Abstract
Transcription factor GATA6 is expressed in the fetal and adult adrenal cortex and has been implicated in steroidogenesis. To characterize the role of transcription factor GATA6 in adrenocortical development and function, we generated mice in which Gata6 was conditionally deleted using Cre-LoxP recombination with Sf1-cre. The adrenal glands of adult Gata6 conditional knockout (cKO) mice were small and had a thin cortex. Cytomegalic changes were evident in fetal and adult cKO adrenal glands, and chromaffin cells were ectopically located at the periphery of the glands. Corticosterone secretion in response to exogenous ACTH was blunted in cKO mice. Spindle-shaped cells expressing Gata4, a marker of gonadal stroma, accumulated in the adrenal subcapsule of Gata6 cKO mice. RNA analysis demonstrated the concomitant upregulation of other gonadal-like markers, including Amhr2, in the cKO adrenal glands, suggesting that GATA6 inhibits the spontaneous differentiation of adrenocortical stem/progenitor cells into gonadal-like cells. Lhcgr and Cyp17 were overexpressed in the adrenal glands of gonadectomized cKO vs control mice, implying that GATA6 also limits sex steroidogenic cell differentiation in response to the hormonal changes that accompany gonadectomy. Nulliparous female and orchiectomized male Gata6 cKO mice lacked an adrenal X-zone. Microarray hybridization identified Pik3c2g as a novel X-zone marker that is downregulated in the adrenal glands of these mice. Our findings offer genetic proof that GATA6 regulates the differentiation of steroidogenic progenitors into adrenocortical cells.
Adrenocortical cells arise from a specialized region of coelomic epithelium, the adrenogonadal primordium, that also gives rise to gonadal steroidogenic cells (1–3). The adrenal anlagen form when adrenocortical progenitors in the adrenogonadal primordium delaminate from the epithelium, invade underlying mesenchyme, and associate with neural crest-derived precursors of adrenal medulla (3). The fetal adrenal cortex in humans consists of a large inner zone, known as the fetal zone, and a thin outer rim of immature cells termed the definitive zone (4). The fetal zone produces adrenal androgens, which the placenta converts to estrogens that maintain pregnancy (4). After birth, the fetal zone atrophies, and the definitive zone partitions into functionally distinct layers: the zona glomerulosa (zG), zona fasciculata (zF), and zona reticularis (zR), which produce mineralocorticoids, glucocorticoids, and adrenal androgens, respectively (4). In the mouse adrenal gland, the zG and zF are well defined, but the zR is difficult to discern (5). The postnatal mouse adrenal cortex contains an additional layer, the X-zone, which develops adjacent to the adrenal medulla. The X-zone is derived from the fetal zone (4, 6, 7) and disappears at puberty in males and during the first pregnancy in females (5). Cyp17, a gene required for the synthesis of sex steroids and cortisol, is expressed in the mouse fetal adrenal but is normally absent from the adrenals of postnatal mice (8); consequently, the mouse adrenal does not produce androgens and secretes corticosterone as its major glucocorticoid.
The definitive adrenal cortex is a dynamic organ in which steroidogenic cells undergo constant turnover. Stem/progenitor cells in the adrenal capsule and subcapsule differentiate and centripetally repopulate the cortex (1). Lineage tracing studies suggest that these stem/progenitor cells originate from the fetal cortex and capsule (1–3). The proliferation, differentiation, and survival of adrenocortical stem cells and their progeny are influenced by a diverse array of extracellular signaling molecules, including ACTH, angiotensin-II, insulin-related growth factors, sonic hedgehog, and Wnt proteins (3, 9–11). Endocrine and paracrine factors traditionally associated with the function of gonadal steroidogenic cells, such as LH, activins, and inhibins, also influence the differentiation of adrenocortical cells (9, 12).
Several transcription factors have been shown to be critical for adrenocortical homeostasis. The prototype of these transcriptional regulators is steroidogenic factor 1 (SF1, Nr5a1), which is expressed in the fetal and adult adrenal cortex and serves to promote cell growth, limit apoptosis, and activate genes involved in steroidogenesis (13–16). The activity of SF1 is modulated by Dax1 (Nr0b1), an X-linked gene that encodes a repressor of steroidogenic gene expression (17). In response to ACTH, SF1-positive subcapsular progenitors down-regulate Dax1 and differentiate into adrenocorticoid-producing cells. DAX1 deficiency in humans and mice leads to excessive differentiation of subcapsular progenitors and eventual depletion of the stem/progenitor cell compartment (18, 19).
Another transcription factor implicated in adrenocortical development is GATA6 (20), which is expressed in both the fetal and adult cortex (21–24). GATA6 acts in synergy with SF1 and other transcription factors to enhance the expression of genes involved in adrenal steroid biosynthesis (20, 23). In humans, GATA6 is hypothesized to regulate the production of adrenal androgens and possibly glucocorticoids (22, 23). Promoter studies have identified several putative target genes for GATA6 in adrenal cortex, including the steroid biosynthetic genes CYP11A1 (22), CYP17A1 (22, 25), HSD3B2 (26), CYB5 (27), and SULT2A1 (22, 23, 28).
Although considerable circumstantial evidence implicates GATA6 in adrenal steroidogenesis, genetic proof that GATA6 is required for adrenocortical homeostasis is lacking. Heterozygous loss-of-function mutations in human GATA6 have been linked to pancreatic agenesis, cardiac malformations, and biliary tract abnormalities but not primary adrenocortical defects (29–31). Gata6-null mice die early in gestation (32, 33), so the role of this transcription factor in adrenal function cannot be ascertained from these animals. Here, we examine the impact of GATA6 deficiency on adrenal gland development and physiology by conditionally deleting Gata6 in murine adrenocortical cells using Cre-LoxP recombination with Sf1-cre.
Materials and Methods
Experimental mice and surgery
Procedures involving mice were approved by the institutional committee for laboratory animal care and were conducted in accordance with the NRC's publication Guide for Care and Use of Laboratory Animals. Gata6F/F mice (Gata6tm2.1Sad/J) and Sf1-cre mice [FVB-Tg(Nr5a1-cre)2Lowl/J] were obtained from The Jackson Laboratory and genotyped as described (34, 35). Gata6F/F mice were mated with Sf1-cre mice, and the resultant Gata6F/+;Sf1-cre mice were mated with Gata6F/F mice to produce Gata6F/F;Sf1-cre mice. Weanling mice were gonadectomized as described (36).
Histological analyses
Tissues were fixed overnight in 4% paraformaldehyde in PBS, Bouin's solution, or Müller's fixative (37). Paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E) or Masson's trichrome. For morphometric evaluation of cortical area (38), the right adrenal glands from 3 control or mutant mice were fixed in paraformaldehyde, and paraffin sections (5 μm) were prepared from the whole organ. Every 10th section was stained with H&E and digitally photographed at ×50 magnification. The cortical area (zG + zF) of 5 axial sections from each adrenal was measured using ImageJ. Oil-Red-O staining was performed as described (39).
Electron microscopy
Anesthetized mice were perfused with modified Karnovsky's fixative. Adrenal glands were postfixed with 2% OsO4 and embedded in epon. Semi-thin sections (1 μm) were stained with toluidine blue. Thin sections were stained with uranyl acetate and lead citrate and examined by transmission electron microscopy (EM) (40).
Immunostaining
Immunoperoxidase staining was performed as described (41, 42). The primary antibodies were goat anti-GATA4 (sc-1237, 1:200; Santa Cruz Biotechnology, Santa Cruz, California), rabbit anti-proliferating cell nuclear antigen (sc-7907, 1:200), goat anti-activated caspase-3 (sc-1225, 1:200), goat anti-GATA6 (AF1700 1:100; R&D Systems, Minneapolis, Minnesota), and rabbit anti-tyrosine hydroxylase (TH) (AB152, 1:500; Chemicon, Temecula, California). Secondary antibodies were donkey antigoat biotinylated IgG (1:1000; Jackson Immunoresearch, West Grove, Pennsylvania) and goat antirabbit biotinylated IgG (NEF-813, 1:2000; NEN Life Science, Boston, Massachusetts). The avidin-biotin immunoperoxidase system (Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, California) and diaminobenzidine were used to visualize bound antibody. A Cy3-conjugated goat antirabbit secondary antibody (1:800; Jackson Immunoresearch) was used for immunofluorescence microscopy, and slides were mounted with 4′,6-diamidino-2-phenylindole mounting media (Vector Laboratories). Fluorescent staining of endogenous biotin using fluorescein isothiocyanate (FITC)-streptavidin was performed as described (43).
Assessment of adrenal and reproductive function
For plasma corticosterone and ACTH measurements, 8-week-old mice were decapitated at 8:00 to 9:00 am with minimum handling, and trunk blood was collected in EDTA (44). Restraint stress experiments were performed as described (44). For the ACTH stimulation tests, the hypothalamic-pituitary-adrenal (HPA) axis was suppressed by sc injection of 5 mg/kg dexamethasone in sesame oil at 6:00 pm 1 day before and at 8:00 am on the day of testing (45). Mice were then anesthetized with 1%–2% isoflurane in 50% oxygen/50% nitrogen, and an external jugular venous catheter was placed to facilitate serial blood sampling. At 9:00 to 10:00 am, 1 mg/kg ACTH1–24 (Bachem, Torrance, California) was injected ip, and 40-μL blood samples were collected at varying times after ACTH injection for corticosterone measurements (45).
To assess the impact of HPA axis suppression on adrenocortical apoptosis, mice were injected with 5 mg/kg dexamethasone every 12 hours for 4 days (46). Adrenal glands were harvested the following morning and immunostained for activated caspase-3.
Steroid hormone measurements were made using commercial kits [estrone sulfate ELISA kit from Endocrine Technologies, Newark, California; testosterone, 17α-OH-progesterone, corticosterone, and cortisol RIA kits from Siemens Healthcare Diagnostics (Erlangen, Germany); and ACTH RIA kit from MP Biomedicals (Santa Ana, California)]. Whole adrenal aldosterone measurements were performed as described (47). Na+ measurements were made on heparinized blood using a Radiometer ABL90 Flex blood gas analyzer. Plasma norepinephrine was measured with a single isotope derivative method (48).
Fertility was evaluated by housing male and female mice with wild-type C57BL/6 mice and measuring litter sizes (49). Sperm motility was evaluated as described (50).
Quantitative RT-PCR
Total RNA was isolated and subjected to quantitative RT-PCR (qRT-PCR) analysis as described (51). Expression was normalized to the housekeeping genes Actb and Gapdh. Primer pairs are listed in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Microarray analysis
RNA was isolated from whole adrenal glands using the RNeasy Mini Kit (QIAGEN, Valencia, California), amplified using the TotalPrep RNA amplification kit (Illumina, San Diego, California), and hybridized on an Illumina Mouse6v2 oligonucleotide array. Hybridization was performed by the GTAC Microarray Core facility according to standard protocols. Results have been deposited at the GEO database (GSE40398).
