Abstract
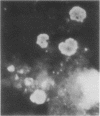
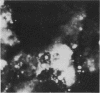
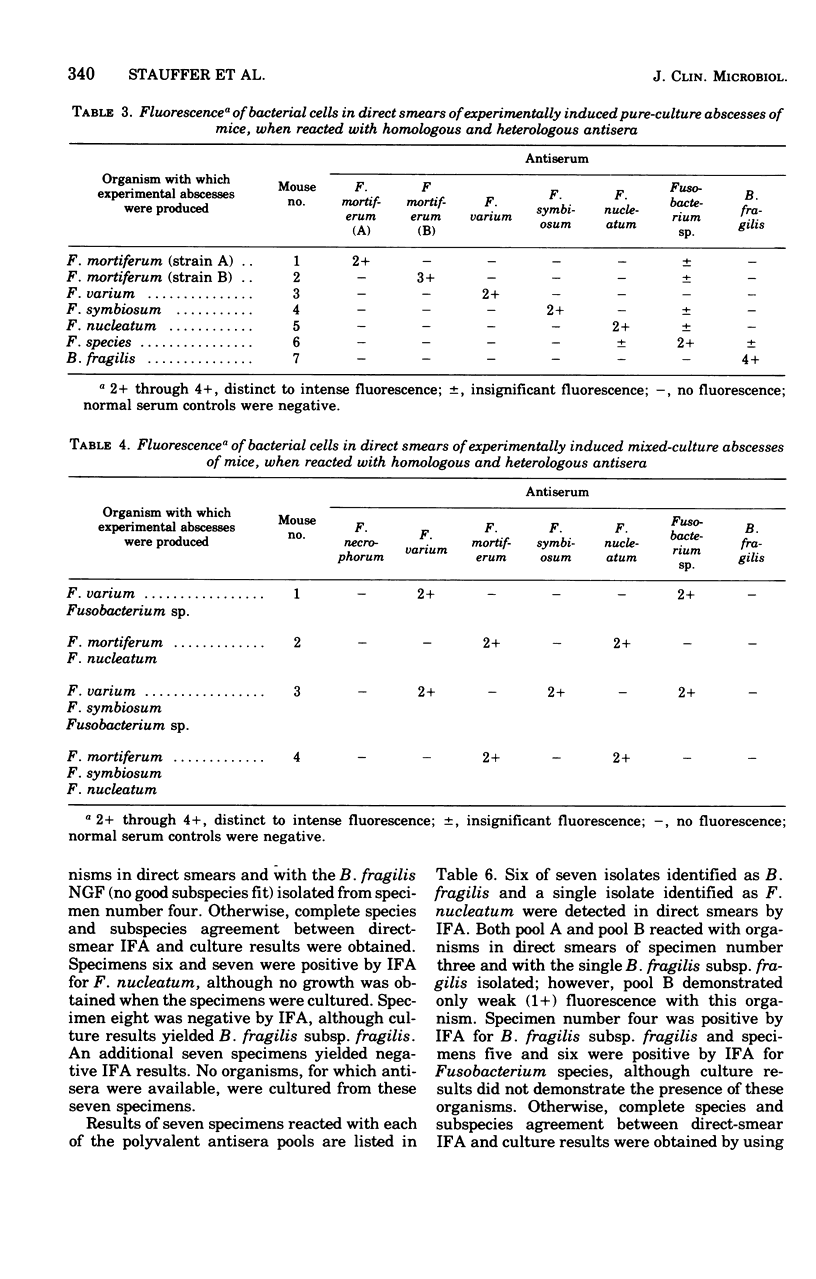
An indirect fluorescent antibody (IFA) technique was evaluated as a procedure for rapid detection and identification of members of the Bacteroidaceae. Antisera were prepared against 31 members of this family, including species of Bacteroides and Fusobacterium commonly isolated from human infections. The antisera had demonstrated species and/or subspecies specificity. Thirty clinical specimens were studied. Of 13 specimens yielding Bacteroidaceae, for which antisera were available, 23 were presumptively diagnosed by IFA to contain subspecies of B. fragilis and/or Fusobacterium species. Of 17 specimens yielding negative culture results, two were positive by IFA on direct smear. Frequently the in vivo morphology of cells detected in direct smears by this procedure closely mimicked that of cellular debris, tissue cells, and leukocytes. Polyvalent antisera pools facilitated use of the IFA procedure as a practical tool for rapid diagnosis of infections involving the Bacteroidaceae.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altemeier W. A., Hill E. O., Fullen W. D. Acute and recurrent thromboembolic disease: a new concept of etiology. Ann Surg. 1969 Oct;170(4):547–558. doi: 10.1097/00000658-196910000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson D., Lambe D. W., Jr, Persson S. The immune response in a patient to an infection with Bacteroides fragilis ss. fragilis and Clostridium difficile. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):709–712. doi: 10.1111/j.1699-0463.1972.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Feingold D. S. Biology and pathogenicity of microbial spheroplasts and l-forms. N Engl J Med. 1969 Nov 20;281(21):1159–1170. doi: 10.1056/NEJM196911202812106. [DOI] [PubMed] [Google Scholar]
- Finegold S. M. Infections due to non-sporeforming anaerobic bacteria. Del Med J. 1970 Nov;42(11):298–passim. [PubMed] [Google Scholar]
- Finegold S. M., Rosenblatt J. E. Practical aspects of anaerobic sepsis. Medicine (Baltimore) 1973 Jul;52(4):311–322. doi: 10.1097/00005792-197307000-00010. [DOI] [PubMed] [Google Scholar]
- Futch C., Zikria B. A., Neu H. C. Bacteroides liver abscess. Surgery. 1973 Jan;73(1):59–65. [PubMed] [Google Scholar]
- Garcia M. M., Neil D. H., McKay K. A. Application of immunofluorescence to studies on the ecology of Sphaerophorus necrophorus. Appl Microbiol. 1971 May;21(5):809–814. doi: 10.1128/am.21.5.809-814.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M. H. Fluorescent antibody techniques in the identification of the gram-negative nonsporeforming anaerobes. Health Lab Sci. 1970 Apr;7(2):78–83. [PubMed] [Google Scholar]
