Abstract
Somatic sensory signals provide a major source of feedback to motor cortex. Changes in somatosensory systems after stroke or injury could profoundly influence brain computer interfaces (BCI) being developed to create new output signals from motor cortex activity patterns. We had the unique opportunity to study the responses of hand/arm area neurons in primary motor cortex to passive joint manipulation in a person with a long-standing brain stem stroke but intact sensory pathways. Neurons responded to passive manipulation of the contralateral shoulder, elbow, or wrist as predicted from prior studies of intact primates. Thus fundamental properties and organization were preserved despite arm/hand paralysis and damage to cortical outputs. The same neurons were engaged by attempted arm actions. These results indicate that intact sensory pathways retain the potential to influence primary motor cortex firing rates years after cortical outputs are interrupted and may contribute to online decoding of motor intentions for BCI applications.
Keywords: brain machine interfaces, decoding, stroke, plasticity
prior work has established that spiking activity driven by attempted movement remains in the primary motor cortex (M1) arm area after stroke or spinal cord injury (Donoghue et al. 2007; Hochberg et al. 2006, 2012; Kim et al. 2008, 2011; Simeral et al. 2011). In these studies, people with paralysis have been able to point and click using a computer cursor or move a robot arm based on spiking activity, providing proof of concept that M1 spiking could provide control signals for brain computer interface (BCI) applications. In the participants studied to date, those with spinal cord injury lacked somatic sensory feedback, whereas the participants with amyotrophic lateral sclerosis (ALS) and with brain stem stroke had intact somatic sensation, based on clinical assessment. Although similar cursor control has been accomplished across the participants, regardless of whether sensory feedback was intact, studies in animals have shown that changes in limb posture can influence the functional organization of M1 (Gellhorn and Hyde 1953; Graziano et al. 2004; Lemon 1981a; Sanes et al. 1992; Scott and Kalaska 1997). Furthermore, monkeys are better able to control the movement of cursors driven with M1 cortical activity when they are provided kinesthetic feedback that matches the movement of the cursor compared with a situation in which sensory feedback is not congruent with the intended movement (Suminski et al. 2010). These data suggest that sensory inputs to motor cortex could play an important role in augmenting BCIs (Hatsopoulos and Suminski 2011). Therefore, effective BCI design will require an understanding of the sensory influences on M1 of humans and their responses after diseases or damage to various neural pathways.
Single-unit studies in nonhuman primates have shown M1 receives abundant somatic sensory input (Albe-Fessard and Liebeskind 1966; Hore et al. 1976; Lemon and Porter 1976; Lucier et al. 1975; Picard and Smith 1992; Strick and Preston 1978a, 1978b; Tanji and Wise 1981; Wiesendanger 1973; Wong et al. 1978). In comparison, knowledge about the organization of somatic sensory inputs to human M1 or the response of this organization to injury is limited because the opportunity to study sensory responses at the single-unit level in humans has been rare (Goldring et al. 1970; Goldring and Ratcheson 1972).
The availability of a person with intact somatic sensation who was part of the BrainGate pilot clinical trial provided a specific opportunity to study sensory input to motor cortex. We examined the influence of passive joint motion on neural responses in M1 of a 55-yr-old woman with a chronically implanted 96-microelectrode array 12 yr after onset of tetraplegia following a brain stem stroke that left sensory pathways intact.
MATERIALS AND METHODS
The BrainGate Neural Interface System pilot clinical trial.
Data for this study were obtained with the assistance of a single participant in a pilot clinical trial studying the preliminary safety and feasibility of people with tetraplegia controlling external devices by imagining the movement of their own hand. The research is performed under an Investigational Device Exemption (IDE) granted by the U.S. Food and Drug Administration (CAUTION: Investigational Device limited by Federal Law to Investigational Use). The IDE for the pilot trial of the BrainGate Neural Interface System (BrainGate) system was previously held by Cyberkinetics Neurotechnology Systems, Inc. The IDE is now administered by Massachusetts General Hospital. The Institutional Review Boards of Rhode Island Hospital, Spaulding Rehabilitation Hospital, and Massachusetts General Hospital reviewed and approved the study for this participant.
Participant description.
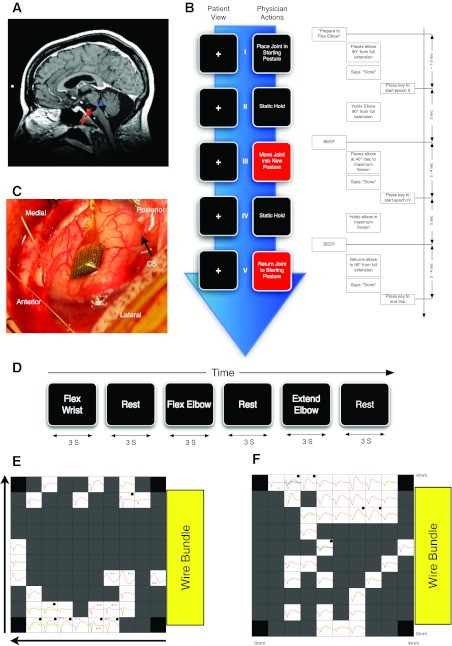
The subject of this study is a 52-yr-old (at time of implant in 2005) woman (BrainGate trial participant S3) who had developed locked-in syndrome secondary to bilateral pontine infarction (Fig. 1A, arrow). S3 initially had classic locked-in syndrome and subsequently recovered to regain full ocular and head movement, with good CN-VII and CN-VIII function but chronically impaired CN IX–XII function. S3 was enrolled in the pilot trial of the BrainGate Neural Interface System in November 2005, 9 yr after the pontine stroke. She is anarthric and functionally tetraplegic. However, she is able to generate biceps and triceps power through a limited range bilaterally over a period of several seconds upon attempt of any type of arm action. S3 has an intermittent, trace handgrip and no finger extension bilaterally, and marked spasticity of the biceps and triceps, moderate spasticity of the shoulder, and no spasticity at the wrist. She also demonstrates occasional (1 or 2 out of 5 power, MRC) knee extension and foot plantar flexion, but other leg movements are absent. Standard neurological clinical exam shows that S3 has normal sensation including kinesthetic sense in all four limbs. She communicates by assistive technology through a computer using a single head motion-activated switch that she also uses to control her powered wheelchair. When an assistant is available, she communicates by spelling words on an alphabet board using eye gaze. S3 was receiving regular physical therapy and intrathecal baclofen to control spasticity during two data collection periods in March 2007 and March 2008.
Fig. 1.
Methods. A: brain MRI before implantation showing location of lesion. The participant suffered a massive pontine stroke (red arrow) that affected most of the ventral descending tracts. The dorsal and lateral aspects of the pons where the spinal sensory pathways pass through that level of the pons were intact (blue arrowhead). B: timeline of 1 trial of the sensory manipulation task. The task was a block design with each block consisting of either 5 trials (session 1) or 10 trials (session 2) of 1 manipulation from the 15 different manipulations performed during the study. Arrow indicates passage of time. Left, the sequence of instructions relayed to the experimenter. Right, an exploded view of the timeline of 1 trial of elbow flexion block describing the auditory relay of instructions to the experimenter, the details of the experimenter performance of sensory manipulation, and the technician actions to mark the transitions between epochs of a trial. C: array placement during surgery (CS, central sulcus). D: details of attempted movement task as described in experimental procedures. E and F: maps of the average triggered waveforms on the array as viewed from above the cortical surface with the electrodes pointing down. Yellow box represents the position of the exit of the wire bundle. Horizontal arrow points in the anterior direction; vertical arrow points from lateral to medial. Each box represents the responses recorded from 1 electrode located at the corresponding spatial location. No electrodes were located at each of the 4 corners of the array, marked by black boxes. Dark gray boxes represent locations where an electrode was present but no waveforms could be isolated. On some channels, more than 1 unit was isolated and the average waveforms for those units are represented by different colors. All boxes have x-axis representing time with a range of 0 to 1.6 ms; the y-axis represents voltage. Two scales were used for the y-axis: boxes marked with a dot have a y-scale of ±100 μV; all other boxes have y-scales of ±50 μV. E shows data collected in session 1 (3/14/2007); in this session there were 3 cross-talk artifacts on channels labeled XT-1 through XT-3. F shows data collected in session 2 (3/19/2008).
Implant procedure and signal acquisition.
