Abstract
In rat testes, the ectoplasmic specialization (ES) at the Sertoli-Sertoli and Sertoli-spermatid interface known as the basal ES at the blood-testis barrier and the apical ES in the adluminal compartment, respectively, is a testis-specific adherens junction. The remarkable ultrastructural feature of the ES is the actin filament bundles that sandwiched in between the cisternae of endoplasmic reticulum and apposing plasma membranes. Although these actin filament bundles undergo extensive reorganization to switch between their bundled and debundled state to facilitate blood-testis barrier restructuring and spermatid adhesion/transport, the regulatory molecules underlying these events remain unknown. Herein we report findings of an actin filament cross-linking/bundling protein palladin, which displayed restrictive spatiotemporal expression at the apical and the basal ES during the epithelial cycle. Palladin structurally interacted and colocalized with Eps8 (epidermal growth factor receptor pathway substrate 8, an actin barbed end capping and bundling protein) and Arp3 (actin related protein 3, which together with Arp2 form the Arp2/3 complex to induce branched actin nucleation, converting bundled actin filaments to an unbundled/branched network), illustrating its role in regulating actin filament bundle dynamics at the ES. A knockdown of palladin in Sertoli cells in vitro with an established tight junction (TJ)-permeability barrier was found to disrupt the TJ function, which was associated with a disorganization of actin filaments that affected protein distribution at the TJ. Its knockdown in vivo also perturbed F-actin organization that led to a loss of spermatid polarity and adhesion, causing defects in spermatid transport and spermiation. In summary, palladin is an actin filament regulator at the ES.
In the mammalian testis, spermatogenesis takes place in the seminiferous epithelium, involving extensive cell junction restructuring (1–4). Cell junctions in the seminiferous epithelium of the testis appear differently from all other epithelia at the ultrastructural level, in which extensive bundles of actin filaments are noted at the Sertoli cell-cell interface at the blood-testis barrier (BTB) near the basement membrane and also at the Sertoli-spermatid interface (step 8–19 spermatids in rats) (5–7). These actin filament bundles are the hallmark ultrastructure of the testis-specific adherens junction known as the ectoplasmic specialization (ES) (5–7). In short, actin filament bundles at the ES that lie perpendicular to the apposing plasma membranes of Sertoli-Sertoli cells known as the basal ES at the BTB and Sertoli cell-spermatid known as the apical ES, are sandwiched in between cisternae of endoplasmic reticulum and the plasma membranes. The only ultrastructural difference between the apical ES and the basal ES is that in the former, the actin filament bundles are restricted only to the Sertoli cell and similar ultrastructure is absent in the spermatid, whereas these actin filament bundles are found on both sides of the apposing Sertoli cells at the basal ES.
Also, once apical ES appears at the Sertoli-spermatid (step 8) interface, it persists until step 19 spermatids; it is the only anchorage device that confers adhesion and polarity to the developing spermatids, and regulates spermatid transport (2, 6). However, basal ES coexists with tight junction (TJ) and gap junction, which together with desmosome constitute the BTB (5, 8). Although these actin filament bundles confer the unusual adhesive strength to the ES (9, 10), the ES restructures cyclically during spermatogenesis to facilitate preleptotene spermatocyte transport across the BTB at stage VIII of the cycle and the transport of spermatids across the epithelium at spermiogenesis (11, 12). Without the timely transport of these germ cells across the epithelium, spermatogenesis fails, leading to infertility. Few reports are found in the literature to study the regulation of actin filament bundles at the ES or the underlying regulatory molecules. Recent studies have shown that the stage-specific and spatiotemporal expression of actin filament bundling and barbed end-capping protein, Eps8 (epidermal growth factor receptor pathway substrate 8) (13), and branched actin polymerization protein Arp3 [actin related protein 3, which together with actin related protein 2 (Arp2) and actin-related protein component (ARPC) 1–5, form a 7 subunit Arp2/3 complex that converts the bundled actin filaments to a debundled/branched state] (14) are working in concert to regulate the organization of these actin filaments, converting from their bundled to their debundled state and vice versa during the epithelial cycle to facilitate germ cell transport (15–17).
Palladin is an approximately 95-kDa protein originally discovered as a scaffolding protein in epithelial and muscle cells, but subsequent studies have shown that it is an actin cross-linking protein that regulates cell movement in many epithelial cells (18, 19). Palladin displays a high binding affinity for F-actin via its Ig-like interacting domain, and it also structurally interacts with Eps8 (20). These features thus implicate that palladin, if found in the testis, may play an important role in regulating actin filament bundles at the ES via its intrinsic actin cross-linking and bundling activity, perhaps in conjunction with other actin regulatory proteins, such as Eps8. We thus sought to examine whether palladin is an integrated component of the ES and the likely involvement of palladin in ES dynamics.
Materials and Methods
Animals
Sprague Dawley rats were obtained from Charles River Laboratories (Kingston, New York). The use of rats reported herein was approved by the Rockefeller University Institutional Animal Care and Use Committee with Protocol number 12-506.
Treatment of rats with adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide
Adult rats, approximately 280–300 g body weight (b. w.), were treated with a single dose of adjudin (50 mg/kg b.w.) by gavage (21). This is an in vivo model to study anchoring junction dynamics in which adjudin was shown to induce anchoring junction restructuring, most notably the apical ES, causing spermatid depletion from the epithelium (21–24). Rats at time 0 without adjudin treatment served as controls. Rats were euthanized by CO2 asphyxiation at specified time points (n = 3–4 rats for each time point), and testes were removed, snap frozen in liquid nitrogen, and stored at −80°C.
Primary cultures of testicular cells
Total germ cells were isolated from adult rat testes, cultured in serum-free F12/DMEM, and terminated for lysate preparation within 16 hours (25). Sertoli cells were isolated from testes of 20-day-old rats and cultured in serum-free F12/DMEM with supplements (26). At this age and under the conditions used herein, Sertoli cells were differentiated and ceased to divide (27) unless serum was included in the media (28, 29). Furthermore, these Sertoli cells were capable of establishing a functional TJ-permeability barrier that mimicked the BTB in vivo (26). Sertoli cells were seeded on Matrigel (BD Biosciences, Bedford, Massachusetts)-coated coverslips, 12-well culture dishes, or Millicell HA cell culture inserts (EMD Millipore, Billerica, Massachusetts) at 0.04, 0.4, and 1.0 × 106 cells/cm2, respectively, for the corresponding experiments for the following: 1) dual-labeled immunofluorescence analysis, 2) lysate preparation for immunoblotting, and 3) assessing the Sertoli cell TJ-permeability barrier function by quantifying transepithelial electrical resistance (TER) across the cell epithelium (24, 30, 31). These cell densities were used based on findings in preliminary experiments so that Sertoli cells were evenly spaced to assess changes in protein distribution at the cell-cell interface for dual-labeled immunofluorescence analysis (experiment 1); to have enough Sertoli cells to obtain sufficient proteins for immunoblotting (experiment 2); and to have sufficient Sertoli cells to cover the entire bicameral units to assess TJ barrier function without any leak (experiment 3). It is noted that each coverslip was then placed in each well of a 12-well dish containing 2 mL F12/DMEM for cultures. Sertoli cells cultured on 12-well dishes for immunoblotting contained 2 mL F12/DMEM for each well. Each bicameral unit was placed in a well of a 24-well dish, and the apical and basal compartment contained 0.5 mL F12/DMEM. Each treatment time point vs controls had at least triplicate coverslips, culture dishes, or bicameral units, and each experiment was repeated at least 3 times.
Knockdown of palladin in Sertoli cells in vitro
The sequence of small interfering RNA (siRNA) duplexes that specifically targeted palladin were 5′-CUACUCCGCUGUCACAUUAUU-3′ and 5′-CAAACGUCUUCAACAUCCAUU-3′ as earlier reported (32), which were purchased from Dharmacon (Thermo Fisher Scientific, Waltham, Massachusetts). Nontargeting siRNA control duplexes (Silencer Select Negative Control 1 siRNA) was obtained from Ambion (catalog number 4390844; Ambion, Austin, Texas), which served as the negative control. For in vitro palladin knockdown, Sertoli cells cultured alone for 3 days were transfected with palladin siRNA duplexes vs nontargeting control siRNA duplexes at 100 nM using RiboJuice siRNA transfection reagent (Novagen; EMD Bioscience) for 24 hours. About 6 hours thereafter, Sertoli cells were harvested for lysate preparation or for dual-labeled immunofluorescence analysis (ie, ∼6 hours the day after transfection was completed). To assess successful transfection, Sertoli cells transfected with the corresponding siRNA duplexes were cotransfected with 1 nM siGLO red transfection indicator (catalog number D-001630-02; Dharmacon). For studies that assessed the effects of RNA interference (RNAi) on the Sertoli cell TJ-permeability barrier function, 150 nM siRNA duplexes were used for the palladin knockdown vs controls for transfection in Sertoli cells after 2 days of cultures, and cells were transfected for 36 hours. These conditions were established based on initial pilot/preliminary experiments, which were shown to yield identifiable phenotypes.
