Abstract
Absolute visual thresholds are limited by “dark noise,” which in Drosophila photoreceptors is dominated by brief (∼10 ms), small (∼2 pA) inward current events, occurring at ∼2/s, believed to reflect spontaneous G protein activations. These dark events were increased in rate and amplitude by a point mutation in myosin III (NINAC), which disrupts its interaction with the scaffolding protein, INAD. This phenotype mimics that previously described in null mutants of ninaC (no inactivation no afterpotential; encoding myosin III) and an associated protein, retinophilin (rtp). Dark noise was similarly increased in heterozygote mutants of diacylglycerol kinase (rdgA/+). Dark noise in ninaC, rtp, and rdgA/+ mutants was greatly suppressed by mutations of the Gq α-subunit (Gαq) and the major light-sensitive channel (trp) but not rhodopsin. ninaC, rtp, and rdgA/+ mutations also all facilitated residual light responses in Gαq and PLC hypomorphs. Raising cytosolic Ca2+ in the submicromolar range increased dark noise, facilitated activation of transient receptor potential (TRP) channels by exogenous agonist, and again facilitated light responses in Gαq hypomorphs. Our results indicate that RTP, NINAC, INAD, and diacylglycerol kinase, together with a Ca2+-dependent threshold, share common roles in suppressing dark noise and regulating quantum bump generation; consequently, most spontaneous G protein activations fail to generate dark events under normal conditions. By contrast, quantum bump generation is reliable but delayed until sufficient G proteins and PLC are activated to overcome threshold, thereby ensuring generation of full-size bumps with high quantum efficiency.
Keywords: TRP channels, phototransduction, retinophilin, INAD, NINAC
phototransduction in drosophila is mediated by a PLC cascade, culminating in activation of two distinct Ca2+-permeable channels encoded by the transient receptor potential (trp) and trp-like (trpl) genes (reviews: Hardie 2012; Katz and Minke 2009; Montell 2012). The light response is characterized by high sensitivity, rapid kinetics, and wide dynamic range, in part achieved by the ultracompartmentalization inherent in the microvillar design of the photoreceptors (Fain et al. 2010; Hardie and Postma 2008; Yau and Hardie 2009). Photoisomerization of 1 rhodopsin results in generation of a quantum bump ∼10 pA in amplitude representing the opening of ∼15 TRP channels in a single microvillus of the light-absorbing rhabdomere (Henderson et al. 2000). Ca2+ influx via TRP channels is essential for both amplification and rapid kinetics (Hardie 1991; Henderson et al. 2000; Ranganathan et al. 1991). In hypomorphic G protein (guanine nucleotide-binding protein) or PLC mutants, bump amplitudes are reduced severalfold, indicating amplification also requires activation of multiple G proteins and PLC. Bump amplitudes in such mutants can be restored to wild-type levels by an additional mutation in the retinal degeneration A (rdgA) gene, which encodes diacylglycerol kinase (DGK), suggesting a role for this enzyme in regulating the supply of excitatory second messenger (Hardie et al. 2002).
To maximize sensitivity, photoreceptors must minimize noise caused by channel activity in the dark. Such “dark noise” can result from spontaneous activation of molecules at any phototransduction stage and sets a fundamental limit on absolute sensitivity (e.g., Aho et al. 1988; Rieke and Baylor 1996). In Drosophila, spontaneous isomerizations of rhodopsin are rare (<1/min), and dark noise is dominated by small (∼2 pA) bumplike events at rates of 2–3/s. These are eliminated in mutants of the Gq protein α-subunit (Gαq), suggesting they originate from spontaneous G protein activation (Elia et al. 2005; Hardie et al. 2002).
An increase in spontaneous dark noise was first reported in mutants of ninaC (neither inactivation nor afterpotential C; Hofstee et al. 1996), which encodes rhabdomeric and cytosolic isoforms of myosin III (Montell and Rubin 1988; Porter et al. 1992). Recently, a similar noise phenotype was found in rtp mutants lacking retinophilin (Mecklenburg et al. 2010), a novel rhabdomeric protein with homologies to junctophilins (Mecklenburg 2007; Takeshima et al. 2000). RTP protein, which associates physically with NINAC (Venkatachalam et al. 2010), was undetectable in ninaC-null mutants, suggesting the increased dark noise in ninaC was due to lack of RTP (Mecklenburg et al. 2010).
The present study presents a detailed investigation of the determinants of dark noise, which both confirms and significantly extends some of these earlier findings. We show that dark noise is increased by a point mutation in NINAC that disrupts its interaction with the INAD scaffolding protein and also in rdgA/+ heterozygotes with reduced DGK function. In addition, we show that dark noise is critically dependent on cytosolic Ca2+ in the submicromolar range. We confirm that dark noise in wild-type photoreceptors as well as the enhanced dark noise in various mutant backgrounds is Gq protein-dependent. We show that it is mediated primarily by TRP channels and unaffected by genetic elimination of rhodopsin. The results allow an in vivo estimate of the rate of spontaneous G protein activations and suggest that under normal conditions only a small fraction of these lead to channel activation. Importantly, we also show that the various mutations that increase dark noise (ninaC, rtp, and rdgA) all increase the amplitude of the small quantum bumps in Gαq and no receptor potential A (norpA; gene encoding PLC) hypomorphic backgrounds, suggesting that dark noise and quantum bumps are regulated by common mechanisms involving RTP, NINAC, and DGK.
MATERIALS AND METHODS
Fly strains.
Flies were reared on standard cornmeal-agar diet at 25°C in the dark. The wild-type strains included both white-eye (w1118) and red-eye Oregon with no difference being observed between them. Mutants used included:
Gαq1, a hypomorph of the Gαq expressing ∼1% of wild-type protein levels (Scott et al. 1995);
norpAP16, PLC hypomorph (Pearn et al. 1996);
rdgA1 (also known as rdgABS12), severe allele of rdgA encoding DGK (Masai et al. 1993);
rdgAKS60, near protein-null allele of rdgA used for validating RDGA antibody (Masai et al. 1993);
ninaEI17, null mutant for the rhodopsin of R1–6 (Rh1);
rtp, null mutant of RTP, generated by combining the Df(3R)rtp1 deletion chromosome with the same chromosome rescued by transgenes for two other genes affected by the deficiency as previously described (Mecklenburg et al. 2010);
trpl302 and trp343, null mutants of the TRPL and TRP channel, respectively (Niemeyer et al. 1996; Scott et al. 1997);
calx (calxA), severe-loss-of-function mutant of CalX, the Na+-Ca2+ exchanger (NCX; Wang et al. 2005);
pCalX, flies overexpressing wild-type calx gene under the ninaE promoter enhancing NCX activity in the photoreceptors approximately five- to eightfold (Wang et al. 2005);
ninaCP235, a null mutant of the NINAC myosin III, and ninaCI1501E, a transgene with a point mutation in the COOH terminus on ninaCP235-null background (Montell and Rubin 1988; Wes et al. 1999). Seven further transgenic mutants of ninaC (mutagenized transgenes on null ninaCP235 background) were tested, but none showed a dark noise phenotype. These were: ninaCKD (deletion in kinase domain); ninaC45KR (point mutation in kinase domain); ninaCΔ132 (lacking P132 cytosolic isoform); ninaCΔC1 and ninaCΔC2 (lacking calmodulin binding domains); and ninaC1015.4 (4-amino acid deletion in myosin domain) and ninaC174PS [mutations of 2 putative PKC phosphorylation sites (Li et al. 1998; Porter et al. 1995; Porter and Montell 1993)].
Double-mutant combinations were generated as required by standard genetic strategies.
Electrophysiology.
Dissociated ommatidia were prepared as previously described (Hardie et al. 2002) from newly eclosed adult flies and transferred to the bottom of a recording chamber on an inverted Nikon Diaphot microscope. Standard bath contained (in mM): 120 NaCl, 5 KCl, 10 TES, 4 MgCl2, 1.5 CaCl2, 25 proline, and 5 alanine, pH 7.15. The intracellular pipette solution was (in mM): 140 K-gluconate, 10 TES, 4 Mg-ATP, 2 MgCl2, 1 NAD, and 0.4 Na-GTP, pH 7.15. For manipulation of NCX equilibrium, the intracellular pipette was adjusted to contain either 10 or 20 mM Na-gluconate (and 130 or 120 mM K-gluconate, respectively). Extracellular NaCl was substituted with LiCl (see individual figures), and solutions were applied from a closely positioned puffer pipette. Linolenic acid (LNA) was kept as a 20 mM DMSO stock at −20°C and diluted 1:1,000 in appropriate bath solution. All chemicals were obtained from Sigma-Aldrich. Whole cell voltage clamp recordings were made at room temperature (20 ± 1°C) at −70 mV (including correction for −10-mV junction potential) using electrodes of resistance approximately 10–15 MΩ. Series resistance values were generally <30 MΩ. Series compensation of >80% was applied for macroscopic responses but not for sampling quantum bumps and dark noise (when series resistance errors are negligible). Data were collected and analyzed using Axopatch 200 or 2D amplifiers and pCLAMP8, 9, or 10 software (Molecular Devices, Union City, CA). Quantum bumps and spontaneous dark events were analyzed using the Mini Analysis Program (Jaejin Software, Leonia, NJ) with a threshold criterion of 0.5 pA. Event rates were analyzed automatically, but for event amplitudes and waveforms, all events were individually scrutinized before acceptance. Photoreceptors were stimulated via green light-emitting diode; intensities were calibrated in terms of wild-type effectively absorbed photons by counting quantum bumps at low intensities in wild-type cells.
Western immunoblotting.
