Abstract
The avian auditory papilla contains two classes of sensory receptor, tall hair cells (THCs) and short hair cells (SHCs), the latter analogous to mammalian outer hair cells with large efferent but sparse afferent innervation. Little is known about the tuning, transduction, or electrical properties of SHCs. To address this problem, we made patch-clamp recordings from hair cells in an isolated chicken basilar papilla preparation at 33°C. We found that SHCs are electrically tuned by a Ca2+-activated K+ current, their resonant frequency varying along the papilla in tandem with that of the THCs, which also exhibit electrical tuning. The tonotopic map for THCs was similar to maps previously described from auditory nerve fiber measurements. SHCs also possess an A-type K+ current, but electrical tuning was observed only at resting potentials positive to −45 mV, where the A current is inactivated. We predict that the resting potential in vivo is approximately −40 mV, depolarized by a standing inward current through mechanotransducer (MT) channels having a resting open probability of ∼0.26. The resting open probability stems from a low endolymphatic Ca2+ concentration (0.24 mM) and a high intracellular mobile Ca2+ buffer concentration, estimated from perforated-patch recordings as equivalent to 0.5 mM BAPTA. The high buffer concentration was confirmed by quantifying parvalbumin-3 and calbindin D-28K with calibrated postembedding immunogold labeling, demonstrating >1 mM calcium-binding sites. Both proteins displayed an apex-to-base gradient matching that in the MT current amplitude, which increased exponentially along the papilla. Stereociliary bundles also labeled heavily with antibodies against the Ca2+ pump isoform PMCA2a.
Keywords: chicken, hair cells, calcium buffers, electrical tuning, mechanotransduction
the auditory papilla of birds, exemplified by the chicken, contains two types of hair cell, tall hair cells (THCs) and short hair cells (SHCs), apparently analogous to the mammalian inner and outer hair cells (Tanaka and Smith 1978). Columnar THCs overlying the fibrocartilaginous plate receive the major afferent innervation, whereas flattened SHCs above the basilar membrane are innervated predominantly by efferent but not afferent neurons (Fischer 1992). The relative roles of these two classes of hair cell and the origin of frequency selectivity has been the source of speculation, and it has been argued that SHCs, like the mammalian outer hair cells, may possess some motor function that is recruited to extend the frequency range to 5 kHz or more (Gleich and Manley 2000; Köppl 2011; Manley and Köppl 1998). Despite the extensive anatomical data on the avian papilla (Hirokawa 1978; Tanaka and Smith 1978; Tilney and Saunders 1983), less is known about the physiological properties of the hair cells. The proportion of SHCs to THCs grows toward the high-frequency end of the papilla (Hirokawa 1978), which would be consistent with their increased contribution at high frequencies. To achieve tuning at low frequencies, the avian cochlea utilizes electrical resonance based on voltage-dependent Ca2+ and K+ channels, especially BK channels, in THCs (Fuchs et al. 1988). As in turtle (Art and Fettiplace 1987; Art et al. 1995), there is a tonotopic gradient in the BK channel kinetics (Duncan and Fuchs 2003) and density (Samaranayake et al. 2004) to change the electrical resonant frequency (Ramanathan and Fuchs 2002). Earlier studies of SHCs focused on their efferent inhibition by ACh (Fuchs and Murrow 1992), and although they possess an inactivating K+ current (Murrow and Fuchs 1990), little else is known about their electrical properties or whether they possess electrical tuning. Although changes in mechanotransducer (MT) currents during embryonic development have been examined (Si et al. 2003), there has been no systematic study of the MT channels or their adaptation, Ca2+ sensitivity, or tonotopic variation in either class of avian hair cell.
The aim of our work was to develop a basilar papilla preparation, optimizing both isolation and recording conditions, for examining the electrical properties of the hair cells, especially the sparsely studied SHCs. As an indication of the condition, a significant criterion was the ability to record large MT currents similar to those seen in other hair cell epithelia, including the turtle (Ricci and Fettiplace 1997), mouse (Kros et al. 1992), and rat (Kennedy et al. 2003). Original recordings from chicken hair cells were carried out on enzymatically dissociated cells (Fuchs et al. 1988; Fuchs and Evans 1990; Murrow 1994), yielding good voltage-dependent currents, although there may be damage to the hair bundle and transduction apparatus during the dissociation procedure. Intact basilar papillar preparations were subsequently explored (Levic et al. 2007; Pantelias et al. 2001; Spassova et al. 2001), but the prime objective in these works was characterizing the voltage-dependent K+ and Ca2+ currents and no attention was paid to mechanotransduction. We shall show that large MT currents can be measured with their amplitudes increasing dramatically toward the basal end. Apart from the MT currents, an ancillary question concerns the change with location in the electrical resonant frequency (FE). A mapping of the THC FE was given in Pantelias et al. (2001), but the range is too small and inconsistent with the characteristic frequency (CF) maps derived from auditory nerve fiber measurements (Chen et al. 1994; Jones and Jones 1995; Jones et al. 2006; Manley et al. 1987). Our results show that over the apical half of the papilla FE fairly closely matches the nerve fiber maps. The work provides a framework for exploring other SHC properties, including a prospective motor function that may underlie amplification and tuning in the chicken papilla, which is the topic of a subsequent paper (Beurg et al., manuscript in preparation).
MATERIALS AND METHODS
Preparation
Recordings were made from hair cells in the isolated basilar papilla of embryonic [embryonic day (E)18–E21] or newly hatched [postnatal day (P)0] chickens (Gallus gallus domesticus, White Leghorn). Auditory sensitivity in the chicken increases substantially in the last two embryonic days, and by E19–E20 both acoustic threshold and frequency range are not far off that of the adult (Jones et al. 2006; Saunders et al. 1973). Fertilized eggs (Sunnyside Hatchery) were stored in a refrigerator with a customized temperature range of 14–16°C prior to transfer to the incubator. For incubation, the eggs were maintained in the self-turning Sportsman 1502 incubator (GQF Manufacturing) until the day of the experiment. The incubator temperature was kept at 38°C and the humidity at 60–65%. Animals were killed by decapitation with methods approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison according to current National Institutes of Health guidelines. The procedure for isolating the basilar papilla was modified from that previously described (Fuchs et al. 1988) with the aim of improving the condition and survival of the preparation. The skull was split along the midline, the brain removed, and the papilla dissected out and transferred to an oxygenized dissection saline containing (in mM) 153 NaCl, 5 KCl, 0.15 CaCl2, 1 MgCl2, 10 HEPES, 0.05 pyridoxal phosphate-6-azophenyl-2',4'-disulfonic acid (PPADS), 8 glucose and 2 Na-pyruvate (pH 7.4, 320 mosM, T = 23°C). PPADS (Sigma-Aldrich, St. Louis, MO) was added to block P2X receptors (Ralevic and Burnstock 1998) and inhibit Ca2+ loading of the supporting cells, which in a poor preparation were often the first to deteriorate, and was accompanied by ejection of the SHCs from the surface of the epithelium as occurs in noise damage to the chick ear (Cotanche and Dopyera 1990). The tegmentum vasculosum and the macula lagena at the apex were removed to expose the intact hair cell epithelium. The length of the comma-shaped basilar papilla was measured and found to increase with age from 3.59 ± 0.17 mm (n = 91; E19) to 3.80 ± 0.14 mm (n = 47; P0) (means ± SD). The isolated basilar papilla was then transferred to the recording chamber, where it was secured, hair bundles uppermost, by two strands of dental floss on either side of the desired recording location. Dissection saline containing 100 μg/ml protease type XXIV (Sigma-Aldrich) was briefly perfused through a 10-μm-diameter pipette along the edge of the papilla to ensure that it was exposed to only just sufficient enzyme to lift up the tectorial membrane at the abneural (inferior) edge. The tectorial membrane was then removed and the enzymatic digestion terminated by exchange with saline containing 50 mg/ml bovine serum albumin (BSA).
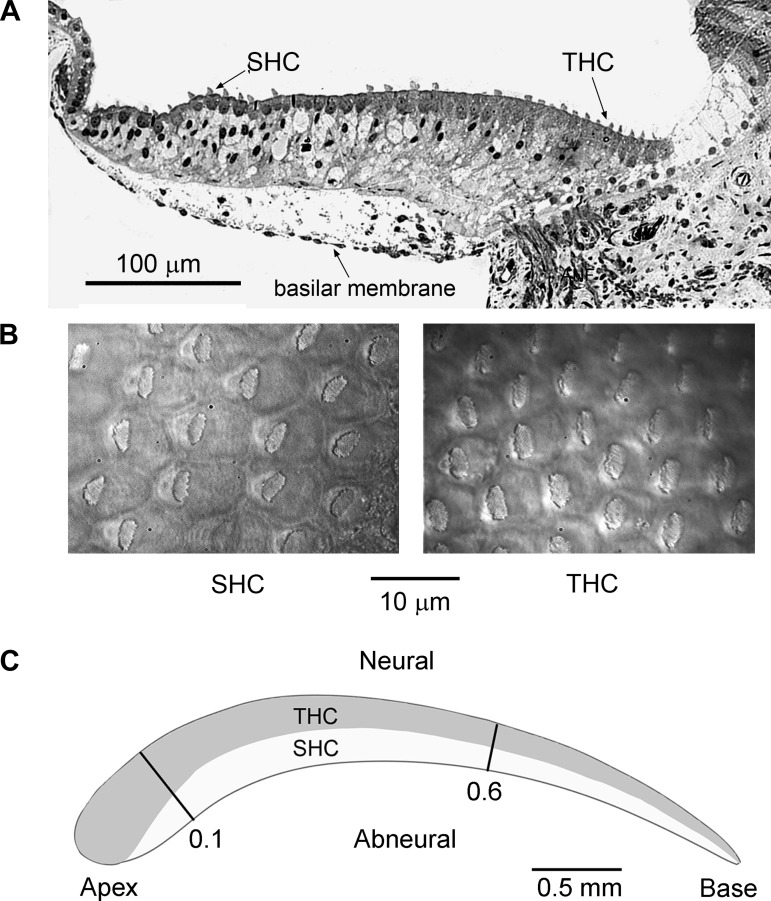
The experimental chamber holding the preparation was transferred to a Leica DMLFS fixed-stage microscope, where it was viewed through a long-working-distance ×63 water-immersion objective (numerical aperture 0.9), a ×1.5 optivar, and a Hamamatsu CCD camera. The chamber was perfused with oxygenated saline of the following composition (in mM): 151 NaCl, 5 KCl, 1.5 or 2.5 CaCl2, 8 glucose, 2 Na-pyruvate, 10 HEPES, pH 7.4 (320 mosM). The saline was heated as it traveled through a glass perfusion tube wrapped in a nichrome heating coil, the bath temperature in most experiments being held at 33–34°C by feedback from a thermocouple placed next to the preparation. Measurements were also made at room temperature (23°C) with no heating. In some experiments, the solution around the hair bundle was controlled by flow from a 10-μm pipette and was changed to artificial endolymph (Sauer et al. 1999) containing (in mM) 156 NaCl, 5 KCl, 0.24 CaCl2, 2 Na-pyruvate, 8 glucose, and 10 K-HEPES, pH 7.4. Blocking agents were introduced either via the top perfusion tube (FM1–43; Invitrogen) or by addition to the entire bath [TEA, 4-aminopyridine (4-AP), Sigma-Aldrich; paxilline, apamin, Tocris]. Apamin (0.3 μM) was sometimes added to the external solution, especially for SHC recording, to block any SK conductance associated with efferent transmission (Yuhas and Fuchs 1999). SHCs were identified by lower surface density (Fig. 1, A and B) and position <10 hair cell rows from the abneural edge (Hirokawa 1978). SHCs are also marked by the eccentric placement of the hair bundle (Fig. 1B; Hirokawa 1978). THCs have a higher surface density and, because they overlie the fibrocartilaginous plate (Fig. 1A), present a less bright image. The recording location was marked at the end of each experiment, and the distance of the recording site from the apical (distal) low-frequency end of the papilla was measured. This distance was normalized by the total length of the papilla and is given as d, the fractional distance from apex. Almost all recordings were made between d = 0.1 and d = 0.6 (Fig. 1C).