Knockdown of palladin in the testis in vivo
Based on the findings in vitro, palladin was also knocked down in the rat testis in vivo using adult rats (n = 5 rats). In short, rats were transfected with siRNA duplexes via intratesticular injection using a 28-gauge needle as described (33–36) on day 0, in which a testis of the same rat received 150 nM the nontargeting control siRNA duplexes in which the other testis received the palladin-specific siRNA duplexes shown to be effective to perturb the Sertoli cell TJ-permeability barrier in vitro. siRNA duplexes at desired concentration (150 nM) were constituted in a transfection mix composed of 11.25 μl Ribojuice siRNA transfection reagent (EMD Millipore, Darmstadt, Germany) in 180 μL Opti-MEM (Invitrogen, Carlsbad, California) in a final volume of approximately 200 μL. Thus, each testis received 200 μL of this solution for transfection, and the volume of each testis was assumed to be approximately 1.6 mL to obtain the desired concentration of the siRNA duplexes at 150 nM. On day 0 and day 1, each testis of the same rat was transfected using their nontargeting control or palladin-specific siRNA duplexes, and as such, each rat testis was transfected twice. Rats were euthanized by CO2 asphyxiation on day 2 (n = 3 rats) and day 3 (n = 2 rats) to assess any changes in phenotypes regarding the status of spermatogenesis, and similar phenotypes were detected in these rats at both termination time points. Testes were immediately removed from these rats, snap frozen in liquid nitrogen, and stored at −80°C until used. All samples including treatment and control groups were examined in a single experimental session to avoid interexperimental variations.
Immunoblotting and coimmunoprecipitation (Co-IP)
Lysates were obtained from adult rat testes, Sertoli cells (from 20 day old rat testes and cultured for 4 days), and germ cells (from adult rat testes and cultured for ∼16 hours) (14, 30, 31), and protein concentrations were estimated using the BioRad DC kits (Bio-Rad Laboratories, Hercules, California). Antibodies used for immunoblotting or Co-IP are listed in Table 1. Co-IP was performed (14, 30, 31) using lysates of adult rat testes (500 μg protein) and corresponding primary antibodies. Chemiluminescence was performed using a kit prepared in-house (37).
Table 1.
Antibodies Used for Different Experiments in This Study
| Antibody | Host Species | Vendor | Catalog Number | Working Dilution |
|
|---|---|---|---|---|---|
| IB | IF | ||||
| Palladin | Rabbit | Protein Tech Group | 10853-1-AP | 1:1000 | 1:100 |
| Arp3 | Mouse | Sigma-Aldrich | A5979 | 1:3000 | 1:200 |
| Eps8 | Mouse | BD Biosciences | 610143 | 1:5000 | 1:100 |
| ARPC2 | Rabbit | Abcam | ab96779 | 1:2000 | |
| N-Cadherin | Rabbit | Santa Cruz Biotechnology | sc-7939 | 1:200 | |
| N-Cadherin | Mouse | Invitrogen | 33-3900 | 1:100 | |
| α-Actinin | Goat | Santa Cruz Biotechnology | sc-7453 | 1:200 | |
| α-Catenin | Rabbit | Santa Cruz Biotechnology | sc-7894 | 1:200 | |
| α-Catenin | Mouse | Invitrogen | 13-9700 | 1:100 | |
| β-Catenin | Rabbit | Invitrogen | 71-2700 | 1:250 | 1:100 |
| JAM-C | Rabbit | Invitrogen | 40-8900 | 1:250 | |
| JAM-C | Goat | Santa Cruz Biotechnology | sc-23006 | 1:100 | |
| β1-Integrin | Rabbit | Santa Cruz Biotechnology | sc-8978 | 1:200 | |
| β1-Integrin | Rabbit | Millipore | ab1952 | 1:100 | |
| ZO-1 | Rabbit | Invitrogen | 61-7300 | 1:250 | 1:100 |
| ZO-1-FITC | Mouse | Invitrogen | 33-9111 | 1:100 | |
| Occludin | Rabbit | Invitrogen | 71-1500 | 1:250 | 1:100 |
| JAM-A | Rabbit | Invitrogen | 36-1700 | 1:250 | |
| c-Src | Mouse | Santa Cruz Biotechnology | sc-8056 | 1:200 | |
| p-Src-Tyr418 | Rabbit | Millipore | 07–909 | 1:1000 | |
| FAK | Rabbit | Santa Cruz Biotechnology | sc-558 | 1:200 | |
| FAK | Mouse | Abcam | ab33141-100 | 1:100 | |
| p-FAK-Tyr397 | Rabbit | Invitrogen | 44625G | 1:1000 | |
| p-FAK-Tyr397 | Rabbit | Abcam | ab4803 | 1:100 | |
| p-FAK-Tyr407 | Rabbit | Invitrogen | 44650G | 1:1000 | |
| PKB | Rabbit | Cell Signaling Technology | 9272 | 1:1000 | |
| p-PKB-Ser473 | Rabbit | Cell Signaling Technology | 4060S | 1:1000 | |
| Vimentin | Mouse | Santa Cruz Biotechnology | sc-6260 | 1:300 | |
| Actin | Goat | Santa Cruz Biotechnology | sc-1616 | 1:300 | |
| GAPDH | Mouse | Abcam | ab8245 | 1:2000 | |
Abbreviations used: IB, immunoblotting; IF, immunofluorescence microscopy; FITC, fluorescein isothiocyanate; p, phosphorylated. Locations for manufacturers are as follows: Abcam, Cambridge, Massachusetts; Santa Cruz Biotechnology, Santa Cruz, California; Cell Signaling Technology, Beverly, Massachusetts.
Dual-labeled immunofluorescence analysis
Dual-labeled immunofluorescence analysis was performed using frozen cross-sections of testes at 7 μm (in thickness) (14, 30, 31) and the corresponding primary antibodies (see Table 1). Negative controls included the use of normal IgG of the corresponding host animals, mouse, rabbit, or goat, depending on the source of the antigen (Table 1), diluted in blocking solution to substitute the primary antibody. Testes sections or cells were then incubated with Alexa Fluor-conjugated secondary antibodies (Alexa Fluor 555 for red fluorescence; Alexa Fluor 488 for green fluorescence; Invitrogen) at a dilution of 1:250 for testes section and 1:100 for Sertoli cells. For F-actin staining, testis sections or cells were incubated with fluorescein isothiocyanate-conjugated phalloidin (Sigma-Aldrich, St Louis, Missouri) at a dilution of 1:70 along with the secondary antibody. Sections or cells were mounted in Prolong Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; a blue fluorescence nuclei stain; Invitrogen) for fluorescence microscopy. Fluorescence images were obtained in an Olympus BX61 fluorescence microscope with a built-in Olympus DP70 12.5-MPx digital camera and analyzed using the Olympus MicroSuite FIVE software (version 1.224; Olympus Soft Imaging Solutions Corp, Tokyo, Japan) and Adobe Photoshop in Adobe Creative Suite (version 3.0; San Jose, California) (14, 30, 31), such as for image overlay to assess colocalization.
Semiquantitative analysis on the efficacy of palladin knockdown in vivo
To assess whether a knockdown had indeed occurred in testes transfected with the palladin siRNA duplexes vs nontargeting control duplexes in vivo, the intensity of palladin fluorescence signals in cross-sections of rat testes was quantified using ImageJ 1.45 (National Institutes of Health, Bethesda, Maryland; http://rsbweb.nih.gov/ij) in both palladin knockdown vs the nontargeting control groups. We opted to use this approach instead of performing immunoblotting as used in the in vitro knockdown experiments because the effects of palladin knockdown on the F-actin organization and the spatiotemporal expression or distribution of actin regulatory proteins (eg, Eps8, Arp3) were most obvious at stage VII-early VIII tubules, which accounted for approximately 25% of all the tubules in adult rat testes (38), and that the efficacy of these in vitro and in vivo was estimated to be approximately 55%. These stages were selected because palladin and the pertinent actin-regulatory proteins or the apical ES proteins were mostly expressed at the apical ES in these tubules. In fact, the knockdown of palladin in late stage VI–VIII tubules that led to defects in spermiation or spermatogenesis were detected in stage IX–X tubules in which spermatids were found to be retained and/or entrapped in the epithelium, failing to be released at spermiation in late stage VIII. In short, at least 20 stage VI, VII, and VIII tubules (and IX–X tubules were also selected in analysis to assess defects in spermiation) from each rat testis were randomly selected (ie, 80 tubules), and the palladin signals from treatment vs the control group were quantified with 5 rats.
