Abstract
Damage to the cerebellum can cause significant problems in the coordination of voluntary arm movements. One prominent idea is that incoordination stems from an inability to predictively account for the complex mechanical interactions between the arm's several joints. Motivated by growing evidence that corrective feedback control shares important capabilities and neural substrates with feedforward control, we asked whether cerebellar damage impacts feedback stabilization of the multijoint arm appropriate for the arm's intersegmental dynamics. Specifically, we tested whether cerebellar dysfunction impacts the ability of posterior deltoid to incorporate elbow motion in its long-latency response (R2 = 45–75 ms and R3 = 75–100 ms after perturbation) to an unexpected torque perturbation. Healthy and cerebellar-damaged subjects were exposed to a selected pattern of shoulder-elbow displacements to probe the response pattern from this shoulder extensor muscle. The healthy elderly subjects expressed a long-latency response linked to both shoulder and elbow motion, including an increase/decrease in shoulder extensor activity with elbow flexion/extension. Critically, cerebellar-damaged subjects displayed the normal pattern of activity in the R3 period indicating an intact ability to rapidly integrate multijoint motion appropriate to the arm's intersegmental dynamics. However, cerebellar-damaged subjects had a lower magnitude of activity that was specific to the long-latency period (both R2 and R3) and a slightly delayed onset of multijoint sensitivity. Taken together, our results suggest that the basic motor pattern of the long-latency response is housed outside the cerebellum and is scaled by processes within the cerebellum.
Keywords: long-latency reflex, multijoint dynamics, feedback
the cerebellum is a key neural structure for achieving coordinated arm movements as evidenced by distinctive abnormalities following its degeneration or damage from trauma (Bastian 2002; Ebner et al. 2011; Holmes 1939). Clinical signs of cerebellar damage include curved and more variable hand paths due to poor elbow-shoulder synchronization and termination errors marked with overshoots, undershoots, and oscillations (Bastian et al. 1996, 2000; Cooper et al. 2000; Day et al. 1998; Gilman et al. 1976; Massaquoi and Hallett 1996; Timmann et al. 2001; Topka et al. 1998a, 1998b). These abnormalities are markedly worse when moving several joints of the arm compared with when motion is physically restricted to a single joint (Bastian et al. 2000; Brown et al. 1990; Hallett et al. 1975; Hore and Flament 1986; Hore and Vilis 1984), likely because of the greater complexity of multijoint dynamics (Fig. 1A). For example, when the limb is unconstrained one must actively stabilize the shoulder in order to produce motion at just the elbow, otherwise the shoulder will passively rotate opposite the exerted elbow torque. Healthy subjects account for these interactions by appropriately timing and scaling shoulder monoarticular activity during intended elbow movements (Almeida et al. 1995; Bastian et al. 2000; Boose et al. 1999; Gribble and Ostry 1999). In contrast, anticipatory shoulder monoarticular activity by cerebellar-damaged subjects is poorly timed and scaled for these interactions, which results in shoulder excursions several times larger than normal (Bastian et al. 2000; Boose et al. 1999).
Fig. 1.
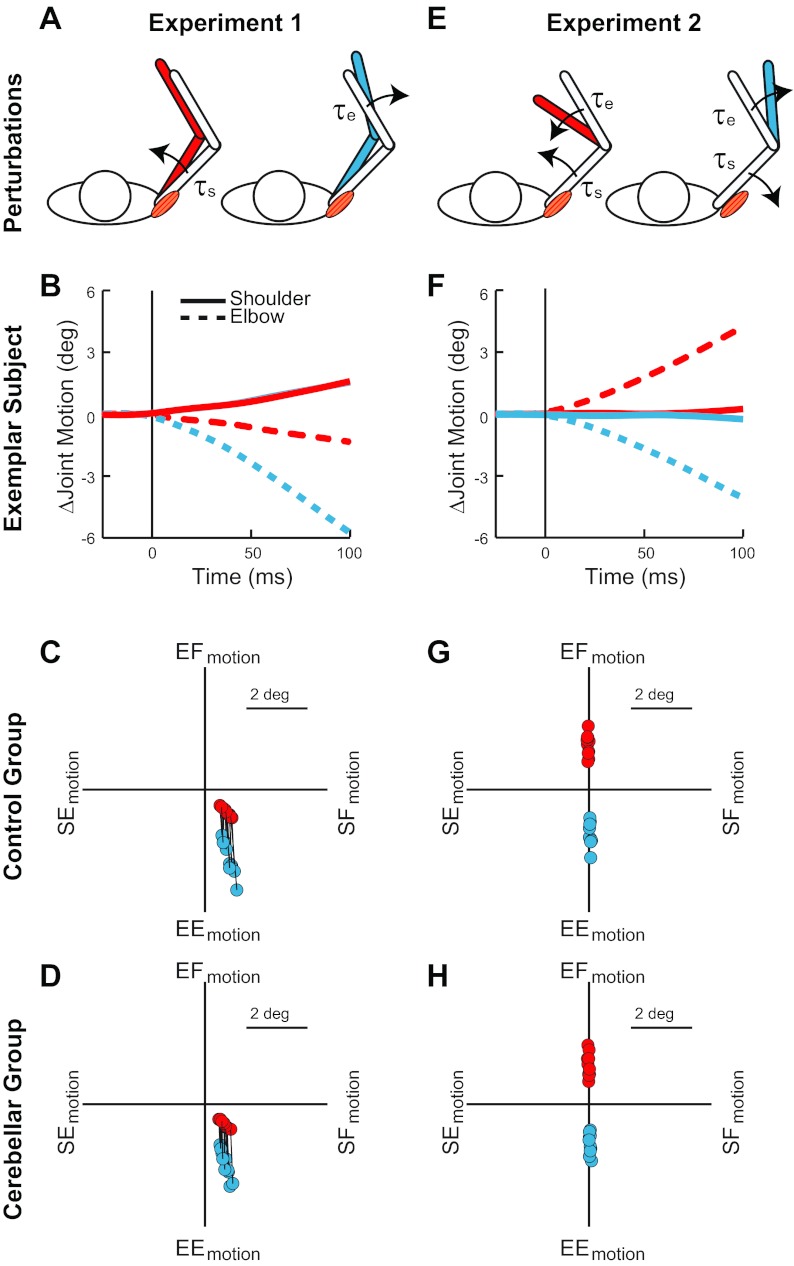
Limb dynamics and torque conditions. A: conditions of experiment 1 where shoulder flexion torque (red-filled arm) and elbow extension torque (blue-filled arm) displace the limb in different trials. The shoulder extensor muscle needs to provide more compensation during the shoulder torque condition than the elbow torque condition. B: data from an exemplar healthy subject showing that the 2 perturbing torques of experiment 1 initially cause a similar change in shoulder motion (solid lines) but clearly different elbow motion (dashed lines). C: pattern of joint displacement 50 ms after the perturbing torques of experiment 1. Data are shown for each control subject, with black lines connecting their data from the 2 conditions. EF, elbow flexion; SF, shoulder flexion; EE, elbow extension; SE, shoulder extension. D: data from the cerebellar-damaged subjects in the same format as in C. E: conditions of experiment 2 where flexion torque is applied at both joints (red-filled arm) and extension torque is applied at both joints (blue-filled arm) on different trials. The 2 perturbations require increased compensation and decreased compensation from the shoulder extensor muscle, respectively. F: data from the same exemplar healthy subject showing the substantial elbow motion and negligible shoulder motion induced by the perturbations. G: pattern of joint displacement 50 ms after the perturbing torques of experiment 2. Data from each control subject are shown. H: data from the cerebellar-damaged subjects in the same format as in G.
Appropriate timing and modulation of muscle activity in anticipation of the arm's intersegmental dynamics has long been considered a signature of healthy feedforward control. However, accumulating research indicates that some feedback processes have a knowledge of limb dynamics similar to feedforward control (Gritsenko et al. 2009; Wagner and Smith 2008), possibly reflecting a functional overlap that is fundamental to motor coordination (Scott 2004; Shadmehr and Krakauer 2008; Todorov and Jordan 2001). The long-latency response (LLR) is a prime candidate for examining altered feedback control following cerebellar damage as it is one of our fastest corrective actions (occurring 45–100 ms after a mechanical perturbation) (for review see Matthews 1991; Pruszynski and Scott 2012; Shemmell et al. 2010) and incorporates motion information from several joints appropriate for the arm's intersegmental dynamics (Gielen et al. 1988; Koshland et al. 1991; Latash 2000; Soechting and Lacquaniti 1988).
A relevant example is the posterior deltoid's excitatory LLR following a perturbation causing pure elbow flexion (Kurtzer et al. 2008, 2009; Pruszynski et al. 2011). The fast reaction of the shoulder muscle accounts for the mechanical interactions between the elbow and the shoulder joints and helps counter the underlying cause of pure elbow motion—torque applied at both joints. Alternatively, if subjects only generated an elbow muscle response to pure elbow motion then they would not create a balance of forces, their shoulder joint would move into flexion, and their arm would not come to rest. Note that the described LLR of the posterior deltoid is not direct compensation of the imposed shoulder torque since this monoarticular muscle is neither stretched nor shortened and none of its intramuscular sensors is altered. The fast response from the posterior deltoid to elbow joint motion must be based on sensory information outside that muscle, presumably those muscles spanning the elbow joint.
Previous studies have shown that cerebellar damage can impair some functions of LLRs such as the ability to scale response magnitude to the expected duration of a perturbation (Horak and Diener 1994; Hore and Vilis 1984; Timmann and Horak 1997; Timmann et al. 2000). However, the few previous studies on the upper limb either used electrical nerve stimulation (Claus et al. 1986; Friedmann et al. 1987) or restricted limb motion to a single joint (Hore and Vilis 1984; Marsden et al. 1977b), so they could not address whether cerebellar integrity is required for LLRs to incorporate motion from several joints appropriate for the arm's intersegmental dynamics. To address this important gap in our knowledge we examined feedback control of the multijoint arm in cerebellar-damaged subjects and healthy age-matched control subjects, using a paradigm previously employed with healthy young adults (Kurtzer et al. 2008).
We hypothesized that cerebellar damage would eliminate the ability of the posterior deltoid's LLRs to respond to elbow motion (and account for the intersegmental dynamics) but not alter its response to shoulder motion (Kurtzer et al. 2008, 2009), i.e., an impairment in “heteronymous reflexes” but not “homonymous reflexes.” Figure 2 illustrates the predictions for the two experiments. For conditions causing both shoulder and elbow motion, cerebellar-damaged subjects were predicted to have shoulder LLRs based only on shoulder motion. Likewise, for conditions causing only elbow motion, cerebellar-damaged subjects were predicted to have no evoked activity in their shoulder LLRs. We were surprised to find that cerebellar damage did not alter the ability of the posterior deltoid's LLR to respond to both elbow and shoulder motion. However, cerebellar damage did result in significant changes from normal, as their LLR had a diminished magnitude and slight delay. Our results suggest that the basic motor pattern of the LLR is housed outside the cerebellum and that the cerebellum contributes to the timing and scaling of this basic pattern.
