Abstract
Objective
To adapt and automate the medication regimen complexity index (MRCI) within the structure of a commercial medication database in the post-acute home care setting.
Materials and Methods
In phase 1, medication data from 89 645 electronic health records were abstracted to line up with the components of the MRCI: dosage form, dosing frequency, and additional administrative directions. A committee reviewed output to assign index weights and determine necessary adaptations. In phase 2 we examined the face validity of the modified MRCI through analysis of automatic tabulations and descriptive statistics.
Results
The mean number of medications per patient record was 7.6 (SD 3.8); mean MRCI score was 16.1 (SD 9.0). The number of medications and MRCI were highly associated, but there was a wide range of MRCI scores for each number of medications. Most patients (55%) were taking only oral medications in tablet/capsule form, although 16% had regimens with three or more medications with different routes/forms. The biggest contributor to the MRCI score was dosing frequency (mean 11.9). Over 36% of patients needed to remember two or more special instructions (eg, take on alternate days, dissolve).
Discussion
Medication complexity can be tabulated through an automated process with some adaptation for local organizational systems. The MRCI provides a more nuanced way of measuring and assessing complexity than a simple medication count.
Conclusions
An automated MRCI may help to identify patients who are at higher risk of adverse events, and could potentially be used in research and clinical decision support to improve medication management and patient outcomes.
Keywords: medication complexity, home health care, MRCI
Background and significance
Poor adherence to recommended medication treatment plans has been linked to adverse consequences for patients and higher costs.1 Missing doses, not taking medications at the correct time or not following the correct administration instructions can result in the patient receiving a suboptimal clinical outcome. Lack of adherence to medication has been estimated to cause at least 10% of hospital admissions in the USA.2 The World Health Organization suggests that improving adherence would result in more health benefits than by developing new medical treatments.3
Multiple studies have identified a link between management complexity of a medication regimen and non-adherence.4–6 A higher number of medications and complicated schedules or special instructions (eg, time of day, food interactions) can all contribute to greater patient difficulty or interest in following treatment recommendations. Complexity is one of the main root causes of patients’ non-adherence. Simplification of complexity and/or greater attention to managing complexity are potentially remedial factors for poor adherence. However, before remedial action can be taken, patients with complex management regimens must be identified.
In 2004, George and colleagues7 developed a medication regimen complexity index (MRCI), a tool for quantifying multiple features of drug regimen complexity. The MRCI was built on the concepts and factors developed for the medication complexity index8 by assigning weights to dosage forms, dosing frequencies and additional instructions. The aim was to create a reliable tool to quantify regimen management complexity using information found in patient charts and prescriptions for research and practice applications.7
To validate the MRCI, George and colleagues7 reviewed 134 charts of patients using the paper-based method. The interest in the MRCI is evident in efforts made to translate the tool into other languages, the additional validation efforts as well as in a number of other studies that have incorporated the use of the tool in recent years.4 9–13 The investigators of these initiatives have continued to code the MRCI manually.
Objectives
In this article we describe the process of adapting the MRCI for electronic use in a large home health organization. We detail the steps used to translate the MRCI tool for use within an existing electronic health record (EHR) system, which incorporates a commercially available and widely used electronic medication database. We also provide the first comprehensive quantitative data on medication regimen complexity in a large population of post-acute care patients characterized by multiple comorbid conditions and medical complexity. Automation of the MRCI will allow investigators and clinicians a more efficient and structured way to identify patients who may be at higher risk of management difficulties, non-adherence, experiencing suboptimal clinical benefit, and potentially preventable emergent care needs. The MRCI has the potential to serve as a tool in clinical decision support systems.
Materials and methods
The original MRCI
The index includes weighted components of (A) dosage form, (B) dosing frequency and (C) additional administration instructions.7 The minimum MRCI index score for someone on a medication is 1.5, which represents a single tablet or capsule taken once a day as needed; there is no established maximum as the score increases with the number of medications.
