] [PubMed] [Google Scholar]
- 29. Pazos A, Probst A, Palacios JM. β-Adrenoceptor subtypes in the human brain: autoradiographic localization. Brain Res. 1985;358:324–328 [DOI] [PubMed] [Google Scholar]
- 30. van Waarde A, Visser TJ, Elsinga PH, et al. Imaging β-adrenoceptors in the human brain with (S)-1′-[18F]fluorocarazolol. J Nucl Med. 1997;38:934–939 [PubMed] [Google Scholar]
- 31. Black DJ, Fregly MJ, Thrasher TN, Moreland AF. Reduced β-adrenergic responsiveness in rats treated with estrogenic agents. J Pharmacol Exp Ther. 1976;197:362–370 [PubMed] [Google Scholar]
- 32. Thawornkaiwong A, Preawnim S, Wattanapermpool J. Upregulation of β1-adrenergic receptors in ovariectomized rat hearts. Life Sci. 2003;72:1813–1824 [DOI] [PubMed] [Google Scholar]
- 33. Wu Q, Zhao Z, Sun H, Hao YL, Yan CD, Gu SL. Oestrogen changed cardiomyocyte contraction and β-adrenoceptor expression in rat hearts subjected to ischaemia-reperfusion. Exp Physiol. 2008;93:1034–1043 [DOI] [PubMed] [Google Scholar]
- 34. Dent MR, Tappia PS, Dhalla NS. Gender differences in β-adrenoceptor system in cardiac hypertrophy due to arteriovenous fistula. J Cell Physiol. 2011;226:181–186 [DOI] [PubMed] [Google Scholar]
- 35. Dent MR, Tappia PS, Dhalla NS. Gender related alterations of β-adrenoceptor mechanisms in heart failure due to arteriovenous fistula. J Cell Physiol. 2012;227:3080–3087 [DOI] [PubMed] [Google Scholar]
- 36. Kam KW, Qi JS, Chen M, Wong TM. Estrogen reduces cardiac injury and expression of β1-adrenoceptor upon ischemic insult in the rat heart. J Pharmacol Exp Ther. 2004;309:8–15 [DOI] [PubMed] [Google Scholar]
- 37. Biegon A, Reches A, Snyder L, McEwen BS. Serotonergic and noradrenergic receptors in the rat brain: modulation by chronic exposure to ovarian hormones. Life Sci. 1983;32:2015–2021 [DOI] [PubMed] [Google Scholar]
- 38. Petrovic SL, McDonald JK, De Castro JC, Snyder GD, McCann SM. Regulation of anterior pituitary and brain β-adrenergic receptors by ovarian steroids. Life Sci. 1985;37:1563–1570 [DOI] [PubMed] [Google Scholar]
- 39. Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav. 2001;40:169–177 [DOI] [PubMed] [Google Scholar]
- 40. Herbison AE, Simonian SX, Thanky NR, Bicknell RJ. Oestrogen modulation of noradrenaline neurotransmission. Novartis Found Symp. 2000;230:74–85; discussion 85–93 [DOI] [PubMed] [Google Scholar]
- 41. Petitti N, Etgen AM. α1-Adrenoceptor augmentation of β-stimulated cAMP formation is enhanced by estrogen and reduced by progesterone in rat hypothalamic slices. J Neurosci. 1990;10:2842–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wagner HR, Crutcher KA, Davis JN. Chronic estrogen treatment decreases β-adrenergic responses in rat cerebral cortex. Brain Res. 1979;171:147–151 [DOI] [PubMed] [Google Scholar]
- 43. Wilkinson M, Bhanot R, Wilkinson DA, Brawer JR. Prolonged estrogen treatment induces changes in opiate, benzodiazepine and β-adrenergic binding sites in female rat hypothalamus. Brain Res Bull. 1983;11:279–281 [DOI] [PubMed] [Google Scholar]
- 44. Atz M, Walsh D, Cartagena P, et al. Methodological considerations for gene expression profiling of human brain. J Neurosci Methods. 2007;163:295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meitzen J, Luoma JL, Stern CM, Mermelstein PG. ß1-Adrenergic receptors activate two distinct signaling pathways in striatal neurons. J Neurochem. 2011;116:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Groth RD, Weick JP, Bradley KC, et al. D1 dopamine receptor activation of NFAT-mediated striatal gene expression. Eur J Neurosci. 2008;27:31–42 [DOI] [PubMed] [Google Scholar]
