Abstract
Cortical networks display persistent activity in the form of periods of sustained synchronous depolarizations (‘UP states’) punctuated by periods of relative hyperpolarization (‘DOWN states’), which together form the slow oscillation. UP states are known to be synaptically generated and are sustained by a dynamic balance of excitation and inhibition, with fast ionotropic glutamatergic excitatory and GABAergic inhibitory conductances increasing during the UP state. Previously, work from our group demonstrated that slow metabotropic GABA receptors also play an important role in terminating the UP state, but the effects of other neuromodulators on this network phenomenon have received little attention. Given that persistent activity is a neural correlate of working memory and that signalling through dopamine receptors has been shown to be critical for working memory tasks, we examined whether dopaminergic neurotransmission affected the slow oscillation. Here, using an in vitro model of the slow oscillation in rat medial entorhinal cortex, we showed that dopamine strongly and reversibly suppressed cortical UP states. We showed that this effect was mediated through D1-like and not D2-like dopamine receptors, and we found no evidence that tonic dopaminergic transmission affected UP states in our model.
Keywords: cortex, slow oscillation, UP states
Introduction
Neuronal networks display persistent activity, an important form of circuit dynamics thought to underlie phenomena such as working memory (reviewed by Major & Tank, 2004). One example of persistent activity is seen during the slow oscillation, where neurons oscillate between periods of sustained depolarization and firing (‘UP states’) punctuated by epochs of hyperpolarization and reduced firing (‘DOWN states’). The slow oscillation can be observed in vivo in anaesthetized (Steriade et al., 1993) or naturally sleeping (Contreras et al., 1996) states as well as during quiet wakefulness (Petersen, 2003). UP and DOWN states (UDS) can also be observed using reduced in vitro preparations such as slices containing the visual cortex (Sanchez-Vives & McCormick, 2000) or entorhinal cortex (Cunningham et al., 2006; Mann et al., 2009). Insights from these in vitro studies show that UP states are associated with an increase in both excitatory and that inhibitory neurotransmission (Sanchez-Vives & McCormick, 2000), and that inhibitory conductances dynamically scale to balance excitatory conductances, both in vitro (Shu et al., 2003) and in vivo (Haider et al., 2006).
Most studies of the circuit dynamics involved in generating UDS have focussed on fast, ionotropic neurotransmission and less on the role played by slower metabotropic receptors. We previously reported that fast GABAA receptor-mediated inhibition balances the UP state, whereas slow GABAB receptor-mediated inhibition is important for the termination of the UP state (Mann et al., 2009), but whether other neuromodulators affect the slow oscillation has yet to be determined. Working memory and its neural correlate in the prefrontal cortex, persistent activity, are known to be dependent upon dopamine (Brozoski et al., 1979). In the prefrontal cortex, dopamine can reduce extracellular GABA concentrations (Grobin & Deutch, 1998) via a mechanism involving both D1-like and D2-like dopamine receptors (Bouthenet et al., 1987; Gao et al., 2001). Much like the prefrontal cortex, the medial entorhinal cortex (mEC) receives multimodal input from many brain regions. The mEC forms one of the main input and output systems to the hippocampus (Canto et al., 2008), so persistent activity in the mEC may be involved in cognitive processes such as working memory and spatial navigation. Diffuse neuromodulatory systems, such as dopaminergic projections, might therefore also be expected to modulate the persistent activity of UDS in the mEC.
Using a model of UDS in submerged mEC slices (Mann et al., 2009), we sought to determine whether the dopaminergic system affected the slow oscillation. We found that dopamine rapidly and reversibly suppressed the incidence of UP states in rat mEC, and that this effect was mediated through D1-like dopamine receptors. We found no evidence for the involvement of D2-like receptors in the dopamine-mediated suppression of persistent activity.
Materials and methods
Animals
All experiments were conducted in accordance with the UK Animals Scientific Procedures Act (1986) and were subject to local ethical review by the University of Oxford and the University of Cambridge. Wistar rats (Harlan, UK) were used in all experiments.