Fig. 2.
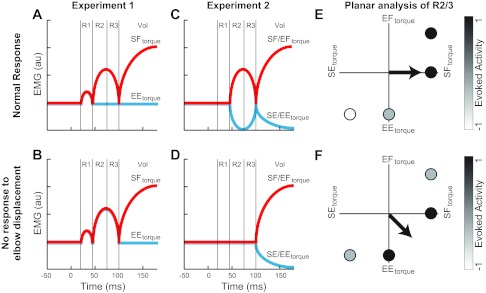
Contrasting predictions of long-latency response (LLR) of the shoulder extensor muscle following cerebellar damage. A: cartoon of the normal response of the shoulder extensor to the perturbations in experiment 1 where shoulder flexor (SF) torque and elbow extensor (EE) torque cause similar amounts of shoulder motion and different amounts of elbow motion. A similar R1 is evoked, whereas later periods show larger activity for the shoulder torque than the elbow torque perturbation. The same pattern is predicted if LLRs are minimally impacted by cerebellar damage. au, Arbitrary units. B: predicted pattern of shoulder extensor LLR if cerebellar damage eliminates the ability to respond to elbow motion: LLR would be identical for the 2 conditions with the normal magnitude when responding to shoulder torque. C: cartoon of the normal muscle response in experiment 2: flexor torque at both joints (SF/EF) and extensor torque at both joints (SE/EE) cause substantial elbow motion and a negligible amount of shoulder motion. Normally, subjects have no evoked R1, whereas the later periods show reciprocal increases and decreases in activity for the flexor torque and extensor torque perturbations. D: predicted pattern of the shoulder extensor's LLR if cerebellar damage eliminates the ability to respond to elbow motion: LLR would not be evoked for the 2 conditions. E: diagram of the predicted “preferred torque direction” (PTD) for control subjects shown in shoulder-elbow torque space. Graph depicts the predicted activity of LLR to the 4 torque conditions of experiments 1 and 2 (SF, EE, SF/EF, and SE/EE); white shading shows maximal inhibitory responses, whereas black shading shows maximal excitatory responses. The arrow indicates the plane-fit to the activity and is directed toward shoulder flexor torque at 0°. F: predicted PTD for cerebellar-damaged subjects. The arrow indicates the plane-fit and is directed toward midway between shoulder flexor torque and elbow extensor torque at 315°.
MATERIALS AND METHODS
Subjects.
We studied 20 subjects in the experiment, which lasted ∼1.5 h. Ten subjects had clinically significant cerebellar damage (Table 1). Most had either known genetic cerebellar degeneration (e.g., spinocerebellar ataxia type 6) or sporadic cerebellar degeneration. All subjects were clinically examined to rule out any signs of extracerebellar involvement in the arm, including spasticity or abnormal muscle tone, fine touch sensory loss (by Semmes-Weinstein monofilament testing), and proprioceptive deficits. Proprioception was assessed with a standard clinical technique while the individual's eyes were closed and the index finger of each hand was rotated by the examiner about the metacarpophalangeal joint ≈5° in either a flexion or extension direction. During this procedure, subjects were instructed to remain passive and identify movement direction.
Table 1.
Subject demographics
| Subject | Age, yr | Lesion | Limb Ataxia | Posture/Gait Ataxia |
|---|---|---|---|---|
| CBL01 | 75 | SCA6 | 19 | 25 |
| CBL02 | 67 | Sporadic | 18 | 29 |
| CBL03 | 43 | Sporadic | 26 | 24 |
| CBL04 | 53 | SCA6 | 20 | 10 |
| CBL05 | 72 | SCA6 | 26 | 23 |
| CBL06 | 68 | SCA6 | 4 | 3 |
| CBL07 | 37 | SCA8 | 20 | 14 |
| CBL08 | 54 | Sporadic | 25 | 22 |
| CBL09 | 50 | Sporadic | 19 | 10 |
| CBL10 | 56 | SCA6 | 23 | 29 |
SCA, spinocerebellar ataxia; Sporadic, sporadic cerebellar ataxia; Limb Ataxia, limb kinetic subscore from the International Cooperative Ataxia Rating Scale (ICARS); Posture/Gait Ataxia, posture and gait subscore from the ICARS.
We were vigilant about screening all of the individuals. The severity of movement dysfunction was assessed with the International Cooperative Ataxia Rating Scale (ICARS), where higher scores indicate greater impairment (Trouillas et al. 1997). The ICARS is organized into the following categories: posture and gait, limb control (kinetic), speech, and eye movements. Ten control subjects were matched for age (range = 38–75 yr, mean = 55.8 yr), sex (7 female, 3 male), the arm utilized (9 right and 1 left), and the magnitude of torque applied to the arm. All subjects gave informed consent and were paid for their time, and all procedures were reviewed and approved by the ethics committee at Johns Hopkins University.
Apparatus.
As described in previous studies (Kurtzer et al. 2008; Prusyznski et al. 2008; Scott 1999), we utilized a robotic exoskeleton (KINARM, BKIN Technologies, Kingston, ON, Canada) that permits flexion/extension movements of the shoulder and elbow in the horizontal plane and can selectively apply torques to each joint; shoulder angle is measured relative to the frontal plane, and elbow angle is measured between the forearm and upper arm; 0° is full extension. Visual targets and a hand-aligned cursor were presented in the same plane as the limb movement via a virtual-reality system while a cloth bib and a metal partition obscured direct vision of the subject's arm.
Muscle recording.
The experiments focused on the surface EMG from each subject's posterior deltoid (a monoarticular shoulder extensor). We also collected data from the pectoralis major (a monoarticular shoulder flexor) and report its responses as a secondary matter. Note that when we refer to a shoulder extensor muscle or a shoulder flexor muscle we only mean these two monoarticulars, not biarticular muscles or other muscles crossing the shoulder joint. We employed procedures similar to our earlier papers (Kurtzer et al. 2008; Pruszynski et al. 2008): a two-bar electrode (DE-2.1 Delsys, Boston, MA) was affixed to the muscle belly, and a ground electrode was placed on the subject's ankle after light abrasion of the overlying skin surfaces with alcohol.
Experimental task.
We examined corrective actions in a group of older adults, using procedures we previously applied to a population of young adults (Kurtzer et al. 2008; Pruszynski et al. 2008). Subjects maintained their arm's position against a background torque and a subsequent step torque that was unpredictable in time and direction. There was one perturbation per trial, and all trials proceeded in the following manner. Subjects stabilized their hand-aligned cursor (0.4-cm radius) within the center of a 2-cm-radius target that corresponded to a 45° shoulder angle and a 75° elbow angle. Subjects were asked to keep their hand in the target and to quickly return if displaced. A shoulder flexor torque was ramped up over 500 ms and remained constant to elicit background activity in the subject's posterior deltoid. After a random hold of 1–4 s, a step torque was applied to the subject's arm. The target turned green or red to indicate accurate or inaccurate performance depending on whether the subject stayed within the target for 1,000 ms of the subsequent 1,500 ms. Finally, the total applied torque ramped down to zero and remained off during an intertrial period of 1 s.
We employed four different step torques: shoulder flexion torque (1 Nm, 0 Nm), elbow extension torque (0 Nm, −1 Nm), shoulder-elbow flexion torque (1 Nm, 1 Nm), and shoulder-elbow extension torque (−1 Nm, −1 Nm). The absolute magnitude of both the background torque and the step torque were scaled by a single value (range = 1.25–3) so that cerebellar-damaged subjects could consistently compensate the applied torques and avoid fatigue; control subjects (matched for age and sex) countered the same magnitude of torque as the corresponding subjects with cerebellar damage. Note that the four perturbations were randomly intermingled, but we discuss them in two groups according to the pattern of joint motion that they induce.
The dynamics of the arm-robot system includes the properties of the robot and joint velocities. However, we can gain some insight into our experimental design by considering just the subject's arm (as the robot's exoskeleton has an inertia largely parallel to the enclosed limb), neglecting velocity terms (which are low immediately after the perturbation) and assuming the elbow angle equals 90° (which is nearby 75°). In this case, the complex nonlinear dynamic equations of the shoulder and elbow (from Soechting and Lacquaniti 1988) simplify to
where θ̈e and θ̈s are elbow and shoulder acceleration, respectively, Te and Ts are elbow and shoulder torque, respectively, and If and Ia are forearm and arm inertia, respectively.
These formulae highlight two features of the arm's dynamics that are independent of its particular mass distribution. First, if two torques are applied individually to either shoulder or elbow, the same shoulder acceleration will be induced if the two torques are opposite in sign and equal in magnitude. Second, if the two torque perturbations have the same sign and are equal in magnitude, then only the elbow will be accelerated. The four torque perturbations that we selected were appropriate to leverage these basic features.
Experiment 1: feedback response to same shoulder motion, different underlying torque.
Because of the arm's intersegmental dynamics, torque applied at a single joint will induce motion at multiple joints when the limb is free to move (Craig 2005; Graham et al. 2003; Hollerbach and Flash 1982) (Fig. 1A). For example, either shoulder flexion torque or elbow extension torque will cause the shoulder to move into flexion. We selected magnitudes of these two perturbations to induce the same amount of initial shoulder flexion and, hence, stretch of the posterior deltoid (Fig. 1, B and C). This allowed us to test whether this muscle's feedback response is only sensitive to its own muscle stretch as it would respond identically to the two perturbations. Alternatively, feedback control of this shoulder monoarticular could account for the arm's intersegmental dynamics by combining elbow and shoulder motion to generate a response countering the imposed torque. Here the shoulder extensor would generate a larger response during the condition with shoulder flexion torque than with elbow extensor torque; this differential pattern would reflect the net contribution of similar amounts of excitatory homonymous LLRs and different amounts of inhibitory heteronymous LLRs. A cartoon of the normal pattern of evoked activity is shown in Fig. 2A and depicts that the earliest muscle response (R1) is only sensitive to displacement of the shoulder, whereas subsequent muscle responses [R2, R3, and Voluntary (Vol)] incorporate shoulder and elbow motion appropriate for the limb's intersegmental dynamics. Figure 2B shows the predicted response for subjects with cerebellar damage, R2/3 only sensitive to shoulder motion.
Experiment 2: feedback response to pure elbow motion, underlying shoulder torque.