Heads from flies aged 0–24 h posteclosion were prepared by decapitating flies cooled on ice. Samples were homogenized in 2× SDS-PAGE sample buffer followed by boiling at 90°C for 1 min. Samples were separated using SDS-PAGE and electroblotted onto supported nitrocellulose membrane (Hybond-P or Hybond-C Extra; GE Healthcare). The uniformity of transfer onto membranes was checked by staining with Ponceau S. Following blocking in 5% nonfat milk (Santa Cruz Biotechnology, Santa Cruz, CA), blots were incubated overnight at 4°C in appropriate dilutions of primary antibodies [rabbit anti-α-tubulin (1:1,000 dilution; ab15246; Abcam), rabbit anti-Gαq (1:3,000 dilution), rabbit anti-RTP (1:500 dilution), and rabbit anti-RDGA (1:500 dilution)]. In each case, antibody specificity was confirmed by lack of staining in respective mutants. Immunoreactive protein was visualized after incubation in appropriate dilution of secondary antibodies. The anti-rabbit IgG ECL HRP-Linked Secondary Antibodies (GE Healthcare) were used with 1:5,000 dilution for RTP and α-tubulin blot and 1:10,000 for Gαq. The RDGA blot was probed with 1:10,000 dilution of anti-rabbit IgG coupled to horseradish peroxidase (Jackson ImmunoResearch Laboratories). For Gαq, RTP, and α-tubulin, bands were visualized by the Amersham ECL Plus detection regents (GE Healthcare) and quantitated by G:BOX iChemi imaging system (Syngene). For RDGA, the blots were developed with ECL (GE Healthcare) using LAS 4000 instrument (GE Healthcare), and the immunoblots were quantified using Quantity One 1-D Analysis Software (Bio-Rad).
RESULTS
Spontaneous dark noise in rdgA/+ heterozygotes.
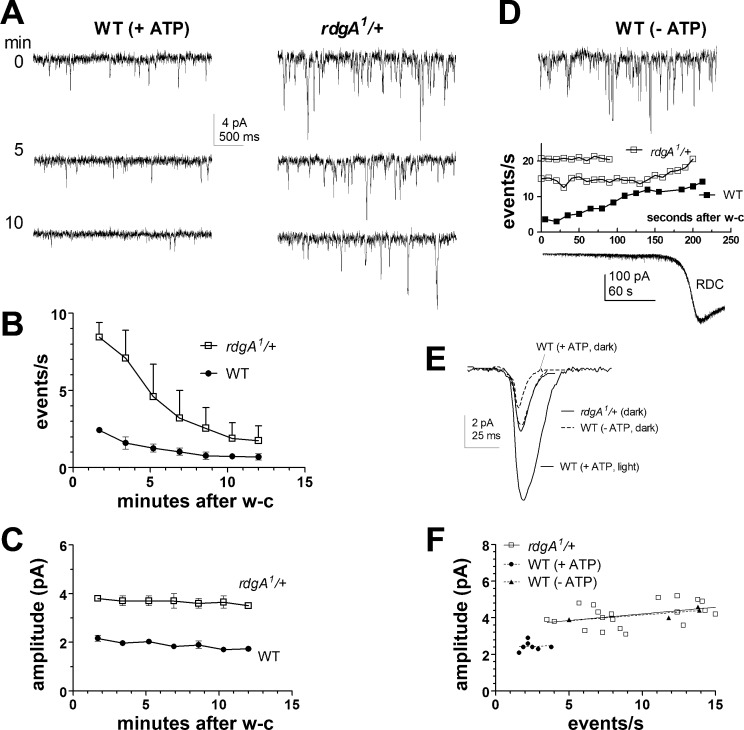
The light-sensitive TRP and TRPL channels are constitutively active in rdgA mutants lacking DGK, indicating that DGK, which converts diacylglycerol (DAG) to phosphatidic acid (PA), is required to prevent spontaneous channel activation (Raghu et al. 2000). However, the uncontrolled Ca2+ influx through constitutively active channels in rdgA mutants results in severe retinal degeneration and loss of light response, leaving it unclear whether DGK plays any role in controlling dark noise under physiological conditions. To address this, we investigated the effect of reducing DGK gene dosage in photoreceptors from rdgA1/+ heterozygotes (Fig. 1). These had normal morphology and sensitivity to light (Fig. 2C); however, immediately after establishing the whole cell configuration in rdgA1/+ photoreceptors, we detected a conspicuously high rate of spontaneous events in complete darkness, consisting of small, quantum bumplike inward currents (Fig. 1A). For quantitative analysis, we used event detection software to calculate the rates and amplitudes of dark events over the 1st 100 s after establishing the whole cell configuration. Despite cell-to-cell variation (Fig. 1F), event rates in rdgA1/+ were on average four times faster than in wild-type (9.5 ± 0.8 events per second, mean ± SE, n =20), and dark event amplitudes were approximately twice as large (4.3 ± 0.2 pA). The differences were statistically highly significant (P < 10−5, 2-tailed unpaired t-test), and a scatterplot of event rates vs. amplitudes for all cells showed no overlap in distributions (Fig. 1F). As in wild-type, the dark event rate (but not amplitude) gradually subsided over a recording period of 10–15 min (Fig. 1, B and C).
Fig. 1.
Increase in dark noise in heterozygote mutants of diacylglycerol kinase (rdgA/+) or by ATP depletion. A: spontaneous noise recorded in wild-type (WT) and rdgA1/+ photoreceptors with normal electrode solution (+ATP) in complete darkness at specified time [minutes after establishing whole cell (w-c) recording configuration]. Dark events were larger and more frequent in rdgA1/+ mutants. In both cases, the frequency but not amplitude of spontaneous events subsided over the recording period, as plotted in B, the amplitude, and C, event rate of spontaneous dark events (mean ± SE, rdgA1/+ n = 2, WT n = 3). D, top: spontaneous dark noise recorded immediately (<10 s) after establishing the w-c mode in WT photoreceptor with electrode containing no nucleotide additives (−ATP). The plot shows event rate increased rapidly during the 1st few minutes (n = 4; error bars not shown) before events fused to form rundown current (RDC; example trace shown below). Also plotted (open symbols) are event rates in 2 rdgA1/+ photoreceptors recorded without ATP. E: averaged dark event waveforms (average of ≥60 events in a representative cell of each genotype aligned by the rising phase) in rdgA1/+ and WT (−ATP) were ∼2 times larger than events in WT (+ATP) but smaller than light-induced quantum bumps. F: summary scatterplot of event rate against mean event amplitude for rdgA1/+ cells (n = 20) and WT (with ATP, n = 8; without ATP, n = 4) obtained during the 1st 100 s of w-c recording. All rdgA1/+ and WT (−ATP) photoreceptors are distinct from miniature events in WT control (+ATP); regression line shows no correlation between event rate and amplitude.
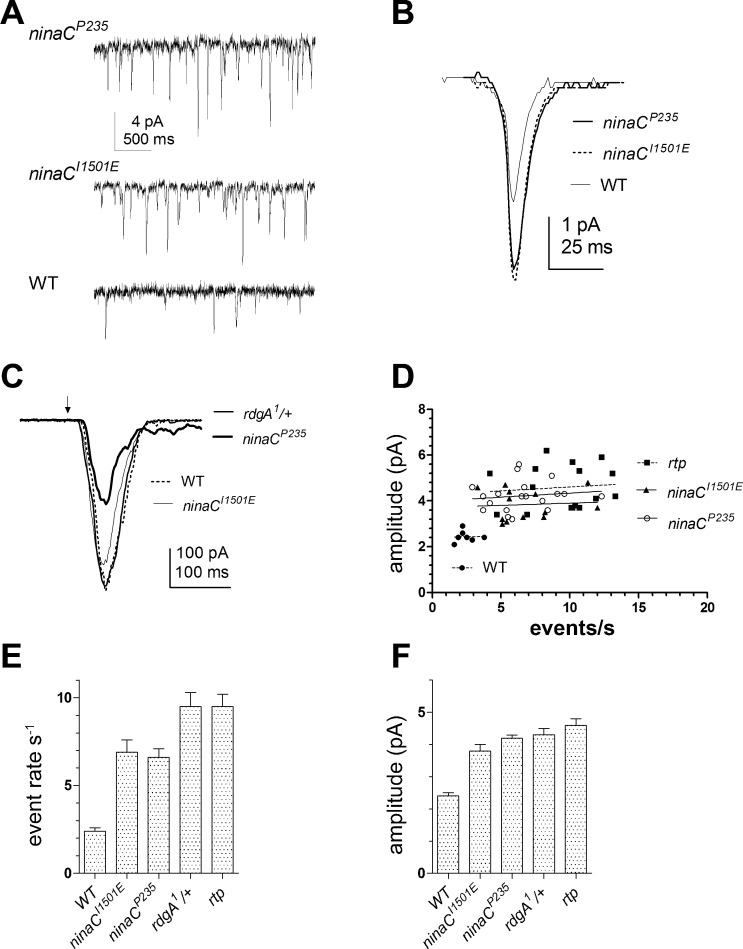
Fig. 2.
High rates of dark noise in ninaC (no inactivation no afterpotential C; gene encoding myosin III) mutants. A: a high rate of spontaneous dark events was observed in ninaCP235 and ninaCI1501E mutants relative to WT (bottom trace). B: the average dark event waveforms (≥100 events in a representative cell of each genotype, aligned by the rising phase) in both ninaC alleles are larger than in WT (see also F). C: macroscopic responses to brief flashes (∼100 photons; arrow) in ninaCI1501E and rdgA1/+ were similar to WT. As previously described, ninaCP235 has reduced sensitivity and a deactivation defect (Hofstee et al. 1996). D: summary scatterplots of event rate against mean event amplitude (regression line shows no correlation) for: ninaCP235 (n = 19), ninaCI1501E (n = 13), WT (n = 8), and retinophilin (rtp; n = 16; data from Mecklenburg et al. 2010). E and F: summary bar graphs showing mean event rate (E) and dark event amplitude (F) in ninaC alleles, rdgA1/+ and rtp mutants. Seven further alleles of ninaC (see materials and methods) had low levels of dark noise similar to WT (data not shown).
