Abstract
Objective
To evaluate an online disease management system supporting patients with uncontrolled type 2 diabetes.
Materials and methods
Engaging and Motivating Patients Online With Enhanced Resources for Diabetes was a 12-month parallel randomized controlled trial of 415 patients with type 2 diabetes with baseline glycosylated hemoglobin (A1C) values ≥7.5% from primary care sites sharing an electronic health record. The intervention included: (1) wirelessly uploaded home glucometer readings with graphical feedback; (2) comprehensive patient-specific diabetes summary status report; (3) nutrition and exercise logs; (4) insulin record; (5) online messaging with the patient's health team; (6) nurse care manager and dietitian providing advice and medication management; and (7) personalized text and video educational ‘nuggets’ dispensed electronically by the care team. A1C was the primary outcome variable.
Results
Compared with usual care (UC, n=189), patients in the intervention (INT, n=193) group had significantly reduced A1C at 6 months (−1.32% INT vs −0.66% UC; p<0.001). At 12 months, the differences were not significant (−1.14% INT vs −0.95% UC; p=0.133). In post hoc analysis, significantly more INT patients had improved diabetes control (>0.5% reduction in A1C) than UC patients at 12 months (69.9 (95% CI 63.2 to 76.5) vs 55.4 (95% CI 48.4 to 62.5); p=0.006).
Conclusions
A nurse-led, multidisciplinary health team can manage a population of diabetic patients in an online disease management program. INT patients achieved greater decreases in A1C at 6 months than UC patients, but the differences were not sustained at 12 months. More INT than UC patients achieved improvement in A1C (>0.5% decrease).
Trial registered in clinical trials.gov: #NCT00542204
Keywords: Diabetes Mellitus Type 2, Disease Management, Personal Health Record, Health Information Technology, Electronic Health Record, Telemedicine
Background
The rising incidence of diabetes has far-reaching implications for population health status and healthcare costs.1 2 Despite improvements in the treatment of diabetes, glycemic control of diabetes remains suboptimal, with an estimated 56.8% of diabetic patients having their glycosylated hemoglobin (A1C) controlled at <7%.3 At the same time, demand on physicians’ time is increasing, as the population ages and physicians are under pressure to manage larger panels of patients while achieving better outcomes. New methods for healthcare professionals to partner patients in managing their diabetes are needed.
The Chronic Care Model emphasizes the use of multidisciplinary healthcare teams and an activated patient.4 5 Integrated personal health records (PHRs) can improve patients’ access to their data and facilitate communication with their professional healthcare team.6–8 Unlike episodic office visits, remote monitoring technologies and automated alerting and communication capabilities can support greater continuity of care.
The Palo Alto Medical Foundation (PAMF) has developed an online disease management system to support patients with diabetes as part of its personalized healthcare program (PHCP). The PHCP incorporates several features of effective disease management programs, including multidisciplinary team-based care, use of nurse care managers (NCMs) authorized to change medication, patient self-management tools, and an online communication channel between patients and their healthcare team.9 10
We present the results of a randomized controlled trial of online disease management of diabetes, which we called Engaging and Motivating Patients Online With Enhanced Resources for Diabetes (EMPOWER-D).
Materials and methods
The study was conducted at PAMF, a not-for-profit healthcare organization with approximately 1000 multispecialty physicians serving over 800 000 patients. After conducting α and β pilot tests, we designed a two-arm randomized controlled trial to evaluate the PHCP for type 2 diabetes, which includes: (1) wireless glucometer upload system that transmits home glucometer readings to PAMF's electronic health record (EHR); (2) diabetes summary status report, a comprehensive, patient-specific ‘dashboard’ of the status of a patient's personalized action plan and treatment goals, diabetes complications risk, monitoring tests, medications, and health maintenance schedule; (3) nutrition log; (4) insulin record; (5) exercise log; (6) online messaging system for communicating with members of the patient's healthcare team; (7) NCMs who provide advice and make protocol-based changes to medications; and (8) patient-specific text and video educational ‘nuggets’ dispensed electronically by NCMs.
The study was reviewed and approved annually by the Institutional Review Board of the PAMF Research Institute, and informed, written consent was obtained from each participant. EMPOWER-D is a registered clinical trial in clinicaltrials.gov.
Participant identification and recruitment
Participants were recruited from March 2008 through December 2009. We reviewed PAMF's EHRs to identify potential study participants based on the following criteria:
Age ≥18 years
Diagnosis of type 2 diabetes mellitus
A1C≥7.5%
Patient seen within the past 12 months
Eligible patients approved by their primary care provider were invited to participate in an online screening survey, which assessed the following exclusion criteria:
Initial diagnosis of type 2 diabetes mellitus within the last 12 months
Inability to speak or read English
Lack of regular internet access with email capabilities
Unwillingness to perform any self-monitoring at home, including blood glucose
Diagnosis of a terminal illness and/or entry into hospice care
Pregnancy, planning a pregnancy, or currently lactating
Current enrollment in a care management program at PAMF or elsewhere
Family household member enrolled in EMPOWER-D study
Resident of a long-term care facility
Plans to discontinue primary care at PAMF during the study period
Uninsured
Participants who met all the screening requirements were asked to complete an online baseline questionnaire and a visit with a research assistant. Clinical measurements were taken, including blood pressure and weight. Laboratory tests were ordered unless a test was ordered and resulted within the past 30 days. Participants with A1C≥7.5% were enrolled.
