Abstract
Testosterone has been shown to suppress the acute stress-induced activation of the hypothalamic-pituitary-adrenal axis; however, the mechanisms underlying this response remain unclear. The hypothalamic-pituitary-adrenal axis is regulated by a neuroendocrine subpopulation of medial parvocellular neurons in the paraventricular nucleus of the hypothalamus (PVN). These neurons are devoid of androgen receptors (ARs). Therefore, a possibility is that the PVN target neurons respond to a metabolite in the testosterone catabolic pathway via an AR-independent mechanism. The dihydrotestosterone metabolite, 5α-androstane-3β,17β-diol (3β-diol), binds and activates estrogen receptor-β (ER-β), the predominant ER in the PVN. In the PVN, ER-β is coexpressed with oxytocin (OT). Therefore, we tested the hypothesis that 3β-diol regulates OT expression through ER-β activation. Treatment of ovariectomized rats with estradiol benzoate or 3β-diol for 4 days increased OT mRNA selectively in the midcaudal, but not rostral PVN compared with vehicle-treated controls. 3β-Diol treatment also increased OT mRNA in the hypothalamic N38 cell line in vitro. The functional interactions between 3β-diol and ER-β with the human OT promoter were examined using an OT promoter-luciferase reporter construct (OT-luc). In a dose-dependent manner, 3β-diol treatment increased OT-luc activity when cells were cotransfected with ER-β, but not ER-α. The 3β-diol–induced OT-luc activity was reduced by deletion of the promoter region containing the composite hormone response element (cHRE). Point mutations of the cHRE also prevented OT-luc activation by 3β-diol. These results indicate that 3β-diol induces OT promoter activity via ER-β–cHRE interactions.
The hypothalamic-pituitary-adrenal (HPA) axis is a neuroendocrine system that maintains homeostasis through a cascade of hormonal signals originating in the paraventricular nucleus of the hypothalamus (PVN). In rodents, hormonal stress responsivity of the HPA axis is higher in females than in males. This may be partly due to the divergent actions of testosterone and estradiol (E2). Testosterone, and its nonaromatizable metabolite dihydrotestosterone (DHT), inhibit whereas E2 enhances stress responsiveness (1–3). Because neuroendocrine neurons of the PVN are devoid of androgen receptors (ARs) (4), actions of DHT on the HPA axis have been proposed to involve an indirect, multisynaptic pathway (5, 6).
An alternative hypothesis suggests a more direct route involving a DHT metabolite (3, 7). Dihydrotestosterone can be converted to 5α-androstane-3β,17β-diol (3β-diol) in multiple tissues (8–11). Given that cells in the PVN express the enzymes for androgen conversion into 3β-diol (7), it is possible that DHT is metabolized into 3β-diol in the PVN. Although 3β-diol has only weak AR binding activity, it can activate estrogen receptor (ER)-β (12–15).
ER-β is expressed by a number of neuronal phenotypes in the PVN. Among these, oxytocin (OT) neurons comprise the most significant population (16–18), raising the possibility of a direct action of 3β-diol on the OT gene via ER-β. Indeed, evidence points to species-dependent effects of E2 on OT. Studies using an ER-β–null mouse model suggest that E2 increases OT mRNA via ER-β (19, 20). By contrast, in the rat, various effects of E2 on OT mRNA have been reported (21–25), including stimulation and inhibition. Nonetheless, ER-β appears to play a role in the hormone regulation of the Ot gene in the PVN.
The finding that in the presence of 3β-diol ER-β coimmunoprecipitates with chromatin in the region of the proximal Ot promoter suggests that ER-β regulation is direct (26). However, the Ot proximal promoter does not contain a classical palindromic estrogen response element. Thus, the mechanism of estrogen-induced OT upregulation (27, 28) appears to not involve specific DNA binding by ER homodimers in the proximal promoter region. Instead, expression is regulated by binding at a composite hormone response element (cHRE), one that is bound by a number of other nuclear receptors (29). The physiological consequences of ER-β interactions with OT neurons have recently come to light. Notably, OT, 3β-diol, and the ER-β agonist diarylpropionitrile (DPN) are anxiolytic (32–34). Furthermore, intracerebroventricular administration of OT (34) or stereotaxic placement of pellets containing 3β-diol or DPN immediately above the PVN (7) inhibits neuroendocrine stress reactivity in rodents. ER, but not AR, antagonists prevent the inhibiting actions of 3β-diol on neuroendocrine response to a stressor (7). These studies suggest the potential for a common pathway involving 3β-diol, ER-β, and OT in regulation of HPA stress reactivity and behavior.
In the present study, we investigated the regulation of the Ot gene via 3β-diol using in vivo and in vitro approaches. Specifically, we examined the in vivo effects of 3β-diol on OT mRNA levels in the rat PVN and the functional regulation of the human Ot promoter by 3β-diol through ER-α or -β using in vitro approaches that focused on the activation of the cHRE.
Materials and Methods
In vivo experiments
Animals
Fifty-six adult female Sprague-Dawley rats, 50 to 60 days of age, were obtained from Charles Rivers Laboratories (Wilmington, Massachusetts). Rats were double housed and maintained on a 12-hour light, 12-hour dark light schedule (lights on at 7:00 am) with ad libitum access to food and water. Animals were maintained in a temperature- and humidity-controlled environment at the Arizona State University Department of Animal Care and Technologies. All animal protocols were previously approved by the Institutional Animal Care and Use Committee at Arizona State University under subcontract with the University of Arizona and were carried out in accordance with the guidelines of the National Institutes of Health and the Association for Assessment and Accreditation of Laboratory Animal Care International.
Two separate groups of animals were used to examine the effects of hormone treatment on OT mRNA levels in the hypothalamus in vivo. An initial group of animals (n = 32) was used to determine the mRNA levels in the PVN by microdissection followed by quantitative RT-PCR (qRT-PCR) (experiment 1), whereas the second group of animals (n = 24) was used to examine the mRNA levels using in situ hybridization histochemistry (ISHH) (experiment 2).
Ovariectomy and hormone treatments
Animals were bilaterally ovariectomized under isoflurane anesthesia.
For experiment 1, beginning 4 days after ovariectomy, animals were injected sc with vehicle (safflower oil, n = 8), E2 benzoate (10 μg/kg body weight, n = 8; Steraloids, Newport, Rhode Island), or 3β-diol dipropionate (low vs high doses, 5 and 15 mg/kg, respectively; n = 8 per dose; Steraloids) daily for 4 days.