Assessing spermatogenesis status after palladin knockdown
To assess any changes and/or defects in spermatogenesis after palladin knockdown, testes were used for fluorescence microscopy with cell nuclei stained with DAPI. Because palladin is an actin cross-linking/bundling protein, its knockdown was anticipated to impede F-actin organization of F-actin at the ES. Furthermore, we also examined changes in the spatiotemporal expression of actin regulatory proteins [eg, Eps8, Arp3, focal adhesion kinase (FAK)] or integral membrane proteins [eg, junctional adhesion molecule (JAM)-C and β1-integrin specific to the apical ES] in the epithelium. In this study, we focused on the apical ES because studies in assessing changes in the stage-specific and spatiotemporal expression of palladin in the epithelium indicated that its localization at the apical ES displayed more subtle changes during the epithelial cycle. The following 3 parameters after palladin knockdown were assessed: 1) changes in the spatiotemporal expression of the selected proteins at the apical ES; 2) changes in spermatid polarity; and 3) defects in spermiation. For the first parameter, sections of testes from 5 rats was examined; for the second and third parameters, changes were assessed by examining approximately 400 randomly selected tubules from cross-sections of a rat testis, and a total of 5 rats were examined (∼80 tubules were scored from each rat testis). A tubule was scored and annotated as defective if it met one of the following criteria: 1) loss of spermatid polarity: it was defined by the presence of approximately 5 spermatids/cross-section of a tubule in which spermatids displayed polarity defects when spermatid heads were no longer pointing toward the basement membrane but greater than 90° deviated from the normal orientation vs the control rat testis; 2) defects in spermiation: it was defined by the presence of at least 5 elongating/elongated spermatids that were retained in and/or entrapped within the seminiferous epithelium in a stage IX or X tubule when spermiation had occurred at stage VIII. Data were expressed as a percentage of the defective tubules in the testes from rats transfected with palladin siRNA duplexes vs nontargeting control duplexes.
Statistical analysis
Statistical analysis was performed using GB-STAT (version 7.0; Dynamic Microsystems, Silver Spring, Maryland). For multiple comparisons, ANOVA was used to be followed by Dunnett's test. A Student's t test was used for paired comparisons with 2 experimental groups.
Results
Palladin is expressed by Sertoli and germ cells in the rat testis, colocalizing with actin filaments in Sertoli cells
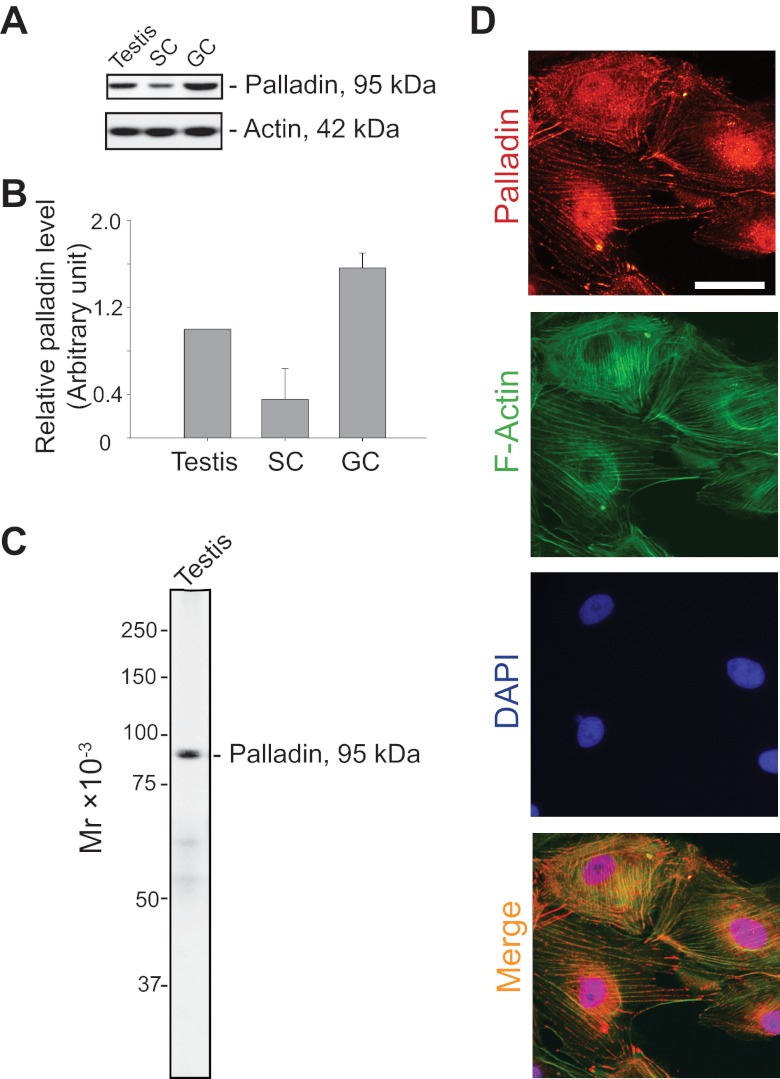
Using an antibody against palladin (Table 1) for immunoblotting, palladin was shown to be expressed by Sertoli and germ cells in the rat testis (Figure 1, A and B). The specificity of this antibody was assessed by immunoblotting using lysates of adult rat testes (Figure 1C). This antibody was then used to assess cellular distribution of palladin in Sertoli cells cultured in vitro for 4 days, illustrating that palladin was associated with the actin filaments, colocalized in part with F-actin (Figure 1D). These findings also illustrate that palladin, an actin cross-linker and an actin filament bundling protein, is a component of the Sertoli cell F-actin cytoskeleton.
Figure 1.
Expression of palladin by Sertoli and germ cells in the rat testis. A, Lysates of testes from adult rats (∼300 g b.w.), Sertoli cells (SC; isolated from 20 day old rat testes and cultured for 4 days in F12/DMEM), and germ cells (GC; isolated from adult rat testes and cultured for ∼16 hours in F12/DMEM) were used for immunoblotting to assess the steady-state protein level of palladin. Actin served as a protein loading control. B, Results of immunoblotting, such as those shown in A, were summarized in this histogram and normalized against actin. Each bar is a mean ± sd of 3 samples. The relative protein level of palladin in the testis was arbitrarily set at 1 against which comparison was performed. C, The specificity of the antipalladin antibody (Table 1) was assessed by immunoblotting using lysates of testes from adult rat testis (15 μg protein). Protein markers used to assess the apparent Mr of palladin was from Bio-Rad (Precision Plus dual color protein standards, catalog number 161-0374). D, Dual-labeled immunofluorescence was performed to assess the colocalization of palladin (red fluorescence) with F-actin (green fluorescence) in Sertoli cells. Sertoli cell nuclei were visualized by DAPI. Bar, 50 μm, which applies to all other micrographs in this panel.
Expression and localization of palladin in the seminiferous epithelium is stage specific
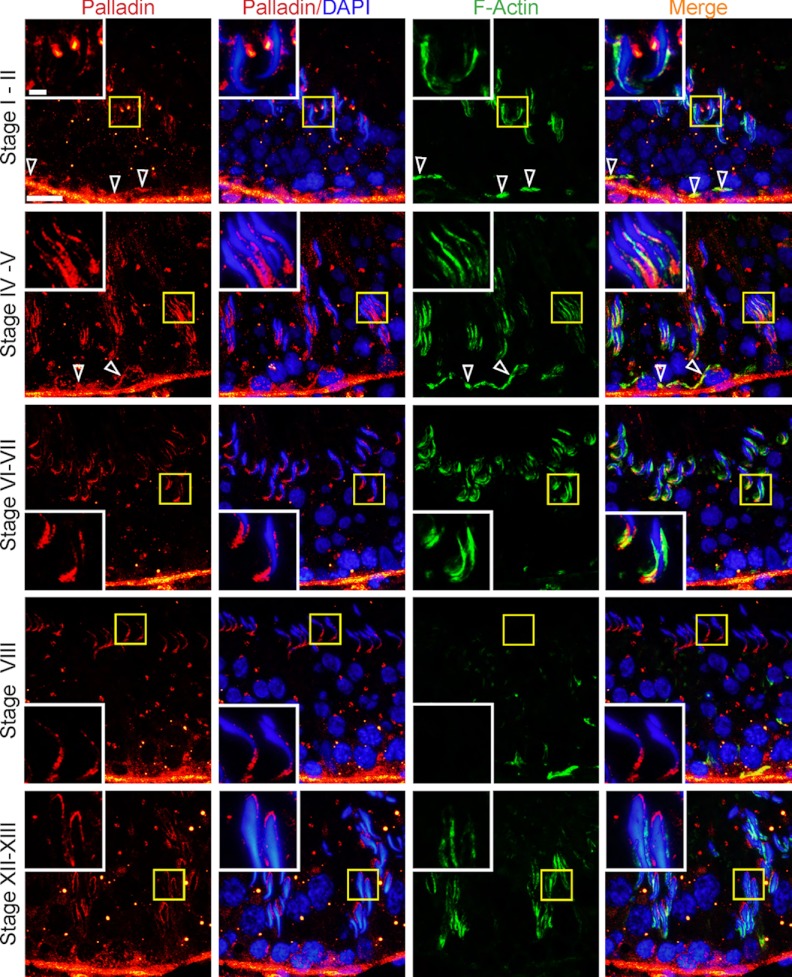
Expression and cellular localization of palladin in the seminiferous epithelium were examined by dual-labeled immunofluorescence analysis (Figure 2). Palladin was highly expressed at the tunica propria, likely by peritubular myoid cells, and its expression at this site was not stage specific. However, palladin was also detected prominently at the apical ES, initially surrounding the head of spermatids in stage I–III tubules, colocalized with F-actin, but its localization at the apical ES shifted to and became predominant at the concave side of spermatid heads at stage IV–V and also colocalized with F-actin (Figure 2). At stage VI–VIII, palladin was localized mostly at the tip of the spermatid head predominantly to the concave side of the head and colocalized with F-actin at stage VII but no longer at late stage VIII when F-actin staining was considerably diminished to prepare for spermiation (Figure 2). By stage X–XIV, as shown in XII–XIII herein, palladin localization further shifted to the back of the spermatid head in step 12–13 spermatids (Figure 2). This pattern of changes in localization of palladin during the epithelial cycle illustrates that this actin cross-linker/bundler is being used to alter the organization of actin filament bundles at the apical ES, which is necessary to accommodate spermatid transport in the epithelium during spermiogenesis. In addition to apical ES, palladin was also detected at the basal ES, colocalized with F-actin near the basement membrane, consistent with its localization at the BTB (Figure 2). Palladin was found to be highly expressed at the basal ES at stage I–V but diminished at stage VII–VIII when the BTB underwent restructuring to facilitate preleptotene spermatocyte transport (Figure 2).