DGK has previously been proposed as a critical ATP-dependent enzyme in regulating bump amplification because rdgA mutations leads to amplification of the small quantum bumps in norpA- and Gαq-hypomorphic mutants, a phenotype that can be mimicked by omission of nucleotide additives (ATP, NAD, and GTP) from the recording electrode (Hardie et al. 2002). It is also known that under ATP-deprived conditions, TRP and TRPL channels become spontaneously activated, leading within minutes to an inward rundown current (RDC) associated with high-frequency channel noise (Agam et al. 2000; Hardie et al. 2003; Hardie and Minke 1994). We reexamined the development of the RDC by recording from wild-type photoreceptors without ATP or other nucleotide additives in the recording pipette, concentrating on the initial phase of the RDC. Immediately after establishing the whole cell configuration, small, spontaneous dark events similar to those using normal (ATP-containing) electrode solution were observed. These then rapidly increased in rate and amplitude reaching rates of up to 10–20 events per second within ∼2 min, after which the events fused to form a noisy inward current, heralding the onset of the full RDC (Fig. 1D, bottom). This pronounced time-dependent rise in spontaneous dark event rates might be explained by progressively reduced function of DGK as endogenous ATP is depleted. In support of this, in rdgA1/+ heterozygotes recorded under the same ATP-depleted conditions, there was now no obvious increase in dark event rate over time, but a stable high rate of ∼20 events per second was observed from the moment of establishing the whole cell configuration until the onset of the full RDC (Fig. 1D). We suggest that this apparently saturated event rate is likely to approximate the true rate of spontaneous G protein activations and that most of these fail to generate a dark event under normal conditions in wild-type photoreceptors (see discussion).
Spontaneous dark noise in alleles of ninaC.
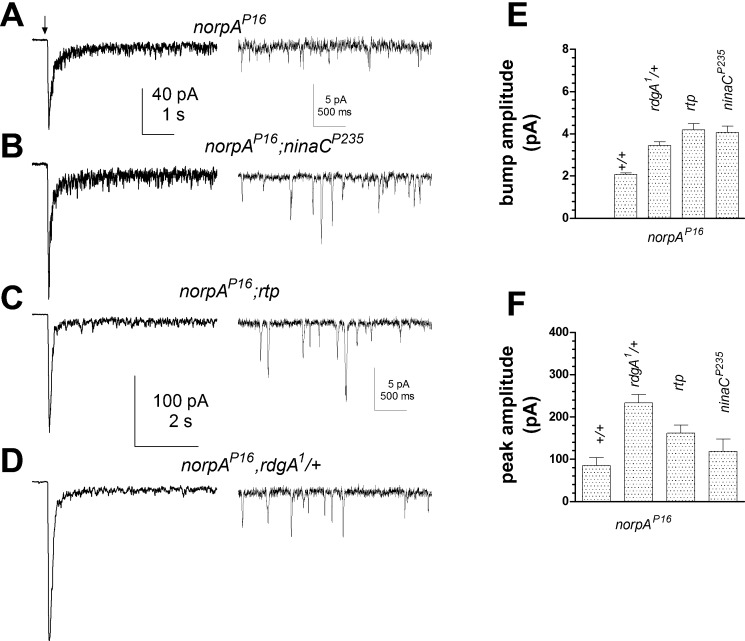
An increased spontaneous dark noise phenotype was first reported in ninaC-null mutants (ninaCP235) and ninaCΔ174 mutants lacking the rhabdomeric p174 isoform of the NINAC protein (Hofstee et al. 1996). NINAC is a multifunctional nonconventional myosin III with a kinase domain, two calmodulin binding domains, and a myosin domain (review: Montell 1999). To determine which part of the protein was important for regulating dark noise, we recorded from nine transgenic ninaC lines, expressing constructs with targeted deletions or mutations in different domains (see materials and methods). Apart from ninaCP235 and ninaCΔ174, the only other line reproducing the dark noise phenotype was ninaCI1501E, a point mutation in the COOH-terminal PDZ-domain binding motif that has been reported to anchor NINAC p174 to the INAD scaffolding protein (Wes et al. 1999). ninaCI1501E photoreceptors displayed high levels of spontaneous dark events (Fig. 2) that were indistinguishable from those in ninaCP235-null mutants in terms of both amplitude (mean, 3.8 ± 0.2 pA; n = 13; 2-tailed unpaired t-test, P = 0.1) and event rates (mean, 6.9 ± 0.7 events per second; t-test, P = 0.7). None of the other physiological phenotypes of the ninaC-null or ninaCΔ174 mutants, which include reduced sensitivity and a prolonged response decay (Hofstee et al. 1996; Porter et al. 1995), were reproduced in ninaCI1051E (Fig. 2C).
We recently reported that dark noise was also substantially increased in mutants (rtp) of a novel rhabdomeric protein, RTP (Mecklenburg et al. 2010), which coimmunoprecipitates with NINAC (Venkatachalam et al. 2010). Comparison of dark noise in rtp mutants (Fig. 2, D–F, rtp data replotted from Mecklenburg et al. 2010) indicated that it was very similar to that in ninaCP235, ninaCI1501E, and rdgA1/+. Because RTP protein was undetectable in Western blots of ninaCP235-null mutants (Mecklenburg et al. 2010; Venkatachalam et al. 2010), we had previously concluded that the increase in noise in ninaC mutants was most likely attributable to loss of RTP protein (Mecklenburg et al. 2010). Interestingly, however, RTP is expressed at near wild-type levels in the ninaCI1501E point mutant (Fig. 3 and Venkatachalam et al. 2010), leading us to propose that it is the association of RTP and NINAC with the INAD complex that is essential for suppressing dark noise.
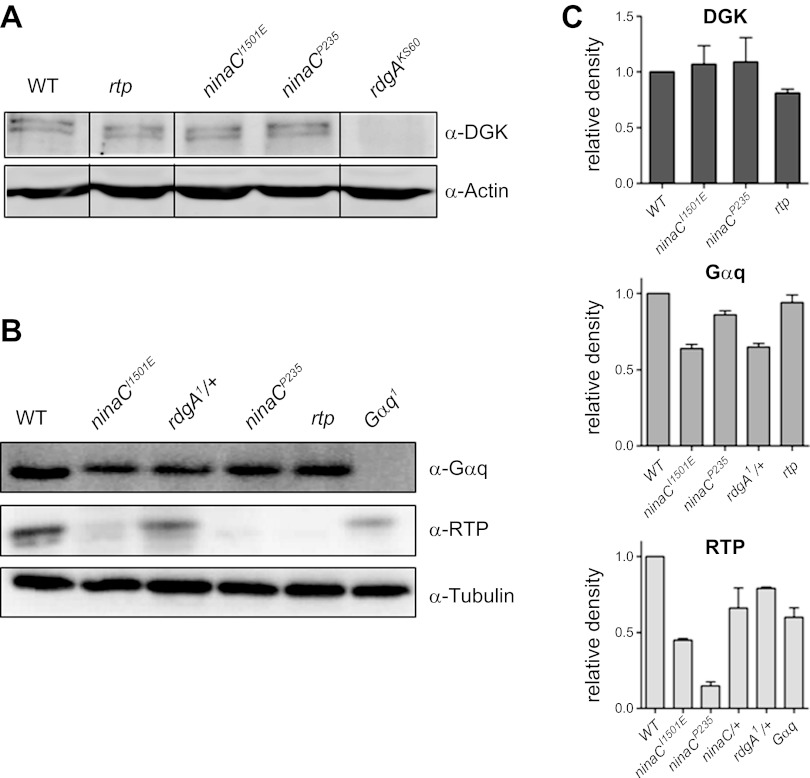
Fig. 3.
Western blots of diacylglycerol kinase (DGK), Gq α-subunit (Gαq), and RTP. Representative Western blots in wild-type, wild-type (w1118), ninaCI1501E, rdgA1/+, ninaCP235, rtp, and Gαq1 probed with antibodies for DGK (A) and Gαq and RTP (B) and loading controls of either actin (A) or tubulin (B). Gαq, RTP, and DGK cannot be reliably detected in Gαq1, rtp, or rdgAKS60 respective null/near null controls. C: quantitation (relative density) of Western blots relative to WT controls normalized to tubulin/actin standards (means ± SE, n = 3).
Gαq, RTP, and DGK protein levels.
To control for the possibility that some of the dark noise phenotypes reported in this study might be due to be compensatory regulation of one or more of the genes implicated in controlling spontaneous noise, we ran Western blots to estimate the level of expression of DGK, Gαq, and RTP in heads from various mutant backgrounds. As shown in Fig. 3, DGK was normally expressed in ninaCP235, ninaCI1501E, and rtp mutants; Gαq was expressed normally in rdgA/+, ninaCP235, ninaCI1501E, and rtp mutants. RTP was expressed normally in rdgA1/+ and essentially eliminated in ninaCP235 as previously reported (Mecklenburg et al. 2010). RTP levels in ninaCI1501E were somewhat reduced (∼45%), most likely because NINAC protein was similarly reduced in this transgenic line (in which only 1 copy of the NINACI1501E was expressed). To test whether the reduced levels of NINAC and/or RTP might have contributed to the ninaCI1501E phenotype, we recorded from photoreceptors from ninaCP235/+ heterozygotes (which have similarly reduced RTP protein levels). We found no significant increase in dark event rate (3.1 ± 0.4 events per second, n = 12) or amplitude (2.3 ± 0.1 pA) above wild-type levels (P ≥ 0.15 for rate, and P ≥ 0.25 for amplitude).
Dark noise is Gαq-dependent but independent of rhodopsin.
