Abstract
The responsiveness of sensory neurons to muscle metabolites is altered under the conditions of insufficient limb blood supply in some diseases, such as peripheral artery disease. The purpose of this study was to examine ATP-induced current with activation of purinergic P2X subtypes P2X3 and P2X2/3 in dorsal root ganglion (DRG) neurons of control limbs and limbs with 24 h of femoral artery occlusion using whole cell patch-clamp methods. Also, dual-labeling immunohistochemistry was employed to determine existence of P2X3 expression in DRG neurons of thin-fiber afferents. DRG neurons from 4- to 6-wk-old rats were labeled by injecting the fluorescence tracer DiI into the hindlimb muscles 4–5 days before the recording experiments. Transient (P2X3), mixed (P2X3 and P2X2/3), and sustained (P2X2/3) current responses to α,β-methylene ATP (a P2X receptor agonist) are observed in small and medium DRG neurons, and size distribution of DRG neurons is similar in control and occluded limbs. However, the peak current amplitude of DRG neuron induced by stimulation of P2X3 and/or P2X2/3 is larger in occluded limbs than that in control limbs. Moreover, the percentage of DRG neurons with P2X3 transient currents is greater after arterial occlusion compared with control. In addition, a rapid desensitization was observed in DRG neurons with transient currents, but not with sustained currents in control and occluded groups. Furthermore, results from immunofluorescence experiments show that femoral artery occlusion primarily augments P2X3 expression within DRG neurons projecting C-fiber afferents. Overall, these findings suggest that 1) greater ATP-induced currents with activation of P2X3 and P2X2/3 are developed when hindlimb arterial blood supply is deficient under ischemic conditions and 2) increased P2X3 expression is largely observed in C-fibers of DRG neurons after hindlimb vascular insufficiency.
Keywords: muscle afferent nerve, P2X, peripheral arterial disease, hindlimb ischemia
the thin-fiber/group iii and iv afferent nerves arising from contracting skeletal muscle contribute to sympathetic nerve activity (SNA) and cardiovascular responses during exercise via a reflex neural mechanism, termed “exercise pressor reflex” (Kaufman et al. 1983, 1984a, 1984b; Mitchell et al. 1983). Exercise induces the production of muscles-metabolites, such as ATP, in the activated muscle (Kaufman et al. 1996; Kaufman and Hayes 2002; Sinoway and Li 2005). These metabolites are released in the interstitial space of muscles and stimulate group III and IV muscle afferents, the free endings of which situate in the interstitium (Kaufman et al. 1996; Kaufman and Hayes 2002; Sinoway and Li 2005). Metabolite-sensitive receptors, including ATP-sensitive purinergic P2X, located on the muscle afferent nerves are excited by those muscle by-products (Hanna et al. 2002; Hanna and Kaufman 2003, 2004; Kindig et al. 2006, 2007; Li and Sinoway 2002). Through these actions, cardiovascular nuclei in the brain stem are activated, the sympathetic nervous system is activated, blood pressure (BP) and heart rate (HR) are further increased, and the exercise pressor reflex is evoked (Kaufman et al. 1996; Sinoway and Li 2005). These reflex mechanisms that process muscle afferent signals via sensory nerve receptors are altered when limb blood supply is insufficient in some cardiovascular diseases (Gao et al. 2007; Sinoway and Li 2005; Smith et al. 2006; Tsuchimochi et al. 2010; Xing et al. 2008a).
A restriction of lower limb blood flow is commonly observed in peripheral arterial disease (PAD) (Critchley and Capewell 2003; Muir 2009; Ouriel 2001). The most common symptom of this disease is intermittent claudication, which frequently occurs during physical activity but is relieved promptly by rest (Rejeski et al. 2008). When the exercise pressor reflex is activated in patients with PAD, increases in SNA, BP, and HR are exaggerated (Baccelli et al. 1999; Bakke et al. 2007). A rat model of femoral artery ligation has been employed to study PAD in humans (Waters et al. 2004). A prior study has used this model to demonstrate that the BP response to static muscle contraction is amplified in occluded rats compared with control rats (Tsuchimochi et al. 2010).
ATP is increased in exercising muscle and/or moderately ischemic tissues (Hellsten et al. 1998; Li et al. 2003; Mo and Ballard 2001). Additionally, injecting α,β-methylene ATP (α,β-meATP, a receptor agonist to P2X) into the arterial blood supply of hindlimb muscles to stimulate P2X of muscle afferent nerves increases SNA and BP to a greater degree in occluded rats (Liu et al. 2011). Notably, P2X3 expression is upregulated in dorsal root ganglion (DRG) neurons innervating the hindlimb muscles with the occluded femoral artery (Liu et al. 2011). Note that among P2X subtypes, P2X3 and P2X2/3 are found predominantly on DRG neurons (Cook et al. 1997; Grubb et al. 1999; Lewis et al. 1995; Ueno et al. 1999) and likely play a key role in regulating sympathetic and cardiovascular responses to stimulation of metabolically sensitive muscle afferent nerves. Nevertheless, the precise mechanisms by which P2X is engaged in the exaggerated SNA and BP responses in rats with the hindlimb ischemia are unclear. Therefore, in this report, whole cell patch-clamp methods were employed to characterize ATP-induced current responses with activation of P2X3 and P2X2/3 in DRG neurons of control limbs and limbs whose femoral artery was ligated for 24 h. Also, P2X3 and P2X2/3 receptor desensitizations were examined to study whether femoral occlusion likely affects mechanisms of neuronal transmission. In addition, dual immunofluorescence techniques were employed to examine localization of P2X3 within DRG neurons with two different types of fibers, A- and C-fibers (representing mechanically and metabolically sensitive group III/IV, respectively) in control limbs and occluded limbs. It should be noted that group III/IV afferent fibers are physiological designations based on conduction velocity, for which there is no availability of immunohistochemical marker. The hypothesis was that femoral occlusion amplifies amplitude of ATP-induced currents and increases expression of P2X3 within DRG neurons of C-fibers.
METHODS
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Pennsylvania State College of Medicine and complied with the National Institutes of Health guidelines.
Labeling DRG neurons innervating hindlimb muscle.