For experiment 2, beginning 4 days after ovariectomy, animals were injected sc daily for 4 days with vehicle (safflower oil, n = 8), E2 benzoate (10 μg/kg, n = 8), or 3β-diol (5 mg/kg, n = 8). The dose of 3β-diol was chosen on the basis of a previous study showing regulation of another related gene, arginine vasopressin, in the rat (35).
In both studies, hormones were administered in a volume of 2 mL/kg per injection at 1000 hours. One hour after injections on the fourth day, animals were killed under isoflurane anesthesia and brains were removed, flash frozen in 2-methylbutane (−30°C), and stored at −80°C until processing. This timeframe of 1 hour survival period was chosen based on previous study showing hormone regulation of the OT mRNA (26).
Quantitative RT-PCR
For experiment 1, a series of 150-μm-thick coronal tissue sections through PVN (bregma −1.3 to −2.2 mm) and supraoptic nucleus (SON) (bregma −0.8 to −1.3 mm) were collected following a previously described protocol (36).
For the in vitro time course studies using N38 cells, cells were plated in 6-well plates at 100 000 cells per well. Cells were then treated with vehicle, 100nM E2, 100nM DPN, or 100nM 3β-diol for 1, 4, or 24 hours. Forty-eight hours after plating, cells were trypsinized at 70%–80% confluency, rinsed with sterile PBS, and collected by centrifuging for 5 minutes.
RNA was purified according to previously published protocol (37). RNA quantity and purity were measured using an EPOCH spectrophotometer (BioTek, Winooski, Vermont), and samples were reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, California) according to the manufacturer's protocol. Gene expression was measured with a LightCycler 480 (Roche Diagnostics, Indianapolis, Indiana) with 1 μl of cDNA, using SYBR green mix (Roche Diagnostics) according to the manufacturer's protocol.
For experiment 1, the following primers were designed to amplify specific regions within the coding sequence of the rat Ot: forward primer, TGGATCTCGGACTGAACACCAA, and reverse primer, TTCTCCTCCTGGCAGCGCA. For the in vitro study, primers designed to target the region of the mouse oxt described in a previous study (26) were used. Absolute target mRNA was calculated as described previously (36). Values were quantified spectrophotometrically using the EPOCH spectrophotometer.
In situ hybridization
A series of coronal 16-μm sections were taken between bregma −0.8 to −2.2 mm to include the PVN and SON (38). Sections were prepared and processed following an established protocol (32, 35) with some modifications. Briefly, an oligonucleotide probe was used to detect OT mRNA and was radiolabeled with [35S]dATP (5′-AAGCAGGCAGCAAGCGAGACTGGGGCAGGCCATGGCGTTGGTGTTCAG-3′) using terminal deoxynucleotide transferase (New England Biolabs, Ipswich, Massachusetts). The radiolabeled probe was diluted to a concentration of 5 × 106 counts/min/ml. This diluted concentration was empirically determined, because the OT signal obtained from a routinely used concentration is very intense and the signal can be too saturated to easily detect treatment differences. Sections were next incubated in hybridization solution overnight at 37°C, and hybridization was examined by opposing slides to 35S-sensitive Biomax MR film (Kodak, Rochester, New York) for 72 hours. The need for a 72-hour exposure was empirically determined for a probe of this specific activity.
Image analysis and quantification of mRNA expression
Image analysis was performed using ImageJ software (version 1.31, Windows 7). For each section, the approximate shape of the PVN was outlined around the region with OT signals. The average pixel density of the left and right side of the PVN was measured and divided by the area of interest to achieve a measure of integrated density units. After subtraction of background hybridization (determined in an adjacent area devoid of labeling), 2 values averaged across rostral PVN (bregma −1.3 to −1.8 mm) and across midcaudal PVN (bregma −1.8 to −2.2 mm) and 4 values averaged across total PVN (bregma −1.3 to −2.2 mm) and across SON (bregma −0.8 to −1.3 mm) were used for statistical analysis.
In vitro experiments
Cell culture
The male mouse-derived hypothalamic cell line N-38 (CELLutions Biosystems, Inc, Toronto, Canada) or the mouse-derived hippocampal cell line HT-22 (generously provided by Dr. Dave Schubert, Salk Institute, San Diego, California) were used for reporter gene assays. Both cell lines were maintained as previously described (39).
Reporter constructs and expression vectors
An 1180-bp fragment of the human OT reporter construct (full-length OT) cloned into the pSGG vector containing a luciferase reporter element was purchased from Switchgear Genomics (Menlo Park, California). For some studies, constructs were made with successive deletions of the 5′-end by restriction enzyme digestion upstream from the transcription start site (TSS) at positions −602, −406, −224, and −50, yielding constructs with OT fragment spanning nucleotides +260 to −602 (−602 OT), +260 to −406 (−406 OT), +260 to −224 (−224 OT), and +260 to −50 (−50 OT) from TSS, respectively (see Figure 5A). The −602 OT and −406 OT were blunted, and all constructs were ligated by T4 ligase, transformed, and amplified. The deletion constructs were confirmed by DNA sequencing (Operon, Huntsville, Alabama). The pRL-CMV renilla luciferase reporter construct (Promega, Madison, Wisconsin) was used as an internal control for plasmid transfection efficiency. A full-length human ER-β cDNA, cloned into a pCMV6-XL4 vector, was purchased from Origene (Rockville, Maryland). The rat ER-β and the human ER-α plasmids used in this study were described previously (39).
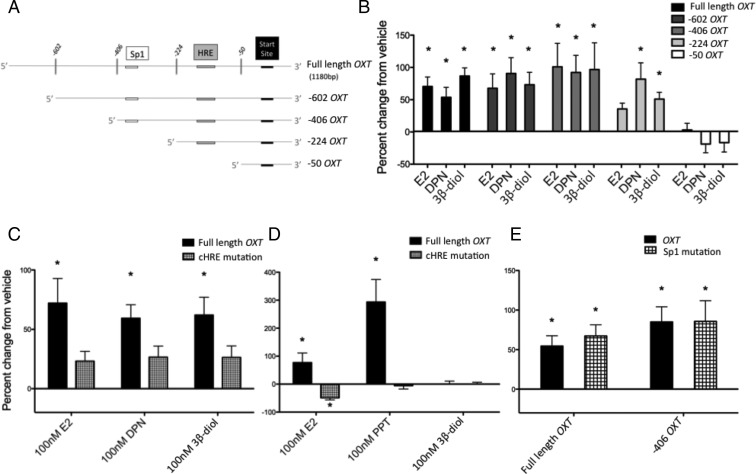
Figure 5.