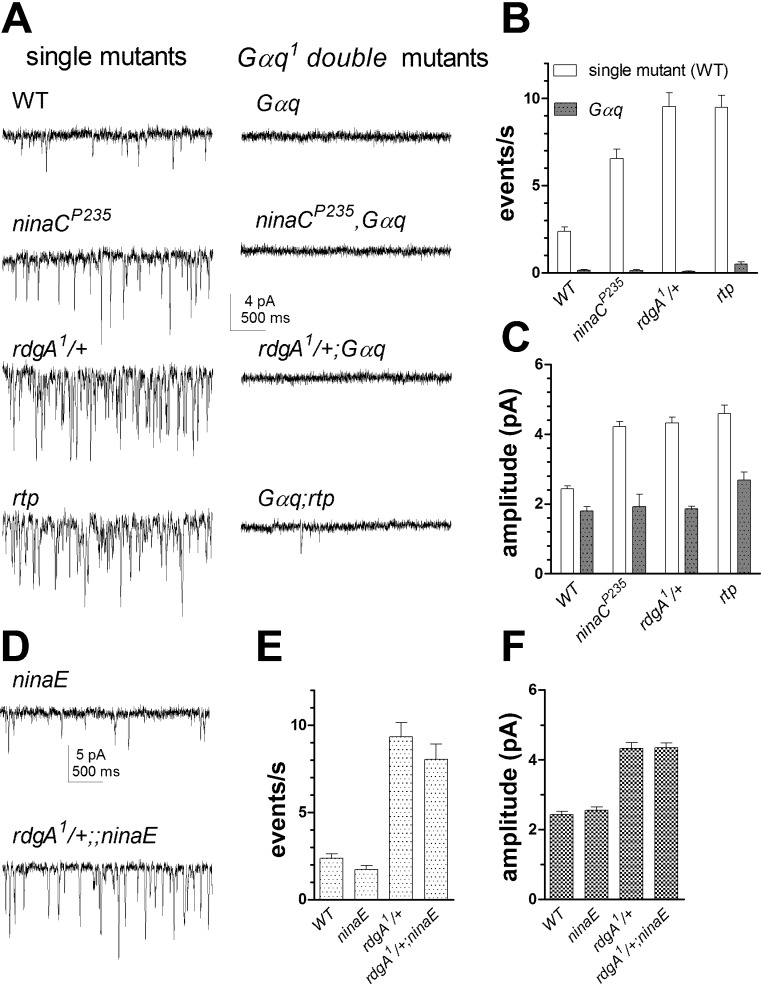
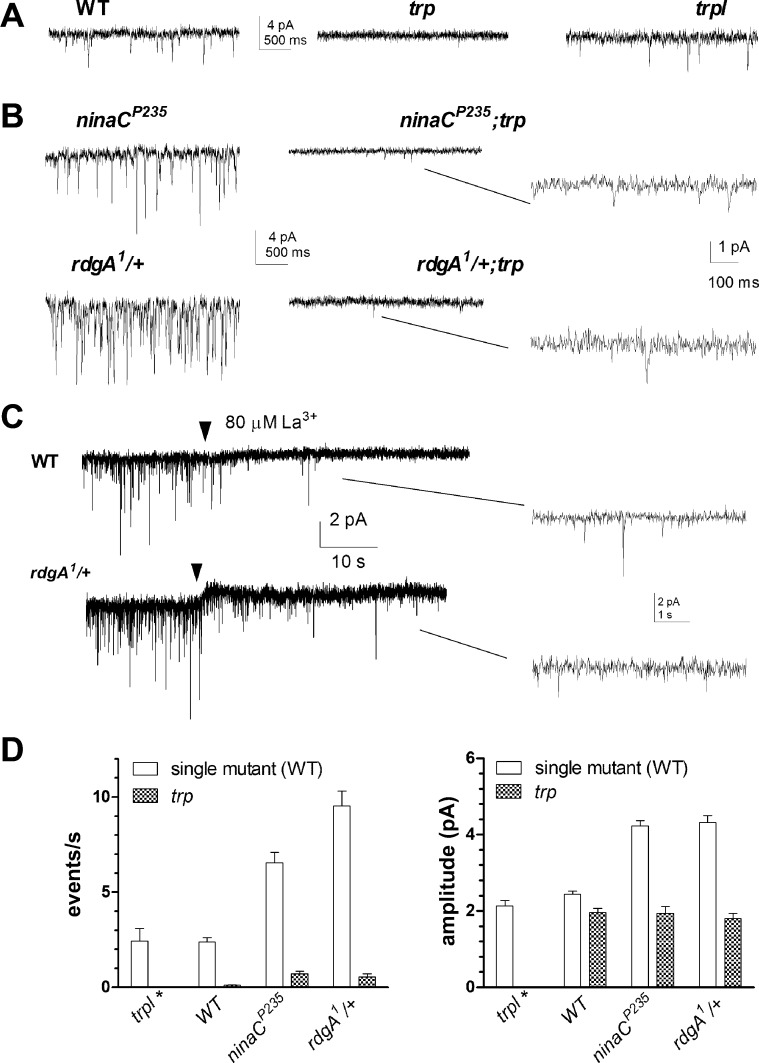
Dark noise events in wild-type photoreceptors are proposed to reflect spontaneous G protein activation and are effectively eliminated in Gαq-hypomorphic mutants (Elia et al. 2005; Hardie et al. 2002). To test whether the same is true for the enhanced noise in rdgA1/+, ninaC, and rtp photoreceptors, we introduced each genotype into a Gαq1-hypomorphic background. Indeed, dark noise was almost completely eliminated in rdgA1/+;Gαq1, ninaCP235,Gαq1, and Gαq1;rtp double mutants. In all double mutants, the mean amplitude of any rare residual dark events was approximately halved to ∼2 pA compared with respective single mutants (Fig. 4, A and B).
Fig. 4.
Dark noise is eliminated in Gαq1 but not in rhodopsin (ninaE) mutants. A: example traces of dark noise. Left in WT and single mutants: ninaCP235, rdgA1/+, and rtp. Right, same genotypes on Gαq1-hypomorphic background: dark events were virtually eliminated, leaving only very occasional events (e.g., Gαq1;rtp). B and C: summary bar graphs showing event rates (B) and amplitude (C) in Gαq1 (n = 5–8), ninaCP235;Gαq1 (n = 4–19), rdgA1/+;Gαq1 (n = 11–20), and Gαq1;rtp (n = 15) along with single mutant (or WT) controls. D: example traces of dark noise events in ninaEI17 and rdgA1/+;ninaEI17 mutants. E and F: summary bar graphs showing mean event rate (E) and amplitude (F) of spontaneous dark events in WT, ninaEI17, and rdgA1/+ on WT (n = 8) and ninaEI17 backgrounds (n = 11).
In vertebrate rods, the G protein (transducin) can be activated with greatly reduced efficiency by bleached opsin (reviewed in Fain 2001). To ask whether Gq protein activation in the dark in Drosophila was truly spontaneous or whether it might result from rare activation by rhodopsin molecules in a nonactivated state, we investigated null mutants of the rhodopsin (Rh1) gene, ninaE (O'Tousa et al. 1985). Recordings from ninaEI17 mutants also revealed small, spontaneous miniature events in complete darkness that were indistinguishable from those seen in wild-type photoreceptors (Fig. 4, D–F). To test whether rhodopsin is also dispensable for the enhanced dark noise production in rdgA1/+, we generated rdgA1/+;ninaEI17 double mutants and found that the phenotype (high rate of spontaneous dark events of larger amplitude) was similar to rdgA1/+ controls (Fig. 4, D–F). These results are consistent with a previous study reporting that enhanced dark noise in Gβ/+ heterozygotes could still be observed in flies reared on a vitamin A-deprived diet (Elia et al. 2005) and confirm that Gαq-dependent dark noise is generated independently of rhodopsin.
Dark noise is predominantly mediated by TRP channels.
The wild-type light-induced conductance is mediated by two distinct Ca2+-permeable cation channels, TRP and TRPL (Hardie and Minke 1992; Niemeyer et al. 1996; Reuss et al. 1997), both activated downstream of Gαq and PLC. To test which are responsible for dark noise, we recorded from trp- and trpl-null mutants to isolate the respective channels (Fig. 5A). Miniature dark events similar to those in wild-type photoreceptors were observed in trpl mutants lacking TRPL channels, showing no statistically significant difference in event rate or amplitude. In marked contrast, spontaneous dark events were largely absent in null trp343 mutants lacking the highly Ca2+-permeable TRP channels, with at most 0.1 events per second (n = 6). We also generated rdgA1/+;trp343 and ninaCP235;trp343 double mutants; both again resulted in greatly reduced frequency of dark events (Fig. 5, B and D), whereas their mean amplitude was reduced to ∼2 pA. We also perfused wild-type and rdgA1/+ heterozygote photoreceptors with La3+, which completely blocks all TRP channel activity (Hardie and Minke 1992; Niemeyer et al. 1996; Reuss et al. 1997). La3+ (80 μM) effectively eliminated most dark events within seconds, leaving only occasional small, residual events presumably mediated by TRPL channels (Fig. 5C).
Fig. 5.
Dark noise is mediated by transient receptor potential (TRP) channels. A: spontaneous dark noise was present in WT (left) and trp-like (trpl; right) photoreceptors but almost entirely absent in trp mutants (middle). B: high rate of dark noise seen in rdgA1/+ and ninaCP235 mutants (left) was abolished on trp mutant background (middle). Right: closer inspection of favorable recordings revealed infrequent residual dark bumps presumably mediated by TRPL channels. C: 80 μM La3+ (perfusion onset marked by arrowhead) rapidly suppressed dark noise in WT (n = 3) and rdgA1/+ (n = 2) photoreceptors, although some miniature dark bumps were retained. D: bar graph (means ± SE) for event rates (left) and bump amplitude (right) in trpl (n = 3; *no dark events were detected in trpl;trp double mutants), WT (n = 8), trp (n = 6), ninaCP235 (n = 19), ninaCP235;trp (n = 5), rdgA1/+ (n = 21), and rdgA1/+;trp (n = 4).
These results indicate that dark noise is predominantly mediated by TRP channels under the conditions of our experiments. Assuming a single-channel conductance of ∼8 pS (Henderson et al. 2000) corresponding to single-channel current of 0.65 pA at resting potential, each miniature dark event of ∼2 pA in amplitude in wild-type and trpl mutants can be interpreted as the summed opening of 2–3 TRP channels, whereas in trp mutants, the residual dark bumps probably represent the opening of single TRPL channels that have an estimated single-channel current of 2.5 pA, based on an effective single-channel conductance of 35 pS (Henderson et al. 2000).