Randomization was performed by-patient. An analyst sequestered from the research assistants entered the participants into a randomization program based on Pocock's ‘minimization’ procedure, which assures better-than-chance group balance for the following key variables: primary care site, age, gender, A1C levels, systolic blood pressure, low-density lipoprotein (LDL) cholesterol, and pre-enrollment use of our patient portal.11 Participants were then sent letters about study assignment (intervention (INT) vs usual care (UC)); INT patients were contacted by an NCM to begin the INT. UC patients continued to receive standard-of-care treatment, including reminders about annual and preventive guideline-based laboratory tests and screening. UC participants received no EMPOWER-D treatment INTs.
Sample size calculation
Our study design is powered to detect net A1C improvements of 0.5% or larger. A sample of 200 participants per arm was calculated to provide 91% power to detect an effect size of 0.36, assuming a 15% loss in 12-month follow-up.
Study participation
Participants randomized to the INT group had three in-person visits: (1) a 90 min group visit introducing the online tools; (2) a 90 min 1 : 1 consultation visit with the NCM to develop a shared care plan for the participant; and (3) a 60 min visit with a registered dietitian. A pharmacist reviewed all INT participants’ charts, made recommendations about medication management, and was consulted by the care team as needed throughout the INT. Participants in both groups completed online questionnaires and were seen by a blinded research assistant at 6 and 12 months for data collection.
Intervention
Universal models of behavior change that include individual difference, perceived severity of a health threat, relevant values, skills and perceived barriers to action guided design of the INT. One of PHCP's core objectives is to empower participants with a better understanding of their disease processes and prompt them to take a more active role in self-management. Diabetes self management education incorporates the needs, goals, and life experiences of people with diabetes and is guided by evidence-based standards.12 Goal setting, using motivational interviewing techniques, and being guided by the Chronic Care Model helped patients identify and reach realistic health goals. Participants were also provided with wireless remote monitoring tools and enhanced patient portal functions to support self-management of diabetes.
To simplify data capture of glucometer readings for participants, PAMF collaborated with Numera (Mountain View, California, USA) to develop a Bluetooth adaptor that wirelessly transmitted annotated (eg, before meals) glucose readings from Lifescan's (Milpitas, California, USA) OneTouch Ultra2 glucometer to a Palm (Sunnyvale, California, USA) Treo smartphone, which uploaded the information to the PAMF EHR, EpicCare, by Epic (Verona, Wisconsin, USA). Glucose data were immediately available to view by the patient via the PHR and were analyzed by the PHCP system according to patient-specific parameters. No data were available on the smartphone.
Participants were also provided with the capability to log information relevant to diabetes management online, such as dietary intake, physical activity, home blood pressure, insulin doses, and weight. Interactive visual displays of these data facilitated tracking progress towards goals and correlated glucose control with medication compliance or lifestyle changes.
The NCMs and registered dietician (RD) primarily used secure messaging to communicate with participants. Check-in times were tailored to individual patient needs. Participants were given timely, regular feedback about their clinical variables, such as blood glucose readings, food intake, and medication doses. NCMs independently adjusted medication on the basis of EMPOWER-D protocols, which were based on the American Diabetes Association recommendations (goals: A1c<7%, blood pressure<130/80, LDL cholesterol<100 mg/dl). NCMs functioned as part of the primary care team. Primary care physicians were kept up to date about clinical changes through the shared EHR.