Laser microdissection
Adrenal cryosections (10 μm) were collected on PEN-Membrane slides (2.0 μm; Leica Microsystems, Wetzlar, Germany). Tissue sections were fixed in ethanol (1 minute at −20°C), stained with Gram's crystal violet, and dehydrated by passage through successively higher concentrations of ethanol followed by xylene. Laser microdissection (LMD) was performed using a Leica LMD6000 microscope. Dissectates were collected in RNA extraction buffer (RNeasy Mini Kit) for qRT-PCR analysis.
In situ hybridization
Digoxigenin-labeled riboprobes were prepared as described (52). In situ hybridization was performed on paraformaldehyde-fixed, paraffin-embedded sections (53).
Statistical methods
The Student's t test (two-tailed) was used for statistical analysis, and significance was set at P < .05.
Results
Conditional deletion of Gata6 in SF1-positive cells results in mice that are viable and fertile
The 129.B6 mice bearing a floxed allele of Gata6 (Gata6F) (34) were crossed with FVB mice harboring an Sf1-cre transgene (35). Studies have shown that Cre-mediated recombination converts Gata6F into a null allele (54, 55). As demonstrated by Rosa26 reporter analysis (http://cre.jax.org/Nr5a1/Nr5a1-creNano.html), Sf1-cre is expressed in both the fetal and adult adrenal cortex; extra-adrenal sites of Sf1-cre expression include gonadal somatic cells, hypothalamus, and pituitary. Intercrosses of Gata6F/F and Gata6F/+;Sf1-cre mice yielded Gata6F/F;Sf1-cre mice, hereafter referred to as Gata6 conditional knockout (cKO) mice, in the expected Mendelian ratio (42 cKO of 173 total ≈ 1:4) and the expected sex ratio (21 male and 21 female = 1:1). Gata6F/F or Gata6F/+;Sf1-cre mice were used as controls for the analyses described below; neither of these strains had an abnormal phenotype.
Immunohistochemistry and RNA analyses documented the reduced expression of Gata6 in the adrenal glands of cKO mice. Consistent with published reports (1, 21, 56), nuclear GATA6 immunoreactivity was observed in capsular, subcapsular, and scattered vascular cells in the adrenal cortex of adult control mice (Figure 1A). In cKO mice, there was decreased GATA6 immunostaining in subcapsular cells, where Sf1-cre is active (3, 57), but persistent GATA6 immunostaining in capsular and vascular cells, where Sf1-cre is inactive (Figure 1B). In situ hybridization confirmed that Gata6 mRNA was markedly reduced in the adrenal subcapsule of adult cKO mice compared with controls (Figure 1, C and D). qRT-PCR analysis of adrenal glands from female mice of varying ages [embryonic day 17.5 (E17.5), postnatal days 10 and 21 (P10 and P21), and adult] showed that Gata6 mRNA, normalized to β-actin mRNA, was significantly reduced in the mutant adrenals at all ages examined (Figure 1E). Similarly, qRT-PCR analysis of whole adrenal gland RNA demonstrated that Gata6 mRNA was lower in 1-month-old cKO male mice than in age-matched controls (0.02 ± 0.01 vs 0.14 ± 0.06, respectively; n = 4 per group; P < .05).
Figure 1.
Conditional mutagenesis of Gata6 in SF1-positive cells results in mice that grow normally but have small adrenal glands. A and B, Adrenal glands from 3-month-old control (A, Ctrl) or cKO (B) parous female mice were subjected to GATA6 immunoperoxidase staining and then counterstained with toluidine blue. In control mice, nuclear GATA6 immunoreactivity was evident in capsular cells, subcapsular cells, and scattered endothelial cells. In cKO mice, there was reduced GATA6 immunoreactivity in subcapsular cells, where Sf1-cre is known to be active, but persistent GATA6 staining in capsular cells (arrowhead) and endothelial cells, where Sf1-cre is inactive. The arrow highlights ectopic medulla cells. Scale bars, 30 μm. Adrenal glands from 2-month-old control (C) or cKO (D) virgin female mice were subjected to in situ hybridization using a digoxigenin-labeled Gata6 antisense riboprobe and alkaline phosphatase-conjugated antidigoxigenin antibody Fab fragments, followed by counterstaining with nuclear fast red. Note the reduced levels of GATA6 mRNA in subcapsular cells of the cKO adrenals. Scale bars, 30 μm. E, qRT-PCR analysis of Gata6 mRNA in whole adrenal glands from control and cKO female mice at varying ages. Results were normalized to β-actin mRNA (*P < .05, n = 3–4 per group). Similar results were obtained when results were normalized to Gapdh mRNA (data not shown). F, Growth curves for control and cKO male mice. G, Growth curves for control and cKO virgin female mice. H, Average adrenal masses of 3-month-old male, virgin female, and parous female mice (*P < .05, n = 3 per group). The inset shows the gross morphology of adrenal glands from 3-month-old control (left) and cKO (right) male mice.
Gata6 cKO mice were viable and appeared healthy. The growth curves of cKO and control mice were indistinguishable (Figure 1, F and G). Although Gata6 is expressed in gonadal somatic cells (20, 58, 59) and Gata6 mRNA levels were lower in the ovaries and testes of cKO mice than in age-matched controls (Supplemental Figure 1, A and B), cKO mice had no obvious reproductive phenotype. At 3 months of age, ovarian mass (Supplemental Figure 2A), plasma estrone sulfate levels (Supplemental Figure 2B), testicular mass (Supplemental Figure 2C), sperm motility (Supplemental Figure 2, D and E), plasma testosterone levels (Supplemental Figure 2F), and seminal vesicle mass (a proxy for circulating testosterone; Supplemental Figure 2G) were similar in cKO and control mice. When mated with wild-type mice, both female and male cKO mice produced viable pups at rates similar to control mice (Supplemental Figure 2, H and I). Gonadal histology appeared normal in the cKO mice (data not shown). The absence of a reproductive phenotype suggests that pituitary gonadotrope function is intact in cKO mice, which is relevant to interpretation of the adrenocortical phenotype described below.
Adrenal glands of Gata6 cKO mice are small and have a thin cortex with cytomegalic changes
Although gonadal development appeared unperturbed in the Gata6 cKO mice, adrenal development was grossly abnormal. At 3 months of age, adrenal gland mass was significantly decreased in cKO mice compared with controls (Figure 1H). This decrease, which was replicated in cKO males, virgin females, and parous females, was attributable to cortical thinning (Supplemental Figure 3). A reduction in adrenal mass was also evident at 1 month of age in cKO males (1.8 ± 0.0 vs 0.8 ± 0.03 mg, P < .05) and females (2.0 ± 0.25 vs 0.9 ± 0.19 mg, P < .05). At E17.5, however, there was no discernible difference in adrenal gland size between cKO and control mice (Figure 2, C and D and data not shown). Theoretically, the reduced size of the adrenal glands in Gata6 cKO could result from decreased cell proliferation; however, adult cKO and control female adrenals showed no differences in immunostaining for the proliferation marker proliferating cell nuclear antigen (data not shown).
Figure 2.
Adrenocortical cytomegaly and ectopic chromaffin cells in Gata6 cKO mice. A and B, Toluidine blue-stained semi-thin (1 μm) sections of adrenal glands from 2-month-old control (Ctrl) and cKO female mice, respectively. zF spongiocytes were larger in cKO than in control adrenal glands, and the fascicular arrangement of spongiocytes was disordered. C and D, Gross morphology of adrenal glands (circled) from female E17.5 control and cKO mice, respectively. E and F, H&E-stained adrenal glands from female E17.5 control (E) and cKO (F) mice. Note the cytomegalic changes (large cells with large nuclei and prominent nucleoli) in the fetal adrenal cortex of the cKO mice. G–J, TH immunoperoxidase staining of 3-month-old male control (G and I) and cKO (H and J) adrenal glands. Note the ectopic islands and finger-like projections of immunoreactive chromaffin cells in the mutant adrenal glands (J, arrowhead). K and L, Chromaffin staining of 1.5-month-old virgin control (K) and cKO (L) adrenal glands using Müller's reagent. The arrowhead highlights a cluster of ectopic medullary cells in the cKO adrenal. Scale bars, 2 μm (A and B), 0.5 mm (C and D), 10 μm (E and F), 200 μm (G and H), 50 μm (I and J), and 100 μm (K and L). m, medulla.
Light microscopy (Figures 2, A and B, and 3, A and B) and EM (Figure 3, C and D, and Supplemental Figure 4, A and B) showed that differentiated steroidogenic cells were present in the zG and zF of adult Gata6 cKO mice, but the adrenocortical architecture was abnormal. The fascicular arrangement of zF spongiocytes was disrupted, and cytomegalic changes were evident (Figure 2, A and B). Cytomegaly, a hallmark of adrenal dysfunction associated with Dax1 deficiency (17, 18, 60) and other genetic disorders (61–66), is thought to be a compensatory response to a reduced number of cortical cells or to progenitor cell exhaustion (19). Cytomegalic changes were also seen in E17.5 Gata6 cKO adrenals (Figure 2, E and F, and Supplemental Figure 5), implying that deletion of Gata6 in SF1-positive cells impacts both fetal and postnatal adrenocortical development. The finding of cytomegaly in the fetal adrenal excludes the possibility that the phenotype is secondary to an HPA axis defect, because although Sf1-cre is expressed in the hypothalamus/pituitary, pituitary secretion of ACTH and other proopiomelanocortin derivatives is not required for prenatal adrenocortical development in the mouse (66, 67).
