Abstract
Gastrointestinal stromal tumors (GISTs) are thought to originate from the electrically active pacemaker cells of the gastrointestinal tract. Despite the presence of synaptic-like vesicles and proteins involved in cell secretion it remains unclear whether GIST cells possess regulated release mechanisms. The GIST tumor cell line GIST882 was used as a model cell system, and stimulus-release coupling was investigated by confocal microscopy of cytoplasmic free Ca2+ concentration ([Ca2+]i), flow cytometry, and luminometric measurements of extracellular ATP. We demonstrate that GIST cells have an intact intracellular Ca2+-signaling pathway that regulates ATP release. Cell viability and cell membrane integrity was preserved, excluding ATP leakage due to cell death and suggesting active ATP release. The stimulus-secretion signal transduction is at least partly dependent on Ca2+ influx since exclusion of extracellular Ca2+ diminishes the ATP release. We conclude that measurements of ATP release in GISTs may be a useful tool for dissecting the signal transduction pathway, mapping exocytotic components, and possibly for the development and evaluation of drugs. Additionally, release of ATP from GISTs may have importance for tumor tissue homeostasis and immune surveillance escape.
Keywords: Gastrointestinal stromal tumor, GIST, ATP release, Ca2+, flow cytometry, confocal microscopy
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms in the gastrointestinal tract and are highly resistant to conventional radio- and chemotherapy [1, 2]. The GIST tumorigenesis is associated with well-established gain-of-function mutations in the proto-oncogene KIT (a stem cell factor receptor) or PDGFRA (platelet-derived growth factor alpha), resulting in ligand-independent signal transduction [3, 4]. In 2001, imatinib mesylate, a small-molecule tyrosine kinase inhibitor (TKI), improved molecular targeted therapy substantially when it was introduced as a first-line treatment for advanced and metastatic GISTs [5, 6]. TKI treatment have significantly prolonged patient survival; however, local recurrence, metastasis, and tumor resistance remain major therapeutic challenges [7].
GISTs are believed to originate from the interstitial cells of Cajal (ICCs), the pacemaker cells of the intestine [8, 9]. GIST cells and ICCs share several characteristics, e.g. the highly specific markers CD117 and DOG1 [10, 11]. ICCs are functionally well characterized, though data for GISTs are scarce.
ICCs show a strong spontaneous rhythmic oscillation pattern that is dependent on Ca2+ influx and cytoplasmic free Ca2+ concentration ([Ca2+]i) [12]. Generation of pacemaker currents in ICCs can be modulated by different drugs. Thapsigargin increases [Ca2+]i by blocking the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) pump and by activating store-operated Ca2+ entry (SOCE) via transient receptor potential canonical type ion channels (TRPCs) in the cell membrane. TRPCs play a role in generating the pacemaker currents in ICCs and have been identified in GIST [12–15]. In ICCs pretreated with Ca2+-free extracellular solution, the pacemaker currents are abolished when thapsigargin is added [16, 17]. ICCs also have cell surface receptors for serotonin (5-hydroxytryptamine; 5-HT), acetylcholine (ACh) and substance P, which convey signals that increase [Ca2+]i, and thereby modulate pacemaker activity in the intestine [18–24].
A potential functional similarity between GIST and ICC has been identified, wherein GIST cells possess oscillation-like spontaneous activity and contain several of the ion channels that are necessary for pacemaker activity in ICCs [13]. K+ and Ca2+ channels in GISTs were identified in a proteomic study attempting to develop prognostic markers in GISTs as well as in a micro-array-based gene expression study [25, 26].
In many cell systems ATP is stored at high concentrations within the secretory vesicles and co-released during exocytosis, and can be used as a universal tracer of cellular secretion events [27, 28]. A good example is the pancreatic β-cell where ATP is co-localized within the insulin containing vesicles and co-secreted together with insulin upon stimulation [29–31]. Similar observations of stimulated ATP release have been described in several other cells and tumor types [32–37]. Extracellular ATP itself plays an important role in the tumor microenvironment [38].
The intracellular signal transduction in GIST cells is poorly comprehended, and identification of signaling pathways play an important role in understanding the mechanism of cellular response to external stimuli. Electron microscopy, immunohistochemistry, Western blot, quantitative PCR and confocal laser scanning microscopy revealed that GIST cells contain synaptic-like vesicles and the exocytotic proteins required for cell secretion [39–41]. Despite an indication of a neuroendocrine phenotype, it remains uncertain if GIST cells possess regulated secretory pathways. In this study, we have used an ATP release assay to characterize the cellular response in GIST cells.