The perturbations used in this experiment were flexor torques applied to both joints or extensor torques applied to both joints (Fig. 1, E and F). These multijoint perturbations were selected to balance the interaction torque from the elbow to the shoulder joint so that pure elbow motion resulted—elbow flexion motion induced by a multijoint flexion perturbation and elbow extension motion induced by a multijoint extension perturbation. If feedback control of the posterior deltoid is only sensitive to its own muscle stretch then it should exhibit no response, i.e., no stretch of the shoulder monoarticular resulting in no response by the shoulder monoarticular. Alternatively, if feedback control of the posterior deltoid accounts for the mechanical interaction between the shoulder and elbow joint then a reciprocal pattern of muscle activity would be elicited—an increase in its activity following the multijoint flexion perturbation and a decrease following the multijoint extension perturbation—to help counter the underlying shoulder torque; this differential pattern would reflect the net contribution of no homonymous LLR and an excitatory and inhibitory heteronymous LLR, respectively. A cartoon of the normal pattern of evoked activity is shown in Fig. 2C and depicts that the earliest muscle response is only sensitive to its own stretch, whereas subsequent muscle responses (R2, R3, and Vol) incorporate shoulder and elbow motion appropriate for the limb's intersegmental dynamics. Figure 2D shows the predicted response for subjects with cerebellar damage, R2/3 only sensitive to shoulder motion.
Data analysis.
Trials were repeated 17–40 times for each condition (depending on the subject expressing fatigue), for a total ranging between 70 and 200 trials. These analyses described below showed similar results if we utilized the lowest trial repeat for everyone or used all the trials from each individual. Kinematic and electromyographic data were processed according to procedures described in our earlier papers (Kurtzer et al. 2008; Pruszynski et al. 2008). Angular positions of the shoulder and elbow were low-pass filtered (25 Hz, 2 pass, 6th-order Butterworth). EMG signals were amplified (gain = 10 K), band-pass filtered (20–450 Hz), digitally sampled at 1,000 Hz (PCI 6071E, National Instruments, Austin, TX), rectified, and normalized by the posterior deltoid's mean activity in the 100 ms prior to the perturbation. Accordingly, the activity of both shoulder muscles was normalized by the subject's posterior deltoid since it was preexcited by the background shoulder flexion torque. This approach assumes that the emg-force relation was largely similar between the two muscles. Note that all intrasubject comparisons are unaffected (similar to using unnormalized data) since it introduces a common factor to the subject.
Joint kinematics were examined shortly after the perturbations (50 ms) and during a later period of postural stabilization (500–1,000 ms after the perturbation). Accuracy during this later period was characterized by the net positional deviations from the starting angle and the net angular velocity (zero velocity at rest):
Repeated-measures ANOVAs and t-tests contrasted the kinematics of the control and cerebellar-damaged groups.
Muscle activity was examined in several periods based on earlier reports (Crago et al. 1976; Kurtzer et al. 2008; Lee and Tatton 1982; Prusyznski et al. 2008): R1 = 20–45 ms; R2 = 45–75 ms; R3 = 75–100 ms; and Vol = 120–180 ms. Hence, R2 and R3 comprise the early and late portions of the LLR.
Our experimental design allowed us to make straightforward comparisons between conditions to determine whether feedback responses of posterior deltoid reflected displacement of just the shoulder joint or incorporated elbow motion appropriate for the underlying shoulder torque. Paired t-tests determined changes from baseline and/or changes between conditions. Repeated-measures ANOVAs examined trends across different response periods.
We also used a planar regression to examine the pattern of posterior deltoid and limb displacement resulting from the four step torques of experiments 1 and 2. Specifically, we examined how the evoked muscle activity and limb displacement were related to a linear combination of imposed shoulder-elbow torque. Note that this simple equation accounts for the magnitude and pattern of the imposed torques:
The orientation of the best-fitting plane, termed a “preferred torque direction” or PTD, describes the relative sensitivity of muscle activity to the perturbing shoulder and elbow torque during a particular epoch (Herter et al. 2009; Kurtzer et al. 2005, 2008):
If shoulder torque is the x-axis, elbow torque is the y-axis, and flexion is positive, then maximal excitation to shoulder flexion translates to a PTD of 0°; maximal activity to elbow flexion, shoulder extension, and elbow extension torques would result in PTDs at 90°, 180°, and 270°, respectively.
Another important aspect of the plane-fit is the steepness of the plane, which describes the overall sensitivity of the evoked response, or “response magnitude,” to the perturbation torque.
Units are EMG/Nm after normalizing to the baseline activity with the background torque.
A final measure was the amount of simultaneous activity by the antagonist pair of shoulder muscles, i.e., the amount of cocontraction. Cocontraction can help counter unexpected perturbations and can be flexibly sculpted to the environmental instabilities and motor noise (Burdet et al. 2001; Selen et al. 2009). We examined the ratio of evoked activity in the pectoralis major versus evoked activity in the posterior deltoid:
Positive or negative values would indicate an excitatory or inhibitory evoked activity in the pectoralis major relative to its baseline compared with the evoked activity in the posterior deltoid. Positive values near 100 indicate a similar increase in activity/high cocontraction, small values near 0 indicate small evoked activity in pectoralis compared with posterior deltoid/low cocontraction, and negative values near −100 indicate changes in activity that are equal in magnitude but of opposite sign.
One prediction is that the shoulder extensor's LLR is based on just shoulder motion. The perturbation torque was chosen to induce identical shoulder motion across conditions (experiment 1, single-joint torques) and substantial elbow motion with no shoulder motion (experiment 2, multijoint torques). Accordingly, the PTD of the posterior deltoid would be 315°. Regressing the measured shoulder displacement at 50 ms after perturbation against the imposed torque yielded a PTD of 313.5°(0.8), which shows how closely the observed initial displacement approached the expected initial displacement. We used this latter value for the prediction of the shoulder extensor response based on just shoulder motion (Θ).
A contrasting prediction is that the shoulder extensor's LLR incorporates shoulder and elbow motion appropriate to counter the underlying torque perturbation because negating the step torque is ultimately needed to bring the limb to rest. Under this hypothesis, relative to the findings expected if the LLR is driven purely by shoulder motion, experiment 1 is predicted to show a reduction in EMG in response to pure elbow extension torque relative to that for pure shoulder flexion torque. Experiment 2 is predicted to show an increase in EMG response to simultaneous flexion torques at the shoulder and elbow and to show reduced EMG in response to simultaneous extension torques at the shoulder and elbow. For the planar regression, these changes translate, respectively, into reduced evoked activity in the 270° direction relative to the 360° direction, greater activity in the 45° direction and activity in the 225° direction. Together these differences correspond to a PTD that is shifted toward 360° and away 315°.
In principle, the PTD under this hypothesis could be 360°, i.e., maximal EMG sensitivity to pure flexion torque applied at the shoulder. However, this PTD is unlikely because of several biomechanical complexities including biarticular muscles. Biarticular muscles likely play an important role in shaping multijoint limb responses to imposed loads by generating passive and active torque across several joints (Franklin and Milner 2003; Hogan 1985). Their contribution also requires concomitant changes in the monoarticular muscles leading to a rotation of the PTDs of monoarticular muscles. For example, recruitment of a shoulder extensor muscle is needed during elbow extensor torque in order to counter the shoulder flexion torque generated by a biarticular flexor muscle (biceps brachii). The presence of the biarticular extensor muscle (triceps longus) could also allow the posterior deltoid to generate no muscle activity during shoulder flexor torque. There have been extensive reports of bias in a muscle's activity away from its line of mechanical action; however, these effects are not so strong that compensating the biarticulars could be considered the primary role of monoarticulars (Buchanan et al. 1989; Kurtzer et al. 2006a, 2006b; Nozaki et al. 2005; van Zuylen et al. 1988). We considered the PTDs during steady-state postural maintenance from a separate experiment to better approximate the ideal pattern than a PTD of 360° (Kurtzer et al. 2008). We found that the PTDs were modestly biased away from pure shoulder flexion torque toward elbow extension torque at 354°. We used this value to predict the response to the underlying shoulder torque (τ). Note that this remains an approximation and does not account for the impact of muscle viscosity or stiffness.
Comparisons of the measured and predicted PTDs were conducted by calculating the circular mean and variance (Batschelet 1981). Statistical significance was met when the 95% confidence interval of the measured PTD (circular C.I.) did not overlap with the predicted PTD. Comparisons of the PTD and response sensitivity between the control and cerebellar-damaged groups utilized the Watson-Williams test and t-test, respectively. Note that we only included subjects who had a significant plane-fit. Significance for all tests was set at P < 0.05. We selected one-sided or two-sided tests for the normal subjects on the basis of the pattern of results observed in our previous studies. We predicted that cerebellar subjects would show a selective deficit in the long-latency reflex (see Fig. 2).
We used the receiver-operator characteristic (ROC) technique to examine the evolution of multijoint responsiveness on a moment-by-moment basis (Green and Swets 1966; Kurtzer et al. 2010; Prusyznski et al. 2008). For each time step (1 ms), we determined the probability that an ideal observer could discriminate between two conditions based on the two sets of muscle activity that the subject expressed. Values of 0 and 1 indicate perfect discrimination, whereas a value of 0.5 indicates performance at chance. We contrasted the muscle activity from the two conditions in experiment 1 (shoulder flexion torque vs. elbow extension torque) and experiment 2 (multijoint flexion torque vs. multitorque extension torque). After separately generating probability trajectories for each subject, we obtained a group probability trajectory by averaging their trajectories together. The first expression of multijoint responsiveness was identified when the group average probability of discrimination increased from 0.5 (equal likelihood) to 3 SD above its nominal variability (observed −50 to 0 ms before torque onset) and remained above this threshold for 5 consecutive samples/ms.
RESULTS
Torque perturbations and patterns of joint motion.
Experiment 1 comprised two conditions, a shoulder flexion torque and an elbow extension torque (Fig. 1B), which caused simultaneous flexion of the shoulder and extension of the elbow (Fig. 1C). Critically, the two perturbations led to a similar amount of initial shoulder motion, whereas the amount of initial elbow extension was substantially different. On average, the shoulder and elbow motion were ≈10% and ≈210% greater in the first 50 ms after the imposed elbow torque compared with shoulder torque. Note that each subject showed this pattern despite the wide range in the absolute size of joint displacement across subjects because of their differently sized arms and step torques. Moreover, the cerebellar-damaged and control groups did not differ in the absolute amount of initial joint displacement [shoulder: F(1,18) = 2,37, P > 0.1; elbow: F(1,18) = 2.08, P > 0.15] (Fig. 1D).
Experiment 2 imposed flexion torque at both joints and extension torque at both joints (Fig. 1E). In each subject, the combined flexion (extension) torques induced substantial flexion (extension) motion at the elbow and little shoulder motion (Fig. 1F). Despite the wide range of absolute joint motion due to their differently sized arms, the shoulder displacement was consistently <3% of the size of the elbow displacement for both conditions (Fig. 1, G and H). In addition, the absolute size of elbow motion did not significantly differ between the cerebellar-damaged and control groups [elbow: F(1,18) = 0.37, P > 0.2]; there was a reliable difference in the very small amount of shoulder motion between groups, as even less shoulder motion occurred for the cerebellar group: F(1,18) = 4.85, P = 0.04.