Description of the BrainGate sensor and the surgical implantation procedures for this trial are described in other reports (Hochberg et al. 2006). The sensor implanted in S3 consists of 100 1.5-mm-long silicon microelectrodes uniformly arranged in a 10 × 10 square array; the base platform supporting the electrodes is 4.2 × 4.2 mm (Guillory and Normann 1999). Ninety-six of these electrodes provide signals. At manufacture, electrodes had impedances of 310 ± 125 kΩ (mean ± SD). After the participant provided informed consent and underwent medical and surgical screening procedures, the sensor was implanted into the “knob” region of her left precentral gyrus (Yousry et al. 1997) with the use of a pneumatic inserter device (Hochberg et al. 2006; Rousche and Normann 1992; Suner et al. 2005). The knob region was targeted using preoperative MRI visualization and guidance (Fig. 1A). This region has been identified in able-bodied humans as the M1 hand/arm area using functional MRI and confirmed in two prior participants by neuronal ensemble activity patterns as a precentral locus of neurons engaged when upper limb actions are imagined or attempted (Hochberg et al. 2006). For data conditioning and processing, signals are relayed from the array by fine gold wires to an external titanium percutaneous connector affixed by titanium screws to the participant's skull at the time of implantation using standard neurosurgical procedures. Recordings of neural signals began in December 2005.
The data presented in this report derive from two sessions, the first on trial day 469 (March 2007) and the second on trial day 840 (March 2008). The two experiments were separated by 371 days. Analog recorded data were filtered between 0.3 Hz (1st-order Butterworth filter) and 7,500 Hz (3rd-order Butterworth filter), digitized at 30,000 samples/s, and recorded on a 128-channel Neural Signal Processing Assembly data acquisition system (Cyberkinetics, now BlackRock Microsystems).
Sensory task.
Responses of the M1 population were recorded during passive joint movement and during attempted actions of the same joints. Joints were moved passively by one individual while cued to move by another. The use of automated physical devices to activate joints or test receptive fields was outside the permitted scope of the research. A clinical trial technician initiated movement instructions to the clinical investigator through earphones so that instructions were not audible to the participant. A computer running a behavioral control program (TG2) delivered cues for joint movement type and timing and managed the flow of the task. The computer logged and signaled the start of each passive movement to the clinical investigator, and the technician logged the end of passive movement events on the data acquisition system when signaled. All behavioral events were digitally time-stamped on the same clock that digitized the neural signals for later temporal alignment of the data. Data were recorded both during the instructed passive movement, e.g., wrist flexion, and during the passive return of the limb to its original position.
The task was divided into blocks (Fig. 1B). One passive manipulation selected from Table 1, initially unknown to the participant, was executed during each block. In both experiments, movements began with shoulder joints and proceeded to move each joint in a proximal-to-distal order. A block consisted of either 5 trials (session 1) or 10 trials (session 2). All trials were divided into four behavioral epochs as described below and in Fig. 1. Throughout the trial, the participant was instructed to focus gaze on a crosshair displayed on a black background on a 17-in. LCD screen placed 50 cm from the participant's eyes. The monitor was positioned so that the participant's head was turned 120° from the shoulder ipsilateral to the manipulated joints. This prevented the participant from observing her own joint manipulations. The participant was specifically instructed not to imagine or attempt movement of her limbs. We used auditory cues delivered to the experimenter through headphones at a volume that could not be detected by the participant. Before the start of the first trial of each block, the experimenter was cued to prepare to perform the appropriate manipulation (e.g., elbow flexion) by positioning the appropriate joint (e.g., elbow) in a predefined position based on the residual range of motion of the participant (e.g., midway between flexion and extension). Exact starting location varied from joint to joint and from session to session due to the variability in the participant's limb spasticity. Before each session, the experimenter examined the participant and determined the joint start position for each movement. When the experimenter positioned the arm in the starting position, he verbally signaled the technician to start the next epoch of the trial. The first epoch was a static hold lasting 5 s. At the end of this epoch, an auditory cue (a 1-kHz sinusoid, 250 ms long) was delivered to the experimenter to start the passive manipulation. Note that the start of passive movement was delayed relative to the go cue by the intentionally slightly delayed reaction time of the experimenter to the auditory cue. In the second epoch, the experimenter moved the joint in the instructed direction as far as the participant's comfortable range of motion permitted, as determined by a physical examination before the experiment. Manipulation speed varied with level of spasticity of the joint and the range of passive movement. Angular manipulation speed varied between 5 and 20°/s. These angular speeds were estimated and not directly measured. The experimenter signaled the end of the epoch to the technician, who then marked it in the recorded data. The third epoch comprised another 5-s holding epoch (e.g., full elbow flexion) followed by another auditory cue signaling the start of the fourth epoch. During this last epoch, the experimenter returned the joint to the starting position and then signaled the technician to mark the end of the trial.
Table 1.
Response statistics to passive and attempted movement in both sessions during passive movement, static posture, and attempted movement
| Session 1 |
Session 2 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D |
S |
A |
D |
S |
A |
||||||||
| Joint | Movement | n | % | n | % | n | % | n | % | n | % | n | % |
| Right wrist | WF* | 13 | 37 | 5 | 14 | 7 | 20 | 14 | 26 | 4 | 8 | 19 | 36 |
| WE* | 10 | 29 | 7 | 20 | 4 | 11 | 14 | 26 | 8 | 15 | 17 | 32 | |
| WU* | 7 | 20 | 10 | 29 | 6 | 17 | 15 | 28 | 8 | 15 | 17 | 32 | |
| WR* | 4 | 11 | 7 | 20 | 14 | 40 | 4 | 8 | 3 | 6 | 22 | 42 | |
| WP | 11 | 31 | 8 | 23 | 10 | 19 | 5 | 9 | 17 | 32 | |||
| WS | 10 | 29 | 11 | 31 | 11 | 21 | 2 | 4 | 16 | 30 | |||
| Any wrist | 23 | 66 | 24 | 69 | 19 | 54 | 26 | 49 | 18 | 34 | 26 | 49 | |
| Right elbow | EE | 10 | 29 | 4 | 11 | 14 | 26 | 4 | 8 | 11 | 21 | ||
| EF* | 6 | 17 | 7 | 20 | 7 | 20 | 19 | 36 | 6 | 11 | 23 | 43 | |
| Any elbow | 12 | 34 | 9 | 26 | 7 | 20 | 23 | 43 | 9 | 17 | 24 | 45 | |
| Right shoulder | SF | 7 | 20 | 6 | 17 | 4 | 8 | 5 | 9 | 7 | 13 | ||
| SE | 3 | 9 | 6 | 17 | 11 | 21 | 2 | 4 | 4 | 8 | |||
| SD | 5 | 14 | 8 | 23 | 9 | 17 | 4 | 8 | 9 | 17 | |||
| SB | 2 | 6 | 10 | 29 | 4 | 8 | 6 | 11 | 7 | 13 | |||
| Any shoulder | 11 | 31 | 16 | 46 | 14 | 26 | 11 | 21 | 12 | 23 | |||
| Left wrist | LP | 7 | 20 | 7 | 13 | ||||||||
| Right knee | RK | 0 | 0 | 0 | 0 | ||||||||
| Left knee | LK | 0 | 0 | 0 | 0 | ||||||||
Values are numbers of units (n) and percentages of units responding in both sessions 1 and 2 during passive movement (D), static posture (S), and attempted movement (A). Right-side manipulations: WF, wrist flexion; WE, wrist extension; WU, wrist ulnar deviation; WR, wrist radial deviation; WP, wrist pronation; WS, wrist supination; EF, elbow flexion; EE, elbow extension; SF, shoulder flexion; SE, shoulder extension; SD, shoulder adduction; SB, shoulder abduction; and RK, right knee extension. Left-side manipulations (ipsilateral to array): LP, left wrist pronation; LK, left knee extension. In session 1, due to time constraints, 8 trials were presented of the 5 movements marked with an asterisk. In session 2, participants performed 10 trials of each movement.
Attempted movement task.