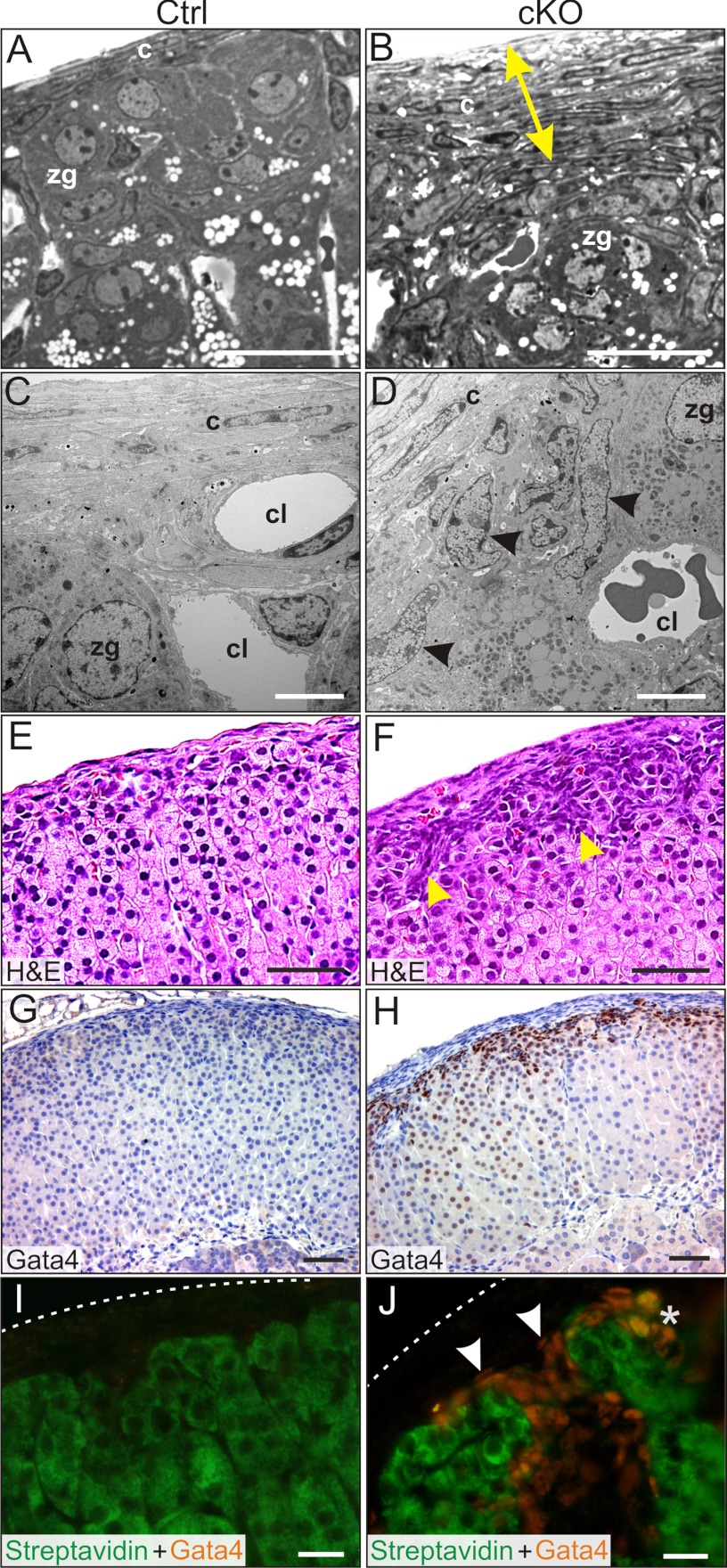
Figure 3.
Capsular and subcapsular changes in the adrenal glands in Gata6 cKO mice. A and B, Semi-thin sections of adrenal gland from 2-month-old control (A) and cKO (B) virgin female mice. Note the thickened capsule in the mutant adrenal gland (yellow arrow). C and D, EM of adrenal glands from 2-month-old control (C) and cKO (D) virgin female mice. Note the accumulation of type A cells (arrowheads) beneath the capsule. E and F, H&E-stained control (G) and cKO (H) adrenal glands from 3-month-old parous female mice. Note the thickened capsule and the accumulation of basophilic type A cells in the subcapsule (arrowheads). G and H, GATA4 immunoperoxidase staining of adrenal glands from 3-month-old control (G) and cKO (H) parous female mice. Note the intense nuclear immunoreactivity in the subcapsule of the cKO adrenal gland. GATA4 immunoreactivity was also evident in some zF cells of the cKO mice. I and J, Adrenal glands from 2-month-old control (I) and cKO (J) virgin female mice stained with FITC-steptavidin and anti-GATA4 (Cy3-labeled secondary antibody). Dashed lines indicate the surface of the capsule. FITC-streptavidin labels steroidogenic and presteroidogenic adrenocortical cells (43). Nonsteroidogenic type A cells exhibit nuclear GATA4 immunoreactivity but do not stain with streptavidin (J, arrowheads). *, Note the rare subcapsular cells that stain with both streptavidin and anti-GATA4 (J) and are surmised to have steroidogenic potential. Abbreviations: c, capsule cell; cl, capillary lumen. Scale bars, 10 μm (A, B, and E–H), 2 μm (C and D), and 30 μm (I and J).
Oil-Red-O staining, which in steroid-producing tissues detects primarily cholesteryl esters, was increased in the zF of Gata6 cKO mice (Supplemental Figure 4, C–F). Increased Oil-Red-O staining of zF cells has been reported in mice with impaired steroidogenesis (39, 68, 69).
Ectopic chromaffin cells in the adrenal glands of Gata6 cKO mice
Adrenocortical dysplasia in the Gata6 cKO mice was accompanied by disorganization of the adrenal medulla. In adult cKO mice, ectopic islands of chromaffin cells were evident in the periphery of the adrenal glands, as highlighted by immunostaining for TH (Figure 2, G–J) and by Müller's chromaffin stain (Figure 2, K and L). In some regions of the cKO adrenals, finger-like projections of chromaffin cells extended to capsule. Ectopic chromaffin cells were observed in cKO mice between the ages of 1 and 6 months. Misplaced chromaffin cells also were evident in the adrenals of E17.5 cKO mice (Supplemental Figure 5), reinforcing the premise that ablation of Gata6 in SF1-positive cells impacts both fetal and postnatal adrenal development. Although chromaffin cells were ectopically located in the cKO mice, qRT-PCR analysis showed that TH mRNA levels, normalized to β-actin, were similar in adrenal glands of cKO and control mice (0.32 ± 0.9 and 0.30 ± 0.8, respectively, n = 4 per group). Plasma norepinephrine levels were comparable in cKO and control mice (1.36 ± 0.36 and 1.40 ± 0.25 ng/mL, respectively, n = 5 per group).
Accumulation of dysplastic cells in the adrenal subcapsule of Gata6 cKO mice and concomitant upregulation of gonadal-like markers
The adrenal capsule of Gata6 cKO mice appeared thicker than that of controls (Figure 3, A–H), which could reflect decreased stretching of the capsule by the smaller cortex. Additionally, the cKO adrenals had an expanded subcapsular cell compartment. Adrenal subcapsular cell hyperplasia is a common finding in aged mice (70) and in certain inbred strains after gonadectomy (9) but is uncommon in nongonadectomized young mice. Subcapsular cell hyperplasia was consistently observed in nongonadectomized male and female cKO mice as young as 1 month of age. The hyperplastic subcapsular cells (Figure 3D, arrowheads) had the morphologic features of type A cells (9), nonsteroidogenic cells that accumulate in the subcapsular region of gonadectomized mice and resemble the gonadal stroma of postmenopausal ovaries (71). Type A cells are spindle-shaped, basophilic cells that stain with anti-GATA4 and contain few lipid droplets (Figure 3, D, F, H, and J) (9, 72). FITC-streptavidin, which labels endogenous biotin in steroidogenic and presteroidogenic cells, does not stain type A cells (Figure 3, I and J) (43). The accumulation of spindle-shaped, basophilic, GATA4-positive cells in the adrenal subcapsule has been reported in other genetically engineered mice (46, 73, 74) and is hypothesized to reflect a block in adrenocortical cell differentiation. Weak GATA4 immunoreactivity was also seen in some zF spongioblasts (Figure 3, G and H) and in a subset of subcapsular cells that colabeled with FITC-streptavidin, surmised to be cells with steroidogenic potential (Figure 3J).
qRT-PCR confirmed that Gata4 mRNA levels were significantly greater in the adrenal glands of 1-month-old female cKO mice than in age-matched control mice (Supplemental Figure 6). Other gonadal-like transcripts significantly upregulated in the cKO adrenals included the type A cell marker Amhr2 (36, 75), the subunits of activin and inhibin (Inha, Inhba, and Inhbb), and forkhead transcription factor Fkhl18 (Foxs1) (76). Tcf21 (Pod1/capsulin/epicardin), a transcription factor expressed in adrenal capsule (77) and gonadal somatic cells (78) where it serves to repress SF1, was upregulated, too. There was significantly reduced expression of the zG differentiation marker Cyp11b2 in the cKO adrenals and a trend toward reduced expression of two zF differentiation markers (Cyp11b1 and Cyp21a1), although the changes in the latter 2 markers did not reach statistical significance. There was no significant change in the expression of Star, Cyp11a1, Sf1, or Dax1, pansteroidogenic genes required for steroid biosynthesis in both adrenocortical and gonadal cells. Other genes implicated in adrenocortical function that showed no change in expression included the following: Pbx1, a transcription factor gene that regulates adrenal growth (79, 80), Pref1 (Dlk1/FA1/PG2/ZOG), a gene that is expressed in the subcapsule (81, 82) and is hypothesized to regulate stem/progenitor cells (2), and Mcm4, a chromosome maintenance gene (73, 83).
Functional consequences of Gata6 deletion in adrenocortical cells
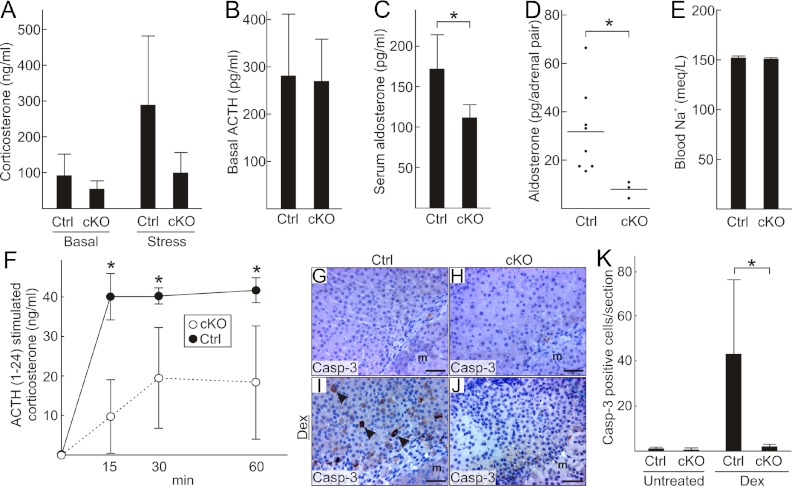
Basal and stress-induced corticosterone levels appeared lower in 2-month-old cKO than in control mice, but these results did not reach statistical significance (Figure 4A). Basal ACTH levels were indistinguishable in Gata6 cKO and control mice (Figure 4B). Serum aldosterone levels (Figure 4C) and whole adrenal aldosterone content (Figure 4D) were significantly reduced in cKO mice compared with controls, although no differences in blood sodium levels (Figure 4E) were detected. ACTH1–24-induced corticosterone secretion after overnight dexamethasone suppression of the HPA axis, a sensitive measure of adrenal steroidogenic capacity (45), was significantly reduced in the cKO mice (Figure 4F).