Male Sprague-Dawley rats (4–6 wk old) were anesthetized by inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen). The skin was incised and pulled away from underlying muscle tissue, and the fluorescent retrograde tracer DiI (60 mg/ml) was injected into the white portion of the gastrocnemius muscle (Xing et al. 2008a, 2008b). The injection volume of 1 μl was administered, and injection was repeated three times at different locations. The injection needle was left in the muscle for 5–10 min to prevent leakage of tracer. The skin overlying the muscle was then sutured. The animals were returned to their cages for 4–5 days to permit the retrograde tracer to be transported to DRG neurons. At the end of each experiment, the gastrocnemius muscle was dissected, frozen, and sectioned to confirm that DiI was located in the white portion of the gastrocnemius muscle. Note that data were included only if injections were located within the white portion of the muscle.
Ligation of the femoral artery.
At 24 h before the recording experiments, the rats that previously received DiI injections were anesthetized with an isoflurane-oxygen mixture. The femoral artery on one limb was then surgically exposed, dissected, and ligated ∼3 mm distal to the inguinal ligament as previously described (Liu et al. 2010; Xing et al. 2008a). The same procedures were performed on the other limb, except that a suture was placed below the femoral artery but was not tied; this served as the control.
Examination of DRG neurons responsiveness.
The rats were anesthetized with an isoflurane oxygen mixture followed by cervical dislocation and decapitation. The L4–L6 DRGs were quickly removed and transferred immediately into Dulbecco's modified Eagle's medium (DMEM). The DRGs were minced, and the ganglion fragments were processed to obtain dissociated DRG neurons as described previously (Xing et al. 2008a, 2008b). The cell suspension was centrifuged to remove the supernatant, and the cell pellet was resuspended in DMEM. The cells were then plated onto a 35-mm culture dish containing precoated coverslips.
Next, patch recordings were performed within 6 h after dissociation (Xing et al. 2008a, 2008b). Neurons were first visualized using a combination of epifluorescent illumination and differential interference contrast (DIC; ×20–40) optics on an inverted microscope (Nikon TE2000). Under DIC, images of DiI-positive neurons were displayed on a video monitor. Neurons were patched in the whole cell configuration and recorded at a holding potential of −70 mV using a MultiClamp 700B amplifier. Seals (1–10 GΩ) between the glass electrode (resistance 2–5 MΩ) and the cell were established in a modified Tyrode solution (Xing et al. 2008a, 2008b). After the whole cell configuration was established, the cell membrane capacitance and series resistance were electronically compensated. All experiments were then conducted. Signals were acquired using pClamp 9.0 software, and experimental data were analyzed using the Clampfit software program. Neurons were considered ATP-sensitive if α,β-meATP solution elicited an inward current of >50 pA in peak amplitude.
Drugs stored in stock solutions were diluted in extracellular solution immediately before being used and were held in a series of independent syringes connected to corresponding fused silica columns (inner diameter 200 μm) (Xing et al. 2008a, 2008b). The ends of the parallel columns were connected to a common silica column. The distance from the column mouth to the examined cell was 100 μm. Cells in the recording chamber were continuously bathed in Tyrode solution. The gravity-fed solutions containing each drug were delivered to the cells by controlling the corresponding valve switch (WP Instruments).
A total of 124 DRG neurons for control and 153 DRG neurons for 24 h of femoral artery ligation were included in this study. All DRG neurons used in this report were DiI positive. Diameters of all neurons recorded were <40 μm (small and medium size).
Immunohistochemistry.
The rats were anesthetized with an isoflurane-oxygen mixture and then transcardially perfused with 200 ml of ice-cold saline containing 1,000 units of heparin followed by 500 ml of 4% freshly prepared ice-cold paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4). L4–L6 DRGs of control and occluded limbs were immediately dissected out and immersed in the same fixative at 4°C for 2 h. The tissues were then stored in PBS containing 30% sucrose overnight, and a cryostat was used to obtain DRG sections (10 μm).
DRG sections on slides were fixed in 4% paraformaldehyde in PBS for 10 min at room temperature. After being washed with PBS, the tissue was permeabilized, blocked in 0.3% Triton X-100 in PBS supplemented with 5% goat serum for 1 h, and then incubated with the rabbit polyclonal anti-P2X3 primary antibody (1:100; Neuromics) overnight at 4°C. After being washed in PBS, the sections were incubated with the goat anti-rabbit fluorescein isothiocyanate (FITC)-labeled secondary antibody (1: 200; Invitrogen Molecular Probes) for 2 h at room temperature.
To examine P2X3 localization within C- and A-fiber DRG neurons, sections were incubated with the second primary antibody (mouse anti-peripherin at 1:200, Sigma; or anti-NF200 at 1:200, Abcam) overnight. Peripherin and NF200 are used to label neurons with C- and A-fibers, respectively. The sections were then washed in PBS and incubated for 2 h at room temperature with a secondary antibody (Alexa Fluor 594-conjugated goat anti-mouse IgG at 1:200). After that, the sections were washed in PBS and coverslipped.
FITC- and Alexa Fluor 594-labeled DRG neurons were examined using a Nikon Eclipse 80i microscope with appropriate filters, and the images were stored digitally on a computer. As described previously (Liu et al. 2010), at least five sections containing L4–L6 DRGs per rat were randomly chosen, coded, and examined for FITC and Alexa Fluor 594 staining intensity in a blinded fashion. A threshold value of staining intensity was set according to the mean staining intensity of background using the Nikon Nis-Elements software. Cells with labeling >1.75 times background intensity were considered positive. The number of total P2X3 and peripherin/NF200-positive neurons was counted in each section. Percentages of double (FITC and peripherin/NF200)-labeled neurons were calculated: (total number of double-labeled cells × 100)/(total number of peripherin/NF200-positive cells). The majority of DRG neurons showed a clear nucleus and perimeter, and they were counted. To minimize the possibility of counting a single neuron more than once, DRG sections were collected on five glass slides in series, and only one of the slides was assessed for immunocytochemical analysis.
Statistical analysis.
Experimental data were analyzed using two-way repeated-measures analysis of variance (ANOVA). As appropriate, Tukey's post hoc tests were used. All values are means ± SE. For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed using SPSS for Windows version 15.0.
RESULTS
Characteristics of P2X currents in DRG neurons.