Component A: dosage form
This component incorporates a weighting scheme for dosage form (eg, tablet vs spray vs gel), route of administration, and, in some instances, body part. More complex combinations of form, route and body part result in higher weights. For example, a liquid oral medication is given a weight of 2 while a liquid eye drop is given a weight of 3, and an injectable liquid medication receives a weight of 3 (if the syringe is prefilled) or 4 (if in vial or ampoule). The MRCI developers provided weights for 32 form/route/body part combinations. Representative combinations are presented in table 1A (top section).
Table 1.
Alignment of EHR data to MRCI components
| MRCI component A: form/route | MRCI component B: dosing frequency | MRCI component C: special instructions | |||||
|---|---|---|---|---|---|---|---|
| Selected form/route combinations from original MRCI developers | Selected dosing frequency combinations from original MRCI developers | Selected special directions from original MRCI developers | |||||
| Dosage Form | Route | Weight | Frequency 1 | Frequency 2 | Weight | Direction | Weight |
| Tablet | Oral | 1.0 | Once a day | As needed | 0.5 | Take/use at specific times | 1.0 |
| Spray | Topical | 1.0 | Once a day | 1.0 | Take/use in relation to food | 1.0 | |
| Gel | Topical | 2.0 | At bedtime | 1.0 | Multiple units at one time | 1.0 | |
| Spray | Nasal | 2.0 | Every other day | 2.0 | Break or crush tablet | 1.0 | |
| Drop | Oral | 2.0 | Three times a day | 3.0 | Tapering/increasing dose | 2.0 | |
| Drop | Ophthalmic | 3.0 | Every 8 h | 3.5 | Alternating dose | 2.0 | |
| Accuhaler | Inhalation | 3.0 | Every 8 h | As needed | 2.0 | ||
| Ampoule pen | Subcutaneous | 3.0 | Every 6 h | 4.5 | |||
| Ampoule | Subcutaneous | 4.0 | Every 6 h | As needed | 2.5 | ||
| Additional form/route weights established by committee for this EHR application | Examples of additional ‘& as needed’ frequency weights established for this EHR application | Additional special instruction established by committee for this EHR application | |||||
| Liquid | Intravenous | 3.0 | Every 8 h | & as needed | 4.0 | Take/use based on sliding scale | 2.0 |
| Implant | Subcutaneous | 1.0 | Every 6 h | & as needed | 5.0 | ||
| MRCI instructions: A given form/route combination is counted only once within a regimen. For example, if a patient's regimen solely consists of five tablets orally their component A subscore=1 EHR translation: A total of 460 form/route data field combinations was identified—all could be collapsed and linked with the established MRCI weights with two exceptions: medication implants and medications administered via intravenous therapy |
MRCI instructions: Frequency weights are tabulated to account of all medications. For example, if a patient is on five medications with a frequency of ‘once a day’, that patient's MRCI component B subscore=5 EHR translation: The EHR had two drop-down menus to specify frequency and some special instructions; 376 data field combinations were reviewed and coded based on the MRCI developer specified weights with one exception: if the 2nd frequency field indicated ‘& as needed’ the weight was increased by 0.5 |
MRCI instructions: A weight is given for each instruction per medication. If a patient is on a single medication that needs to be crushed and taken with meals, the component C subscore for that medication=2 EHR translation: Special instructions were obtained from multiple fields in the EHR, including the frequency field that had indicators such as to be taken at bedtime, in the morning, with meals and the comments field with was scanned for words like crushed, sliding scale along with other terms |
|||||
EHR, electronic health record; MRCI, medication regimen complexity index.
Component B: dosing frequency
This component includes 23 weights ranging from 0.5 for a once daily PRN (as needed) to 12.5 for a medication that is prescribed to be taken every 2 h. Table 1B (top section) presents selected frequency weights established by the MRCI developers.
Component C: additional directions
This component provides weights for 10 additional directions a patient may need to follow in adhering to a prescribed regimen. Table 1C (top section) presents selected examples and their assigned weights.
The bottom sections of table 1A–C outline the scoring schema created by the MRCI developers along with information on how we adapted the MRCI in our automation described in detail below. A simple regimen of two tablets, one prescribed to be taken once a day at bedtime and the other to be taken two times a day with meals would have a MRCI score of 6; component A=1 (each dosage form present in a regimen is scored only once under this component)+component B=3 (once daily=1; twice daily=2)+component C=2 (take at a specified time=1; take in relation to food=1).