Figure 2.
Stage-specific expression of palladin in the seminiferous epithelium during the epithelial cycle of spermatogenesis. Dual-labeled immunofluorescence analysis was performed using frozen sections of adult rat testes and stained for palladin (red fluorescence) and F-actin (green fluorescence). It was noted that palladin was abundantly found in the tunica propria, illustrating its expression by peritubular myoid cells and/or endothelial cells of the lympathic vessels in virtually all stages of the epithelial cycle. Palladin was also highly expressed at the apical ES, displaying a stage-specific and spatiotemporal expression pattern surrounding the head of developing elongate spermatids. In stage I–II tubules, palladin was found at the apical ES, surrounding the entire head of elongating spermatids and colocalized with F-actin, and its staining shifted to and was more predominant at the concave side of the spermatid head at stage IV–V tubules and also colocalized with F-actin. At stage VI–VII, palladin was most prominent at the concave side of the spermatid head (and colocalized with F-actin), and palladin was restricted to this site by stage VIII; and at stage XII–XIII, it was localized mostly to the middle and rear end of the spermatid head. Furthermore, palladin was also detected at the basal ES, colocalized with F-actin (annotated by open arrowheads), consistent with its localization at the BTB in stage I–II and IV–V, but not in stage VII-VIII at the time of BTB restructuring. Bar, 50 μm and bar in inset, 10 μm, which apply to all other micrographs and insets in this panel.
Palladin is a component of the ES and its interactions with other actin regulatory proteins
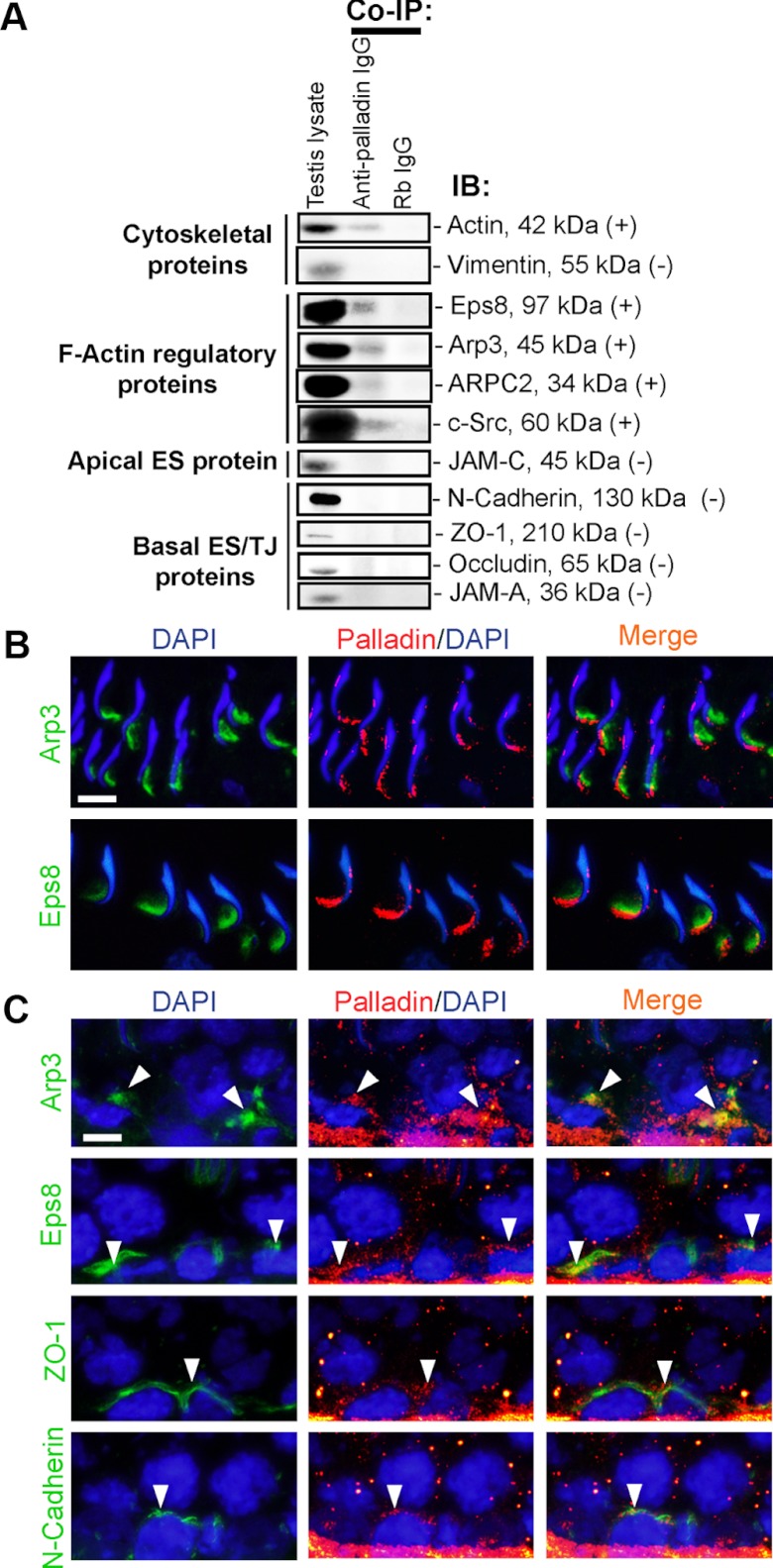
To further confirm whether palladin is indeed a component of the ES, a study by Co-IP using lysates of adult rat testes was performed (Figure 3A). It was noted that palladin indeed structurally interacted with actin and Eps8, consistent with earlier findings in other mammalian cells (20, 39). More important, it was shown that palladin also structurally interacted with Arp3, ARPC2, and also c-Src [an apical ES regulatory protein known to affect F-actin organization (40, 41)] but not other apical ES (eg, JAM-C) or basal ES/TJ [eg, occludin, JAM-A, zonula occludens-1 (ZO-1), N-cadherin] proteins (Figure 3A). The structural interaction of palladin with the actin-regulatory proteins Eps8 and Arp3 at the apical ES (Figure 3B) and the basal ES (Figure 3C) was further confirmed by dual-labeled immunofluorescence analysis. In stage VII tubules when the expression of Arp3 and Eps8 was high, palladin was found to colocalize with these 2 actin-regulatory proteins at the apical ES (Figure 3B) and the basal ES (Figure 3C). Furthermore, palladin also colocalized with TJ adaptor protein ZO-1 and basal ES protein N-cadherin at the BTB (Figure 3C).
Figure 3.
Palladin is an integrated component of the apical and the basal ES in the rat testis. A, To further confirm whether palladin is indeed a component of the apical ES, and the basal ES at the BTB, Co-IP using lysates of testes was performed with approximately 500 μg protein for each assay tube to identify the binding partner of palladin as shown in A. It was noted that, consistent with earlier findings in other epithelia, palladin indeed interacted structurally with actin and Eps8. However, palladin was also found to interact with Arp3, ARPC2, and c-Src in the rat testis but not other apical ES (eg, JAM-C) and basal ES/TJ (eg, N-cadherin, ZO-1, occludin, JAM-A) proteins. B and C, To further confirm data from the Co-IP experiment, dual-labeled immunofluorescence analysis was performed in which palladin (red fluorescence) was found to colocalize with apical ES proteins Arp3 (green) and Eps8 (green) (B) and also with basal ES/TJ proteins Arp3 (green), Eps8 (green), ZO-1 (green), and N-cadherin (green) (annotated by white arrowheads) (C). Bar, 10 μm in B or C, which applies to other micrographs in the same panel.