The cHRE plays an important role in the hormone regulation of the OT promoter. A, Schematic diagram of the 5′-end deletion constructs. Full-length (1180 base pairs) OT-luc construct was progressively deleted from the 5′-end of the promoter using single- or double-enzyme restriction digests. Note that the cHRE of the OT promoter is not present in the −50 OT construct. B, N38 cells were cotransfected with the OT constructs and ER-β 24 hours before hormone treatment (vehicle or 100nM E2, DPN, or 3β-diol) and luciferase activity was measured 24 hours later. C–E, N38 cells were cotransfected with ER-β (C and E) or ER-α (D) and full-length OT or OT-luc construct with site-directed mutagenesis of the cHRE (C and D) or the Sp-1 (E) sites, and 24 hours later, cells were treated with vehicle or 100nM E2, DPN, or 3β-diol. Cells were lysed and assayed for dual luciferase activity 24 hours after hormone treatment. Values are expressed as mean percent change of OT-luc activity from vehicle treatment +/- SEM. *, Significantly different from vehicle group, P < .05.
Transient transfections and reporter assay
Cells were plated at a density of 30 000 cells per well in 24-well plates to achieve a final confluency of 70%–80%. All treatment groups were transfected in triplicate, and each transfection assay was repeated a minimum of 3 times. Sixteen hours after cell plating, transfections were carried out using Fugene6 or XtremeGENE (Roche Molecular Biomedical, Indianapolis, Indianapolis) according to the manufacturer's instructions. Cells were incubated with transfection reagent and plasmid vectors for 24 hours, followed by hormone treatments in DMEM containing 10% dextran-charcoal–stripped fetal bovine serum (Hyclone Laboratories, Logan, Utah) to ensure steroid-free culture conditions. Twenty-four hours after hormone treatments, luciferase activity was measured using the dual-luciferase assay according to the manufacturer's protocol (Promega). Relative light units were measured using a 20/20 TD luminometer (Turner Designs, Sunnyvale, California).
Hormone treatments
The following compounds were diluted in 100% ethanol and used at a final concentration of 10−13M to 10−7M in 0.001% ethanol (vehicle control): E2 benzoate and 3β-diol (Steraloids), ER-α agonist propylpyrazoletriol (PPT) (Tocris, Ellisville, Missouri), and the ER-β agonist S-DPN, the more potent form of the racemic DPN that was synthesized and separated previously (33, 40).
Site-directed mutagenesis
The putative cHRE located at −164 from the TSS of the human OT promoter fragment was mutated using the QuikChange II XL kit according to the manufacturer's instructions (Stratagene, La Jolla, California). For the cHRE mutation, the antisense primer sequence 5′-GACCATTAGCCAACGCAGTGCCCACGTCGCCGGCCCAGGCCCTGC-3′ was designed to mutate 6 sites (shown in bold) of the cHRE (GCCACTGGAACTGGG → GTCACGGGTGCAGCG). For the specificity protein 1 (Sp1) site mutation, located at −387 from TSS of the human OXT promoter fragment, the primer sequence 5′-AGGCTCTGGCAGGATACTACCACCCTCTTGGCCC-3′ was designed to mutate 3 sites (shown in bold) of the Sp1 (TGGGCGGATC → TGGTAGTATC). The presence of the mutation was confirmed by DNA sequencing (Operon).
Statistics
For the in vivo experiments, data were analyzed by one-way ANOVA. If significant, post hoc analysis was performed using Fisher's protected least significant difference test. For the in vitro experiments, data were analyzed by one-way ANOVA. For experiments examining the cHRE and Sp1 mutation, 2-way ANOVA was used. If ANOVA was significant, post hoc analysis using the Bonferroni's multiple-comparison test was performed. Differences were considered significant when P < .05. All transfection data are represented as percent change against vehicle or percent change compared with vehicle-treated, empty vector controls.
Results
E2 benzoate and 3β-diol treatment increases OT mRNA in the PVN of ovariectomized rats in vivo
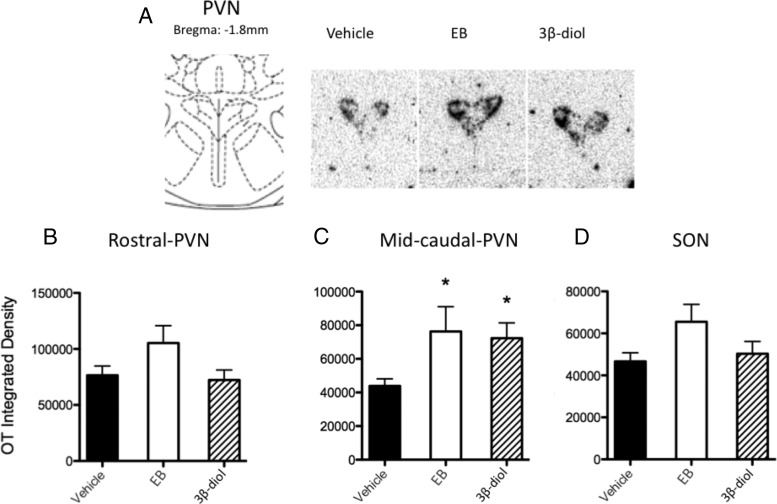
In experiment 1, analysis of qRT-PCR showed no effects of treatment on OT mRNA in microdissected PVN (data not shown). In experiment 2, we performed a more detailed analysis of the rostrocaudal distribution of the OT mRNA in the PVN using ISHH (Figure 1A). Results showed significant increases after both E2 benzoate and 3β-diol treatment, selectively in the midcaudal PVN but not in total or rostral PVN regions. One-way ANOVA of data from the midcaudal PVN showed a significant main effect of hormone treatment (F2,19 = 3.632, P = .0462), and post hoc test revealed significant differences between vehicle and E2 benzoate (P = .0346) and 3β-diol (P = .0132) (Figure 1C). In contrast, there were no significant differences in OT mRNA signal in rostral PVN (Figure 1B) or SON (Figure 1D) between any treatment groups.
Figure 1.
E2 benzoate and 3β-diol increase OT mRNA expression in the rat midcaudal PVN. Ovariectomized rats were treated with vehicle (safflower oil), E2 benzoate (EB), or 3β-diol daily for 4 days before brains were processed for OT mRNA analysis using qRT-PCR or ISHH. A, Left panel shows the rat atlas (38) of the region of PVN that corresponds to the representative in situ hybridization histochemistry signal of the OT mRNA on the right. B–D, OT mRNA integrated density from ISHH analysis of the rostral PVN (B), midcaudal PVN (C), and SON (D). The values are expressed as mean arbitrary integrated density ± SEM. *, Significantly different from vehicle group, P < .05.