Automating the MRCI using an electronic database: methods
Setting and sample
We implemented this initiative at the Visiting Nurse Service of New York (VNSNY), the largest, non-profit Medicare/Medicaid-certified home health organization in the USA. We used medication data on all 2008 new admissions to VNSNY's adult acute care program to inform translation of the paper-based MRCI tool to an automated MRCI process. The data file included 89 645 patient cases involving 679 327 medications. The study was approved by the VNSNY institutional review board.
EHR and medication data source
As part of usual practice, VNSNY home care nurses use tablet computers, a mobile point of care platform that runs a secure EHR. The EHR is a largely an agency-developed system. Information on new referrals and continuing patients is regularly updated and wirelessly communicated between the tablet and VNSNY's mainframe. Three key modules in this patient care record system inform nurses’ clinical practice: (1) the plan of care; (2) the visit module; and (3) the medications module. Medication data are entered at start of care and each time a medication change is made by the patient's prescribing provider. This module incorporates the first databank National Drug Data File (FDB), a commercial drug database application. FDB is used to standardize medication documentation, flag potential medication duplication or drug interactions, and serve as a drug reference for field staff. Medications in FDB are linked with individual permanent numeric identifiers that represent a unique combination of product or generic name, route of administration, dosage form, strength, and strength unit of measure. Nurses select from this comprehensive database to populate the EHR medication data for each patient.
To capture the frequency of dosing and some prescription directions, the agency EHR uses two data entry fields with drop-down menus. Each menu has 28 frequency choices to indicate both the number of times to take the medication over a specific time period (eg, TID=three times a day; TIW=three times a week) and specific instructions (eg, AC=before meals; HS=at bedtime).
A free-text comment field linked to each medication allows clinicians to enter additional information they feel important to record (eg, prescribed insulin dose based on blood sugar sliding scale parameters).
Translating the MRCI for use with the electronic data
To estimate the content and scope of available medication data that could contribute to the MRCI index tabulation, data were extracted on dosage form and route, dosage frequency and the text comment fields and imported into spreadsheets. A six-member interdisciplinary committee with nursing, pharmacology and research expertise reviewed the spreadsheets and aligned the medication data with the MRCI components and weighting schema—described in more detail below. The committee met five times over a 3-month period to develop an algorithm and to achieve consensus on adjudicating unusual situations.
Translating MRCI component A: dosage form
A total of 460 distinct form/route combinations was present among medications in the agency dataset. The high number of raw data combinations was partly due to the very detailed form/route data fields in the FDB system. For example, oral tablets/capsules, which have a weight of 1 and are the most frequent route/form of prescribed medications, are expressed in 53 ways; an oral tablet could be expressed simply as ‘tablet oral’ or as ‘tab. SR12H oral’ or ‘tab. SR24H oral’. At the same time, while collapsing many expressions of oral tablet, we had to be careful not to collapse all oral medications because an oral tablet is weighted differently than an oral liquid medication. Furthermore, some medications are available as a combined packet containing different forms (eg, tablet and lozenge in a prescribed packet). In these cases, each form required a weight assignment. In developing the automated algorithm, we chose to crosswalk each of the 460 form/route combinations manually with the MRCI weights to facilitate programing.
The worksheet created for committee review included a column for form (eg, capsule, drops) and a column for route (oral, ophthalmic), corresponding to columns A and B in supplementary appendix table S1 (available online only). The committee matched each FDB combination to a code that represented one or more of the 32 MRCI weights (see supplementary appendix table S4, available online only). The completed list was used as a reference table in the component A portion of the automated MRCI application developed for use at the agency.
Translating MRCI component B: dosing frequency
While identifying a given medication may help to narrow down the potential dosing frequency, it does not provide information about prescribed dosing frequency for a specific individual. Therefore, frequency is not an automatic property linked with a medication in FDB; it must be collected and stored separately. Nurses use the agency-created drop-down fields to record the prescribed frequency for each medication. We empirically identified 376 distinct frequency combinations entered by agency nurses in the study sample. These combinations were reviewed and assigned one or more of the 23 weights established by the MRCI developers (see supplementary appendix table S2, available online only). The committee reviewed each combination of the frequency fields to make sure that the translation to the MRCI weights was done appropriately.