Adjudin-induced spermatid loss from the epithelium is associated with a mislocalization and down-regulation of palladin at the apical ES
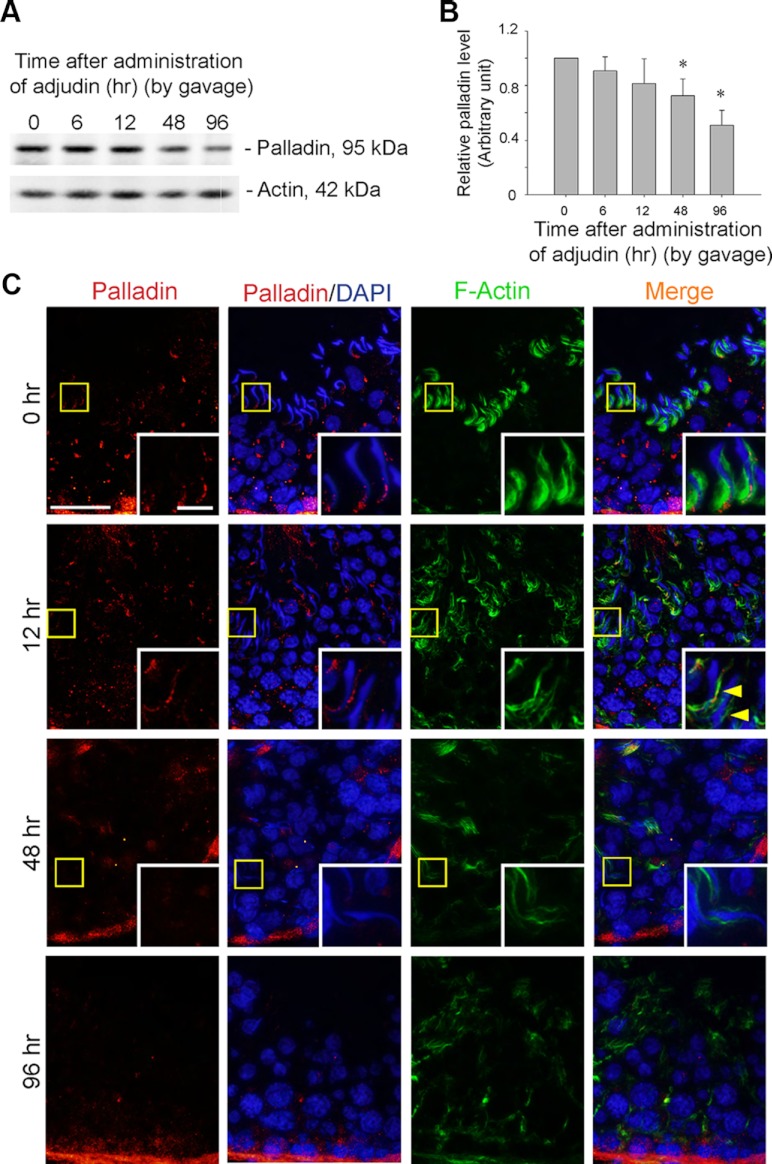
To examine the likely functional role of palladin at the ES, a model of apical ES disruption in which rats treated with a single dose of adjudin, which is known to induce apical ES disruption within approximately 6–9 hours after treatment, was used (21). However, it is noted that the basal ES is not perturbed until approximately 4 weeks after treatment when the BTB is transiently disrupted (23). Consistent with the concept that palladin is important to confer actin filament bundles at the apical ES, treatment of rats with adjudin was found to down-regulate palladin expression in the testis (Figure 4, A and B). When cellular localization of palladin in these adjudin-treated rats were examined by fluorescence microscopy, palladin at the apical ES in tubules of stage VII displayed a considerably diminished expression and mislocalization in which palladin no longer restricted to the concave side of spermatid heads vs the control rat testes at 0 hour; instead, palladin was either diminished or localized to the convex side of spermatid heads (Figure 4C; see yellow arrowheads). By 48 and 96 hours after adjudin treatment when spermatids were being depleted from the epithelium, palladin was no longer detected at the apical ES (Figure 4C), thereby failing to maintain the actin filament bundles at the apical ES to anchor spermatids onto the Sertoli cell, leading to spermatid detachment.
Figure 4.
Adjudin-induced spermatid loss from the seminiferous epithelium is associated with a down-regulation in the expression and mislocalization of palladin at the apical ES. A and B, To further examine the functional significance of palladin at the apical ES, an in vivo model of anchoring junction disruption, in particular apical ES, was used to examine changes in the steady-state level of palladin by immunoblotting. Adjudin-induced spermatid loss from the seminiferous epithelium occurred by as early as 12 hours when spermatids were found in tubule lumen; by 48 hours, few elongating/elongated spermatids were detected in the seminiferous epithelium; and by 96 hours, virtually no elongating/elongated spermatids were found in any tubules (see panel C). A time-dependent and significant down-regulation on the expression of adjudin was detected by immunoblotting, wherein actin served as a protein loading control (A). The data shown in A were summarized in B with each bar representing a mean ± sd of 3 rats, and the level of palladin at time 0 normalized against actin was arbitrarily set at 1 against which statistical comparison was performed. *P < .05. C, In stage VII tubules at 0 hour, such as the one shown herein, palladin was detected at the apical ES, at the tip and the concave side of the spermatid head, colocalized with F-actin. However, by 12 hours, palladin staining was considerably diminished and mislocalized, no longer tightly associated with the apical ES, and for palladin detected at the ES, it was no longer highly expressed at the concave side of spermatid heads; instead, it was shifted to the convex side of the spermatid head (see yellow arrowheads), and these changes were associated with their premature release of elongating/elongated spermatids from the epithelium. Boxed areas in micrographs were magnified and shown in insets to better illustrate the localization of palladin and F-actin at the apical ES. Bar, 50 μm, and bar in inset, 10 μm, which apply to all other micrographs and insets.
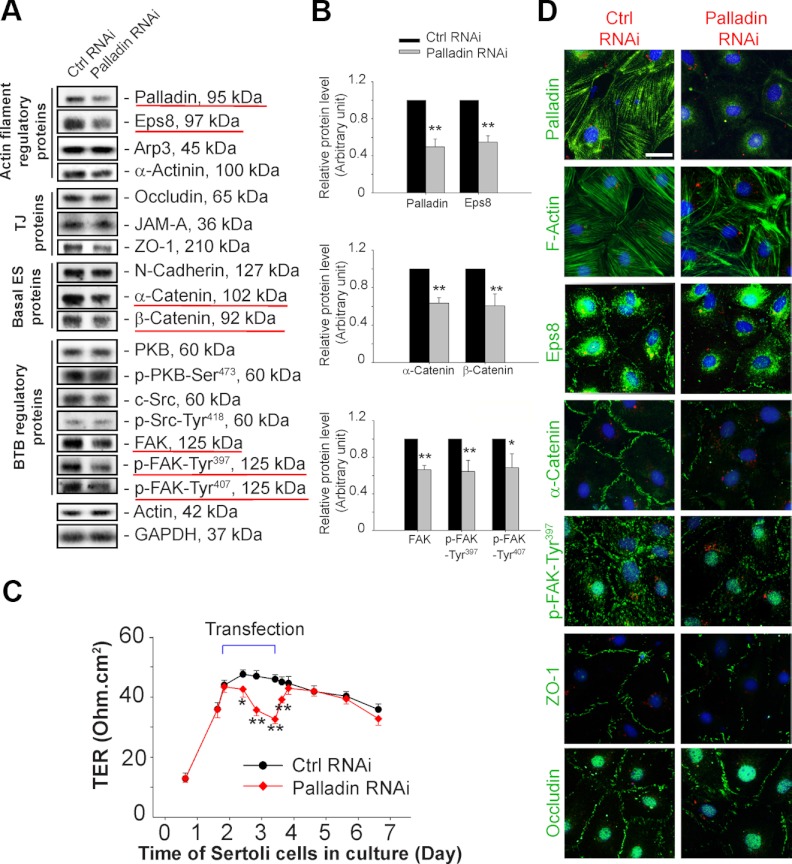
Knockdown of palladin in Sertoli cells perturbs the TJ barrier and induces disorganization of F-actin and mislocalization of BTB proteins
To further explore the function of palladin at the ES, such as the basal ES, a Sertoli cell BTB model was used in which Sertoli cells were cultured in vitro for 2 days with an established TJ-permeability barrier as manifested by the presence of a stable TER across the Sertoli cell epithelium (Figure 5) as earlier described (26, 42). Also, ultrastructures of basal ES, TJ, gap junction, and desmosome were found in these cell cultures when examined by electron microscopy (43, 44). Thereafter Sertoli cells were transfected with palladin-specific siRNA duplexes vs nontargeting control siRNA duplexes at 150 nM using RiboJuice (EMD Bioscience) as the transfection medium for 1.5 days. It was noted that when palladin was knocked down by approximately 55%, the levels of actin bundling/barbed end-capping protein Eps8 (but not the branched actin polymerization-inducing protein Arp3 or the actin cross-linking protein α-actinin, even though it was mildly reduced, but the effect was statistically insignificant), basal ES proteins α-/β-catenin, and BTB regulatory protein FAK and the two activated phosphorylated FAK-Tyr397 and -Tyr407 forms were also down-regulated but not several other BTB-associated proteins (Figure 5, A and B). More important, a knockdown of palladin was found to associate with a transient disruption of the Sertoli cell TJ-permeability barrier (Figure 5C). The knockdown of palladin was further confirmed by staining Sertoli cells transfected with the palladin siRNA duplexes vs control duplexes with palladin (Figure 5D vs Figure 5A). The most notable disruption after palladin knockdown was the disorganization of the actin filaments in Sertoli cells, in which filament bundles were found to be truncated and actin filaments no longer distributed evenly across the cell cytosol but became mislocalized and truncated/defragmented (Figure 5D), thereby failing to support the Sertoli TJ barrier function as noted in Figure 5C. Furthermore, α-catenin, ZO-1, and occludin were mislocalized in the palladin knockdown cells, no longer restrictively localized to the Sertoli cell-cell interface vs control cells. It was also noted that Eps8 no longer localized to the cell-cell interface, which was likely needed to maintain actin filaments at the site to confer cell adhesion (Figure 5D). Most importantly, more phosphorylated FAK-Tyr397 was found at the cell-cell interface (Figure 5D), and this finding is consistent with a recent report that overexpression of phosphorylated FAK-Tyr397 perturbs the integrity of Sertoli cell BTB (31). The changes depicted in Figure 5D thus destabilized the Sertoli cell TJ-barrier, contributing to its disruption as shown in Figure 5C.