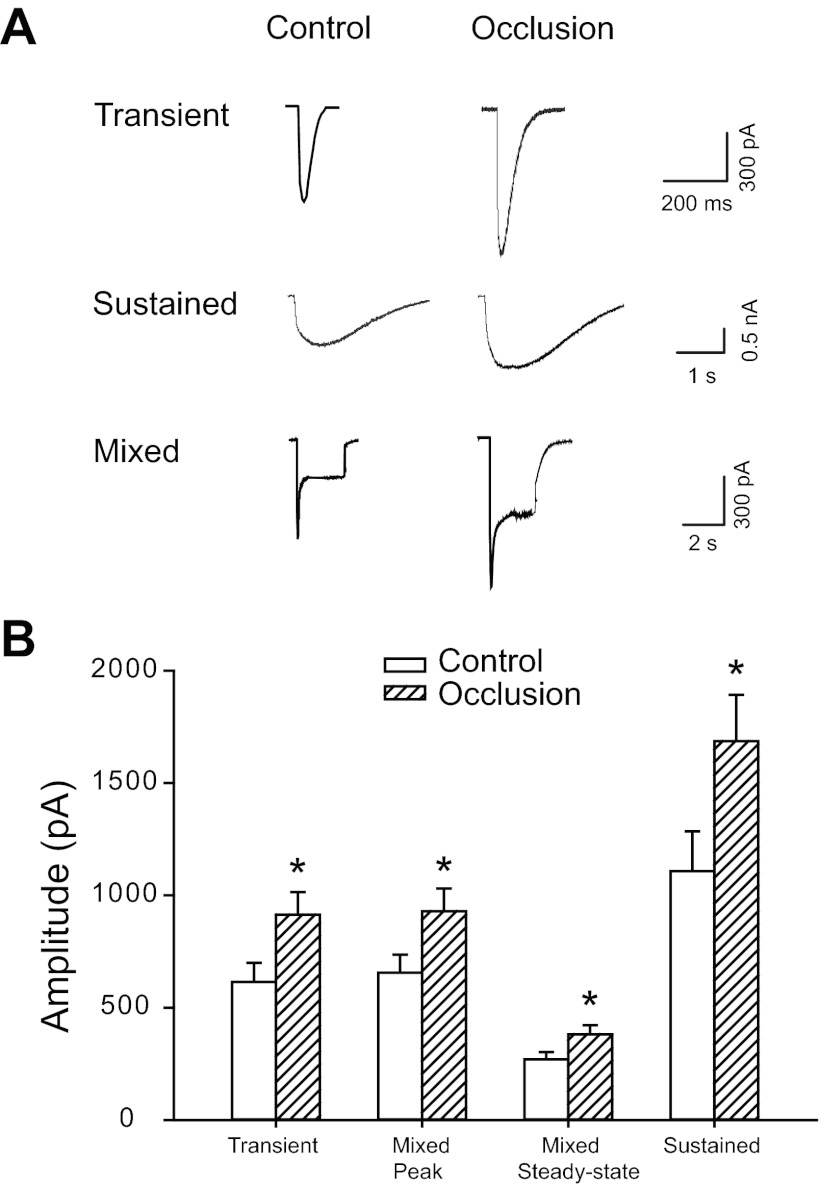
Three distinct types of inward currents were elicited when 30 μM α,β-meATP was applied onto DRG neurons (Fig. 1A). The first type was a transient current that had a short rise time and a rapid inactivating rate (Fig. 1A, top). The rise times of transient currents were 6.4 ± 0.8 ms in control (n = 30) and 6.2 ± 0.7 ms in occlusion (n = 52, P > 0.05 vs. control; shown in Table 1). The decay kinetics of the transient currents was best fitted with a sum of two exponentials with two different decay times, τ1d and τ2d. τ1d was 42.3 ± 5.4 ms in control and 40.2 ± 6.1 ms in occlusion (P > 0.05 vs. control). τ2d was 298.2 ± 69.0 ms in control and 326.1 ± 77.3 ms in occlusion (P > 0.05 vs. control). The second type was a sustained inward current that reached a peak slowly and desensitized slowly (Fig. 1A, middle). Rise times of sustained currents were 68.6 ± 8.5 ms in control (n = 29) and 71.7 ± 10.0 ms in occlusion (n = 41, P > 0.05 vs. control; shown in Table 1). Single-exponential curve fitting was applied to the inactivation phase of the sustained currents with monoexponential decay time, τd. τd was 2,582.7 ± 374.6 ms in control and 2,791.0 ± 478.3 ms in occlusion (P > 0.05 vs. control). The third type of response showed a mixed current that exhibited a fast response followed by a steady-state response (Fig. 1A, bottom). Like the transient current, the mixed responses were characterized by a rapid rise time and a distinct two-phase decay time. The fast inactivation phase had a time constant similar to τ1d of the transient current. However, the slow inactivation phase had a time constant that was similar to the feature of the slow α,β-meATP responses. Note that 24 h of femoral artery ligation did not change the kinetics of the currents in DRG neurons innervating muscle evoked by α,β-meATP.
Fig. 1.
Whole cell patch-clamp methods were employed to study effects of 24-h femoral arterial occlusion on P2X currents induced by α,β-methylene ATP (α,β-meATP). A: original traces showing currents elicited by 30 μM α,β-meATP in dorsal root ganglion (DRG) neurons of control limbs and limbs with femoral artery ligation. Three different P2X currents were observed in both the control and occlusion groups, including the fast transient (top), sustained (middle), and mixed currents (bottom). The amplitudes of the 3 types of currents in occlusion limbs were much larger than those obtained in control limbs. B: averaged data show mean amplitudes of currents activated by α,β-meATP. Twenty-four hours of arterial occlusion significantly increased amplitudes of transient currents, both peak and steady-state of mixed currents, and sustained currents compared with control. *P < 0.05 vs. control.
Table 1.