Kinetics of estrogen treatment in regulating OT mRNA in the N38 cells
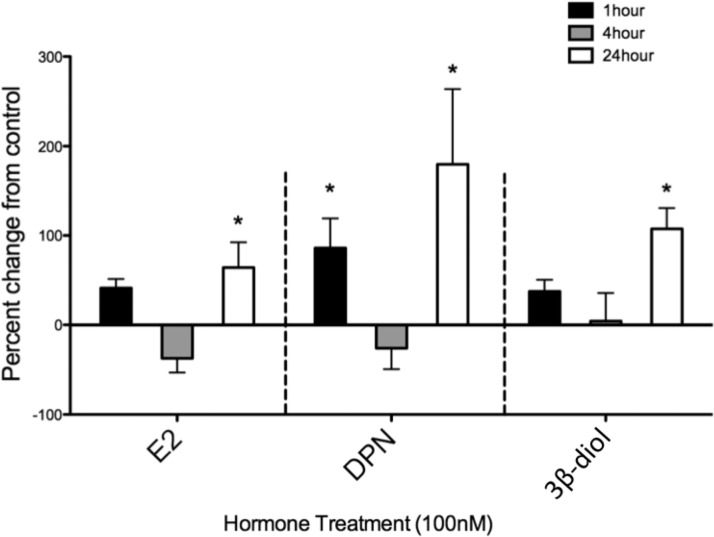
The N38 cells were treated with 100nM E2, DPN, or 3β-diol for 1, 4, or 24 hours (Figure 2). One-way ANOVA across time points in the E2 group showed significant main effect (F3,28 = 5.329; P = .0049) and a post hoc test revealed significant differences between vehicle and E2 at 24 hours. In the DPN group, a significant main effect was found (F3,23 = 8.17; P = .0004), and a post hoc test revealed significant differences between vehicle and DPN at 1 hour and at 24 hours. In the 3β-diol group, a significant main effect was found (F3,31 = 8.375; P = .0003), and a post hoc test showed significant differences between vehicle and 3β-diol at 24 hours. For the rest of the studies examining hormone effects on OT-luc activity, a 24-hour treatment was used.
Figure 2.
Hormone-induced OT mRNA induction in the N38 cell line reveals a complex time course. N38 cells were plated in 6-well plates at a density of 100 000 per well. After plating, cells were treated with vehicle, 100nM E2, 100nM DPN, or 100nM 3β-diol for 1, 4, or 24 hours. All cells were then harvested by trypsinization and processed for OT mRNA using qRT-PCR, and n = 4 to 10 per group. Values are expressed as mean percent change of OT mRNA from vehicle ± SEM. *, Significantly different from vehicle group, P < .05.
Ligand-independent activities of ERs on OT-luc
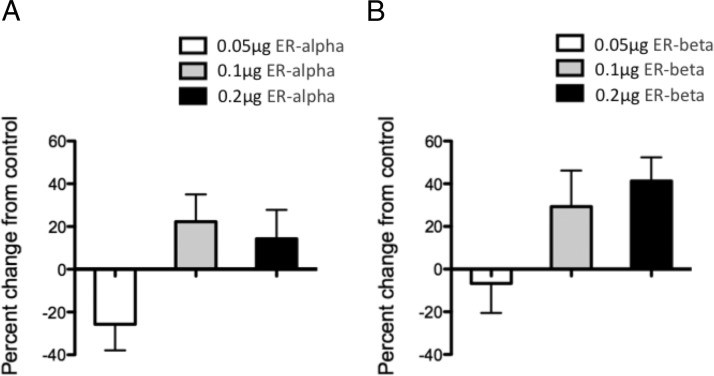
To determine whether ER-α and ER-β have ligand-independent activity on the OT promoter, we used HT-22 cells, which reportedly lack endogenous ER-α/β expression (39, 41–43). The HT-22 cells were cotransfected with OT-luc and 0 (control), 0.05, 0.1, or 0.2 μg of ER-α or ER-β and treated with vehicle. Despite a significant main effect of ER-α concentration after analysis by 1-way ANOVA (F3,12 = 3.596; P = .0463), a post hoc test showed no significant differences between vehicle- and hormone-treated groups (Figure 3A). Similarly, 1-way ANOVA showed a significant main effect of ER-β concentration (F3,12 = 3.498, P = .0497); however, a post hoc test showed no significant differences between vehicle- and hormone-treated groups (Figure 3B).
Figure 3.
ER-α (A) and ER-β (B) constitutively activate OT-luc. HT22 cells were cotransfected with OT-luc and varying concentrations of ER-α or ER-β. Twenty-four hours later, cells were treated with vehicle and harvested 24 hours later. Values are expressed as mean percent change of OT-luc activity from negative control (cells that were cotransfected with OT-luc and empty vectors for ER-α/β) ± SEM. *, Significantly different from vehicle group, P < .05.
Dose response of hormone-induced OT-luc activity via ER-β
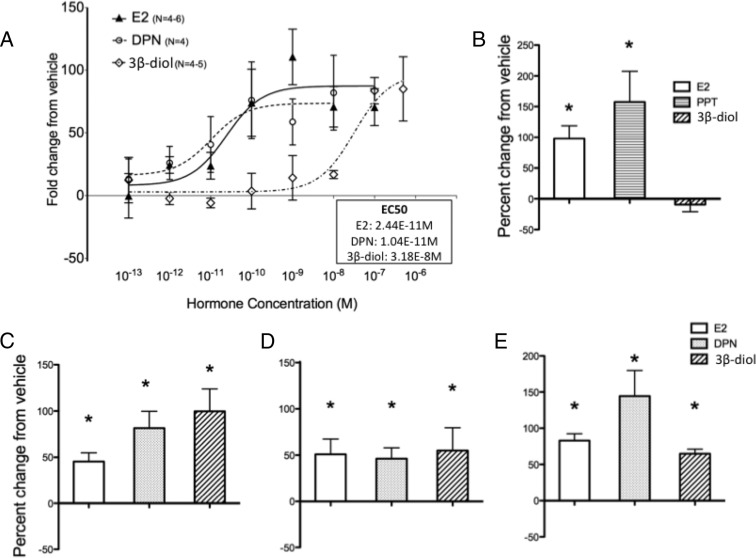
To assess whether 3β-diol activates OT-luc activity via ER-β, we cotransfected OT-luc and a rat ER-β expression vector into the N38 cells, which express endogenous OT mRNA. A dose response of the hormone treatments ranging from 1−13M to 1−6M revealed that the half-maximal effective concentrations (EC50) of E2-, DPN-, and 3β-diol–induced OT-luc activation were 2.44 × 10−11M, 1.04 × 10−11M, and 3.18 × 10−8M, respectively (Figure 4A).