Patients also received personalized educational ‘nuggets’ (brief text or videos) delivered by NCMs through the PHR in response to updated patient data, such as uploaded blood glucose readings, patient messages, exercise and food data, laboratory data, and other clinical measurements. The PHCP queues up these materials for NCMs by matching the patient's clinical scenario (derived from the EHR and patient-generated data) to a predefined library of 500 diabetes scenarios. For example, in response to a hypoglycemic reading submitted via the remote glucometer device, the PHCP platform queues up a personalized video nugget educating about the dangers and prevention of hypoglycemia.
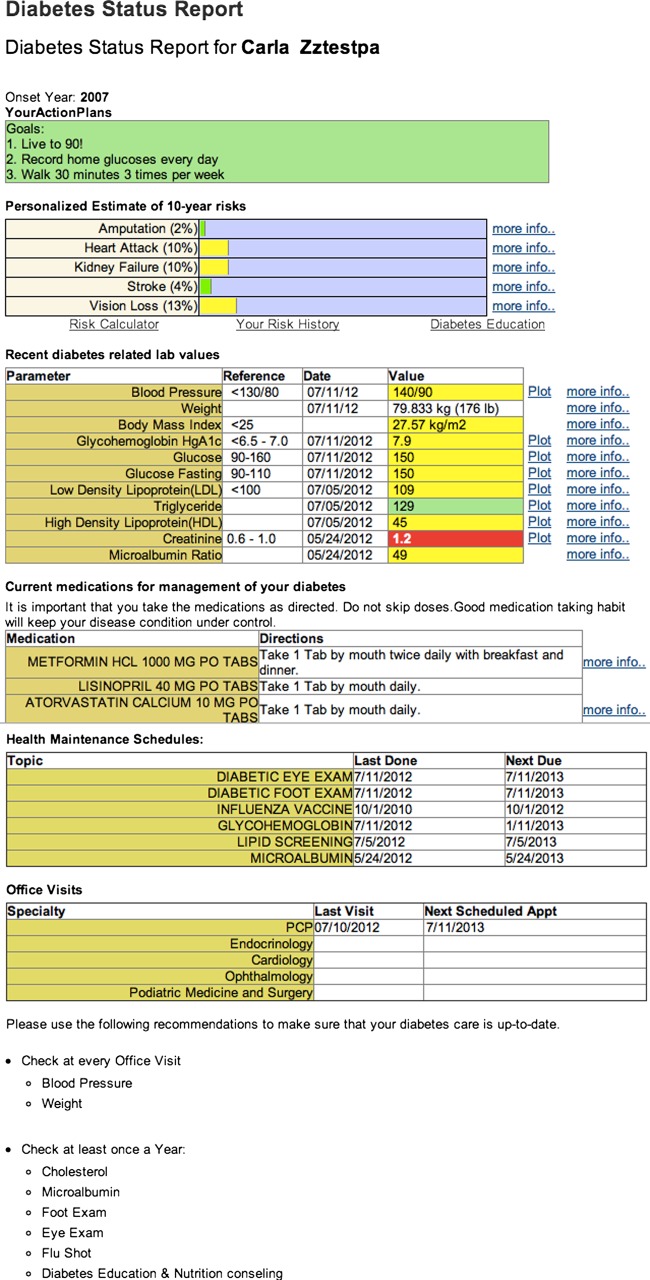
One of the most significant barriers to diabetes self-management is the burden of tracking and collating all of the important elements to manage the disease. Consequently, we developed a diabetes status report (figure 1), an organized, consolidated summary of key parameters of diabetes care, enabling patients to better understand the relationship of these data to diabetes management and their risk of long-term complications. The diabetes status report (figure 1) displayed the following data to the patient in the PHR:
An action plan—leveraging the principles of the Chronic Care Model4; the patient's customized action plans developed in collaboration with the NCM appear at the top of the report
A morbidity risk calculator—Using a verified disease model for complications of diabetes (Diabetes S.E.T. for Success V.2.0; Medicom Digital and GlaxoSmithKline) populated by the patient's data, the interactive risk calculator provides both a current personalized estimate of the diabetes complications and an opportunity to project how behavioral modifications such as smoking cessation can reduce risk
Vital signs—link to the biometric parameters stored in the EHR
Laboratory test results—selection of the test results most relevant to diabetes management
Diabetes-related medications—emphasizing the medications most important to risk reduction of diabetes complications
Relevant health maintenance recommendations—reminders of preventive medicine and disease management recommendations that reduce the risk of diabetic complications.
Upcoming diabetes-related clinical appointments.
Figure 1.
Diabetes status report.
Data collection and reporting
Clinical measurements, adverse event reports, and online questionnaires were collected from all participants at 6 and 12 months by a research assistant blinded to randomization status.
Measures
The primary outcome was glucose control, measured by A1C, over a 12-month period. A clinically significant decrease in A1C was defined as a decline of ≥0.5%, a standard described in many analyses.13 Secondary outcomes included blood pressure, LDL cholesterol, 10-year Framingham cardiovascular risk, satisfaction, and psychosocial well-being. Healthcare utilization of all participants was documented. Participant self-reported data were collected through online questionnaires, including:
Diabetes Knowledge Test—a 14-item assessment of knowledge about diet, glycemic control, glucose testing, complications, and insulin-use.
Problem Areas in Diabetes—measures diabetes-related stress in response to 20 common situations.14
Patient Health Questionnaire (PHQ-9)—depression screening tool.15
Diabetes Treatment Satisfaction Questionnaire (DTSQ)—the eight-item status version (DTSQs) was used for the baseline assessment of total diabetes treatment satisfaction, treatment satisfaction in specific areas, and perceived frequencies of hypo- and hyper-glycemia. An eight-item change version (DTSQc), administered at the 6- and 12-month follow-up, was adapted to assess changes in satisfaction.
CAHPS Clinician and Group Survey assessed patient experience in access to care, clinician communication, shared decision making, and cost of care.
Statistical methods
Patients were analyzed by group assignment at randomization in an intention-to-treat analysis. For participants with missing data, all data collected before attrition were used in the analysis. We also performed a sensitivity analysis to address attrition using the last-observation-carried-forward approach to missing data, which showed similar results to the primary analysis and was not reported.