Figure 4.
Assessment of adrenocortical function in Gata6 cKO mice. A, Morning plasma corticosterone levels under basal conditions and after 30 minutes of restraint-induced stress in 8-week-old female control and cKO mice (n = 4–6 per group). B, Morning plasma ACTH levels in 8-week-old male control and cKO mice (n = 4–14 per group). C, Serum aldosterone levels in 8-week-old female control and cKO mice (*P < .05, n = 6–7 per group). D, Aldosterone content in whole adrenal glands from 8-week-old male control and cKO mice. E, Blood Na+ concentration in 8-week-old male control and cKO mice (n = 10 per group). F, Plasma corticosterone levels in 8-week-old male control and cKO mice after overnight dexamethasone suppression and administration of ACTH1–24 at time 0 (*P < .05, n = 3 per group). G–J, Immunoperoxidase staining for cleaved caspase-3 (Casp-3), a marker of apoptosis, in the inner cortical cells of 5-month-old female control (G and I) and cKO (H and J) treated with vehicle alone (G and H) or dexamethasone (I and J) for 4 days. K, Quantification of apoptotic cells (Casp-3 positive) in the adrenal cortex of untreated or dexamethasone (Dex)-treated mice of both genotypes (*P < .05, n = 6 per group).
Inner cortical cells of the mouse adrenal normally undergo apoptosis in response to prolonged dexamethasone suppression of the HPA axis. Interestingly, cortical cells of cKO mice were resistant to dexamethasone-induced apoptosis (Figure 4, G–K), a phenotype previously reported in older wild-type rats (84) and in a mouse model of Carney complex characterized by ectopic adrenocortical expression of Cyp17 and circulating cortisol (46). At 3 months of age, Gata6 cKO mice had plasma 17α-OH-progesterone and cortisol levels below the limit of detection (<0.1 ng/mL and <2 μg/dL, respectively, n = 5 per group).
Absence of an X-zone in the adrenal glands of Gata6 cKO mice
The adrenal X-zone first appears at 2 weeks of age as clusters of eosinophilic cells at the corticomedullary junction. In males, the X-zone soon ceases to grow and disappears by the onset of puberty (85). In females, the X-zone continues to enlarge until 5 weeks of age, eventually undergoing fatty degeneration in older, nulliparous females (85). During the first pregnancy, the X-zone rapidly involutes (85). Growth of the X-zone is enhanced by LH, whereas apoptosis of this zone is triggered by activin (86, 87). Orchiectomy of weanling male mice induces a secondary X-zone (88).
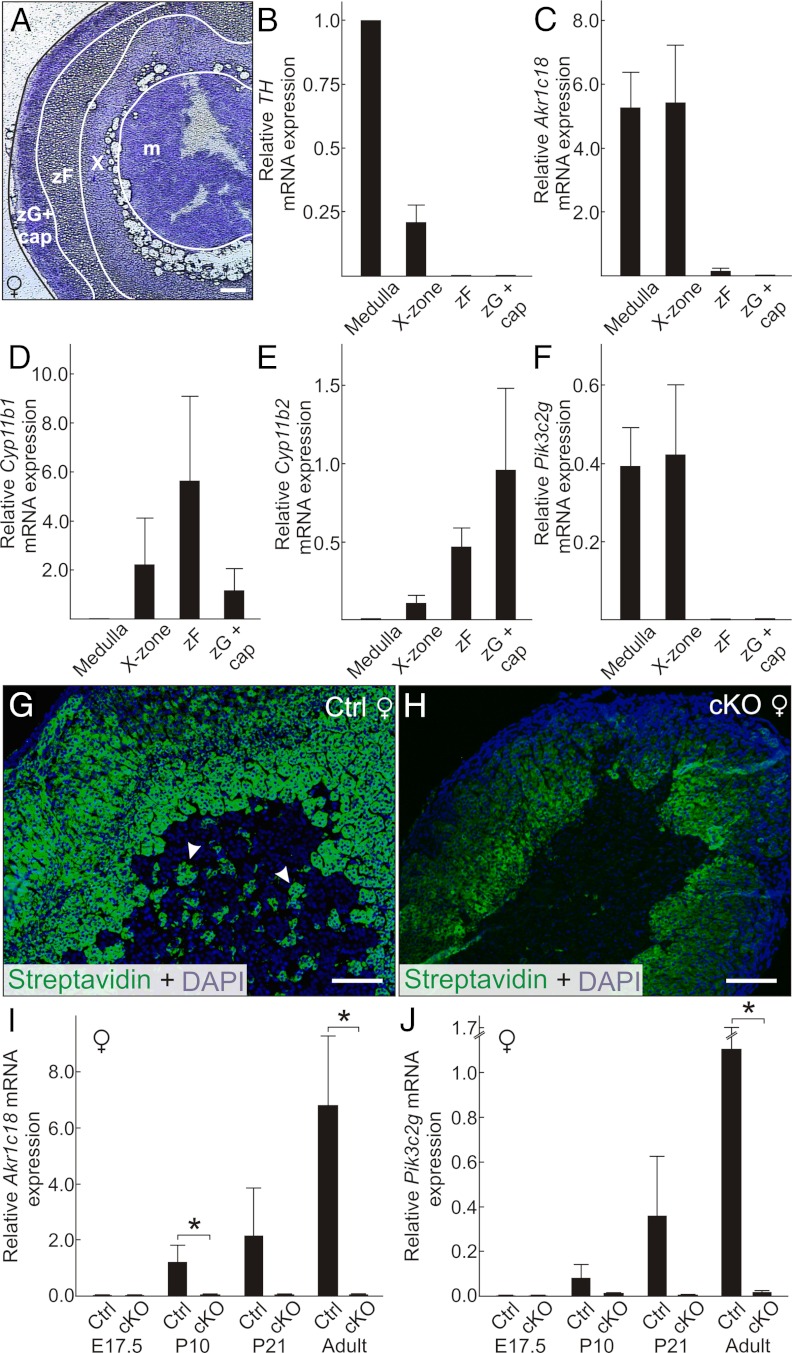
Light microscopy showed that young virgin female cKO mice lacked an X-zone (Figure 5, A and B). Moreover, there was no evidence of adipocyte accumulation at the corticomedullary junction suggestive of fatty degeneration of a preexisting X-zone (Supplemental Figure 7, A and B). EM of the juxtamedullary region of control virgin female mice demonstrated cells with the hallmarks of the X-zone, including dense cytoplasm, abundant smooth endoplasmic reticulum (ER), and peculiar mitochondrial complexes consisting of flattened mitochondria alternating with ER cisternae (Figure 5, C and E) (85). In contrast, the adrenal glands of age-matched virgin female cKO mice lacked EM evidence of X-zone cells. Instead, cells with ultrastructural features of zR (85), such as electron-lucent lipid droplets and numerous ellipsoidal mitochondria, abutted the medulla (Figure 5, D and F). Orchiectomy induced a secondary X-zone in control mice (Figure 5, G and I) but not in cKO mice (Figure 5, H and J). Consistent with the morphologic findings, qRT-PCR analysis showed significantly decreased expression of the X-zone marker Akr1c18, which encodes 20α-hydroxysteroid dehydrogenase (89), in nulliparous female and orchiectomized male cKO mice (Supplemental Figure 7C).
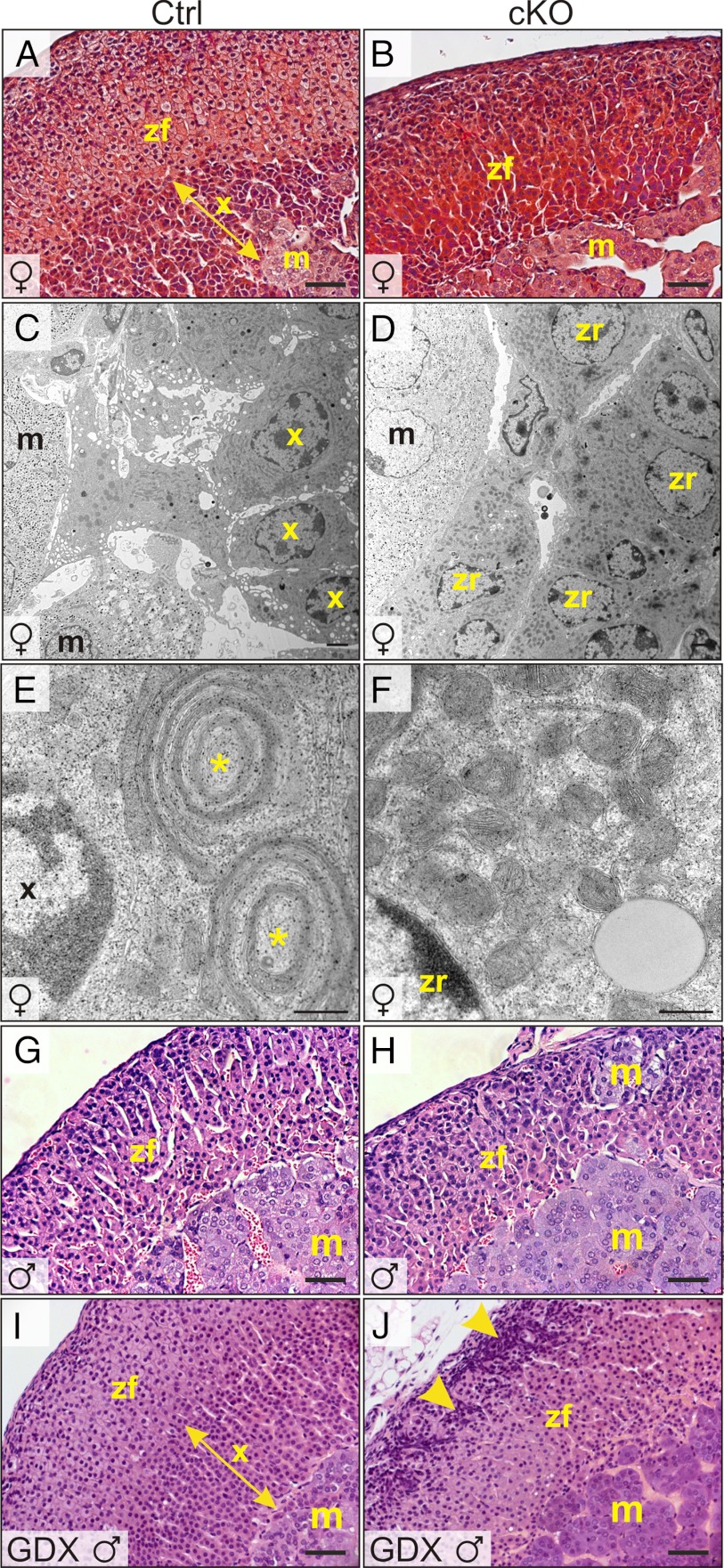
Figure 5.