To compare attempted and passive movement responses for each neuron, S3 was instructed to attempt the various single joint movements listed in Table 1 in response to random cues displayed on a computer. The task was split into trials each consisting of two epochs (Fig. 1B). The epochs were announced by displaying text describing the desired action on a 17-in. LCD computer screen in white letters against a black background (20-point font, subtending an approximate visual angle of 2°). The first epoch was cued by the display of the word “rest” on the screen for 3 s. During this epoch, the participant was instructed to relax and focus on the word “rest” in the middle of the screen. At the end of the rest period, the word “rest” was replaced with a written description of a randomly chosen movement from Table 1 by the software. S3 was instructed to start attempting the described movement when the cue appeared and to continue attempting the movement until the word “rest” appeared again. The participant was instructed to “attempt moving the joint as instructed on the screen in one direction.” This “move” epoch lasted 3 s. The exact instruction given to the participant before the start of the experiment was “Please attempt to produce the movement indicated on the computer screen and continue to attempt the movement in the same direction as long as the instruction is displayed. When the word ‘rest’ appears please relax completely.” The participant was given a chance to practice a few trials of the task before starting the experiment. In session 1, due to time constraints, we presented eight trials of the five movements marked with an asterisk in Table 1. In session 2, we asked the participant to perform 10 trials of each movement in Table 1.
Cell sorting.
After digitization, analog data was high-pass filtered at 500 Hz with a 4th-order Butterworth filter to separate single- and multiunit spiking activity from lower frequency local field potential (LFP) activity. The signal on each channel was then thresholded with a subjectively determined level to allow separation of unit activity from background noise. Each threshold crossing on a channel triggered the acquisition of a 1.6-ms (48 samples) waveform. This procedure yielded a mixture of single neuronal units and multiunit waveforms on each channel. We examined these waveforms for possible cross talk between channels. In session 1, we identified cross talk between 9 channels using cross-correlation methods and rejected all but one of each set of the resultant redundant units. No cross talk was observed in session 2 because the channels that were showing cross talk in the first session did not record any units. The waveforms were then sorted with an automated offline-sorting program (Offline Sorter; Plexon).
Tuning to joint motion.
For each of the test trials listed in Table 1, we constructed the peristimulus time histograms (PSTH) using 50-ms bins aligned on onset of the passive manipulation cue. We compared the mean firing rate before and after this cue. We determined statistical significance using a nonparametric test random permutation (Good 2005). Under the null hypothesis that the unit's rate is the same before and after onset of passive movement, we expect that the observed difference of means should not be significantly different from a difference of mean rate, calculated around any other time point. We therefore randomize the event times and calculate the mean difference of firing rate around these random event times. We repeated this process 1,000 times to generate a distribution of possible difference of mean rates under the null hypothesis that events do not modulate the neuron activity. We tested the observed difference of means for significance against this distribution using an alpha level of 0.01. We elected to use this method instead of more standard statistics because it requires fewer assumptions about the underlying process. Most importantly, the two samples under comparison are not independent, because the prestimulus neural activity is produced by the same neuron producing the poststimulus activity. This makes the use of statistics that assume independence of samples less appropriate. Furthermore, with random permutation we test the specific hypothesis that the neural activity changed around the cue time and avoid the confounding effect of the properties of the neural activity generating process.
Tuning to static joint position.
To test the tuning to static joint position, we computed the number of spikes in a time window from 1 s after end of the passive movement until the end of hold epoch. Statistical significance was tested using random permutation of trial labels. We randomly permuted the trials among the possible joint positions and calculated a mean for each joint position, with 1,000 iterations as described above. We tested the observed mean number of spikes fired by a unit for each static joint position against the relevant random-permutation generated distribution using an alpha level of 0.01, corrected for multiple comparisons (Benjamini and Hochberg 1995).
Decoding.
We evaluated the information available from the engaged neural populations by testing whether a decoder built on neuronal ensemble activity was able to predict the passively moved or actively attempted actions. All decoding reported here used discrete classification by linear discriminant analysis (Hastie et al. 2001). To test the accuracy of the resulting classifiers, we used a standard “leave-one-out” cross-validation strategy. We remove one trial from the set and build a decoder using data from the remaining trials, and then we use the resulting decoder to classify the data from the left-out trial. Repeating this process for every trial in the database leads to a success rate (number of correctly classified trials divided by total number of trials) for the classifier. We tested the significance of the success rate using random permutation. We randomly shuffled the neural activity among trials (e.g., binned spikes from a wrist flexion condition were swapped with binned activity from elbow flexion). For each of the shuffled trials we computed the classification percentage using a leave-one-out strategy. We repeated the random shuffling 1,000 times to generate a distribution of expected classification percentage of random neural data. We tested the observed classification rate against this distribution at an alpha level of 0.01 All analysis was performed using Matlab (The MathWorks, Natick, MA).
RESULTS
Data summary.