Slice preparation and electrophysiology
Horizontal slices (400 μm) containing the mEC were prepared from postnatal day 14–21 rats of both sexes after decapitation under deep isoflurane-induced anaesthesia. Slices were cut in ice-cold (< 4 °C) standard artificial cerebrospinal fluid containing (in mm): 126 NaCl, 3–3.5 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2 and 26 NaHCO3, and were incubated at room temperature (22 − 26 °C) for 1 h in interface conditions with standard artificial cerebrospinal fluid, before being transferred to modified artificial cerebrospinal fluid with reduced MgSO4 (1 mm) and CaCl2 (1.2 mm). Slices were maintained in interface conditions prior to recording; they were then mounted on a coverslip (coated with 0.1% poly-l-lysine in ultrapure H2O) and transferred to a submerged-style recording chamber where they were superfused with modified artificial cerebrospinal fluid at 4–5 mL/min at 32–34 °C. The maintenance of slices in interface conditions was critical for the development of spontaneous UDS, and the use of poly-l-lysine coverslips allowed slices to be mechanically stable under high perfusion rates and also improved laminar flow across the slice; these conditions are known to promote spontaneous network activity in submerged slices (Hajos et al., 2009).
Whole-cell current-clamp recordings were made from principal cells in layer 3 of the mEC, using glass pipettes pulled from standard borosilicate glass (6–8 MΩ) containing (in mm): 110 K gluconate, 40 HEPES, 2 ATP-Mg, 0.3 GTP, 4 NaCl and 2–4 mg/mL biocytin (pH 7.2–7.3, osmolarity 275–290 mosmol/L). Membrane potential values were not corrected for the liquid junction potential.
Data acquisition and analysis
Data were recorded using an Axon Multiclamp 700B amplifier (Molecular Devices) and low-pass filtered at 2 kHz. The signal was digitized at 5 kHz using an Instrutech ITC-18 on a PC running Igor Pro (Wavemetrics Inc., Lake Oswego, OR, USA) using procedures written in-house. UDS transitions were monitored automatically using an algorithm that detected changes in DC membrane potential and membrane potential fluctuations using a moving average window method (Craig, 2011). All detected UP states were confirmed by visual inspection. Statistical comparisons were made using anova with post hoc Bonferroni multiple-comparison correction, or Student's two-sample and paired t-tests as appropriate. Unless otherwise stated, all values are given as mean ± SEM. All data and statistical analyses were carried out using either Igor Pro or GraphPad Prism (GraphPad, San Diego, CA, USA).
Drugs and chemicals
Raclopride and SCH23390 were purchased from Tocris Bioscience. All other chemicals were purchased from Sigma-Aldrich.
Results
UP states occur spontaneously in the medial entorhinal cortex and are suppressed by bath application of dopamine
Whole-cell current-clamp recordings made from principal cells in layer 3 of the mEC were used to monitor UDS. UP states occurred spontaneously with an incidence of 2.9 ± 0.3/min and a mean duration of 5.2 ± 0.4 s (n = 37 slices, Fig. 1A and C). The mean spiking frequency of principal cells during UP states was 0.6 ± 0.2 Hz. The incidence of UP states was stable for at least 40 min after whole-cell configuration was achieved (Fig. 1B). Bath application of 10 μm dopamine reduced the incidence of UP states (Fig. 2A and B); after 10 min, spontaneous UP states were almost entirely suppressed (UP state incidence, baseline vs. 10 μm dopamine: 3.3 ± 0.67/min vs. 0.04 ± 0.03/min; t4 = 4.8, P = 0.0089; n = 5; paired t-test; Fig. 2C). Bath application of dopamine caused a small but significant hyperpolarization of the resting membrane potential (baseline vs. 10 μm dopamine: −62.1 ± 0.16 vs. −64.3 ± 0.19 mV; t4 = 2.9, P = 0.044; n = 5; two-tailed paired t-test). We next asked which type of dopamine receptor, D1-like or D2-like, mediates the effects of dopamine on UDS.
FIG. 1.