Ca2+ dependence of spontaneous dark noise.
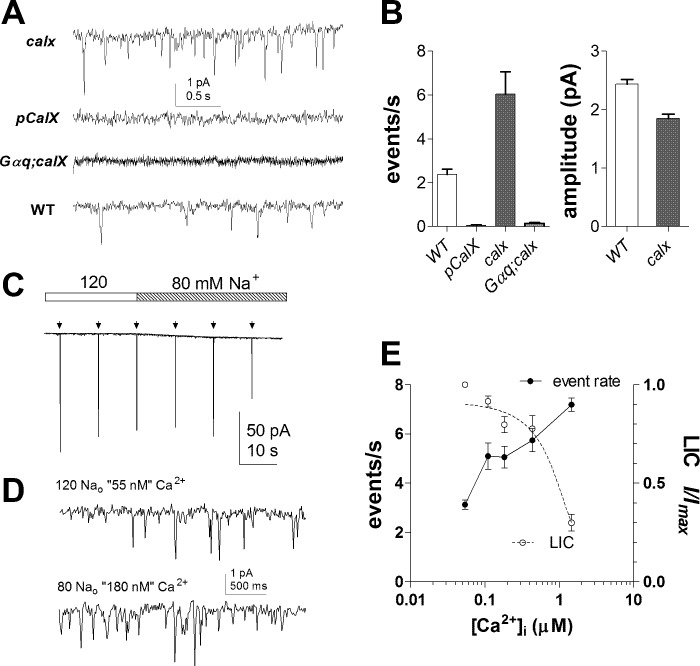
Ca2+ is critical in determining the shape and size of quantum bumps (Henderson et al. 2000), and recently it was reported that dark events were essentially eliminated in the absence of external Ca2+ in the bath (Katz and Minke 2012). To investigate the Ca2+ dependence of dark noise more quantitatively and in the physiological range, we exploited the NCX encoded by the calx gene (Schwarz and Benzer 1997). This is the major mechanism for Ca2+ extrusion in the photoreceptors, and dark resting cytosolic Ca2+ levels in calx mutant photoreceptors are elevated to ∼400 nM (Wang et al. 2005). As shown in Fig. 6, calx mutants invariably displayed a high frequency of spontaneous miniature events with rates at least twice as fast as in wild-type (mean, 6.0 ± 1.4 events per second; n = 6; P = 0.002 cf. wild-type). The events again appeared to represent spontaneous G protein activation as they were essentially eliminated in a Gαq1;calx double mutant. Conversely, overexpression of CalX in pCalX transgenic flies, which can be expected to lower the resting microvillar Ca2+ to levels below wild-type levels (normally ∼150 nM), resulted in the near elimination of dark noise. In contrast to the effects of ninaC, rtp, and rdgA/+ mutations, the mean amplitude of dark events in calx mutants was slightly but significantly smaller (P = 0.0002) than in wild-type (Fig. 6B). This is consistent with the known inhibition of the light-sensitive channels by Ca2+ (Gu et al. 2005; see also Fig. 6E) and implies that the effects of the ninaC, rtp, or rdgA/+ mutations (all of which increase dark event amplitude) are not mediated indirectly by an effect on Ca2+ levels.
Fig. 6.
Ca2+ dependence of dark noise. A: relative to WT (bottom trace), dark noise was increased in calx mutants (severe-loss-of-function mutants of CalX, the Na+-Ca2+ exchanger; top) but virtually absent in pCalx photoreceptors overexpressing calX (2nd trace) and in Gαq;calx double mutants (3rd trace). B: summary of dark event rate and amplitude in WT (n = 8), pCalX (n = 6), Gαq;calx (n = 11), and calx (n = 6). Amplitudes for pCalx and Gαq;calx are not shown due to negligible event count. C: responses to brief (5-ms) flashes (∼100 effective photons; arrows) in WT photoreceptors were slightly (∼20%) reduced as the control bath solution containing 120 mM Na+ was substituted for 80 mM Na+ [10 mM intracellular Na ([Na]i) predicted intracellular Ca2+ ([Ca]i) at −70 mV, 55 nM, and 180 nM, respectively]. D: on expanded scale, the rate of spontaneous dark events was increased following perfusion with 80 mM extracellular Na ([Na]o). E: Ca2+ dependence of dark noise (event rate) and macroscopic light-induced current (LIC). Nominal [Ca2+] was calculated from Eq. 1 with internal solution containing either 10 or 20 mM [Na]i (holding potential of −70 mV); peak responses (I/Imax) were normalized to responses immediately before Na+ substitution. Mean ± SE, n = 7–18 cells per data point.
To quantify the Ca2+ dependence of miniature dark events, we used ion substitution to control the transmembrane Na+ gradient ([Na]i/[Na]o) and thereby manipulate intracellular Ca2+ ([Ca]i) according to the NCX equilibrium (Gu et al. 2005; Wang et al. 2005):
| (1) |
where E is potential difference in volts, R is the gas constant, T is temperature in kelvin, and F is Faraday's constant.
In wild-type photoreceptors recorded with 10 mM [Na]i and 120 mM [Na]o (i.e., control bath, predicted [Ca]i at −70 mV, 55 nM), the dark event rate of 3.1 ± 0.2 events per second (n = 16; Fig. 6, C and E) was slightly above controls (t-test, P = 0.03) without added internal [Na+]i in the electrode. As [Na]o was lowered (substituted for Li+), the rate of dark events increased two- to threefold as [Ca]i was raised (nominally) from 55 nM to 1.5 μM (Fig. 6, C–E). Dark event amplitude was significantly decreased (e.g., 2.23 ± 0.05 pA, n = 16 at 55 nM, cf. 1.86 ± 0.14 pA at 180 nM, n = 8; t-test, P = 0.00005), although not as much as the light-induced current, which was inhibited with an IC50 of ∼1 μM as previously reported (Gu et al. 2005). Nominal Ca2+ concentrations beyond 1 μM were not tested due to deteriorating signal-to-noise ratio that prevented discrete events from being reliably resolved.
Ca2+ sensitizes TRP channels.
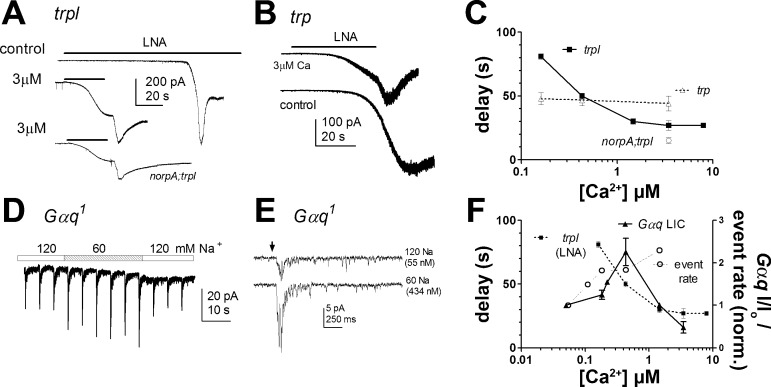
In principle, there are several mechanisms by which Ca2+ could increase the rate of dark events. For example, we previously speculated that Ca2+ influx may sensitize the light-sensitive channels by shifting their effective dose-response function for excitatory second messenger(s) (Hardie et al. 2002). The identity of the second messenger is still debated (e.g., Delgado and Bacigalupo 2009; Hardie and Franze 2012; Huang et al. 2010; Lev et al. 2012); however, exogenously applied polyunsaturated fatty acids (PUFAs) such as LNA are effective agonists, which act downstream of PLC probably acting directly on the channel or their lipid environment (Chyb et al. 1999; Hardie et al. 2003; Parnas et al. 2009). We therefore investigated the sensitivity of the light-sensitive channels to LNA as a function of cytosolic Ca2+ manipulated, as above, by exploiting the NCX equilibrium (Fig. 7). To assess sensitivity, we applied a standard concentration (20 μM) of LNA by a puffer pipette and measured the time taken for a criterion current of 10 pA to develop. Sensitivity of TRP and TRPL channels were investigated separately using trp and trpl mutants. In trp mutants, where only TRPL channels are present, the time taken to activate a criterion current was ∼50 s and independent of [Ca]i in the range tested. By contrast, the time required to activate TRP channels (in trpl mutants) was very sensitive to imposed cytosolic [Ca]i, accelerating from ∼80 s at (nominally) 100 nM to ∼20 s at concentrations >1 μM with an EC50 of ∼300 nM. A similar sensitization to LNA by raised Ca2+ was also observed in a norpAP24;trpl double mutant indicating that the effect was mediated independently of PLC and most likely at the level of the channel (Fig. 7).
Fig. 7.
Ca2+ dependence of TRP channel sensitivity to LNA and light response in Gαq mutants. A: activation of TRP channels (in trpl mutant photoreceptor) by perfusion of 20 μM linolenic acid (LNA; bars): under control conditions (top; 1 mM [Na]i, 120 mM [Na]o) and with [Ca]i nominally at 3 μM (middle; perfusion with 60 mM Na; 20 mM [Na]i). Activation was greatly accelerated at the higher [Ca]i. Activation in no receptor potential A (norpA; gene encoding PLC);trpl (norpA;trpl) under the same conditions (bottom) was similarly fast. B: by contrast, activation of TRPL channels (in trp mutants) occurred with a similar delay under both conditions. In both cases (A and B), the high Ca2+ also induced Ca2+-dependent inhibition of the TRP (or TRPL) channels, and on reperfusion with 120 mM Na (end of bar) extrusion of Ca2+ by the CalX exchanger relieved inhibition inducing an increase in current. C: dose-response (mean ± SE, n = 4–6 cells per point) expressed as perfusion time (delay) required to activate a criterion (10-pA) current, plotted against nominal Ca2+ concentration (Eq. 1). D: responses to brief (0.5-ms) flashes (∼3 × 105 WT effective photons) in Gαq1 hypomorphs were reversibly increased ∼2-fold during perfusion with 60 mM [Na]o (10 mM [Na]i), nominally increasing Ca2+ from 55 to 434 nM. E: example responses to light flash (arrow) before and during perfusion with 60 mM [Na]o. F: dose-response function for LIC in Gαq1 [solid line; peak response normalized (norm.) to value before perfusion with low [Na]o; n = 3–5 cells per data point]. The Ca2+ dependence of TRP channel activation by LNA is replotted from C (trpl LNA) for comparison along with the increase in dark event rate (normalized to value before perfusion) from Fig. 5E. All showed significant facilitation in a similar submicromolar range.