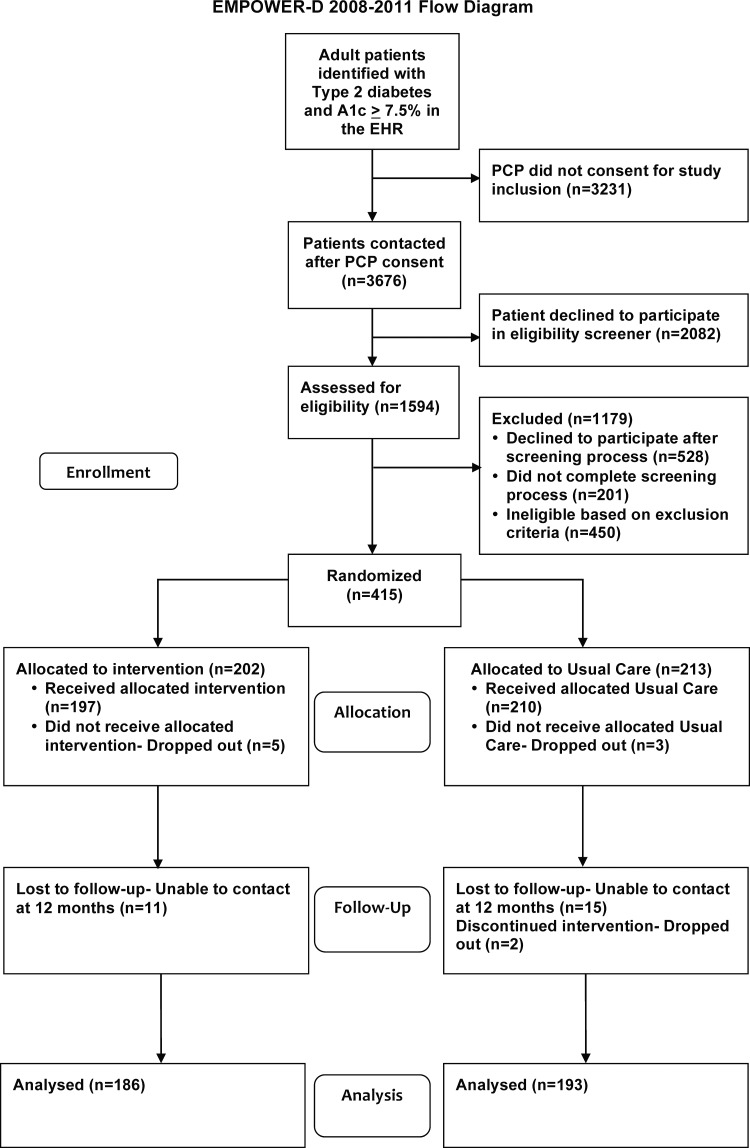
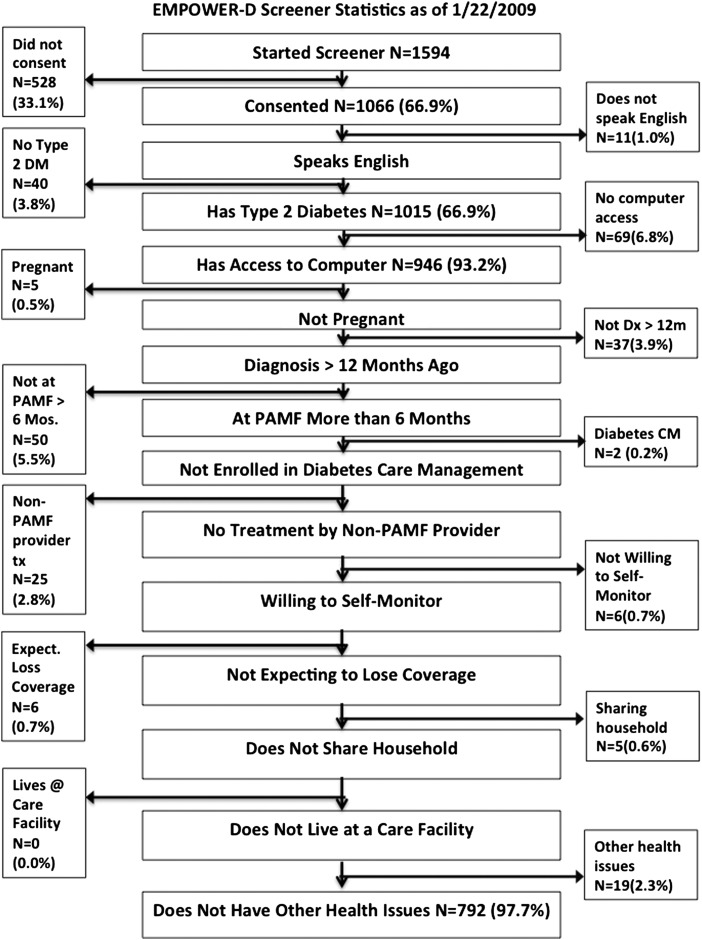
Two-sample t tests and χ2 tests were used to test for differences in demographic and clinical characteristics at baseline between the INT and UC groups. For each dependent variable, mixed-effects regression models were used with 6- and 12-month follow-up with adjustment for the baseline value. For each model, a random effect for patient was included to account for within-patient correlation. In the models, time was included as a categorical variable, with time, INT status, and their interaction included in the model as fixed effects. Appropriate contrasts were used to estimate and test the INT effect at each time point. All analyses were performed using SAS V.9.2. A recruitment diagram is shown in figure 2. Figure 3 shows losses and exclusions during recruitment.
Figure 2.
Recruitment flow diagram. PCP, primary care provider.
Figure 3.
Patient exclusion by criterion. CM, care management; DM, diabetes mellitus; Dx, diagnosis; PAMF, Palo Alto Medical Foundation; Tx, treatment.
For certain post hoc analyses, we categorized patient outcomes into four groups based on change in A1C values at 6 and 12 months. Improvement was defined as 0.5% decrease in A1C, and we performed a sensitivity analysis using 1.0% decrease in A1C. χ2 tests and analysis of variance were applied to test for differences in demographic and INT-related measures (logins, uploads, glucose readings) between the four improvement groups for the INT patients. For several of these comparisons, where the data were highly skewed, the Kruskal–Wallis test was applied instead of analysis of variance.
Results
Of the 6907 potential study participants identified through the EHR and approved by their physician to be invited to participate, 1594 were contacted and agreed to complete the online screening questionnaire. Of the patients screened, 768 (48.2%) met the inclusion criteria and 415 consented to participate in the study. Two hundred and two (48.7%) patients were randomly assigned to INT, and 213 (51.3%) were assigned to UC.
Of the 415 enrolled patients, 10 formally dropped out of the study (2.4%). Of the remaining 405 patients, 351 (87%) completed all of the 12-month data collection. An additional 28 patients completed their 12-month A1C measurements, but not other aspects of data collection. The primary outcome variable, the 12-month A1C level, was collected for 379 (91%) patients. The demographic characteristics and baseline clinical measures were comparable between INT and UC (table 1).
Table 1.