Absence of an adrenal X-zone in Gata6 cKO mice. Adrenal glands from control (A, C, E, G, and I) or cKO mice (B, D, F, H, and J) were processed for light (A, B, G, and H) or EM (C–F). A and B, Trichrome staining of 1-month-old female adrenal glands highlights the lack of an X-zone in the mutant. C–F, Adrenal glands from 2-month-old virgin female control mice contain cells with the ultrastructural features of the X-zone, including dense cytoplasm, abundant smooth ER, and distinctive mitochondrial complexes consisting of flattened mitochondria alternating with ER cisternae (*). The 2-month-old virgin female cKO adrenal glands lack cells with the hallmarks of X-zone; instead, cells with ultrastructural features of zR, including electron-lucent lipid droplets and numerous ellipsoidal mitochondria, abut chromaffin cells in the adrenal medulla. G and H, H&E staining of adrenal glands from 2-month-old control or cKO male mice. I and J, H&E staining of adrenal glands from 2-month-old male mice that were subjected to orchiectomy at 3 weeks of age. Note the secondary X-zone in the orchiectomized control mice. In contrast, the adrenal glands of orchiectomized cKO mice lack an X-zone and have pronounced subcapsular cell hyperplasia (arrowheads). Scale bars, 10 μm (A, B, and G–J), 250 nm (C and D), and 25 nm (E and F). Abbreviations: m, medulla; x, X-zone.
Overexpression of sex steroidogenic differentiation markers in the adrenal glands of gonadectomized Gata6 cKO mice
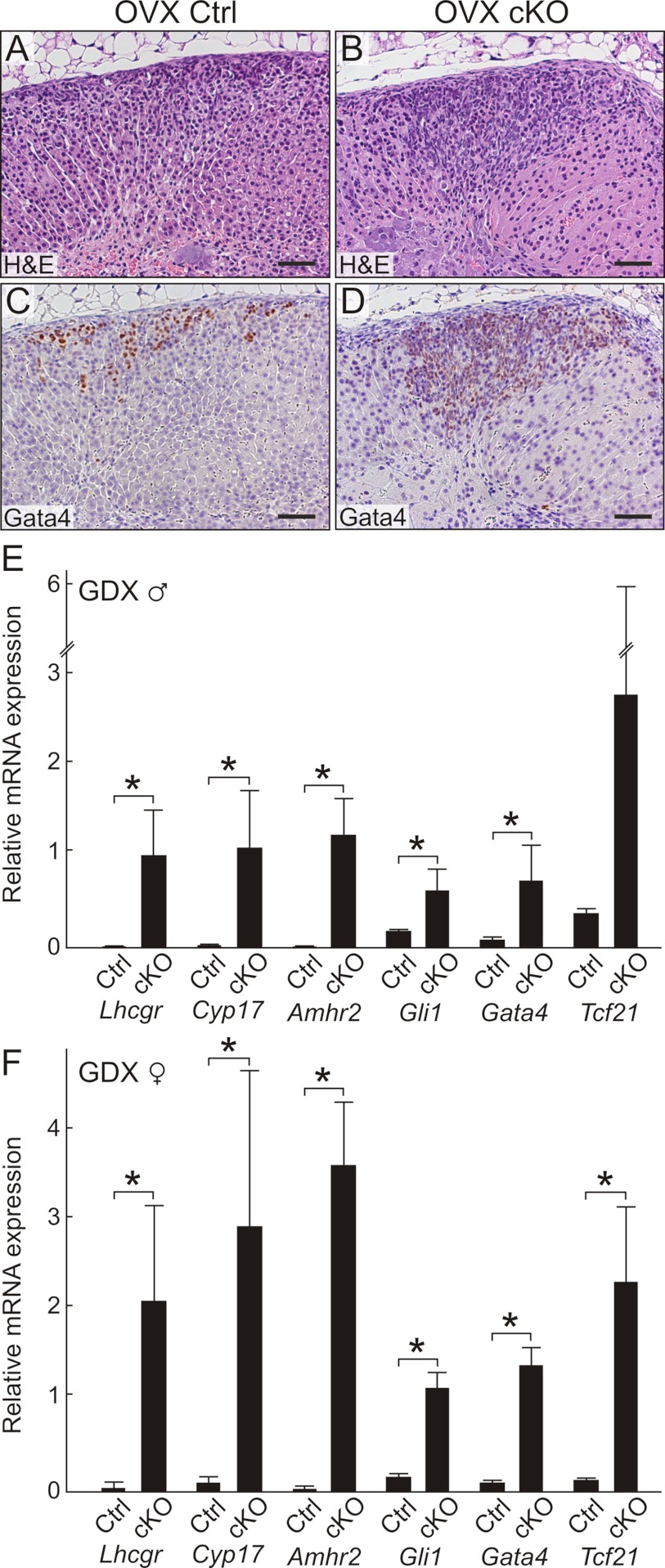
In genetically susceptible strains of mice, the hormonal changes associated with gonadectomy can elicit subcapsular accumulation of not only nonsteroidogenic type A cells but also sex steroidogenic type B cells (9). When Gata6 cKO and control mice were orchiectomized to monitor secondary X-zone formation, we observed expansion of the adrenal subcapsular cell compartment in cKO compared with control animals (Figure 5, G and H). Similarly, ovariectomy induced pronounced subcapsular cell hyperplasia in the adrenal glands of cKO vs control mice (Figure 6, A and B). GATA4 immunostaining demonstrated large, wedge-shaped lesions of gonadal-like cells in the cKO adrenal glands (Figure 6D). Only small collections of GATA4 immunoreactive cells were seen in control adrenals (Figure 6C). qRT-PCR analysis of orchiectomized and ovariectomized mice showed that type A (Amhr2 and Gata4) and type B (Cyp17 and Lhcgr) differentiation markers were significantly overexpressed in adrenal glands from cKO vs control mice (Figure 6, E and F). Gli1, a gene normally expressed in the inner capsule (3), also was overexpressed in the adrenal glands of gonadectomized cKO mice (Figure 6, E and F). Additionally, Tcf21 was overexpressed in the adrenal glands of gonadectomized cKO mice (Figure 6, E and F), and in situ hybridization showed that Tcf21 mRNA localized to gonadal-like cells in the subcapsular region (Supplemental Figure 8). Although the sex steroidogenic markers Cyp17 and Lhcgr were markedly upregulated in the adrenal glands of the cKO mice 3 to 6 months after ovariectomy, there was no histological evidence of ectopic sex steroid production (75); submaxillary glands had no sign of androgen stimulation, and uteri were hypoestrogenic.
Figure 6.
Gonadectomy promotes differentiation of gonadal-like cells in the adrenal glands of Gata6 cKO mice. A and B, H&E stain of adrenal glands from control (A) or cKO (B) mice that were ovariectomized 3 months earlier. Note the large, wedge-shaped, subcapsular lesion in the cKO adrenal. C and D, GATA4 immunoperoxidase staining highlights gonadal-like cells in the subcapsular region of the control (C) and cKO (D) mice. The cKO adrenals contained many more GATA4 immunoreactive cells than control adrenals. Scale bars, 100 μm (A–D). E and F, Relative expression, normalized to β-actin, of transcripts in whole adrenal glands from gonadectomized cKO vs control mice. Shown are results for male (E) and female (F) mice. The female mice were 3 months after ovariectomy, whereas the male mice were 40 days after orchiectomy (*P < .05, n = 3–4 per group).
Microarray hybridization identifies Pik3c2g as a novel X-zone marker that is underexpressed in the adrenal glands of Gata6 cKO mice
To identify other genes dysregulated by conditional mutagenesis of Gata6, we performed microarray hybridization with mRNA isolated from whole adrenal glands of 2-month-old virgin female cKO (n = 3) and control (n = 3) mice. Consistent with aforementioned qRT-PCR results, microarray analysis showed upregulation of Amhr2 (9-fold), Tcf21 (3-fold), and Fkhl18 (2-fold) and downregulation of Akr1c18 (9-fold) in the cKO adrenal glands. Interestingly, conditional deletion of Gata6 was associated with marked (∼40-fold) downregulation of Pik3c2g, a phosphoinositide-3 kinase (PI3K), in 3 probe sets on the microarray. qRT-PCR analysis confirmed that the ratio of expression of Pik3c2g in 1-month-old female cKO vs control adrenals was 0.01 (P = .008; n = 3 per group).
qRT-PCR analysis of whole adrenal glands from control mice showed that Pik3c2g expression correlated with the presence of an X-zone; ie, Pik3c2g mRNA was detected in adrenal glands from virgin females, gonadectomized females, and gonadectomized males but not from postpubertal males or parous females (Supplemental Figure 7D). To confirm that Pik3c2g is expressed specifically in the X-zone, we performed LMD on adrenal glands from virgin female control mice (Figure 7, A–F). qRT-PCR analysis showed that expression of Pik3c2g, like that of Akr1c18, localized to the X-zone fraction and to a lesser extent the medulla fraction (Figure 7, C and F). Expression of Akr1c18 (and Pik3c2g) in the medulla fraction presumably reflects the well-documented phenomenon of infiltration of the medulla by X-zone cells (4, 6, 7, 43, 85). FITC-streptavidin staining (43) demonstrated such misplaced X-zone cells in the medulla of control but not cKO mice (Figure 7, G and H). As further proof that Pik3c2g is expressed in X-zone cells, we compared Pik3c2g and Akr1c18 transcript levels at different stages of adrenal development (Figure 7, I and J); the age-dependent expression of Pik3c2g in whole adrenals from control and cKO mice mirrored that of the prototypical X-zone marker, Akr1c18. We conclude that Pik3c2g is a novel marker of the adrenal X-zone.
Figure 7.
Pik3c2g, a gene downregulated in the adrenal glands of Gata6 cKO mice, is a novel marker of the X-zone. A–F, qRT-PCR analysis of gene expression in microdissected adrenal zones. Cyrosections of adrenal glands from 1.5-month-old nulliparous female control mice were stained with crystal violet and subjected to LMD. Dissectates were collected from 4 regions [medulla (m), X-zone (X), zF, and zG plus capsule (cap)] (A) and then subjected to qRT-PCR (B–F). The zone-specific markers examined included TH (medulla), Akr1c18 (X-zone), Cyp11b1 (zF), and Cyp11b2 (zG). Results were normalized to β-actin and TH expression (*P < .05, n = 3 per group). The zonal expression pattern of Pik3c2g is identical to that of the prototypical X-zone marker Akr1c18. The detection of Akr1c18 (and Pik3c2g) mRNA in the medulla fraction partly reflects infiltration of the medulla by X-zone cells. G and H, FITC-streptavidin staining demonstrates such misplaced X-zone cells in the medulla of control (G, arrowheads) but not cKO (H) mice. Scale bars, 150 μm (I and J). qRT-PCR analysis of Akr1c18 (I) and Pik3cg2 (J) in whole adrenal extracts of nulliparous female control or cKO mice of varying ages. Results were normalized to β-actin expression (*P < .05, n = 3–4 per group). The developmental expression pattern of Pik3c2g mirrors that of Akr1c18.
