Abstract
Sensory feedback is critical for normal locomotion and adaptation to external perturbations during movement. Feedback provided by group Ia afferents influences motor output both directly through monosynaptic connections and indirectly through spinal interneuronal circuits. For example, the circuit responsible for reciprocal inhibition, which acts to prevent co-contraction of antagonist flexor and extensor muscles, is driven by Ia afferent feedback. Additionally, circuits mediating presynaptic inhibition can limit Ia afferent synaptic transmission onto central neuronal targets in a task-specific manner. These circuits can also be activated by stimulation of proprioceptive afferents. Rodent locomotion rapidly matures during postnatal development; therefore, we assayed the functional status of reciprocal and presynaptic inhibitory circuits of mice at birth and compared responses with observations made after 1 wk of postnatal development. Using extracellular physiological techniques from isolated and hemisected spinal cord preparations, we demonstrate that Ia afferent-evoked reciprocal inhibition is as effective at blocking antagonist motor neuron activation at birth as at 1 wk postnatally. In contrast, at birth conditioning stimulation of muscle nerve afferents failed to evoke presynaptic inhibition sufficient to block functional transmission at synapses between Ia afferents and motor neurons, even though dorsal root potentials could be evoked by stimulating the neighboring dorsal root. Presynaptic inhibition at this synapse was readily observed, however, at the end of the first postnatal week. These results indicate Ia afferent feedback from the periphery to central spinal circuits is only weakly gated at birth, which may provide enhanced sensitivity to peripheral feedback during early postnatal experiences.
Keywords: presynaptic inhibition, development, proprioceptive, spinal cord, GABA
proprioceptive feedback is critical for normal locomotion. Primary proprioceptive neurons provide information to motor circuits in the spinal cord by virtue of their different sensory endings in the periphery. The effects of feedback provided by group Ia proprioceptive afferents have been the subject of study for many years (Baldissera et al. 1981; Eccles et al. 1957; Prochazka 1996; Proske 2006; Zehr and Stein 1999). Stretch-activated channels associated with the annulospiral terminations of these afferents on intrafusal muscle fibers provide the molecular substrate for high sensitivity to changes in muscle length (Banks 2005; Simon et al. 2010). These sensory signals are transmitted directly to motor neurons (MNs) and interneurons related to motor control in the spinal cord. Readily observable deficits in coordinated movements of various genetic mutants lacking Ia afferent feedback illustrate the critical importance of this sensory modality in voluntary locomotion (Arber et al. 2000; Levanon et al. 2002; Tourtellotte and Milbrandt 1998).
Group Ia afferent proprioceptive feedback affects MN output and consequent locomotion, via both direct and indirect excitation and indirect inhibition of target MNs (Eccles et al. 1957; Eccles and Lundberg 1958; McCrea et al. 1995). Direct communication with MNs is accomplished through the monosynaptic stretch reflex, in which Ia afferents that convey information from a particular muscle in the periphery establish glutamatergic excitatory connections with MNs projecting back to the same muscle or with MNs innervating close synergists, but do not synapse on MNs that project to antagonist muscles (Eccles et al. 1957; Mears and Frank 1997). The indirect inhibitory pathway of Ia afferent feedback that has been studied most extensively is that of reciprocal inhibition, which acts to prevent co-contraction of antagonist muscles (Jankowska 1992). This effect is mediated by a class of glycinergic interneurons termed Ia inhibitory interneurons (IaINs) located near MN pools in the ventral horn of the spinal cord (Curtis et al. 1968; Jankowska and Lindstrom 1972). Distinct subsets of these interneurons receive monosynaptic input from axon collaterals of Ia afferents carrying sensory information from either flexor or extensor muscles and act to inhibit MNs innervating antagonist muscles (Eccles and Lundberg 1958). For example, extensor Ia afferents, such as those innervating the knee extensor quadriceps muscle group, activate subsets of IaINs that inhibit MNs projecting to flexor muscles of the knee.
Both direct and indirect Ia afferent feedback can be limited by presynaptic inhibition, which acts to reduce synaptic transmission between Ia afferents and their target neurons. Activation of axo-axonic contacts arising from GABAergic interneurons onto Ia afferent terminals results in depolarization of the synaptic terminal and limits sensory neurotransmitter release (Alvarez 1998; Hughes et al. 2005; Rudomin and Schmidt 1999). Presynaptic inhibitory control of synaptic transmission can be highly selective, affecting only subsets of terminals arising from a single Ia afferent collateral (Lomeli et al. 1998; Quevedo et al. 1997). Furthermore, studies of adult locomotion indicate the degree of presynaptic inhibition can change rapidly, in concert with ordered muscle contraction during locomotion (Capaday et al. 1995; Gossard et al. 1989, 1990; Gossard and Rossignol 1990; Rossignol et al. 2006; Stein 1995).
The development of direct and indirect Ia feedback pathways, as well as the gating of Ia afferent feedback by presynaptic inhibition, is of fundamental interest in understanding the acquisition of normal motor skills. Are these circuits effective in altering MN output at birth, as neonates begin to develop purposeful motor control? Studies attempting to answer this question have focused primarily on analysis of developing rodents. The answer is clear for the direct monosynaptic connections of the stretch reflex circuit. This pathway is first observed at E19.5 in rats (Kudo and Yamada 1985) and at E17.5 in mice (Mears and Frank 1997). While the strength of the reflex pathway increases over the first postnatal week in rodents, stimulation of Ia afferents, even in embryonic stages, can lead to action potentials in MNs (Mears and Frank 1997). Reciprocal inhibition can also be detected at birth, but initial reports suggest the strength of inhibition may increase over the first postnatal week (Wang et al. 2008). Presynaptic inhibition has been measured primarily through detection of primary afferent depolarization (PAD) in the form of a dorsal root potential (DRP) that originates on central terminals of afferents in the spinal cord and then is conducted antidromically into the periphery along sensory axons. DRPs are readily detected from early neonatal rodent preparations (Hayes et al. 2012; Vinay and Clarac 1999). Nevertheless, dorsal roots contain a variety of proprioceptive and cutaneous afferents, and it is unclear whether presynaptic inhibition of Ia afferents, or of proprioceptive afferents in general, is functional in early neonatal mice.
In this study, we investigated the status of reciprocal inhibition and of presynaptic inhibition of group Ia afferents at birth and at the end of the first postnatal week in mice. Using an isolated spinal cord preparation and extracellular physiological assays focusing on antagonistic knee flexor and extensor motor groups, we found reciprocal inhibition to be as robust, stable, and effective at birth as at 1 wk of age. These results suggest that Ia afferent feedback control of flexor and extensor MNs through reciprocal inhibition is likely to be fully functional at birth. In contrast, proprioceptive evoked gating of Ia afferent input by presynaptic inhibitory circuits is not sufficiently strong at birth to alter direct activation of MNs by Ia afferents. By the end of the first postnatal week, however, robust presynaptic inhibition of Ia afferent synaptic transmission is consistently observed.
MATERIALS AND METHODS
Spinal cord preparation.