Although the cerebellar-damaged and control groups experienced nearly identical patterns of initial arm displacement, their ensuing behavior was qualitatively different. In experiment 1 (Fig. 3A) and experiment 2 (Fig. 3E) the exemplar control subject stabilized his arm consistently from trial to trial, had either small or no terminal oscillations, and eventually brought the joints in register with the preperturbation configuration. In contrast, stabilization by the exemplar cerebellar-damaged subject (Fig. 3, B and F) was more variable, oscillatory, and inaccurate. For most conditions, the cerebellar-damaged subjects had significantly higher position errors 500–1,000 ms after the perturbation onset than exhibited by the control subjects [t(18) > 2.7, P < 0.01 1-sided] (Fig. 3, C and G); the behavioral difference during the elbow extensor torque was above threshold [t(18) = 1.6, P = 0.06 1-sided]. In addition, the more prominent terminal oscillations of the cerebellar-damaged subjects were evident as an elevated joint velocity occurring 500–1,000 ms from perturbation onset compared with the control group [t(18) > 1.9, P < 0.05 1-sided].
Fig. 3.
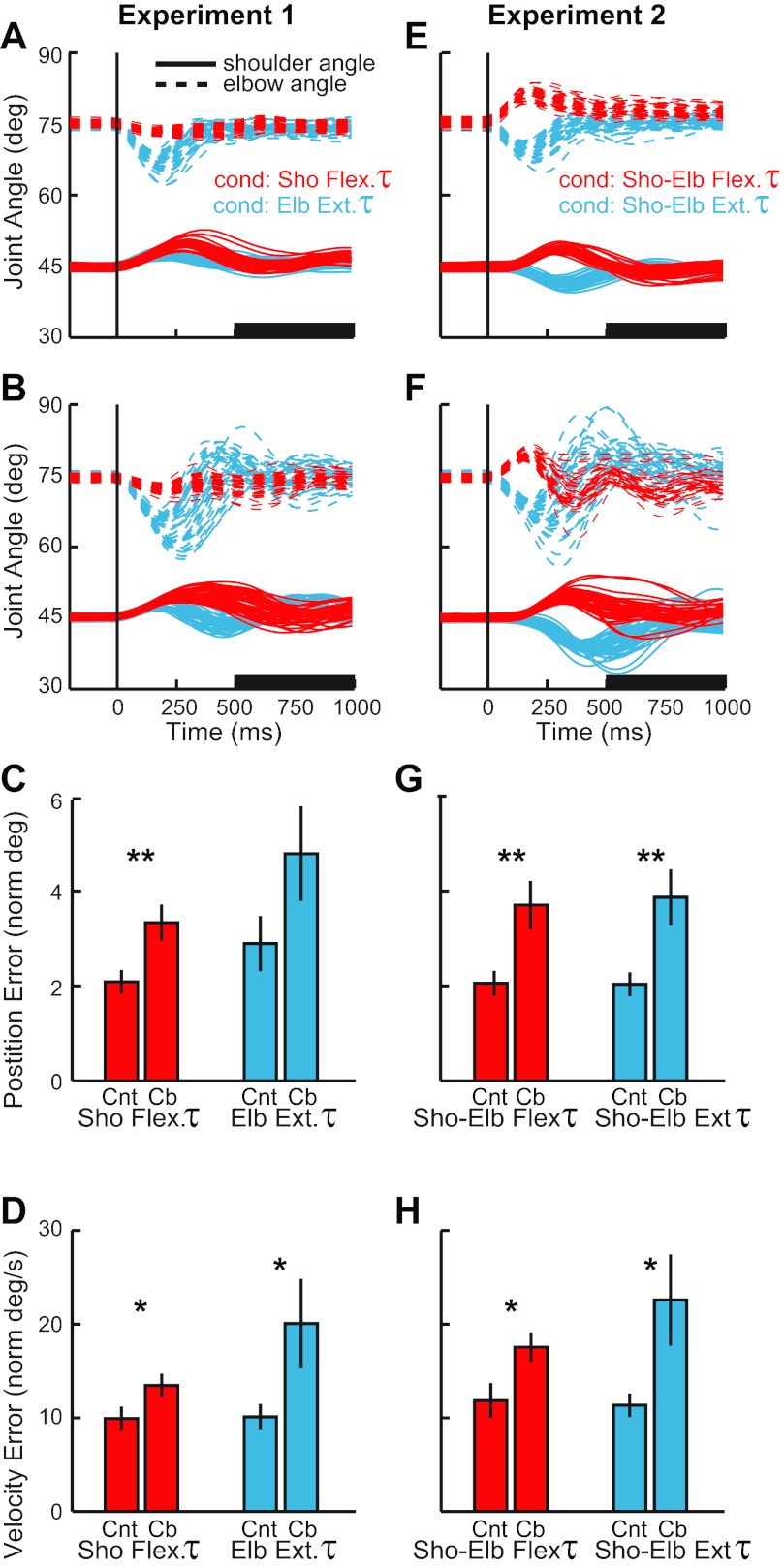
Postural stabilization and final accuracy. A: joint motion from an exemplar control subject after the perturbations of experiment 1, shoulder flexion torque (red) and elbow extensor torque (blue). Shoulder and elbow angles of each trial are depicted with solid and dashed lines, respectively. Vertical line shows perturbation onset. B: joint motion from an exemplar subject with cerebellar damage, same format as in A. C: position error (mean and SE) from 500–1,000 ms after perturbation onset (see black bar on time axis in A and B). Red and blue bars correspond to shoulder torque and elbow torque conditions, respectively. Cnt, normal subjects; Cb, cerebellar subjects. D: velocity error (mean and SE) from 500–1,000 ms after perturbation onset, same format as in C. E–H: joint motion of control subjects and cerebellar subjects during experiment 2; same format as in A–D. Significant difference between cerebellar and control groups for the perturbation condition: *P < 0.05; **P < 0.01.
Experiment 1: evoked shoulder extensor activity to similar shoulder displacement caused by different underlying torques.
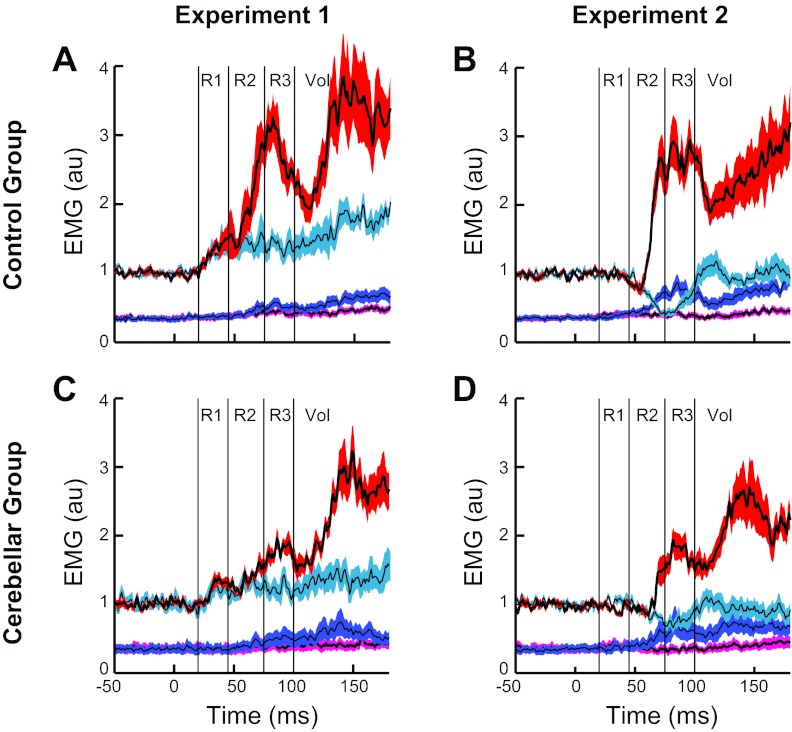
The torque perturbations evoked a stereotyped sequence of activity in the stretched posterior deltoid of normal older subjects (mean age ≈ 55 yr) similar to the pattern we reported earlier in normal young subjects (mean age ≈ 25 yr) (Kurtzer et al. 2008). The earliest evoked response did not differ between conditions until ≈60 ms, when the muscle activity grew larger for the shoulder torque condition than the elbow torque condition, which was appropriate to counter the underlying shoulder torque perturbation. This pattern is visible in the time-varying traces of the exemplar subject (Fig. 4A) and the group average (Fig. 4B) and is confirmed when the data are binned in predefined periods (Fig. 4C); note that these differences in the shoulder extensor activity did not reflect the small difference in shoulder motion across conditions as slightly less shoulder motion occurred with shoulder torque than elbow torque. Excitatory increases from baseline were statistically present in the R1 period [subject's mean t-value > 2.9; group t(9) > 3.0, P < 0.01 1-sided] but did not differ significantly between the two conditions [subject's mean t-value = 0.13; group t(9) = 0.17, P > 0.2 2-sided]. In contrast, R2, R3, and Vol periods expressed greater activity after the shoulder torque than elbow torque condition [subject's mean t-value > 3.4; group t(9) > 3.3, P < 0.005 1-sided].
Fig. 4.
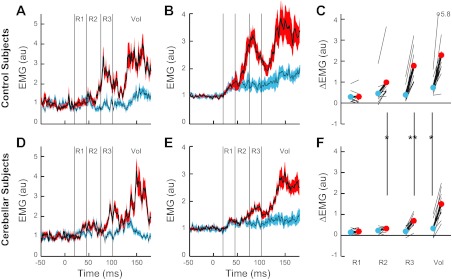
Evoked posterior deltoid activity during experiment 1. A: posterior deltoid activity from the exemplar control subject shown in Fig. 3, A and D. Black lines indicate mean activity, and colored outlines indicate SE after the shoulder flexor torque (red) and elbow extensor torque (blue). Thin vertical lines delineate the different time periods that we examined for compensatory muscle activity (R1, R2, R3, and Vol). B: average muscle activity and SE from normal group of subjects. C: evoked muscle activity within the 4 epochs. Thin black lines depict data from each subject (1 subject's data is out of range and presented numerically). Circles and thick black lines indicate the group mean. Horizontal line at 0 is the preperturbation muscle activity. D–F: posterior deltoid activity from the exemplar cerebellar subject shown in Fig. 3, B and E, and group of cerebellar subjects during experiment 1. Same format as in A–C. Vertical lines and asterisks spanning panels indicate significant difference between cerebellar and control groups for that epoch of activity and perturbation condition: *P < 0.05; **P < 0.01.
The exemplar subject with cerebellar damage (Fig. 4D), group average (Fig. 4E), and binned analysis (Fig. 4F) exhibit some of the patterns shown by the control group. Excitatory responses were generally present in the shoulder extensor's R1 period [subject's mean t-value > 1.8; group t(9) > 3.9, P < 0.005 1-sided] and did not differ between conditions [subject's mean t-value = 0.38; group t(9) = 0.9, P > 0.35 2-sided]. Unlike the control group, the shoulder extensor's activity during the R2 period did not show a strong difference between conditions [subject's mean t-value = 0.91; group t(9) = 1.7, P = 0.11 2-sided]. Greater activity to the shoulder torque condition was observed in the R3 period [subject's mean t-value = 3.6; group t(9) > 9.0, P < 0.001 2-sided] and the Vol period [subject's mean t-value = 6.8; group t(9) > 8.9, P < 0.001 1-sided].