Fig. 1.
Hair cell arrangement in the chicken basilar papilla. A: transverse section through a papilla in the apical region [fractional distance from apex (d) = 0.25] showing the location of the short hair cells (SHCs) above the basilar membrane and the tall hair cells (THCs) overlying the fibrocartilaginous plate containing the auditory nerve fibers; P1 chicken. B: surface views of the intact papilla showing the lower density of the SHCs with their eccentrically placed hair bundles and the higher density of THCs. The low surface density and placement <10 cells from the abneural edge were used to identify SHCs for recording. C: schematic of basilar papilla showing the longitudinal section over which most of the recordings were made from d = 0.1 to 0.6 and the approximate distributions of the THCs and SHCs based upon Hirokawa (1978; Fig. 2b) and the surface morphology of whole mount preparations as in B. In some descriptions, the apical and basal ends of the papilla are referred to as “distal” and “proximal,” respectively, and the neural and abneural edges are termed “superior” and “inferior.”
Electrical Recordings and Stimulation
Hair cell recordings were made with borosilicate patch electrodes introduced through the luminal surface of the epithelium as described for other preparations (Kennedy et al. 2003; Kros et al. 1992; Ricci and Fettiplace 1997). Patch pipettes were filled with an intracellular solution composed of (in mM) 137 KCl, 2.5 MgCl2, 0.5 BAPTA, 3 MgATP, 10 Tris creatine phosphate, and 10 HEPES, pH 7.2, and had starting resistances of 3–5 MΩ. In some experiments, Cs+ was substituted for K+ to block voltage-dependent K+ currents and 1 mM BAPTA or EGTA was used to vary the calcium buffering. For perforated-patch recordings (Ricci et al. 1998), the pipette solution contained (in mM) 135 K-aspartate, 10 KCl, 5 MgATP, 1 EGTA, and 10 HEPES, pH 7.2 with or without nystatin (Calbiochem, San Diego, CA), 2.5 mg of which was dissolved in 10 μl of dry dimethyl sulfoxide and diluted 1:500 in the pipette solution. The patch pipette was tip-filled with antibiotic-free stock solution and back-filled with the nystatin solution. Series resistances in perforated-patch mode were 11–24 MΩ with no added compensation. The patch electrode was connected to an Axopatch 200B amplifier for all voltage-clamp measurements, but to determine a hair cell's FE an Axoclamp-2A voltage follower was used in some recordings because of its better frequency response in current clamp. Series resistance in ruptured-patch recordings with up to 70% compensation was 2–5 MΩ. All membrane potentials are corrected for current flow across the residual series resistance and for junction potentials (−4 mV for CsCl or KCl and −13 mV for K-aspartate intracellular).
Hair bundles were mechanically stimulated by a fluid jet from a pipette, tip diameter ∼10 μm, driven by a 25-mm-diameter piezoelectric disk as previously documented (Johnson et al. 2011). The distance of the pipette tip from the bundle was adjusted to elicit a maximal MT current. Saturating mechanical stimuli were applied as 40- or 50-Hz sinusoids. When the effects of low (0.24 mM)-Ca2+ endolymph were tested the fluid jet pipette was filled with the endolymph, and during stimulus delivery the fluid around the hair bundle was also exchanged for the same low-Ca2+ solution. In a few experiments, hair bundles were deflected with a glass stylus driven by a piezoelectric stack actuator (PA8/12, Piezosystems Jena, Hopedale, MA). The driving voltage to the actuator was filtered at 3–4 kHz, yielding step displacements with a rise time of ∼100 μs. Displacements of the glass probe or hair bundle were calibrated (Crawford and Fettiplace 1985; Ricci et al. 2000) by projecting their image onto a pair of photodiodes (LD 2-5; Centronics, Newbury Park, CA) at ×270 total magnification. Current-voltage relationships and MT current-displacement curves were measured from automated protocols generated by a Cambridge Electronic Design (CED; Cambridge, UK) Power1401 interface driven by a PC. Data were digitized with the CED interface, and the results were analyzed with WaveMetrics IGOR Pro v6.
Immunogold Labeling
Antibodies and protein standards.
A polyclonal calbindin D-28k antibody (CB 38a) against the rat protein, which also recognizes chicken calbindin D-28k, and the recombinant calbindin D-28k protein were both obtained from Swant (Bellinoza, Switzerland). A polyclonal antibody to bullfrog parvalbumin-3 (PV-3; GenBank accession no. AALO992; Heller et al. 2002) and also a sample of the recombinant parvalbumin-3 were kindly provided by Dr. Stefan Heller (Stanford University, Stanford, CA). The bullfrog parvalbumin-3 shows high homology to chicken parvalbumin-3 (74% identity) and to mammalian oncomodulin, and the antibody labels chicken basilar papilla hair cells and also labels a 13-kDa band of chicken basilar papilla lysate (Heller et al. 2002). Plasma membrane Ca2+ pumps (PMCA) were labeled with both a polyclonal anti-peptide antibody (NR2) made against the NH2 terminal of the PMCA2 isoform (NR2, ThermoFisher Scientific, Pittsburgh, PA) and an anti-peptide antibody (F2a) against the COOH terminal of the PMCA2a splice variant (Dumont et al. 2001), which was a gift from Dr. Peter Gillespie (Vollum Institute, Portland, OR). The secondary antibody was a goat anti-rabbit IgG conjugated to 10-nm gold particles (British BioCell, Cardiff, UK).
Fixation, embedding, and postembedding immunogold labeling.
Whole basilar papillae from P1 chickens were isolated as described above and fixed in 4% paraformaldehyde plus 0.1% glutaraldehyde in 0.1 M phosphate buffer (PB, pH 7.4) for 2 h. The fixed papilla was then dehydrated in a graded series of ethanols (70%, 80%, 90%, 100%, and dry 100%) for 15 min in each, infiltrated with LR (London Resin)-White resin (Agar Scientific, Stansted, UK) at room temperature for 24 h, and embedded in pure resin polymerized at 50°C for 24 h in gelatin capsules. Semithin sections (0.5–2 μm) were collected on glass slides, stained with 1% toluidine blue in 1% borax, and photographed with a digital camera (DeltaPix Infinity X, Indigo-scientific) and a ×10 objective Leitz Dialux 20 EB Orthoplan microscope (Ernst Leitz Wetzlar). Ultrathin transverse sections (100 nm) were cut from apical, middle, and basal regions of the papilla on a Leica Ultracut UCT Microtome and collected on 200-mesh thin-bar nickel grids. The three regions of the papilla assayed were centered on d of 0.25 (apex), 0.35 (middle), and 0.70 (base).
The grids containing sections were washed in 0.05 M Tris-buffered saline (TBS, pH 7.4) and nonspecific-labeling blocked with TBS containing 20% goat serum (GS) and 0.2% Tween 20 (TBS-GS-T20) for 30 min at room temperature (∼20°C). Grids were then incubated overnight at 4°C in one of the primary antibodies (calbindin D-28k at a dilution of 1:500, parvalbumin-3 at 1:1,000, NR2 at 1:200, and F2a at 1:100) in TBS containing 1% BSA and 0.2% Tween 20 (TBS-BSA-T20). The antibody concentrations were chosen so that there was no background labeling on the resin. For a negative control, grids containing sections were incubated in TBS-BSA-T20 without the primary antibody. The sections were then incubated in TBS-BSA-T20 (3 × 10 min), nonspecific labeling-blocked in TBS-GS-T20 (15 min), and incubated in goat anti-rabbit IgG (British BioCell) conjugated to 10-nm gold particles diluted 1:20 in TBS-BSA-T20 for 2 h at room temperature. The sections were then washed in TBS followed by distilled water, stained in 2% aqueous uranyl acetate for 20 min, and examined with a JEOL JEM 1230 or a JEOL-100CX transmission electron microscope operated at an accelerating voltage of 100 kV.
Quantification of calcium-binding proteins.
Densities of Ca2+-binding proteins in the stereocilia were semiquantified by calibrating the immunogold particle counts against known concentrations of the relevant protein as previously described (Hackney et al. 2003, 2005). Solutions of the Ca2+-binding protein were made in 10% BSA plus 4% paraformaldehyde in 0.1 M PB (pH 7.4) and were solidified into gels by the addition of 0.1% glutaraldehyde. One- to two-millimeter blocks of each gel standard were dehydrated and embedded in LR-White resin in the same way as for the chicken papilla segments in order to provide protein standards. Ultrathin sections of these protein standard gels were cut onto nickel grids, immunogold labeled with the relevant antibody and 10-nm gold-conjugated goat anti-rabbit IgG diluted 1:20, both in TBS-BSA-T20 under the same conditions and in parallel with transverse sections from the three locations, and stained in 2% aqueous uranyl acetate for 20 min, and all micrographs were taken at ×25,000. Density of gold particles per square micrometer of the protein standard gels was calculated by using two random micrographs for each of the calbindin D-28k protein standards and five micrographs for the parvalbumin-3 protein standard, and the number of gold particles within the entire area of each micrograph was counted. The mean gold particle density on the gel increased linearly with protein concentration (Hackney et al. 2003, 2005) up to a maximum concentration of 0.23 mM, the largest that could be made with the limited amount of protein available. Electron micrographs (n = 10) of stereociliary bundles from THCs and SHCs in the apical, middle, and basal regions were also taken. Micrographs of stereocilia from THCs that were on the neural limbus (neural edge of the basilar papilla) and stereocilia from SHCs that were on the basilar membrane of the abneural edge (∼1/3 width of the basilar papilla) were taken into account for quantification. The area of the stereociliary bundles was estimated by using the area/perimeter measurement tool in ANALYSIS (Olympus) software, and the number of gold particles on the stereocilia and within the spatial resolution (21 nm) external to the stereociliary membrane was counted to calculate the density of gold particles per square micrometer. Matching the particle densities on the standard with those on the tissue allowed the tissue concentration to be ascertained. Since the tissue concentrations were larger than the maximum gel concentration, this required an extrapolation.