Baseline data
| Characteristic | Intervention (n=202) | Usual care (n=213) |
|---|---|---|
| Demographic, n (%) | ||
| Ethnicity | ||
| White | 121 (60) | 123 (58) |
| Black or African-American | 7 (3) | 15 (7) |
| Asian | 42 (21) | 47 (22) |
| Native Hawaiian | 3 (1) | 3 (1) |
| American Indian | 3 (1) | 1 (0) |
| Hispanic | 20 (10) | 19 (9) |
| Declined to state | 6 (3) | 5 (2) |
| Age at first contact | ||
| 18–29 | 1 (0) | 1 (0) |
| 30–39 | 18 (9) | 21 (10) |
| 40–49 | 54 (27) | 48 (23) |
| 50–59 | 69 (34) | 81 (38) |
| 60–69 | 43 (21) | 52 (24) |
| 70–79 | 15 (7) | 10 (5) |
| 80–89 | 2 (1) | 0 (0) |
| Mean=54.0 | Mean=53.5 | |
| SD=10.7 | SD=10.2 | |
| Range=28–85 | Range=26–77 | |
| Sex | ||
| Male | 119 (58.9) | 130 (61) |
| Female | 83 (41.1) | 83 (39) |
| Education | ||
| Grades 1–8 | 1 (0) | 0 (0) |
| Grades 9–11 | 2 (1) | 2 (1) |
| Grade 12 or GED | 18 (9) | 27 (13) |
| College, 1–3 years | 69 (34) | 71 (33) |
| College, 4 years or more | 56 (28) | 57 (27) |
| Postgraduate | 56 (28) | 56 (26) |
| Clinical, mean (SD) | ||
| A1c at baseline (%) | 9.24 (1.59) | 9.28 (1.74) |
| LDL cholesterol (mg/dl) | 98.4 (34.0) | 95.0 (34.8) |
| BP controlled (%) | 87.3 | 83.2 |
| Systolic BP (mm Hg) | 126.1 (12.5) | 127.0 (14.4) |
| Diastolic BP (mm Hg) | 72.7 (9.5) | 72.6 (9.4) |
| Weight (pounds) | 215.3 (49.4) | 218.4 (51.3) |
BP, blood pressure; GED, general education diploma; LDL, low-density lipoprotein.
Primary outcome
Compared with UC (table 2), participants in the INT group had significantly better diabetes control than those in the UC group as measured by A1C at 6 months, adjusted for baseline levels (−1.32% INT vs −0.66% UC; p<0.001). At 12 months, the difference in A1C was not significant between groups (−1.14% INT vs −0.95% UC; p=0.133). Additional models with adjustment for age, gender, race, and ethnicity produced similar results.
Table 2.
Biometric outcomes over time by intervention status
| Usual care | Intervention | ||||
|---|---|---|---|---|---|
| Outcome | N | % or mean (SD) | N | % or mean (SD) | p Value |
| A1C (%) | |||||
| Baseline | 213 | 9.28 (1.74) | 202 | 9.24 (1.59) | 0.791 |
| 6 months | 189 | 8.62 (1.94) | 185 | 7.92 (1.39) | <0.001 |
| 12 months | 193 | 8.33 (1.81) | 186 | 8.10 (1.68) | 0.133 |
| LDL cholesterol (mg/dl) | |||||
| Baseline | 198 | 98.4 (34.0) | 184 | 95.0 (34.8) | 0.341 |
| 6 months | 184 | 96.7 (35.0) | 178 | 90.1 (32.7) | 0.059 |
| 12 months | 189 | 98.4 (32.4) | 183 | 88.9 (33.5) | 0.001 |
| Weight (pounds) | |||||
| Baseline | 213 | 215.3 (49.4) | 202 | 218.4 (51.3) | 0.530 |
| 6 months | 190 | 213.9 (49.2) | 185 | 218.4 (53.0) | 0.757 |
| 12 months | 191 | 215.7 (51.0) | 188 | 218.8 (52.9) | 0.232 |
| Blood pressure control (mm Hg) | |||||
| Systolic | |||||
| Baseline | 213 | 126.1 (12.5) | 202 | 127.0 (14.4) | 0.513 |
| 6 months | 188 | 123.8 (12.7) | 185 | 123.7 (12.5) | 0.658 |
| 12 months | 192 | 120.8 (11.5) | 189 | 119.9 (11.4) | 0.306 |
| Diastolic | |||||
| Baseline | 213 | 72.7 (9.5) | 202 | 72.6 (9.4) | 0.965 |
| 6 months | 188 | 71.6 (9.0) | 185 | 71.1 (9.4) | 0.737 |
| 12 months | 192 | 72.5 (8.3) | 189 | 71.7 (8.9) | 0.374 |
| Framingham risk (%) | |||||
| Baseline | 213 | 5.7 (5.8) | 202 | 5.5 (5.8) | 0.727 |
| 6 months | 184 | 5.4 (5.7) | 179 | 4.9 (5.3) | 0.083 |
| 12 months | 182 | 5.2 (5.7) | 170 | 4.9 (5.4) | 0.051 |
Secondary outcomes
The INT group had significantly better control of their LDL cholesterol at 12 months, compared with the UC group (−6.1 mg/dl INT vs 0.0 mg/dl UC, p=0.001) (table 2). There were no statistically significant differences between the INT and UC groups at 12 months for blood pressure (systolic or diastolic), weight, or Framingham risk. Of the INT patients, 177 (88%) wirelessly uploaded home glucose readings. Significantly more INT than UC patients initiated online messages to providers, mostly NCMs (145 (71.8%) vs 81 (38%); p<0.001).