All procedures for animal experiments were approved by the Wright State University Animal Care and Use Committee. Neonatal mice (C57BL/6J) from two different age groups were used in this study. Mice in the first group were studied on the day of birth or the first postnatal (P) day (P0/P1). A second group of mice were studied after 1 wk of postnatal development at either P7 or P8. Isolated spinal cords dissected in continuity with selected peripheral nerves were prepared as described previously (Mears and Frank 1997). Briefly, mice were anesthetized by hypothermia induction in an ice water bath and then perfused transcardially with 5 ml of ice-cold, oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid [ACSF; containing in mM: 127 NaCl, 1.9 KCl, 1.2 KH2PO4, 1 MgSO4·7H2O, 26 NaHCO3, 16.9 d(+)-glucose monohydrate, and 2 CaCl2]. The spinal column and attached lower limbs were dissected free and immersed in a recirculating bath of cold (16–18°C), oxygenated ACSF. The spinal cord was exposed by dorsal laminectomy of the spinal column and careful removal of the dura. All spinal cords, regardless of the age of the preparation, were hemisected to maximize oxygen penetration into the cord. Previous studies have shown increased function of monosynaptic sensory-motor connections following hemisection (Jiang et al. 1999). The hemisected cord was then removed from the vertebral column together with several peripheral nerves projecting to muscles of interest in the periphery. These included the nerve bundle projecting to the knee flexor muscles posterior biceps and semitendinosus (PBST) and the nerve supplying the knee extensor quadriceps (Quad). The saphenous (Saph), a purely cutaneous nerve, was also dissected free in some preparations. The final preparation was transferred to the recording chamber and allowed to recover for 1 h while the recirculating oxygenated ACSF equilibrated to room temperature (22°C). All recordings were made at room temperature.
Peripheral nerve recordings.
Extracellular recordings of either sensory or motor axon responses were obtained using suction electrodes (see Fig. 1A for diagram). The nerve to be recorded from, usually PBST, was placed in a suction electrode (A-M Systems, Sequim, WA). Responses were recorded with an EX4-400 Quad Channel Differential Amplifier (1,000× gain, 2 Hz low cut, 500 Hz high cut; Dagan, Minneapolis, MN) and digitized at 20 kHz with the use of WinLTP software (WinLTP, Bristol, UK). Compound action potentials (CAPs) in the PBST were evoked by stimulation of afferent fibers in dorsal root L5 (DRL5). DRL5 was cut just proximal to the dorsal root ganglion and carefully pinned to the bottom of a Sylgard-covered recording chamber to allow for stimulation. A matrix electrode [catalog no. MX21AEW(RT1); FHC, Bowdoin, ME] was positioned on the cut DRL5 and triggered using a constant current stimulus isolator (A365; World Precision Instruments, Sarasota, FL). Positive unipolar current pulses (0.1-ms duration) required to elicit the maximal size of the PBST CAP varied between preparations but ranged from 1 to 5 mA. Sweeps where only DRL5 was stimulated were referred to as test pulses (T).
Fig. 1.

Conditioning stimulation of afferents innervating the knee extensor quadriceps muscle group (Quad afferents) produces 2 phases of inhibition of posterior biceps and semitendinosus (PBST) responses that are pharmacologically distinct. A: diagram of experimental model used demonstrates dorsal root L5 (DRL5) afferents were stimulated via a matrix electrode and the response in PBST motor neurons (MNs), termed the “test” response (T), could be recorded in the periphery via a suction electrode. A conditioning stimulus (C) could also be applied to Quad afferents via a separate suction electrode. B: representative average traces illustrating the effect of conditioning stimulation of Quad afferents on PBST response (postnatal day 7; P7). Black trace illustrates compound action potential recorded on the PBST nerve in the periphery following test pulse stimulation of DRL5 afferents (2-mA DRL5 stimulation). Red trace illustrates PBST response when a conditioning pulse of quadriceps nerve is given prior to test pulse (C+T; 200-μA quadriceps stimulation; 22-ms conditioning stimulus interval). The portion of compound action potential (CAP) in the shaded box was rectified and integrated to quantify the effects of the conditioning pulse and to generate a response ratio. C: response ratios measured at different conditioning stimulus intervals from a representative isolated spinal cord preparation from a P8 mouse. Intervals represent time (ms) between the Quad conditioning pulse and the test pulse stimulation on DRL5. Note 2 phases of inhibition were observed, a short- and long-interval inhibitory period. D: response ratios for another representative P7 preparation. E: response ratios for same preparation as in C following addition of 0.4 μM strychnine. F: response ratios for same preparation as in D following addition of 5 μM bicuculline. Test (T; black) and condition + test pulse (C+T; red) example traces are shown at right of C–F. Black circles on response ratio graphs indicate the specific conditioning stimulus interval depicted in example traces. In C–F, data points represent mean response ratio ± SD.
Conditioning pulses of muscle nerves were delivered through suction electrodes. Most commonly, afferents in Quad nerve were stimulated using a second constant current stimulation isolator (200 μA, 0.1-ms duration). The ventral roots of lumbar segments 2–4 were cut to eliminate effects mediated by antidromic stimulation of Quad motor axons (Wang et al. 2008). Sweeps with a test pulse (T) to DRL5 were alternated every 10 s with sweeps in which a conditioning pulse of the Quad preceded a test pulse to DRL5 (C+T) by a predetermined interval (Fig. 1B). Intervals ranged from 0 ms (synchronous stimulation of Quad and DRL5) to 32 ms (Quad stimulation 32 ms before DRL5) in 2-ms increments. Longer conditioning intervals of 40 and 50 ms were also tested. For each interval, alternating T and C+T pulses were presented six times each and the responses were averaged offline for later analysis before a new conditioning interval was tested. In some experiments, the entire series of conditioning intervals were presented multiple times to test the stability of responses over time. In these cases, the order in which intervals were tested was randomized from series to series to control for the possibility of order-dependent effects. Average traces were analyzed using custom routines in MATLAB (The MathWorks, Natick, MA) to rectify and integrate the area of the waveforms of the average CAPs from the initiation of the rise phase to the maximum peak of the hyperpolarization period. Ratios of the resultant areas of the T and C+T CAPs were calculated, referred to as response ratios, and plotted against the conditioning stimulus interval. Latency-to-peak values were measured in WinLTP.
The intensity used for the conditioning stimulation pulse was determined by stimulating the peripheral Quad nerve and recording the response from DRL3 (from which a proportion of quadriceps afferents enter) to determine the threshold for quadriceps afferents. We then ran a full series of T/C+T pulses with Quad stimulation of 200 μA (ranging from 7.75–10T; n = 3). We determined the conditioning intervals associated with maximal short- and long-interval inhibition and re-ran the response ratio paradigm at these conditioning intervals at various multiples of threshold (1.5T, 3T, and 5T). We determined that there was not a significant difference among the degrees of inhibition at the various thresholds studied (1.5T, 3T, 5T, and 7.75–10T) at either the short-interval inhibition (P = 0.95, 1-way ANOVA) or long-interval inhibition (P = 0.97, 1-way ANOVA). Of note, both short- and long-interval inhibitions were observed at 1.5T.