Materials and Methods
Cell lines
Three cell lines were used for the study: GIST882 kindly provided by Professor Jonathan Fletcher at Brigham and Women’s Hospital, Boston, MA, USA; MIN6m9, a mouse insulinoma cell line; and the Human Embryonic Kidney (HEK) 293 cell line.
The imatinib-sensitive GIST cell line GIST882 was used to examine ATP release in GIST.
GIST882 was established from an untreated metastatic human GIST expressing a homozygous missense KIT mutation in exon 13 causing a single amino acid substitution - K642E [42]. Prior to experiments, the presence of the KIT mutation and the c-KIT expression were verified by sequencing and flow cytometry. Cells were cultured in RPMI-1640 medium (GIBCO, cat. no. 11879), supplemented with 15% fetal bovine serum (Biochrom AG, cat. no. S0115), 0.25 mg/ml L-glutamine (Biochrom AG, cat. no. K0282), 100 units/ml penicillin G, 100 µg/ml streptomycin-sulfate and 0.21 µg/ml amphotericin B (Calbiochem, cat. no. 516104) at 5% CO2 and 37 °C.
MIN6m9 [43] was maintained in DMEM (Invitrogen, cat. no. 31885), containing 11 mM glucose supplemented with 10% fetal bovine serum, 100 units/ml penicillin G, 100 µg/ml streptomycin-sulfate and 5 nl/ml β-mercaptoethanol at 5% CO2 and 37 °C. Unless otherwise indicated, cells were split in equal cell numbers into 25 cm2 flasks, and cultured until about 50% or 80% confluence. The HEK 293 cell line was cultured in DMEM media (Invitrogen, cat. no. 41965-039), supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 100 units/ml penicillin G.
DNA Extraction, PCR and Sanger Sequencing
An automated Maxwell® 16 DNA purification system kit AS1020 (Promega Corporation Madison Wisconsin, USA) was used to purify genomic DNA from the GIST882 cell line. An aliquot of ≤ 5 million cells was spun briefly and lysed with lysis solution. Four hundred microliters (400µl) of lysate was transferred to the Maxwell cartridge. The concentration and purity of the eluted genomic DNA were determined in a NanoDrop spectrophotometer ND-1000 (Thermo Scientific, Wilmington, USA). Polymerase chain reaction (PCR) and sequencing were carried out to detect mutations in exons 9, 11, 13, and 17 of the KIT gene, as previously described by Sihto et al [44].
PCR amplicons were purified using the ChargeSwitch® PCR clean-up kit (Life Technologies Corporation, NY, USA)
Sequencing was performed in both directions using the BigDye Terminator version 3.1 cycle sequencing kit and the 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). All sequencing data were analyzed both visually and using the Mutation Surveyor Software version 4.0.5 (SoftGenetics LLC, PA, USA). The numbering of specific mutations was referenced from http://www.ensembl.org and http://www.sanger.ac.uk/genetics/CGP/cosmic/.
Detection of CD117 expression on GIST882 cells
The Miltenyi Biotec’s protocol was used to evaluate c-KIT expression on GIST882 cells. The HEK293 cell line was used as negative control. Cells were harvested, filtered through a 40- µm cell strainer and counted. Two million cells were suspended in 80 µl buffer solution, MACS BSA stock solution (cat. no. 130 091 376), diluted 1:20 with autoMACS rinsing solution (cat. no. 130 091 222) and incubated with 20 µl FcR blocking reagent and 20 µg/ml of antibody, either human CD117 (c-KIT) or mouse IgG1 as a negative control, for 10 min at 4 °C in the dark. The cells were then washed with buffer solution, centrifuged, and resuspended in 300 µl buffer solution before analysis. Allophycocyanin (APC)-coupled antibody against human CD117 (cat. no. 130 091 733) was obtained from Miltenyi Biotec GmbH (Bergisch Gladbach, Germany). For isotype control experiments, an APC-coupled mouse IgG1 (cat. no. 345818) was obtained from Becton Dickinson GmbH (Franklin Lakes, NJ, USA). Cell fluorescence was determined by flow cytometry, using the Gallois instrument (Beckman Coulter), and data were analyzed with Kaluza flow cytometry analysis software v1.1 (Beckman Coulter).