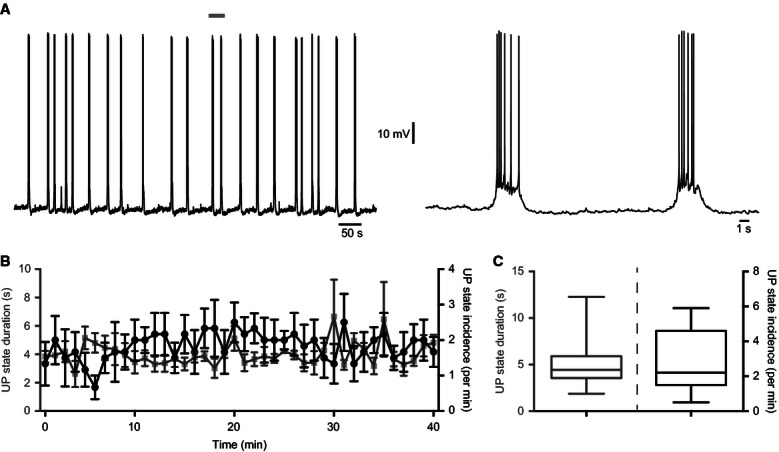
UP states in the mEC are stable over time. (A) Whole-cell current-clamp recording from a layer 3 principal cell showing that UDS are stable over long periods. Area marked by grey bar is shown on the right on an expanded time scale. (B) The duration (grey squares) and incidence (black circles) of UP states did not change over time (n = 6). (C) Distribution of mean UP state duration (left) and incidence (right). The mean UP state duration was 5.2 ± 0.4 s and the mean UP state incidence was 2.9 ± 0.3/min (n = 37 slices). Whiskers on the box plots represent the maximum and minimum values.
FIG. 2.
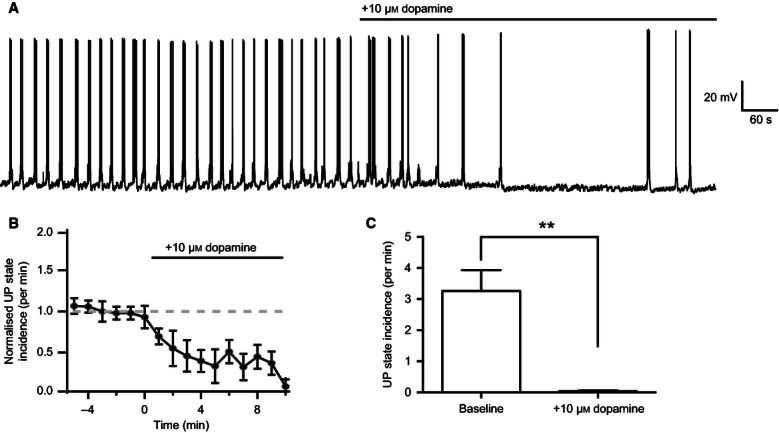
Dopamine suppresses the incidence of UP states. (A) Example recording showing the reduction in the incidence of UP states after bath application of 10 μm dopamine. (B) Time course of the depression of UP states over 10 min after start of wash-in (n = 14). (C) After 10 min, dopamine had significantly reduced the frequency of UP states (n = 5). **P < 0.01, paired t-test.
Dopaminergic suppression of UP states is mediated by D1-like dopamine receptors
To investigate the dopamine receptor subtype involved in suppressing persistent activity, we repeated the previous experiment in the presence of either the D1-like receptor antagonist SCH23390 or the D2-like receptor antagonist raclopride. Bath application of 10 μm SCH23390 prior to bath application of dopamine prevented the suppression of UP states (Fig. 3A), with no significant effect on either the duration (baseline vs. +10 μm SCH23390 vs. +10 μm dopamine: 5.5 ± 0.94 vs. 6.4 ± 1.10 vs. 3.9 ± 0.33 s; F2,5 = 4.9, P = 0.077; n = 6; repeated-measures anova; Fig. 3B) or incidence (baseline vs. +10 μm SCH23390 vs. +10 μm dopamine: 4.8 ± 0.58/min vs. 5.3 ± 0.40/min vs. 3.9 ± 0.61/min; F2,5 = 2.3, P = 0.19; n = 6; repeated-measures anova; Fig. 3C) of UP states. Conversely, bath application of 200 nM raclopride failed to prevent the subsequent suppression of UP states after the application of dopamine (Fig. 3A), which significantly reduced UP state duration (baseline vs. +200 nm raclopride vs. +10 μm dopamine: 5.1 ± 1.17 vs. 3.7 ± 0.68 vs. 0.4 ± 0.44 s; F2,5 = 15.5, P = 0.0091; n = 6; multiple comparisons after repeated-measures anova; Fig. 3D) and incidence (baseline vs. +200 nm raclopride vs. +10 μm dopamine: 2.7 ± 0.73/min vs. 3.2 ± 0.74/min vs. 0.05 ± 0.05/min; F2,5 = 16.6, P = 0.0082; n = 6; multiple comparisons after repeated-measures anova; Fig. 3E). We observed no significant relationship between the baseline UP state incidence and the change in UP state incidence after bath application of dopamine alone (r = −0.50, P = 0.39; n = 5; Pearson's correlation test), dopamine and raclopride (r = 0.62, P = 0.18; n = 6) or dopamine and SCH23390 (r = 0.06, P = 0.92, n = 6; Pearson's correlation test). We conclude that dopamine-induced suppression of persistent activity is mediated through D1-like receptors.