In preparations designed to measure primary afferent depolarization responses, all dorsal roots of the lumbar spinal cord (L1–L6) were kept intact, whereas all of the corresponding ventral roots (L1–L6) were cut to eliminate any contribution from motor axon activation. No test pulses were given. Sensory afferent responses in the PBST nerve were measured using a suction electrode following stimulation of Quad or other nerves. Stimulation pulses (200 μA) were presented at 0.1 Hz.
Statistical comparisons between small samples were performed using the nonparametric Wilcoxon rank sum test. Comparisons between larger groups were performed using Student's t-test. Data are reported as means ± SE, except in Figs. 1 and 5, in which data points plotted in the graphs represent the mean ± SD. Results were considered to be significant if P ≤ 0.05.
Fig. 5.

Conditioning stimulation of Quad afferents produces only 1 phase of inhibition of PBST responses at birth. A: response ratios from representative preparation at P1. T (black) and C+T (red) example traces from preparation are displayed at right (22-ms conditioning intervals as indicated by black circle in graph). B: response ratios from representative preparation at P0. Example traces from 22-ms conditioning latency are shown at right. C: response ratios from same preparation as in A, but after addition of 0.4 μM strychnine. Inhibition was blocked and replaced by facilitation of PBST response at some conditioning intervals. Example traces (as in A) from the same preparation following addition of 0.4 μM strychnine are displayed at right. D: response ratios from same preparation as in B, but after addition of 5 μM bicuculline. Note that the latency and degree of inhibition are not altered. Example traces from the same preparation following addition of 5 μM bicuculline are displayed at right. Data points represent mean response ratio ± SD.
Pharmacology.
To determine the relative contributions of glycinergic and GABAergic pathways in inhibiting MN responses, oxygenated ACSF containing either 5 μM bicuculline or 0.4 μM strychnine was allowed to recirculate in the bath for 5 min before recordings began to allow time for the drugs to take effect. Concentrations were chosen to maximize specificity for their respective receptors, as described previously (Wang et al. 2008). All chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
RESULTS
We adapted an extracellular recording assay utilizing the isolated spinal cord preparation to measure changes in motor output evoked by proprioceptive afferents via interneuron-mediated pathways (Wang et al. 2008). Motor neurons in the L5 lumbar spinal segment were activated through monosynaptic inputs from Ia afferents by electrical stimulation of the L5 dorsal root (see Fig. 1A for diagram). Stimulation of the entire dorsal root activates Ia afferents projecting to various muscles and results in broad activation of MNs projecting to many muscles in the hindlimb. To observe effects on selected MN pools, recordings were made from identified muscle nerves in the periphery where the area of the CAP is a function of the number of MNs driven to threshold by activation of DRL5 Ia afferents that project to that particular muscle. We chose to record the CAP response of MNs innervating two major knee flexor muscles, the posterior biceps and semitendinosus (PBST), which together are supplied by a distinct muscle nerve bundle that branches from the common sciatic nerve.
To test the effectiveness of interneuron-mediated proprioceptive pathways in modulating the PBST CAP, conditioning pulses to afferents supplying knee extensor muscles, Quad, were applied at varying intervals before the DRL5 test pulse. Stimulation of Quad nerve alone evoked no CAP response in the PBST nerve, confirming previous reports that Quad afferents are not monosynaptically connected to PBST MNs (data not shown) (Baldissera et al. 1981; Eccles and Lundberg 1958; Wang et al. 2008). Trials utilizing a Quad conditioning pulse followed by the DRL5 test pulse (C+T trials) were interleaved with test pulse-only trials (T trials) at a frequency of 0.1 Hz (Fig. 1B). An increase or reduction in CAP area during C+T trials indicates greater or fewer PBST MNs reach threshold compared with T-only trials, respectively. PBST CAP area thus serves as a quantitative measure of proprioceptive modulation of MN output via interneuron pathways.
Efficacy of inhibitory circuits at 1 wk of age.
In P7/P8 mice, varying the interval between the conditioning and test pulse (conditioning pulse given 0–50 ms before the test pulse; see materials and methods for details on stimulation protocols) produced two distinct phases of inhibition of PBST motor output (Fig. 1, C and D). One reached a maximum degree of inhibition (70.6 ± 8.8%; n = 10) at short intervals (6.5 ± 0.8 ms), and the second reached a maximum degree of inhibition (51.6 ± 12.3%) at longer intervals (22.8 ± 0.5 ms). Conditioning pulse intervals between these two regions of greatest inhibition still evoked inhibition of the test pulse, but to a lesser extent, suggesting separate circuits may mediate the two phases of inhibition. Previously published results indicate that the short-interval inhibition observed here was consistent with classic disynaptic reciprocal inhibition elicited by activation of antagonist Ia afferents (Wang et al. 2008). We found that application of the glycine receptor antagonist strychnine (0.4 μM) abolished short-interval inhibition of the PBST nerve, consistent with the fact that reciprocal inhibition is mediated by glycinergic IaINs (Fig. 1E; n = 5) (Fyffe 1991). Application of strychnine also appeared to result in a general facilitation, likely due to disinhibition of MNs (control: 49.9 ± 5.5% inhibition; 0.4 μM strychnine: 27.9 ± 13.4% facilitation; P = 0.01, Wilcoxon rank sum test; n = 5). The longer interval component of inhibition was unaffected by strychnine (control: 44.5 ± 16.4% inhibition; 0.4 μM strychnine: 45.7 ± 5.5% inhibition; P =0.81, Wilcoxon rank sum test; n = 5), however, indicating this proprioceptive pathway is mediated by nonglycinergic interneurons (Fig. 1E). In separate preparations, we found that long-interval inhibition was blocked by addition of the GABAA receptor antagonist bicuculline (control: 87.6 ± 3.0% inhibition; 5 μM bicuculline: 16.6 ± 5.7% inhibition; P < 0.05, Wilcoxon rank sum test; n = 3), whereas short-interval inhibition was unaffected (Fig. 1F; control: 92.3 ± 2.4% inhibition; 5 μM bicuculline: 73.6 ± 9.4% inhibition; P = 0.12, Wilcoxon rank sum test; n = 3). These experiments provided evidence for two pharmacologically distinct interneuronal pathways through which proprioceptive information can modulate motor output.