Despite the similar overall pattern of the posterior deltoid response by the control and cerebellar-damaged groups, there is a visible difference in the overall size of their responses (Fig. 4, C and F). A significant main effect was present for the shoulder torque condition [F(1,18) = 6.1, P < 0.05] but not the elbow torque condition [F(1,18) = 1.7, P > 0.20]. Likewise, group contrasts of particular epochs indicated that the control subject had significantly larger activity in the R2 and R3 epochs after the shoulder torque [t(18) > 2.2, P < 0.05 2-sided] and in the Vol epoch after elbow torque [t(18) = 2.4, P < 0.05 2-sided]; no other contrasts were significant (P > 0.10).
Experiment 2: evoked shoulder extensor activity to pure elbow displacement caused by underlying shoulder torques.
Pure elbow displacement evoked a distinct pattern of activity in the unstretched posterior deltoid of normal older subjects (Fig. 5, A–C) that matches the pattern of activity from normal young subjects (Kurtzer et al. 2008). Shoulder extensor activity in the R1 period was unchanged from baseline after either pure elbow flexion or pure elbow extension [subject's mean t-value < |0.2|; group t(9) < |0.9|, P > 0.20 2-sided]. Changes from baseline were apparent in the R2 period with excitatory and inhibitory responses to pure elbow flexion and pure elbow extension, respectively [subject's mean t-value > |4.8|; t(9) > |4.7|, P < 0.001 1-sided]; for both conditions a small and brief decrease in activity was also observed immediately prior to the larger and more sustained reciprocal response at ≈60 ms. Greater responses to elbow flexion than extension were also present in the shoulder extensor's R3 and Vol periods [subject's mean t-value > 7.9; t(9) > 4.0, P < 0.005 1-sided], which was appropriate for the shoulder extensor muscle to counter the underlying flexor torque perturbation.
Fig. 5.
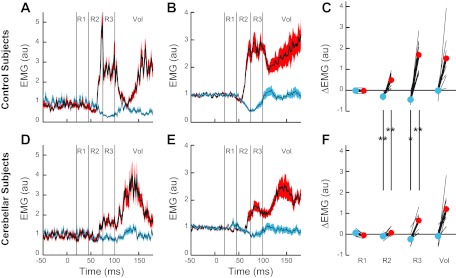
Evoked posterior deltoid activity during experiment 2. A–C: posterior deltoid activity from the exemplar healthy subject and group of healthy subjects during experiment 2. Black lines indicate mean activity, and colored outlines indicate SE after flexor torque at both joints (red) and extensor torque at both joints (blue). B: average muscle activity during experiment 2 from normal group of subjects. C: evoked muscle activity within the 4 epochs. Thin black lines depict data from each subject. Circles and thick black lines indicate the group mean. Horizontal line at 0 is the preperturbation muscle activity. D–F: posterior deltoid activity from the exemplar cerebellar subject and group of cerebellar subjects during experiment 2. Same format as in A–C. Vertical lines and asterisks spanning C and F indicate significant difference between cerebellar and control groups for that epoch of activity and perturbation condition: *P < 0.05; **P < 0.01.
The cerebellar-damaged group shared some of these response patterns (Fig. 5, D–F). They did not exhibit a significant evoked response of the shoulder extensor in the R1 period to pure elbow extension or flexion [subject's mean t-value < |0.4|; group t(9) < 1.7, P > 0.10 2-sided]. In the R2 period their muscle activity was either unchanged from baseline [subject's mean t-value = 1.0; elbow flexion: group t(9) = 1.2, P > 0.20 2-sided] or just at threshold [subject's mean t-value = −1.5; elbow extension: group t(9) = −2.3, P = 0.05 2-sided]. Activity during R2 did not differ between conditions [subject's mean t-value = 1.5; group t(9) = 2.0, P = 0.07 2-sided]. It was only during the R3 period that excitatory and inhibitory responses were reliably observed [subject's mean t-value > |3.4|; group t(9) > |2.7|, P < 0.05 2-sided] along with greater activity to pure elbow flexion than extension [subject's mean t-value = 6.1; group t(9) = 6.9, P < 0.001 2-sided]. Shoulder extensor activity during the Vol period also had greater activity to pure elbow flexion than extension [subject's mean t-value = 8.1; group t(9) = 5.5, P < 0.001 1-sided].
The posterior deltoid responses of the control and cerebellar-damaged groups to pure elbow motion show a visible difference in the overall size of their responses (Fig. 5, C and F). Significant main effects for group were obtained for both elbow flexion [F(1,22) = 3.2, P < 0.05] and extension [F(1,22) = 1.9, P < 0.05], and similar to experiment 1, group contrasts of particular epochs showed significant differences in the R2 epoch [t(18) > |3.5|, P < 0.001 1-sided] and the R3 epoch [t(18) > |2.0|, P < 0.05 1-sided]; no other contrasts were significant (P > 0.15).
Pattern of evoked posterior deltoid activity across experiments.
For each subject, we combined the results from the two experiments by regressing the change in shoulder extensor activity against the imposed shoulder and elbow torques (see Data analysis). The subsequent analyses only included individuals with a significant plane-fit (P < 0.05). Almost all exclusions came from the R1 period (3 cerebellar-damaged and 3 control subjects) as the evoked responses were quite small; no control subjects were excluded from the remaining periods, and 1 cerebellar subject was excluded in the R2 period.
The orientation of the best-fitting plane indicates the combination of shoulder-elbow torques for which the response is maximally sensitive (“preferred torque direction” or PTD). Consistent with our earlier experiment with young control subjects (Kurtzer et al. 2008), we found that the R1 activity of older control subjects (Fig. 6A) was aligned with pure shoulder motion at 313.5° [R1PTD mean(SD) = 316°(3.5); P > 0.05, circular C.I.]. All later periods [R2PTD = 337°(13.5); R3PTD = 351°(14.5); VolPTD = 333°(7)] showed a significant bias away from pure shoulder motion toward the steady-state postural activity at 354° (P < 0.01, circular C.I.), although alignment only occurred in the R3 period (P > 0.1, circular C.I.).
Fig. 6.
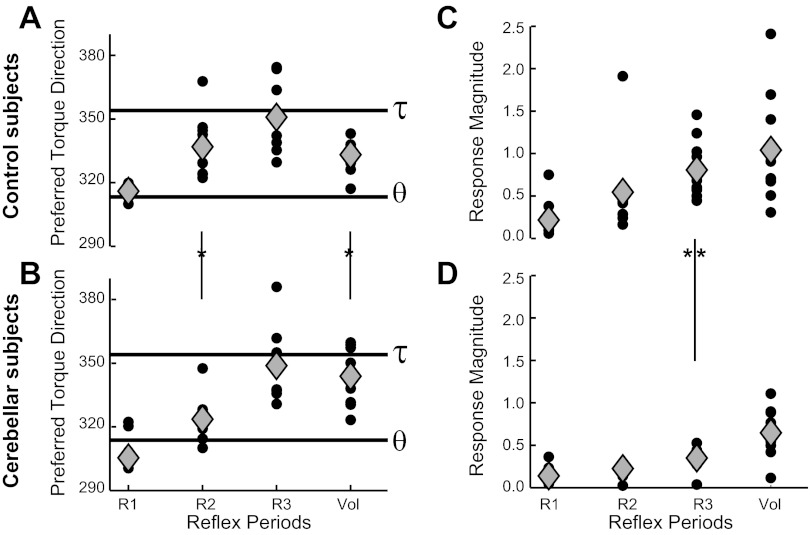
Directional tuning of the posterior deltoid to the applied shoulder-elbow torque. A: PTDs from the shoulder extensor muscle of normal subjects during different response epochs. Small circles show PTDs of individual subjects having significant plane-fits (P < 0.05), whereas gray diamonds show the average PTD from these individuals. The 2 horizontal lines are the predicted PTDs if responses only reflect shoulder motion (θ) or the empirically determined postural tuning when countering applied torque (τ) (see Data analysis). B: PTDs of the shoulder extensor muscle of cerebellar-damaged subjects; same format as in A. C: response magnitude from the shoulder extensor muscle of normal subjects during different epochs. Small circles and gray diamonds show individual subjects with significant plane-fits and the group average, respectively. D: response sensitivity of cerebellar-damaged subjects; same format as in C. Asterisk between the grouped icons indicates significant difference between cerebellar and control groups for that epoch of muscle activity (P < 0.05).
The cerebellar-damaged group showed a pattern similar to that of the control group (Fig. 6B). PTDs in the R1 period were aligned with pure shoulder motion [R1PTD = 305.5°(15.5); P > 0.1, circular C.I.]. PTDs in the R2 period had a moderate bias from pure shoulder motion [R2PTD = 323.5°(10); P < 0.05, circular C.I.], whereas subsequent epochs had stronger biases [R3PTD = 349°(15), VolPTD = 343°(12); P < 0.01, circular C.I.]. Only during the R3 period were the PTDs aligned with steady-state postural activity (P > 0.1, circular C.I.).
Comparisons between the PTDs of the two groups (Watson-Williams test) did not reveal a statistically significant difference in the R1 period [F(1,12) = 1.8, P = 0.12] or the R3 period [F(1,18) = 0.1, P > 0.20]. However, the control group had a greater bias toward steady-state postural activity during the R2 period [F(1,17) = 5.4, P < 0.05] and less bias during the Vol period [F(1,18) = 5.3, P < 0.05].
A second measure obtained from the planar analysis is the overall sensitivity of posterior deltoid activation to the imposed shoulder-elbow torques (“response magnitude”), which corresponds to the steepness of the plane-fit (Fig. 6, C and D). Linear regressions indicated a significant trend of increasing response magnitude in later periods for both the control group (F = 17.9, P < 0.001) and the cerebellar-damaged group (F = 19.8, P < 0.001). A comparison of the two groups shows that the response magnitude for the cerebellar group tended to be lower than the control group in all periods, but the contrast only reached statistical significance in the R3 period [t(18) = 3.9, P < 0.005 2-sided]; contrasts of the R1, R2, and Vol periods did not achieve significance (P > 0.05). To further ensure that the lower response magnitude of the cerebellar R3 period did not reflect a weak overall depression of activity, we normalized the response magnitudes of each period by that in the Vol period. Even after the normalization, the cerebellar-damaged group continued to show a significantly lower response magnitude in the shoulder extensors' R3 period [t(18) = 2.2, P < 0.05].
Moment-by-moment analysis of posterior deltoid's multijoint sensitivity.