In the agency's EHR, only one of the two available frequency fields is mandatory. Most often, the first mandatory frequency field indicated the number of prescribed doses within a timeframe (eg, BID=twice a day), while the second optional frequency field was used to indicate additional instructions (eg, QAM=every morning; PRN=as needed). When the combination was potentially contradictory, the committee set up decision rules. For example, one field could indicate a medication was taken ‘4 times a day’ while the other field indicated it was taken ‘at bedtime’. In these potentially conflicting situations, the committee made a decision rule to include the higher frequency indicated. As was done for component A, the committee created a crosswalk by assigning a code to represent the MRCI component B weight for each of the 376 combinations—supplementary appendix 2 (available online only). This completed list was used as a reference table for the automated MRCI application.
Translating MRCI component C: additional directions
The agency's EHR includes several data fields that were used to identify medication management instructions that qualified for MRCI scoring. First, the agency-developed drop-down lists used to record frequency data were scanned, as they contained indicators to take medications at specific times (eg, bedtime, in the morning, before or after meals, etc). The frequency fields also include indicators of taking medications less often than daily, which qualifies for component C scoring. Second, we scanned a field in the agency EHR that indicates the number of dosing units to be taken each time a medication is taken. Multiple doses prescribed to take at one time qualifies for component C weighting. Third, we scanned the free-text ‘comment’ fields for a selected set of text patterns corresponding to component C categories. The simple parsing text pattern search was implemented using regular expressions as implemented in Oracle 10 g. For example, we searched for words such as ‘crush’, ‘dissolve’, and ‘taper’ that would indicate the kinds of additional instructions qualifying for MRCI scoring. In developing the text patterns, we reviewed lists of comments captured by each regular expression to identify and modify as necessary to avoid unintended matches. In general, we only accounted for the most common misspellings or abbreviations to avoid excessive customization to a few nurses’ charting tendencies. Expressions that returned unintended matches were modified or paired with exclusionary expressions developed to maximize selectivity; expressions that could not be generally modified to exclude unintended matches were dropped. Supplementary appendix table S3 (available online only) presents the final list of expressions along with the corresponding MRCI section C component, as implemented in our application.
Key decisions and modifications from the original MRCI
The study agency database lacked some information used by the MRCI developers; at the same time, supplementary information about patients’ medication regimens was available in the EHR that is particularly relevant to the home health setting. Consequently, the committee made several key decisions in the adaptation of the original MRCI for the new automated approach and service setting. The committee's approach was to try to capture as much of the management complexity that the electronic data could provide. Specifically, the following questions were addressed:
What should be done about missing information? The original MRCI includes several weights to indicate oxygen use and frequency. However, oxygen use is not recorded by the study agency in the electronic medication fields, so we proceeded without it for the scoring of the index.
Should over-the-counter (OTC) medications, available through FDB but not included in the original MRCI, be added to the complexity index? As part of usual practice at the study agency, nurses record both prescribed and OTC medications. OTC medications are included in the home care record because they can play an important role in a patient's recommended treatment regimen; they also have the potential to interact with prescribed medications. In such instances, there may be a need for nurse review and/or instruction. The committee concluded that if part of the patient's medication management routine includes OTC medications, then they should be included in the MRCI calculation.
Should additional dosage form/route information available at the study agency be considered? If yes, how should it be coded? Agency medication data included two forms/routes that did not have corresponding MRCI weights: medication implants (eg, etonogestrel implant) and medications administered through intravenous therapy. Although these represented a small number of medications, the committee felt it was important to consider them. While these medications may not affect a patient's day to day management of medications, they may require the patient to monitor side effects or attend physician appointments, having some impact on medication management responsibilities. Therefore, implants were assigned a weight of 1 for the dosage form. Intravenous medications were assigned the same weight established for prefilled injectable medications.