Figure 4.
3β-Diol increases OT-luc activity via ER-β but not ER-α. A, Dose-response curves of OT-luc activity treated for 24 hours with vehicle or varying concentrations of E2, DPN, or 3β-diol. N38 cells were cotransfected with OT-luc and ER-β, and 24 hours later, cells were lysed and assayed for luciferase activity. B and C, N38 cells were cotransfected with OT-luc and ER-α (B) or ER-β (C), 24 hours before hormone treatment (vehicle or 100nM E2, DPN, or 3β-diol), and 24 hours later, cells were lysed and assayed for dual-luciferase activity. D, Identical experiment as C performed with human ER-β. E, Identical experiment as C performed in HT22 cells, a cell line derived from mouse embryonic hippocampal cells. Values are expressed as mean percent change of OT-luc activity from vehicle treatment ± SEM. *, Significantly different from vehicle group, P < .05.
3β-Diol increases OT-luc activity via ER-β but not ER-α
Using higher doses of hormone treatment (100nM), we performed a series of experiments to explore hormone-induced regulation of OT-luc via ER-α and ER-β. First, we examined the effects of overexpression of ER-α vs ER-β in the N38 cells. When cells were cotransfected with ER-α, a significant activation of OT-luc after E2 and PPT treatment was found (Figure 4B); however, there was no difference between vehicle and 3β-diol treatment on OT-luc activity. One-way ANOVA showed a significant main effect of hormone treatment (F2,11 = 6.973, P = .0127), and a post hoc test revealed significant differences between vehicle and E2 and PPT. In contrast, E2, DPN, and 3β-diol all increased OT-luc when cells were cotransfected with OT-luc and ER-β (Figure 4C). One-way ANOVA showed a significant main effect of treatment (F3,43 = 10.11, P < .0001), and a post hoc test revealed significant differences between vehicle and E2, DPN, and 3β-diol.
The experiments described thus far were conducted using a human OT-luc construct with cotransfected rodent ER-β or human ER-α; thus, to obviate issues concerning species differences, we also examined the responses to human ER-β. Similar effects on OT-luc activation were found when the human ER-β was transfected in the N38 cell line (Figure 4D); albeit with a different pattern, the effect of 3β-diol and DPN were not greater than E2. One-way ANOVA showed a significant main effect of treatment (F3,14 = 4.160, P = .0249), and a post hoc test revealed significant differences between vehicle and E2, DPN, and 3β-diol.
We also found significant differences when HT-22 cells were cotransfected with OT-luc and ER-β (Figure 4E). One-way ANOVA showed a significant main effect of treatment (F3,22 = 10.12, P = .0042), and a post hoc test showed significant differences between vehicle and E2, DPN, and 3β-diol.
The OT promoter region containing the cHRE is important for ligand-bound ER-β–induced activation of the OT-luc
To detail the potential regulatory sites of 3β-diol/ER-β interaction, a series of progressive 5′-end deletions of the OT promoter fragment was analyzed (Figure 5, A and B). As expected, 1-way ANOVA in the full-length OT group showed significant main effect (F4,23 = 17.4; P < .0001), and a post hoc test revealed significant differences between vehicle and E2, DPN, and 3β-diol. Similar differences between vehicle and E2, DPN, and 3β-diol were found in the −602 OT (F3,18 = 6.780; P = .003, 1-way ANOVA) and −406 OT (F3,18 = 5.678; P = .0064, 1-way ANOVA). In contrast, a post hoc test revealed significant differences only between vehicle and DPN and 3β-diol, but not E2, in the −224 OT (F3,17 = 7.364; P = .0023, 1-way ANOVA). Finally, the hormone effects were completely abolished in the −50 OT, because 1-way ANOVA showed no significant effects.
Because the −50 OT construct was unresponsive to hormone treatment, we mutated the cHRE to analyze the contributions of cHRE nucleotides to the ligand responses (Figure 5C). Two-way ANOVA showed significant main effect of hormone (F3,40 = 7.193, P = .0006), and mutation (F1,40 = 12.23, P = .0012), and a post hoc test revealed significant differences in full-length OT activity in E2, DPN, and 3β-diol compared with vehicle. No significant differences were found in OT activity between any of the ligands compared with vehicle when the cHRE was mutated. Moreover, mutation of the cHRE also blocked the E2- and PPT-induced OT-luc activity when ER-α was overexpressed (Figure 5D). Two-way ANOVA showed significant main effects of interaction (F3,40 = 12.77, P < .0001), hormone (F3,40 = 12.74, P < .0001), and mutation (F1,40 = 20.9, P < .0001). Post hoc analysis showed significant differences in the full-length OT activity in E2 and PPT but not in 3β-diol compared with vehicle. There was also a significant decrease in the E2-induced OT activity when cHRE was mutated. No significant differences were found in OT activity between vehicle and PPT or 3β-diol when cHRE was mutated.
Mutagenesis of a putative Sp1 regulatory element in the OT promoter
Because E2-induced luciferase activity via ER-β was selectively blocked in the −224 OT construct, which maintains the cHRE, we further analyzed the promoter region within and upstream of this construct between −406 and −224. Investigation of the sequence revealed that there is a putative Sp1 binding site (Figure 5A). Given previous studies showing that Sp1 can mediate ER actions in a target gene promoter, including ovine Ot (44, 45), we performed site-directed mutagenesis of this site. Mutation of the Sp1 site in the full-length OT showed that E2-induced luciferase activity remained unchanged in both full-length OT and the −406 OT (Figure 5E). E2-induced luciferase activity also remained unchanged when the Sp1 site in the −406 OT construct was mutated, suggesting that a regulatory site besides Sp1, but within the region of the promoter between TSS −406 and −224 is responsible for E2-induced OT activity.
Discussion
ERs, similar to other nuclear receptors, can be activated by a variety of ligands, albeit not necessarily to the same extent as their cognate ligand. For example, some androgens can also interact with ERs and may act preferentially through ER-β (12–15, 32, 39, 46, 47). In the present studies, we have determined that 3β-diol interacts with ER-β to activate the OT gene, thereby providing additional evidence that androgen metabolites may regulate a physiological pathway by activating an ER (12–15).
These results raise the possibility of a pathway regulating HPA axis reactivity that involves the local metabolism of DHT to 3β-diol and subsequent binding to ER-β. This idea is consistent with observations showing that ER-β is found in PVN neurons that coexpress OT, that OT can directly modulate the HPA axis function, and that 3β-diol induces recruitment of ER-β to the OT promoter (26, 34, 48–50).