Figure 5.
A knockdown of palladin by RNAi in Sertoli cell epithelium perturbs the TJ-permeability barrier via its effects on the organization of actin filament bundles and the distribution of proteins at the Sertoli cell-cell interface. A and B, Sertoli cells isolated from 20-day-old rat testes were cultured alone for approximately 2 days when a functional TJ-barrier was established as manifested by a relative stable TER across the cell epithelium (see panel C). These cells then transfected with palladin-specific siRNA duplexes (palladin RNAi) to knock down its expression by approximately 60% vs nontargeting control siRNA duplexes (Ctrl RNAi) (B), and the effects of palladin knockdown on the expression of different target proteins were assessed by immunoblotting (A), and those target proteins that were down-regulated were underlined by red and shown in panel B. Each bar in panel B is a mean ± SD of 3 independent experiments. The level of the corresponding marker protein including palladin in cells transfected with nontargeting control siRNA duplexes was arbitrarily set at 1 against which statistical comparison was performed. *P < .05; **P < .01. C, Changes in the Sertoli cell TJ-permeability barrier after palladin knockdown vs control were assessed by quantifying TER across the Sertoli cell epithelium at specified time points. Each data point is a mean ± SD of 4 bicameral units from a representative experiment, and this experiment was repeated 3 times using different batches of Sertoli cells and yielded similar results. *P < .05; **P < .01. D, Changes in the expression and/or localization of palladin and different marker proteins in Sertoli cells or at the cell-cell interface after palladin knockdown vs controls were assessed by fluorescence microscopy. It was noted that the fluorescence signal of palladin (green fluorescence) was considerably diminished after its knockdown, consistent with findings shown in panels A and B. A knockdown of palladin also led to changes in the organization/configuration of F-actin in which the actin filaments in Sertoli cells were truncated and mislocalized, thereby perturbing the Sertoli cell-permeability barrier, supporting the findings shown in panel C. Furthermore, Eps8, α-catenin, ZO-1, and occludin were mislocalized, failing to support the Sertoli cell TJ-permeability barrier that led to a disruption of the barrier function shown in panel C. More important, phosphorylated FAK-Tyr397, a regulator of Sertoli cell TJ-barrier function (31), was mislocalized and more concentrated to the Sertoli cell-cell interface, and its expression at this site was recently shown to disrupt the TJ-barrier function (31). Sertoli cell nuclei were visualized by DAPI (blue). Sertoli cells transfected with palladin siRNA or nontargeting control siRNA duplexes at 100 nM were also cotransfected with 1 nM siGLO (red fluorescence; Dharmacon) red transfection indicator to track successful transfection. Bar, 20 μm in the first micrograph, which applies all other micrographs in this panel.
Knockdown of palladin in the testis in vivo perturbs spermatid polarity, leading to defects in spermatid adhesion and spermiation
When palladin was knocked down in vivo by transfecting testes with the palladin-specific siRNA duplexes vs the nontargeting control siRNA duplexes, a considerably loss of palladin signals was detected in the seminiferous epithelium (Figure 6, A and B), consistent with data in vitro (Figure 5). In our studies, the effects of palladin knockdown were detected mostly in stage VII–IX tubules in which the loss of this actin cross-linking and actin bundling protein at the apical ES disrupted spermatid adhesion and transport as well as spermatid polarity, which are the functions conferred by the apical ES (2, 5, 45). For instance, in late-stage IX tubules, such the one shown in Figure 6C (left panel) manifested by the presence of elongating spermatids transformed from round spermatid (see Figure 6C, green arrowheads), in which groups of elongated spermatids were still found near the luminal edge of the tubule (Figure 6C, white arrowheads), illustrating defects in spermatid adhesion, and some spermatids were retained and/or entrapped in the epithelium because of a disruption in spermatid transport that led to defects in spermiation (see Figure 6C, yellow and green boxed areas, which were enlarged and shown below the micrograph). In stage VII tubules (Figure 6C, right panel), some elongated spermatids were found to undergo premature release from the epithelium, and some spermatids were also entrapped in the epithelium (Figure 6C, yellow and green boxed areas and enlarged and shown below the micrograph), and some of these spermatids were embedded at the base of the tubule, near the basement membrane, which were not a typical feature in stage VII tubules (see Figure 6C, red arrowheads and also blue boxed area and enlarged below), illustrating defects in spermatid adhesion (Figure 6C). Also, many spermatids lost their polarity (see Figure 6C, yellow arrowheads) as shown in this stage VII tubule (Figure 6C). These defects in spermatogenesis were summarized in Figure 6D when the data in the palladin knockdown group were normalized against the nontargeting control group. Furthermore, after the knockdown of palladin in the testis in vivo, a loss of β-catenin and ZO-1 was detected at the BTB (Figure 6E).
Figure 6.
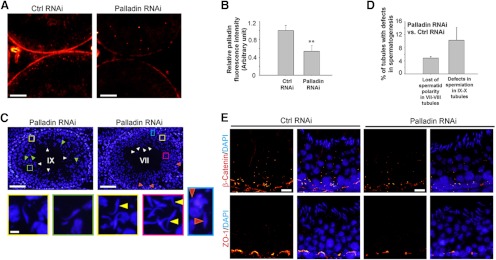
Effects of palladin knockdown on the status of spermatogenesis in the testis in vivo. Rat testes were transfected with palladin siRNA duplexes (Palladin RNAi) vs nontargeting control siRNA duplexes (Ctrl RNAi) on day 0 and day 1 (2 consecutive transfections) (see Materials and Methods) before rats were terminated on day 2 (n = 3 rats) (two additional rats were terminated on day 3, displaying similar phenotypes; thus a total of 5 rats were examined) to examine changes in the status of spermatogenesis using frozen cross-sections of testes. It was noted that the effects of the knockdown of palladin were most obvious in tubules at stage VI–VIII regarding F-actin organization and protein distribution in the seminiferous epithelium (see Figure 7), which in turn led to defects in spermiation detected in stage IX–X tubules when spermatids were retained and/or entrapped in the seminiferous epithelium. A, After the knockdown of palladin in the testis in vivo, a considerable decline in the palladin (red fluorescence) signal in the epithelium was noted. Scale bar, 50 μm. B, Intensity of the palladin signals shown in micrographs such as those shown in panel A was scanned and shown here with each bar indicating the mean ±SD of approximately 20 randomly selected VII–VIII tubules from a rat testis, and testes from a total of 5 rats were analyzed. **P < .01. C, Both tubules are representative tubules from rat testes transfected with palladin siRNA duplexes for palladin knockdown. The left panel is a stage IX tubule, illustrating defects in spermiation that occurred at stage VIII of the epithelial cycle. This is a late stage IX tubule because elongating spermatids (step 9 spermatids) began to appear in the seminiferous epithelium (see green arrowheads), yet a number of spermatozoa (and/or step 19 elongated spermatids) remained attached to the Sertoli cell in the epithelium (see white arrowheads). Furthermore, the yellow and green boxed areas illustrate spermatids entrapped in the epithelium and were magnified below the micrograph. The right panel is a stage VII tubule; however, some elongated spermatids were no longer tightly attached to the Sertoli cell in the epithelium and were depleting from the epithelium (see white arrowheads), and some spermatids were also entrapped in the epithelium (see red arrowheads; and also the blue boxed rectangle, which was magnified below the micrograph, illustrating 2 entrapped elongated spermatids, were found close to the basement membrane in this stage VII tubule). The yellow and red boxed areas were also magnified and shown below this micrograph, illustrating spermatids that lost their polarity with their heads pointing almost 180° away from the basement membrane, opposite to that of other spermatids in these insets (annotated by yellow arrowheads). Scale bar is 100 μm in the top panel and 10 μm in the enlarged image in the lower panel, which applies to remaining micrographs in this panel. D, This histogram illustrating the percentage of tubules in Palladin RNAi group vs the Ctrl RNAi group, displaying defects in spermatogenesis with each bar a mean ±SD of approximately 400 randomly selected tubules from 5 rats (∼80 tubules were randomly scored from one rat testis). E, After the knockdown of palladin, a considerable loss of β-catenin (a basal ES adaptor protein at the BTB and also at the apical ES) and ZO-1 (a TJ adaptor protein at the BTB) was detected at the basal ES at the BTB, which is likely the result of a loss of palladin at the site via its knockown that failed to confer actin filament bundles to anchor these proteins at the BTB, leading to their mislocalizatioin, moving away from the BTB. Scale bar, 20 μm, which applies to other micrographs in the control and palladin RNAi panel.