Should additional dosing frequency information be considered? If yes, how should it be coded? The EHR data identified one commonly seen frequency field combination about which the MRCI instructions did not provide clear instructions—a defined frequency on the number of times to take daily along with the ‘and as needed’ indicator. For example, weights are provided for the frequency of ‘twice daily’ (=2) and ‘twice daily as needed’ (=1) but not ‘twice daily and as needed’. In these instances, the committee decided to add the weight specified for the number of times to take daily and the weight the MRCI developers assigned the general ‘as needed’ instructions (=0.5) in the calculation of the component B score.
Should additional administration instructions be considered? If yes, how should they be weighted? The committee created an additional direction not represented on the original MRCI—sliding scale instructions for insulin administration—to accommodate the significant prevalence of home healthcare patients with diabetes whose medication regimens include sliding scale insulin. The same weight assigned by the developers to tapering dose special directions was applied to sliding scale directions.
Automating the index
We chose to use the existing technology infrastructure at the agency to implement the algorithm in order to streamline its availability to clinical workflows already in place. We developed an application using the study agency's existing Oracle database through the PL/SQL code, automating the calculation of a MRCI score for each patient based on the adapted MRCI model using the information available in the EHR. The automated algorithm referenced the hard-coded crosswalk lists created for each of the three MRCI components as described above to generate a final score. The application de-duplicated and consolidated the medication list so that medications listed multiple times (eg, to indicate a sliding scale prescription or differing am/pm dosing) were counted only once. MRCI scores were calculated on all new home care admissions in 2008 having at least one medication. The use of existing production systems afforded rapid integration and availability for ongoing use.
Validation of automated components
Scores for components A and B were tabulated by direct mapping to electronic fields that store this information. Information on form, frequency and some special instructions was easily ‘translatable’ and did not require any subjective decisions by the coder. Selected regimens were abstracted and manually compared to make sure the programing was working as expected. Free-text comment field review to capture some of the potential additional directions to be accounted for in component C is subject to greater coder discretion. To evaluate the reliability of the automation process for this more subjective portion of component C, we compared the automated coding results to coding results produced by a clinician. The clinician had been oriented to the MRCI component C additional directions and the sliding scale direction incorporated by the study investigators. The automated algorithm was applied to a validation dataset from 2009. A stratified, random sample of 274 comments containing text assigned to one or more of the 11 MRCI component C additional directions was then drawn, along with a sample of comments that remained unclassified by the algorithm. The clinician separately coded the comments into the MRCI component C additional direction categories. Individual special direction kappa coefficients ranged from 0.79 to 1.00 with an overall kappa of 0.89. Additional information about the interrater reliability test procedures can be found in supplementary appendix table S5 (available online only).
Results
MRCI home care patient results
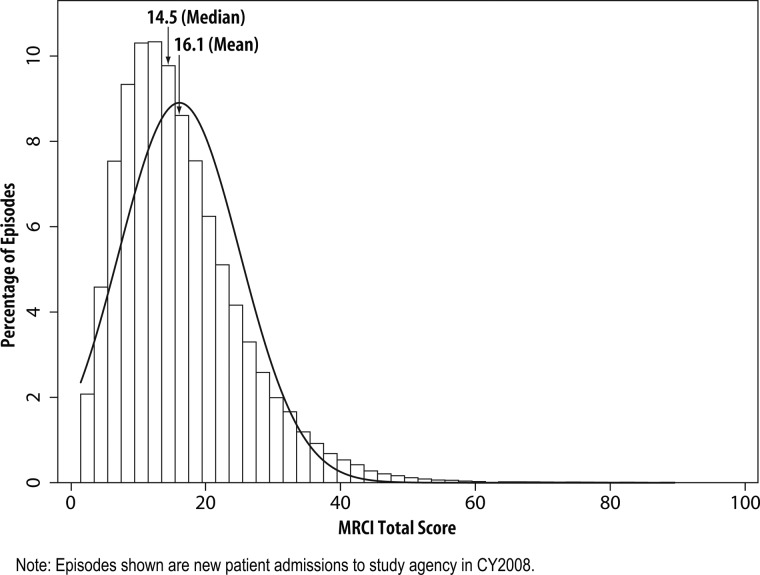
The data file included 89 645 newly admitted patient cases involving 679 327 medication entries. Demographics of the patient population and data on the number of medications they take is shown in table 2. MRCI total scores ranged from 1.5 to 88.5 with a mean average of 16.1 (SD 9.0) (table 2 and figure 1). Most patients (55%) were taking medications in only one route/form (almost all in tablet/capsule form), although 16% were dealing with regimens that had three or more medications with different routes/forms (table 3). The majority of medications were prescribed to be taken once (63%) daily. Seventeen per cent of the medications were prescribed to take twice daily, of which 3% were prescribed specifically to take every 12 h; 10% of the medications were prescribed to be taken three or more times daily, of which 4% were prescribed at certain time intervals. Others were prescribed to be taken as needed once or more often daily (7%) or on alternating days or less frequently (3%) (data not on tables). The biggest contributor to the MRCI score was dosing frequency, with a mean component B score of 11.9 (SD 6.5), which ranged from 0.5 (representing a single PRN dosing regimen) to 64.5 (representing multiple medications with multiple dosing patterns). Based on the EHR, 65% of the patients had to follow at least one special administration instruction; with many being instructed to take the medications at a specific time (eg, at bedtime) (49%) or to take multiple pills of the same medication at the same time (21%). Over 36% of patients needed to remember two or more special instructions (eg, take on alternate days, dissolve).