- 50. Meitzen J, Grove DD, Mermelstein PG. The organizational and aromatization hypotheses apply to rapid, nonclassical hormone action: neonatal masculinization eliminates rapid estradiol action in female hippocampal neurons. Endocrinology. 2012;153:4616–4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci. 2000;20:266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stern CM, Luoma JI, Meitzen J, Mermelstein PG. Corticotropin releasing factor-induced CREB activation in striatal neurons occurs via a novel Gβγ signaling pathway. PLoS One. 2011;6:e18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mermelstein PG, Deisseroth K, Dasgupta N, Isaksen AL, Tsien RW. Calmodulin priming: nuclear translocation of a calmodulin complex and the memory of prior neuronal activity. Proc Natl Acad Sci U S A. 2001;98:15342–15347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fels E, Bosch LR. Effect of prenatal administration of testosterone on ovarian function in rats. Am J Obstet Gynecol. 1971;111:964–969 [DOI] [PubMed] [Google Scholar]
- 55. Lamond DR. Alteration of ovarian function of immature mice treated with androgen after birth. J Endocrinol. 1969;43:319–320 [DOI] [PubMed] [Google Scholar]
- 56. Hara M, Fukui R, Hieda E, et al. Role of adrenoceptors in the regulation of dopamine/DARPP-32 signaling in neostriatal neurons. J Neurochem. 2010;113:1046–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shelton DL, Reichardt LF. Studies on the expression of the β nerve growth factor (NGF) gene in the central nervous system: level and regional distribution of NGF mRNA suggest that NGF functions as a trophic factor for several distinct populations of neurons. Proc Natl Acad Sci U S A. 1986;83:2714–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martínez HJ, Dreyfus CF, Jonakait GM, Black IB. Nerve growth factor promotes cholinergic development in brain striatal cultures. Proc Natl Acad Sci U S A. 1985;82:7777–7781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chesley LC, Talledo E, Bohler CS, Zuspan FP. Vascular reactivity to angiotensin II and norepinephrine in pregnant women. Am J Obstet Gynecol. 1965;91:837–842 [DOI] [PubMed] [Google Scholar]
- 60. Riemer RK, Wu YY, Bottari SP, Jacobs MM, Goldfien A, Roberts JM. Estrogen reduces β-adrenoceptor-mediated cAMP production and the concentration of the guanyl nucleotide-regulatory protein, Gs, in rabbit myometrium. Mol Pharmacol. 1988;33:389–395 [PubMed] [Google Scholar]
- 61. Monjo M, Rodríguez AM, Palou A, Roca P. Direct effects of testosterone, 17β-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinology. 2003;144:4923–4930 [DOI] [PubMed] [Google Scholar]
- 62. Pinos H, Collado P, Rodríguez-Zafra M, Rodríguez C, Segovia S, Guillamón A. The development of sex differences in the locus coeruleus of the rat. Brain Res Bull. 2001;56:73–78 [DOI] [PubMed] [Google Scholar]
- 63. Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153:5373–5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Di Paolo T, Rouillard C, Bédard P. 17β-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur J Pharmacol. 1985;117:197–203 [DOI] [PubMed] [Google Scholar]
- 65. Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Forlano PM, Woolley CS. Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol. 2010;518:1330–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Meitzen J, Pflepsen KR, Stern CM, Meisel RL, Mermelstein PG. Measurements of neuron soma size and density in rat dorsal striatum, nucleus accumbens core and nucleus accumbens shell: differences between striatal region and brain hemisphere, but not sex. Neurosci Lett. 2011;487:177–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct. 2011;215:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS. Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology. 2011;61:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wagner HR, Davies JN. Decreased β-adrenergic responses in the female rat brain are eliminated by ovariectomy: correlation of [3H]dihydroalprenolol binding and catecholamine stimulated cyclic AMP levels. Brain Res. 1980;201:235–239 [DOI] [PubMed] [Google Scholar]