The data sets used for these analyses comprised two sessions in participant S3 recorded from the array placed in the knob region of precentral gyrus, which corresponds to the anterior, surface-exposed arm/hand region of primary motor cortex (M1). Thirty-five units were recorded from 27 channels in session 1 and 53 units from 36 channels in session 2. Average waveforms of units after sorting and ranking are shown in Fig. 1, E and F, overlaid on a map of the electrode array to show their spatial organization. We cannot determine whether waveforms recorded in sessions 1 and 2 originated from the same cells, but the session interval of longer than 1 yr and sampling of a partially nonoverlapping set of channels (Fig. 1, E and F) suggest that substantial overlap is unlikely. Therefore, we treated the data from each session as separate and independent data sets.
Unit responses to passive movement.
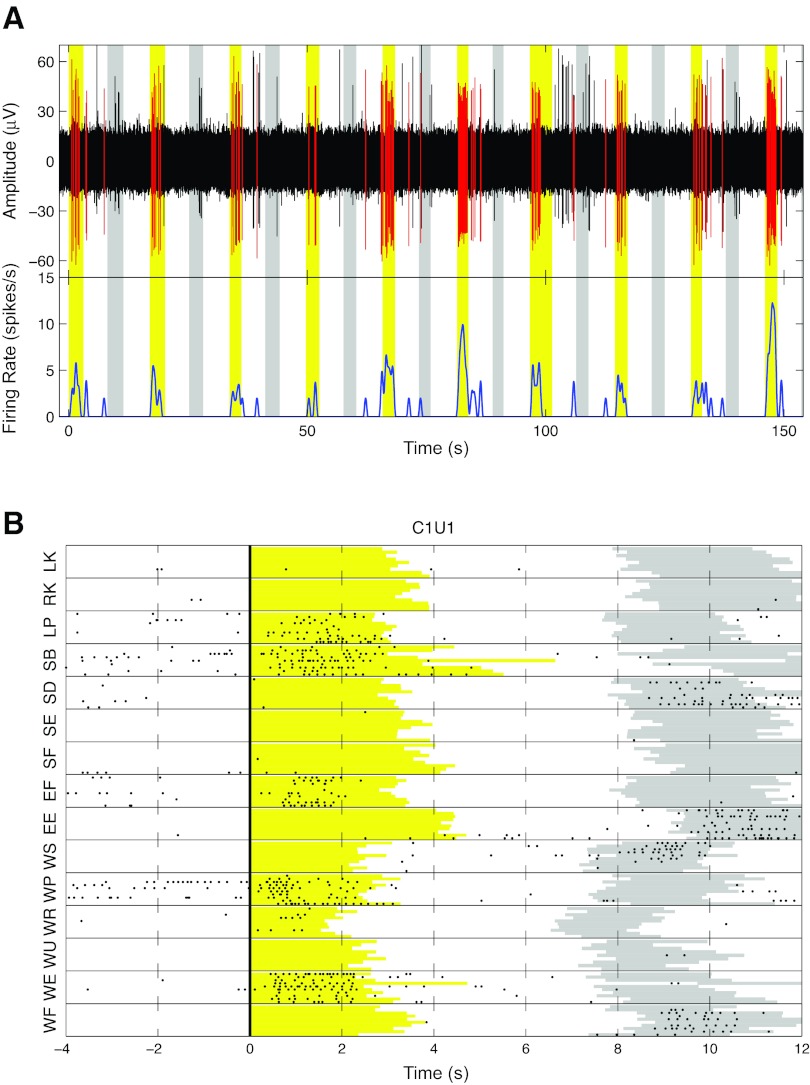
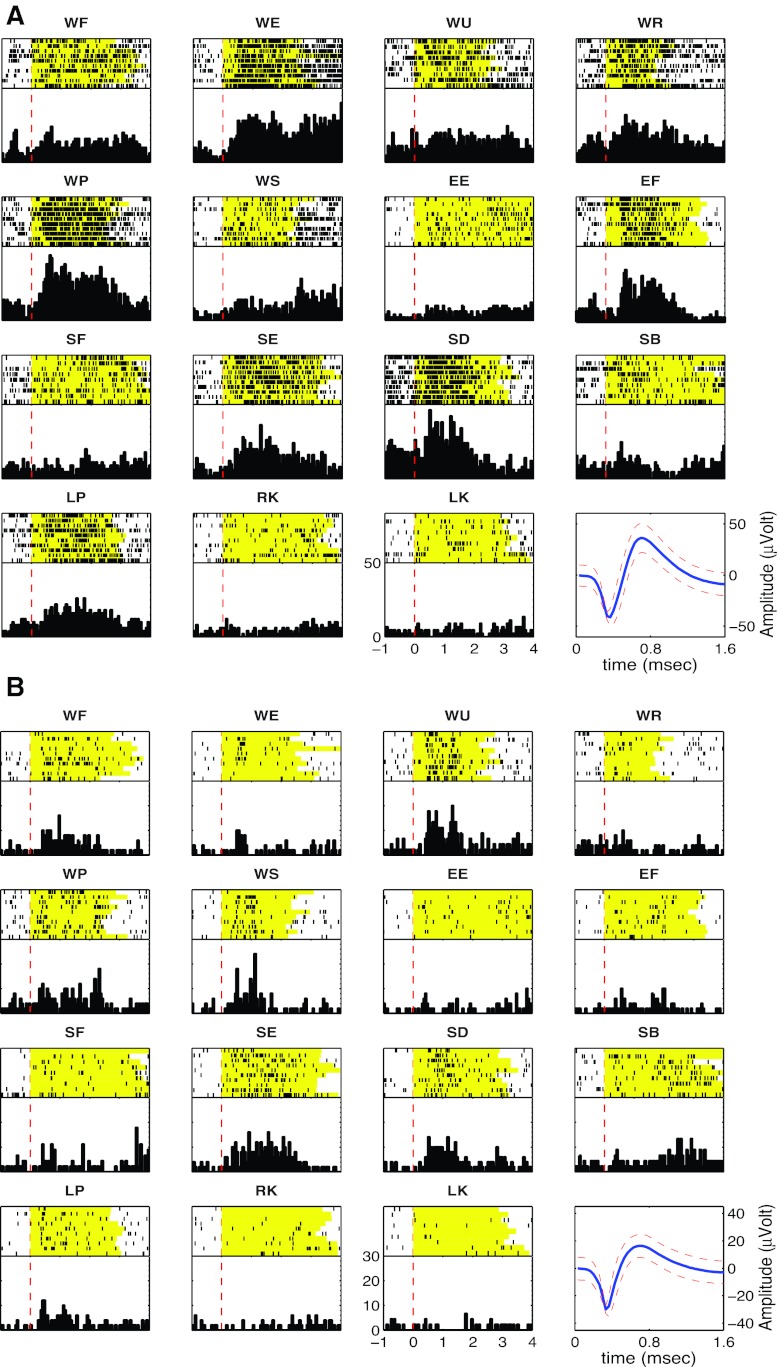
Units that responded reliably to passive joint motion were readily identified in both sessions. In both cases more than half of all units detected responded to joint movements. Of 35 units studied in session 1, 69% (n = 24) responded to passive motion of one or more joints, whereas in session 2, 58% (n = 31) of the 53 units responded to passive motion of one or more joints. Representative examples of unit responses are shown in Figs. 2 and 3. Figure 2 shows one example of activity from a well-isolated multijoint unit from session 2. This unit responded to passive wrist extension, pronation, elbow flexion, and shoulder abduction of the right arm. The cell was largely silent outside the passive movement phase. In addition, the unit fired during return epochs of the blocks of trials corresponding to wrist flexion, supination, elbow extension, and shoulder adduction (Fig. 2B). The unit was completely silent during the manipulation of either knee but weakly responded to left wrist pronation (ipsilateral to the recorded cortex), suggesting that responses were dominated by contralateral arm inputs. Two further examples of neurons, shown in Fig. 3, also responded to motion around multiple joints. The unit in Fig. 3A responded to passive motion about all contralateral (right) arm joints. Note that the increase in firing rate correlated with contralateral arm wrist extension and pronation, elbow flexion, and shoulder extension and adduction. The second unit (Fig. 3B) had a multijoint response field that spanned two joints, increasing its rate in response wrist flexion, ulnar deviation, pronation, and supination. It did not respond to any passive movement of the elbow but increased firing rate when the shoulder was extended or adducted.
Fig. 2.
Responses of 1 unit to passive joint manipulation. A, top: continuously recorded activity on 1 electrode filtered with a 4-pole Butterworth filter between 500 and 3,000 Hz to reveal spiking activity. Waveforms assigned to unit 1 on this channel are shown in red. Bottom, firing rate of the unit marked in red, calculated by convolving spike waveform with a hamming window 1 s long. Yellow rectangles mark the epochs during which the experimenter passively extended the wrist. Gray rectangles mark the epochs during which the joint was flexed back to its starting position. B: raster plot of the responses from the same unit. Each dot represents a detected action potential. Horizontal lines separate passive manipulation blocks. In each block a row represents 1 trial of the passive movement indicated on the vertical axis. All trials were aligned on the go cue delivered to the experimenter (thick vertical line). Yellow and gray regions represent movement epochs in the direction indicated on the vertical axis and back to starting position, respectively. Right-side manipulations: WF, wrist flexion; WE, wrist extension; WU, wrist ulnar deviation; WR, wrist radial deviation; WP, wrist pronation; WS, wrist supination; EF, elbow flexion; EE, elbow extension; SF, shoulder flexion; SE, shoulder extension; SD, shoulder adduction; SB, shoulder abduction; RK, right knee extension. Left-side manipulations (ipsilateral to array): LP, left wrist pronation; LK, left knee extension.
Fig. 3.
Example of rasters and peristimulus histograms (PSTH) from simultaneously recorded units with multiple joint responses to passive manipulation. The top part of each subpanel is a raster, with each row representing a trial and each dot representing a spike from the neuron. The yellow background indicates the duration of passive movement. Black dots represent discriminated spikes. All trials were centered at the time of auditory cue presentation to the experimenter (red dashed line). Bottom right subpanel shows the discriminated unit along with a confidence interval of 2 SD surrounding each amplitude time point of the waveform. A: unit (C8U1) shows responses to wrist, elbow, and shoulder. B: unit (C12U1) shows responses to wrist and shoulder only.
Qualitatively similar responses were routinely observed in the two sessions. Table 1 summarizes the number of responses to each of the 15 passive joint movements tested. In each session wrist responses were most prevalent, comprising more than half the cells that responded. In session 1, 66% (n = 23), 35% (n = 12), and 32% (n = 11) of units responded to wrist, elbow, and shoulder manipulation, respectively. In session 2, 50% (n = 26), 44% (n = 23), and 27% (n = 14) of units responded to wrist, elbow, and shoulder manipulation, respectively. Moreover, in session 1, only one unit had a response field that did not include the wrist. In session 2, 5 of the 31 responding units (16%) had response fields that did not include the wrist. Responses involving only one joint were rare. The majority of responding cells were modulated by more than one joint of the right arm. Sixty-three percent (n = 15) and 68% (n = 21) of units responded to passive movement of two or more joints in sessions 1 and 2, respectively (Table 2).
Table 2.
Unit receptive field size measured by the number of joints that elicited a positive unit response during passive joint movement
| 1 Joint |
2 Joints |
3 Joints |
None |
|||||
|---|---|---|---|---|---|---|---|---|
| Session No. | n | % | n | % | n | % | n | % |
| 1 | ||||||||
| Dynamic | 9 | 26 | 8 | 23 | 7 | 20 | 11 | 31 |
| Static | 11 | 31 | 7 | 20 | 8 | 23 | 9 | 26 |
| 2 | ||||||||
| Dynamic | 10 | 19 | 10 | 19 | 11 | 21 | 22 | 42 |
| Static | 19 | 36 | 5 | 9 | 3 | 6 | 26 | 49 |
Values are numbers (n) and percentages of units that responded positively during the dynamic and static epochs of passive movement.