To assess the stability of the two phases of inhibition, in several experiments conditioning stimulation intervals were randomized and the whole range of values were tested repeatedly for a total of five series, encompassing more than 3 h of total recording time. We consistently observed that the short-interval maximal glycinergic inhibition was robust and highly stable throughout the entire recording period (Fig. 2, A–C; 1st series: 70.6 ± 8.8%; 5th series: 70.8 ± 6.3%; n = 10; P = 0.95). Interestingly, we observed that the long-interval GABAergic inhibition was more variably effective in its ability to inhibit the PBST. In more than half of the preparations (n = 6/10), long-interval maximal GABAergic inhibition had an initially low degree of inhibition (1st series: 29.8 ± 14.8%) that would increase upon repetitive proprioceptive stimulation (Fig. 2, A and C; 2nd series: 43.0 ± 13.4%; 3rd series: 53.7 ± 12.6%; 4th series: 63.8 ± 12.1%; 5th series: 62.7 ± 12.1%; P < 0.005, 1st vs. 5th series). In the remainder of preparations (n = 4/10), however, the long-interval maximal GABAergic inhibition initially had a higher degree of inhibition (1st series: 84.2 ± 1.4%) that was reduced upon repetitive Quad stimulation (Fig. 2, B and C; 2nd series: 75.2 ± 2.0%; 3rd series: 71.1 ± 2.6%; 4th series: 71.6 ± 4.8%; 5th series: 69.8 ± 4.6%; P < 0.05, 1st vs. 5th series). Overall, these data point to the stability of the short-interval glycinergic inhibition compared with the variability of the long-interval GABAergic inhibition, in response to repetitive proprioceptive activity from the Quad, observed at the first postnatal week of development.
Fig. 2.
Long-interval inhibition at P7 is variably modulated by long-term repetitive proprioceptive stimulation. A and B: response ratio graphs for 2 representative P7 preparations. Note the colored boxes at top of A indicate the respective time course of each series and their respective response ratios in A and B. In both preparations (A and B), short-interval inhibition was consistent throughout all series. In some preparations (A), long-interval inhibition increased over the course of the series presented. In other preparations (B), the degree of long-interval inhibition decreased. C: quantitation of short- and long-interval percent inhibition from multiple preparations. Average responses for long-interval inhibition are presented in 2 groups: those with increasing (↑) inhibition over time (as illustrated by example in A) and those with decreasing (↓) inhibition over time (as illustrated by example in B). *P ≤ 0.05. Int., interval; S1, series 1; S5, series 5.
We next sought to determine the number of synaptic linkages involved in mediating these pharmacologically distinct windows of inhibition by making a series of measurements of the times required to generate various aspects of the responses that were recorded. Response latencies in our experiments are the combination of both peripheral and central conduction times of the various elements in the proprioceptive pathways. We began by measuring the latency of known monosynaptic connections between Ia afferents and MNs at two relevant locations in the lumbar spinal cord.
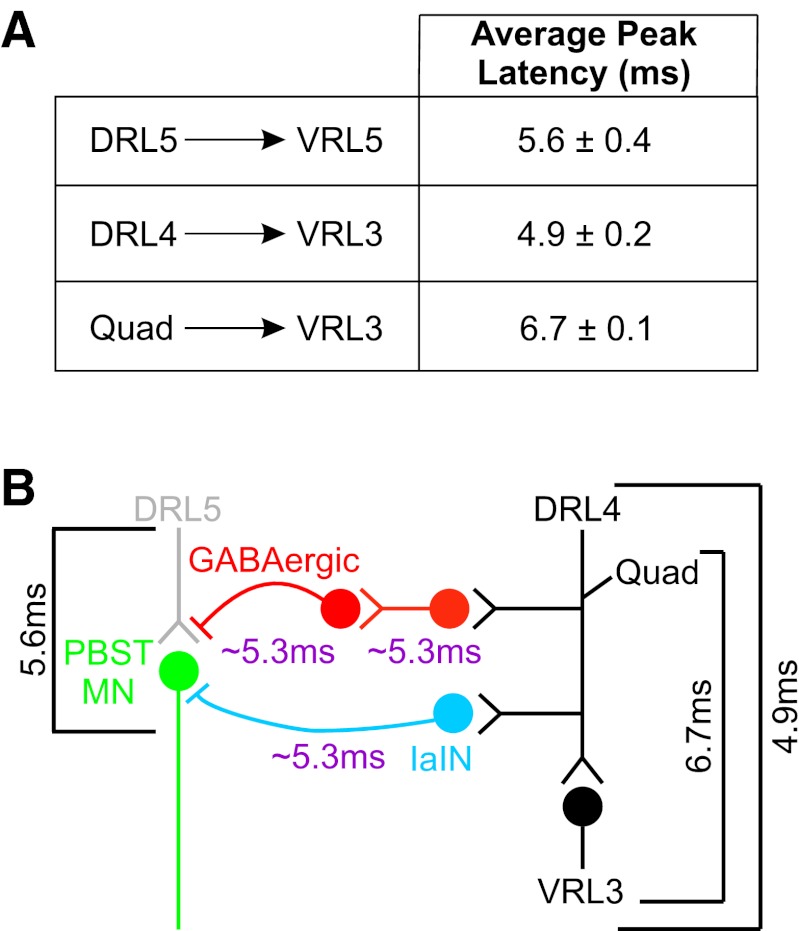
First, we measured the time required to elicit a monosynaptic response at ventral root L5 (VRL5) following stimulation of DRL5. Strong, synchronous activation of DRL5 afferents provided by electrical stimulation of the whole dorsal root elicits excitatory postsynaptic potentials (EPSPs) in MNs that lead to action potentials, and both of these responses can be recorded on the ventral root (Kudo and Yamada 1987; Shneider et al. 2009; Wang et al. 2008; Whelan et al. 2000). Although measuring the onset of the EPSP in MNs provides insight into the time required to initiate a monosynaptic response in MNs, the time required to generate an action potential response in the postsynaptic neuron is of greater importance in our current experiments because information will only be passed to the next neuron in a pathway after an action potential is generated. The latency to the peak of the action potential response at VRL5 recorded at room temperature was determined to be 5.6 ± 0.4 ms (Fig. 3A; n = 4). Because the latency-to-peak response is influenced by the fluctuating state of excitability of MNs in L5, some trial-to-trial variability might be expected in these measurements. We found, however, the standard deviation of the latency-to-peak recorded at VRL5 (0.055 ms) to be in agreement with that reported for monosynaptic connections in other studies measuring only the onset latency variability (Vrieseling and Arber 2006; Wang et al. 2008). Similarly, we found the monosynaptic latency-to-peak measured at VRL3 following DRL4 stimulation to be 4.9 ± 0.2 ms (Fig. 3A; n = 4). Again, variability between trials was low (SD = 0.10 ms) and in line with reports for monosynaptic onset latencies derived from intracellular recordings (Wang et al. 2008). On the basis of these measurements, we estimated the average latency-to-peak required for the monosynaptic reflex in the lumbar spinal cord at room temperature to be ∼5.3 ms (average of 5.6 and 4.9 ms).
Fig. 3.
Predicted durations of individual stages of proposed pathways mediating inhibition of PBST responses based on measurements of monosynaptic connectivity between Ia afferents and MNs at P7/8. A: latency-to-peak values (means ± SE) of various monosynaptic connections measured in lumbar spinal cord. B: schematic of predicted proprioceptive pathways that mediate Quad afferent effects on PBST MN responses. Average measured durations (black brackets) are shown for various stimulus and recording combinations. Estimated duration of 1 central synaptic relay (purple) was used to predict central latencies of the Ia interneuron (IaIN)-mediated reciprocal inhibitory pathway (blue) and GABAergic presynaptic inhibitory pathway (red). VR, ventral root.