Comparison of kinetics of transient and sustained currents evoked by α,β-meATP in DRG neurons from control limbs and limbs with femoral artery occlusion
| Transient |
Sustained |
||||
|---|---|---|---|---|---|
| Group | Tr, ms | τ1d, ms | τ2d, ms | Tr, ms | τd, ms |
| Control | 6.4 ± 0.8 | 42.3 ± 5.4 | 298.2 ± 69.0 | 68.6 ± 8.5 | 2,582.7 ± 374.6 |
| Occlusion | 6.2 ± 0.7 | 40.2 ± 6.1 | 326.1 ± 77.3 | 71.7 ± 10.0 | 2,791.0 ± 478.3 |
Data are means ± SE for rise time (Tr) and decay time constants (τd); n = no. of neurons. Kinetics were measured in control group dorsal root ganglion (DRG) neurons with transient currents (n = 30) and with sustained currents (n = 29) and in occlusion group neurons with transient currents (n = 52) and with sustained currents (n = 41) evoked by α,β-methylene ATP (α,β-meATP). No significant differences were seen in Tr and τd values between the control and occlusion groups (P > 0.05).
DRG neurons from both control and occluded limbs exhibited all three types of responses. However, the amplitudes were different (Fig. 1, A and B). Peak amplitudes of all three types of inward currents responsive to α,β-meATP were significantly greater in DRG neurons of ligated limbs than in those of control limbs; i.e., transient currents: 614.1 ± 85.0 pA in control (n = 30) vs. 913.2 ± 101.4 pA in occlusion (n = 52, P < 0.05 vs. control); peak of mixed currents: 656.2 ± 79.9 pA in control (n = 34) vs. 929.0 ± 101.2 pA in occlusion (n = 46, P < 0.05 vs. control); steady-state of mixed currents: 270.0 ± 31.8 pA in control (n = 34) vs. 380.9 ± 41.1 pA in occlusion (n = 46, P < 0.05 vs. control); and sustained currents: 1,106.9 ± 177.6 pA in control (n = 29) vs. 1,685.5 ± 206.5 pA in occlusion (n = 41, P < 0.05 vs. control).
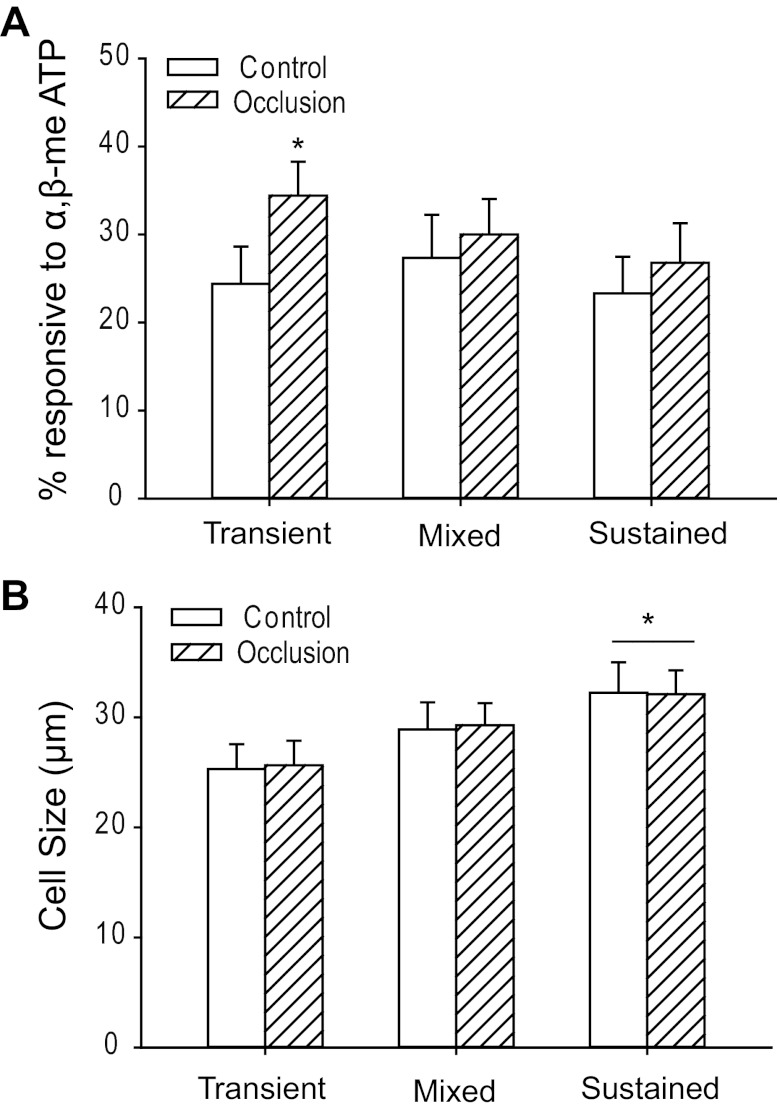
Overall, the percentage of the total number of DRG neurons responsive to α,β-meATP was 75.0% in control limbs and 91.2% in occluded limbs. Specifically, the percentage of DRG neurons with transient currents responding to α,β-meATP was significantly larger in ligated limbs (34.4 ± 3.8%) than in control limbs (24.4 ± 4.2%, P < 0.05 vs. control; shown in Fig. 2A). However, as shown in Fig. 2A, there were no significant differences between control and occlusion groups in percentage of DRG neurons with mixed (27.3 ± 4.9% in control to 30.0 ± 4.0% in occlusion, P > 0.05 vs. control) and sustained currents (23.3 ± 4.2% in control to 26.8 ± 4.5% in occlusion, P > 0.05 vs. control) in response to α,β-meATP.
Fig. 2.
Percentage and size distribution of DRG neurons responding to α,β-meATP. A: percentage of DRG neurons innervating muscle with 3 different types of currents responsive to α,β-meATP in control limbs and limbs with occlusion. A larger percentage of DRG neurons with transient currents was observed after 24 h of arterial occlusion than in control. *P < 0.05 vs. control. The percentages of cells with mixed currents (P = 0.99, control vs. occlusion) and sustained currents (P = 0.98, control vs. occlusion) were not significantly changed by 24-h femoral occlusion. B: diameters of DRG neurons innervating muscle responding to α,β-meATP in control limbs and limbs with occlusion. Averaged data show mean diameters of cells with fast-inactivating currents were significantly smaller than those of cells with sustained currents in both control and occlusion groups. *P < 0.05, transient vs. sustained currents for both control and occlusion groups. There were no significant differences of cell size of DRG neurons between the groups with transient currents and mixed currents (P = 0.48 in control; P = 0.23 in occlusion) and between the groups with mixed currents and sustained currents (P = 0.58 in control; P = 0.57 in occlusion). No differences were observed for the average size of DRG neurons that responded to α,β-meATP with fast, mixed, or sustained currents between both control and occlusion groups.