FIG. 3.
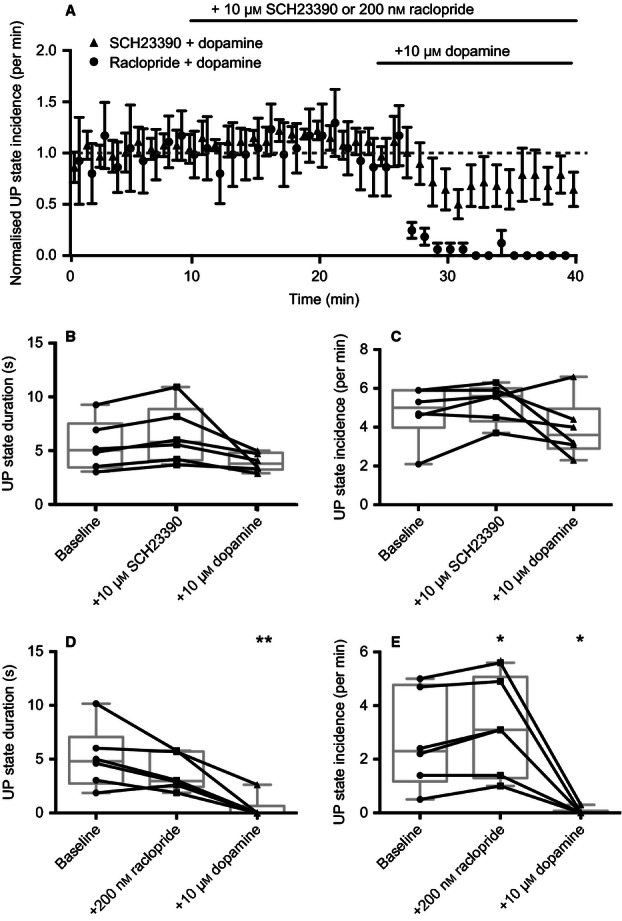
Dopamine-induced suppression of persistent activity is mediated via D1-like receptors. (A) The D1-like receptor antagonist SCH23390 prevented dopamine-induced suppression of UP states, whereas the D2-like receptor antagonist raclopride did not. (B) In the presence of SCH23390, no significant difference was observed in UP state duration upon application of dopamine. (C) In the presence of SCH23390, no significant difference was observed in UP state incidence upon application of dopamine. (D) In the presence of raclopride, dopamine significantly reduced the duration of UP states. (E) In the presence of 200 nm raclopride, dopamine also significantly reduced the incidence of UP states (n = 6). *P < 0.05, **P < 0.01; repeated-measures anova.
Application of a D1-like receptor antagonist can reverse dopamine-induced suppression of persistent activity
Given that the D1-like receptor antagonist SCH23390 could prevent dopamine from suppressing UP states, we next sought to determine whether this suppression was reversible. After recording spontaneous UDS for 10 min, 10 μm dopamine was bath-applied. Subsequently, we then applied either 10 μm SCH23390 or 200 nm raclopride. Application of SCH23390 significantly reversed the suppression of UP states, whereas raclopride had no effect (normalized incidence of UP states at 25 min after washing antagonist, 10 μm SCG23390 vs. 200 nm raclopride: 1.4 ± 0.26/min vs. 0.30 ± 0.14/min; t10 = 3.7, P = 0.004; n = 6; two-tailed Student's t-test; Fig. 4A). As well as reversing the dopamine-induced suppression of UP states, it appeared that 10 μm SCH23390 might have increased the incidence above baseline values. However, this increase in UP state incidence was not statistically significant (t5 = 1.5, P = 0.18; one-sample t-test; h0 = normalized UP state incidence after SCH22390 application was 1).