Discussion
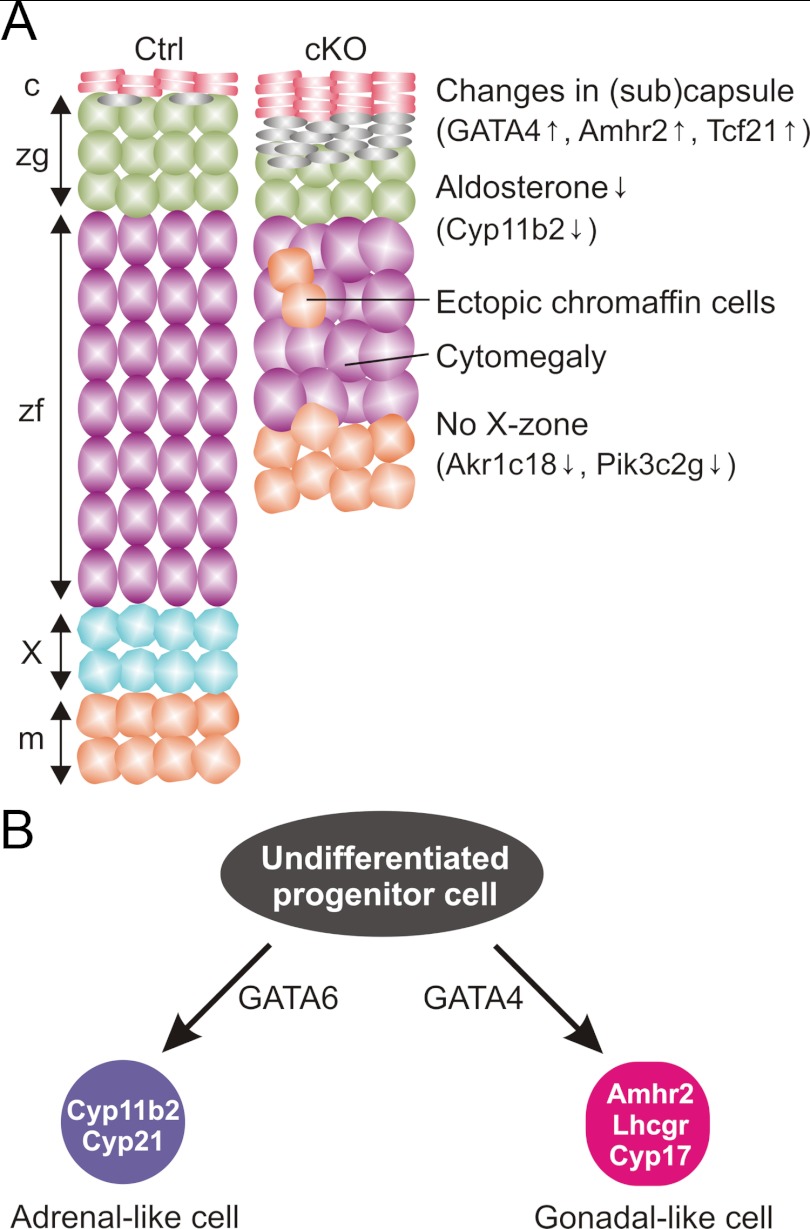
Like other epithelial tissues, the adrenal cortex regenerates through activation of endogenous stem cells, but the molecular pathways controlling the maintenance and differentiation of adrenocortical stem/progenitor cells are not fully understood (2, 3, 13, 90). Cells in the adrenal capsule and subcapsule are essential components of the adrenocortical stem/progenitor cell niche (2, 3). Our findings suggest that GATA6, a transcription factor expressed in the adrenal capsule and subcapsule, regulates adrenal homeostasis and function by influencing the differentiation of adrenocortical stem/progenitor cells. As summarized in Figure 8A, conditional deletion of Gata6 in SF1-positive cells produces a pleiotropic phenotype that includes 1) a thin, cytomegalic adrenal cortex, 2) decreased expression of the zG differentiation marker Cyp11b2 and an attendant reduction in serum aldosterone and whole adrenal aldosterone content, 3) blunted production of corticosterone in response to exogenous ACTH, and 4) the spontaneous accumulation of nonsteroidogenic cells expressing gonadal-like markers including Gata4 and Amhr2. At least one of these phenotypic changes, cytomegaly, reflects loss of Gata6 function in fetal tissue, because it is evident prenatally. The remaining changes could reflect loss of GATA6 function in either fetal or postnatal adrenocortical tissue, because SF1-positive cells in the fetal adrenal contribute to long-term repopulating cells in the adult cortex (4).
Figure 8.
A, Schematic depiction of phenotypic changes in the adrenal glands of young adult Gata6 cKO mice. This cartoon was adapted from drawings by Martinez and colleagues (46, 92) summarizing the phenotype of mice with an adrenocortical-specific deletion in Prkara1. Abbreviations: c, capsule; m, medulla, X, x-zone. B, Adrenocortical and gonadal steroidogenic cells arise from a common pool of progenitors. GATA6 promotes adrenocortical differentiation, whereas GATA4 promotes gonadal-like differentiation. Targeted mutagenesis of Gata6 is associated with spontaneous and gonadectomy-induced expression of gonadal-like markers.
It has been proposed that stem/progenitor cells in the adrenal capsule and subcapsule are multipotential, capable of differentiation into either adrenocortical lineages or gonadal-like cells (Figure 8B) (2, 9, 46, 56, 90). Certain genetic factors or hormonal manipulations, such as gonadectomy, have been shown to favor gonadal-like differentiation in the adrenal cortex (2, 9, 46, 56, 90). The accumulation of cells expressing Gata4 and Amhr2 in the adrenal subcapsule of young Gata6 cKO mice suggests that GATA6 inhibits the spontaneous differentiation of stem/progenitor cells into gonadal-like cells. The marked overexpression of Lhcgr and Cyp17 in the adrenal glands of gonadectomized Gata6 cKO mice compared with controls implies that GATA6 also limits sex steroidogenic cell differentiation in response to the hormonal changes that follow gonadectomy. Whereas GATA6 appears to inhibit sex steroidogenic differentiation, GATA4 is thought to promote this process. Gata4 is expressed in the gonadal-like cells that accumulate in the adrenal subcapsule of gonadectomized mice (9), and targeted mutagenesis of Gata4 attenuates the ovariectomy-induced accumulation of sex steroidogenic cells in the adrenal subcapsule of mice (75). Collectively, these findings offer genetic proof of the longstanding hypotheses that GATA6 promotes adrenocortical differentiation, whereas GATA4 drives gonadal-like differentiation (2, 9, 56).
Although young Gata6 cKO mice exhibit both a decreased capacity to produce glucocorticoid in response to exogenous ACTH and decreased basal serum aldosterone levels, the mice do not have overt adrenocortical insufficiency. It is conceivable that the hormonal abnormalities associated with conditional mutagenesis of Gata6 will worsen with age, as is the case in DAX1 deficiency. In both humans and mice, loss-of-function mutations in DAX1 cause excessive differentiation of subcapsular progenitors and eventual depletion of the stem/progenitor cell compartment (18). The adrenal glands of older DAX1-deficient males are characterized by a disorganized steroidogenic cortex that contains cytomegalic cells and impaired basal glucocorticoid production (19, 91). Other mouse mutants with aberrant adrenocortical differentiation overproduce adrenocorticoids as they age (46, 92); for example, constitutive activation of β-catenin signaling enhances differentiation of zG cells at the expense of zF cells, resulting in age-dependent hyperaldosteronism (74). Future experiments will assess age-related changes in the phenotype of the Gata6 cKO mice.
Along with cortical abnormalities, the adrenal glands of fetal and adult Gata6 cKO mice exhibit medulla defects, which manifest as chromaffin cells in the periphery of the gland (Figure 8A). Ectopic chromaffin cells have been reported in mice with alterations in adrenocortical β-catenin signaling (37), sonic hedgehog signaling (66), and SF1 function (47). Comparing a series of conditional mouse models generated with Sf1-cre, Huang et al (37) concluded that the adrenal cortex is dispensable for chromaffin cell differentiation but necessary for the subsequent growth and organization of the medulla. These same investigators noted that balanced β-catenin signaling in SF1-positive cortical cells is critical for proper organization of medulla cells in the developing adrenal gland (37). Loss of GATA6 function has been linked to aberrant β-catenin signaling in other tissues, such as pulmonary epithelium (93, 94), raising the possibility that β-catenin signaling is dysregulated in the adrenal glands of cKO mice.
Another distinctive feature of the Gata6 cKO adrenal is absence of an X-zone (Figure 8A). The X-zone is analogous to the fetal zone of the human adrenal. Both zones are located in the juxtamedullary region and regress with age (4). Activin induces apoptosis in the mouse X-zone (86) and human fetal zone (95). Lineage tracing studies indicate that the X-zone is a remnant of the mouse fetal adrenal cortex (6, 7), and mutations in the gene encoding DAX1 in mice and humans (96) cause lack of regression of the X-zone and fetal zone, respectively. Abnormalities in X-zone formation or regression have been reported in other mutant mice. Like Gata6 cKO mice, acd/acd mice lack an X-zone and have adrenocortical dysplasia, although the developmental defects in acd/acd mice are more severe and are associated with frank adrenocortical insufficiency (61). Pbx1 haploinsufficiency reduces the size of the X-zone but does not eliminate it (80). In female Prop1−/− mice, the X-zone is underdeveloped and undergoes early regression (44). Mice harboring an Sf1 mutation that eliminates sumoylation exhibit persistence of the X-zone (47). Similarly, parous female mice with an adrenocortical-specific deletion in Prkara1, encoding the type 1 α-regulatory subunit of cAMP-dependent protein kinase A, display persistence of a large X-like zone (46, 92). Whether the absence of an X-zone in Gata6 cKO mice reflects a lack of progenitor proliferation or early degeneration of a nascent zone is unclear. Gata6 is not expressed in the X-zone of postnatal mice (Supplemental Figure 7, E–H), arguing that the effect of Gata6 ablation on X-zone development may be indirect. The increased expression of Inhba and Inhbb in the cKO adrenal raises the possibility that local production of activins by gonadal-like cells causes precocious X-zone degeneration.