- 72. Pisani A, Bonsi P, Centonze D, et al. Activation of β1-adrenoceptors excites striatal cholinergic interneurons through a cAMP-dependent, protein kinase-independent pathway. J Neurosci. 2003;23:5272–5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McKittrick CR, Abercrombie ED. Catecholamine mapping within nucleus accumbens: differences in basal and amphetamine-stimulated efflux of norepinephrine and dopamine in shell and core. J Neurochem. 2007;100:1247–1256 [DOI] [PubMed] [Google Scholar]
- 74. Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168 [DOI] [PubMed] [Google Scholar]
- 75. Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451 [DOI] [PubMed] [Google Scholar]
- 76. Rommelfanger KS, Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson's disease. Biochem Pharmacol. 2007;74:177–190 [DOI] [PubMed] [Google Scholar]
- 77. Harris GC, Hedaya MA, Pan WJ, Kalivas P. β-Adrenergic antagonism alters the behavioral and neurochemical responses to cocaine. Neuropsychopharmacology. 1996;14:195–204 [DOI] [PubMed] [Google Scholar]
- 78. Lindenbach D, Ostock CY, Eskow Jaunarajs KL, Dupre KB, Barnum CJ, Bhide N, Bishop C., et al. Behavioral and cellular modulation of l-DOPA-induced dyskinesia by β-adrenoceptor blockade in the 6-hydroxydopamine-lesioned rat. J Pharmacol Exp Ther. 2011;337:755–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cornil CA, Ball GF. Interplay among catecholamine systems: dopamine binds to α2-adrenergic receptors in birds and mammals. J Comp Neurol. 2008;511:610–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Malenka RC, Nicoll RA. Dopamine decreases the calcium-activated afterhyperpolarization in hippocampal CA1 pyramidal cells. Brain Res. 1986;379:210–215 [DOI] [PubMed] [Google Scholar]
- 81. Zhang W, Klimek V, Farley JT, Zhu MY, Ordway GA. α2C adrenoceptors inhibit adenylyl cyclase in mouse striatum: potential activation by dopamine. J Pharmacol Exp Ther. 1999;289:1286–1292 [PubMed] [Google Scholar]
- 82. Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812 [DOI] [PubMed] [Google Scholar]
- 83. Küppers E, Krust A, Chambon P, Beyer C. Functional alterations of the nigrostriatal dopamine system in estrogen receptor-α knockout (ERKO) mice. Psychoneuroendocrinology. 2008;33:832–838 [DOI] [PubMed] [Google Scholar]
- 84. Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498 [DOI] [PubMed] [Google Scholar]
- 85. Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172 [DOI] [PubMed] [Google Scholar]
- 86. Le Saux M, Morissette M, Di Paolo T. ERβ mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology. 2006;50:451–457 [DOI] [PubMed] [Google Scholar]
- 87. Parellada E, Lomeña F, Catafau AM, et al. Lack of sex differences in striatal dopamine D2 receptor binding in drug-naive schizophrenic patients: an IBZM-SPECT study. Psychiatry Res. 2004;130:79–84 [DOI] [PubMed] [Google Scholar]
- 88. Zappia M, Annesi G, Nicoletti G, et al. Sex differences in clinical and genetic determinants of levodopa peak-dose dyskinesias in Parkinson disease: an exploratory study. Arch Neurol. 2005;62:601–605 [DOI] [PubMed] [Google Scholar]
- 89. Carpentier AF, Bonnet AM, Vidailhet M, Agid Y. Improvement of levodopa-induced dyskinesia by propranolol in Parkinson's disease. Neurology. 1996;46:1548–1551 [DOI] [PubMed] [Google Scholar]
- 90. Zappia M, Albanese A, Bruno E, et al. Treatment of essential tremor: a systematic review of evidence and recommendations from the Italian Movement Disorders Association. J Neurol. 2013;260:714–740 [DOI] [PubMed] [Google Scholar]