FIG. 4.
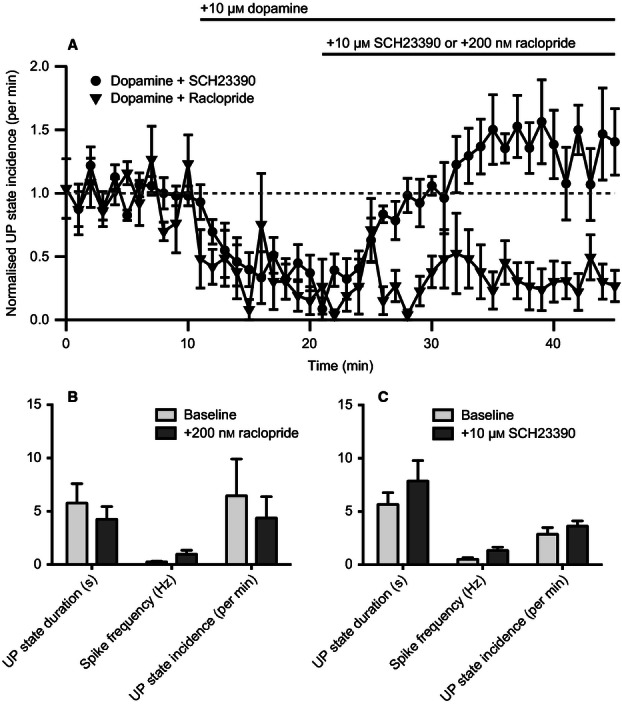
A D1-like antagonist can reverse dopamine-induced suppression of persistent activity. (A) Application of SCH23390 could reverse dopamine-induced suppression of UP states, but application of raclopride could not (n = 6). (B and C) SCH233390 and raclopride alone had no significant effect on UP state duration, incidence or firing frequency (n = 6). Repeated-measures anova.
In the previous experiment, application of 200 nm raclopride appeared to cause a small but significant increase in UP state incidence (Fig. 3E). To determine whether there was any tonic dopaminergic activity affecting the UP states, we bath-applied either 10 μm SCH23390 or 200 nm raclopride for 40 min to observe the effect on UDS properties. Raclopride had no significant effect on UP state duration (baseline vs. 200 nm raclopride: 5.8 ± 1.8 vs. 4.3 ± 1.2 s; t5 = 1.6, P = 0.17; n = 6; two-tailed paired t-test; Fig. 4B), incidence (baseline vs. 200 nm raclopride: 6.5 ± 3.5/min vs. 4.4 ± 2.1/min; t5 = 1.4, P = 0.23; n = 6; two-tailed paired t-test; Fig. 4B) or spiking frequency (baseline vs. 200 nm raclopride: 0.25 ± 0.07 vs. 0.97 ± 0.37 Hz; t5 = 1.9, P = 0.12; n = 6; two-tailed paired t-test; Fig. 4B). Similarly, SCH23390 had no significant effect on UP state duration (baseline vs. 10 μm SCH23390: 5.7 ± 1.1 vs. 7.9 ± 1.9 s; t5 = 2.1, P = 0.092; n = 6; two-tailed paired t-test; Fig. 4C), incidence (baseline vs. 10 μm SCH23390: 2.9 ± 0.62/min vs. 3.6 ± 0.49/min; t5 = 1.9, P = 0.12; n = 6; two-tailed paired t-test; Fig. 4C) or spiking frequency (baseline vs. 10 μm SCH23390: 0.52 ± 0.15 vs. 1.3 ± 0.32 Hz; t5 = 2.3, P = 0.070; n = 6; two-tailed paired t-test; Fig. 4C).
These data suggest that there was no detectable tonic effect of dopamine in our preparation, and we conclude that phasic application of dopamine can powerfully but reversibly block persistent activity in the mEC through a mechanism mediated by D1-like dopamine receptors.