In addition, diameters of DRG neurons innervating muscle responding to α,β-meATP with different types of currents were examined in control limbs and limbs with femoral occlusion (Fig. 2B). Overall, no differences were seen in average diameter of DRG neurons that responded to α,β-meATP with fast, mixed, or sustained currents between control and occlusion groups. Averaged data further showed that mean diameters of cells with fast-inactivating currents were much smaller than those of cells with sustained currents [in control: 25.3 ± 2.2 μm with transient currents (n = 30) to 32.2 ± 2.8 μm with sustained currents (n = 29, P < 0.05 vs. transient); in occlusion: 25.6 ± 2.2 μm with transient currents (n = 52) to 32.1 ± 2.2 μm with sustained currents (n = 41, P < 0.05 vs. transient)]. There were no significant differences in cell size of DRG neurons between the groups with transient currents and with mixed currents [in control: 25.3 ± 2.2 μm with transient currents (n = 30) to 28.9 ± 2.5 μm with mixed currents (n = 34, P > 0.05 vs. transient); in occlusion: 25.6 ± 2.2 μm with transient currents (n = 52) to 29.3 ± 2.0 μm with mixed currents (n = 46, P > 0.05 vs. transient)]. Likewise, no significant differences were observed in cell size of DRG neurons between the groups with mixed currents and with sustained currents [in control: 28.9 ± 2.5 μm with mixed currents (n = 34) to 32.2 ± 2.8 μm with sustained currents (n = 29, P > 0.05 vs. mixed currents); in occlusion: 29.3 ± 2.0 μm with mixed currents (n = 46) to 32.1 ± 2.2 μm with sustained currents (n = 41, P > 0.05 vs. mixed currents)].
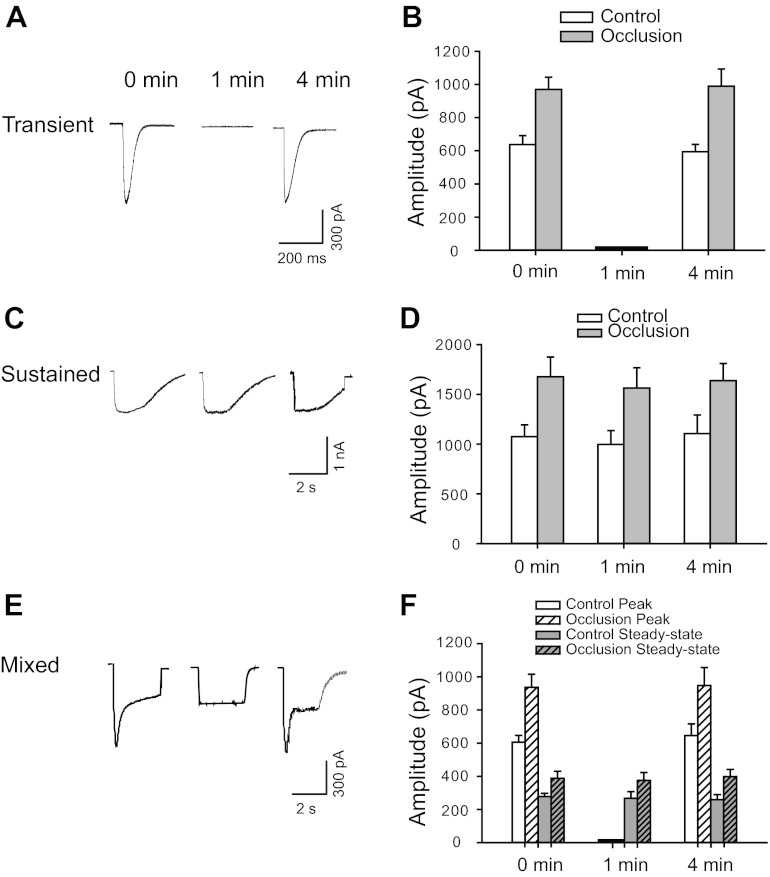
Moreover, desensitization of ATP-induced currents in DRG neurons innervating muscle was examined (Fig. 3). Original traces obtained from DRG neurons of control group were presented to show characteristics of three types of currents activated by the application of α,β-meATP at different time intervals (Fig. 3, A, C, and E). Also, representative traces and averaged data from control limbs and occluded limbs showed that transient current desensitized rapidly (Fig. 3, A and B). Small or no second responses were observed when α,β-meATP was reapplied within 1 min following the first application. The original peak amplitude would be restored only at intervals longer than 4 min between consecutive α,β-meATP applications. Unlike the transient responses, the sustained current recovered relatively quickly, could be evoked repeatedly, and reached its original amplitude at 1-min intervals (Fig. 3, C and D). In the neurons exhibiting mixed responses, the peak component returned to its original amplitude 4 min after the first application of α,β-meATP, which was similar to the transient response (Fig. 3, E and F). The steady-state component of mixed currents demonstrated behavior similar to that of pure sustained currents (Fig. 3, E and F). As shown in Fig. 3, B, D, and F, 24 h of arterial occlusion did not change the desensitizing properties of the currents elicited by α,β-meATP.
Fig. 3.
Desensitization of ATP-induced currents in DRG neurons innervating muscle. Original traces from control group (A, C, and E) and averaged data (B, D, and F) from control and occlusion groups show currents of DRG neurons activated by application of 30 μM α,β-meATP at different time intervals. A and B: transient current desensitized rapidly. The original peak amplitude was restored at intervals longer than 4 min between consecutive α,β-meATP applications (P = 0.73 in control and P = 0.76 in occlusion between 0 and 4 min). C and D: sustained current was elicited repeatedly and reached its original amplitude at 1-min intervals (P = 0.56 in control and P = 0.63 in occlusion among intervals). E and F: in the cells exhibiting mixed responses, the peak component exhibited desensitization characteristics similar to those of transient currents. The steady-state component of mixed currents demonstrated behavior similar to that of pure sustained currents. Overall, no significant difference was observed in the desensitizing property of the currents elicited by α,β-meATP between control and 24 h of arterial occlusion.
Immunolabeling of P2X3 within DRG neurons with C- and A-fibers.