Last, we measured the time required to evoke a monosynaptic response at VRL3 following stimulation of the Quad nerve from the periphery (Fig. 3A; 6.7 ± 0.1 ms; n = 4). This pathway includes a greater peripheral conduction component, in this case Ia sensory axons carrying the signal to the spinal cord via the Quad nerve. This latency was measured at DRL4 following stimulation of the Quad nerve as 2.0 ± 0.2 ms (n = 6). The distance from Quad to the recording site at DRL4 was also measured to estimate peripheral conduction velocity of group I sensory afferents at this age (2.52 ± 0.36 m/s; n = 4). Subtracting the peripheral conduction time would leave an average central monosynaptic latency of Quad to VRL3 of 4.7 ms (6.7 ms − 2.0 ms = 4.7 ms), a value in general agreement with measurements made directly from DRL4 to VRL3.
We then inferred the sum of two central monosynaptic latencies would result in a reasonable value for a central disynaptic latency, or the two-step relay between the Ia afferent and an interneuron and then to the motor neuron target (Fig. 3B; 5.3 ms + 5.3 ms = 10.6 ms). Does this estimate agree with our observations that the interval between Quad and DRL5 stimulation producing maximum inhibition of the test pulse in PBST is 6.5 ± 0.8 ms? If our estimate for disynaptic linkage (10.6 ms) is added to the time required for conduction of the signal along Quad sensory axons from the periphery (2.0 ms), then we would estimate that maximal inhibition of PBST MNs would likely occur ∼12.6 ms following stimulation of the Quad nerve. Monosynaptic excitation of L5 MNs, including PBST MNs, by DRL5 Ia afferents requires only 5.6 ms. Therefore, if Quad were stimulated 6.5 ms before DRL5, then monosynaptic excitation of PBST MNs would occur ∼12.1 ms after stimulation of Quad, which falls within the time window in which reciprocal inhibition would be expected when evoked by Quad stimulation. A study of another disynaptic pathway in spinal cord, again recorded at room temperature, reported disynaptic inputs occurred with a latency about 5 ms longer than monosynaptic inputs (Machacek and Hochman 2006). In addition, other reports using similar methodologies have demonstrated disynaptic reciprocal inhibition mediated by IaINs is likely the active pathway mediating the short-interval inhibition observed in our experiments (Talpalar et al. 2011; Wang et al. 2008).
We employed similar logic to estimate the number of synaptic relays in circuits that may mediate the long-interval inhibition observed in our experiments. Inhibition mediated by two central interneurons in a trisynaptic pathway would have a latency of ∼15.9 ms (5.3 ms + 5.3 ms + 5.3 ms), based on our measurements of monosynaptic communication in the spinal cord (Fig. 3B). Accounting for Quad peripheral conduction time (2.0 ms), this would suggest that trisynaptic inhibition of PBST MN output would begin at ∼18 ms. Analysis of preparations where short-interval inhibition is blocked by addition of 0.4 μM strychnine suggests that the long-interval inhibition begins to be observed at intervals of 16–20 ms (average of 16.8 ± 0.8 ms; n = 5; see Fig. 1E for example). These observations are then consistent with a model where the long-interval inhibition observed in our experiments is mediated by a trisynaptic response elicited by Quad afferent activation.
Long-interval inhibition is mediated via GABAergic presynaptic interneurons.
Activation of trisynaptic presynaptic inhibitory circuits by stimulation of Quad afferents could potentially reduce the PBST CAP evoked by stimulation of the DRL5 by blocking transmission of DRL5 Ia afferents on PBST MNs. We investigated this possibility by modifying a common strategy to measure PAD. PAD is often measured at the dorsal root and evoked by stimulation of an adjacent dorsal root (Bautista et al. 2012; Bos et al. 2011). In our preparation, we instead recorded antidromically conducted action potentials in the PBST nerve in the periphery following stimulation of the Quad nerve (Fig. 4A). All ventral roots were cut in these experiments to eliminate possibilities for contamination from MN axon responses, meaning that all responses in the PBST would be from sensory axons and evoked by stimulation of Quad sensory afferents via projections in the L3 and L4 dorsal roots. In all preparations at P7 (n = 5), a cluster of CAPs was observed in the PBST in each sweep following Quad stimulation at 0.1 Hz (Fig. 4B, top trace). These responses were greatly attenuated at increased stimulation frequencies (1.0–5.0 Hz), excluding the possibility the CAPs resulted from direct activation of PBST sensory afferents (data not shown). We found PBST primary afferent CAPs were blocked by bath application of 5 μM bicuculline (n = 3). In all three tests in which bicuculline was applied, no CAPs were observed under any stimulus condition (Fig. 4B, bottom trace). CAPs were observed again after 1 h of washout with ACSF (data not shown). Overall, these results strongly support the likelihood that long-interval Quad afferent inhibition of sensory-evoked activation of PBST MNs is likely mediated by GABAergic presynaptic inhibition of DRL5 primary afferent terminals via a trisynaptic circuit.
Fig. 4.
Sensory afferent action potentials in peripheral nerves evoked by primary afferent depolarization at P7/P8 are blocked by bicuculline. A: schematic diagram of experiment to record action potentials in primary sensory afferents antidromically conducted into the periphery following stimulation. A suction electrode was placed on the Quad nerve for stimulation to elicit primary afferent depolarization in the spinal cord. Afferent responses were recorded via a second suction electrode placed on the peripheral PBST nerve. All ventral roots were cut to prevent stimulation or recording of motor axon responses. B: representative trace illustrating action potential responses recorded from PBST afferents following stimulation of Quad afferents in a P7 preparation (top trace; n = 5). These responses were blocked by 5 μM bicuculline (bottom trace; n = 3).
Efficacy of proprioceptive circuits at birth.
Previous studies have clearly demonstrated that monosynaptic connections between Ia afferents and target neurons in the spinal cord, including MNs and IaINs, exist at birth (Kudo and Yamada 1987; Pinco and Lev-Tov 1993; Wang et al. 2008; Ziskind-Conhaim 1990). Therefore, we used our CAP analysis assay to determine the functional efficacy of reciprocal inhibition and presynaptic inhibition in reducing PBST motor output in early neonates (P0/P1). In contrast to the two phases of short- and long-interval inhibition observed at P7, only one phase of inhibition was observed at P0/P1 with maximum inhibition at an interval of 20.0 ± 0.9 ms (Fig. 5, A and B; n = 6). Quadriceps group I sensory afferent conduction velocities were measured as being only 0.58 ± 0.04 m/s (n = 3), so the longer interval required for maximum inhibition is likely a function of slower action potential conduction velocities. We wondered if the latencies for activation of both reciprocal inhibition and presynaptic inhibition could be less discrete than at P7, and whether these two circuits might be interacting at birth to generate a single phase of inhibition. To test this idea, we applied 5 μM strychnine to block glycinergic transmission and found the reduction of PBST CAP was completely blocked (Fig. 5C). Similar to the response at P7, facilitation was observed at birth upon strychnine application (n = 3; control: 50.4 ± 5.1% inhibition; 0.4 μM strychnine: 66.0 ± 9.9% facilitation; P < 0.05, Wilcoxon rank sum test). Interestingly, a greater degree of facilitation was observed, in strychnine, at birth than at P7 (P < 0.0005, Wilcoxon rank sum test). In addition, the single phase of inhibition was not blocked by bicuculline (Fig. 5D; n = 3; control: 52.2 ± 7.03%; 5 μM bicuculline: 46.5 ± 11.3%; P = 0.83, Wilcoxon rank sum test). These manipulations suggest only disynaptic reciprocal inhibition may be strong enough to block activation of MNs at birth.