Because sensory input from ipsilateral limbs has been reported in humans (Goldring and Ratcheson 1972) and monkeys M1 (Lemon and Porter 1976; Wong et al. 1978), we tested the ability to engage M1 with a few selected ipsilateral (left) limb movements. Time constraints did not allow us to test all ipsilateral arm or leg joints. No units in either session responded to passive ipsilateral wrist pronation exclusively. Twenty percent (n = 7) and 13% (n = 7) of units, in sessions 1 and 2, respectively, responded weakly, but significantly, to pronation of the ipsilateral wrist. In both sessions, all seven units that responded to ipsilateral wrist pronation also responded to passive right wrist manipulation. In contrast, we did not find any unit, from either session, that responded to passive extension of right or left knee, supportive of maintenance of general arm-leg M1 segregation in this individual.
Unit responses to static joint posture.
Postural effects were investigated by responses related to static limb position. Effects related to holding a new static position were defined as postural effects. To test unit responses to static joint posture, we measured spiking activity as the right arm was held for 5 s at the extremes of each passive movement about the wrist, elbow, and shoulder (except left wrist pronation and knee extension). Postural effects were measured by comparing firing rates during the 4-s period starting 1 s after the end of each passive movement, to allow for settling. Figure 4, A and B, shows examples of two units that responded to static joint position. The unit in Fig. 4A responded during the dynamic phase of elbow flexion and in the static flexed posture. In contrast, the unit in Fig. 4B showed mixed static joint effects in that it increased its firing rate to maintained shoulder abduction and wrist extension and decreased its rate when the elbow was held in a flexed posture. Note that this unit exhibited very different responses during the passive movement (yellow region in Fig. 4) and the static holding period (white region in Fig. 4) for each joint and movement direction. For example, the unit fired strongly to dynamic wrist pronation, but it was not significantly modulated during the static holding of wrist pronation. Rate increased during dynamic elbow flexion but then decreased during maintained elbow flexion. Increases also occurred during dynamic and static wrist extension, but only during static shoulder abduction. Table 1 summarizes all joint posture responses. Across the entire population tested, 74% (n = 26) and 51% (n = 27) of units responded to static position in sessions 1 and 2, respectively. Generally, the number of units responding during static posture was lower than during passive joint movement. For example, 26% (n = 14) of units responded to dynamic wrist flexion, whereas only 8% (n = 4) of units responded to static holding of full wrist flexion posture in session 2, and 37% (n = 13) and 14% (n = 5), respectively, in session 1. Similar to passive motion, the majority of units responded to static wrist position [session 1: 69% (n = 24), session 2: 34% (n = 18) of units]. In session 2, 36% (n = 19) of the units responded to specific static joint postures, whereas only 8% (n = 15) of units responded to more than one arm joint. However, we observed the reverse relationship in session 1, where more units responded to static position of more than one joint (43%, n = 15) than to holding only one joint in a static posture (31%, n = 11).
Fig. 4.
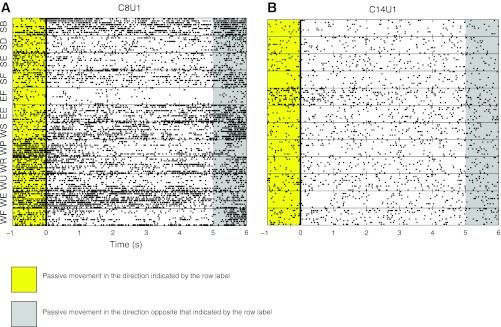
Example of units with static responses. Each horizontal block is a raster plot of neural activity from a block of 10 trials of the movement indicated on the y-axis. Each row in each block represents a passive movement trial. All rasters are centered on the time onset of the second hold epoch in which the joint was held at the extreme of the range of movement for that block. Yellow periods indicate passive movement in the direction indicated on the vertical axis. Gray periods indicate passive movement in the opposite direction. For example, in the WF block, the joint was flexed during the yellow period, held fully flexed during the following 5-s period (white background) that starts at the solid black line at time 0, and then returned to a neutral position during the gray period. A: C8U1 is an example of a unit with multijoint receptive fields. B: C14U1 had a single-joint receptive field. Both units were recorded in session 2.
Unit responses to attempted movement.
M1 neurons firing upon request to attempt specific actions were regularly encountered, as previously reported for this participant and others with long-standing tetraplegia (Hochberg et al. 2006, 2012; Kim et al. 2008; Simeral et al. 2011). Figure 5 shows examples of these volitional activation responses (black) and their passive movement responses (green). All of these sensory units responded to at least one attempted joint movement. Note that all of these units showed reproducible firing rate modulation from trial to trial despite each trial being intermingled among 12 distinct requested actions cued in random order. Table 2 summarizes the unit responses during attempted movement (column titled M). More than half of the recorded units in session 1 (54%, n = 19) and session 2 (53%, n = 28) responded to at least one instructed attempted movement. Of those, a larger percentage responded to attempted wrist movement (54 and 49% in sessions 1 and 2, respectively), and fewer responded to the elbow (20 and 45% in the respective session) and shoulder (23% for session 2, not tested in session 1; see Table 1 for details). The majority of these units responded to attempted movement about more than one joint. In session 2, 9% (n = 5) of units only responded to attempted movement around a single joint, but 43% (n = 23) responded to two or three joints. In session 1, we only tested attempted movement responses related to two joints. In this set more units responded to attempted movement of either elbow or wrist joint (34%, n = 12) compared with the number of units that responded to attempted movement of both wrist and elbow joints (20%, n = 7).
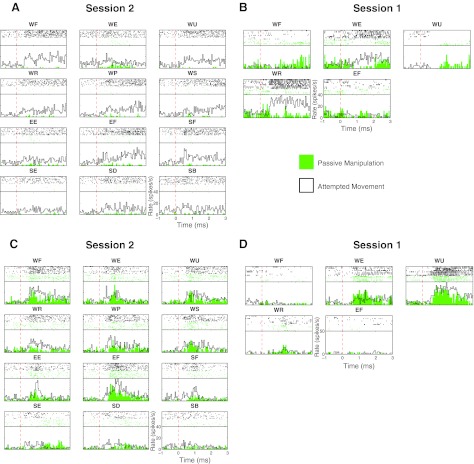
Fig. 5.
Comparison of sensory and attempted movement responses. PSTH and rasters were calculated from passive joint manipulations (green) and attempted movement (black outline with no fill) of the same joint and same direction of the passive manipulation. The movement direction is indicated above each raster and PSTH. A: responses of a unit in session 2 (C42U1) that was unresponsive during passive manipulation but responded strongly to attempted movement. Each subpanel shows the result of 10 trials of each attempted and passive movement. B: responses from a unit (C75U1) in session 1 showing differential responses to passive and attempted movement. In this session only 5 movements were attempted, and there were only 5 trials of each passive movement and 8 trials of each attempted movements. C: responses of a unit in session 2 (C13U2). D: responses from a unit in session 1 (C83U2). Both the units in C and D show very similar responses to passive and attempted movement.
Comparing attempted and passive movement responses.
We observed a range of relationships between the responses of the units to passive and attempted movements. Figure 5, A and B, shows two examples of units with different relationships between their response to attempted movement and passive manipulation. Figure 5A illustrates a unit that increased its firing rate mainly during attempted movement. This unit was modulated by attempted wrist flexion, ulnar and radial deviation, pronation, supination, elbow flexion, and shoulder flexion. However, during passive manipulation (green) modulation was limited to wrist extension. The unit in Fig. 5B, recorded in session 1, was only tested for response to five attempted movements. It decreased firing during passive (P) wrist extension and radial deviation but increased its rate when movements were attempted (A, active condition). Neurons exhibiting this response pattern, termed A-opp-P (i.e., active response is the opposite of passive, consistent with stretching the muscle engaged during A) are common in nonhuman primates (Evarts 1973; Evarts and Tanji 1976). By contrast, only 8 of the 316 responses (3%) were A-opp-P, whereas A-same-P were common (Fig. 5, C and D).
To quantify the joint similarity of responses, we classified units on the basis of congruency of receptive fields in each condition. Because the participant attempted all 12 attempted movements in the second session only, we restricted this analysis to that session. As summarized in Table 3, 39 (74%) of all recorded units responded with at least one movement during either passive or attempted movement. Among these 39 units, those responding to A and P were about evenly split with those responding during A or P (51 vs. 49%). To further quantify these relationships, we used the following classes: 1) congruent units responded to P and A exclusively around the same joints; 2) congruent-plus units responded to A of all joints to which it responded during P, in addition to responding during A around joints to which it did not respond during P; 3) congruent-minus units responded with A for a subset of the joints it responded to during P; and 4) noncongruent units had entirely different P and A responses. This last category includes units that respond in one condition but not the other. Among the group of 39 cells responsive in any way, roughly equal numbers were of one of the congruent classes or noncongruent (37 vs. 36%), with the congruent units similarly distributed (13, 15, and 9% in the congruent, congruent-plus, and congruent-minus classes). Finally, 26% (n = 14) of the units did not respond to either passive or attempted movement.