Table 2.
Characteristics of home health patient episodes* (N=89 645)
| Patient characteristics | Mean (SD) or N (%) | Range |
|---|---|---|
| Age, years | 71 (16) | 18–110 |
| Female sex, N (%) | 55 483 (62%) | |
| Race/ethnicity, N (%) | ||
| White | 44 508 (50%) | |
| Black | 21 669 (24%) | |
| Hispanic | 19 011(21%) | |
| Asian | 4100 (5%) | |
| Other | 357 (<1%) | |
| MRCI profilea | ||
| Count of unduplicated medications | 7.6 (3.8) | 1.0–35.0 |
| MRCI total score | 16.1 (9.0) | 1.5–88.5 |
| MRCI component A score (form/route) | 3.1 (2.8) | 1.0–24.0 |
| MRCI component B score (frequency) | 11.9 (6.5) | 0.5–64.5 |
| MRCI component C score (cirections) | 1.2 (1.5) | 0–18.0 |
*Episodes, new patient admissions to study agency in CY2008.
MRCI, Medication regimen complexity index.
Figure 1.
Distribution of medication regimen complexity index (MRCI) scores (N=89 645).
Table 3.
Medication regimen characteristics
| No of different medication forms/routes | No/% of cases | No of different special instructions | No/% of cases |
|---|---|---|---|
| 0 | 31 722 (35) | ||
| 1 | 48 877 (55) | 1 | 25 240 (28) |
| 2 | 26 516 (30) | 2 | 15 127 (17) |
| 3 | 9890 (11) | 3 | 8151 (9) |
| 4 | 3178 (4) | 4 | 4310 (5) |
| 5+ | 1184 (1) | 5+ | 5095 (6) |
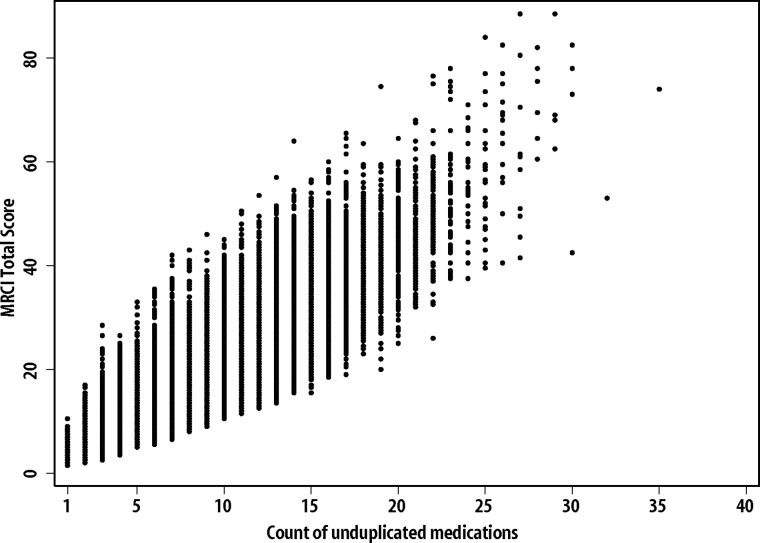
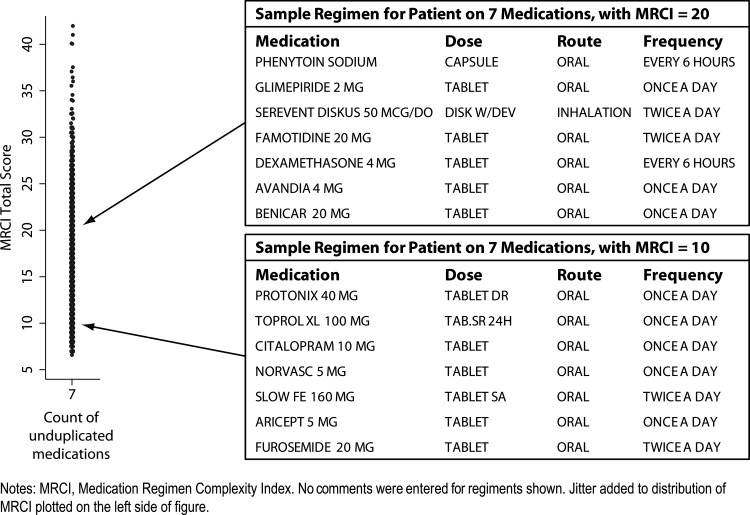
As one would expect, there was a strong correlation between the number of medications and the MRCI score (figure 2). Nevertheless, there was wide variation in MRCI scores within regimens with the same number of medications. For example, among patients on seven medications, MRCI scores ranged from 6.5 to 42.0 (figure 3). The regimen listed in the bottom box in figure 3 is less complex than is typical for patients with seven medications—the MRCI score of 10 is approximately 33% lower than average, while the top box in figure 3 shows a regimen with a MRCI score of 20, which is approximately 33% higher than average.
Figure 2.
Scatterplot of medication regimen complexity index (MRCI) scores and count of medications (N=89 645).
Figure 3.
Example regimens: below and above average complexity, among patients taking seven medications (N=9457).
Discussion