Reduction in the area of the CAP in a peripheral nerve such as the PBST indicates fewer MNs are driven to threshold and fire action potentials in response to a given stimulus, in this case via activation of monosynaptically connected Ia afferents from DRL5. As a consequence, this assay can be utilized to quantify the effectiveness of a particular proprioceptive pathway in modulating MN output. We found that the percent reduction of the PBST CAP through disynaptic reciprocal inhibition was very similar at P0/P1 (n = 6) and at P7 (n = 10) (65.4 ± 5.7% reduction at P0/P1 and 70.6 ± 8.8% at P7; P = 0.63, 2-tailed, unpaired t-test). In a subset of these P0/P1 preparations (n = 3) we tested the stability of reciprocal inhibition over three full series of conditioning latencies and found glycinergic reciprocal inhibition was indeed stable (1st series: 54.5 ± 13.5%; 3rd series: 47.3 ± 9.1%; P = 0.83, Wilcoxon rank sum test), similar to the observation at P7. This suggests that reciprocal inhibition is a functionally relevant and effective pathway through which MN activation can be modulated even at birth.
GABAergic presynaptic inhibition of proprioceptive afferents is weak at birth.
Our finding that conditioning stimulation via Quad afferents evokes only a strychnine-sensitive inhibition of PBST MN activation at birth suggests presynaptic inhibition of DRL5 Ia afferents may be ineffective at this developmental stage. Nevertheless, GABAergic PAD can be elicited by stimulating and recording from adjacent dorsal roots in neonatal rats (Vinay and Clarac 1999), indicating that a large nerve bundle containing a variety of proprioceptive and cutaneous sources is capable of eliciting PAD. To investigate potential differences between these two results, we employed our modified PAD assay to attempt to record antidromic action potentials in sensory afferents of the PBST (n = 9; Fig. 6A). In contrast to what was observed at P7, we found stimulation of Quad afferents in P0/P1 preparations failed to evoke sensory afferent action potentials in the PBST nerve (Fig. 6B). Even very high stimulation intensities (>0.5 mA), which likely recruit many classes of sensory afferents, failed to induce action potentials in the PBST (data not shown). Interestingly, activation of afferents in the purely cutaneous saphenous nerve were capable of evoking some sensory afferent action potentials in the same preparation (Fig. 6C). Nevertheless, robust antidromic sensory action potentials could only be recorded in the PBST nerve following stimulation of the entire DRL4 (Fig. 6D). Our results with broad stimulation of DRL4 are consistent with previous reports demonstrating dorsal root potentials at birth, responses that we could observe also in the preparations (Fig. 6E). In three of the nine experiments, all of the stimulation paradigms mentioned were conducted in single preparations (Fig. 6, B–E). Together, these experiments suggest stimulation of proprioceptive afferents at birth fails to elicit presynaptic inhibition of other Ia proprioceptive afferents but that this circuitry becomes effective by the end of the first postnatal week.
Fig. 6.
Sensory afferent action potentials in peripheral nerves evoked by primary afferent depolarization at birth. A: schematic diagram of experimental setup similar to that described in Fig. 4. Afferent responses were recorded via a suction electrode placed on the peripheral PBST nerve or on DRL5. Stimulation pulses were presented via a suction electrode on the Quad or saphenous (Saph) nerve or on DRL4. All ventral roots were cut to prevent stimulation or recording of motor axon responses. B–D: recordings from PBST afferents following stimulation of Quad (B) or Saph nerves (C) or the entire DRL4 (D). E: dorsal root potential recorded from DRL5 following stimulation of DRL4. Data in B–E were recorded from the same preparation.
DISCUSSION
Proprioceptive information provides critical feedback to spinal circuits that can be used to alter motor output in response to perturbations in the periphery. Significant processing of proprioceptive information is conducted via interneuron-based pathways, yet relatively little is known regarding the functional status of interneuronal pathways in early postnatal animals. In this study, we assayed the ability of two interneuronal pathways to block motor neuron output using an acute in vitro isolated spinal cord preparation derived from early postnatal mice. We found that disynaptic reciprocal inhibition circuitry is as effective at birth as at P7 in blocking MN activation. We also found that proprioceptive afferents do not evoke presynaptic inhibition of Ia afferents at birth. In contrast, by P7 proprioceptive afferents are able to elicit presynaptic inhibition as has been reported in adult animals. These results suggest the mechanism of presynaptic inhibition, which acts to gate the flow of proprioceptive information into the circuits of the spinal cord, is only weakly established at birth but increases by the end of the first postnatal week.
Our finding that reciprocal inhibition of PBST via Quad afferents was as effective at blocking PBST activation at birth as at P7 differs from results published by Wang et al. (2008). They reported minimal reciprocal inhibition effectiveness at P0 (reduction of test pulse response of ∼12%). By P3, however, reciprocal inhibition strength was similar to that found at P7 (∼42% and ∼45% reductions, respectively). In contrast, intracellular recordings made from PBST MNs in the same study indicated the amplitude of reciprocal inhibition potentials was essentially unchanged between P0 and P7 (Wang et al. 2008). Differences in extracellular recording strategies may explain these apparently contradictory findings. Wang et al. recorded the compound response of all MNs exiting VRL5, whereas we recorded specifically the response of PBST MNs via measurements of the PBST CAP in the periphery. Their strategy focused on selective afferent stimulation (PBST afferents), with the converse trade-off of nonspecific recording from the whole ventral root. In contrast, our approach focused on selective recording of PBST MN responses activated by stimulation of a larger population of afferents, including synergists of PBST, via whole dorsal root stimulation. At birth, stimulation of only PBST afferents may be insufficient to drive many MNs to threshold, thus reducing the response measured at VRL5. In fact, Wang et al. reported EPSPs were difficult to obtain with this stimulation paradigm at P0/P1 (Wang et al. 2008). On the other hand, robust CAP responses were always obtained in our preparations at P0/P1, likely a consequence of activation of PBST afferents together with other synergistic DR afferents. In our experiments, PBST CAP amplitude was consistently reduced by conditioning stimulation of Quad afferents at appropriate intervals. This effect was sensitive to strychnine, indicating it is mediated by glycinergic transmission. These results strongly suggest that reciprocal inhibition is functional at P0/P1 and can effectively block MN activation even at this early postnatal stage.