Insulin Secretion
Insulin release was evaluated using static incubation. MIN6m9 cells were seeded at a density of 60,000 cells/cm2 in 24-well tissue culture plates and cultured for 48 h in a CO2 incubator at 37 °C. After 48 h the cultures had reached approximately 70–80% confluence. Cells were washed twice with pre-warmed Hanks buffered salt solution (HBSS), containing (in mM): 1.26 CaCl2, 0.49 MgCl2-6H20, 0.41 MgSO4-7H20, 5.33 KCl, 0.44 KH2PO4, 4.17 NaHCO3, 137.93 NaCl, 0.34 Na2HPO4, 3 D-glucose and incubated with the same buffer for 20 min in a water bath at 37 °C. Next, the buffer was removed and the cells were stimulated with HBSS buffer containing varying glucose concentrations. After 20 min, the conditioned buffer was collected on ice and centrifuged (5000 g) at 4 °C for 5 min. The supernatant was stored at −20 °C for subsequent insulin measurements using the ArcDia 2-photon fluorescence excitation microparticle fluorometry (TPX) assay for insulin content (Arc-Dia Group, Turku, Finland). The assay was performed according to the manufacturer’s instructions, using a recombinant human insulin standard to determine the insulin concentration, as previously described by Leibiger et al. [45].
Measurement of Adenosine 5’-Triphosphate (ATP) Release
ATP release from the cultured cell lines was measured with a commercially available rLuciferase/Luciferin (rL/L) reagent assay (Promega Enliten, Madison, WI, USA). This bioluminescence detection reagent is cell membrane-impermeable and designed to measure extracellular ATP in the 10−11 to 10−16 mole range. When ATP is released from cells within this range, it serves as the limiting reagent in the luciferase reaction. Measurements of the emitted light intensity with an Envision 96-well plate reader enabled the quantification of the extracellular ATP in the supernatant, given that light intensity was proportional to ATP level. An ATP standard curve was constructed using HBSS to dilute the ATP standard and all samples were measured in duplicate. To ensure a low background, a “blank” containing only rL/L reagent and HBSS was analyzed to determine the level of “background” relative light units (RLUs).
In brief, GIST882 cells were seeded in 25 cm2 flasks in equal cell numbers for 48 h until approximately 80% confluence. The cells were washed four times with pre-warmed HBSS and statically incubated for 5 minutes in HBSS (control) or HBSS including either 1 µM carbachol, 60 mM K+, 2 µM thapsigargin, 50 µM serotonin, 1 µM substance P or 2 µM thapsigargin without extracellular Ca2+. Experiments were performed in triplicates and each sample was measured twice.
Similarly, MIN6m9 cells were seeded in 25 cm2 flasks in equal cell numbers and cultured for 48 h until about 50% or 80% confluence. The cells were washed four times with HBSS (supplemented with 3 mM glucose) and incubated in the same buffer for 20 minutes at 37 °C. After starvation, the cells were re-rinsed and stimulated with HBSS containing either 3 or 16 mM glucose, for 5 minutes. Supernatant was collected from all flasks, while being kept upside down to lower the risk of detaching cells, and centrifuged to ensure a cell-free sample. The supernatant was then analyzed immediately (within 5 min of collection) with the 2103 EnVision Multilabel Plate Reader (Perkin Elmer, CT, USA), at the 560 nm emission wavelength. Fifty microliters (50 µl) of the sample was mixed three times with equal amounts of reagent (50 µl rL/L). A 5-second delay time and a 10-second RLU signal integration time was used. All samples were read twice.
Cell Viability and Permeability
GIST882 cells were cultured for four days in 25 cm2 flasks until approximately 80% confluence. On the experimental day each flask was washed four times with pre-warmed HBSS containing 5.56 mM D-glucose. Cells were then incubated for 5 minutes in culture medium, HBSS or HBSS supplemented with 2 µM thapsigargin. Each flask was then trypsinized for 6 minutes, centrifuged, and aliquoted in two parts. One portion was processed to determine cell viability by light microscopy trypan blue exclusion staining and the other portion was used to assess cell membrane permeability using the nucleic acid dye 7-aminoactinomycin D (7-AAD, Via-Probe BD Biosciences), according to the manufacturer’s instructions. Cellular uptake of 7-AAD was assessed by flow cytometry (BD FACS Canto II) where 7-AAD fluorescence was detected in the far red range of the spectrum (at wavelengths > 650 nm).
Lactate Dehydrogenase Assay
Flasks were prepared using the same protocol as when measuring ATP and were incubated with either HBSS or HBSS including 2 µM thapsigargin for 5 min. The supernatant was collected and cells were lysed with 0.1% triton-X-100 for 30 min during intermittent shaking, while kept on ice. Lysed cells were collected and cell debris was excluded after centrifugation at 250g for 4 min. The LDH concentration in the supernatant and from lysed cells was measured at the routine clinical chemistry laboratory at Karolinska University Hospital. Experiments were carried out in triplicates.