PMCA2 labeling in the stereociliary bundle was quantified by determining the areal density (gold particles/μm2). For each location, 10 micrographs of stereociliary bundles of both THCs and SHCs were analyzed. To determine the variation in parvalbumin-3 labeling across the papilla, low-magnification images of the basilar papilla (middle region) from five serial sections, all from the same grid, were taken and every hair cell visible on the section plane (excluding the hair cells obscured by grid bars) was identified and matched in all five sections. Micrographs of stereocilia visible on the section planes and not obscured by grid bars were taken from all five sections, and the labeling density (gold particles/μm2) was calculated. For determining the variation in PMCA2 across the papilla, only a single section was used.
Statistical Analysis
For the electrophysiological measurements, values are quoted as means ± SD and differences were evaluated with a two-tailed t-test. For immunolabeling, values are quoted as means ± SE and differences were evaluated with a Wilcoxon signed-rank test or a Kruskal-Wallis test depending on the distribution of data points.
RESULTS
Electrical Tuning in THCs and SHCs
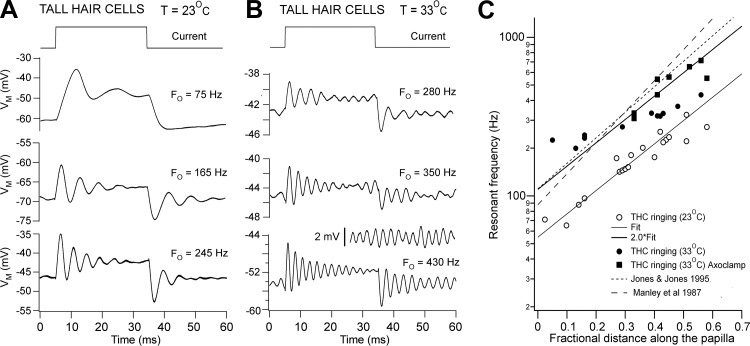
Damped oscillations in membrane potential indicative of electrical tuning (Crawford and Fettiplace 1981; Fuchs et al. 1988; Hudspeth and Lewis 1988) could be evoked in most THCs by depolarizing current steps (Fig. 2, A and B). More sharply tuned and symmetrical responses were observed at the onset and termination of the current when the temperature was raised from 23°C to 33°C, and at the higher temperature spontaneous oscillations were sometimes observed at rest (Fig. 2B, inset). Unlike previous reports (Fuchs and Evans 1990; Pantelias et al. 2001), action potentials were not seen in low-frequency hair cells at either temperature. The evoked resonance can be characterized in terms of the frequency of the damped voltage oscillations and the time constant of their decay. The FE for a given cell was defined as the frequency at which the tuning was sharpest, corresponding to the largest number of oscillations. Measured FE values ranged from ∼190 to 700 Hz at 33°C. The sharpness of tuning can be quantified by the Q3dB, given by Q3dB ≈ π·FE·τE, where τE is the time constant of decay in oscillation amplitude (Crawford and Fettiplace 1981): the slower the decay time constant, the larger the Q3dB. For the three examples shown in Fig. 2B, Q3dB values were 7, 9, and 13, which are similar to the sharpest frequency selectivity seen in turtle hair cells (Art and Fettiplace 1987; Crawford and Fettiplace 1981). Tuning in THCs was maximal at membrane potentials between −50 and −40 mV (mean = −45.3 ± 4.7 mV; T = 33°C). How this relates to the resting potential is discussed below.
Fig. 2.
Electrical resonance in THCs. A: voltage (VM) responses of cells at 3 locations to extrinsic current steps; the resonant frequency at current onset (Fo) is shown above each of the averaged traces; T = 23°C. B: responses of 3 other cells at different locations to extrinsic current steps; Fo is shown above each of the averaged traces; T = 33°C. Inset: spontaneous oscillations in membrane potential for cell at bottom. In A and B, each voltage trace is the average of 10 presentations. C: collected results of resonant frequency plotted against THC location, expressed as the distance from the apical (distal) end normalized to the total length of the papilla. ○, T = 23°C; ● and ■, T = 33°C. The line through the points for T = 23°C is an exponential fit: FE = A·exp(d/λ), where A = 51 Hz and λ = 0.3 for 0 ≤ d ≤ 1. The fit was extended to the points at T = 33°C by multiplying by 2 (A = 102), which corresponds to Q10° = 2. All measurements were made on E19–E20 birds. ■, Obtained from recordings with Axoclamp-2A. Dashed lines are maps of characteristic frequency (CF) from auditory nerve fiber tuning curves from Manley et al. (1987) in P2 intact chickens and Jones and Jones (1995) in E19 birds at T = 39°C.
At both temperatures FE increased exponentially with distance along the papilla (Fig. 2C), but at the higher temperature there appeared to be a frequency limit at ∼400 Hz. This may be an artifact of the limited frequency response of the Axopatch 200 (Magistretti et al. 1996), because higher frequencies were measured if current-clamp recordings were made with the Axoclamp-2A, which is a voltage follower (compare filled circles and squares in Fig. 2C). An exponential fit to the frequency map at 23°C could be extended to 33°C by assuming a doubling of FE for a 10° rise in temperature (Fig. 2C). This temperature dependence is similar to that previously reported for electrical tuning in both chicken and turtle hair cells (Fuchs and Evans 1990; Wu et al. 1995) and also for the acoustic CF of avian nerve fibers (Schermuly and Klinke 1985). The tonotopic map for THC electrical tuning can be compared with the maps derived in intact chickens by measuring the CFs of auditory nerve fibers labeled so that the site of origin along the papilla can be traced (Fig. 2C, discontinuous lines). The map is not very different from those determined in E19 chickens (Jones and Jones 1995) and P2 and P21 chickens (Manley et al. 1987), both at T = 39°C, but the FE values at given locations are smaller than the equivalent acoustic CFs in adults at T = 41°C (Chen et al. 1994). However, given the different temperatures between the various measurements, the THC FE values accord well with the auditory nerve fiber CFs at least over the apical half of the papilla. For example, at d = 0.5, the interpolated CFs are 800–900 Hz (39°C; Jones and Jones 1995; Manley et al. 1987) and 1,200 Hz (41°C; Chen et al. 1994). These may be compared to the FE value of 600 Hz (33°C; Fig. 2C), which, when corrected for the temperature difference, gives values of 912 Hz at 39°C and 1,050 at 41°C, in good agreement with the CFs at these temperatures. Owing to the greater vulnerability of the basal hair cells, it was not possible to assay systematically their properties or frequency tuning.
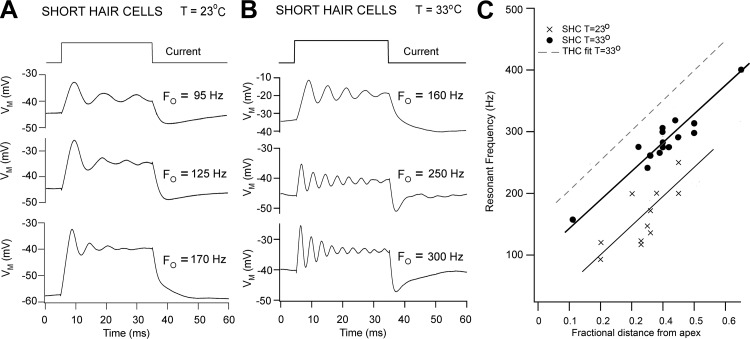
The SHCs also displayed electrical tuning, and as in the THCs it was more prominent and sharply tuned at 33°C (Fig. 3, A and B), with Q3dB values comparable to those of the THCs. However, in contrast to the THCs, the ringing in SHCs was rarely symmetrical at the onset and termination of the current step and the membrane potential for maximal tuning was more depolarized in the range −40 to −20 mV. Furthermore, although the FE was tonotopically mapped, the values were less than those of the THCs at any given location (Fig. 3C). Some of these differences may be artifactual and attributable to the greater fragility of these cells. Nevertheless, they clearly demonstrate that SHCs are electrically tuned.
Fig. 3.
Electrical resonance in SHCs. A: voltage responses of SHCs at 3 locations to extrinsic current steps; Fo is given above each of the averaged traces; T = 23°C. B: responses of 3 other SHCs at different locations to extrinsic current steps; Fo is given above each of the averaged traces; T = 33°C. C: collected results of resonant frequency plotted against SHC location as in Fig. 2C. ×, T = 23°C; ●, T = 33°C. The dashed line is the fit to the THCs at T = 33°C in Fig. 2C. Note that the SHC resonant frequencies are slightly less than those of the THCs. In A and B, each voltage trace is the average of 10 presentations. Measurements were made on E18–E19 birds.
Ionic Currents in THCs and SHCs
Depolarizing voltage steps in THCs (d = 0.3–0.6) evoked a large, fast voltage-dependent outward K+ current, which was blocked by 1 mM TEA and was absent if K+ was replaced by Cs+ in the intracellular solution (not shown). These features are consistent with it being a BK Ca2+-activated K+ current that has been extensively documented in isolated THCs (Duncan and Fuchs 2003; Fuchs and Evans 1990; Pantelias et al. 2001). In the most apical THCs (d < 0.2), a sizable inward-rectifying K+ channel was also evident and the outward K+ current had slower onset kinetics and was blocked by 1 mM 4-AP as reported previously (Fuchs and Evans 1990; Pantelias et al. 2001). In some of the more basal THCs a residual slow outward current also blocked by 4-AP was sometimes present, and it can be speculated that this is a developmental remnant, as it diminishes the sharpness of the electrical tuning endowed by the fast BK channel.