Using microarray hybridization, the Pik3c2g gene, which encodes a class II PI3K containing a C2 domain (97), was found to be markedly downregulated in the adrenal gland of nulliparous female Gata6 cKO mice. LMD established that Pik3c2g is a novel marker of the X-zone. The precise biological function of Pik3c2g in the adrenal X-zone or in other organs is unknown, but this subset of PI3Ks is known to phosphorylate phosphatidylinositol (PI) and PI4P but not PI(4,5)P2 (97). C2 domains are Ca2+-dependent phospholipid-binding motifs that mediate translocation of proteins to membranes.
In summary, conditional mutagenesis of Gata6 in SF1-positive cells of the mouse results in a complex adrenal phenotype, highlighted by aberrant differentiation of adrenocortical stem/progenitor cells into gonadal-like cells, ectopic chromaffin cells, and absence of an X-zone. By studying the abnormalities in these mice, insight may be gained into the regulation of normal and pathological adrenocortical cell development. Based on analogous conditional mutagenesis studies of Gata6 in pulmonary (93, 94) and intestinal epithelia (55, 98), we speculate that GATA6 may regulate the balance between stem/progenitor cell expansion and differentiation in the adrenal cortex.
Acknowledgments
We thank Ann-Kathrin Löbs for assistance with in situ hybridization, Kris Jehti and Phil Cryer for help with catecholamine measurements, Bob Heuckeroth for providing TH antibody and technical advice, and members of the histology, laser microscopy, EM, and microarray cores for their assistance.
This work was supported by National Institutes of Health Grants DK52574, DK75618, and HD047349; Children's Discovery Institute; McDonnell Center for Systems Neuroscience; Academy of Finland; and Sigrid Jusélius Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- cKO
- conditional knockout
- E17.5
- embryonic day 17.5
- EM
- electron microscopy
- ER
- endoplasmic reticulum
- FITC
- fluorescein isothiocyanate
- H&E
- hematoxylin and eosin
- HPA
- hypothalamic-pituitary-adrenal
- LMD
- Laser microdissection
- P10
- postnatal day 10
- P21
- postnatal day 21
- PI
- phosphatidylinositol
- PI3K
- phophoinositide-3 kinase
- qRT-PCR
- quantitative RT-PCR
- SF1
- steroidogenic factor 1
- TH
- tyrosine hydroxylase
- zF
- zona fasciculata
- zG
- zona glomerulosa
- zR
- zona reticularis.
References
- 1. Wood MA, Hammer GD. Adrenocortical stem and progenitor cells: unifying model of two proposed origins. Mol Cell Endocrinol. 2011;336:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simon DP, Hammer GD. Adrenocortical stem and progenitor cells: implications for adrenocortical carcinoma. Mol Cell Endocrinol. 2012;351:2–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laufer E, Kesper D, Vortkamp A, King P. Sonic hedgehog signaling during adrenal development. Mol Cell Endocrinol. 2012;351:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morohashi K, Zubair M. The fetal and adult adrenal cortex. Mol Cell Endocrinol. 2011;336:193–197 [DOI] [PubMed] [Google Scholar]
- 5. Dunn TB. Normal and pathologic anatomy of the adrenal gland of the mouse, including neoplasms. J Natl Cancer Inst. 1970;44:1323–1389 [PubMed] [Google Scholar]
- 6. Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26:4111–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zubair M, Oka S, Parker KL, Morohashi K. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol Endocrinol. 2009;23:1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keeney DS, Jenkins CM, Waterman MR. Developmentally regulated expression of adrenal 17α-hydroxylase cytochrome P450 in the mouse embryo. Endocrinology. 1995;136:4872–4879 [DOI] [PubMed] [Google Scholar]
- 9. Bielinska M, Kiiveri S, Parviainen H, Mannisto S, Heikinheimo M, Wilson DB. Gonadectomy-induced adrenocortical neoplasia in the domestic ferret (Mustela putorius furo) and laboratory mouse. Vet Pathol. 2006;43:97–117 [DOI] [PubMed] [Google Scholar]
- 10. Bielinska M, Parviainen H, Kiiveri S, Heikinheimo M, Wilson DB. Origin and molecular pathology of adrenocortical neoplasms. Vet Pathol. 2009;46:194–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151:1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Looyenga BD, Wiater E, Vale W, Hammer GD. Inhibin-A antagonizes TGFβ2 signaling by down-regulating cell surface expression of the TGFβ coreceptor betaglycan. Mol Endocrinol. 2010;24:608–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gardiner JR, Shima Y, Morohashi K, Swain A. SF-1 expression during adrenal development and tumourigenesis. Mol Cell Endocrinol. 2012;351:12–18 [DOI] [PubMed] [Google Scholar]
- 14. Bland ML, Fowkes RC, Ingraham HA. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol. 2004;18:941–952 [DOI] [PubMed] [Google Scholar]
- 15. Bland ML, Jamieson CA, Akana SF, et al. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci U S A. 2000;97:14488–14493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buaas FW, Gardiner JR, Clayton S, Val P, Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development. 2012;139:4561–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lalli E, Melner MH, Stocco DM, Sassone-Corsi P. DAX-1 blocks steroid production at multiple levels. Endocrinology. 1998;139:4237–4243 [DOI] [PubMed] [Google Scholar]
- 18. Achermann JC, Meeks JJ, Jameson JL. Phenotypic spectrum of mutations in DAX-1 and SF-1. Mol Cell Endocrinol. 2001;185:17–25 [DOI] [PubMed] [Google Scholar]
- 19. Scheys JO, Heaton JH, Hammer GD. Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology. 2011;152:3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol. 2008;22:781–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiiveri S, Liu J, Westerholm-Ormio M, Narita N, Wilson DB, Voutilainen R, Heikinheimo M. Differential expression of GATA-4 and GATA-6 in fetal and adult mouse and human adrenal tissue. Endocrinology. 2002;143:3136–3143 [DOI] [PubMed] [Google Scholar]
- 22. Jimenez P, Saner K, Mayhew B, Rainey WE. GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis. Endocrinology. 2003;144:4285–4288 [DOI] [PubMed] [Google Scholar]
- 23. Nakamura Y, Xing Y, Sasano H, Rainey WE. The mediator complex subunit 1 enhances transcription of genes needed for adrenal androgen production. Endocrinology. 2009;150:4145–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakamura Y, Suzuki T, Sasano H. Transcription factor GATA-6 in the human adrenocortex: association with adrenal development and aging. Endocr J. 2007;54:783–789 [DOI] [PubMed] [Google Scholar]
- 25. Flück CE, Miller WL. GATA-4 and GATA-6 modulate tissue-specific transcription of the human gene for P450c17 by direct interaction with Sp1. Mol Endocrinol. 2004;18:1144–1157 [DOI] [PubMed] [Google Scholar]
- 26. Martin LJ, Taniguchi H, Robert NM, Simard J, Tremblay JJ, Viger RS. GATA factors and the nuclear receptors SF-1/LRH-1 are key mutual partners in the regulation of the human HSD3B2 promoter. Mol Endocrinol. 2005;19:2358–2370 [DOI] [PubMed] [Google Scholar]
- 27. Huang YH, Lee CY, Tai PJ, et al. Indirect regulation of human dehydroepiandrosterone sulfotransferase family 1A member 2 by thyroid hormones. Endocrinology. 2006;147:2481–2489 [DOI] [PubMed] [Google Scholar]
- 28. Saner KJ, Suzuki T, Sasano H, et al. Steroid sulfotransferase (SULT2A1) gene transcription is regulated by steroidogenic factor 1 (SF1) and GATA-6 in the human adrenal. Mol Endocrinol. 2004;19:184–197 [DOI] [PubMed] [Google Scholar]
- 29. Allen HL, Flanagan SE, Shaw-Smith C, et al. GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nat Genet. 2012;44:20–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maitra M, Koenig SN, Srivastava D, Garg V. Identification of GATA6 sequence variants in patients with congenital heart defects. Pediatr Res. 2010;68:281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonnefond A, Sand O, Guerin B, et al. GATA6 inactivating mutations are associated with heart defects and, inconsistently, with pancreatic agenesis and diabetes. Diabetologia. 2012;55:2845–2847 [DOI] [PubMed] [Google Scholar]
- 32. Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732 [PubMed] [Google Scholar]
- 33. Morrisey EE, Tang Z, Sigrist K, et al. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sodhi CP, Li J, Duncan SA. Generation of mice harbouring a conditional loss-of-function allele of Gata6. BMC Dev Biol. 2006;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dhillon H, Zigman JM, Ye C, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203 [DOI] [PubMed] [Google Scholar]
- 36. Bielinska M, Parviainen H, Porter-Tinge SB, et al. Mouse strain susceptibility to gonadectomy-induced adrenocortical tumor formation correlates with the expression of GATA-4 and luteinizing hormone receptor. Endocrinology. 2003;144:4123–4133 [DOI] [PubMed] [Google Scholar]
- 37. Huang CC, Liu C, Yao HH. Investigating the role of adrenal cortex in organization and differentiation of the adrenal medulla in mice. Mol Cell Endocrinol. 2012;361:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weber MM, Fottner C, Schmidt P, et al. Postnatal overexpression of insulin-like growth factor II in transgenic mice is associated with adrenocortical hyperplasia and enhanced steroidogenesis. Endocrinology. 1999;140:1537–1543 [DOI] [PubMed] [Google Scholar]
- 39. Lee G, Makhanova N, Caron K, et al. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology. 2005;146:2650–2656 [DOI] [PubMed] [Google Scholar]
- 40. Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 is required for sex steroidogenic cell development in the fetal mouse. Dev Dyn. 2007;236:203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anttonen M, Ketola I, Parviainen H, Pusa AK, Heikinheimo M. FOG-2 and GATA-4 are coexpressed in the mouse ovary and can modulate Mullerian-inhibiting substance expression. Biol Reprod. 2003;68:1333–1340 [DOI] [PubMed] [Google Scholar]
- 42. Bielinska M, Genova E, Boime I, et al. Gonadotropin-induced adrenocortical neoplasia in NU/J nude mice. Endocrinology. 2005;146:3975–3984 [DOI] [PubMed] [Google Scholar]
- 43. Paul A, Laufer E. Endogenous biotin as a marker of adrenocortical cells with steroidogenic potential. Mol Cell Endocrinol. 2011;336:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nasonkin IO, Ward RD, Bavers DL, et al. Aged PROP1 deficient dwarf mice maintain ACTH production. PLoS One. 2011;6:e28355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Winnay JN, Xu J, O'Malley BW, Hammer GD. Steroid receptor coactivator-1-deficient mice exhibit altered hypothalamic-pituitary-adrenal axis function. Endocrinology. 2006;147:1322–1332 [DOI] [PubMed] [Google Scholar]