Table 3.
Congruency of fields between attempted and passive movements
| Attempted Movement |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| W | E | S | W,E | W,S | E,S | W,E,S | None | Total | |
| Passive movement | |||||||||
| W | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 6 |
| E | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 4 |
| S | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| W,E | 1 | 1 | 0 | 3 | 0 | 0 | 0 | 2 | 7 |
| W,S | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| E,S | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| W,E,S | 0 | 0 | 0 | 6 | 0 | 0 | 4 | 1 | 11 |
| None | 2 | 0 | 1 | 2 | 0 | 0 | 3 | 14 | 22 |
| Total | 3 | 1 | 1 | 12 | 0 | 0 | 11 | 25 | 53 |
Rows correspond to the joints being passively moved and columns correspond to the joint the participant was instructed to attempt moving. W, wrist; E, elbow; S, shoulder. Values indicate the number of units that showed responses to passive and attempted movement of the joints indicated in the corresponding row and column, respectively.
Decoding attempted and passive movement responses.
We tested whether the population activity could distinguish actions about different joints or different actions about the same joint using discrete offline classification. Using unit activity, we attempted to classify the passive or attempted movements during the following three experimental epochs: 1) dynamic, representing activity from 1 to 3 s after the start of passive movement cue; 2) static, representing the activity of units during the static hold period from 1 to 4 s after the end of the dynamic passive movements; and 3) attempted movement, representing the activity from 0 to 3 s after delivery of the attempted movement cue to the participant. In sessions 1 and 2, during the dynamic epoch, 85 and 79.8% of passive movements, respectively, were classified accurately (chance = 8.5%). Classification of static posture was less accurate, reaching 45 and 44.5% accuracy in sessions 1 and 2, respectively (chance = 8.5%). For attempted movement in session 1, where we tested only 5 attempted movements (4 wrist and 1 elbow), decoding accuracy was 82.5% (chance = 20%). In session 2, with 12 tested movements, decoding accuracy was 55% (chance = 8.5%). These results indicate that small, local M1 populations contain significant information about the entire limb posture, as well as intended actions.
Decoding also provides a direct test of whether the information available during attempted actions is the same as during passive actions. That is, if the activity were the same, a decoder generated from the activity in one condition would accurately predict activity during the other condition. For this analysis we used activity from all units recorded in the session to calibrate the decoder. Classification rate of attempted movements using a decoder calibrated with neural data recorded during passive movement was not significant at 8% (95% CI = 3–15%). Similarly, the decoder trained using the data collected during attempted movement was not successful at classification of the passive movements (classification rate = 17%, 95% CI = 8–25%). The inability to predict active movement attempts from passive manipulation is evidence that these two conditions produce different spatiotemporal activation patterns.
DISCUSSION
We have demonstrated for the first time, in a person with tetraplegia secondary to long-standing bilateral pontine stroke, that local populations of neurons in the arm region of M1 retain substantial sensory responses related to both joint position and joint angle changes. Responses from wrist, elbow, and shoulder joints were encountered within this local population, with inputs from the contralateral limb predominating. Decoding methods showed that the ensemble contained considerable information about the configuration of the entire limb that was different from the activity pattern engaged when limb motion was attempted. These observations were made twice in two separate experimental sessions that occurred 1 yr apart. Although the data in this study are limited by being acquired from one subject, the results provide unique evidence that the human M1 has passive and active proprioceptive sensory responses closely related to motor output, and that these topographically appropriate responses are retained years after M1 is largely disconnected from bulbar and spinal targets. These observations have important implications for the understanding of cortical plasticity in humans, for the design of BCIs where sensation remains, and potentially for stroke rehabilitation strategies.
Precentral responses to passive manipulation.
Our results reveal abundant sensory responses in human M1 when the arm is moved or held statically at a specific position, despite a stroke that severely damaged corticospinal axons in the pons. Properties of the sensory responses and their topographic arrangement in this participant with a stroke are remarkably similar to those previously observed for the arm M1 of able-bodied monkeys (Fetz et al. 1980; Fromm et al. 1984; Hore et al. 1976; Lemon 1981b; Lemon and Porter 1976; Picard and Smith 1992; Rosén and Asanuma 1972; Strick and Preston 1978b; Wise and Tanji 1981; Wong et al. 1978) and humans (Goldring et al. 1970; Goldring and Ratcheson 1972), suggesting that there has been no major reorganization in response to the stroke.
One consistent finding in both sessions is that wrist joint manipulation dominated the response types observed. In sessions 1 and 2, 96% and 84%, respectively, of the total population of neurons that were activated by sensory input included the wrist. One explanation for this dominance could be based on the participant's different level of spasticity around different joints. In contrast to other joints, the wrist joint showed no clinically appreciable spasticity, and this was reflected in the speed of the passive movement of this joint compared with other movements (compare the length of movement period in yellow in Figs. 2 and 3). Experimental evidence suggest that increase in primary muscle spindle discharge is correlated with muscle stretch (Matthews 1972), and hence it is possible that we were able to encounter more cells that responded to passive wrist movement. Alternatively, this bias toward wrist could indicate that the 4 × 4-mm array was placed in the wrist representation of M1. However, most of the responsive neurons were also activated by shoulder or elbow motions, consistent with the highly intermingled joint movement relationships of neurons in M1 in able-bodied monkeys (Vargas-Irwin et al. 2010).
We found a few (13 and 20% in sessions 1 and 2, respectively) neurons that were activated by the ipsilateral wrist. Our results could easily underestimate the total number of units with bilateral inputs because we did not explore all joints of the ipsilateral arm. Ipsilateral sensory fields have been encountered in M1 of nonparalyzed humans (Goldring et al. 1970; Goldring and Ratcheson 1972) and intact nonhuman primates (Lemon and Porter 1976; Wong et al. 1978) at about the same prevalence as encountered in this study, suggesting that able-bodied human and nonhuman primates have similar patterns of sensory input organization and that these responses are not the result of the lesion.
We also observed responses to static limb configuration in this participant. However, more units in this participant responded to passive limb movements rather than static position. Decoding results show that the population of units in both sessions contained more information about the dynamic portion of passive movement than the static portion. Our results agree with results from able-bodied monkey studies that show that static responses are less frequently seen in M1 than dynamic responses (Fetz et al. 1980; Fromm et al. 1984; Wise and Tanji 1981).
In this study, most M1 neurons we encountered had peripheral fields that responded to passive movement of more than one upper limb joint. We found that ∼40% of passively driven neurons responded to two or three joints. This is in contrast to the observations on neurons recorded from nonhuman primate M1 where the majority of units reportedly responded to passive movement of a single joint. Previous work in able-bodied monkeys suggested that about 15–30% of M1 units respond to multiple joints (Fetz et al. 1980; Lemon and Porter 1976; Wong et al. 1978). Lemon et al. (1981b) reported that 35% of neurons encountered in one penetration responded to input from multiple joints, suggesting that our results might be attributed to unit intermingling on the recording of one electrode. However, approximately the same proportion of multijoint input (45%) was evident when we repeated the analysis using only well-isolated single units. In addition, there was no relationship between the number of units on a channel and the number of joints to which they responded, lending further evidence that the observed propensity of multijoint cells is not the result of collection artifact. This difference between our observation and those in intact nonhuman primates suggests that whereas the gross functional organization of the M1 is retained, convergence of multiple joints onto single motor cortex cells might be a result of postlesion reorganization. On the other hand, recent data utilizing arm perturbation in monkeys suggest that M1 neurons receiving information about applied torque across different joints are more common than those that receive information from single joints (Herter et al. 2009; Pruszynski et al. 2011). Whether our results are due to pathological changes or are indeed inherent to the natural function of the neurons will require further study with additional participants.
Relationship between passive and attempted movement.