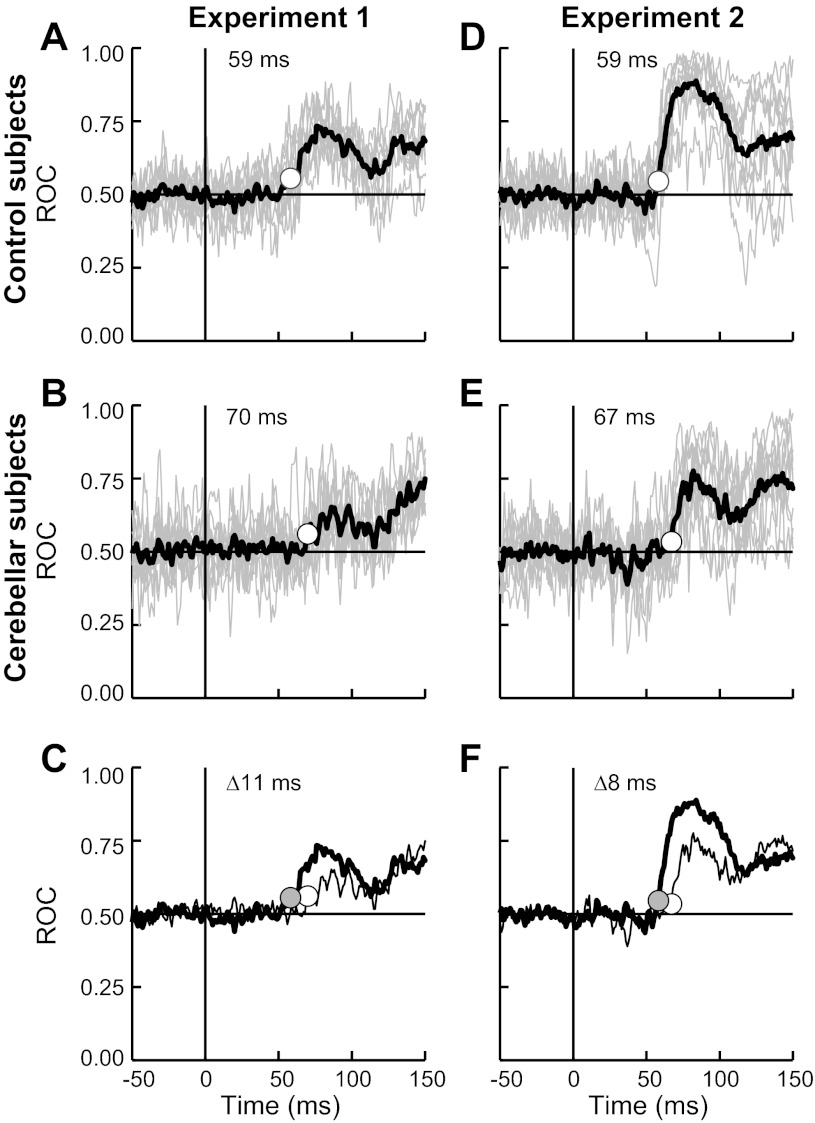
All previous analyses utilized predefined time epochs that broadly correspond to different functional periods but can smear the precise evolution of muscle activity. We performed a moment-by-moment ROC analysis on each experiment to address when the shoulder extensor evoked activity switched from only reflecting shoulder motion to reflecting shoulder and elbow motion (see Data analysis); chance discrimination occurs at 0.05, and perfect discrimination occurs at 1. Although each subject's data are quite variable over time (owing to the small bin size of 1 ms), the mean ROC of the control group shows sustained trends above chance. In experiment 1 the control group's mean ROC achieves threshold at 59 ms after perturbation (Fig. 7A). The mean ROC of the cerebellar-damaged group also achieves threshold in experiment 1 but at 70 ms after perturbation (Fig. 7B), 11 ms later than the normal group (Fig. 7C). In experiment 2, the control group again had a mean ROC that passed threshold at 59 ms and preceded the cerebellar-damaged group at 67 ms (Fig. 7, D–F). Hence, the cerebellar group exhibited slightly delayed multijoint responses compared with normal subjects.
Fig. 7.
Evolving multijoint sensitivity of the posterior deltoid response. A: moment-by-moment statistical discrimination of the shoulder extensor's activity occurring with the shoulder flexor torque and elbow extensor torque conditions of experiment 1; data from the group of normal subjects. If its muscle activity is only linked to shoulder motion then discrimination between these 2 conditions will be at chance levels, 0.5. Instead, a multijoint response would lead to discrimination greater than chance; perfect discrimination occurs at 1.0. Individual and group average receiver-operator characteristic (ROC) curves are depicted by thin gray lines and thick black lines, respectively. White dot and value indicate the time when the average ROC passes threshold (>3 SD). B: ROC curves from the group of cerebellar-damaged subjects in experiment 1. Same format as in A. C: mean ROC curves from the normal group (thick line) and the cerebellar-damaged group (thin line). Difference in the threshold time (Δ) of the 2 groups is also indicated. D–F: moment-by-moment discrimination of muscle activity occurring with combined flexion torque and combined extension torque conditions in experiment 2; same format as A–C.
A difference in the onset of multijoint sensitivity may account for some of our previous observations. The later onset in the cerebellar-damaged group (68.5 ms vs. 59 ms on average) could account for their PTDs having less bias toward steady-state postural activity during the R2 period (lasting 45–75 ms). A delay in feedback processing could also account for the lower response magnitude from the cerebellar group, since response magnitude increases with time. To test these possibilities, we examined a 25-ms window following the ROC threshold of each group. The corresponding PTDs from the cerebellar-damaged and control groups did not differ within this group-specific epoch [F(1,17) = 0.18, P > 0.20], and hence the difference in the R2 period (Fig. 6, A and B) could be explained by the small delay in multijoint processing. In contrast, the response magnitude of the cerebellar-damaged group remained significantly lower than normal and did not reflect a shift in the time-dependent increase in gain [t(21) = 2.9, P < 0.01 2-sided].
Relation between response magnitude of posterior deltoid and severity of ataxia.
The most consistent difference in the shoulder extensor activity between the control and cerebellar-damaged groups was a lowered response magnitude in the R3 epoch. To determine whether the lowered gain of the posterior deltoid was related to the severity of motor dysfunction, we correlated the R3 gain to the clinically assessed ataxic score of the upper limb. The correlation between the two variables did not achieve significance (R = −0.21, df = 8, P > 0.20 1-sided). Normalizing the R3 response magnitude by activity in the Vol epoch may be a more appropriate measure of clinical change by accounting for intersubject differences in motor vigor. However, no significant correlation was obtained with this measure (P > 0.2), the response magnitude during a combined R2/R3 epoch (P > 0.2), or the gain from the 25-ms epoch following multijoint responsiveness (P > 0.2).
We also tested whether the delayed onset of multijoint responses (determined from the ROC) had a positive association with the degree of ataxia. The correlation did not reach significance (R = 0.40, df = 8, P > 0.10 1-sided), nor did a regression that included both the delay and gain (P > 0.10).
Evoked activity by pectoralis major in both experiments.
Although our experiments focused on the posterior deltoid, we also collected data from the pectoralis major (Fig. 8), a monoarticular shoulder flexor shown to exhibit fast feedback responses that incorporate shoulder and elbow information (Kurtzer et al. 2008). The pectoralis major of both the control and clinical groups had low preperturbation activity compared with the posterior deltoid, consistent with the need to counter a background shoulder flexor torque. The subsequent evoked response of the shoulder flexor muscle was opposite the pattern expressed by the shoulder extensor muscle. The control group's pectoralis major expressed greater activity to elbow extensor torque than shoulder flexor torque from the R3 period onward (Fig. 8A) [subject's mean t-value > |2.1|; group t(9) > |3.2|, P < 0.01 1-sided]. Their pectoralis major also had greater responses to combined extensor torque than combined flexor torque from the R2 period onward (Fig. 8B) [subject's mean t-value > |1.6|; group t(9) > |2.3|, P < 0.05 1-sided]. The shoulder flexor of the cerebellar-damaged group showed a similar, though less reliable, pattern (Fig. 8, C and D)—greater activity to elbow extensor torque than shoulder flexor torque during the Vol period [subject's mean t-value = |3.2|; group t(8) = −2.5, P < 0.05 1-sided] and greater activity with combined extensor torque than with combined flexor torque from R2 onward [subject's mean t-value > |1.9|; group t(8) > |2.1|, P < 0.05 1-sided].
Fig. 8.
Evoked activity of the pectoralis major during experiments 1 and 2. A: evoked muscle activity by normal subjects to torque perturbations of experiment 1. Black lines with red and light blue surrounds indicate the posterior deltoid's activity to shoulder flexor torque and elbow extensor torque (same data and color scheme shown in Fig. 4B). Black lines with pink and navy blue surrounds depict the pectoralis major's activity to the same 2 perturbations. Hence muscular activity during shoulder flexor torque is indicated by red and pink coloration, whereas muscular activity during elbow extensor torque is indicated by light blue and navy blue. C: evoked muscle activity by cerebellar-damaged subjects to the torque perturbations of experiment 1; same format as normal subjects in A. B: evoked muscle activity by the normal subjects to torque perturbations of experiment 2. Black lines with red and light blue surrounds indicate the posterior deltoid's activity to combined flexor torque and combined extensor torque (same data and color scheme shown in Fig. 5B). Black lines with pink and navy blue surrounds depict the pectoralis major's activity to the same 2 perturbations. Hence muscular activity during combined flexor torque is indicated by red and pink coloration, whereas muscular activity during combined extensor torque is indicated by light blue and navy blue. Data for posterior deltoid also shown in Fig. 4E. D: evoked muscle activity by cerebellar-damaged subjects to the torque perturbations of experiment 2; same format as normal subjects in B. Data for posterior deltoid also shown in Fig. 5E.
Another notable feature is the low level of coactivity by the pair of shoulder monoarticulars. This is most evident for the two conditions that evoked the greatest level of posterior deltoid activity, shoulder flexor torque and combined flexor torque. On average, the control group's pectoralis major had 4% and 5% of the level of their posterior deltoid during the R2/3 epoch. Similarly, the cerebellar group's pectoralis major had 2% and 5% of the level expressed by their posterior deltoid during the R2/3 epoch. Note that this ratio was formed subject by subject and yielded an identical result whether we employed their normalized or unnormalized data since the normalization introduced a common factor to the activity of both muscles. Differences in the force-emg profile of the two muscles (due to differences in physical efficacy along with distortion by fatty tissue and location parameters) could lead to an underestimation of the degree of cocontraction. However, even a fourfold difference would not alter the observed trend of weak cocontraction.
DISCUSSION
Research over the past century has established that damage to the cerebellum creates significant problems in the voluntary control of multijoint arm movements (Holmes 1939). Disordered feedback processing has also been reported after cerebellar damage, but, to our best knowledge, our study is the first to examine the impact on LLRs by the multijoint arm. We expected that cerebellar damage would impair the ability of LLRs to account for the arm's intersegmental dynamics similar to how cerebellar damage impairs the ability of feedforward commands to account for the arm's intersegmental dynamics. Specifically, we predicted that cerebellar damage would eliminate the ability of the posterior deltoid to generate LLRs based on elbow motion (Fig. 2). Instead, we found a normal overall pattern of its LLRs. The PTDs in the LLR were indistinguishable from normal in the R3 epoch and in a fixed 25-ms window after each group expressed multijoint integration (∼59 ms in normal subjects vs. ∼69 ms in cerebellar subjects).