The ability to facilitate dark events and LNA-induced channel activity by raising Ca2+ in this manner contrasts with the effect of Ca2+ on the wild-type light response, where raising [Ca]i via the NCX equilibrium only inhibited the response (Gu et al. 2005). We suspected that this might be because quantum bumps in wild-type photoreceptors already represent an effectively near-saturating response at the level of individual microvilli: i.e., quantum efficiency (Q.E.) is near 100%, and the majority of TRP channels in a microvillus are activated following successful absorption of a photon. We therefore also investigated the effect of raising Ca2+ in Gαq1 hypomorphs, where Q.E. is reduced, with many photon absorptions failing to reach threshold for bump generation (Hardie et al. 2002; see also discussion). Indeed, in Gαq1, the response to light was facilitated two- to threefold by [Ca]i in a narrow, submicromolar (approximately 100–400 nM) range similar to the Ca2+ dependence of dark event rate (Fig. 7, D–F). The effect appeared to be largely due to an increase in Q.E. as bump amplitudes were unaffected or slightly reduced, again presumably because of the competing effect of Ca2+-dependent inhibition. At higher concentrations, Ca2+-dependent inhibition dominated, resulting in suppression of the light-induced currents as found in wild-type photoreceptors.
Mutations that enhance dark noise also facilitate light responses in norpA and Gαq mutant backgrounds.
As previously reported, hypomorphic Gαq and norpA mutants show a severalfold reduction in quantum bump amplitude (Hardie et al. 2002) and variable reduction (up to ∼1,000-fold in Gαq1) in effective Q.E. (Hardie et al. 2002; Scott et al. 1995). This implies that normal bump generation and amplification requires the activation of multiple G proteins. Since dark events were amplified ∼2-fold in rdgA1/+, ninaC, and rtp mutants, we asked whether light-evoked quantum bumps in Gαq1 and norpAP16 hypomorphs would be similarly enhanced in these backgrounds.
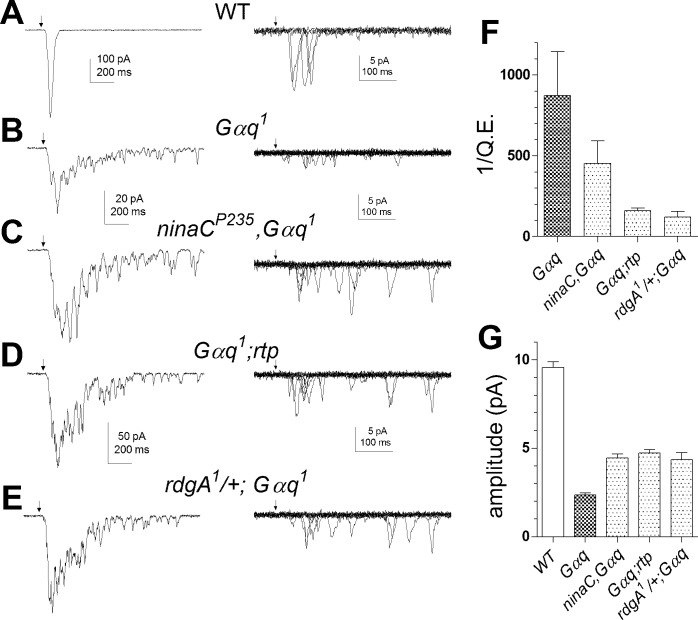
In agreement with an earlier study (Hardie et al. 2002), we confirmed that quantum bump amplitudes in rdgA1/+;Gαq1 and rdgA1/+,norpAP16 were increased ∼2-fold relative to the respective Gαq1 or norpAP16 single mutants. New here, we found that bump amplitudes in both ninaCP235,Gαq1 and Gαq1;rtp (Fig. 8) as well as norpAP16;ninaCP235 and norpAP16;rtp (Fig. 9) were also all similarly increased, supporting roles for DGK, NINAC, and RTP in regulating bump amplification as well as noise suppression. The average waveform and amplitude of quantum bumps in Gαq1 and norpAP16 double mutants were indistinguishable from dark events in each respective single mutant (i.e., rdgA1/+, rtp, or ninaC) genotype. Significantly, Q.E. was enhanced in all three Gαq1 double mutants with respect to Gαq1 single mutants (Fig. 8). Improvement in Q.E. in these flies implies that rdgA1/+, ninaC, and rtp mutations all resulted in a higher probability that activated G proteins in Gαq1 successfully generate a bump and conversely that in Gαq hypomorphs many (single) G protein activations fail to generate a quantum bump (see also Hardie et al. 2002). This suggests that reduction/removal of DGK, NINAC, and RTP lowers the effective threshold that G proteins and PLC must overcome for bump generation. The increase in bump amplitude can be explained similarly: lowering the threshold for channel activation allows more channels to be recruited per bump.
Fig. 8.
Facilitation of light responses in Gαq by ninaCP235, rtp, and rdgA/+ mutations. A–E, left: responses to brief flashes (arrow) containing ∼100 effective photons in WT and ∼7 × 104 photons in Gαq mutants, ninaCP235,Gαq, Gαq;rtp, and rdgA/+;Gαq double mutants. Right: quantum bumps induced by brief flashes (arrows) in the same fly strains (superimposed traces selected for single bumps). Both macroscopic responses and quantum bumps in Gαq1 were increased on the ninaCP235 rtp and rdgA/+ backgrounds (note different scales). F and G: bar graphs showing reciprocal of quantum efficiency (F) relative to WT (1/Q.E.) and bump amplitude (G) in WT (n = 11), Gαq1 (n = 6), ninaCP235,Gαq1 (n = 8), Gαq1;rtp (n = 14), and rdgA/+;Gαq1 (n = 3) double mutants.
Fig. 9.
Facilitation of light responses in PLC hypomorph norpAP16 by rdgA1/+, ninaCP235, and rtp mutations. A–D: responses in norpAP16 hypomorph were enhanced on ninaCP235, rdgA1/+, and rtp backgrounds. Left: macroscopic responses elicited by flashes (arrow) containing ∼7.5 × 105 WT effective photons. Right: representative samples of quantum bumps in darkness after decay of the current to baseline. E and F: bar graphs summarizing the effect of rdgA/+, ninaCP235, and rtp backgrounds on bump amplitude (E) and peak response to flashes containing ∼7.5 × 105 WT effective photons (F) compared with single norpAP16 mutant controls (labeled +/+). (P16, n = 6–14; ≥50 bumps in each cell; double mutants, n = 5–19 cells).
DISCUSSION
Spontaneous, dark noise in photoreceptors sets a fundamental limit to absolute visual thresholds (Aho et al. 1988; Rieke and Baylor 1996) and in Drosophila is manifest as small (∼2 pA), quantum bumplike events occurring at rates of approximately 2–3/s. Previous studies indicated that these dark events reflect spontaneous G protein activations (Hardie et al. 2002) and that a suprastoichiometric Gβ-/Gα-subunit ratio (∼2-fold excess of Gβ) is required to keep the rate even this low (Elia et al. 2005). Our results strengthen and extend this conclusion by showing that dark events are generated independently of rhodopsin but are Gαq-dependent in a variety of mutant backgrounds and mediated predominantly by the major light-sensitive channel (TRP). Our results suggest that spontaneous G protein activations are at least ∼10 times more frequent than indicated by the wild-type dark noise but that potential dark noise is normally suppressed by mechanisms involving NINAC, RTP, the INAD scaffolding protein, DGK, and a Ca2+-dependent threshold for channel activation. Our results further indicate that all of these mechanisms regulating dark noise are also involved in light-induced quantum bump amplification.
Spontaneous G protein activation.
Little information is available on spontaneous G protein activation rates in vivo in any system (e.g., Siekhaus and Drubin 2003). Photoreceptors, with their high density of signaling components, would seem well-suited for studying spontaneous G protein activation. However, dark noise in vertebrate photoreceptors is dominated by spontaneous activation of phosphodiesterase or spontaneous thermal isomerization of rhodopsin (Luo et al. 2011; Rieke and Baylor 1996, 2000). In Drosophila, thermal isomerizations are rare (<1/min; Henderson et al. 2000), and although PLC has measurable basal activity, it is less than ∼1/1,000th of the G protein-activated PLC activity and does not normally overcome threshold for channel activation (Hardie et al. 2004). Fly photoreceptors thus afford a rare opportunity to study spontaneous G protein activation rates. In wild-type photoreceptors, the spontaneous dark event rate of 2–3/s could be accelerated in various mutant backgrounds by ATP depletion or raising cytosolic Ca2+. It seems unlikely that these diverse manipulations directly affected the G protein activation rate, and hence the wild-type dark event rate probably greatly underestimates the underlying rate of spontaneous G protein activation.
This implies that most single G protein activation events normally fail to overcome threshold for channel activation. A similar conclusion was also reached in a recent independent study (Katz and Minke 2012) and first proposed in an earlier study showing that Q.E. is greatly reduced in Gαq hypomorphs (Hardie et al. 2002). We suggest that the true rate of spontaneous G protein activation is of the order of the maximum we observed (∼20/s in rdgA1/+ heterozygotes under ATP-deprived conditions). Assuming 2 × 106 G proteins per cell (Hardie and Raghu 2001), this represents an average lifetime (per G protein molecule) of ∼30 h. Heterotrimeric G proteins would thus seem rather stable, albeit much less so than the visual pigment rhodopsin, which can have a theoretical half-life of hundreds of years (Baylor et al. 1980)!
Dark-noise suppression by DGK, NINAC, and RTP.