- Ingham H. R., Selkon J. B., Codd A. A., Hale J. H. A study in vitro of the sensitivity to antibiotics of Bacteroides fragilis. J Clin Pathol. 1968 Jul;21(4):432–436. doi: 10.1136/jcp.21.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny J. F. Bacterial variants in central nervous system infections in infants and children. J Pediatr. 1973 Oct;83(4):531–542. doi: 10.1016/s0022-3476(73)80211-2. [DOI] [PubMed] [Google Scholar]
- Kislak J. W. The susceptibility of Bacteroides fragilis to 24 antibiotics. J Infect Dis. 1972 Mar;125(3):295–299. doi: 10.1093/infdis/125.3.295. [DOI] [PubMed] [Google Scholar]
- Lambe D. W., Jr Determination of Bacteroides melaninogenicus serogroups by fluorescent antibody staining. Appl Microbiol. 1974 Oct;28(4):561–567. doi: 10.1128/am.28.4.561-567.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh D. A. Clinical importance of infections due to Bacteroides fragilis and role of antibiotic therapy. Br Med J. 1974 Jul 27;3(5925):225–228. doi: 10.1136/bmj.3.5925.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Gardner M., Washington J. A., 2nd In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972 Feb;1(2):148–158. doi: 10.1128/aac.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J. Isolation and indentification of anaerobic bacteria in the clinical laboratory. A 2-year experience. Mayo Clin Proc. 1974 May;49(5):300–308. [PubMed] [Google Scholar]
- Pearson H. E., Anderson G. V. Bacteroides infections and pregnancy. Obstet Gynecol. 1970 Jan;35(1):31–36. [PubMed] [Google Scholar]
- Quick J. D., Goldberg H. S., Sonnenwirth A. C. Human antibody to Bacteroidaceae. Am J Clin Nutr. 1972 Dec;25(12):1351–1356. doi: 10.1093/ajcn/25.12.1351. [DOI] [PubMed] [Google Scholar]
- SALIBI B. BACTEROIDES INFECTION OF THE BRAIN. SUCCESSFUL MANAGEMENT OF CASE WITH THREE INTRACRANIAL ABSCESSES. Arch Neurol. 1964 Jun;10:629–634. doi: 10.1001/archneur.1964.00460180095009. [DOI] [PubMed] [Google Scholar]
- Spiers M. Classification systems of the Bacteroides group. Med Lab Technol. 1971 Oct;28(4):360–366. [PubMed] [Google Scholar]
- Zabransky R. J. Isolation of anaerobic bacteria from clinical specimens. Mayo Clin Proc. 1970 Apr;45(4):256–264. [PubMed] [Google Scholar]
- Zierdt C. H., Wertlake P. T. Transitional forms of Corynebacterium acnes in disease. J Bacteriol. 1969 Feb;97(2):799–805. doi: 10.1128/jb.97.2.799-805.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]