The most dramatic impact of cerebellar damage on the LLR of the shoulder monoarticular was its smaller size. Activity in the R2 and R3 epochs was lower than normal for most conditions (6 of 8 comparisons), and the response magnitude of the planar fit was lower than normal in the R3 epoch. The attenuated LLR of cerebellar subjects did not reflect a general downscaling since their R1 response was not altered, nor did it reflect an increase in tonic inhibition since their inhibitory LLR to elbow extension was also smaller than normal. Changes in the “central set”—communicated verbally (Crago et al. 1976; Rothwell et al. 1980) or implicitly (Evarts and Tanji et al. 1976; Pruszynski et al. 2008)—can also have dramatic changes on LLR magnitude and could underlie the smaller LLRs by the cerebellar group if the subjects adopted a less vigorous “central set” than the control group. However, the magnitude of their voluntary reaction did not differ from normal, indicating that the lower magnitude of evoked activity following cerebellar damage was specific to the LLR epoch.
The conserved pattern of the posterior deltoid's LLR to imposed shoulder-elbow displacements suggests that its basic motor pattern is not housed by the cerebellum. Rather, the cerebellum is necessary for the proper expression of the basic motor pattern. This broad conclusion has been made previously by several authors (Dichgans and Fetter 1993; Holmes 1939; Jo and Massaquoi 2004; MacKay and Murphy 1979), and our results extend this idea to fast feedback corrections of the multijoint arm.
Cerebellar damage appears to degrade the predictive ability of the sensorimotor system in a general manner rather than disrupting computations for a specific set of conditions. For example, predictive synchronization of eye-hand coordination depends on the cerebellum (Miall et al. 2001; Vercher and Gauthier 1988), the cerebellum is essential for translating sensory errors into motor learning (Martin et al. 1996; Tseng et al. 2007), and recordings of single Purkinje neurons also demonstrate that cerebellar activity is linked to the predicted state of the arm, not the exerted motor commands (Palasar et al. 2006). Cerebellar damage also has been shown to impair the predictive capabilities of the LLR. Cerebellar-damaged subjects cannot scale their postural LLRs to the amplitude of surface displacement (Horak and Diener 1994; Timmann and Horak 1997) or whether the surface rotates upward or displaces backward (Nashner 1976). In a similar vein, cerebellar cooling in monkeys interferes with their ability to generate LLRs appropriate for torque pulses and torque steps (Hore and Vilis 1984). If feedback gain is modulated by prediction accuracy to ensure stability, as some authors have suggested (Crevecoeur et al. 2010), then a degradation of prediction accuracy from cerebellar damage should result in lower feedback gains. Likewise, the discrepancy between previous studies that cerebellar LLRs are greater than normal (Claus et al. 1986; Friedemann et al. 1987; Horak and Diener 1994) or lower than normal (Bakker et al. 2006; Kung et al. 2009; Marsden et al. 1977b) may reflect different demands on prediction in their different paradigms.
Subjects with cerebellar damage also exhibited a slight delay (≈10 ms) of their posterior deltoid in responding to both shoulder and elbow motion. Previous findings in cerebellar patients have been mixed regarding the timing of the LLR, even when excluding clinical signs of sensory loss: some studies have reported delayed LLRs (≈5 ms) (Lee et al. 2003), some studies found no LLR delays (Diener et al. 1984; Friedemann et al. 1987), and some studies report that delays are occasionally expressed (Claus et al. 1986). We interpret the delay here as a result of cerebellar damage but cannot rule out subclinical somatosensory abnormalities. That being said, this timing abnormality could not fully explain the lower feedback gain in the cerebellar group.
Previous studies that cooled the cerebellum resulted in a loss of predictive feedback (Hore and Flament 1986; Hore and Vilis 1984), and the coimpact of delay and lowered gain can lead to instabilities akin to cerebellar ataxia (Hore and Flament 1986; Jo and Massaquoi 2004). However, we did not find a significant correlation of these measures during the LLR with ataxia. We also did not address whether muscle spindle or cutaneous sensors are the peripheral afferents relaying multijoint information since both sources were affected by the mechanical perturbations. The few studies on this topic found no change in LLRs when cutaneous information was abolished (Bawa and McKenzie 1981; Grey et al. 2001; Matthews 1984), suggesting that muscle spindles play the greatest role.
Cerebellar damage may impact the LLRs through its connections to primary motor cortex (Middleton and Strick 2000), a region that has been consistently shown to support LLRs (Cheney and Fetz 1984; Day et al. 1991; Marsden et al. 1977a; Matthews et al. 1990) and voluntary control (Porter and Lemon 1993; Scott 2003). Our recent study of healthy human subjects and behaving monkeys also provided clear evidence that primary motor cortex contributes to the multijoint integration exhibited by the LLR (Pruszynski et al. 2011). The cerebellum makes extensive connections to subcortical pathways via the red nucleus (Keifer and Houk 1994) and recticular formation (Asunama et al. 1983), which may provide an additional contribution to long-latency reflexes (Miller and Brooks 1981; Shemmell et al. 2009). Future work is needed to identify the relative role of these cortical and subcortical regions and whether they exhibit a lowered response magnitude similar to the LLR.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant R01 HD-040289, the National Science and Engineering Research Council (NSERC) of Canada, and the Canadian Institute of Health Research (CIHR).
DISCLOSURES
S. H. Scott is associated with BKIN Technologies, which commercializes the KINARM robot used in this study.
AUTHOR CONTRIBUTIONS
Author contributions: I.L.K., S.H.S., and A.J.B. conception and design of research; I.L.K., P.T., R.J.R., N.H.B., and A.J.B. performed experiments; I.L.K., P.T., R.J.R., and N.H.B. analyzed data; I.L.K., S.H.S., and A.J.B. interpreted results of experiments; I.L.K., S.H.S., and A.J.B. prepared figures; I.L.K. drafted manuscript; I.L.K., S.H.S., and A.J.B. edited and revised manuscript; I.L.K., S.H.S., and A.J.B. approved final version of manuscript.
REFERENCES
- Almeida GL, Hong DA, Corcos D, Gottlieb GL. Organizing principles for voluntary movement: extending single-joint rules. J Neurophysiol 74: 1374–1381, 1995 [DOI] [PubMed] [Google Scholar]
- Asanuma C, Thach WT, Jones EG. Brainstem and spinal projections of the deep cerebellar nuclei in the monkey, with observations on the brainstem projections of the dorsal column nuclei. Brain Res 286: 299–322, 1983 [DOI] [PubMed] [Google Scholar]
- Bakker M, Allum JH, Visser JE, Gruneberg C, van de Warrenburg BP, Kremer BH, Bloem BR. Postural responses to multidirectional stance perturbations in cerebellar ataxia. Exp Neurol 202: 21–35, 2006 [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Cerebellar limb ataxia: abnormal control of self-generated and external forces. Ann NY Acad Sci 978: 16–27, 2002 [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol 76: 492–509, 1996 [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Zackowski KM, Thach WT. Cerebellar ataxia: torque deficiency or torque mismatch between joints? J Neurophysiol 83: 3019–3030, 2000 [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics in Biology. New York: Academic, 1981 [Google Scholar]
- Bawa P, McKenzie DC. Contribution of joint and cutaneous afferents to longer-latency reflexes in man. Brain Res 211: 185–189, 1981 [DOI] [PubMed] [Google Scholar]
- Boose A, Dichgans J, Topka H. Deficits in phasic muscle force generation explain insufficient compensation for interaction torque in cerebellar patients. Neurosci Lett 261: 53–56, 1999 [DOI] [PubMed] [Google Scholar]
- Brown SH, Hefter H, Mertens M, Freund HJ. Disturbances in human arm movement trajectory due to mild cerebellar dysfunction. J Neurol Neurosurg Psychiatry 53: 306–313, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TS, Rovai GP, Rymer WZ. Strategies for muscle activation during isometric torque generation at the human elbow. J Neurophysiol 62: 1201–1212, 1989 [DOI] [PubMed] [Google Scholar]
- Burdet E, Osu R, Franklin DW, Milner TE, Kawato M. The central nervous system stabilizes unstable dynamics by learning optimal impedance. Nature 414: 446–449, 2001 [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol 349: 249–272, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus D, Schocklmann HO, Dietrich HJ. Long latency muscle responses in cerebellar diseases. Eur Arch Psychiatry Neurol Sci 235: 355–360, 1986 [DOI] [PubMed] [Google Scholar]
- Cooper SE, Martin JH, Ghez C. Effects of inactivation of the anterior interpositus nucleus on the kinematic and dynamic control of multijoint movement. J Neurophysiol 84: 1988–2000, 2000 [DOI] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol 39: 925–935, 1976 [DOI] [PubMed] [Google Scholar]
- Craig JJ. Introduction to Robotics. Upper Saddle River, NJ: Pearson Prentice Hall, 2005 [Google Scholar]
- Crevecoeur F, McIntyre J, Thonnard JL, Lefevre P. Movement stability under uncertain internal models of dynamics. J Neurophysiol 104: 1301–1313, 2010 [DOI] [PubMed] [Google Scholar]
- Day BL, Riescher H, Struppler A, Rothwell JC, Marsden CD. Changes in the response to magnetic and electrical stimulation of the motor cortex following muscle stretch in man. J Physiol 433: 41–57, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Thompson PD, Harding AE, Marsden CD. Influence of vision on upper limb reaching movements in patients with cerebellar ataxia. Brain 121: 357–372, 1998 [DOI] [PubMed] [Google Scholar]
- Dichgans J, Fetter M. Compartmentalized cerebellar functions upon the stabilization of body posture. Rev Neurol (Paris) 149: 654–664, 1993 [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Bacher M, Guschlbauer B. Characteristic alterations of long-loop “reflexes” in patients with Friedreich's disease and late atrophy of the cerebellar anterior lobe. J Neurol Neurosurg Psychiatry 47: 679–685, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner TJ, Pasalar S. Cerebellum predicts the future motor state. Cerebellum 7: 583–588, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner TJ, Hewitt AL, Popa LS. What features of limb movements are encoded in the discharge of cerebellar neurons? Cerebellum 10: 683–694, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV, Tanji J. Reflex and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol 39: 1069–1080, 1976 [DOI] [PubMed] [Google Scholar]
- Franklin DW, Milner TE. Adaptive control of stiffness to stabilize hand position with large loads. Exp Brain Res 152: 211–220, 2003 [DOI] [PubMed] [Google Scholar]