In this experiment, we further determined if P2X3 exists within DRG neurons that project C- and A-fiber afferents. Dual immunofluorescence techniques were used to examine colocalization of fluorescent P2X3 and peripherin/NF200 immunoreactivity in DRG neurons from control limbs and occluded limbs (Figs. 4 and 5). The appearance of P2X3 and peripherin/NF200 within DRG neurons is characterized by fluorescent green and red color, respectively (Figs. 4 and 5).
Fig. 4.
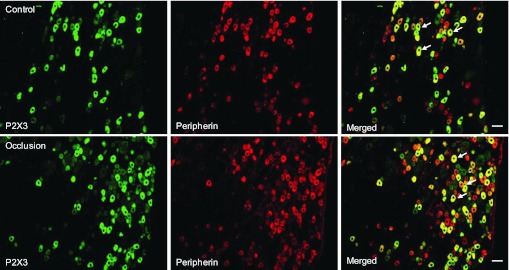
Immunofluorescence was employed to examine double labeling for P2X3 and peripherin. Peripherin was used to label DRG neurons that project thin C-fibers. Representative photomicrographs show P2X3 and peripherin staining in DRG neurons of a control limb (top) and an occluded limb (bottom). Arrows indicate representative cells positive for both P2X3 and peripherin after the images were merged. The number of double-labeled DRG neurons is greater in occluded limbs than in control limbs. Scale bar, 50 μm.
Fig. 5.
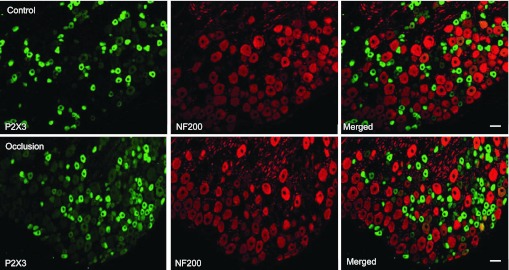
Immunofluorescence was employed to examine double labeling for P2X3 and NF200. NF200 was used to identify A-fibers of DRG neurons. Representative photomicrographs show P2X3 and NF200 staining in DRG neurons of a control limb (top) and an occluded limb (bottom). Very few DRG neurons appeared to be both P2X3 and NF200 positive. No differences in the number of DRG neurons double-stained for P2X3 and NF200 were observed in the control and occluded groups. Scale bar, 50 μm.
The photomicrographs show that P2X3 staining appears in C-fibers of DRG neurons in both control and occlusion groups, but very little P2X3 staining can be found in A-fibers of DRG neurons in both groups (Figs. 4 and 5). A large proportion of DRG neurons with C-fiber were P2X3 positive. Figure 4 further shows that more C-fiber neurons containing P2X3 were found in an occluded limb (bottom) compared with a control limb (top). The percentage of neurons double-labeled with P2X3 and peripherin was significantly greater in occluded limbs than in controls: 32 ± 2% in the controls (n = 5) and 50 ± 3% in the ligation group (n = 5, P < 0.05 vs. control). Figure 5 demonstrates that few A-fibers of DRG neurons include P2X3 staining in both the control and ligation groups. The percentage of neurons double-labeled with P2X3 and NF200 was similar in both experimental groups: ∼1% in controls (n = 6) and in the ligation group (n = 6, P > 0.05 vs. control). There was no significant difference in number of peripherin- and NF200-positive DRG neurons between the two experimental groups.
DISCUSSION
The P2X receptors in DRG neurons are predominantly P2X3-containing oligomers of homomers (P2X3) or heteromers (P2X2/3). In general, stimulation of P2X3 produces a rapidly desensitizing transient response whereas activation of P2X2/3 produces a relatively persistent response (Lewis et al. 1995). The response to ATP in a single DRG neuron is either one or a mixture of these response types depending on the complement of receptors that are presented in the cell (Cook et al. 1997; Grubb et al. 1999; Lewis et al. 1995). In addition, a prior study suggests that small-diameter DRG neurons express P2X3 receptors whereas medium diameter neurons express P2X2/3 receptors (Ueno et al. 1999). Overall, the data of this study demonstrate that in DRG neurons with nerve endings in the hindlimb muscles, P2X3 and P2X2/3 receptors represent the majority of currents elicited by ATP, which is in a range that is relevant to exercising muscle and/or hindlimb ischemia (Light et al. 2008). Additionally, a greater current response with activation of P2X3 and P2X2/3 is observed as the arterial blood supply to the hindlimb is deficient under ischemic conditions. Note that the size of DRG neurons that have P2X3 currents is typically small and the size of DRG neurons that have P2X2/3 is medium. However, the size distribution is similar in control and occluded limbs. Also, the percentage of DRG neurons with P2X3 transient currents is greater after arterial occlusion compared with control. Nevertheless, a rapid desensitization observed in DRG neurons with transient currents is not altered by arterial occlusion. The results of immunohistochemical experiments further suggest that P2X3 appears in C-fibers of DRG neurons and barely is observed in A-fibers of DRG neurons, and that femoral artery occlusion largely increases expression of P2X3 in DRG neurons that project C-fiber afferents.
Previous studies suggest that the glycolytic muscle plays a major role in reflex muscle responses evoked by static contraction (Wilson et al. 1995). Furthermore, a prior study using electrophysiological methods has demonstrated that DRG neurons with nerve endings in the white portion of the gastrocnemius muscle develop greater inward current responses to metabolic stimulation (Xing et al. 2008b). Therefore, in the present study, ATP-induced currents were recorded on rat DRG neurons innervating the white portion of the gastrocnemius muscles identified by retrograde labeling with the fluorescent dye DiI.
Muscle ATP concentrations increase from ∼0.3 μM at rest to ∼5 μM with contraction (Hellsten et al. 1998; Li et al. 2003, 2005; Mo and Ballard 2001). In the previous studies (Li et al. 2003, 2005), microdialysis methods were employed and ATP concentrations in dialysate were reported. The recovery rate of ∼25–30% for microdialysis probes should be taken into consideration. Thus the actual concentration of ATP during muscle contraction is about 17–20 μM (estimated as ∼5 μM per 25–30% of recovery rate). In addition, 10–500 μM α,β-meATP injected into the arterial blood supply of the hindlimb muscles increases discharge of the muscle afferents and increases BP (Hanna et al. 2002; Hanna and Kaufman 2004; Li and Sinoway 2002). Note that these compounds (i.e., α,β-meATP) are partly metabolized as they are injected the tissues. According to those data, in the present study 30 μM α,β-meATP was used to evoke P2X currents in the DRG neurons. Similar dosages of α,β-meATP have also been reported to effectively induce P2X currents in sensory neurons (Ma et al. 2005; Xu and Huang 2002).
