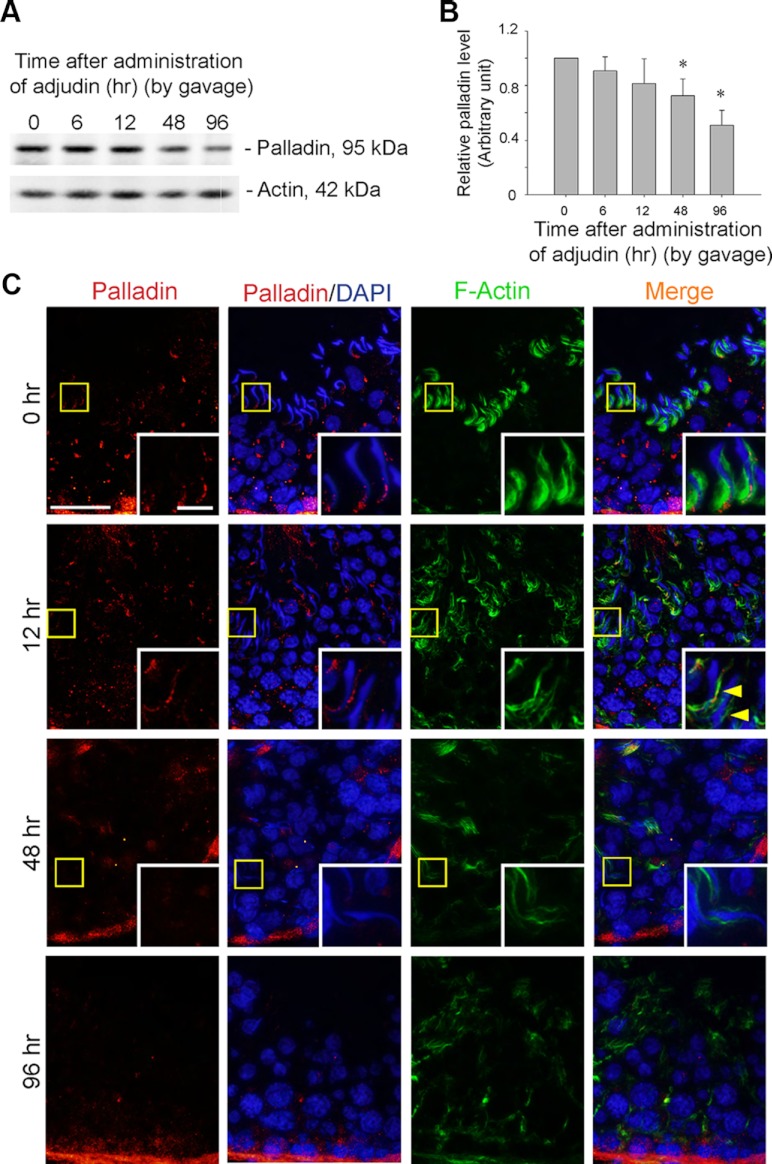
Figure 4.
Adjudin-induced spermatid loss from the seminiferous epithelium is associated with a down-regulation in the expression and mislocalization of palladin at the apical ES. A and B, To further examine the functional significance of palladin at the apical ES, an in vivo model of anchoring junction disruption, in particular apical ES, was used to examine changes in the steady-state level of palladin by immunoblotting. Adjudin-induced spermatid loss from the seminiferous epithelium occurred by as early as 12 hours when spermatids were found in tubule lumen; by 48 hours, few elongating/elongated spermatids were detected in the seminiferous epithelium; and by 96 hours, virtually no elongating/elongated spermatids were found in any tubules (see panel C). A time-dependent and significant down-regulation on the expression of adjudin was detected by immunoblotting, wherein actin served as a protein loading control (A). The data shown in A were summarized in B with each bar representing a mean ± sd of 3 rats, and the level of palladin at time 0 normalized against actin was arbitrarily set at 1 against which statistical comparison was performed. *P < .05. C, In stage VII tubules at 0 hour, such as the one shown herein, palladin was detected at the apical ES, at the tip and the concave side of the spermatid head, colocalized with F-actin. However, by 12 hours, palladin staining was considerably diminished and mislocalized, no longer tightly associated with the apical ES, and for palladin detected at the ES, it was no longer highly expressed at the concave side of spermatid heads; instead, it was shifted to the convex side of the spermatid head (see yellow arrowheads), and these changes were associated with their premature release of elongating/elongated spermatids from the epithelium. Boxed areas in micrographs were magnified and shown in insets to better illustrate the localization of palladin and F-actin at the apical ES. Bar, 50 μm, and bar in inset, 10 μm, which apply to all other micrographs and insets.