Discussion
The slow oscillation is a synaptically-driven oscillation, where UP states are generated by recurrent activity mediated via a dynamic balance of excitatory and inhibitory conductances (Sanchez-Vives & McCormick, 2000; Shu et al., 2003; Cunningham et al., 2006; Haider et al., 2006). Whereas the contributions of fast, ionotropic excitatory (Sanchez-Vives & McCormick, 2000; Compte et al., 2003; Shu et al., 2003) and inhibitory (Hasenstaub et al., 2005; Haider et al., 2006) conductances to generating neocortical UP states are well understood, the effects that neuromodulators have in modulating persistent activity are less so. Previously, work from our group showed that slow GABAB receptors are involved in both the spontaneous and evoked termination of UP states (Mann et al., 2009), and here we have shown that dopamine can strongly suppress UP states.
We found that blockade of D1-like receptors with the antagonist SCH23390 prevented the dopamine-induced suppression of UP states, although there still appeared to be a small, but non-significant reduction in UP state incidence (Fig. 3A). SCH23390 is also an agonist at 5-HT1C and 5-HT2C receptors so it is possible that activation of these receptors may have had an effect on network oscillations. However, we saw no evidence for significant modulation of UP states by application of dopaminergic antagonists alone, suggesting that the off-target activation of 5HT1C and 5HT2C receptors had a minimal effect in our model.
Although it has been shown that, in the striatal spiny neurons, activation of D2 receptors can truncate UP states (Plotkin et al., 2011), we found that the effects of dopamine on mEC UP states were mediated via D1-like receptors. As dopamine is necessary for working memory in the prefrontal cortex and can lower extracellular GABA concentrations (Brozoski et al., 1979; Grobin & Deutch, 1998), one might have expected dopamine to increase excitation and thus enhance persistent activity, yet we saw the opposite result. However, even within the prefrontal cortex, the effects of dopamine can appear contradictory and dependent upon experimental conditions (Seamans & Yang, 2004). We chose a concentration of dopamine (10 μm) that was at the lower end of those reported in the literature, but that should still have activated both D1-like and D2-like receptors. There are a number of potential mechanisms that could explain why dopamine suppresses persistent activity. First, an early study in the entorhinal cortex found that dopamine could inhibit layer 5 pyramidal cells by acting via D1-like receptors to increase IH (Rosenkranz & Johnston, 2006), and a similar effect could potentially act on layer 3 principal cells in our study. D1-like receptors also depolarize neocortical fast-spiking interneurons (Towers & Hestrin, 2008); a shift in the excitatory/inhibitory balance caused by dopamine could perturb network activity. Other studies report that activation of D1-like receptors can actually reduce evoked inhibitory postsynaptic currents in the prefrontal cortex (Gonzalez-Islas & Hablitz, 2001). D1/D5 receptors have also been shown to increase the N-methyl-d-aspartate component of excitatory postsynaptic currents in prefrontal cortex pyramidal cells (Seamans et al., 2001), which could also affect persistent activity by altering the excitatory/inhibitory balance of the network. Dopamine's effects on network activity are likely to vary substantially from one region of the brain to another, depending on both the cellular and laminar distribution of D1-like vs. D2-like receptors. An additional degree of variability may arise due to differential expression of the intracellular targets of activated dopamine receptors between different areas of the cortex.
A more recent study reported that, in prefrontal cortex slices, bath application of dopamine selectively increased inhibitory over excitatory currents and, using calcium imaging, dopamine was observed to inhibit the spread of local activity via D1-like dopamine receptor activation (Bandyopadhyay & Hablitz, 2007). Dopamine also inhibits carbachol-induced gamma oscillations in rat hippocampal slices, through a mechanism that also acts via D1-like dopamine receptors (Weiss et al., 2003). Taken together with the results from our study, it would seem that dopamine can strongly inhibit network activity by acting through D1-like receptors. Further questions remain, e.g. are the actions of dopamine mediated through a specific type of interneuron, or do they come from a general increase in inhibitory tone? These questions have functional implications; understanding the effects of dopamine on the network, as opposed to neuronal level, may help give insights into the progression and treatment of brain disorders.