Recording antidromically conducted afferent responses from the PBST muscle nerve allows for observation of effects of presynaptic inhibition on proprioceptive afferents. In contrast, recording dorsal root potentials from the whole dorsal root prohibits identification of sensory modality as well as the peripheral targets of afferents that contribute to the signal (Vinay and Clarac 1999). Admittedly, recordings from whole muscle nerves are still blind to the relative contributions of afferent subtypes (group Ia, Ib, and II) to the response. Nevertheless, our results demonstrate that these afferents are influenced by presynaptic inhibitory circuits differently at P0 vs. P7. Conditioning stimulation of Quad muscle nerve afferents fails to elicit action potentials in PBST afferents at birth but can evoke a strong response at P7. Thus proprioceptive afferent stimulation appears not to engage presynaptic inhibitory circuits at early postnatal stages.
A limitation of recording sensory responses from a nerve in the periphery is that only action potentials can be observed. Subthreshold PAD signals will not be observed in the periphery. Thus it remains a possibility that presynaptic inhibition of proprioceptive afferents is present at birth but that our peripheral PAD measurements are not well suited to detect it. Indeed, stimulation of the whole dorsal root, which synchronously activates many proprioceptive and cutaneous afferents, does generate sufficiently strong PAD to generate action potentials in PBST afferents. Yet simultaneous activation of all afferents in a dorsal root is not likely to occur in the normally behaving animal.
Recent anatomic analysis of the development of presynaptic inhibitory circuits lends support to our findings that presynaptic inhibition increases in efficacy from birth to P7. Betley et al. (2009) used mouse genetic approaches to demonstrate that although axons of GABAergic presynaptic inhibitory neurons are found in the ventral horn at birth, their synaptic terminals lack significant expression of GAD65 and GAD67, glutamic acid decarboxylase (GAD) isoforms that in combination specifically identify presynaptic inhibitory terminals (Hughes et al. 2005). By P7 terminals contacting Ia afferents are enriched with GAD65/67 (Betley et al. 2009). Whereas GAD67 provides basal levels of GABA synthesis, GAD65 is localized to synaptic terminals and supports additional synthesis during high-frequency firing (Patel et al. 2006; Tian et al. 1999). The lack of both of these enzymes in presynaptic inhibitory neuron terminals at P0 suggests presynaptic inhibition of proprioceptive afferents is minimal at this early postnatal stage. Afferent responses to GABA may also change during development. For example, differences in sensitivity to GABA antagonists as well as differences in GABAA receptor subunit expression have been demonstrated in embryonic and adult dorsal root ganglion neurons (Maddox et al. 2004; Valeyev et al. 1996, 1999).
Nevertheless, whole dorsal root stimulation can evoke PAD at birth, probably even in proprioceptive afferents. This implies that some synaptic terminals receive active presynaptic inhibition. Betley et al. (2009) did not investigate the status of GAD65/67 expression at presynaptic inhibitory terminals in more dorsal laminae of the spinal cord; therefore, the possibility remains that presynaptic inhibitory circuits may be more mature at birth on terminals in the intermediate zone of the spinal cord. If this were the case, conditioning stimulation of Quad should be able to initiate antidromically propagated action potentials in PBST afferent collaterals terminating at more dorsal positions in the cord. Because no action potentials were recorded, however, it is more likely that presynaptic inhibition is weak at birth and that responses can only be generated if large enough groups of axons are synchronously activated.
It should be noted that the present results can only provide information regarding presynaptic inhibition status on Ia afferent connections with MNs. Recent studies utilizing an isolated spinal cord and hindlimb preparation in neonatal rats (P1–P4) reported robust, phasic DRP evoked in contralateral dorsal root during supported locomotion (Hayes et al. 2012). The authors suggest these responses may be initiated by Ib afferents in the contralateral limb, particularly toe muscle afferents, and that the likely target of PAD, and hence the source of contralateral DRP, are Ib afferents (Devanandan et al. 1965; Hayes et al. 2012). Although our experiments cannot confirm or disprove this hypothesis, the general lack of PAD-evoked antidromic action potentials observed in the periphery following stimulation of Quad afferents suggests PAD on other proprioceptive afferents, including Ib afferents, is weaker at birth than at P7.
At the onset of a voluntary contraction, presynaptic inhibition of Ia afferent terminals on MNs projecting to the contracting muscle is reduced, whereas presynaptic inhibition of Ia afferent terminals on nonparticipating muscles is increased (Hultborn et al. 1987). Selective gating of Ia afferent input allows sensory feedback to influence MN activity to compensate for unpredictable length and/or velocity differences (Meunier and Pierrot-Deseilligny 1989). Multiple descending systems exert control on the interneurons involved in presynaptic inhibition (Rudomin et al. 1983; Rudomin and Schmidt 1999). In the developing system studied presently, both the local circuits that effect presynaptic inhibition as well as descending systems that selectively increase and/or decrease the strength of presynaptic inhibition during voluntary movements are immature. Thus, before both of these systems mature, Ia afferent signals on MNs are not gated in the way they are in adults. This situation may be advantageous for neonatal animals as they learn to interact with the environment. Proprioceptive feedback from either passive or intentional limb movements are unfiltered by presynaptic inhibition and may serve to maximize sensitivity or to calibrate various feedback-sensitive pathways, including the stretch reflex and reciprocal inhibition, to respond to the widest range of signal intensity. This may be particularly important when considering the fact that the response profiles of muscle spindle afferents during early postnatal development are still somewhat immature in coding signals of muscle stretch (Vejsada et al. 1985). Analysis of the acquisition of locomotor skills in genetic experiments in mice with reduced or increased presynaptic inhibition may provide additional insight into its role in postnatal development.
GRANTS
Support for this work was provided by National Institute of Neurological Disorders and Stroke Grant NS-072454 (to D. R. Ladle).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.M.S. and D.R.L. conception and design of research; P.M.S. and D.R.L. performed experiments; P.M.S. and D.R.L. analyzed data; P.M.S. and D.R.L. interpreted results of experiments; P.M.S. and D.R.L. prepared figures; P.M.S. and D.R.L. drafted manuscript; P.M.S. and D.R.L. edited and revised manuscript; P.M.S. and D.R.L. approved final version of manuscript.