Regarding medication management, there was a significant difference in the two groups at 12 months in the number of medication orders to initiate a new medication or change an existing medication (1312 INT vs 1158 UC, p=0.02) and number of insulin orders (336 INT vs 170 UC, p=0.002). Refills of existing medications were excluded from this analysis. Intensification of diabetes treatment, as defined by either addition of a new diabetes treatment or increase in dose of an existing medication, was increased in the INT group compared with the UC group (563 vs 401, p=0.001). For patients already receiving insulin, the INT group significantly increased the doses of insulin (227 vs 90, p=0.001).
In terms of healthcare utilization, there were no significant differences in the number of total physician visits (3.5 (3.4) vs 3.3 (2.9); p=0.53) or physician visits for diabetes (2.4 (2.0) vs 2.3 (1.9); p=0.46) between the INT and UC groups.
For all survey scales, INT and UC participants had statistically similar baseline raw scores. At 12 months, participants in the INT group had significantly lower treatment-distress scores compared with those in the UC group (0.6 (0.8) vs 1.0 (1.0), p<0.001). Other subscales of diabetes distress (food, social support) and results of depression screening were not different between the two groups at 12 months. Compared with UC, INT participants had better knowledge about blood glucose testing (1.8 (0.4) vs 1.6 (0.6), p=0.004) and an understanding of diabetes (4.9 (1.0) vs 4.3 (1.3), p<0.001) at 12 months. INT patients also had greater overall treatment satisfaction (27.7 (6.1) vs 24.5 (7.4), p<0.001) and willingness to recommend treatment to others (5.1 (1.4) vs 4.2 (1.6), p<0.001) at 12 months.
Post hoc analysis
To explore the temporal effects of the INT at 6 and 12 months, we conducted a post hoc categorization of study participants into four primary outcome categories for 6-month and 12-month A1C improvement (defined as a reduction in A1C>0.5%): (1) no improvement at 6 or 12 months; (2) improvement at 6 months alone; (3) improvement at 12 months alone; (4) improvement at 6 and 12 months. Participants whose A1C improved during the INT period significantly obtained more glucose readings and initiated more uploads compared with those patients who did not improve (table 3). Significantly more patients in the INT group improved diabetes control than UC at both 6 months (70.3 (95% CI 63.6 to 76.9) vs 53.4 (95% CI 46.3 to 60.6); p=0.002) and 12 months (69.9 (95% CI 63.2 to 76.5) vs 55.4 (95% CI 48.4 to 62.5); p=0.006). The percentage of patients in each group improving ≥ 1% was not significantly different.
Table 3.
Comparison of intervention patients with 0.5% change in A1c
| No improvement (n=35) | Improvement at 6 months, regression at 12 months (n=21) | Improvement at 12 months only (n=23) | Improvement at 6 and 12 months (n=107) | p Value | |
|---|---|---|---|---|---|
| No of glucose readings at 6 months | 134.3 (140.3) | 164.9 (148.6) | 189.8 (195.0) | 237.4 (197.1) | 0.013 |
| No of glucose readings between 6 and 12 months | 48.9 (120.0) | 90.4 (156.1) | 113.7 (140.4) | 161.9 (193.3) | <0.001 |
| Base A1C (%) | 8.7 (1.1) | 9.1 (1.4) | 8.5 (1.0) | 9.6 (1.7) | 0.001 |
Values are mean (SD).
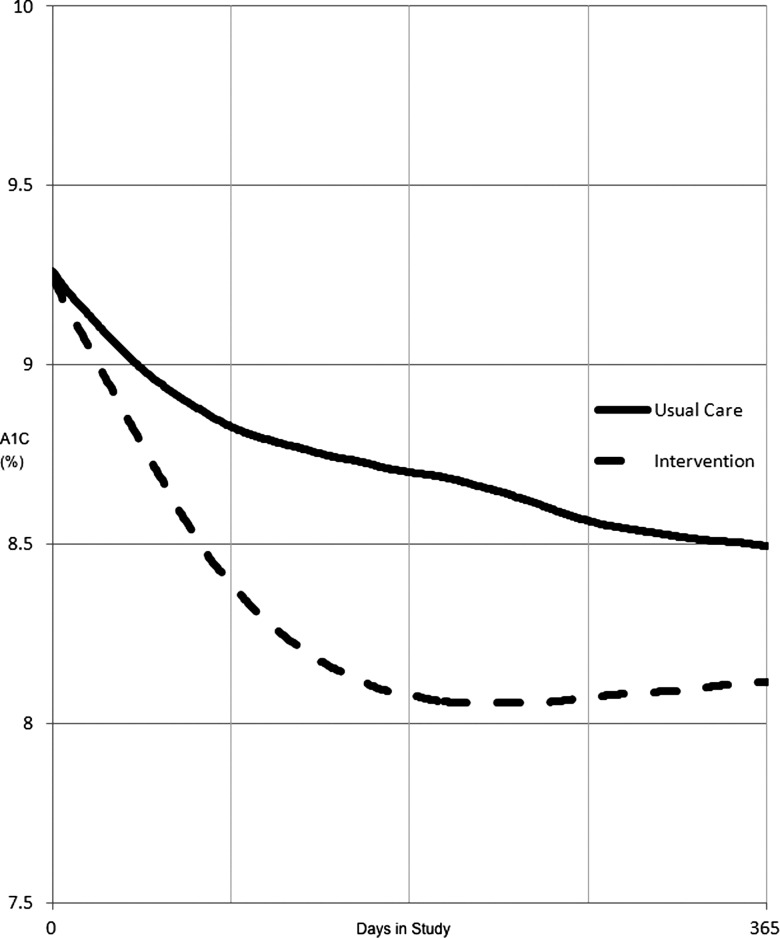
Figure 4 shows a graph of the estimated daily A1C values for patients in each group during study participation. Since the percentage of glycosylated hemoglobin varies slowly over time as a function of the ‘average’ serum glucose concentration, we estimated the daily A1C values in between actual laboratory readings using a linear interpolation. We then plotted the average of all patients’ interpolated daily A1C levels during their 12-month study period.