In our initial studies, we compared the effects of 3β-diol to E2 on the rat OT mRNA in vivo. Amico and colleagues (21, 22, 24, 51) had shown, through a series of elegant studies, that a strict regimen of hormone treatment is required for in vivo E2 induction of rat OT mRNA; a chronic exposure to both E2 and progesterone followed by progesterone withdrawal, was most effective. Chronic exposure to E2 alone was ineffective (24). In contrast, Shughrue and colleagues (23) reported that 6, 12, and 24, but not 48, hours of E2 exposure (without progesterone) to ovariectomized rats decreased OT mRNA in the PVN. In comparison, we found that 4 days of E2 or 3β-diol increased OT mRNA selectively in midcaudal PVN. To our knowledge, this is the first report of 3β-diol regulation of OT mRNA in the rat PVN. The difference between the result of the current studies and those of the previous studies may be due to the duration of the hormone treatment. The duration of treatment in the current studies (4 days) was in between those of Shughrue and colleagues (6–48 hours after a single injection) and Amico and colleagues (2+ weeks). Therefore, these studies suggest that an initial decrease may occur with acute treatment, followed by an increase in PVN OT mRNA after longer treatment. The present study also highlights the importance of subregion-specific analysis, because we and others (27, 52) have shown that E2-induced upregulation of OT mRNA was not detected when the whole PVN was analyzed using qRT-PCR and ISHH. A detailed analysis of the rostrocaudal subdivisions using ISHH was required to reveal treatment effects.
The effect of E2 benzoate on OT mRNA was found in regions of the PVN that include both the parvocellular and the magnocellular populations. The parvocellular neurons are generally distributed in rostral-mid PVN, whereas magnocellular populations are found in midcaudal regions, as are preautonomic neurons of the PVN. The absence of a hormone effect on OT mRNA in the rostral PVN reduces the probability that the E2 benzoate effect is in the hypophysiotrophic neurons. However, the somatotopical organization of these neurons is not absolute, and further studies are required to determine the potential selective effect of E2 benzoate on OT in these distinct populations of PVN neurons.
Experiments designed to investigate the kinetics of estrogen regulation of OT mRNA in vitro also revealed a complex interaction between ER and the OT promoter. Sharma and colleagues (26) reported a complex response to hormones with significant increases noted in OT mRNA in N38 cells at 30 minutes and 1 hour after E2 and 3β-diol treatments, followed by a return to baseline by 2 and 4 hours. We found similar initial hormone-induced increases of OT mRNA after 1 hour of treatment with DPN, followed by a return nearly to baseline after 4 hours. There was a trend to increase after 1 hour of E2 or 3β-diol treatments, which did not reach statistical significance in these studies.
The present study also examined a longer time point after hormone treatment. When sampled after 24 hours of treatment, all 3 compounds increased OT mRNA levels. The similarity between E2, DPN, and 3β-diol effects on OT mRNA levels suggests an ER-β–mediated effect. Indeed, in the presence of 3β-diol, there is a physical association between ER-β and the region of the Ot promoter and a similar time course of association between the same region and coregulator proteins (26). Thus, a functional complex involving ER-β and coregulators appear to contribute to Ot promoter activity in the presence of 3β-diol.
Regulation of the Ot promoter via E2 and the ERs has been extensively studied (27, 28, 53–55). The Ot promoter lacks a consensus estrogen response element, and transcriptional activation of the Ot gene by estrogen instead involves a complex sequence motif, cHRE (28, 29). Of particular interest, the cHRE in the Ot promoter is responsive to E2 in vitro using cell lines transfected with Ot promoter-reporter constructs (27–29, 55–57).
Previous studies of the Ot promoter cHRE reported results inconsistent with those from the present study. A mutation or deletion of the cHRE reduced, but did not completely eliminate, the E2-dependent induction of bovine OT using a breast cancer cell line (53) or of human OT using a neuroblastoma cell line (55) via ER-α. These differences may be related to the fact that those studies used cell lines that do not endogenously express the OT gene and thus may be lacking transcription factors and regulatory proteins necessary for OT gene regulation. As a result, we used the N38 cell line, which is of mouse hypothalamic origin and endogenously expresses OT (26, 58). We found that the ER-β–dependent OT promoter activation by E2, DPN, and 3β-diol requires an intact cHRE, suggesting that 3β-diol acts on the OT promoter through a functional interaction between ER-β, and another protein-protein complex, and the cHRE. Depending on the environment of the cell line used, availability and presence of the regulatory proteins may influence E2 regulation of OT promoters.
Individual hormones interact with the ER to recruit unique sets of intranuclear regulatory proteins, which may help explain why 3β-diol and ER-β regulation of the OT promoter is fundamentally distinct from that of E2 binding to ER-β. Because we found no significant ligand-independent activity of ER-α or ER-β on OT promoter activity and neither ER-α nor ER-β seems to have direct physical interaction with the cHRE when treated with E2 (28), E2 action on the OT promoter may involve recruitment of regulatory proteins that interact with the regions outside of the cHRE that may cooperate with the cHRE to confer responsiveness when E2 is bound with ER-β.
To examine the possibility that E2-bound ER-β interacts with regulatory regions outside of the cHRE, we also examined the Sp1 element in the OT promoter. We chose the Sp1 site in the OT promoter, because deletion constructs containing this site were activated via E2, whereas the OT constructs lacking the Sp1 site lost E2 responsiveness. Sp1 is a transcription factor shown to interact with GC-rich sequences found on various promoters. Several genes, including ovine Ot, are induced by estrogens through interaction between ER/Sp1 and the GC-rich Sp1 site (44, 45). However, in the present study, mutagenesis of the Sp1 site did not affect E2 responsiveness, suggesting that this site is not required for E2 regulation of the OT promoter. Rather, E2 actions on OT promoter may involve recruitment of other yet unidentified transcription factors or interactions with other DNA sites.
Our results also demonstrated subtle differences between ER-β and ER-α interactions with the cHRE in the OT promoter. Although mutation of the cHRE in the OT promoter reduced E2 activation of OT-luc through ER-β, via ER-α reduced it even further. One explanation for this inhibition is a potential compensating action resulting from interactions between E2-bound ER-α and corepressor protein complexes that activate a regulatory element outside of the cHRE. Such an interaction between ER and repressor protein complexes has been demonstrated (59). Thus, the regulation of OT promoter activity may depend on the cell-specific expression of other transcription factors or coregulatory proteins.