Given the diversity of neuronal functions that can be attributed to dopamine, which vary depending on the location and time course of release (Schultz, 2007), in addition to the number and diversity of dopamine receptors present throughout the brain, understanding the effects of dopamine on network activity can be challenging. Although we have shown here that dopamine can strongly suppress persistent network activity in the mEC, further work will be needed to determine the mechanism(s) by which this effect is mediated.
Acknowledgments
This work was supported by the Wellcome Trust OXION initiative (M.T.C. and O.P.) and a National Institute of Child Health and Human Development (NICHD) intramural award (C.J.M.). M.T.C. held a Wellcome Trust Prize Studentship. E.W.M. is supported by the NIH MD/PhD Partnership Training programme and by the Rhodes Trust.
Glossary
Abbreviations
- mEC
medial entorhinal cortex
- UDS
UP and DOWN states
Conflict of interests
The authors declare that they do not have any competing interests.
References
- Bandyopadhyay S, Hablitz JJ. Dopaminergic modulation of local network activity in rat prefrontal cortex. J. Neurophysiol. 2007;97:4120–4128. doi: 10.1152/jn.00898.2006. [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Martres MP, Sales N, Schwartz JC. A detailed mapping of dopamine D-2 receptors in rat central nervous system by autoradiography with [125I]iodosulpride. Neuroscience. 1987;20:117–155. doi: 10.1016/0306-4522(87)90008-x. [DOI] [PubMed] [Google Scholar]
- Brozoski T, Brown R, Rosvold H, Goldman P. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Canto CB, Wouterlood FG, Witter MP. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast. 2008;2008:381243. doi: 10.1155/2008/381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (< 1 Hz) and wave propagations in a cortical network model. J. Neurophysiol. 2003;89:2707–2725. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- Contreras D, Timofeev I, Steriade M. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J. Physiol. 1996;494:251–264. doi: 10.1113/jphysiol.1996.sp021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MT. University of Oxford, Oxford: University Thesis: Department of Physiology, Anatomy and Genetics; 2011. The cortical slow oscillation: the role of slow GABAergic inhibition in mediating the UP-to-DOWN state transition. [Google Scholar]
- Cunningham MO, Pervouchine DD, Racca C, Kopell NJ, Davies CH, Jones RS, Traub RD, Whittington MA. Neuronal metabolism governs cortical network response state. Proc. Natl. Acad. Sci. USA. 2006;103:5597–5601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W-J, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc. Natl. Acad. Sci. USA. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J. Neurophysiol. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Deutch AY. Dopaminergic regulation of extracellular gamma-aminobutyric acid levels in the prefrontal cortex of the rat. J. Pharmacol. Exp. Ther. 1998;285:350–357. [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J. Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, Freund TF, Paulsen O. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur. J. Neurosci. 2009;29:319–327. doi: 10.1111/j.1460-9568.2008.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr. Opin. Neurobiol. 2004;14:675–684. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Mann EO, Kohl MM, Paulsen O. Distinct roles of GABAA and GABAB receptors in balancing and terminating persistent cortical activity. J. Neurosci. 2009;29:7513–7518. doi: 10.1523/JNEUROSCI.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC. The barrel cortex - integrating molecular, cellular and systems physiology. Pflugers Arch. 2003;447:126–134. doi: 10.1007/s00424-003-1167-z. [DOI] [PubMed] [Google Scholar]
- Plotkin JL, Day M, Surmeier DJ. Synaptically driven state transitions in distal dendrites of striatal spiny neurons. Nat. Neurosci. 2011;14:881–888. doi: 10.1038/nn.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex. J. Neurosci. 2006;26:3229–3244. doi: 10.1523/JNEUROSCI.4333-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat. Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc. Natl. Acad. Sci. USA. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers SK, Hestrin S. D1-like dopamine receptor activation modulates GABAergic inhibition but not electrical coupling between neocortical fast-spiking interneurons. J. Neurosci. 2008;28:2633–2641. doi: 10.1523/JNEUROSCI.5079-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Veh RW, Heinemann U. Dopamine depresses cholinergic oscillatory network activity in rat hippocampus. Eur. J. Neurosci. 2003;18:2573–2580. doi: 10.1046/j.1460-9568.2003.02970.x. [DOI] [PubMed] [Google Scholar]