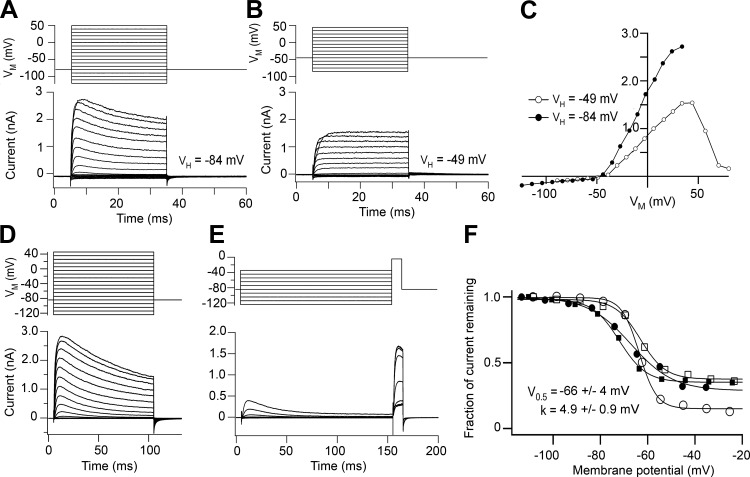
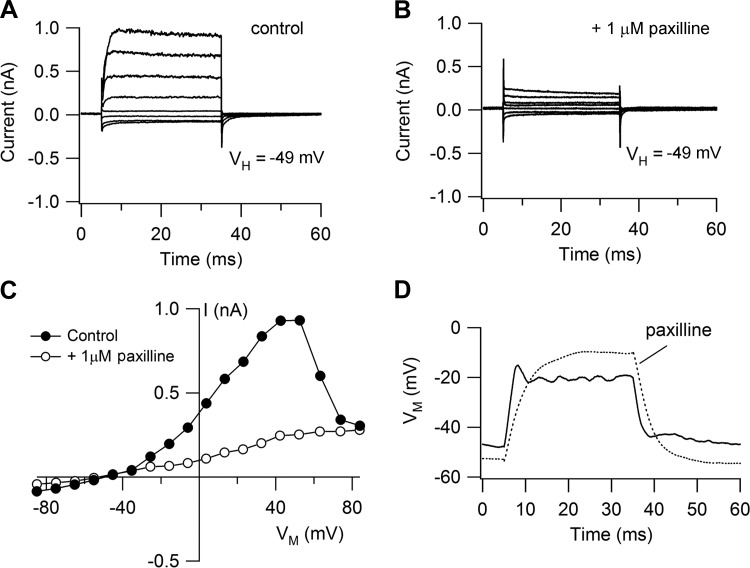
Depolarizing voltage steps in SHCs elicited a fast voltage-dependent K+ current that was abolished with Cs+-based intracellular solution. It could be dissected into two components of roughly equal amplitude by changing the holding potential. From −84 mV the current was partially inactivating, but from −49 mV the inactivating component disappeared and the current was sustained (Fig. 4, A–C). The inactivating component was characterized with a conditioning and test pulse protocol (Fig. 4, D–F), from which its half-inactivation was deduced as −66 ± 4 mV with a slope factor of 4.9 ± 0.9 mV (N = 4), similar to a previous report (Murrow 1994). It was also blocked by 5 mM 4-AP (not shown) and had an inactivating time constant of ∼10 ms (range 7–50 ms). These properties are consistent with an A current, thought to flow through Kv4.2 channels (Sokolowski et al. 2004). The other component, a sustained current, was identified as a BK Ca2+-activated K+ current on the basis of its distinctive nonlinear current-voltage relationship and its being blocked (Fig. 5, A–C) by 1 μM paxilline (Sanchez and McManus 1996). The outward current increased with depolarization, peaked, and then decreased, presumably reflecting the reduction in Ca2+ influx with the largest voltage steps (Fuchs et al. 1990). This dose of paxilline reduced the peak outward current at a holding potential of −49 mV by 78 ± 8% (N = 4) and also abolished any ringing in current clamp (Fig. 5D). The peak sustained outward current was 1.6 ± 0.7 nA in 31 SHCs compared with 3.3 ± 0.7 nA in 11 THCs for papillar locations of d = 0.3–0.6. The differences in current size may reflect different basolateral membrane areas between the two cell types, which is quantified in a subsequent section. We suggest that the sustained component is a BK current that will support electrical tuning provided the membrane potential is positive to where the A current is inactivated. As shown below (see Fig. 10C), the transition from THCs to SHCs is not abrupt but occurs gradually at the edge of the basilar membrane via cells of intermediate dimensions and properties (Gleich and Manley 2000). These intermediate cells also lacked the A current. We did not examine the voltage-dependent Ca2+ current that has been extensively studied in the THCs (Fuchs et al. 1990; Martinez-Dunst et al. 1997; Spassova et al. 2001), but we did note that the BK current in SHCs was absent with low-Ca2+ endolymph perfusion of the entire bath (as opposed to just local perfusion of the hair bundles).
Fig. 4.
Voltage-dependent currents in SHCs. A: outward currents for depolarizing voltage steps from a holding potential (VH) of −84 mV, showing an inactivating component. B: when the same cell is held at −49 mV, the inactivating component in the outward current is lost. C: current-voltage relationships for the peak current in A (●) and B (○). D: outward currents in another SHC for depolarizing voltage steps from a holding potential of −84 mV. E: characterization of inactivating component using a long conditioning step of variable amplitude and a short step of fixed amplitude. F: inactivation relationships from records in E (○) and for 3 other SHCs. y-Axis, current remaining normalized to the maximum current at negative potentials. Each set of points was fitted with a Boltzmann relationship, with mean half-inactivating potential (V0.5) and slope k as shown. Cells were from E19 animals.
Fig. 5.
Paxilline blocks the sustained current in SHCs. A: outward currents from holding potential of −49 mV are sustained. B: currents are attenuated by 1 μM paxilline, which at this concentration specifically blocks BK Ca2+-activated K+ channels. C: current-voltage relationships from records for control (A) and with paxilline (B). D: current-clamp responses with and without 1 μM paxilline, which abolishes ringing and increases the input resistance.
Fig. 10.
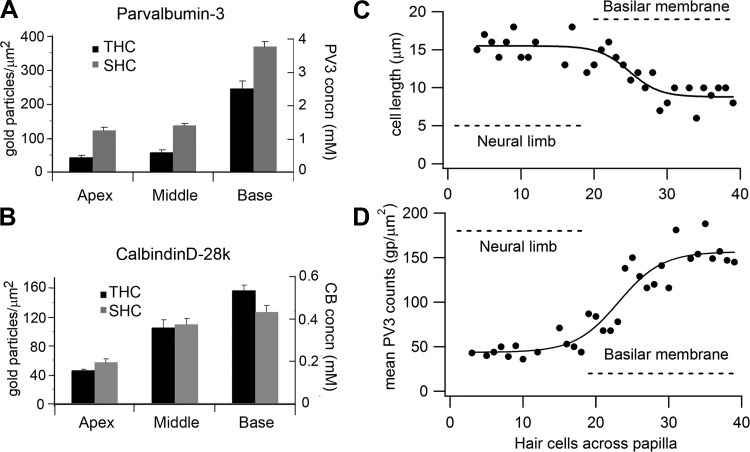
Quantification of hair bundle immunogold labeling for Ca2+-binding proteins. A: mean gold particle counts and protein concentration for parvalbumin-3 (PV3) in THCs and SHCs at apex, middle, and base. B: mean gold particle counts and protein concentrations for calbindin D-28k (CB) in THCs and SHCs at apex, middle, and base. In A and B, each bar is the mean ± SE of 10 micrographs and the papilla locations were as in Fig. 8. C: transverse gradient in length of hair cell body: cell length (L) is plotted vs. the cell number from the edge of the neural limb (R), and the points are fitted with the sigmoidal equation L = L1 + L2/{1 + exp[−(R − R0.5)/RS]}, where L1 = 15.5 μm, L1 + L2 = 8.8 μm, R0.5 = 25 hair cells across, and slope RS = 2.1. D: transverse gradient in hair bundle labeling for parvalbumin-3: the mean gold particle count (average of 5 sections) is plotted vs. the position of the cell across the papilla from the neural edge. Note that some positions have no counts because the hair bundle was not measurable in the section. Parvalbumin-3 counts (P) vs. hair cell number (R) fitted with a sigmoidal equation: P = P1 + P2/{1 + exp[−(R − R0.5)/RS]}, where P1 = 44 counts, P1 + P2 = 157 counts, R0.5 = 23 hair cells across, and RS = 3.0. In both C and D, the extent of the fibrocartilaginous plate and the basilar membrane are denoted by the dashed lines. Note that the changes are gradual over several hair cells and begin at the edge of the basilar membrane. Both C and D were from a papilla location from the apex, d = 0.35.
Mechanotransducer Currents in SHCs
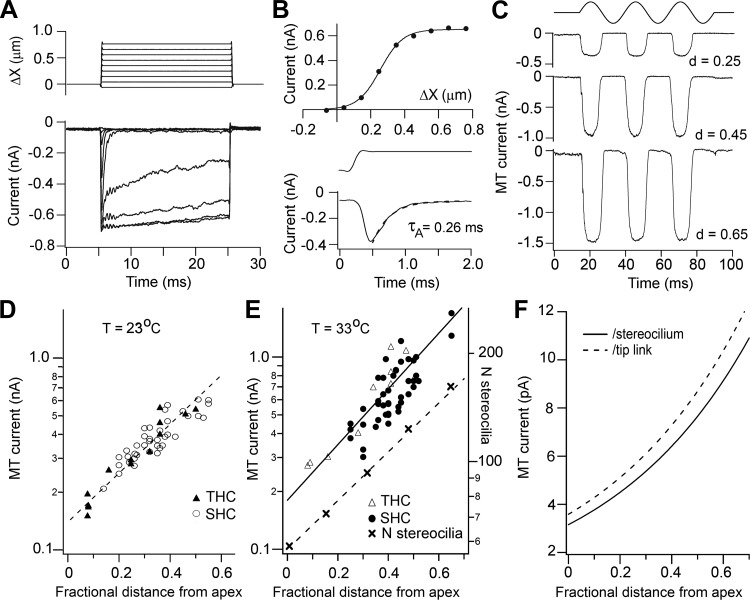
MT currents recorded from both THCs and SHCs were characterized with both sinusoidal fluid jet stimuli and step deflections of the hair bundle implemented by a glass probe driven by a piezoactuator (see materials and methods). With the latter type of stimulus, MT currents displayed properties similar to those delineated in other species (Beurg et al. 2006; Kennedy et al. 2003; Ricci et al. 1998), including fast adaptation, saturation for stimuli exceeding a few tenths of a micrometer (Fig. 6, A and B), and block by external Ca2+. For example, for eight SHCs located at d = 0.39–0.41, with peak transducer currents of 0.4–0.66 nA (mean = 0.49 ± 0.09 nA), the fast adaptation time constant was 0.23 ± 0.09 ms. For most experiments, however, deflections of the hair bundle were achieved with a fluid jet stimulator (Kros et al. 1992), useful for assaying current size without inflicting damage on the bundles. Under these conditions submicrometer bundle displacements, calibrated by imaging the hair bundle on a pair of photodiodes, produced a saturating MT current the peak-to-peak amplitude of which increased exponentially along the papilla between d = 0.2 and 0.65 from 0.3 to 1.6 nA (33°C; Fig. 6, C and E). Although fewer THCs were studied, the amplitudes of the MT currents overlapped with those of the SHCs. A similar exponential map was observed at room temperature (23°C; Fig. 6D), but the current amplitudes were reduced by a factor of 1.32–1.54 (the fitted line on the logarithmic plot was not quite parallel to that at 33°C), the mean Q10 therefore being 1.43.