Confocal Microscopy and Measurements of [Ca2+]i
Black 96-well Microplates (BD Falcon™, ref. 353219) with a clear flat bottom were imaged using a BD Pathway™ 855 High Content Bioimager (Becton Dickinson Biosciences, CA, USA), equipped with 560/55-nm and 645/75-nm excitation and emission filters, respectively. Images were acquired from the periphery of each well with an Olympus UApo 20x/0.75 dry objective. An automated laser focus was used and wells were exposed for 0.25 milliseconds (gain 255) to acquire spinning disc confocal images with a scale binning of 1×1 and 0.31 µm/pixel. To segment GIST882 cells from each other, an image algorithm was used based on local intensity thresholds. Staining was carried out manually while stimulation solutions were added using the BD Pathway 855 robotic workstation which also acquired images automatically.
In brief, the GIST882 cells were seeded in equal numbers of approximately 104 cells per well in 96-well plates and cultured for 24–72 h. The wells were then washed with HBSS and incubated for 30 min with 4 µM cell-permeant Ca2+ indicator X-rhod-1 AM (Invitrogen™, Molecular Probes, X-14210). This single wavelength dye with 580/602-nm excitation/emission maxima was used because it yielded superior loading compared to other commonly used dyes, such as Fura-2 (data not shown). Wells were washed three times after dye loading with HBSS before the experiments were performed. Aliquots of 25 µl HBSS, including the test compound, were added to a final volume of 225 µl in each well at final concentrations of 2 µM thapsigargin and 60 mM K+, respectively. A baseline was acquired for two min with a total acquisition time of 5 min.
Data from the BD Pathway 855 Bioimager were initially organized using the BD Attovision Software System and were then exported to a Microsoft® Excel file containing raw fluorescence data. Background, calculated as a mean of five regions of interest, was subtracted and each cell was then normalized to itself, divided by a mean of 30 s prior to stimulation. A mean value of all cells within each experiment was then calculated. Cells with a ratio increase of more than 10% after stimulation were considered to be responding cells.
Statistical Analysis
Statistical significance was determined using unpaired Student’s t test or one-way analysis of variance (ANOVA), and P-values of <0.05 were considered significant. Data are expressed as average ± standard error of the mean throughout the manuscript.
Results
GIST882 Mutational Analysis and Phenotype
Sequencing of mutational hot spot exons of KIT in the GIST882 cell line confirmed the presence of the homozygous exon 13 missense mutation (Supplementary Figure S1). No additional mutations were revealed in exons 9, 11, 13 or 17. To confirm the phenotype of the GIST882 cell line, expression of the cell surface marker CD117 was compared with the HEK293 cell line. The gating scheme utilized for assessment of CD117-positive cells is shown in Supplementary Figure S2. We demonstrated that the GIST882 cell line expresses CD117 in 98.6% of cells, thus verifying a pure GIST882 cell population.
Insulin Secretion and ATP Release in β-Cells
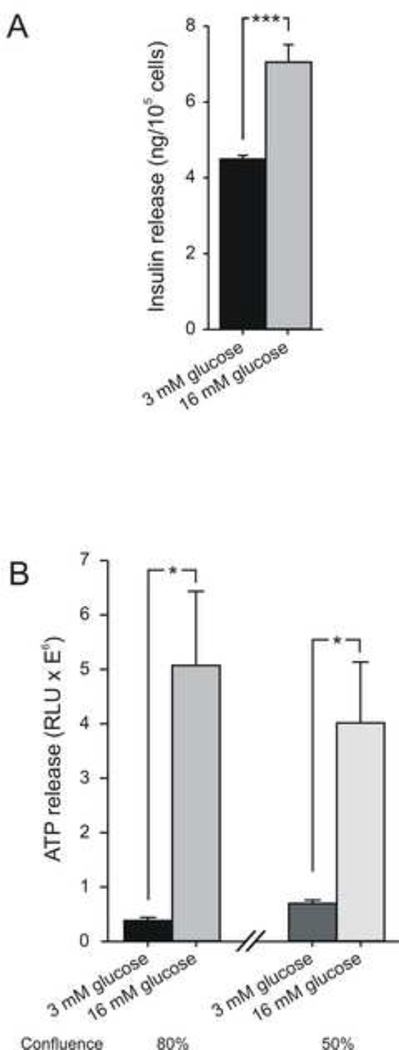
To prove a well-functioning experimental setup, before continuing with the GIST882 cell line, the extracellular secretion of insulin and co-release of the purinergic-signaling molecule ATP was examined in the MIN6m9 cell line, established from pancreatic β-cells and known to secrete insulin. Increasing the extracellular glucose concentration from 3 mM to 16 mM evoked a significant (P = 0.00041) insulin release in MIN6m9 cells with about 80% confluence (Figure 1A). The accumulation of ATP in the extracellular medium was also significantly increased by glucose stimulation both in flasks that were 80% confluent (P = 0.016) and 50% confluent (P = 0.045), Figure 1B. This confirms that the insulin secretagogue glucose elicits the release of both insulin and ATP and that the experimental protocol to measure extracellular ATP is robust.