- 46. Sahut-Barnola I, de Joussineau C, Val P, et al. Cushing's syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. PLoS Genet. 2010;6:e1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee FY, Faivre EJ, Suzawa M, et al. Eliminating SF-1 (NR5A1) sumoylation in vivo results in ectopic hedgehog signaling and disruption of endocrine development. Dev Cell. 2011;21:315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shah SD, Tse TF, Clutter WE, Cryer PE. The human sympathochromaffin system. Am J Physiol. 1984;247:E380–E384 [DOI] [PubMed] [Google Scholar]
- 49. Kyronlahti A, Vetter M, Euler R, et al. GATA4 deficiency impairs ovarian function in adult mice. Biol Reprod. 2011;84:1033–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kyronlahti A, Euler R, Bielinska M, et al. GATA4 regulates Sertoli cell function and fertility in adult male mice. Mol Cell Endocrinol. 333:85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Slott VL, Suarez JD, Poss PM, Linder RE, Strader LF, Perreault SD. Optimization of the Hamilton-Thorn computerized sperm motility analysis system for use with rat spermatozoa in toxicological studies. Fundam Appl Toxicol. 1993;21:298–307 [DOI] [PubMed] [Google Scholar]
- 52. Heikinheimo M, Scandrett JM, Wilson DB. Localization of transcription factor GATA-4 to regions of the mouse embryo involved in cardiac development. Dev Biol. 1994;164:361–373 [DOI] [PubMed] [Google Scholar]
- 53. Val P, Jeays-Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev Biol. 2006;299:250–256 [DOI] [PubMed] [Google Scholar]
- 54. van Berlo JH, Elrod JW, van den Hoogenhof MM, et al. The transcription factor GATA-6 regulates pathological cardiac hypertrophy. Circ Res. 2010;107:1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Beuling E, Baffour-Awuah NY, Stapleton KA, et al. GATA factors regulate proliferation, differentiation, and gene expression in small intestine of mature mice. Gastroenterology. 2011;140:1219–1229 e1211–e1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Looyenga BD, Hammer GD. Origin and identity of adrenocortical tumors in inhibin knockout mice: implications for cellular plasticity in the adrenal cortex. Mol Endocrinol. 2006;20:2848–2863 [DOI] [PubMed] [Google Scholar]
- 57. Kim AC, Reuter AL, Zubair M, et al. Targeted disruption of β-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135:2593–2602 [DOI] [PubMed] [Google Scholar]
- 58. Heikinheimo M, Ermolaeva M, Bielinska M, et al. Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology. 1997;138:3505–3514 [DOI] [PubMed] [Google Scholar]
- 59. Bennett J, Wu YG, Gossen J, Zhou P, Stocco C. Loss of GATA-6 and GATA-4 in granulosa cells blocks folliculogenesis, ovulation, and follicle stimulating hormone receptor expression leading to female infertility. Endocrinology. 2012;153:2474–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zazopoulos E, Lalli E, Stocco DM, Sassone-Corsi P. DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature. 1997;390:311–315 [DOI] [PubMed] [Google Scholar]
- 61. Beamer WG, Sweet HO, Bronson RT, Shire JG, Orth DN, Davisson MT. Adrenocortical dysplasia: a mouse model system for adrenocortical insufficiency. J Endocrinol. 1994;141:33–43 [DOI] [PubMed] [Google Scholar]
- 62. Keegan CE, Hutz JE, Else T, et al. Urogenital and caudal dysgenesis in adrenocortical dysplasia (acd) mice is caused by a splicing mutation in a novel telomeric regulator. Hum Mol Genet. 2005;14:113–123 [DOI] [PubMed] [Google Scholar]
- 63. Shuman C, Beckwith JB, Smith AC, Weksberg R. 1993 Beckwith-Wiedemann syndrome. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, eds. GeneReviews. Seattle, WA: University of Washington; 2000. http://www.ncbi.nlm.nih.gov/books/NBK1394/. Updated December 14, 2010 [Google Scholar]
- 64. Pedreira CC, Savarirayan R, Zacharin MR. IMAGe syndrome: a complex disorder affecting growth, adrenal and gonadal function, and skeletal development. J Pediatr. 2004;144:274–277 [DOI] [PubMed] [Google Scholar]
- 65. Arboleda VA, Lee H, Parnaik R, et al. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nat Genet. 2012;44:788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106:21185–21190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Karpac J, Ostwald D, Bui S, Hunnewell P, Shankar M, Hochgeschwender U. Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology. 2005;146:2555–2562 [DOI] [PubMed] [Google Scholar]
- 68. Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci U S A. 1997;94:11540–11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hu MC, Hsu NC, El Hadj NB, et al. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol. 2002;16:1943–1950 [DOI] [PubMed] [Google Scholar]
- 70. Firth CH, Dunn TB. Tumours of the adrenal gland. IARC Sci Publ. 1994;111:595–609 [PubMed] [Google Scholar]
- 71. Jabara S, Christenson LK, Wang CY, et al. Stromal cells of the human postmenopausal ovary display a distinctive biochemical and molecular phenotype. J Clin Endocrinol Metab. 2003;88:484–492 [DOI] [PubMed] [Google Scholar]
- 72. Johnsen IK, Slawik M, Shapiro I, et al. Gonadectomy in mice of the inbred strain CE/J induces proliferation of sub-capsular adrenal cells expressing gonadal marker genes. J Endocrinol. 2006;190:47–57 [DOI] [PubMed] [Google Scholar]
- 73. Hughes CR, Guasti L, Meimaridou E, et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. 2012;122:814–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Berthon A, Sahut-Barnola I, Lambert-Langlais S, et al. Constitutive β-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19:1561–1576 [DOI] [PubMed] [Google Scholar]
- 75. Krachulec J, Vetter M, Schrade A, et al. GATA4 Is a critical regulator of gonadectomy-induced adrenocortical tumorigenesis in mice. Endocrinology. 2012;153:2599–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sato Y, Baba T, Zubair M, et al. Importance of forkhead transcription factor Fkhl18 for development of testicular vasculature. Mol Reprod Dev. 2008;75:1361–1371 [DOI] [PubMed] [Google Scholar]
- 77. Kim AC, Barlaskar FM, Heaton JH, et al. In search of adrenocortical stem and progenitor cells. Endocr Rev. 2009;30:241–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lu J, Richardson JA, Olson EN. Capsulin: a novel bHLH transcription factor expressed in epicardial progenitors and mesenchyme of visceral organs. Mech Dev. 1998;73:23–32 [DOI] [PubMed] [Google Scholar]
- 79. Beuschlein F, Mutch C, Bavers DL, et al. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology. 2002;143:3122–3135 [DOI] [PubMed] [Google Scholar]
- 80. Lichtenauer UD, Duchniewicz M, Kolanczyk M, et al. Pre-B-cell transcription factor 1 and steroidogenic factor 1 synergistically regulate adrenocortical growth and steroidogenesis. Endocrinology. 2007;148:693–704 [DOI] [PubMed] [Google Scholar]
- 81. Halder SK, Takemori H, Hatano O, Nonaka Y, Wada A, Okamoto M. Cloning of a membrane-spanning protein with epidermal growth factor-like repeat motifs from adrenal glomerulosa cells. Endocrinology. 1998;139:3316–3328 [DOI] [PubMed] [Google Scholar]
- 82. Okamoto M, Takemori H, Halder SK, Nonaka Y, Hatano O. Implication of ZOG protein (zona glomerulosa-specific protein) in zone development of the adrenal cortex. Endocr Res. 1998;24:515–520 [DOI] [PubMed] [Google Scholar]
- 83. Gineau L, Cognet C, Kara N, Lach FP, et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. 2012;122:821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Almeida H, Matos L, Ferreira J, Neves D. Age-related effects of dexamethasone administration in adrenal zona reticularis. Ann N Y Acad Sci. 2006;1067:354–360 [DOI] [PubMed] [Google Scholar]
- 85. Hirokawa N, Ishikawa H. Electron microscopic observations on postnatal development of the X zone in mouse adrenal cortex. Z Anat Entwicklungsgesch. 1974;144:85–100 [DOI] [PubMed] [Google Scholar]
- 86. Beuschlein F, Looyenga BD, Bleasdale SE, et al. Activin induces X-zone apoptosis that inhibits luteinizing hormone-dependent adrenocortical tumor formation in inhibin-deficient mice. Mol Cell Biol. 2003;23:3951–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Deacon CF, Mosley W, Jones IC. The X zone of the mouse adrenal cortex of the Swiss albino strain. Gen Comp Endocrinol. 1986;61:87–99 [DOI] [PubMed] [Google Scholar]
- 88. Hirokawa N, Ishikawa H. Electron microscopic observations on the castration-induced X zone in the adrenal cortex of male mice. Cell Tissue Res. 1975;162:119–130 [DOI] [PubMed] [Google Scholar]
- 89. Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20α-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148:976–988 [DOI] [PubMed] [Google Scholar]
- 90. Berthon A, Martinez A, Bertherat J, Val P. Wnt/β-catenin signalling in adrenal physiology and tumour development. Mol Cell Endocrinol. 2012;351:87–95 [DOI] [PubMed] [Google Scholar]
- 91. Guo W, Mason JS, Stone CG, Jr, et al. Diagnosis of X-linked adrenal hypoplasia congenita by mutation analysis of the DAX1 gene. JAMA. 1995;274:324–330 [PubMed] [Google Scholar]
- 92. de Joussineau C, Sahut-Barnola I, Levy I, et al. The cAMP pathway and the control of adrenocortical development and growth. Mol Cell Endocrinol. 2012;351:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang Y, Goss AM, Cohen ED, et al. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tian Y, Zhang Y, Hurd L, et al. Regulation of lung endoderm progenitor cell behavior by miR302/367. Development. 2011;138:1235–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Spencer SJ, Rabinovici J, Mesiano S, Goldsmith PC, Jaffe RB. Activin and inhibin in the human adrenal gland. Regulation and differential effects in fetal and adult cells. J Clin Invest. 1992;90:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Muscatelli F, Strom TM, Walker AP, et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372:672–676 [DOI] [PubMed] [Google Scholar]
- 97. Misawa H, Ohtsubo M, Copeland NG, Gilbert DJ, Jenkins NA, Yoshimura A. Cloning and characterization of a novel class II phosphoinositide 3-kinase containing C2 domain. Biochem Biophys Res Commun. 1998;244:531–539 [DOI] [PubMed] [Google Scholar]
- 98. Beuling E, Aronson BE, Tran LM, et al. GATA6 is required for proliferation, migration, secretory cell maturation, and gene expression in the mature mouse colon. Mol Cell Biol. 2012;32:3392–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]