Fig. 6.
Mechanotransducer (MT) currents in SHCs and THCs. A: SHC MT currents in response to step deflections of the hair bundle; stimulus monitor shown at top, holding potential −84 mV, T = 33°C. ΔX, displacement. B, top: peak current-displacement relationship from records in A; points fitted with a single Boltzmann with maximum current = 0.63 nA and 10–90% working range of 0.29 μm. Bottom: a low-level response can be seen on an expanded timescale to illustrate fast adaptation, which has a time constant τA of 0.26 ms. The current onset is limited by the time course of the stimulus shown above. C: MT currents in 3 SHCs in response to fluid jet deflections of the hair bundle; holding potential = −84 mV. Sinusoidal driving voltage to the fluid jet piezoelectric disk is shown at top. Cell locations are indicated beside traces, expressed as fractional distance from the apical end of the papilla (d); T = 33°C. D: collected peak amplitudes of MT currents vs. location for THCs and SHCs at T = 23°C. E: collected peak amplitudes of MT currents vs. location for THCs and SHCs at T = 33°C. Line is exponential fit to all points. E19–E21 animals. The variation in the number of stereocilia per bundle (right-hand axis) with cell location is taken from the data of Tilney and Saunders (1983). F: MT current per stereocilium was derived from the data in B by dividing the fit to the experimental MT currents by the fit to the number of stereocilia per bundle. MT current per tip link was obtained by dividing the solid line by 0.88, the approximate ratio of tip links to stereocilia in a bundle (see text).
Two factors may underlie the growth in MT current along the tonotopic axis: a change in the number of MT channels per bundle and an increase in the single-channel conductance. The number of stereocilia per bundle is known to change from ∼60 to 300 along the entire papilla (Tilney and Saunders 1983), and the number is plotted against location for the range studied here in Fig. 6E. The MT current per stereocilium can then be evaluated by dividing the current amplitude by the number of stereocilia at each location (Fig. 6E). The analysis can be refined on the assumption that channels are present only at the lower end of the tip links (Beurg et al. 2009). The chicken hair bundles consist of between 7 and 10 rows of stereocilia (Tilney and Saunders 1983), so the fraction of tip links to stereocilia therefore varies between 0.86 and 0.9. A mean of 0.88 was used to derive the current per tip link, which increases exponentially from 4 pA to 12 pA from d = 0.1 to 0.7 (Fig. 6F). If there are a fixed number of channels per tip link (usually 2), then the single-channel conductance must increase from apex to base.
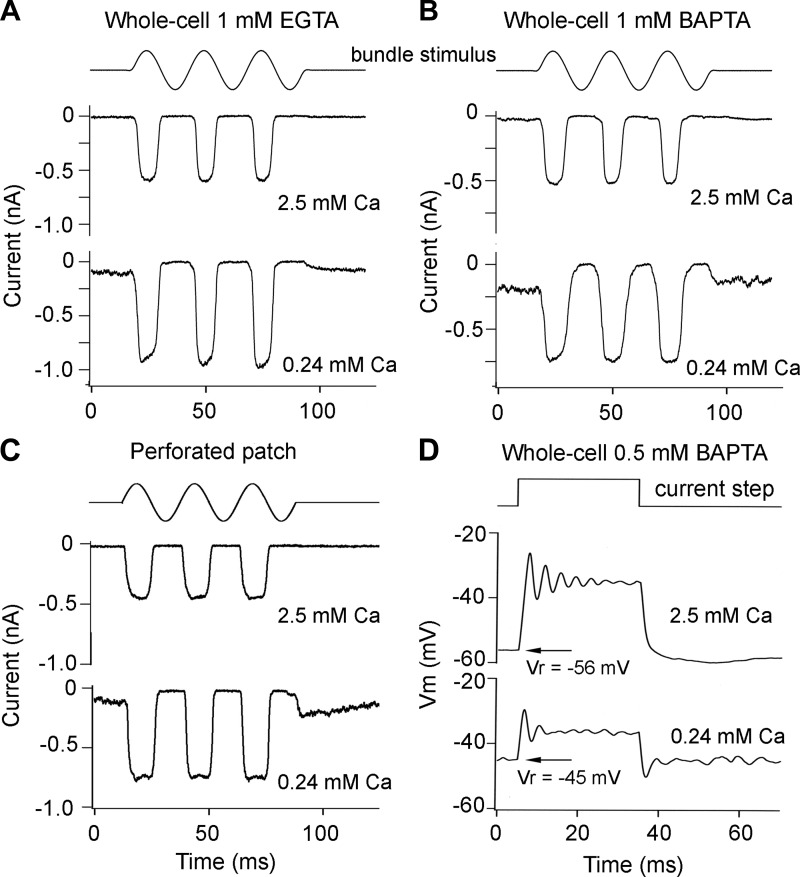
As in other hair cell preparations (Beurg et al. 2006, 2010; Ricci et al. 1998), the MT current amplitude increased ∼50% (mean increase = 1.56 ± 0.3, N = 18 SHCs) when the Ca2+ concentration around the hair bundle was reduced from that in perilymph (2.5 mM) to 0.24 mM, which is the concentration reported for endolymph (Sauer et al. 1999). This is indicative of a relief of block of the channel by external Ca2+, which is regarded as a permeant blocker. A second consequence of exposing the bundle to endolymph Ca2+ was an increase in the fraction of current activated at the resting position of the bundle (Fig. 7, A and B). The extent of the increase depended on the speed and concentration of the mobile intracellular Ca2+ buffer (Ricci et al. 1998; Roberts 1994), which determine the gradient in cytoplasmic concentration away from the channel. In whole cell recording with 1 mM EGTA as the mobile buffer (Fig. 7A) the probability of MT channel opening at rest (POR) was 0.12 ± 0.01 (N = 3), whereas with 1 mM BAPTA as the mobile buffer (Fig. 7B) POR was 0.32 ± 0.01 (N = 4). EGTA has a much slower Ca2+ binding (ON) rate constant compared with BAPTA, which is thought to account for the difference in effect on POR. With a lower BAPTA concentration of 0.5 mM, POR was 0.28 ± 0.04 (N = 21; d = 0.27–0.45). This value of POR corresponded to a standing inward current of 195 ± 87 pA in these cells.
Fig. 7.
Effects of mobile intracellular Ca2+ buffer on MT current. A: MT currents in response to fluid jet stimuli during hair bundle perfusion with perilymphatic 2.5 mM Ca2+ (top) and endolymphatic 0.24 mM Ca2+ (bottom). Intracellular solution contains 1 mM EGTA as Ca2+ buffer. B: MT currents in response to fluid jet stimuli during hair bundle perfusion with 2.5 mM Ca2+ (top) and 0.24 mM Ca2+ (bottom). Intracellular solution contains 1 mM BAPTA as Ca2+ buffer. In both cases, lowering Ca2+ increases the MT current, but with BAPTA ∼30% of channels are open at rest in low Ca2+. C: with perforated patch recordings, lowering extracellular Ca2+ increases the MT current and the fraction of channels open at rest. D: lowering extracellular Ca2+ to 0.24 mM depolarizes hair cells in current clamp and voltage ringing becomes more symmetrical; 0.5 mM BAPTA in intracellular solution. Vr, resting potential. Time course of driving voltage to fluid jet piezo is shown at top in A–C. Measurements were made on E19 SHCs; T = 33°C.
When recordings were performed with perforated-patch electrodes (Fig. 7C), in which the mobile proteinaceous buffer cannot pass through the nystatin-formed channels in the membrane patch and is therefore retained in the cytoplasm, POR was 0.26 ± 0.07 (N = 4; d = 0.35–0.45) in SHCs and 0.28 ± 0.07 (N = 6; d = 0.28–0.47) in THCs. Thus, within the error of the measurements, the mobile buffer is approximately equivalent to 0.5 mM BAPTA, and there is no significant difference between SHCs and THCs. This concentration was used for most of the recordings to characterize hair cell properties. A partial opening of the MT channels at rest generates a standing inward transduction current that acts to depolarize the hair cells (Fig. 7D). For SHCs recorded with a pipette solution containing 0.5 mM BAPTA as the Ca2+ buffer, the resting potential was −57 ± 3 mV (N = 11) with bundles exposed to 2.5 mM Ca2+ perilymph and −39 ± 2 mV (N = 12) with bundles exposed to 0.24 mM Ca2+ endolymph. With perforated-patch recordings, the resting potential was −38 mV in two SHCs. We suggest that the SHC resting potential in vivo may be close to −40 mV.