George and colleagues7 created a measure of medication complexity that takes into account more factors than a simple medication count. We developed an automated tool used within our EHR to allow additional testing of the MRCI. This effort involved evaluation of data fields available through a large, commercial medication database along with data fields that were part of the EHR unique to the health service organization. The approach described in this article provides a framework for vendors and organizations to begin automation processes in other environments.
We opted to include OTC medications because they are commonly used in this population as a whole, and may add considerably to the practical complexity faced by patients as they manage their regimens. OTC medications are also relevant to promote patient safety as a review of a full medication list may reveal unintended duplications or potential drug–drug interactions. Our tabulation of special instructions may be an under-representation of the number of instructions a patient really needs to remember as there are limitations on how the available data fields could be used. Furthermore, EHR data do not capture directions that a patient may have received verbally from healthcare providers or written material provided to the patient.
An additional limitation is the hard-coded crosswalk approach taken to map FDB and agency EHR data to MRCI component elements. While this approach simplified programming and contributed to the transparency of the translation from the original MRCI to the final automated scores, it requires ongoing maintenance. As the dosage form/route combinations are derived from FDB data on currently available medications, these fields are subject to modification over time, as new medications enter the market. Approximately 6 months after the initial development, we found a total of three new dosage form/route combinations appearing in agency data.
Once the automated algorithm is set up, the ability to generate MRCI scores for a large population is considerable. Previously published research calculated paper-based MRCI scores on samples ranging from 20 to 320.4 7 9–13 After development of this project, MRCI scores were tabulated for almost 90 000 patient cases. We revealed a wide range of medication complexity scores in the population of home healthcare patients. A complexity score is a more systematic way of providing information to clinicians then a simple ‘eyeballing’ of the regimen to identify individual patients who may require more intense or specialized medication management.