Figure 4.
Plot of average estimated daily hemoglobin A1C for intervention versus usual care. Estimated daily A1C levels were calculated using a linear interpolation between actual A1C measurements.
Serious adverse events
All adverse event reports were reviewed by the study physician and clinical pharmacist, and were reported to the Institutional Review Board and Data Safety and Monitoring Board. There were no deaths in the study population. There was no significant difference in the number of serious adverse events or adverse events such as hypoglycemia between the two groups, and none of the adverse events were attributed to study participation.
Discussion
The INT group was able to achieve a sustained and rapid reduction in population mean A1C at 6 and 12 months after randomization. However, the difference in A1C reduction compared with UC was not statistically significant at 12 months. The cause of the slower and delayed drop in A1C in the UC group is uncertain. A number of possibilities could contribute to the improvement under UC. First, the physicians and patients in UC, like the INT group, received results of three A1C test during the study, according to the protocol. For both groups, the mean time to A1C testing decreased markedly (180 days vs 148 for UC and 174 vs 138 for INT; p<0.001) compared with 14 months before the study. Viewing the results of the A1C testing for patients with uncontrolled diabetes in UC could have stimulated behavioral changes in either physicians or patients. Second, there could have been a ‘Hawthorne effect’ (changes influenced by being observed in a study) as a result of participating in the study. Third, although physicians and their patients who were in another ‘official’ diabetes quality improvement (QI) project were excluded from our study, diabetes has been a focus for PAMF QI projects for over a decade. More than 70% of PAMF's patients with diabetes have A1C levels <7.0%. In addition, PAMF has been using an EHR with extensive clinical decision support, shown to improve care of diabetic patients, and high-performing medical groups that use a robust EHR have a higher rate of improvement.16 Conducting our clinical trial under these circumstances may have limited our ability to detect a statistically significant difference between the INT group and the constantly improving UC group. The magnitude of the overall reduction in A1C (1.14%) might have been more meaningful in a different setting. Finally, regression to the mean may have contributed to A1C reduction in both groups.
In our post hoc analysis, a significantly higher proportion of INT patients improved their diabetes control than those under UC. Some have referred to these patient-specific improvement measures as ‘δ measures’. Active discussion of δ measures is currently taking place at the National Quality Forum and within the US Department of Health and Human Services because delta measures may represent a complementary method of reporting patient-specific improvement that may be more meaningful to consumers.
Recent reviews17 18 of INTs including web-based systems to improve A1Cs concluded that there is no strong evidence supporting their benefit. There are only a few randomized controlled trials of health information technology-based, patient-directed INTs that assess outcomes at 12 months or longer. The largest included 1665 Medicare beneficiaries supported by diabetes center-based case managers which, in an initial analysis, demonstrated a small difference for the INT group at 12 months,19 while in a subsequent analysis, they did not observe any difference in A1C at 12 months, but did demonstrate small differences in years 4 and 5.20 Two other much smaller studies, one with 83 patients in a general medicine setting and A1C >7.0%21 and another with 163 commercially insured patients (118 of whom were in arms comparable to our study) and A1C >7.5%,22 respectively demonstrated a modest (0.7%) and no decrease in A1C at 12 months compared with UC when adjusted for patient characteristics. Another small study with 104 patients that compared older Veteran's Administration patients with A1C levels >9.0% demonstrated a 0.7% decrease in A1C compared with UC.23 None involved use of mobile health tools. Our randomized controlled trial was larger than all of the studies except that of Shea et al19 with 415 patients, and, unlike the study of Shea et al, was contained completely in a primary care setting and focused on patients with suboptimal control (A1C>7.5%). McMahon et al23 were able to demonstrate a larger improvement in A1C in an elderly population of Veteran's with poor control (baseline A1C>9.0%). The pilot study of Ralston et al,21 which allowed care managers to directly alter therapeutic regimens, achieved a somewhat greater improvement in A1C than observed in our study (0.7% vs 0.2%). Although the comparable groups in the study of Quinn et al22 showed a difference in A1C improvement, the difference was not significant when adjusted for baseline A1Cs. Particularly given the limited evidence, our study adds additional insight into the potential value of web-based systems in primary care to improve A1C levels in patients with poorly controlled type 2 diabetes.
Not everyone benefited from the resources included in the EMPOWER-D INT to the same degree. Participants who tested their home glucose and uploaded their results more often were more likely to have improved at 6 and 12 months than those who did not. Active participation in measurement and communication is one measure of patient activation. Even though NCMs received computer-generated notifications when individual patients were not uploading glucose readings as scheduled, not all patients were responsive to NCMs’ encouragement. Studies to understand factors that might predict patient engagement and activation may help in the selection of patients who are more likely to benefit from this specific INT, analogous to the use of genetic testing in personalized medicine to help find the right drug for a specific patient.