Concentrations of Ca2+-Binding Proteins
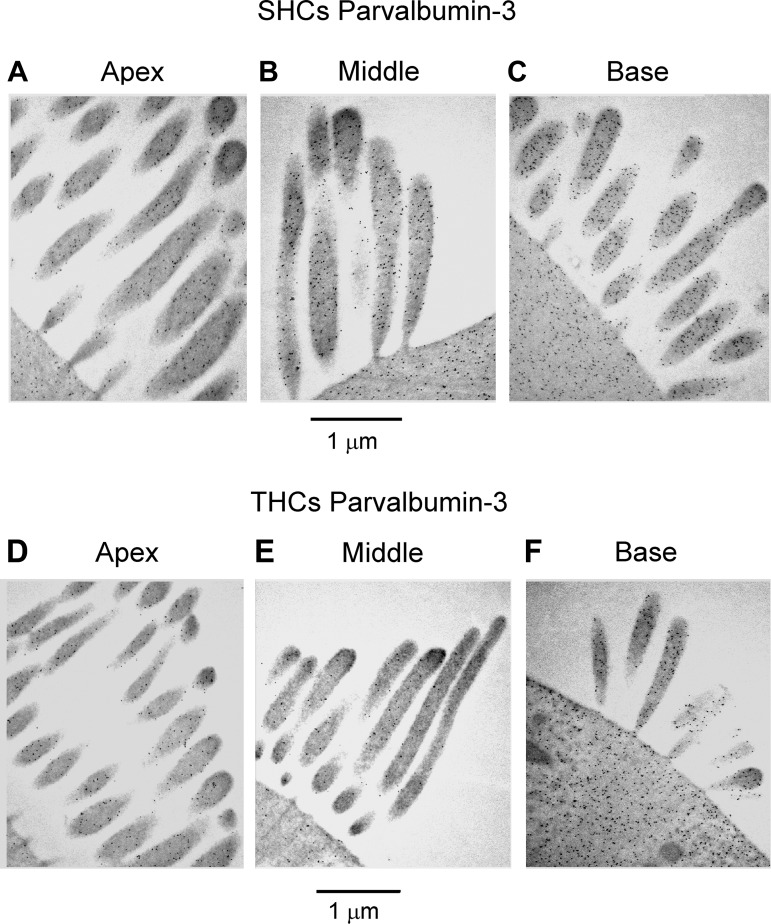
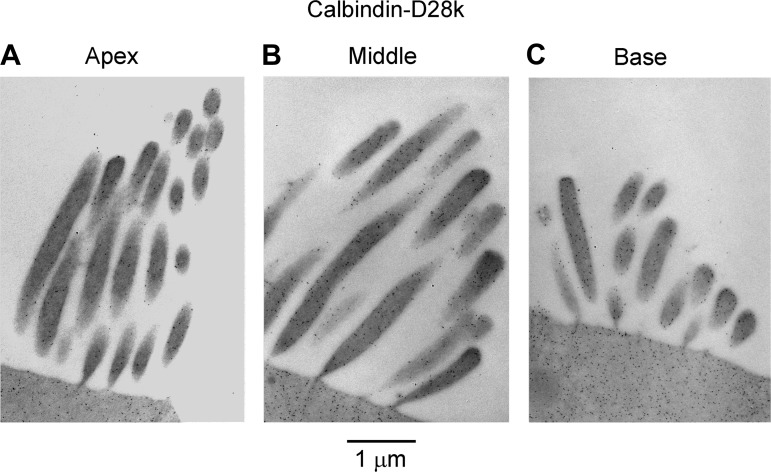
Diffusible Ca2+ buffers in the cytoplasm are usually identified with EF-hand proteins such as calbindin D-28K and parvalbumin, both of which occur at high concentrations in the chicken basilar papilla (Heller et al. 2002; Hiel et al. 2002; Oberholtzer et al. 1988) as well as in turtle auditory hair cells (Hackney et al. 2003) and cochlear outer hair cells (Hackney et al. 2005). Parvalbumin is present as the parvalbumin-3 isoform (Heller et al. 2002), which is equivalent to parvalbumin-β or oncomodulin in mammalian outer hair cells (Sakaguchi et al. 1998). Here we used postembedding immunogold labeling to examine the tonotopic distribution and to quantify these Ca2+-binding proteins in the hair cells. We focused on the stereociliary bundles because the buffer estimates from the recordings used the POR of the MT channel, which is located in the bundle. Immunogold labeling for both proteins was evident in the stereocilia and was more pronounced at the base than at the apex (Figs. 8 and 9). The concentrations of the proteins were determined as previously described (Hackney et al. 2003, 2005) by calibrating the immunogold counts against gels containing the relevant proteins that were labeled in parallel with tissue sections. The gold counts and the concentrations increased two- to sixfold from the apex (d = 0.25) to the base (d = 0.70). For parvalbumin-3 the SHCs had a higher concentration than THCs (Fig. 8, Fig. 10A), the difference being statistically significant (Wilcoxon signed-rank test; P < 0.05 for all 3 locations), but for calbindin D-28k there was no significant difference between the hair cell types at apical and middle locations (Wilcoxon signed-rank test; P > 0.05) but a significant difference was observed in the basal location (Wilcoxon signed-rank test; P = 0.04) (Fig. 10B). At the middle papilla location (d = 0.35), the concentrations inferred for parvalbumin-3 and calbindin D-28k were 0.59 ± 0.09 mM and 0.37 ± 0.04 mM (means ± SE), respectively, for the THCs and 1.39 ± 0.08 mM and 0.39 ± 0.04 mM, respectively, for the SHCs. Parvalbumin-3 has two Ca2+-binding sites, whereas calbindin D-28k has four Ca2+-binding sites (Celio et al. 1996), and therefore the total concentration of Ca2+-binding sites contributed by these proteins is 2.7 ± 0.34 mM in THCs and 4.3 ± 0.32 mM in SHCs at the middle location, which is close to the site where the equivalent buffer concentrations were obtained from perforated-patch recordings. At the other locations the total concentrations of Ca2+-binding sites were as follows: apex, 1.5 ± 0.3 mM in THCs and 3.3 ± 0.6 mM in SHCs; base, 7.2 ± 1.2 mM in THCs and 9.3 ± 1.2 mM in SHCs.
Fig. 8.
Immunogold labeling of hair bundles for parvalbumin-3. A–C: labeling of hair bundles from apical (A), middle (B), and basal (C) SHCs with parvalbumin-3 antibody. D–F: labeling of hair bundles from apical (D), middle (E), and basal (F) THCs with parvalbumin-3 antibody. Note for both cell types that there is an increase in label from apex to base but the SHCs are more heavily labeled at all locations than the THCs. Papilla locations: apex, d = 0.25; middle, d = 0.35; base, d = 0.70. P1 bird.
Fig. 9.
Immunogold labeling of SHC hair bundles for calbindin D-28k: labeling of hair bundles for apical (A), middle (B), and basal (C) SHCs with calbindin D-28k antibody. Labeling intensity increases from apex (d = 0.25) to middle (d = 0.35) to base (d = 0.70). P1 chicken.
We exploited the difference in parvalbumin-3 concentrations between THCs and SHCs to examine the transition between these two cell types. For this analysis, counts on five serial sections were averaged and demonstrated that the changeover was gradual and not abrupt (Fig. 10D). The increase in concentration began for hair cells on the basilar membrane and took about five cells to become maximal. The transverse gradient in parvalbumin-3 concentration correlates well with the change in hair cell length across the papilla from the most neural edge (Fig. 10C). Our observations accord with the notion that there is a continuum in hair cell properties across the papilla between THCs and SHCs (Fischer 1992; Hirokawa 1978) and an intermediate hair cell type exists between the extremes (Gleich and Manley 2000; Tilney and Saunders 1983).
Plasma Membrane Ca2+ pump
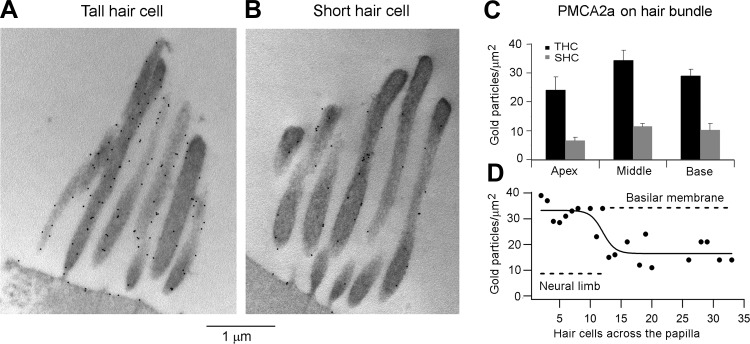
Owing to the high Ca2+ permeability of the MT channel and the role of the divalent cation in MT channel performance, tight control of stereociliary Ca2+ concentration is exerted by the presence of both large amounts of Ca2+-binding proteins and a plasma membrane Ca2+ pump in the stereocilia. In several species including mammals the PMCA2a is preferentially targeted to the stereocilia, where it is the sole isoform (Dumont et al. 2001; Hill et al. 2006). Furthermore, the PMCA2 membrane density is severalfold higher in mammalian outer hair cells than inner hair cells (Chen et al. 2012). The stereocilia and the apical membrane of both THCs and SHCs were labeled with the PMCA2 antibody and also with the antibody against the PMCA2a splice variant (Dumont et al. 2001); labeling for the latter is shown in Fig. 11, A and B. Gold particle counts revealed that the density was up to threefold higher in THCs than in SHCs (Fig. 11C), the difference being significant at all three locations (Wilcoxon signed-rank test, P < 0.05). However, for neither hair cell type was there any significant change along the papilla (Kruskal-Wallis test, P > 0.05). Thus no gradient along the tonotopic axis was found, which is similar to that reported for mammalian outer hair cells (Chen et al. 2012), but there was a significant gradient from neural to abneural edge. We exploited this gradient to also examine the transition from THCs to SHCs as for parvalbumin-3. Despite the scatter in points, there was again a change in the region of the intermediate hair cells at the neural edge of the basilar membrane (Fig. 11D).
Fig. 11.
Immunogold labeling for the plasma membrane Ca2+ pump in the hair bundle. A: labeling of THC hair bundle at the middle location with PMCA2 antibody. B: labeling of SHC hair bundle at the middle location with PMCA2 antibody. Note in A and B that the apical plasma membrane is also labeled. C: mean gold particle density for labeling for PMCA2a (F2A antibody) for apical, middle, and basal THCs and SHCs; each bar is the mean ± SE of 10 micrographs. D: transverse gradient in labeling for PMCA2 using the NR2 antibody: gold particle density (PC) plotted vs. the number of hair cells (R) across the papilla from the neural edge. Smooth curve is fit: PC = PC1 + PC2/{1 + exp[−(R − R0.5)/RS]}, where PC1 = 33 counts, PC1 + PC2 = 17 counts, R0.5 = 12 hair cells across, and slope RS = 1.0.
The higher PMCA2 labeling of the THCs is surprising because it is contrary to that for the buffering proteins and is also opposite to that in the mammalian cochlea, where the stereociliary bundles of outer hair cells, equivalent to the SHCs, are more densely labeled than those of inner hair cells (Chen et al. 2012). One possible explanation for this discrepancy is that the total number of pumps per cell is the same but there is a difference in the area of the hair cell endolymphatic surface, the hair bundle, and apical membrane, which compensates for the dissimilarity in pump density. To address this issue, we calculated the apical and basolateral membrane areas of the two types of hair cell for the middle region of the papilla. The length and diameter of the cells are 9 μm and 7.0 μm, respectively, for the SHCs and 15 μm and 7.7 μm, respectively, for the THCs. The diameters were measured in the intact papilla at the level of the nucleus (SHCs: 7.0 ± 0.3 μm, n = 11; THCs: 7.7 ± 0.3 μm, n = 18; both at d = 0.4), and the lengths were from Fig. 10C (which come from fixed material and therefore might be subject to some shrinkage). Remarkably, both cell types had membrane capacitances (SHCs: 5.6 ± 0.6 pF, n = 41; THCs: 5.7 ± 0.9, n = 8; d = 0.39 −0.41) that were not significantly different (t-test, P > 0.05), which, assuming a specific capacitance of 1 μF/cm2, correspond to a total membrane area of 565 μm2. The cellular dimensions were used to calculate the area of the basolateral membrane as 236 μm2 for the SHCs and 410 μm2 for the THCs. This difference may account for the approximately twofold difference in the BK current between the two cell types maintaining a constant channel density (see above). However, it can also be used to infer the area of the apical membrane plus hair bundle, giving values of 364 μm2 for the SHCs and 147 μm2 for the THCs, the ratio being 2.1. With a 10% shrinkage in the cell length for the fixed material, the ratio increases to 2.6. This calculation implies that the apical membrane area is at least twofold larger for SHCs compared with THCs. It is consistent with the larger apical aspect of the SHCs and also with reported differences in the hair bundle structure (Tilney et al. 1988), THC bundles having a steeper staircase (the increase in height of sequential rows of stereocilia across the bundle) than SHCs. Despite the structural difference, the maximum hair bundle heights of the two cell types were identical (THCs: 5.7 ± 0.3 μm, n = 8; SHCs: 5.8 ± 0.3 μm, n = 5; both at d = 0.4). We conclude that the larger pump density in THCs is offset by a smaller membrane area so the total number of pumps is similar in the two cell types, to handle comparable MT currents and hence similar Ca2+ influxes. It should be emphasized that these calculations are confined to a single papillary location and age range where all the pertinent information is available.