Mechanism by which a knockdown of palladin perturbs spermatid polarity, adhesion, and spermiation
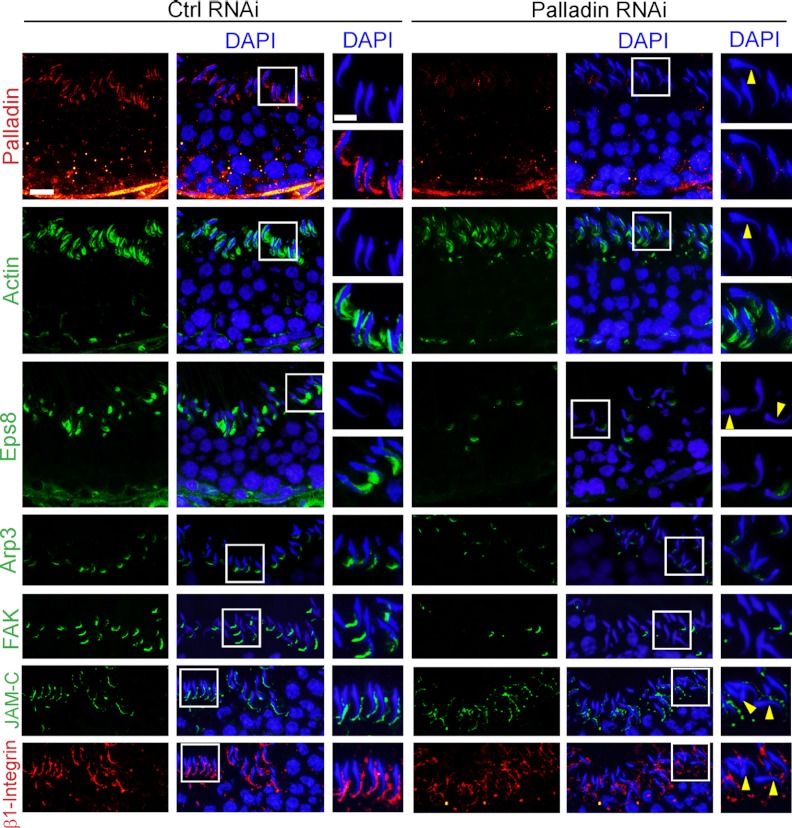
Stage VII was selected herein for analysis because the expression of 2 other actin-regulatory proteins, Eps8 and Arp3, was most abundant at this stage (13, 14). The signals of palladin in the seminiferous epithelium in stage VII tubules after its knockdown were found to be considerably diminished (Figure 7), consistent with findings shown in Figure 6, A and B. Interestingly, palladin that was intensely localized to the head of spermatids in control testes was considerably diminished in the knockdown group, and a loss of spermatid polarity was also noted, likely due to defects in F-actin organization (Figure 7). For instance, F-actin no longer highly expressed at the apical ES, surrounding the spermatid head. These changes in F-actin were likely the result of considerable loss of Eps8 and Arp3, illustrating that a knockdown of palladin also impeded the expression and/or distribution of Eps8 and Arp3 at the apical ES (Figure 7), and Eps8 and Arp3 were shown to be binding partners of palladin (see Figure 3). Furthermore, FAK, a putative apical ES-regulatory protein known to confer apical ES integrity (46, 47), was down-regulated after palladin knockdown (Figure 7), and the localization of JAM-C and β1-integrin was disrupted, with these proteins becoming truncated and/or mislocalized at the apical ES (Figure 7).
Figure 7.
Effects of palladin knockdown in the testis in vivo on the F-actin organization and spatiotemporal expression of actin regulatory proteins and apical ES proteins. After the knockdown of palladin by transfecting testes with palladin specific siRNA duplexes (Palladin RNAi) vs nontargeting control duplexes (Ctrl RNAi), the signals of palladin (red fluorescence) were considerably diminished, such as very few palladin signals were detected at the apical ES, and some step 19 spermatids were shown to have lost their polarity (yellow arrowheads) as shown in these stage VII tubules, when the expression of palladin and other proteins were examined. It is noted that at this stage, the expression of F-actin, Eps8, Arp3, FAK, JAM-C, and β1-integrin was shown to be predominant, but Eps8 and Apr3 were shown to be greatly diminished and almost undetectable in stage VIII tubules, and F-actin was also diminished considerably at the apical ES in stage VIII tubule to facilitate spermiation. After palladin knockdown, F-actin was considerably reduced at the apical ES and it no longer prominently localized to the concave side of the spermatid heads in stage VII tubules. A considerably loss of Eps8, Arp3, and FAK was also detected at the apical ES in stage VII tubules after palladin knockdown. Although the loss of JAM-C and β1-integrin at the apical ES was less obvious vs Eps8, Arp3, and FAK after palladin knockdown in the testis in vivo, these 2 proteins no longer restricted to the convex side of the elongated spermatids; instead, the fluorescence signals of JAM-C and β1-integrin were truncated at the apical ES in most spermatids, and in some spermatids, they were diffused away from the apical ES. These changes thus contributed to a destabilization of the apical ES, causing the loss of spermatid polarity, inducing defects in spermatid transport across the epithelium during spermiogenesis, leading to defects in spermiation. Scale bar, 20 μm, which applies to other micrographs; inset, 10 μm, which applies to other insets.
Discussion
Based on the established functions of palladin in different epithelia, an actin cross-linker and an actin bundling protein in addition to its scaffolding function (18, 48), it is not surprising to find palladin to be associated with the F-actin-rich ultrastructure in the testis, namely the ES. Because the extensive actin filament bundles are the hallmark ultrastructure of the ES, both at the apical ES and the basal ES at the BTB (8, 49–51), palladin is plausibly being used to confer the integrity of actin filament bundles at these sites via its intrinsic actin cross-linking and bundling activity. Palladin was also shown to be structurally interacted with Eps8 in vascular smooth muscle cells (32), and Eps8 is an actin barbed end-capping and bundling protein (15, 16, 52); thus, it is not unusual that palladin forms a working partner with Eps8 to affect actin filament dynamics in cells. However, the findings reported herein that palladin colocalizes and also forms a structural complex with Arp3 and ARPC2 is rather unexpected, but such an interaction with the Arp2/3 complex perhaps is physiologically important. Arp3 and Arp2, in conjunction with ARPC1 to ARPC5, is known to form a 7-subunit Arp2/3 complex and can induce barbed end branching and side branching on existing actin filaments following its activation by N-WASP (neuronal Wiskott-Aldrich syndrome protein), thereby creating a branched actin network, converting the bundled configuration of the actin filaments to an unbundled state, conferring plasticity to epithelial cells to facilitate cell movement (15, 16, 52). It is therefore logical to speculate that palladin is working closely with both Eps8 and the Arp2/3 complex to maintain the flexibility of the actin filament bundles at the ES during spermatogenesis to convert the actin filament bundles from their bundled to debundled/branched state and vice versa cyclically during the epithelial cycle.
At present, the precise molecular mechanism(s) by which palladin, Eps8, and Arp2/3 complex regulate actin filament dynamics at the ES remains unknown, such as the cascade of events that determines the switching of the action between Eps8/palladin that confers actin bundling and the action of the Arp2/3 complex that confers actin branching and debundling. Recent studies have suggested that the two activated forms of FAK, namely phosphorylated FAK-Tyr397 and -Tyr407, may be involved in these events by serving as molecular switches (31, 53) that turn on and off the actin filaments between their bundled and debundled/branched configuration because these two FAK isoforms were shown to have antagonistic effects on the basal ES function in which phosphorylated FAK-Tyr397 perturbs, whereas p-FAK-Tyr407 promotes, Sertoli cell TJ-barrier function (31). Other studies have shown that FAK and c-Src are a dual kinase complex crucial to regulate cell physiological function including actin dynamics (54–56). Thus, phosphorylated FAK-Tyr407 and c-Src may be working in concert with palladin and Eps8 to confer actin filaments in their bundled configuration, whereas phosphorylated FAK-Tyr397 and c-Src may be working together with palladin and the Arp2/3 complex to induce branched actin network, debundling the actin filaments. This possibility is strengthened by the findings that c-Src is a binding partner of palladin. Indeed, early findings have shown that palladin formed a functional protein complex with c-Src to regulate cytoskeletal remodeling in COS-7 and human embryonic kidney-293 cells (57), and FAK is also a binding partner of palladin in HIVS-125 cells to affect F-actin reorganization (58). In short, it is likely that c-Src and FAK activate palladin via their intrinsic phosphorylation activity, which in turn modulates the actin cross-linking and bundling activity of palladin at the ES in response to changes in the epithelium during the epithelial cycle.