Increased dark noise in ninaC and rtp mutants had been reported previously (Hofstee et al. 1996; Mecklenburg et al. 2010) and attributed to loss of RTP since RTP protein is absent in ninaCP235-null mutants (Mecklenburg et al. 2010). Here, we found that a point mutation in the COOH terminus of NINAC (ninaCI1501E), required for its interaction with the INAD scaffolding protein (Wes et al. 1999), fully and uniquely reproduced the ninaCP235-null mutation in increasing dark noise without mimicking any other electrophysiological phenotypes of the null mutant. NINAC and RTP protein are both expressed at near normal levels in ninaCI1501E (Venkatachalam et al. 2010), leading us to propose that incorporation of RTP in the INAD complex via attachment to NINAC is required for suppressing noise. Also new here, we report a dark noise phenotype, similar to that in ninaCI1501E and rtp, in rdgA1/+ heterozygotes expressing reduced levels of DGK.
In principle, DGK, NINAC, and RTP might maintain a low rate of dark events by suppression of spontaneous G protein activation. However, there is no obvious precedent for such a mechanism, and it would be difficult to reconcile the equivalent effect of ninaC, rdgA, and trp mutations on both spontaneous dark noise and bump amplification in Gαq and norpA hypomorphs with such an explanation. We suggest instead that DGK and RTP/NINAC modulate the downstream effects of spontaneous G protein activations either by regulating the availability of excitatory second messenger(s) that lead to channel openings or by modulating the sensitivity of the channels to excitatory messenger.
Thus, following spontaneous G protein activations, we propose that most Gqα-PLC complexes inactivate before generating sufficient excitatory messenger(s) to activate any channels. However, if DGK activity is reduced (in rdgA1/+ heterozygotes), then PLC products [e.g., DAG, protons, and phosphatidylinositol 4,5-bisphosphate (PIP2) reduction] can now overcome threshold more readily, resulting in increased probability of generating a dark event. On one hand, the similar effects seen in ninaC and rtp mutants might be explained if a NINAC-RTP-INAD complex is required to maximize effective DGK activity (e.g., by incorporating DGK itself within the INAD complex). However, because available evidence indicates that DGK is localized outside the rhabdomere (Masai et al. 1997) and because both PLC and TRP are integral components of the INAD scaffolding complex (Chevesich et al. 1997; Huber et al. 1996; Shieh and Zhu 1996; Tsunoda et al. 1997), it can also be speculated that interactions between INAD/NINAC and RTP may be involved in regulating either PLC activity or the sensitivity of the TRP channel to excitatory messenger(s).
At face value, the role of DGK in dark noise suppression supports a role for DAG (and/or downstream PUFA metabolites) in channel activation as previously discussed (Hardie et al. 2003; Leung et al. 2008; Lev et al. 2012; Raghu et al. 2000). A detailed discussion of the mechanism of excitation is beyond the scope of this study; however, a recent study suggested that the light-sensitive channels may be activated by the combination of PIP2 depletion, possibly acting via mechanical effects on the lipid bilayer (Hardie and Franze 2012), and the proton released by the PLC reaction rather than by DAG (Huang et al. 2010). Since DGK product (PA) is a potent positive regulator of PIP 5-kinase (Cockcroft 2009; Jenkins et al. 1994), rdgA1/+ mutants might have significantly impaired ability to rapidly resynthesize PIP2, potentially also accounting for the increased sensitivity and dark noise phenotypes in rdgA1/+ (reviews: Hardie 2012; Raghu and Hardie 2009).
Ca2+ dependence of spontaneous dark noise.
Importantly, we also found that dark event rate, but not amplitude, was increased by raising cytosolic Ca2+ via manipulating NCX (Wang et al. 2005). Conversely, dark noise was almost eliminated in photoreceptors overexpressing CalX, which presumably results in a lower [Ca]i. We again consider it unlikely that [Ca]i affects the spontaneous rate of G protein activation, and therefore these results suggest the existence of an effective Ca2+-dependent threshold, which must be reached for successful generation of a dark event. The failure of raising Ca2+ to increase dark event amplitude is presumably explained by the competing effect of Ca2+-dependent inhibition of the light-sensitive channels, which has an IC50 of 1 μM (Gu et al. 2005).
Positive feedback by Ca2+ is a key feature of phototransduction in microvillar photoreceptors (Hardie 1991), but the underlying mechanisms are poorly understood. There are at least two targets where Ca2+ might act to facilitate channel activation, namely PLC and the channels themselves (see also Katz and Minke 2012). PLC is a Ca2+-dependent enzyme (Rhee 2001), and light-induced PLC activity in Drosophila eyes is facilitated by Ca2+ in the submicromolar range both in vitro (Running Deer et al. 1995) and in vivo (Hardie 2005). However, TRP (but not TRPL) channels can be facilitated by caged Ca2+ on a submillisecond time scale, suggesting a direct effect on the channels themselves (Hardie 1995). In the present study, we found that the sensitivity of TRP (but not TRPL) channels to exogenous agonist (LNA) was enhanced by Ca2+ with an EC50 of ∼300 nM, downstream of PLC (Fig. 8), supporting a previous suggestion that Ca-dependent positive feedback functions by lowering the threshold for channel activation (Hardie et al. 2002). Both mechanisms (facilitation of TRP channels or PLC) clearly have the potential to increase the probability that an activated G protein-PLC complex may activate the channels: on one hand by hydrolyzing more PIP2 and on the other by lowering the threshold of PIP2-derived excitatory messenger required to activate the channels.
Concluding remarks.
Our results indicate roles for DGK and INAD/NINAC/RTP in suppressing dark-noise. Importantly, all mutations that enhanced dark noise also increased Q.E. and bump amplitude in Gαq1 and norpAP16 hypomorphs, suggesting common roles in regulating bump amplification. We propose that DGK, NINAC, and RTP (via INAD) all function to reduce the probability that single activated Gqα-PLC complexes overcome a Ca2+-dependent threshold for channel activation. The downstream mechanisms require further investigation but probably include regulation of the TRP channel sensitivity to excitatory messenger as well as the generation and/or degradation of excitatory messenger. The Ca2+-dependent threshold seems critical to minimize dark noise from spontaneous (single) G protein activation while maximizing the detectability of light-evoked quantum bumps, which require sequential activation of five or so G proteins and PLC molecules within the finite but variable latency period of 20–100 ms (Hardie et al. 2002). More specifically, under physiological conditions, low [Ca]i in the dark sets a high threshold for channel activation: on one hand, this suppresses generation of dark events by single, spontaneous G protein activations; on the other hand, it also delays light-induced quantum bump initiation until sufficient G protein and PLC molecules are activated to allow buildup of excitatory messenger throughout most of the microvillus. Once the first channel opens, Ca2+ influx rapidly floods the entire microvillus, lowering the threshold for the remaining channels, which can then be activated by what were previously only subthreshold (but finite) levels of excitatory messenger, thus generating an amplified, full-size quantum bump. Computational models based on this conceptual model (postulated by Hardie et al. 2002; Henderson et al. 2000) successfully recreate quantum bump kinetics, amplification, and latency distributions (Pumir et al. 2008; Song et al. 2012). Importantly, they also predict that single G protein activations lead to the generation of only small bumps with much reduced probability or Q.E. (Pumir et al. 2008).
GRANTS
This research was supported by the Biotechnology and Biological Sciences Research Council (BBSRC Grant BB/G006865/1 to R. C. Hardie and BBSRC Doctoral Award to B. Chu) and the Cambridge-Nehru Trust (S. Sengupta).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.C. performed most electrophysiological experiments and wrote paper; C.-H.L., Western blot analysis and genetics; S.S., genetics, designing, and performing crosses; A.G., generation of RDGA antibody and Western blot analysis; P.R., generation of RDGA antibody and Western blot analysis; R.C.H., electrophysiology, experimental design, and wrote paper.
ACKNOWLEDGMENTS
We thank Drs. B. Minke, B. Katz, and J. O'Tousa for comments on an earlier version of the manuscript. We also thank Dr. Craig Montell for supplying his collection of transgenic ninaC alleles.