Figure 1. Measurement of insulin and ATP release from the pancreatic β-cell line MIN6m9.
(A) Insulin release increased from 4.49±0.077 ng/105 cells (n = 5) to 7.05±0.44 ng/105 cells (n = 5) in 16 mM glucose-stimulated samples. (B) Using the same buffer conditions as in A, extracellular ATP increased from 0.38±0.058 RLUx106 to 5.1±1.36 RLUx106 in 80% confluent flasks (n = 6, for controls, n = 9, for stimulated samples). Under 50% confluence ATP increased from 0.7±0.062 RLUx106 to 4.01±1.11 RLUx106 (n = 4, for controls, n = 6, for stimulated samples). RLU, Relative light units. Data in all figures are presented as average ± SEM. Asterisks denote statistical significance (Student t test; *P < 0.05, ***P < 0.001).
ATP Measurements in GIST882
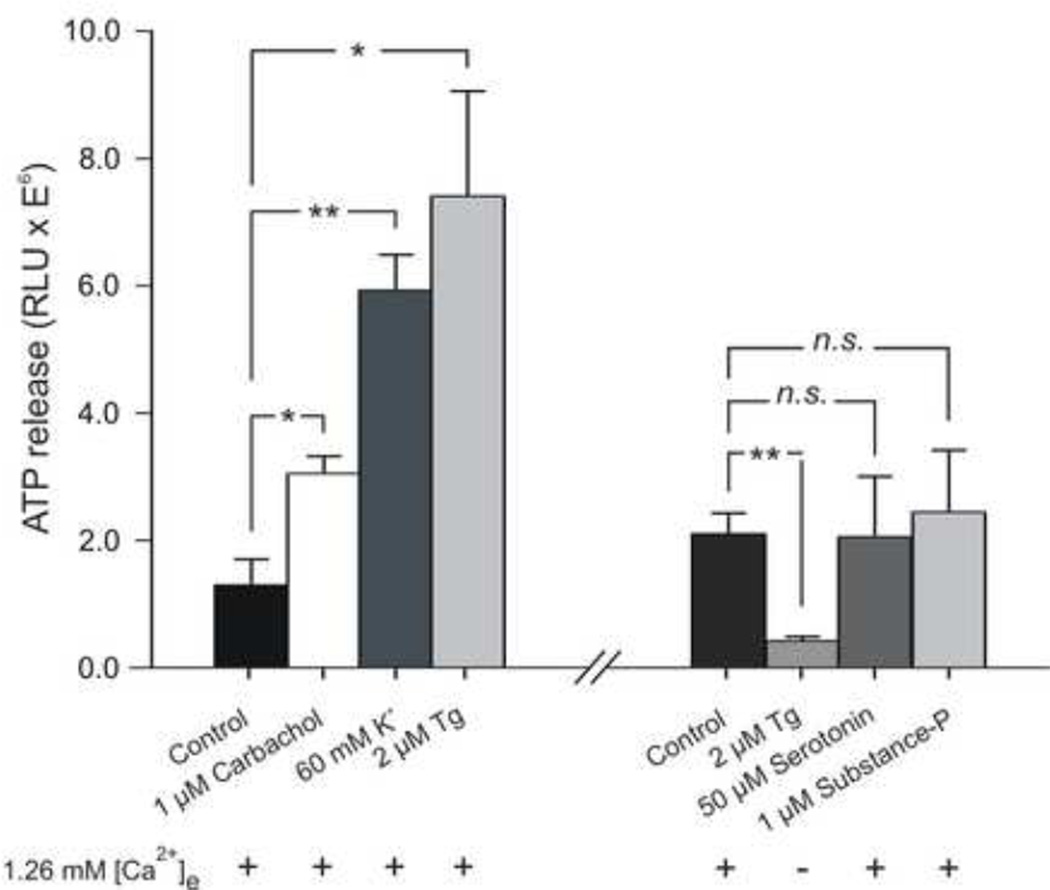
ATP release from GIST882 cells was measured after static incubation in the presence or absence of pharmacologic stimulation (Figure 2). The fractional background level detected in the HBSS buffer was subtracted from all stimulated samples. A close correlation was found between the addition of 1 µM carbachol, 60 mM K+ or 2 µM thapsigargin and the increase in ATP release in all preparations (P < 0.020, n = 3, P < 0.0023, n = 3, and P < 0.022, n = 3, respectively). In contrast, addition of 2 µM thapsigargin in HBSS without Ca2+ resulted in a significant decrease in ATP release compared to basal conditions, (P < 0.0064, n = 3), reflecting a constitutive and Ca2+-dependent ATP release. Exposure to 50 µM serotonin or 1 µM substance P did not affect ATP release (P < 0.97, n = 3, and P < 0.76, n = 3, respectively). A summary of all conditions is shown in Figure 2.