One caveat with studies examining ER-α activation of the OT gene is that ER-α is not expressed in the OT neurons in rat PVN or SON (18, 46), providing little support for a direct action in vivo. The regulation of the human OT gene, however, may be distinct from that of rodents. Both ER-α and ER-β mRNA are found in the human PVN (30, 60), although these studies did not examine the phenotype of the PVN neurons. Another study reported coexpression of ER-β in OT neurons of human PVN (31), but it remains to be seen whether human OT neurons coexpress ER-α.
In summary, the present data add to the growing body of evidence that some androgen metabolites possess estrogenic properties and may be capable of inducing gene transcription via ER-β. This novel concept may be useful in advancing pharmacological treatment of neurological disorders that involve ER-β signaling.
Acknowledgments
We thank Ms Alicia Quihuis for the assistance with animal work.
This work was supported by National Institutes of Health Grants F32-MH093145 (to R.H.) and R01-NS039951 (to R.J.H.).
Current address for R.H.: Department of Psychology, Arizona State University, Tempe, Arizona.
Disclosure Summary: The authors have no potential conflict of interest to disclose.
Footnotes
- AR
- androgen receptor
- cHRE
- composite hormone response element
- DHT
- dihydrotestosterone
- 3β-diol
- 5α-androstane-3β,17β-diol
- DPN
- diarylpropionitrile
- E2
- estradiol
- ER
- estrogen receptor
- HPA
- hypothalamic-pituitary-adrenal
- ISHH
- in situ hybridization histochemistry
- OT
- oxytocin
- PPT
- propylpyrazoletriol
- PVN
- paraventricular nucleus of the hypothalamus
- qRT-PCR
- quantitative RT-PCR
- SON
- supraoptic nucleus
- TSS
- transcription start site
- Sp1
- specificity protein 1.
References
- 1. Gaskin JH, Kitay JI. Adrenocortical function in the hamster. Sex differences and effects of gonadal hormones. Endocrinology. 1970;87:779–786 [DOI] [PubMed] [Google Scholar]
- 2. Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476 [DOI] [PubMed] [Google Scholar]
- 3. Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004;16:272–278 [DOI] [PubMed] [Google Scholar]
- 4. Bingaman EW, Baeckman LM, Yracheta JM, Handa RJ, Gray TS. Localization of androgen receptor within peptidergic neurons of the rat forebrain. Brain Res Bull. 1994;35:379–382 [DOI] [PubMed] [Google Scholar]
- 5. Bingham B, Myung C, Innala L, Gray M, Anonuevo A, Viau V. Androgen receptors in the posterior bed nucleus of the stria terminalis increase neuropeptide expression and the stress-induced activation of the paraventricular nucleus of the hypothalamus. Neuropsychopharmacology. 2011;36:1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williamson M, Bingham B, Gray M, Innala L, Viau V. The medial preoptic nucleus integrates the central influences of testosterone on the paraventricular nucleus of the hypothalamus and its extended circuitries. J Neurosci. 2010;30:11762–11770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β,17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gangloff A, Shi R, Nahoum V, Lin SX. Pseudo-symmetry of C19 steroids, alternative binding orientations, and multispecificity in human estrogenic 17β-hydroxysteroid dehydrogenase. FASEB J. 2003;17:274–276 [DOI] [PubMed] [Google Scholar]
- 9. Jin Y, Penning TM. Steroid 5α-reductases and 3α-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab. 2001;15:79–94 [DOI] [PubMed] [Google Scholar]
- 10. Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3α-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3β-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279:10784–10795 [DOI] [PubMed] [Google Scholar]
- 11. Torn S, Nokelainen P, Kurkela R, et al. Production, purification, and functional analysis of recombinant human and mouse 17β-hydroxysteroid dehydrogenase type 7. Biochem Biophys Res Commun. 2003;305:37–45 [DOI] [PubMed] [Google Scholar]
- 12. Guerini V, Sau D, Scaccianoce E, et al. The androgen derivative 5α-androstane-3β,17β-diol inhibits prostate cancer cell migration through activation of the estrogen receptor β subtype. Cancer Res. 2005;65:5445–5453 [DOI] [PubMed] [Google Scholar]
- 13. Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. The estrogen receptor β subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol. 1998;19:253–286 [DOI] [PubMed] [Google Scholar]
- 14. Oliveira AG, Coelho PH, Guedes FD, Mahecha GA, Hess RA, Oliveira CA. 5α-Androstane-3β,17β-diol (3β-diol), an estrogenic metabolite of 5α-dihydrotestosterone, is a potent modulator of estrogen receptor ERβ expression in the ventral prostrate of adult rats. Steroids. 2007;72:914–922 [DOI] [PubMed] [Google Scholar]
- 15. Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERβ, AR, 5α-androstane-3β,17β-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci U S A. 2002;99:13589–13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor β (ERβ) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. Proc Natl Acad Sci U S A. 1998;95:3281–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hrabovszky E, Kallo I, Hajszan T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-β messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–2604 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki S, Handa RJ. Estrogen receptor-β, but not estrogen receptor-α, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42 [DOI] [PubMed] [Google Scholar]
- 19. Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-β regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res Mol Brain Res. 2002;109:84–94 [DOI] [PubMed] [Google Scholar]
- 20. Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor β in the female mouse hypothalamus. J Neuroendocrinol. 2003;15:787–793 [DOI] [PubMed] [Google Scholar]
- 21. Amico JA, Crowley RS, Insel TR, Thomas A, O'Keefe JA. Effect of gonadal steroids upon hypothalamic oxytocin expression. Adv Exp Med Biol. 1995;395:23–35 [PubMed] [Google Scholar]
- 22. Blyth BJ, Hollingshead DJ, Amico JA. Time course of induction of oxytocin messenger ribonucleic acid levels in the hypothalamic paraventricular nucleus of ovariectomized rats following gonadal steroid administration. Life Sci. 1997;60:2427–2433 [DOI] [PubMed] [Google Scholar]
- 23. Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-β. Prog Brain Res. 2002;139:15–29 [DOI] [PubMed] [Google Scholar]
- 24. Thomas A, Amico JA. Sequential estrogen and progesterone (P) followed by P withdrawal increases the level of oxytocin messenger ribonucleic acid in the hypothalamic paraventricular nucleus of the male rat. Life Sci. 1996;58:1615–1620 [DOI] [PubMed] [Google Scholar]