DISCUSSION
We have developed a preparation of the chicken basilar papilla allowing us to record from both THCs and SHCs and examine their properties in situ. The main findings are that 1) THCs display a high Q electrical resonance with symmetrical ringing when recorded at 33°C, yielding a frequency map over the apical two-thirds of the papilla; 2) SHCs possess electrical tuning underpinned by a BK Ca2+-activated K+ conductance; 3) robust MT currents with peak amplitude also mapped along the papilla in parallel with the concentrations of the diffusible Ca2+-binding proteins parvalbumin-3 and calbindin D-28k; and 4) there is also a gradient in properties across the papilla, but the distinction between the THCs over the neural limb and the abneurally placed SHCs is less than the sharp dichotomy between the inner and outer hair cells of the mammalian cochlea. A complication in considering the gradients in hair cell properties is that the contours of bundle morphology and orientation, which might be interpreted as isofrequency contours, are not strictly transverse but oblique (Tilney et al. 1987). Taken at face value, this implies that at a given longitudinal position, d, the most abneural SHCs have properties, including best frequency, equivalent to more basally located THCs on the neural edge. The significance of this feature is not well understood, and our results may be insufficiently precise to confirm or reject the idea.
Electrical Tuning in THCs
THCs synapse onto the auditory nerve fibers (Fischer 1992), and their tonotopic map (Fig. 2C) shows good agreement with the map of the CFs of the nerve fiber acoustic tuning curves (Chen et al. 1994; Jones and Jones 1995; Manley et al. 1987) when corrected to the avian body temperature of ∼40°C. We were not able to make measurements at that temperature because the viability and longevity of the isolated preparation decreased markedly above 33°C. The electrical resonance in THCs was sharply tuned, with Q3dB values of 7–13 in the midfrequency range. The sharpness of tuning of avian auditory nerve fibers, expressed in terms of the mean Q10dB values of their tuning curves, is 1.5 to 5 in the frequency range up to 0.8 kHz (chicken, Saunders et al. 1996; pigeon, Smolders et al. 1995). The Q3dB value for a second-order resonance is approximately three times the Q10dB, so the nerve fibers would have Q3dB values of 4.5 to 15, very similar to those measured for the THC resonance. The conclusion is that the resonance is sufficient to account for all of the nerve fiber frequency selectivity at least up to 0.8 kHz. Moreover the spontaneous oscillations the THC membrane potential at FE have a counterpart in the periodicity in spontaneous firing of the nerve fibers (Temchin 1988). In contrast to a previous report (Pantelias et al. 2001), there was no discontinuity in the tonotopic map at low frequencies and action potentials were not seen in those low-frequency hair cells. A possible explanation is that action potentials only occur if the resting potential is hyperpolarized to near the K+ equilibrium potential and the input resistance is large (Wu et al. 1995). In our recordings, neither condition is met: the membrane potential was depolarized by the standing current through MT channels open at rest, which also lowered the input resistance. It seems likely that hair cells in the previous report (Pantelias et al. 2001) had little or no MT current.
A limitation of our measurements was the inability to investigate the basal third of the papilla where the high-frequency hair cells, tuned between 1 kHz and 4 kHz, are located. As a consequence, we were not able to address two important questions about the chicken papilla. First, does the electrical resonance still operate in these high-frequency cells? We have previously modeled resonance at high frequencies above 1 kHz, but it requires extreme properties for the BK channels (Wu et al. 1995). Second, is there a developmental change in the tonotopic map that would be most obvious at these high frequencies (Lippe and Rubel 1983; Rübsamen and Lippe 1998)? While nerve fiber recordings have not confirmed the developmental change in the tonotopic map (Jones et al. 2006; Manley et al. 1987), nevertheless the question might be reexamined by cataloging the acquisition of voltage-dependent currents and electrical tuning in the basal third of the chicken papilla during late embryogenesis.
Electrical Tuning in SHCs
Electrical tuning in SHCs is a new observation and validates our preparation because maintaining these cells in a healthy condition is difficult. It is notable that the FE values were smaller than those of the THCs (Fig. 3C), which might reflect deterioration. Isolated SHCs that have been studied previously may not have been in sufficiently good condition to reveal the resonance; they did, however, possess a BK current in addition to the inactivating K+ (A type) current (Murrow 1994). The functional significance of the latter K+ current is unclear, but it would certainly diminish or “short out” the resonance just as the efferent conductance does in turtle hair cells (Art et al. 1985). The attenuation of the resonance by the A current can be circumvented under normal circumstances by the SHCs sitting at a resting potential of −40 mV, where the current is fully inactivated. Furthermore, such a resting potential ensures that the resonance (which is voltage dependent; Crawford and Fettiplace 1981) is most sharply tuned. The same argument was made earlier (Farris et al. 2006) for turtle hair cells, for which the resting potential in vivo is predicted to be about −45 mV to maximize the resonance. As with the turtle (Farris et al. 2006; Ricci et al. 1998), the positive resting potential stems from a standing depolarizing current flowing through MT channels that are partially open at rest when they are exposed to low-Ca2+ endolymph. This behavior is a consequence of the Ca2+ dependence of MT channel adaptation: with a low external Ca2+ concentration and a high intracellular Ca2+ buffer, the channel activation relationship is shifted toward negative bundle positions, increasing the fraction of channels open at the bundle's resting position. Interestingly, both the POR (0.26) and internal Ca2+ buffer equivalent (0.5 mM BAPTA) are similar in turtle and chicken SHCs, as is the complement of Ca2+-binding proteins parvalbumin-3 and calbindin D-28k. For hair cells in both animals, the high Ca2+ buffer also ensures that Ca2+ signals at the two ends of the hair cell, attributable to the MT channel at the apical pole and the efferent ACh receptor and the voltage-dependent Ca2+ current at the basal pole, are independent. None of our work establishes a role for the A current in SHCs, but its negative voltage activation range suggests that it shapes the efferent synaptic response (Murrow and Fuchs 1990).
Ca2+ Buffer Concentrations
Calibration of immunogold counts was used to estimate the cytoplasmic concentrations of calbindin D-28k and parvalbumin-3 in the hair bundles, from which the total concentration of Ca2+-binding sites contributed by these proteins in middle-region SHCs was deduced as ∼4.3 mM. In comparison, the mobile buffer at the same location inferred from perforated-patch measurements was equivalent to 0.5 mM BAPTA, which binds only a single Ca2+ per molecule, in contrast to the proteins, which bind two (parvalbumin-3) or four (calbindin D-28k) divalent ions. The measurements from the perforated-patch experiments and immunolabeling can be compared by assuming that the two major factors dictating the effectiveness of a mobile buffer are the Ca2+-binding rate, kON, and the buffer concentration (Naraghi and Neher 1997). Other properties, such as the Ca2+ dissociation constant and diffusion coefficient, are probably similar for BAPTA and the proteins. kON is not known for parvalbumin-3, but for calbindin D-28k the reported values are 1–8 × 107 M−1·s−1 (Nägerl et al. 2000). The two extremes are for each of the two sets of binding sites, and averaging these gives a kON for calbindin D-28k of 4.5 × 107 M−1·s−1, which is approximately one-tenth of that for BAPTA (4 × 108 M−1·s−1). If we assume similar kON values for calbindin D-28k and parvalbumin-3, the Ca2+-buffering capacity of 0.5 mM BAPTA is equivalent to 5 mM Ca2+-binding sites, not too different from that calculated from immunolabeling (4.3 mM Ca2+-binding sites in the middle region; see above). These comparisons suggest that calbindin D-28k and parvalbumin-3 may together account for much of the cytoplasmic Ca2+ buffering in SHCs.
Mechanotransducer Currents
Our characterization of the MT currents in chicken hair cells is more extensive than previously undertaken, apart from Ohmori (1985), who measured the ionic permeability and block of MT channels in chicken vestibular hair cells. The properties of the MT current resembled those described in other preparations, including submicrometer activation, fast adaptation, and external Ca2+ block. The amplitude of the current, similar in THCs and SHCs, varied exponentially along the papilla because of gradients in both the number of stereocilia per bundle (Tilney and Saunders 1983) and the single-channel conductance (Fig. 6, E and F). The current per tip link varied from 4 to 12 pA, and in the midpapillar region where most of the recordings were made it was ∼8 pA at −84 mV holding potential. Assuming two channels per tip link (Beurg et al. 2006, 2009), the unitary conductance is 50 pS in 2.5 mM Ca2+. This is smaller than in turtle or mammalian auditory hair cells but is close to the size of the single-channel events described by Ohmori (1985). What might be the functional significance of the tonotopic gradient in MT current size? One purpose might be to match a likely gradient in the BK conductance, needed to increase the FE (Art and Fettiplace 1987; Wu et al. 1995). Thus, if the MT channel POR remains constant along the papilla, the standing inward current will increase to keep the resting potential constant as in mammalian outer hair cells (Johnson et al. 2011). Another more intriguing role for the MT current gradient may be to allow greater Ca2+ influx per channel and hence increase the time constant of fast adaptation (Ricci and Fettiplace 1997). If adaptation of the MT channel generates force to move the bundles (Ricci et al. 2000) such an electro-mechanical feedback may contribute to amplification, but its kinetics would need to mesh with a cell's FE in order to optimize the feedback. The chicken auditory papilla, because of its wide frequency range, is an ideal preparation to investigate the interaction between these two tuning processes.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant RO1-DC-01362 to R. Fettiplace. C. Hackney was supported by a grant from Deafness Research UK.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.T., M.B., C.H., S.M., and R.F. performed experiments; X.T., M.B., C.H., S.M., and R.F. analyzed data; M.B. and R.F. conception and design of research; M.B., C.H., S.M., and R.F. interpreted results of experiments; M.B., C.H., S.M., and R.F. prepared figures; M.B., C.H., S.M., and R.F. edited and revised manuscript; M.B., C.H., S.M., and R.F. approved final version of manuscript; R.F. drafted manuscript.
ACKNOWLEDGMENTS
We thank David Furness (Keele University) for help with the immunogold images.