Figure 2. Measurement of ATP release from GIST882 cells before and after stimulation.
(Left) GIST882 cells were exposed for 5 min to the following conditions: HBSS (control), HBSS including either carbachol (1 µM), K+ (60 mM), Tg (2 µM). Conditions after cell re-split. A new control sample was measured. Cells were then exposed to Ca2+ free HBSS including Tg (2 µM), or HBSS including either serotonin (50 µM) or substance P (1 µM). All experiments where performed three times (n = 3), with dual measurement analysis of ATP. With extracellular Ca2+ present (1.26 mM), exposure of GIST882 cells to Tg increased ATP release by 572%, whereas in the absence of extracellular Ca2+, the ATP level decreased to 20% of control. Similarly, high K+ and carbachol increased ATP release by 458%, and 236%, respectively. No effect was seen after exposure to serotonin (98%) and substance P (116%). *P < 0.05, **P < 0.01 indicate statistical significance of difference from the control. Tg, thapsigargin. RLU, Relative light units.
Cell Permeability and Viability
To confirm that the source of ATP is not related to disruption of cell membrane integrity, we evaluated cell viability and plasma membrane permeability with trypan blue exclusion staining and flow cytometric analysis of 7-AAD stained cells.
Under trypan blue examination the following results were obtained: in the culture medium GIST882 had a high viability with virtually no trypan blue uptake (n = 3). In HBSS 0.33±0.33% (n = 3) of the cells were stained, and HBSS containing 2 µM thapsigargin yielded 0.33±0.16% (n = 3) stained cells. Analysis of variance between the three groups showed no significant difference (P < 0.49).
Further evaluation of cell membrane permeability and viability with 7-AAD staining on the GIST882 cells showed a flow cytometric frequency of stained cells in the control group (culture medium) of 0.51±0.048% (n = 3), in HBSS of 0.45±0.022% (n = 3), and in HBSS containing 2 µM thapsigargin, of 0.40±0.037% (n = 3); Figure 3A. Analysis of variance between the three groups showed no significant difference (P < 0.17), thus reflecting preserved cell membrane integrity and viability in samples stimulated with thapsigargin and/or HBSS (Figure 3B).
Figure 3. Cell viability and permeability analysis of GIST882 cells.
(A) Gating strategy for the identification of permeable and non-viable GIST882 cells in unstained, culture medium (control), HBSS and HBSS + 2 µM Tg-stimulated samples, stained with 7-AAD. Characteristic dot plots are depicted. (B) The indicated GIST882 cell permeability was calculated by subtracting the percentage of positive unstained cells (top row in A) from all groups. No change in cell permeability between all groups was seen (P < 0.17, n = 3). Asterisks denote statistical significance (ANOVA; P < 0.05). FSC, forward scatter. SSC, side scatter.
Lactate Dehydrogenase (LDH) Assay
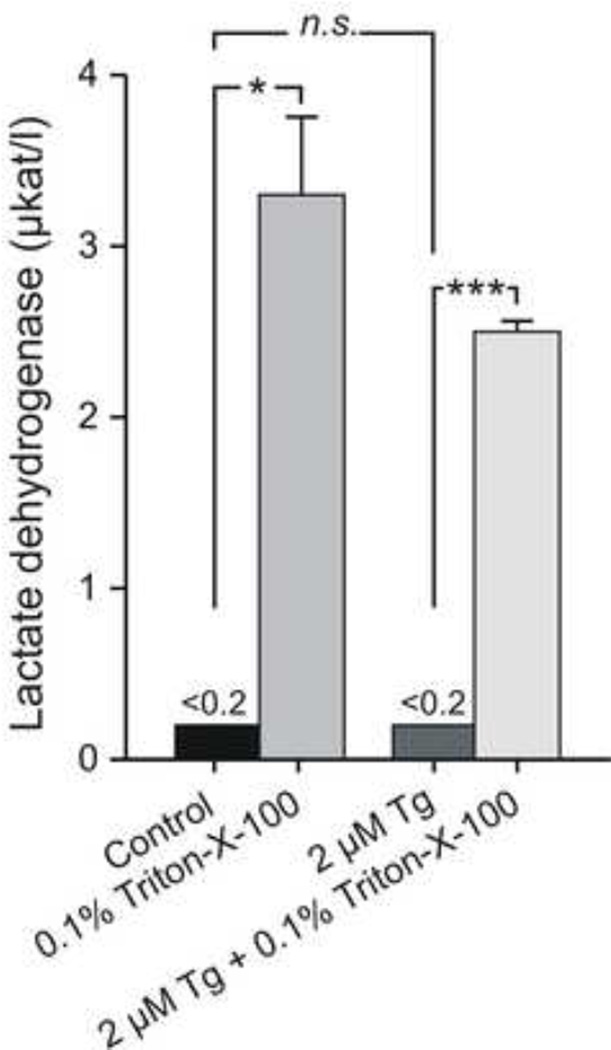
Lactate dehydrogenase (LDH) catalyzes the conversion of lactate to pyruvate. When the cell membrane is disrupted LDH can leak out and therefore serve as a marker of cell lysis. Determination of the extracellular LDH concentration under basal (HBSS) and stimulated conditions (HBSS including 2 µM thapsigargin) was used to further exclude cell lysis as a potential explanation for the increase in extracellular ATP concentration. No difference was observed between the non-stimulated and stimulated groups (n = 3) of cells. Addition of the nonionic detergent Triton-X-100 to lyse all cells, used as positive control, resulted in a significant increase of LDH in all samples (Figure 4).