We directly compared sensory responses with modulation associated with instructed attempted actions. As in our previous work (Hochberg et al. 2006, 2012; Kim et al. 2008; Simeral et al. 2011; Truccolo et al. 2008), we demonstrated that intention or attempting to move was sufficient to activate neurons in M1 years after tetraplegia, in this case following brain stem stroke. We confirmed that responses are largely topographically appropriate in that leg responses were not evident, although because of time limitations and participant endurance, we limited our evaluation to the knee, so other joints might have activated our population. It is noteworthy that our participant showed changes in arm posture (due to changes in spastic tone) during testing. We believe that sensory responses are not a result of these movements for several reasons. First, movements observed were infrequent, bilateral, slow, and stereotyped, whereas we found joint-specific actions with weak and restricted ipsilateral influence. Second, uncorrelated random arm movements alone could not explain the patterns and diversity of the observed responses. However, further studies with clinical trial participants who lack significant motor tone (e.g., people with advanced ALS) may provide better answers to whether the responses to attempted movements are unrelated to actual motor output.
Passive movement and attempted movement fields generally overlapped in our participant, as found in able-bodied primates (Fetz et al. 1980; Goldring and Ratcheson 1972; Lemon et al. 1976; Lemon 1981a; Murphy et al. 1978; Rosén and Asanuma 1972; Strick and Preston 1982; Wise and Tanji 1981). These studies have described units for which passive and active responses were opposite (A-opp-P cells). This pattern, found commonly in monkeys, is consistent with an arrangement that would provide feedback of length/velocity changes to neurons engaged in contracting muscles acting about a particular joint. This observation supports the concept of a “transcortical reflex” (Phillips 1969) action for M1. Whereas A-opp-P neurons are common in M1 of nonhuman primates (Cheney and Fetz 1984; Evarts 1973; Evarts and Tanji 1976; Fetz et al. 1980; Murphy et al. 1978; Wolpaw 1979, 1980), we only found such a relationship in 8 of the 316 responses (3%). In fact, we were not able to identify a consistent relationship between the responses of the cells to passive manipulation and attempted movement. Such complex relationships have been reported (Fetz et al. 1980; Lemon et al. 1976; Lemon 1981a; Murphy et al. 1978; Rosén and Asanuma 1972), albeit less frequently. These differences between our observations and those of previous studies may emerge from experimental conditions (spastic paralysis, long-standing central nervous system insult vs. able-bodied monkeys, imagined vs. electrically stimulated or actual movement), the presence of agents affecting muscle tone (intrathecal baclofen), or a true difference between humans and monkeys. Further study of the M1 organization of additional participants will help further clarify this point. Our study provides a baseline for comparison and approach for forthcoming investigation.
Spatial maps of passive and attempted movements.
We did not observe any spatial structure of response fields. Sensory and motor responses related to different and multiple limb joints of the arm were intermingled and nontopographically organized, at least within the small (4 × 4 mm) cortical area examined presently. Our results indicate that the hand area of the M1 as mapped in studies on intact human (Yousry et al. 1997) retains its gross somatotopic topography relative to other major parts of the body (leg) after pontine stroke and long-standing tetraplegia. In this participant, the M1 arm area responded to arm joint sensory inputs and showed intended arm movement correlates years after paralysis onset, with no apparent reorganization. These observations are in disagreement with findings from studies using both central and peripheral lesions demonstrating M1 representational plasticity (Donoghue and Sanes 1988; Donoghue et al. 1990; Nudo et al. 1996, 2001; Sanes et al. 1990, 1992; Sanes and Donoghue 2000).
The reason for this lack of change is not clear, but it is consistent with the ability for attempted arm actions to activate M1 arm area neurons among clinical trial participants with a variety of neurological impairments including cervical spinal cord injury, pontine infarction such as that of the currently reported participant, or advanced amyotrophic lateral sclerosis. We have not examined sensory responses in any other participant, noting that arm sensory responses were not possible in the participants with complete spinal cord injury.
Influence of sensory inputs on neural interface design.
Our results showed that decoders built using data recorded in response to passive manipulation cannot be used to decode neural responses to attempted movements. If this participant's data are representative of the able-bodied human, they suggest that somatic sensory and motor representations show complex functional interrelationships. Understanding the transformation between these two is likely to reveal mechanisms of cortical sensorimotor computation.
Furthermore, our results showed that precentral neurons changed their firing pattern as a function of posture (Fig. 4). These results, taken together, suggest that a BCI depending on a decoder generated for one limb position may be less effective if the limb position is changed. Indeed, in rats and monkeys, prior studies observed that change in limb configuration can induce immediate and delayed changes in the motor responses induced by stimulation of M1 (Gellhorn and Hyde 1953; Graziano et al. 2004; Sanes et al. 1992). Studies in humans have shown that transcranial magnetic stimulation (TMS) delivered during different arm configuration evoked different EMG patterns in the distal arm (Graziano et al. 2004; Swayne et al. 2006). In monkeys, it was shown that providing kinesthetic feedback that correlated with the visual feedback from the movement of a cursor driven by motor cortical activity resulted in improved performance of the BCI (Suminski et al. 2010). Therefore, BCIs designed to be used in humans with residual sensory feedback would appear to need to account for postural driven effects for people with motor disabilities who have intact kinesthetic sense. It may be useful to incorporate kinesthetic feedback to improve performance.
The population of potential intracortical BCI users who have sensory feedback include people with ALS, incomplete spinal cord injuries, or stroke with spared ascending tracts similar to the participant discussed in this study. Although changes in limb position affect M1, this participant has used closed-loop decoders built early in sessions to reliably perform point-and-click actions over the full session (Hochberg et al. 2012; Kim et al. 2008; Simeral et al. 2011). In these studies limb position was not monitored, so its effect on control could not be evaluated, but these new data suggest that there could be an important effect, particularly if there were no limb movements during the original filter building for that day. It is also important to note that the presence of such robust sensory feedback to the same neurons that participate in intracortically based BCI motor control essentially provides more information to filter building and subsequent real-time decoding. We expect that this feature will be useful for the anticipated direct cortical control of functional electrical stimulation systems (FES) (Chadwick et al. 2011; Peckham and Knutson 2005).
GRANTS
This work is supported by the Rehabilitation Research and Development Service, Office of Research and Development, Department of Veterans Affairs. Additional support is provided by National Institutes of Health Grants N01 HD-53403, RC1 HD-063931, R01 DC-009899, R01 EB-007401, and NS-25074; National Center for Research Resources Grant C06 16549-01A1; the Doris Duke Charitable Foundation; the Massachusetts General Hospital-Deane Institute of Integrated Research on Atrial Fibrillation and Stroke; and the Katie Samson Foundation.
DISCLOSURES
The pilot clinical trial from which these data are derived was sponsored in part by Cyberkinetics Neurotechnology Systems, Inc. J. P. Donoghue is a former Chief Scientific Officer and director of Cyberkinetics Neurotechnology Systems, Inc. (CKI); he held stocks and received compensation. L. R. Hochberg received research support from Massachusetts General and Spaulding Rehabilitation Hospitals, which in turn received clinical trial support from CKI. A. Shaikhouni was a contracted consultant for CKI and received compensation. CKI ceased operations in 2009.
DISCLAIMER
The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
AUTHOR CONTRIBUTIONS
Author contributions: A.S., J.P.D., and L.R.H. conception and design of research; A.S. performed experiments; A.S. analyzed data; A.S. interpreted results of experiments; A.S. prepared figures; A.S. drafted manuscript; A.S., J.P.D., and L.R.H. edited and revised manuscript; A.S., J.P.D., and L.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank participant S3 for her dedication to this research. We thank K. Centrella and G. Friehs for significant contributions to this research.