REFERENCES
- Art JJ, Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol 385: 207–242, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Wu YC, Fettiplace R. The calcium-activated potassium channels of turtle hair cells. J Gen Physiol 105: 49–72, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Crawford AC, Fettiplace R, Fuchs PA. Efferent modulation of hair cell tuning in the cochlea of the turtle. J Physiol 360: 397–421, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Evans MG, Hackney CM, Fettiplace R. A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26: 10992–11000, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R, Nam JH, Ricci AJ. Localization of inner hair cell mechano-transducer channels using high-speed calcium imaging. Nat Neurosci 12: 553–558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Nam JH, Chen Q, Fettiplace R. Calcium balance and mechanotransduction in rat cochlear hair cells. J Neurophysiol 104: 18–34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR, Pauls T, Schwaller B. Guidebook to Calcium-Binding Proteins. New York: Oxford Univ. Press, 1996 [Google Scholar]
- Chen L, Salvi R, Shero M. Cochlear frequency-place map in adult chickens: intracellular biocytin labeling. Hear Res 81: 130–136, 1994 [DOI] [PubMed] [Google Scholar]
- Chen Q, Mahendrasingam S, Tickle JA, Furness DN, Hackney CM, Fettiplace R. The development, distribution and density of the PMCA2 calcium pump in rat cochlear hair cells. Eur J Neurosci 36: 2302–2310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche DA, Dopyera CE. Hair cell and supporting cell response to acoustic trauma in the chick cochlea. Hear Res 46: 29–40, 1990 [DOI] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol 312: 377–412, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol 364: 359–379, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci 21: 5066–5078, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RK, Fuchs PA. Variation in large-conductance, calcium-activated potassium channels from hair cells along the chicken basilar papilla. J Physiol 547: 357–371, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris HE, Wells GB, Ricci AJ. Steady-state adaptation of mechanotransduction modulates the resting potential of auditory hair cells, providing an assay for endolymph Ca2+. J Neurosci 26: 12526–12536, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer FP. Quantitative analysis of the innervation of the chicken basilar papilla. Hear Res 61: 167–178, 1992 [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Evans MG. Potassium currents in hair cells isolated from the cochlea of the chick. J Physiol 429: 529–551, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. Cholinergic inhibition of short (outer) hair cells of the chick's cochlea. J Neurosci 12: 800–809, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Nagai T, Evans MG. Electrical tuning in hair cells isolated from the chick cochlea. J Neurosci 8: 2460–2467, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Evans MG, Murrow BW. Calcium currents in hair cells isolated from the cochlea of the chick. J Physiol 429: 553–568, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich O, Manley GA. The hearing organ of birds and crocodilia. In: Springer Handbook of Auditory Research: Comparative Hearing: Birds and Reptiles, edited by Dooling RJ, Popper AN, Fay RR. New York: Springer, 2000, p. 70–138 [Google Scholar]
- Hackney CM, Mahendrasingam S, Jones EM, Fettiplace R. The distribution of calcium buffering proteins in the turtle cochlea. J Neurosci 23: 4577–4589, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci 25: 7867–7875, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller S, Bell AM, Denis CS, Choe Y, Hudspeth AJ. Parvalbumin 3 is an abundant Ca2+ buffer in hair cells. J Assoc Res Otolaryngol 3: 488–498, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiel H, Navaratnam DS, Oberholtzer JC, Fuchs PA. Topological and developmental gradients of calbindin expression in the chick's inner ear. J Assoc Res Otolaryngol 3: 1–15, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JK, Williams DE, LeMasurier M, Dumont RA, Strehler EE, Gillespie PG. Splice-site A choice targets plasma-membrane Ca2+-ATPase isoform 2 to hair bundles. J Neurosci 26: 6172–6180, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. The ultrastructure of the basilar papilla of the chick. J Comp Neurol 181: 361–374, 1978 [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ, Lewis RS. A model for electrical resonance and frequency tuning in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol 400: 275–297, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Beurg M, Marcotti WM, Fettiplace R. Prestin-driven cochlear amplification is not limited by the outer hair cell membrane time constant. Neuron 70: 1143–1154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Jones TA. The tonotopic map in the embryonic chicken cochlea. Hear Res 82: 149–157, 1995 [DOI] [PubMed] [Google Scholar]
- Jones TA, Jones SM, Paggett KC. Emergence of hearing in the chicken embryo. J Neurophysiol 96: 128–141, 2006 [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Evans MG, Crawford AC, Fettiplace R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci 6: 832–836, 2003 [DOI] [PubMed] [Google Scholar]
- Köppl C. Birds—same thing, but different? Convergent evolution in the avian and mammalian auditory systems provides informative comparative models. Hear Res 273: 65–71, 2011 [DOI] [PubMed] [Google Scholar]
- Kros CJ, Rüsch A, Richardson GP. Mechano-electrical transducer current in hair cells of the cultured neonatal mouse cochlea. Proc Biol Sci 249: 185–193, 1992 [DOI] [PubMed] [Google Scholar]
- Levic S, Nie L, Tuteja D, Harvey M, Sokolowski BH, Yamoah EN. Development and regeneration of hair cells share common functional features. Proc Natl Acad Sci USA 104: 19108–19113, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe W, Rubel EW. Development of the place principle: tonotopic organization. Science 219: 514–516, 1983 [DOI] [PubMed] [Google Scholar]
- Magistretti J, Mantegazza M, Guatteo E, Wanke E. Action potentials recorded with patch-clamp amplifiers: are they genuine? Trends Neurosci 19: 530–534, 1996 [DOI] [PubMed] [Google Scholar]
- Manley GA, Köppl C. Phylogenetic development of the cochlea and its innervation. Curr Opin Neurobiol 8: 468–474, 1998 [DOI] [PubMed] [Google Scholar]
- Manley GA, Brix J, Kaiser A. Developmental stability of the tonotopic organization of the chick's basilar papilla. Science 237: 655–656, 1987 [DOI] [PubMed] [Google Scholar]
- Martinez-Dunst C, Michaels RL, Fuchs PA. Release sites and calcium channels in hair cells of the chick's cochlea. J Neurosci 17: 9133–9144, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrow BW, Fuchs PA. Preferential expression of transient potassium current (IA) by “short” hair cells of the chick's cochlea. Proc Biol Sci 242: 189–195, 1990 [DOI] [PubMed] [Google Scholar]
- Murrow BW. Position-dependent expression of potassium currents by chick cochlear hair cells. J Physiol 480: 247–259, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägerl UV, Novo D, Mody I, Vergara JL. Binding kinetics of calbindin-D(28k) determined by flash photolysis of caged Ca2+. Biophys J 79: 3009–3018, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci 17: 6961–6973, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberholtzer JC, Buettger C, Summers MC, Matschinsky FM. The 28-kDa calbindin-D is a major calcium-binding protein in the basilar papilla of the chick. Proc Natl Acad Sci USA 85: 3387–3390, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol 359: 189–217, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelias AA, Monsivais P, Rubel EW. Tonotopic map of potassium currents in chick auditory hair cells using an intact basilar papilla. Hear Res 156: 81–94, 2001 [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- Ramanathan K, Fuchs PA. Modeling hair cell tuning by expression gradients of potassium channel β subunits. Biophys J 82: 64–75, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R. The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells. J Physiol 501: 111–124, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Wu YC, Fettiplace R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci 18: 8261–8277, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Crawford AC, Fettiplace R. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J Neurosci 20: 7131–7142, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci 14: 3246–3262, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsamen R, Lippe WR. The development of cochlear function. In: Springer Handbook of Auditory Research: Development of the Auditory System, edited by Rubel EW, Popper AN, Fay RR. New York: Springer, 1998, p. 193–270 [Google Scholar]
- Sakaguchi N, Henzl MT, Thalmann I, Thalmann R, Schulte BA. Oncomodulin is expressed exclusively in outer hair cells in the organ of Corti. J Histochem Cytochem 46: 29–39, 1998 [DOI] [PubMed] [Google Scholar]
- Samaranayake H, Saunders JC, Greene MI, Navaratnam DS. Ca2+ and K+ (BK) channels in chick hair cells are clustered and colocalized with apical-basal and tonotopic gradients. J Physiol 560: 13–20, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963–968, 1996 [DOI] [PubMed] [Google Scholar]
- Sauer G, Richter CP, Klinke R. Sodium, potassium, chloride and calcium concentrations measured in pigeon perilymph and endolymph. Hear Res 129: 1–6, 1999 [DOI] [PubMed] [Google Scholar]
- Saunders JC, Coles RB, Gates GR. The development of auditory evoked responses in the cochlea and cochlear nuclei of the chick. Brain Res 63: 59–74, 1973 [DOI] [PubMed] [Google Scholar]
- Saunders JC, Doan DE, Poje CP, Fisher KA. Cochlear nerve activity after intense sound exposure in neonatal chicks. J Neurophysiol 76: 770–787, 1996 [DOI] [PubMed] [Google Scholar]
- Schermuly L, Klinke R. Change of characteristic frequencies of pigeon primary auditory nerve afferents with temperature. J Comp Physiol A 156: 209–211, 1985 [Google Scholar]
- Si F, Brodie H, Gillespie PG, Vazquez AE, Yamoah EN. Developmental assembly of transduction apparatus in chick basilar papilla. J Neurosci 23: 10815–10826, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders JW, Ding-Pfennigdorff D, Klinke R. A functional map of the pigeon basilar papilla: correlation of the properties of single auditory nerve fibres and their peripheral origin. Hear Res 92: 151–169, 1995 [DOI] [PubMed] [Google Scholar]
- Sokolowski BH, Sakai Y, Harvey MC, Duzhyy DE. Identification and localization of an arachidonic acid-sensitive potassium channel in the cochlea. J Neurosci 24: 6265–6276, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova M, Eisen MD, Saunders JC, Parsons TD. Chick cochlear hair cell exocytosis mediated by dihydropyridine-sensitive calcium channels. J Physiol 535: 689–696, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Smith CA. Structure of the chicken's inner ear: SEM and TEM study. Am J Anat 153: 251–271, 1978 [DOI] [PubMed] [Google Scholar]
- Temchin AN. Unusual discharge patterns of single fibers in the pigeon's auditory nerve. J Comp Physiol A 163: 99–115, 1988 [DOI] [PubMed] [Google Scholar]
- Tilney LG, Saunders JC. Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J Cell Biol 96: 807–821, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney MS, Tilney LG, DeRosier DJ. The distribution of hair cell bundle lengths and orientations suggests an unexpected pattern of hair cell stimulation in the chick cochlea. Hear Res 25: 141–151, 1987 [DOI] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Cotanche DA. New observations on the stereocilia of hair cells of the chick cochlea. Hear Res 37: 71–82, 1988 [DOI] [PubMed] [Google Scholar]
- Wu YC, Art JJ, Goodman MB, Fettiplace R. A kinetic description of the calcium-activated potassium channel and its application to electrical tuning of hair cells. Prog Biophys Mol Biol 63: 131–158, 1995 [DOI] [PubMed] [Google Scholar]
- Yuhas WA, Fuchs PA. Apamin-sensitive, small-conductance, calcium-activated potassium channels mediate cholinergic inhibition of chick auditory hair cells. J Comp Physiol A 185: 455–462, 1999 [DOI] [PubMed] [Google Scholar]