Figure 4. Lactate dehydrogenase (LDH) assay.
LDH was analyzed in extracellular solution. Under control conditions, LDH activity was not detected, whereas after cell lysis using Triton-X-100 (0.1%), LDH increased significantly, P < 0.021 and P < 0.00063, respectively. Tg did not increase LDH release compared to control (paired experiments, n = 3). µkat/l – microkatal/liter. Asterisks denote statistical significance (Student t test; *P < 0.05, ***P < 0.001).
Confocal Microscopy and Measurements of [Ca2+]i
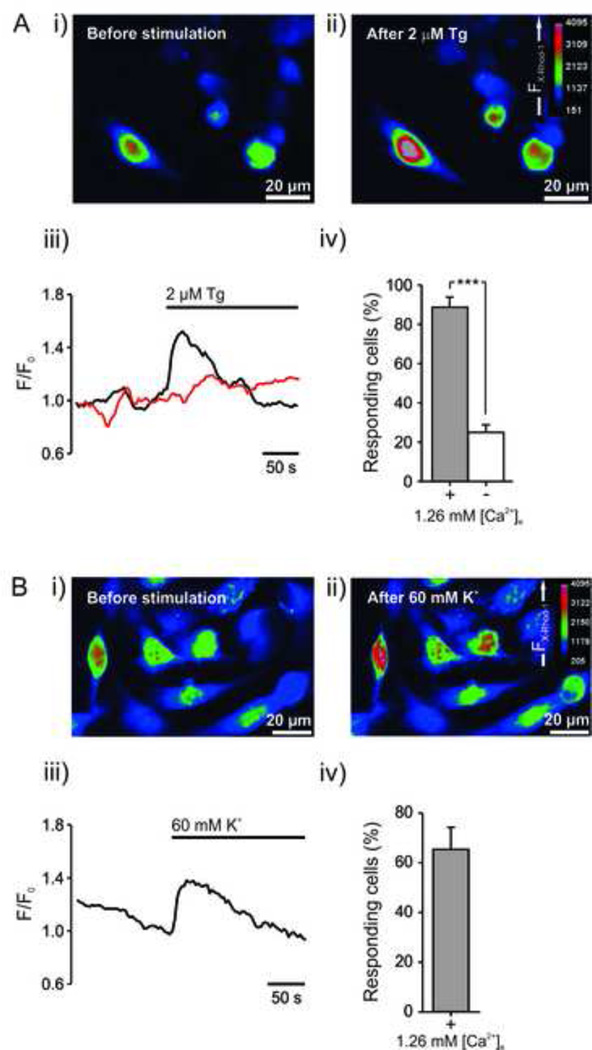
Vesicular secretion is well documented to rely on increased [Ca2+]i. A Ca2+ assay to measure [Ca2+]i utilizing the X-rhod-1 calcium indicator dye was therefore set up in 96-well plates with an automated workflow. We evaluated whether the test compounds that showed the largest luminescence increase in the ATP luciferin/luciferase assay, thapsigargin and K+, also increased [Ca2+]i. Both thapsigargin and K+ increased [Ca2+]i and the majority of cells responded to the stimulation (Figure 5). Excluding extracellular Ca2+ significantly decreased the response during thapsigargin stimulation, reflecting the response to be partly dependent on extracellular Ca2+ (Figure 5A). Also, there was generally a lower peak response in experiments without Ca2+ compared to experiments with Ca2+ present.
Figure 5. Confocal microscopy images and [Ca2+]i measurements in GIST882 cells after stimulation with Tg and high K+.