REFERENCES
- Albe-Fessard D, Liebeskind J. Origine des messages somatosensitifs activant les cellules du cortex moteur chez le singe. Exp Brain Res 1: 127–146, 1966 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 57: 289–289, 1995 [Google Scholar]
- Chadwick EK, Blana D, Simeral JD, Lambrecht J, Kim SP, Cornwell AS, Taylor DM, Hochberg LR, Donoghue JP, Kirsch RF. Continuous neuronal ensemble control of simulated arm reaching by a human with tetraplegia. J Neural Eng 8: 034003, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol 349: 249–272, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Nurmikko A, Black M, Hochberg LR. Assistive technology and robotic control using motor cortex ensemble-based neural interface systems in humans with tetraplegia. J Physiol 579: 603–611, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN. Organization of adult motor cortex representation patterns following neonatal forelimb nerve injury in rats. J Neurosci 8: 3221–3232, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Suner S, Sanes JN. Dynamic organization of primary motor cortex output to target muscles in adult rats. II. Rapid reorganization following motor nerve lesions. Exp Brain Res 79: 492–503, 1990 [DOI] [PubMed] [Google Scholar]
- Evarts EV. Motor cortex reflexes associated with learned movement. Science 179: 501–503, 1973 [DOI] [PubMed] [Google Scholar]
- Evarts EV, Tanji J. Reflex and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol 39: 1069–1080, 1976 [DOI] [PubMed] [Google Scholar]
- Fetz EE, Finocchio DV, Baker MA, Soso MJ. Sensory and motor responses of precentral cortex cells during comparable passive and active joint movements. J Neurophysiol 43: 1070–1089, 1980 [DOI] [PubMed] [Google Scholar]
- Fromm C, Wise SP, Evarts EV. Sensory response properties of pyramidal tract neurons in the precentral motor cortex and postcentral gyrus of the rhesus monkey. Exp Brain Res 54: 177–185, 1984 [DOI] [PubMed] [Google Scholar]
- Gellhorn E, Hyde J. Influence of proprioception on map of cortical responses. J Physiol 122: 371–385, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring S, Aras E, Weber PC. Comparative study of sensory input to motor cortex in animals and man. Electroencephalogr Clin Neurophysiol 29: 537–550, 1970 [DOI] [PubMed] [Google Scholar]
- Goldring S, Ratcheson R. Human motor cortex: sensory input data from single neuron recordings. Science 175: 1493–1495, 1972 [DOI] [PubMed] [Google Scholar]
- Good PI. Permutation, Parametric and Bootstrap Tests of Hypotheses. New York: Springer, 2005 [Google Scholar]
- Graziano MS, Patel KT, Taylor CS. Mapping from motor cortex to biceps and triceps altered by elbow angle. J Neurophysiol 92: 395–407, 2004 [DOI] [PubMed] [Google Scholar]
- Guillory KS, Normann RA. A 100-channel system for real time detection and storage of extracellular spike waveforms. J Neurosci Methods 91: 21–29, 1999 [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction: with 200 Full-Color Illustrations. New York: Springer, 2001 [Google Scholar]
- Hatsopoulos NG, Suminski AJ. Sensing with the motor cortex. Neuron 72: 477–487, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter TM, Korbel T, Scott SH. Comparison of neural responses in primary motor cortex to transient and continuous loads during posture. J Neurophysiol 101: 150–163, 2009 [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485: 372–375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442: 164–171, 2006 [DOI] [PubMed] [Google Scholar]
- Hore J, Preston JB, Cheney PD. Responses of cortical neurons (areas 3a and 4) to ramp stretch of hindlimb muscles in the baboon. J Neurophysiol 39: 484–500, 1976 [DOI] [PubMed] [Google Scholar]
- Kim SP, Simeral JD, Hochberg LR, Donoghue JP, Black MJ. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J Neural Eng 5: 455–476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SP, Simeral JD, Hochberg LR, Donoghue JP, Friehs GM, Black MJ. Point-and-click cursor control with an intracortical neural interface system by humans with tetraplegia. IEEE Trans Neural Syst Rehabil Eng 19: 193–203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. J Physiol 311: 497–519, 1981a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Variety of functional organization within the monkey motor cortex. J Physiol 311: 521–540, 1981b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Hanby JA, Porter R. Relationship between the activity of precentral neurones during active and passive movements in conscious monkeys. Proc R Soc Lond B Biol Sci 194: 341–373, 1976 [DOI] [PubMed] [Google Scholar]
- Lemon RN, Porter R. Afferent input to movement-related precentral neurones in conscious monkeys. Proc R Soc Lond B Biol Sci 194: 313–339, 1976 [DOI] [PubMed] [Google Scholar]
- Lucier GE, Rüegg DC, Wiesendanger M. Responses of neurones in motor cortex and in area 3A to controlled stretches of forelimb muscles in cebus monkeys. J Physiol 251: 833–853, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Mammalian Muscle Receptors and Their Central Actions. London: Edward Arnold, 1972 [Google Scholar]
- Murphy JT, Kwan HC, MacKay WA, Wong YC. Spatial organization of precentral cortex in awake primates. III. Input-output coupling. J Neurophysiol 41: 1132–1139, 1978 [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785–807, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve 24: 1000–1019, 2001 [DOI] [PubMed] [Google Scholar]
- Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng 7: 327–360, 2005 [DOI] [PubMed] [Google Scholar]
- Phillips CG. The Ferrier lecture, 1968. Motor apparatus of the baboon's hand. Proc R Soc Lond B Biol Sci 173: 141–174, 1969 [DOI] [PubMed] [Google Scholar]
- Picard N, Smith AM. Primary motor cortical activity related to the weight and texture of grasped objects in the monkey. J Neurophysiol 68: 1867–1881, 1992 [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature 478: 387–390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén I, Asanuma H. Peripheral afferent inputs to the forelimb area of the monkey motor cortex: input-output relations. Exp Brain Res 14: 257–273, 1972 [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann Biomed Eng 20: 413–422, 1992 [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci 23: 393–415, 2000 [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp Brain Res 79: 479–491, 1990 [DOI] [PubMed] [Google Scholar]
- Sanes JN, Wang J, Donoghue JP. Immediate and delayed changes of rat motor cortical output representation with new forelimb configurations. Cereb Cortex 2: 141–152, 1992 [DOI] [PubMed] [Google Scholar]
- Scott SH, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. I. Activity of individual cells in motor cortex. J Neurophysiol 77: 826–852, 1997 [DOI] [PubMed] [Google Scholar]
- Simeral JD, Kim SP, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J Neural Eng 8: 025027, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Preston JB. Multiple representation in the primate motor cortex. Brain Res 154: 366–370, 1978a [DOI] [PubMed] [Google Scholar]
- Strick PL, Preston JB. Sorting of somatosensory afferent information in primate motor cortex. Brain Res 156: 364–368, 1978b [DOI] [PubMed] [Google Scholar]
- Strick PL, Preston JB. Two representations of the hand in area 4 of a primate. II. Somatosensory input organization. J Neurophysiol 48: 150–159, 1982 [DOI] [PubMed] [Google Scholar]
- Suminski AJ, Tkach DC, Fagg AH, Hatsopoulos NG. Incorporating feedback from multiple sensory modalities enhances brain-machine interface control. J Neurosci 30: 16777–16787, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans Neural Syst Rehabil Eng 13: 524–541, 2005 [DOI] [PubMed] [Google Scholar]
- Swayne O, Rothwell J, Rosenkranz K. Transcallosal sensorimotor integration: effects of sensory input on cortical projections to the contralateral hand. Clin Neurophysiol 117: 855–863, 2006 [DOI] [PubMed] [Google Scholar]
- Tanji J, Wise SP. Submodality distribution in sensorimotor cortex of the unanesthetized monkey. J Neurophysiol 45: 467–481, 1981 [DOI] [PubMed] [Google Scholar]
- Truccolo W, Friehs GM, Donoghue JP, Hochberg LR. Primary motor cortex tuning to intended movement kinematics in humans with tetraplegia. J Neurosci 28: 1163–1178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Irwin CE, Shakhnarovich G, Yadollahpour P, Mislow JM, Black MJ, Donoghue JP. Decoding complete reach and grasp actions from local primary motor cortex populations. J Neurosci 30: 9659–9669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesendanger M. Input from muscle and cutaneous nerves of the hand and forearm to neurones of the precentral gyrus of baboons and monkeys. J Physiol 228: 203–219, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Tanji J. Neuronal responses in sensorimotor cortex to ramp displacements and maintained positions imposed on hindlimb of the unanesthetized monkey. J Neurophysiol 45: 482–500, 1981 [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Electromagnetic muscle stretch strongly excites sensorimotor cortex neurons in behaving primates. Science 203: 465–467, 1979 [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Correlations between task-related activity and responses to perturbation in primate sensorimotor cortex. J Neurophysiol 44: 1122–1138, 1980 [DOI] [PubMed] [Google Scholar]
- Wong YC, Kwan HC, MacKay WA, Murphy JT. Spatial organization of precentral cortex in awake primates. I. Somatosensory inputs. J Neurophysiol 41: 1107–1119, 1978 [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120: 141–157, 1997 [DOI] [PubMed] [Google Scholar]