- Friedemann HH, Noth J, Diener HC, Bacher M. Long latency EMG responses in hand and leg muscles: cerebellar disorders. J Neurol Neurosurg Psychiatry 50: 71–77, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen CC, Ramaekers L, van Zuylen EJ. Long-latency stretch reflexes as co-ordinated functional responses in man. J Physiol 407: 275–292, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Carr D, Hollenberg J. Kinematic effects of deafferentation and cerebellar ablation. Brain 99: 311–330, 1976 [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Song Q, Almeida GL, Hong DA, Corcos D. Directional control of planar human arm movement. J Neurophysiol 78: 2985–2998, 1997 [DOI] [PubMed] [Google Scholar]
- Graham KM, Moore KD, Cabel DW, Gribble PL, Cisek P, Scott SH. Kinematics and kinetics of multijoint reaching in nonhuman primates. J Neurophysiol 89: 2667–2677, 2003 [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966 [Google Scholar]
- Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjaer T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol 534: 925–933, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble PL, Ostry DJ. Compensation for interaction torques during single- and multijoint limb movement. J Neurophysiol 82: 2310–2326, 1999 [DOI] [PubMed] [Google Scholar]
- Gritsenko V, Yakovenko S, Kalaska JF. Integration of predictive feedforward and sensory feedback signals for online control of visually guided movement. J Neurophysiol 102: 914–930, 2009 [DOI] [PubMed] [Google Scholar]
- Hallett M, Shahani BT, Young RR. EMG analysis of patients with cerebellar deficits. J Neurol Neurosurg Psychiatry 38: 1163–1169, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter TM, Korbel T, Scott SH. Comparison of neural responses in primary motor cortex to transient and continuous loads during posture. J Neurophysiol 101: 150–163, 2009 [DOI] [PubMed] [Google Scholar]
- Hogan N. The mechanics of multi-joint posture and movement control. Biol Cybern 52: 315–331, 1985 [DOI] [PubMed] [Google Scholar]
- Hollerbach MJ, Flash T. Dynamic interactions between limb segments during planar arm movement. Biol Cybern 44: 67–77, 1982 [DOI] [PubMed] [Google Scholar]
- Holmes G. The cerebellum of man. Brain 65: 573–571, 1939 [Google Scholar]
- Horak FB, Diener HC. Cerebellar control of postural scaling and central set in stance. J Neurophysiol 72: 479–493, 1994 [DOI] [PubMed] [Google Scholar]
- Hore J, Flament D. Evidence that a disordered servo-like mechanism contributes to tremor in movements during cerebellar dysfunction. J Neurophysiol 56: 123–136, 1986 [DOI] [PubMed] [Google Scholar]
- Hore J, Vilis T. Loss of set in muscle responses to limb perturbations during cerebellar dysfunction. J Neurophysiol 51: 1137–1148, 1984 [DOI] [PubMed] [Google Scholar]
- Jo S, Massaquoi SG. A model of cerebellum stabilized and scheduled hybrid long-loop control of upright balance. Biol Cybern 91: 188–202, 2004 [DOI] [PubMed] [Google Scholar]
- Kawato M, Gomi H. A computational model of four regions of the cerebellum based on feedback-error learning. Biol Cybern 68: 95–103, 1992 [DOI] [PubMed] [Google Scholar]
- Keifer J, Houk JC. Motor function of the cerebellorubrospinal system. Physiol Rev 74: 509–542, 1994 [DOI] [PubMed] [Google Scholar]
- Koshland GF, Hasan Z, Gerilovsky L. Activity of wrist muscles elicited during imposed or voluntary movements about the elbow joint. J Mot Behav 23: 91–100, 1991 [DOI] [PubMed] [Google Scholar]
- Kung UM, Horlings CG, Honegger F, Kremer HP, Bloem BR, van De Warrenburg BP, Allum JH. Postural instability in cerebellar ataxia: correlations of knee, arm and trunk movements to center of mass velocity. Neuroscience 159: 390–404, 2009 [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nat Neurosci 8: 498–504, 2005 [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Herter TM, Scott SH. Nonuniform distribution of reach-related and torque-related activity in upper arm muscles and neurons of primary motor cortex. J Neurophysiol 96: 3220–3230, 2006a [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Herter TM, Scott SH. Primate upper limb muscles exhibit activity patterns that differ from their anatomical action during a postural task. J Neurophysiol 95: 493–504, 2006b [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Scott SH. Long-latency reflexes of the human arm reflect an internal model of limb dynamics. Curr Biol 18: 449–453, 2008 [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Scott SH. Long-latency responses during reaching account for the mechanical interaction between the shoulder and elbow joints. J Neurophysiol 102: 3004–3015, 2009 [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Scott SH. Long-latency and voluntary responses to an arm displacement can be rapidly attenuated by torque offset. J Neurophysiol 103: 3195–3204, 2010 [DOI] [PubMed] [Google Scholar]
- Latash ML. The organization of quick corrections within a two-joint synergy in conditions of unexpected blocking and release of a fast movement. Clin Neurophysiol 111: 975–987, 2000 [DOI] [PubMed] [Google Scholar]
- Lee RG, Tatton WG. Long latency reflexes to imposed displacements of the human wrist: dependence on duration of movement. Exp Brain Res 45: 207–216, 1982 [DOI] [PubMed] [Google Scholar]
- Lee YC, Chen JT, Liao KK, Wu ZA, Soong BW. Prolonged cortical relay time of long latency reflex and central motor conduction in patients with spinocerebellar ataxia type 6. Clin Neurophysiol 114: 458–462, 2003 [DOI] [PubMed] [Google Scholar]
- MacKay WA, Murphy JT. Cerebellar modulation of reflex gain. Prog Neurobiol 13: 361–417, 1979 [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB, Adam J. The effect of lesions of the sensorimotor cortex and the capsular pathways on servo responses from the human long thumb flexor. Brain 100: 503–526, 1977a [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB, Hallet M, Adam J, Rushton DN. Disorders of movement in cerebellar disease in man. In: The Physiological Aspect of Clinical Neurology, edited by Rose F. Oxford, UK: Blackwell, 1977b, p. 179–199 [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119: 1183–1198, 1996 [DOI] [PubMed] [Google Scholar]
- Massaquoi S, Hallett M. Kinematics of initiating a two-joint arm movement in patients with cerebellar ataxia. Can J Neurol Sci 23: 3–14, 1996 [DOI] [PubMed] [Google Scholar]
- Matthews PB. The human stretch reflex and the motor cortex. Trends Neurosci 14: 87–91, 1991 [DOI] [PubMed] [Google Scholar]
- Matthews PB. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol 348: 383–415, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB, Farmer SF, Ingram DA. On the localization of the stretch reflex of intrinsic hand muscles in a patient with mirror movements. J Physiol 428: 561–577, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Reckess GZ, Imamizu H. The cerebellum coordinates eye and hand tracking movements. Nat Neurosci 4: 638–644, 2001 [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar output: motor and cognitive channels. Trends Cogn Sci 2: 348–354, 1998 [DOI] [PubMed] [Google Scholar]
- Miller AD, Brooks VB. Late muscular responses to arm perturbations persist during supraspinal dysfunctions in monkeys. Exp Brain Res 41: 146–158, 1981 [DOI] [PubMed] [Google Scholar]
- Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res 26: 59–72, 1976 [DOI] [PubMed] [Google Scholar]
- Nozaki D, Nakazawa K, Akai M. Muscle activity determined by cosine tuning with a nontrivial preferred direction during isometric force exertion by lower limb. J Neurophysiol 93: 2614–2624, 2005 [DOI] [PubMed] [Google Scholar]
- Pasalar S, Roitman AV, Durfee WK, Ebner TJ. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci 9: 1404–1411, 2006 [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. New York: Oxford Univ. Press, 1993 [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature 478: 387–390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol 100: 224–238, 2008 [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Scott SH. Optimal feedback control and the long-latency stretch response. Exp Brain Res 218: 341–359, 2012 [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature 286: 496–498, 1980 [DOI] [PubMed] [Google Scholar]
- Scott SH. Apparatus for measuring and perturbing shoulder and elbow joint positions and torques during reaching. J Neurosci Methods 89: 119–127, 1999 [DOI] [PubMed] [Google Scholar]
- Scott SH. The role of primary motor cortex in goal-directed movements: insights from neurophysiological studies on non-human primates. Curr Opin Neurobiol 13: 671–677, 2003 [DOI] [PubMed] [Google Scholar]
- Scott SH. Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci 5: 532–546, 2004 [DOI] [PubMed] [Google Scholar]
- Selen LP, Franklin DW, Wolpert DM. Impedance control reduces instability that arises from motor noise. J Neurosci 29: 12606–12616, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res 185: 359–381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemmell J, An JH, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci 29: 13255–13263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemmell J, Krutky MA, Perreault EJ. Stretch sensitive reflexes as an adaptive mechanism for maintaining limb stability. Clin Neurophysiol 121: 1680–1689, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechting JF, Lacquaniti F. Quantitative evaluation of the electromyographic responses to multidirectional load perturbations of the human arm. J Neurophysiol 59: 1296–1313, 1988 [DOI] [PubMed] [Google Scholar]
- Timmann D, Citron R, Watts S, Hore J. Increased variability in finger position occurs throughout overarm throws made by cerebellar and unskilled subjects. J Neurophysiol 86: 2690–2702, 2001 [DOI] [PubMed] [Google Scholar]
- Timmann D, Horak FB. Prediction and set-dependent scaling of early postural responses in cerebellar patients. Brain 120: 327–337, 1997 [DOI] [PubMed] [Google Scholar]
- Timmann D, Richter S, Bestmann S, Kalveram KT, Konczak J. Predictive control of muscle responses to arm perturbations in cerebellar patients. J Neurol Neurosurg Psychiatry 69: 345–352, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. Prog Brain Res 165: 299–321, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci 5: 1226–1235, 2002 [DOI] [PubMed] [Google Scholar]
- Topka H, Konczak J, Dichgans J. Coordination of multi-joint arm movements in cerebellar ataxia: analysis of hand and angular kinematics. Exp Brain Res 119: 483–492, 1998a [DOI] [PubMed] [Google Scholar]
- Topka H, Konczak J, Schneider K, Boose A, Dichgans J. Multijoint arm movements in cerebellar ataxia: abnormal control of movement dynamics. Exp Brain Res 119: 493–503, 1998b [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145: 205–211, 1997 [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- van Zuylen EJ, Gielen CC, Denier van der Gon JJ. Coordination and inhomogeneous activation of human arm muscles during isometric torques. J Neurophysiol 60: 1523–1548, 1988 [DOI] [PubMed] [Google Scholar]
- Vercher JL, Gauthier GM. Cerebellar involvement in the coordination control of the oculo-manual tracking system: effects of cerebellar dentate nucleus lesion. Exp Brain Res 73: 155–166, 1988 [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Smith MA. Shared internal models for feedforward and feedback control. J Neurosci 28: 10663–10673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci 2: 338–347, 1998 [DOI] [PubMed] [Google Scholar]