A number of prior studies have suggested that ATP plays an important role in mediating the exercise pressor reflex (Gao et al. 2007; Hanna et al. 2002; Hanna and Kaufman 2003, 2004; Li and Sinoway 2002). First, static contraction increases the levels of interstitial ATP in the hindlimb muscles, and the increases of ATP are linearly related to muscle tension produced during contraction (Li et al. 2003). Second, α,β-meATP, a P2X receptor agonist, injected into the arterial blood supply of hindlimb muscles reflexively increases BP via its stimulation of metabolically sensitive muscle afferents in anesthetized cats (Hanna et al. 2002; Hanna and Kaufman 2004; Li and Sinoway 2002). Third, blocking P2X receptors attenuates discharge of group IV afferent fibers and BP response during static muscle contraction (Hanna and Kaufman 2003, 2004; Kindig et al. 2006, 2007). In a recent study (McCord et al. 2010), blocking P2X3 and P2X2/3 receptors by injecting A-317491 and RO-3, two structurally different P2X3 and P2X2/3 receptor antagonists, into the arterial circulation of the muscles showed that the pressor response to arterial injection of α,β-meATP was significantly attenuated. This prior study further demonstrated that the pressor response to postcontraction circulatory occlusion, a stimulus of metaboreceptors, is attenuated after blocking of P2X3 and P2X2/3, which suggests that P2X3 and P2X2/3 receptors contribute to the metabolic component of the exercise pressor reflex.
Recent studies have examined the mechanisms responsible for augmented SNA and BP responses during the exercise pressor reflex in PAD (Liu et al. 2011, 2012; Xing et al. 2008a, 2009). When α,β-meATP is injected into the arterial blood supply of hindlimb muscles to stimulate P2X of muscle afferent nerves, SNA and BP are increased to a greater degree in occluded rats (Liu et al. 2011). Also, P2X3 expression is upregulated in DRG neurons innervating the hindlimb muscles with the occluded femoral artery (Liu et al. 2011). Altogether, the data suggest that P2X plays a role in regulating abnormal sympathetic and pressor responses to static exercise observed in PAD. However, precise subtypes of P2X and receptor characteristics that play a functional role in engagement of occlusion-enhanced the reflex responses were not specifically determined in the prior studies.
Given that DRG cells are the primary sensory projections to group III and IV fiber afferent nerves, expression and characteristics of sensory receptors (i.e., P2X3) in DRG neurons are generally examined to study receptor physiology (Cook et al. 1997; Grubb et al. 1999; Lewis et al. 1995). The receptors in question are found in both the peripheral terminals and cell bodies of sensory DRG neurons. Receptor activity and characteristics of the DRG cell body have been used to reflect activity and characteristics of the receptors located at the nerve endings (Cook et al. 1997; Grubb et al. 1999; Lewis et al. 1995).
In the present study, DiI was injected into the hindlimb muscles to label the DRG neurons that innervate control and occluded muscles, and the results of the patch-clamp experiments demonstrated that DiI-labeled DRG neurons with P2X3 currents are small and those with P2X2/3 currents are medium in size. In addition, immunocytochemistry has shown that P2X3 immunolabeling appears in DRG neurons of C-fibers. Importantly, femoral occlusion largely amplifies current responses with stimulation of P2X3 and P2X2/3 and increases receptor expression in small-to-medium size neurons, specifically in DRG neurons that project C-fibers, which are considered to be engaged in the muscle metaboreflex (Kaufman et al. 1983, 1984b). Thus data of the present study are presented for the first time to suggest that femoral artery occlusion augments both P2X3 and P2X2/3 activities and thereby leads to the exaggerated exercise pressor reflex via stimulation of metabolically sensitive C-fiber muscle afferent nerves.
A prior study demonstrate that the levels of extracellular ATP are increased in ischemic tissues (Borst and Schrader 1991). Increased ATP is likely to upregulate P2X and thereby augment receptor activity. Thus additional investigations need to be performed to examine interstitial ATP of the hindlimb muscles at different time points following femoral occlusion to clarify the effects of ATP. It is speculated that following femoral occlusion the concentration of ATP is elevated in the muscle interstitium of occluded limb, and this increases expression of P2X engaged in augmented SNA and BP. A precise mechanism responsible for P2X upregulation in occluded limbs still needs to be determined.
Nevertheless, a prior study has demonstrated that infusion of nerve growth factor (NGF) into the hindlimb muscle of rats increases expression of P2X3 in DRG neurons as well as pressor response induced by stimulation of P2X3 (Liu et al. 2011). Likewise, NGF antibody injected into the hindlimb muscles attenuated P2X3-induced pressor response (Liu et al. 2011). These findings suggest that NGF has a regulatory effect on expression and function of afferent nerves' P2X3, and then P2X3 plays an important role in augmented sympathetic responsiveness via a reflex pathway when blood supply to the hindlimb muscles is insufficient, as seen in PAD. The results of this prior study are consistent with more recent findings (Lu et al. 2012) suggesting the role played by NGF in the exercise pressor reflex after femoral occlusion. In this study (Lu et al. 2012), femoral occlusion augmented the BP response to static muscle contraction in rats and to stimulation of chemically sensitive muscle afferent nerves by lactic acid injected into arterial blood supply of the hindlimb muscles. Moreover, a prior administration of the NGF antibody can effectively neutralize occlusion-increased NGF in DRG neurons and significantly attenuate the reflex pressor response to muscle contraction and lactic acid (Lu et al. 2012).