REFERENCES
- Alvarez FJ. Anatomical basis for presynaptic inhibition of primary sensory fibers. In: Presynaptic Inhibition and Neural Control, edited by Rudomin P, Romo R, Mendell L. New York: Oxford University Press, 1998, p. 13–49 [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell 101: 485–498, 2000 [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Handbook of Physiology. The Nervous System. Bethesda, MD: Am. Physiol. Soc., 1981, p. 509–596 [Google Scholar]
- Banks RW. The muscle spindle. In: Peripheral Neuropathy, edited by Dyck PJ, Thomas PK. Philadelphia, PA: Saunders, 2005, p. 131–150 [Google Scholar]
- Bautista W, Nagy JI, Dai Y, McCrea DA. Requirement of neuronal connexin36 in pathways mediating presynaptic inhibition of primary afferents in functionally mature mouse spinal cord. J Physiol 590: 3821–3839, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Wright CV, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 139: 161–174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R, Brocard F, Vinay L. Primary afferent terminals acting as excitatory interneurons contribute to spontaneous motor activities in the immature spinal cord. J Neurosci 31: 10184–10188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Comeau F. Differential effects of a flexor nerve input on the human soleus H-reflex during standing versus walking. Can J Physiol Pharmacol 73: 436–449, 1995 [DOI] [PubMed] [Google Scholar]
- Curtis DR, Hosli L, Johnston GA, Johnston IH. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res 5: 235–258, 1968 [DOI] [PubMed] [Google Scholar]
- Devanandan MS, Holmqvist B, Yokota T. Presynaptic depolarization of group I muscle afferents by contralateral afferent volleys. Acta Physiol Scand 63: 46–54, 1965 [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol 137: 22–50, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol 144: 271–298, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe RE. Glycine-like immunoreactivity in synaptic boutons of identified inhibitory interneurons in the mammalian spinal cord. Brain Res 547: 175–179, 1991 [DOI] [PubMed] [Google Scholar]
- Gossard JP, Cabelguen JM, Rossignol S. Intra-axonal recordings of cutaneous primary afferents during fictive locomotion in the cat. J Neurophysiol 62: 1177–1188, 1989 [DOI] [PubMed] [Google Scholar]
- Gossard JP, Cabelguen JM, Rossignol S. Phase-dependent modulation of primary afferent depolarization in single cutaneous primary afferents evoked by peripheral stimulation during fictive locomotion in the cat. Brain Res 537: 14–23, 1990 [DOI] [PubMed] [Google Scholar]
- Gossard JP, Rossignol S. Phase-dependent modulation of dorsal root potentials evoked by peripheral nerve stimulation during fictive locomotion in the cat. Brain Res 537: 1–13, 1990 [DOI] [PubMed] [Google Scholar]
- Hayes HB, Chang YH, Hochman S. Stance-phase force on the opposite limb dictates swing-phase afferent presynaptic inhibition during locomotion. J Neurophysiol 107: 3168–3180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabo G, Erdelyi F, Veress G, Szucs P, Antal M, Todd AJ. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc Natl Acad Sci USA 102: 9038–9043, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol 389: 757–772, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38: 335–378, 1992 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lindstrom S. Morphology of interneurones mediating Ia reciprocal inhibition of motoneurones in the spinal cord of the cat. J Physiol 226: 805–823, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816: 493–499, 1999 [DOI] [PubMed] [Google Scholar]
- Kudo N, Yamada T. Development of the monosynaptic stretch reflex in the rat: an in vitro study. J Physiol 369: 127–144, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Yamada T. Morphological and physiological studies of development of the monosynaptic reflex pathway in the rat lumbar spinal cord. J Physiol 389: 441–459, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon D, Bettoun D, Harris-Cerruti C, Woolf E, Negreanu V, Eilam R, Bernstein Y, Goldenberg D, Xiao C, Fliegauf M, Kremer E, Otto F, Brenner O, Lev-Tov A, Groner Y. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J 21: 3454–3463, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli J, Quevedo J, Linares P, Rudomin P. Local control of information flow in segmental and ascending collaterals of single afferents. Nature 395: 600–604, 1998 [DOI] [PubMed] [Google Scholar]
- Machacek DW, Hochman S. Noradrenaline unmasks novel self-reinforcing motor circuits within the mammalian spinal cord. J Neurosci 26: 5920–5928, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox FN, Valeyev AY, Poth K, Holohean AM, Wood PM, Davidoff RA, Hackman JC, Luetje CW. GABAA receptor subunit mRNA expression in cultured embryonic and adult human dorsal root ganglion neurons. Brain Res Dev Brain Res 149: 143–151, 2004 [DOI] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol 487: 527–539, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears SC, Frank E. Formation of specific monosynaptic connections between muscle spindle afferents and motoneurons in the mouse. J Neurosci 17: 3128–3135, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. J Physiol 419: 753–763, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Martin DL, Battaglioli G, Behar KL. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J Neurochem 97: 385–396, 2006 [DOI] [PubMed] [Google Scholar]
- Pinco M, Lev-Tov A. Synaptic excitation of alpha-motoneurons by dorsal root afferents in the neonatal rat spinal cord. J Neurophysiol 70: 406–417, 1993 [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, part I, p. 89–127 [Google Scholar]
- Proske U. Kinesthesia: the role of muscle receptors. Muscle Nerve 34: 545–558, 2006 [DOI] [PubMed] [Google Scholar]
- Quevedo J, Eguibar JR, Lomeli J, Rudomin P. Patterns of connectivity of spinal interneurons with single muscle afferents. Exp Brain Res 115: 387–402, 1997 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006 [DOI] [PubMed] [Google Scholar]
- Rudomin P, Jimenez I, Solodkin M, Duenas S. Sites of action of segmental and descending control of transmission on pathways mediating PAD of Ia- and Ib-afferent fibers in cat spinal cord. J Neurophysiol 50: 743–769, 1983 [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999 [DOI] [PubMed] [Google Scholar]
- Shneider NA, Mentis GZ, Schustak J, O'Donovan MJ. Functionally reduced sensorimotor connections form with normal specificity despite abnormal muscle spindle development: the role of spindle-derived neurotrophin 3. J Neurosci 29: 4719–4735, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Shenton F, Hunter I, Banks RW, Bewick GS. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol 588: 171–185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB. Presynaptic inhibition in humans. Prog Neurobiol 47: 533–544, 1995 [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Endo T, Low P, Borgius L, Hagglund M, Dougherty KJ, Ryge J, Hnasko TS, Kiehn O. Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron 71: 1071–1084, 2011 [DOI] [PubMed] [Google Scholar]
- Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci USA 96: 12911–12916, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourtellotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat Genet 20: 87–91, 1998 [DOI] [PubMed] [Google Scholar]
- Valeyev AY, Hackman JC, Holohean AM, Wood PM, Katz JL, Davidoff RA. GABA-induced Cl− current in cultured embryonic human dorsal root ganglion neurons. J Neurophysiol 82: 1–9, 1999 [DOI] [PubMed] [Google Scholar]
- Valeyev AY, Hackman JC, Wood PM, Davidoff RA. Pharmacologically novel GABA receptor in human dorsal root ganglion neurons. J Neurophysiol 76: 3555–3558, 1996 [DOI] [PubMed] [Google Scholar]
- Vejsada R, Hnik P, Payne R, Ujec E, Palecek J. The postnatal functional development of muscle stretch receptors in the rat. Somatosens Res 2: 205–222, 1985 [DOI] [PubMed] [Google Scholar]
- Vinay L, Clarac F. Antidromic discharges of dorsal root afferents and inhibition of the lumbar monosynaptic reflex in the neonatal rat. Neuroscience 90: 165–176, 1999 [DOI] [PubMed] [Google Scholar]
- Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell 127: 1439–1452, 2006 [DOI] [PubMed] [Google Scholar]
- Wang Z, Li L, Goulding M, Frank E. Early postnatal development of reciprocal Ia inhibition in the murine spinal cord. J Neurophysiol 100: 185–196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O'Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol 84: 2821–2833, 2000 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 58: 185–205, 1999 [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. J Neurosci 10: 125–135, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]