Studies, by using RNAi to silence palladin in Sertoli cell epithelium with a functional TJ barrier in vitro, further support the notion that palladin is crucial to confer actin filament bundles at the ES because its knockdown was found to perturb the actin filaments in Sertoli cells. More important, palladin knockdown also perturbs the localization of Eps8 at the Sertoli cell-cell interface with a considerably loss of Eps8 at the basal ES, but more phosphorylated FAK-Tyr397 was localized to the cell-cell interface, and an earlier study demonstrated that an overexpression of phosphorylated FAK-Tyr397 (via the use of a phosphomimetic mutant FAK Y397E) was found to perturb the Sertoli cell TJ barrier function (31). These changes in distribution of Eps8 and the loss of structural integrity of F-actin at the cell-cell interface thus impeded the localization of ZO-1 and occludin at this site, leading to a disruption of the Sertoli cell TJ barrier. These findings in vitro regarding the physiological significance of palladin in F-actin organization were reproduced in studies in vivo when palladin was knocked down in rat testes. For instance, a loss of Eps8, Arp3, and FAK at the apical ES were detected in the epithelium of rat testes via their down-regulation in expression and/or changes in distribution when palladin was knocked down. These changes thus contribute to a defect in F-actin organization because the expression of F-actin was no longer robust at the apical ES in stage VII tubules after palladin knockdown. This, in turn, leads to defects in the localization of JAM-C and β1-integrin, even though their expression was not considerably different from the control rats. For instance, JAM-C and β1-integrin, both integral membrane proteins at the apical ES, surrounding the spermatid heads, were found to become either truncated or mislocalized, diffused away from the apical ES. These changes thus impeded the apical ES function, leading to a loss of spermatid polarity, perturbing spermatid transport across the epithelium during the epithelial cycle, disrupting spermiation in which elongated spermatids were found to be retained/entrapped inside the epithelium, and failed to be released at spermiation, some of which were found near the base of the tubule in stage VII–IX tubules, close to the basement membrane.
In summary, palladin is a regulator of the ES. Work is now in progress to assess how palladin is working in concert with other actin regulatory proteins, such as Eps8 (13), the Arp2/3 complex (14), drebrin E (59), and filamin A (60) and the nonreceptor protein kinases (eg, c-Src, FAK) to regulate F-actin organization at the ES and the role of testosterone, estradiol-17β, and FSH on this event.
Acknowledgments
This work was supported by grants from the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant R01 HD056034 to C.Y.C.; and Grant U54 HD029990 Project 5 to C.Y.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Arp2
- actin-related protein 2
- Arp3
- actin-related protein 3
- ARPC
- actin-related protein component
- BTB
- blood-testis barrier
- b.w.
- body weight
- Co-IP
- coimmunoprecipitation
- DAPI
- 4′,6-diamidino-2-phenylindole
- Eps8
- epidermal growth factor receptor pathway substrate 8
- ES
- ectoplasmic specialization
- FAK
- focal adhesion kinase
- JAM
- junctional adhesion molecule
- RNAi
- RNA interference
- siRNA
- small interfering RNA
- TER
- transepithelial electrical resistance
- TJ
- tight junction
- ZO-1
- zonula occludens-1.
References
- 1. Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874 [DOI] [PubMed] [Google Scholar]
- 2. Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806 [DOI] [PubMed] [Google Scholar]
- 3. O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Kretser DM, Kerr JB. 1988 The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, eds. The Physiology of Reproduction. Vol 1 New York: Raven Press; 837–932 [Google Scholar]
- 5. Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211 [DOI] [PubMed] [Google Scholar]
- 6. Russell LD. Morphological and functional evidence for Sertoli-germ cell relationships. In: Russell LD, Griswold MD, eds. The Sertoli Cell. Clearwater, FL: Cache River Press; 1993:365–390 [Google Scholar]
- 7. Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Russell LD, Goh JC, Rashed RMA, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod. 1988;39:105–118 [DOI] [PubMed] [Google Scholar]
- 10. Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–359 [DOI] [PubMed] [Google Scholar]
- 11. Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–328 [DOI] [PubMed] [Google Scholar]
- 12. Russell LD. The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat Rec. 1978;190:99–112 [DOI] [PubMed] [Google Scholar]
- 13. Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lie PPY, Chan AY, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435:553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng CY, Mruk DD. Actin binding proteins and spermatogenesis. Some unexpected findings. Spermatogenesis. 2011;1:99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng CY, Lie PPY, Wong EWP, Mruk DD, Silvestrini B. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis. 2011;1:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Otey CA, Rachlin A, Moza M, Arneman D, Carpen O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int Rev Cytol. 2005;246:31–58 [DOI] [PubMed] [Google Scholar]
- 19. Chin YR, Toker A. Akt isoform-specific signaling in breast cancer. Uncovering an anti-migratory role of palladin. Cell Adh Migr. 2011;5:211–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dixon RD, Arneman DK, Rachlin AS, et al. Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. J Biol Chem. 2008;283:6222–6231 [DOI] [PubMed] [Google Scholar]
- 21. Cheng CY, Mruk DD, Silvestrini B, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72:251–261 [DOI] [PubMed] [Google Scholar]
- 22. Mok KW, Mruk DD, Lie PP, Lui WY, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminifeorus epithelium of mammalian testes. Reproduction. 2011;141:571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl. 2012;35:86–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aravindan GR, Pineau C, Bardin CW, Cheng CY. Ability of trypsin in mimicking germ cell factors that affect Sertoli cell secretory function. J Cell Physiol. 1996;168:123–133 [DOI] [PubMed] [Google Scholar]
- 26. Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol. 2011;763:237–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203:485–492 [DOI] [PubMed] [Google Scholar]
- 28. Ahmed EA, Barten-van Rijbroek AD, Kal HB, et al. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod. 2009;80:1084–1091 [DOI] [PubMed] [Google Scholar]
- 29. Chui K, Trivedi A, Cheng CY, et al. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant. 2011;20:619–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109:12562–12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goicoechea SM, Arneman D, Disanza A, Garcia-Mata R, Scita G, Otey CA. Palladin binds to Eps8 and enhances the formation of dorsal ruffles and podosomes in vascular smooth muscle cells. J Cell Sci. 2006;119:3316–3324 [DOI] [PubMed] [Google Scholar]
- 33. Su WH, Wong EW, Mruk DD, Cheng CY. The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis. Endocrinology. 2012;153:6041–6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mok KW, Mruk DD, Silvestrini B, Cheng CY. rpS6 regulates blood-testis barrier dynamics by affecting F-actin organization and protein recruitment. Endocrinology. 2012;153:5036–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mok KW, Mruk DD, Lee WM, Cheng CY. 2013 Rictor/mTORC2 regulates blood-testis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB J 2013;27(3):1137–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab. 2013;304:E145–E159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting. An inexpensive alternative to commercially available kits. Spermatogenesis. 2011;1:121–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hess RA, Schaeffer DJ, Eroschenko VP, Keen JE. Frequency of the stages of the cycle of the seminiferous epithelium in the rat. Biol Reprod. 1990;43:517–524 [DOI] [PubMed] [Google Scholar]
- 39. Endlich N, Schordan E, Cohen CD, et al. Consortium ERcB 2009 Palladin is a dynamic actin-associated protein in podocytes. Kidney Int. 754:214–226 [DOI] [PubMed] [Google Scholar]
- 40. Xiao X, Mruk DD, Cheng FL, Cheng CY. c-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv Exp Med Biol. 2012;763:295–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frame MC. Newest findings on the oldest oncogene: how activated src does it. J Cell Sci. 2004;117:989–998 [DOI] [PubMed] [Google Scholar]
- 42. Grima J, Pineau C, Bardin CW, Cheng CY. Rat Sertoli cell clusterin, α2-macroglobulin, and testins: biosynthesis and differential regulation by germ cells. Mol Cell Endocrinol. 1992;89:127–140 [DOI] [PubMed] [Google Scholar]
- 43. Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047 [DOI] [PubMed] [Google Scholar]
- 44. Lie PP, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42:975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211 [DOI] [PubMed] [Google Scholar]
- 46. Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology. 2003;144:2141–2163 [DOI] [PubMed] [Google Scholar]
- 47. Beardsley A, Robertson DM, O'Donnell L. A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol. 2006;190:759–770 [DOI] [PubMed] [Google Scholar]
- 48. Goicoechea SM, Arneman D, Otey CA. The role of palladin in actin organization and cell motility. Eur J Cell Biol. 2008;87:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–259 [PubMed] [Google Scholar]
- 50. Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127 [DOI] [PubMed] [Google Scholar]
- 51. Lie PP, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ahmed S, Goh WI, Bu W. I-BAR domains, IRSp53 and filopodium formation. Semin Cell Dev Biol. 2010;21:350–356 [DOI] [PubMed] [Google Scholar]
- 53. Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Commun. 2012;3:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bolos V, Gasent JM, Lopez-Tarruella S, Grande E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther. 2010;3:83–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523 [DOI] [PubMed] [Google Scholar]
- 56. Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68 [DOI] [PubMed] [Google Scholar]
- 57. Ronty M, Taivainen A, Heiska L, et al. Palladin interacts with SH3 domain of SPIN90 and Src and is required for Src-induced cytoskeletal remodeling. Exp Cell Res. 2007;313:2757–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jin L, Kern MJ, Otey CA, Wamhoff BR, Somlyo AV. Angiotensin II, focal adhesion kinase, and PRX1 enhance smooth muscle expression of lipoma preferred partner and its newly identified binding partner palladin to promote cell migration. Circ Res. 2007;100:817–825 [DOI] [PubMed] [Google Scholar]
- 59. Li MWW, Xiao X, Mruk DD, et al. Actin binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011;1:123–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Su WH, Mruk DD, Lie PPY, Lui WY, Cheng CY. Filamin A is a regulator of blood-testis barrier assembly during postnatal development in the rat testis. Endocrinology. 2012;153:5023–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]