- 25. Thomas A, Crowley RS, Amico JA. Effect of progesterone on hypothalamic oxytocin messenger ribonucleic acid levels in the lactating rat. Endocrinology. 1995;136:4188–4194 [DOI] [PubMed] [Google Scholar]
- 26. Sharma D, Handa RJ, Uht RM. The ERβ ligand 5α-androstane, 3β,17β-diol (3β-diol) regulates hypothalamic oxytocin (Oxt) gene expression. Endocrinology. 2012;153:2353–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burbach JP, Adan RA, Tol HH, et al. Regulation of the rat oxytocin gene by estradiol. J Neuroendocrinol. 1990;2:633–639 [DOI] [PubMed] [Google Scholar]
- 28. Stedronsky K, Telgmann R, Tillmann G, Walther N, Ivell R. The affinity and activity of the multiple hormone response element in the proximal promoter of the human oxytocin gene. J Neuroendocrinol. 2002;14:472–485 [DOI] [PubMed] [Google Scholar]
- 29. Adan RA, Cox JJ, Beischlag TV, Burbach JP. A composite hormone response element mediates the transactivation of the rat oxytocin gene by different classes of nuclear hormone receptors. Mol Endocrinol. 1993;7:47–57 [DOI] [PubMed] [Google Scholar]
- 30. Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor β (ERβ) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERα mRNA. J Clin Endocrinol Metab. 2000;85:3840–3846 [DOI] [PubMed] [Google Scholar]
- 31. Hrabovszky E, Kallo I, Steinhauser A, et al. Estrogen receptor-β in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. J Comp Neurol. 2004;473:315–333 [DOI] [PubMed] [Google Scholar]
- 32. Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor β by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm Behav. 2008;53:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;146:797–807 [DOI] [PubMed] [Google Scholar]
- 34. Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834 [DOI] [PubMed] [Google Scholar]
- 35. Pak TR, Chung WC, Hinds LR, Handa RJ. Arginine vasopressin regulation in pre- and postpubertal male rats by the androgen metabolite 3β-diol. Am J Physiol Endocrinol Metab. 2009;296:E1409–E1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carbone DL, Zuloaga DG, Hiroi R, Foradori CD, Legare ME, Handa RJ. Prenatal dexamethasone exposure potentiates diet-induced hepatosteatosis and decreases plasma IGF-I in a sex-specific fashion. Endocrinology. 2012;153:295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159 [DOI] [PubMed] [Google Scholar]
- 38. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York, NY: Academic Press; 1998 [Google Scholar]
- 39. Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5α-androstane-3β,17β-diol, is a potent modulator of estrogen receptor-β1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155 [DOI] [PubMed] [Google Scholar]
- 40. Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-β agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Behl C, Widmann M, Trapp T, Holsboer F. 17-β-Estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun. 1995;216:473–482 [DOI] [PubMed] [Google Scholar]
- 42. Green PS, Gridley KE, Simpkins JW. Nuclear estrogen receptor-independent neuroprotection by estratrienes: a novel interaction with glutathione. Neuroscience. 1998;84:7–10 [DOI] [PubMed] [Google Scholar]
- 43. Kim H, Bang OY, Jung MW, et al. Neuroprotective effects of estrogen against β-amyloid toxicity are mediated by estrogen receptors in cultured neuronal cells. Neurosci Lett. 2001;302:58–62 [DOI] [PubMed] [Google Scholar]
- 44. Fleming JG, Spencer TE, Safe SH, Bazer FW. Estrogen regulates transcription of the ovine oxytocin receptor gene through GC-rich SP1 promoter elements. Endocrinology. 2006;147:899–911 [DOI] [PubMed] [Google Scholar]
- 45. Safe S. Transcriptional activation of genes by 17 β-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm. 2001;62:231–252 [DOI] [PubMed] [Google Scholar]
- 46. Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5α-androstane-3β,17β-diol, in modulating oestrogen receptor β-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145:584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ochedalski T, Subburaju S, Wynn PC, Aguilera G. Interaction between oestrogen and oxytocin on hypothalamic-pituitary-adrenal axis activity. J Neuroendocrinol. 2007;19:189–197 [DOI] [PubMed] [Google Scholar]
- 49. Windle RJ, Gamble LE, Kershaw YM, Wood SA, Lightman SL, Ingram CD. Gonadal steroid modulation of stress-induced hypothalamo-pituitary-adrenal activity and anxiety behavior: role of central oxytocin. Endocrinology. 2006;147:2423–2431 [DOI] [PubMed] [Google Scholar]
- 50. Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24:2974–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crowley RS, Insel TR, O'Keefe JA, Kim NB, Amico JA. Increased accumulation of oxytocin messenger ribonucleic acid in the hypothalamus of the female rat: induction by long term estradiol and progesterone administration and subsequent progesterone withdrawal. Endocrinology. 1995;136:224–231 [DOI] [PubMed] [Google Scholar]
- 52. Chung SK, McCabe JT, Pfaff DW. Estrogen influences on oxytocin mRNA expression in preoptic and anterior hypothalamic regions studied by in situ hybridization. J Comp Neurol. 1991;307:281–295 [DOI] [PubMed] [Google Scholar]
- 53. Koohi MK, Ivell R, Walther N. Transcriptional activation of the oxytocin promoter by oestrogens uses a novel non-classical mechanism of oestrogen receptor action. J Neuroendocrinol. 2005;17:197–207 [DOI] [PubMed] [Google Scholar]
- 54. Koohi MK, Walther N, Ivell R. A novel molecular assay to discriminate transcriptional effects caused by xenoestrogens. Mol Cell Endocrinol. 2007;276:45–54 [DOI] [PubMed] [Google Scholar]
- 55. Richard S, Zingg HH. The human oxytocin gene promoter is regulated by estrogens. J Biol Chem. 1990;265:6098–6103 [PubMed] [Google Scholar]
- 56. Adan RA, Burbach JP. Regulation of vasopressin and oxytocin gene expression by estrogen and thyroid hormone. Prog Brain Res. 1992;92:127–136 [PubMed] [Google Scholar]
- 57. Richard S, Zingg HH. Identification of cis-acting regulatory elements in the human oxytocin gene promoter. Mol Cell Neurosci. 1991;2:501–510 [DOI] [PubMed] [Google Scholar]
- 58. Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, Shkreta L. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology. 2004;145:393–400 [DOI] [PubMed] [Google Scholar]
- 59. Miller L, Foradori CD, Lalmansingh AS, Sharma D, Handa RJ, Uht RM. Histone deacetylase 1 (HDAC1) participates in the down-regulation of corticotropin releasing hormone gene (crh) expression. Physiol Behav. 2011;104:312–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Osterlund MK, Grandien K, Keller E, Hurd YL. The human brain has distinct regional expression patterns of estrogen receptor α mRNA isoforms derived from alternative promoters. J Neurochem. 2000;75:1390–1397 [DOI] [PubMed] [Google Scholar]