Most of the extant QI programs in diabetes at PAMF are physician-based. Primary-care physician time is at a premium. Time spent managing common chronic diseases and satisfy US Preventive Services Task Force (USPSTF) recommendations for preventive care would take 18 h every day.24 25 Although the EMPOWER-D INT protocol did not make any recommendations about the frequency of physician visits, future studies should explore whether online disease management occurring between physician visits can reduce the frequency of physician visits, allowing physicians to focus on more complex cases.
Limitations
This study was conducted in a large, integrated group practice. Consequently, the results may not apply to smaller, independent practices. This study purposely focused attention on patients with uncontrolled diabetes with a diagnosis of over a year. Consequently, some of the patients who could potentially benefit from the INT were not eligible to receive it.
Conclusions
We demonstrated that a nurse-led, multidisciplinary health team could manage a population of diabetic patients primarily using online management and communication tools.
While initial improvements in mean A1C control were pronounced, rapidly obtained, and sustained at 12 months, the statistically significant difference from UC present at 6 months was not sustained at 12 months. This appears to be related to significant improvements in the UC group in our setting. More patients in the INT group achieved clinically meaningful improvement in A1C than in the UC group. Patients demonstrating continuous engagement through sustained uploading of glucose readings achieved better results. Further study is required to evaluate whether online disease management by multidisciplinary teams can reduce the frequency of physician visits while improving control of diabetes.
Acknowledgments
This work was supported by the Agency for Healthcare Research and Quality, AHRQ grant #1R18HS017179-01. We thank Elizabeth Silva, Palo Alto Medical Foundation, for her coordination support for the project. We would like to acknowledge the support of Numera, Epic Systems, Palm Corporation, and Sprint for their generous contributions of devices, consultation, and service plans to the study. This research was conducted with support from the Investigator-Initiated Study Program of LifeScan, Inc. We would also like to acknowledge the participation of S Wilson, J Ma, and V Luna, Palo Alto Medical Foundation Research Institute, in the early phase of the study.
Footnotes
Contributors: PCT substantively contributed to study conception, design, implementation, interpretation, and writing of the manuscript, and is the guarantor responsible for the content of the article. NLB contributed to the design, implementation, interpretation, and writing of the manuscript. JMO, BA, ASC, MPE, SLH, SMH, LHK, CJM, LSQ, TAW, LJW and CYY contributed to the implementation, interpretation and writing of the manuscript. AJP contributed to the interpretation and writing of the manuscript. PCT is the guarantor of the manuscript.
Funding: Agency for Healthcare Research and Quality, grant No 1R18HS017179-01.
Competing interests: JMO became an employee of Siemens after the study was completed.
Ethics approval: Palo Alto Medical Foundation Research Institute Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cheung BM, Ong KL, Cherny SS, et al. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med 2009;122:443–53 [DOI] [PubMed] [Google Scholar]
- 2.Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med 2004;140:945–50 [DOI] [PubMed] [Google Scholar]
- 3.Hoerger TJ, Segel JE, Gregg EW, et al. Is glycemic control improving in U.S. adults? Diabetes Care 2008;31:81–6 [DOI] [PubMed] [Google Scholar]
- 4.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q 1996;74:511–44 [PubMed] [Google Scholar]
- 5.Wagner EH. The role of patient care teams in chronic disease management. BMJ 2000;320:569–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang PC, Ash JS, Bates DW, et al. Personal health records: definitions, benefits, and strategies for overcoming barriers to adoption. J Am Med Inform Assoc 2006;13:121–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang PC, Lansky D. The missing link: bridging the patient-provider health information gap. Health Aff (Millwood) 2005;24:1290–5 [DOI] [PubMed] [Google Scholar]
- 8.Halamka JD, Mandl KD, Tang PC. Early experiences with personal health records. J Am Med Inform Assoc 2008;15:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA 2002;288:1775–9 [DOI] [PubMed] [Google Scholar]
- 10.Grimshaw JM, Shirran L, Thomas R, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care 2001;39:II2–45 [PubMed] [Google Scholar]
- 11.Pocock SJ. Clinical trials, a practical approach. Chichester: John Wiley and Sons, 1991 [Google Scholar]
- 12.Funnell MM, Brown TL, Childs BP, et al. National standards for diabetes self-management education. Diabetes Care 2012;35(Suppl 1):S101–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32: 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–60 [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cebul RD, Love TE, Jain AK, et al. Electronic health records and quality of diabetes care. N Engl J Med 2011;365:825–33 [DOI] [PubMed] [Google Scholar]
- 17.Seitz P, Rosemann T, Gensichen J, et al. Interventions in primary care to improve cardiovascular risk factors and glycated haemoglobin (HbA1c) levels in patients with diabetes: a systematic review. Diabetes Obes Metab 2011;13:479–89 [DOI] [PubMed] [Google Scholar]
- 18.Franc S, Daoudi A, Mounier S, et al. Telemedicine and diabetes: achievements and prospects. Diabetes Metab 2011;37:463–76 [DOI] [PubMed] [Google Scholar]
- 19.Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc 2006;13:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shea S, Weinstock RS, Teresi JA, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. J Am Med Inform Assoc 2009;16:446–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ralston JD, Hirsch IB, Hoath J, et al. Web-based collaborative care for type 2 diabetes: a pilot randomized trial. Diabetes Care 2009;32:234–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn CC, Shardell MD, Terrin ML, et al. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011;34:1934–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon GT, Gomes HE, Hickson HS, et al. Web-based care management in patients with poorly controlled diabetes. Diabetes Care 2005;28:1624–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarnall KS, Pollak KI, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health 2003;93:635–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med 2005;3: 209–14 [DOI] [PMC free article] [PubMed] [Google Scholar]