Conclusion
All components of the MRCI—the number of different form/routes a patient needs to manage, the number of doses, special instructions—have been independently found to influence patient adherence.4–6 Adherence and medication management challenges have been linked to adverse drug events and increased health service use.14 15 The automated MRCI scores can potentially be linked with patient outcome data to determine risk thresholds for adverse events. Once established, health service organizations can use the MRCI to help with clinical decision support by identifying high-risk patients who can then be evaluated further to determine the need for medication regimen simplification, medication reminder devices, increased involvement of a caregiver, closer clinical monitoring and/or clinical interventions. The MRCI, therefore, has several important research and clinical applications. The tools developed here can be used to automate the calculation of the MRCI score, and to accelerate the evaluation of its potential.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the significant support the research team received from several members of the Visiting Nurse Service of New York information technology department. In addition, they would like to thank Paula Wilson, BSN, MPH for her contributions to the MRCI automation committee and for a critical review of the manuscript by Yolanda Barron-Vaya, MS.
Footnotes
Funding: This project was supported by grant number R18HS017837 from the Agency for Healthcare Research and Quality (principal investigator: Penny H. Feldman, PhD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Competing interests: None.
Ethics approval: The study was approved by the Visiting Nurse Service of New York institutional review board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Open Access: This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
References
- 1.Viswanathan M, Golin CE, Jones CD, et al. Adherence Interventions: Comparative Effectiveness. Closing the Quality Gap: Revisiting the State of the Science. Evidence Report No. 208. (Prepared by RTI International–University of North Carolina Evidence-based Practice Center under contract no. 290-2007-10056-I.) AHRQ Publication no. 12-E010-EF Rockville, MD: Agency for Healthcare Research and Quality; September 2012. http://www.effectivehealthcare.ahrq.gov/reports/final.cfm(accessed 13 September 2012). [Google Scholar]
- 2.Peterson AM, Takiya L, Finley R. Metaanalysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm 2003;60:657–65 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Adherence to long term therapies: evidence for action. Switzerland: WHO, 2003 [Google Scholar]
- 4.Mansur N, Weiss A, Beloosesky Y. Looking beyond polypharmacy: quantification of medication regimen complexity in the elderly. Am J Geriatr Pharmacother 2012;10(4):223–9. [DOI] [PubMed] [Google Scholar]
- 5.Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med 2008;31:213–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corsonello A, Pedone C, Lattanzio F, et al. Regimen complexity and medication nonadherence in elderly patients. Ther Clin Risk Manag 2009;5:209–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George J, Phun YT, Bailey MJ, et al. Development and validation of the medication regimen complexity index. Ann Pharmacother 2004;38:1369–76 [DOI] [PubMed] [Google Scholar]
- 8.Conn VS, Taylor SG, Kelley S. Medication regimen complexity and adherence among older adults. Image J Nurs Sch 1991;23:231–5 [DOI] [PubMed] [Google Scholar]
- 9.Melchiors AC, Correr CJ, Fernandez-Llimos F. Translation and validation into Portuguese language the medication regimen complexity index. Arq Bras Cardiol 2007;89:210–18 [DOI] [PubMed] [Google Scholar]
- 10.Stange D, Kriston L, Langebrake C, et al. Development and psychometric evaluation of the German version of the Medication Regimen Complexity Index (MRCI-D). J Eval Clin Pract 2012;18:515–22 [DOI] [PubMed] [Google Scholar]
- 11.Cardone KE, Manley HJ, Grabe DW, et al. Quantifying home medication regimen changes and quality of life in patients receiving nocturnal home hemodialysis. Hemodial Int 2011;15:234–42 [DOI] [PubMed] [Google Scholar]
- 12.Correr CJ, Melchiors AC, Fernandez-Llimos F, et al. Effects of a pharmacotherapy follow-up in community pharmacies on type 2 diabetes patients in Brazil. Int J Clin Pharm 2011;33:273–80 [DOI] [PubMed] [Google Scholar]
- 13.Fröhlich SE, Zaccolo AV, da Silva SL, et al. Association between drug prescribing and quality of life in primary care. Pharm World Sci 2010;32: 744–51 [DOI] [PubMed] [Google Scholar]
- 14.Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65: 989–95 [DOI] [PubMed] [Google Scholar]
- 15.Steinman MA, Hanlon JT. Managing medications in clinically complex elders: ‘There's got to be a happy medium’. JAMA 2010;304:1592–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.