In general, receptor desensitization essentially contributes to the modulation of neurotransmitter action (Jones and Westbrook 1996). For example, some P2X receptors are calcium-permeable ion channels, and P2X desensitization can prevent excess intracellular calcium influx (Surprenant et al. 1995), which likely results in neurotoxicity. In the present study, a rapid desensitization was observed in muscle DRG neurons with activation of P2X3 receptors, and femoral artery occlusion did not largely affect the desensitization. Given that P2X3 receptor desensitization appears to be a functionally relevant neuronal transmission, it is unlikely that the mechanisms for the regulation of muscle afferent signaling during the exercise pressor reflex are significantly altered via P2X3 after femoral occlusion. However, amplitudes of P2X3 current responses in engagement of the reflex are amplified under the conditions of hindlimb vascular insufficiency.
In conclusion, results of the present study for the first time demonstrate that DRG response to P2X3 and/or P2X2/3 stimulation(s) is augmented following femoral artery ligation and that amplified responses are especially affected in small/medium-diameter DRG neurons innervating the hindlimb muscles. Additional data suggest that femoral occlusion increases P2X3 expression within DRG neurons of C-fiber afferents. Taken together, the results of the present study suggest that among P2X subtypes, P2X3 and P2X2/3 play a major role in augmented sympathetic responsiveness during activation of the exercise pressor reflex via thin-fiber afferent nerves when hindlimb blood supply is insufficient, as observed in PAD.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant R01 HL-090720, American Heart Association Established Investigator Award 0840130N, and NHLBI Grant P01 HL-096570.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.X., J. Lu, and J. Li conception and design of research; J.X. and J. Lu performed experiments; J.X. and J. Lu analyzed data; J.X., J. Lu, and J. Li interpreted results of experiments; J.X. and J. Lu prepared figures; J.X. and J. Li drafted manuscript; J.X. and J. Li edited and revised manuscript; J.X., J. Lu, and J. Li approved final version of manuscript.
REFERENCES
- Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007 [DOI] [PubMed] [Google Scholar]
- Borst MM, Schrader J. Adenine nucleotide release from isolated perfused guinea pig hearts and extracellular formation of adenosine. Circ Res 68: 797–806, 1991 [DOI] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on painsensing and stretchsensing neurons. Nature 387: 505–508, 1997 [DOI] [PubMed] [Google Scholar]
- Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 290: 86–97, 2003 [DOI] [PubMed] [Google Scholar]
- Gao Z, Xing J, Sinoway L, Li J. P2X receptor-mediated muscle pressor reflex in myocardial infarction. Am J Physiol Heart Circ Physiol 292: H939–H945, 2007 [DOI] [PubMed] [Google Scholar]
- Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur J Neurosci 11: 149–154, 1999 [DOI] [PubMed] [Google Scholar]
- Hanna RL, Hayes SG, Kaufman MP. α,β-Methylene ATP elicits a reflex pressor response arising from muscle in decerebrate cats. J Appl Physiol 93: 834–841, 2002 [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol 96: 1166–1169, 2004 [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol 94: 1437–1445, 2003 [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation 98: 6–8, 1998 [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci 19: 96–101, 1996 [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 10, p 381–447 [Google Scholar]
- Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002 [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984a [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984b [DOI] [PubMed] [Google Scholar]
- Kindig AE, Hayes SG, Hanna RL, Kaufman MP. P2 antagonist PPADS attenuates responses of thin fiber afferents to static contraction and tendon stretch. Am J Physiol Heart Circ Physiol 290: H1214–H1219, 2006 [DOI] [PubMed] [Google Scholar]
- Kindig AE, Hayes SG, Kaufman MP. Purinergic 2 receptor blockade prevents the responses of group IV afferents to post-contraction circulatory occlusion. J Physiol 578: 301–308, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377: 432–435, 1995 [DOI] [PubMed] [Google Scholar]
- Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol 95: 577–583, 2003 [DOI] [PubMed] [Google Scholar]
- Li J, King NC, Sinoway LI. Interstitial ATP and norepinephrine concentrations in active muscle. Circulation 111: 2748–2751, 2005 [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol 283: H2636–H2643, 2002 [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol 299: H1357–H1364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li J, Lu J, Xing J, Li J. Contribution of nerve growth factor to upregulation of P2X3 expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H1070–H1079, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Xing J, Li J. Role for NGF in augmented sympathetic responses to activation of mechanically and metabolically sensitive muscle afferents in rats with femoral artery occlusion. J Appl Physiol 113: 1311–1322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Rong W, Dunn P, Burnstock G. 17β-Estradiol attenuates αβ-meATP-induced currents in rat dorsal root ganglion neurons. Life Sci 76: 2547–2558, 2005 [DOI] [PubMed] [Google Scholar]
- McCord JL, Tsuchimochi H, Kaufman MP. P2X2/3 and P2X3 receptors contribute to the metaboreceptor component of the exercise pressor reflex. J Appl Physiol 109: 1416–1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- Mo FM, Ballard HJ. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP and ATP in dog skeletal muscle. J Physiol 536: 593–603, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir RL. Peripheral arterial disease: pathophysiology, risk factors, diagnosis, treatment, and prevention. J Vasc Nurs 27: 26–30, 2009 [DOI] [PubMed] [Google Scholar]
- Ouriel K. Peripheral arterial disease. Lancet 358: 1257–1264, 2001 [DOI] [PubMed] [Google Scholar]
- Rejeski WJ, Tian L, Liao Y, McDermott MM. Social cognitive constructs and the promotion of physical activity in patients with peripheral artery disease. J Cardiopulm Rehabil Prev 28: 65–72, 2008 [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol 99: 5–22, 2005 [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006 [DOI] [PubMed] [Google Scholar]
- Surprenant A, Buell G, North RA. P2X receptors bring new structure to ligand-gated ion channels. Trends Neurosci 18: 224–229, 1995 [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol 126: 429–436, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol 97: 773–780, 2004 [DOI] [PubMed] [Google Scholar]
- Wilson LB, Dyke CK, Parsons D, Wall PT, Pawelczyk JA, Williams RS, Mitchell JH. Effect of skeletal muscle fiber type on the pressor response evoked by static contraction in rabbits. J Appl Physiol 79: 1744–1752, 1995 [DOI] [PubMed] [Google Scholar]
- Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol 295: H1262–H1269, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol 296: H1380–H1387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Sinoway L, Li J. Differential responses of sensory neurones innervating glycolytic and oxidative muscle to protons and capsaicin. J Physiol 686: 3245–3252, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci 22: 93–102, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]