REFERENCES
- Agam K, von Campenhausen M, Levy S, Ben-Ami HC, Cook B, Kirschfeld K, Minke B. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J Neurosci 20: 5748–5755, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho AC, Donner K, Hyden C, Larsen LO, Reuter T. Low retinal noise in animals with low body-temperature allows high visual sensitivity. Nature 334: 348–350, 1988 [DOI] [PubMed] [Google Scholar]
- Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol 309: 591–621, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevesich J, Kreuz AJ, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron 18: 95–105, 1997 [DOI] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature 397: 255–259, 1999 [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Phosphatidic acid regulation of phosphatidylinositol 4-phosphate 5-kinases. Biochim Biophys Acta 1791: 905–912, 2009 [DOI] [PubMed] [Google Scholar]
- Delgado R, Bacigalupo J. Unitary recordings of TRP and TRPL channels from isolated Drosophila retinal photoreceptor rhabdomeres: activation by light and lipids. J Neurophysiol 101: 2372–2379, 2009 [DOI] [PubMed] [Google Scholar]
- Elia N, Frechter S, Gedi Y, Minke B, Selinger Z. Excess of Gβe over Gqα in vivo prevents dark, spontaneous activity of Drosophila photoreceptors. J Cell Biol 171: 517–526, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL. Dark adaptation. Prog Brain Res 131: 383–394, 2001 [DOI] [PubMed] [Google Scholar]
- Fain GL, Hardie R, Laughlin SB. Phototransduction and the evolution of photoreceptors. Curr Biol 20: R114–R124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Oberwinkler J, Postma M, Hardie RC. Mechanisms of light adaptation in Drosophila photoreceptors. Curr Biol 15: 1228–1234, 2005 [DOI] [PubMed] [Google Scholar]
- Hardie RC. Inhibition of phospholipase C activity in Drosophila photoreceptors by 1,2-bis(2-aminophenoxy)ethane N,N,N′,N′-tetraacetic acid (BAPTA) and di-bromo BAPTA. Cell Calcium 38: 547–556, 2005 [DOI] [PubMed] [Google Scholar]
- Hardie RC. Photolysis of caged Ca2+ facilitates and inactivates but does not directly excite light-sensitive channels in Drosophila photoreceptors. J Neurosci 15: 889–902, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC. Phototransduction mechanisms in Drosophila microvillar photoreceptors. WIRES Membr Transp Signal doi:10.1002/wmts.20, 2012 [Google Scholar]
- Hardie RC. Whole-cell recordings of the light-induced current in Drosophila photoreceptors: evidence for feedback by calcium permeating the light sensitive channels. Proc R Soc Lond B Biol Sci 245: 203–210, 1991 [Google Scholar]
- Hardie RC, Franze K. Photomechanical responses in Drosophila photoreceptors. Science 338: 260–263, 2012 [DOI] [PubMed] [Google Scholar]
- Hardie RC, Gu Y, Martin F, Sweeney ST, Raghu P. In vivo light-induced and basal phospholipase C activity in Drosophila photoreceptors measured with genetically targeted phosphatidylinositol 4,5-bisphosphate-sensitive ion channels (Kir2.1). J Biol Chem 279: 47773–47782, 2004 [DOI] [PubMed] [Google Scholar]
- Hardie RC, Martin F, Chyb S, Raghu P. Rescue of light responses in the Drosophila “null” phospholipase C mutant, norpAP24, by the diacylglycerol kinase mutant, rdgA, and by metabolic inhibition. J Biol Chem 278: 18851–18858, 2003 [DOI] [PubMed] [Google Scholar]
- Hardie RC, Martin F, Cochrane GW, Juusola M, Georgiev P, Raghu P. Molecular basis of amplification in Drosophila phototransduction. Roles for G protein, phospholipase C, and diacylglycerol kinase. Neuron 36: 689–701, 2002 [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. Spontaneous activation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol 103: 389–407, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8: 643–651, 1992 [DOI] [PubMed] [Google Scholar]
- Hardie RC, Postma M. Phototransduction in microvillar photoreceptors of Drosophila and other invertebrates. In: The Senses: A Comprehensive Reference: Vision, edited by Albright TD, Masland R. Oxford, UK: Academic Press, 2008, vol. 1, p. 77–130 [Google Scholar]
- Hardie RC, Raghu P. Visual transduction in Drosophila. Nature 413: 186–193, 2001 [DOI] [PubMed] [Google Scholar]
- Henderson SR, Reuss H, Hardie RC. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+. J Physiol 524: 179–194, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstee CA, Henderson S, Hardie RC, Stavenga DG. Differential effects of NINAC proteins (p132 and p174) on light-activated currents and pupil mechanism in Drosophila photoreceptors. Vis Neurosci 13: 897–906, 1996 [DOI] [PubMed] [Google Scholar]
- Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol 20: 189–197, 2010 [DOI] [PubMed] [Google Scholar]
- Huber A, Sander P, Gobert A, Bahner M, Hermann R, Paulsen R. The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J 15: 7036–7045, 1996 [PMC free article] [PubMed] [Google Scholar]
- Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem 269: 11547–11554, 1994 [PubMed] [Google Scholar]
- Katz B, Minke B. Drosophila photoreceptors and signaling mechanisms. Front Cell Neurosci 3: 2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Minke B. Phospholipase C-mediated suppression of dark noise enables single-photon detection in Drosophila photoreceptors. J Neurosci 32: 2722–2733, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Tseng-Crank J, Kim E, Mahapatra C, Shino S, Zhou Y, An L, Doerge RW, Pak WL. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron 58: 884–896, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Katz B, Tzarfaty V, Minke B. Signal-dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate without activation of phospholipase C: implications on gating of Drosophila TRPL (transient receptor potential-like) channel. J Biol Chem 287: 1436–1447, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Porter JA, Montell C. Requirement for the NINAC kinase/myosin for stable termination of the visual cascade. J Neurosci 18: 9601–9606, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo DG, Yue WW, Ala-Laurila P, Yau KW. Activation of visual pigments by light and heat. Science 332: 1307–1312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai I, Okazaki A, Hosoya T, Hotta Y. Drosophila retinal degeneration A gene encodes an eye-specific diacylglycerol kinase with cysteine-rich zinc-finger motifs and ankyrin repeats. Proc Natl Acad Sci USA 90: 11157–11161, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai I, Suzuki E, Yoon CS, Kohyama A, Hotta Y. Immunolocalization of Drosophila eye-specific diacylgylcerol kinase, rdgA, which is essential for the maintenance of the photoreceptor. J Neurobiol 32: 695–706, 1997 [DOI] [PubMed] [Google Scholar]
- Mecklenburg KL. Drosophila retinophilin contains MORN repeats and is conserved in humans. Mol Genet Genomics 277: 481–489, 2007 [DOI] [PubMed] [Google Scholar]
- Mecklenburg KL, Takemori N, Komori N, Chu B, Hardie RC, Matsumoto H, O'Tousa JE. Retinophilin is a light-regulated phosphoprotein required to suppress photoreceptor dark noise in Drosophila. J Neurosci 30: 1238–1249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Drosophila visual transduction. Trends Neurosci 35: 356–363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol 15: 231–268, 1999 [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. The Drosophila ninaC locus encodes two photoreceptor cell specific proteins with domains homologous to protein kinases and the myosin heavy chain head. Cell 52: 757–772, 1988 [DOI] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell 85: 651–659, 1996 [DOI] [PubMed] [Google Scholar]
- O'Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML. The Drosophila ninaE gene encodes an opsin. Cell 40: 839–850, 1985 [DOI] [PubMed] [Google Scholar]
- Parnas M, Katz B, Lev S, Tzarfaty V, Dadon D, Gordon-Shaag A, Metzner H, Yaka R, Minke B. Membrane lipid modulations remove divalent open channel block from TRP-like and NMDA channels. J Neurosci 29: 2371–2383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearn MT, Randall LL, Shortridge RD, Burg MG, Pak WL. Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutants. J Biol Chem 271: 4937–4945, 1996 [DOI] [PubMed] [Google Scholar]
- Porter JA, Hicks JL, Williams DS, Montell C. Differential localizations of and requirements for the two Drosophila ninaC kinase/myosins in photoreceptor cells. J Cell Biol 116: 683–693, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JA, Minke B, Montell C. Calmodulin binding to Drosophila NinaC required for termination of phototransduction. EMBO J 14: 4450–4459, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JA, Montell C. Distinct roles of the Drosophila ninaC kinase and myosin domains revealed by systematic mutagenesis. J Cell Biol 122: 601–612, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumir A, Graves J, Ranganathan R, Shraiman BI. Systems analysis of the single photon response in invertebrate photoreceptors. Proc Natl Acad Sci USA 105: 10354–10359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu P, Hardie RC. Regulation of Drosophila TRPC channels by lipid messengers. Cell Calcium 45: 566–573, 2009 [DOI] [PubMed] [Google Scholar]
- Raghu P, Usher K, Jonas S, Chyb S, Polyanovsky A, Hardie RC. Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron 26: 169–179, 2000 [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Harris GL, Stevens CF, Zuker CS. A Drosophila mutant defective in extracellular calcium-dependent photoreceptor deactivation and rapid desensitization. Nature 354: 230–232, 1991 [DOI] [PubMed] [Google Scholar]
- Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron 19: 1249–1259, 1997 [DOI] [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70: 281–312, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Baylor DA. Molecular origin of continuous dark noise in rod photoreceptors. Biophys J 71: 2553–2572, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Baylor DA. Origin and functional impact of dark noise in retinal cones. Neuron 26: 181–186, 2000 [DOI] [PubMed] [Google Scholar]
- Running Deer JL, Hurley JB, Yarfitz SL. G protein control of Drosophila photoreceptor phospholipase C. J Biol Chem 270: 12623–12628, 1995 [DOI] [PubMed] [Google Scholar]
- Schwarz EM, Benzer S. Calx, a Na-Ca exchanger gene of Drosophila melanogaster. Proc Natl Acad Sci USA 94: 10249–10254, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Becker A, Sun Y, Hardy R, Zuker C. Gq a protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron 15: 919–927, 1995 [DOI] [PubMed] [Google Scholar]
- Scott K, Sun YM, Beckingham K, Zuker CS. Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell 91: 375–383, 1997 [DOI] [PubMed] [Google Scholar]
- Shieh BH, Zhu MY. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron 16: 991–998, 1996 [DOI] [PubMed] [Google Scholar]
- Siekhaus DE, Drubin DG. Spontaneous receptor-independent heterotrimeric G-protein signalling in an RGS mutant. Nat Cell Biol 5: 231–235, 2003 [DOI] [PubMed] [Google Scholar]
- Song Z, Postma M, Billings SA, Coca D, Hardie RC, Juusola M. Stochastic, adaptive sampling of information by microvilli in fly photoreceptors. Curr Biol 22: 1371–1380, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell 6: 11–22, 2000 [DOI] [PubMed] [Google Scholar]
- Tsunoda S, Sierralta J, Sun YM, Bodner R, Suzuki E, Becker A, Socolich M, Zuker CS. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature 388: 243–249, 1997 [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Wasserman D, Wang X, Li R, Mills E, Elsaesser R, Li HS, Montell C. Dependence on a retinophilin/myosin complex for stability of PKC and INAD and termination of phototransduction. J Neurosci 30: 11337–11345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xu H, Oberwinkler J, Gu Y, Hardie RC, Montell C. Light activation, adaptation, and cell survival functions of the Na+/Ca2+ exchanger CalX. Neuron 45: 367–378, 2005 [DOI] [PubMed] [Google Scholar]
- Wes PD, Xu XZ, Li HS, Chien F, Doberstein SK, Montell C. Termination of phototransduction requires binding of the NINAC myosin III and the PDZ protein INAD. Nat Neurosci 2: 447–453, 1999 [DOI] [PubMed] [Google Scholar]
- Yau KW, Hardie RC. Phototransduction motifs and variations. Cell 139: 246–264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]