GIST882 cells were loaded with X-Rhod-1 for 30 min and spinning disc confocal images acquired during 5 minutes, with a 2 minutes baseline. (A) Upper images (i, ii) show a representative experiment with extracellular Ca2+ present. Left lower traces (iii) show representative experiments of Tg’s stimulatory effect on [Ca2+]i with extracellular Ca2+ present (black trace) and without extracellular Ca2+ (red trace). Lower right bar chart (iv) shows percentage of responding cells from all experiments. In the presence of 1.26 mM extracellular Ca2+, [Ca2+]i intensity was significantly increased in 89±5.1% in a total of 274 analyzed cells (n = 6). Excluding extracellular Ca2+ significantly lowered the response, and only 25±3.8% of 686 analyzed cells (n = 7) responded. (B) A representative experiment after exposure to 60 mM K+ (i-iii), with 65±8.9% responding cells among a total of 555 analyzed cells (iv), n = 6. F/Fo, X-Rhod-1 fluorescence intensity (F) normalized with the average intensity 30 sec before stimulation (Fo).
Discussion
With the advent of TKI treatments, GIST has become a model for modern targeted cancer therapy during the last decade. Detailed studies of ICCs show that these cells have a cell signal transduction involving surface receptors for acetylcholine, serotonin and substance P. Like in many other cell types, [Ca2+]i have an important role since increased levels trigger cell secretion [46]. The signal transduction in GISTs is not completely understood, and it is still unclear whether GIST cells do indeed possess an intact secretory pathway. Findings supporting a secretory pathway in GISTs are the presence of dense core vesicles and vesicular proteins including synaptic vesicle protein 2 (SV2), synaptobrevin, and others [39].
In several cell types ATP serves as a marker for regulated secretion. In the pancreatic β-cells it is co-localized and co-released with insulin [27, 29]. A key feature in the intracellular signal transduction in these cells is [Ca2+]i, where an increased level precedes secretion [47]. Our own experiments verify that glucose stimulation results in secretion of both insulin and ATP in MIN6m9 cells. A similar experimental protocol was then applied to the GIST cell line GIST882, except that glucose was replaced with other stimulators. Here we show that modulators of [Ca2+]i – carbachol, K+, and thapsigargin – have a strong influence on ATP levels detected in extracellular solutions. To confirm that the source of ATP is not related to cell lysis or compromised cell membrane integrity, we performed a number of control experiments. Thapsigargin was chosen for further cell viability experiments because it had the largest effect on ATP release and because of its well-known cytotoxic effects. No changes in cell viability or cell membrane integrity were observed: microscopy of the supernatant did not detect detached cells, and a trypan blue examination confirmed high cell viability; no release of the intracellular cell lysis marker lactate dehydrogenase (LDH) was detected and no changes in the fraction of cells stained with 7-AAD were observed. To further strengthen the hypothesis that ATP release is indeed Ca2+ regulated, we excluded extracellular Ca2+ following the notion that extracellular Ca2+ is required for a normal secretory event. Exclusion of extracellular Ca2+ markedly reduced the stimulatory effect of thapsigargin.
In many electrically-active cells, high extracellular K+ depolarizes the plasma membrane, thereby triggering opening of voltage-gated Na+ and/or Ca2+ channels and resulting in increased [Ca2+]i and cell secretion. Since ICCs are electrically active, and GISTs also exhibit electrical activity [13], it is not unlikely that GIST cells have a stimulus-secretion coupling that is influenced by membrane potential. Exposing GIST cells to high extracellular K+ (60 mM), results in a significant increase in ATP release, further supporting electrically active cell secretion. Furthermore, both thapsigargin and high extracellular K+ cause rapid increase in [Ca2+]i measured using a Ca2+-sensitive dye. The transient effect by thapsigargin (2 µM) can possibly be explained by an incomplete block of the SERCA pump, since other studies have used thapsigargin concentrations between 5–10 µM to achieve complete SERCA inhibition [17, 48, 49]. Similarly, depolarizing the plasma membrane using high K+ causes a transient [Ca2+]i increase. One possible explanation is that depolarization opens voltage-operated Ca2+ channels found in GIST [25], but these channels close in a time dependent manner allowing SERCA pumps to clear the cytosol from Ca2+. Taken together, this further supports our finding that ATP is, at least partly, regulated by an intracellular Ca2+-dependent mechanism.
There is a growing understanding that purines are important contributors to the control of gastrointestinal motility, secretion, blood flow and immune function in the gut. ICC express purinoceptors that may function as a feedback mechanism for pacemaker activity in the intestine under normal physiological conditions [50]. In the tumor microenvironment increased levels of extracellular ATP have been ascribed a potential role for tumor growth, as an important potential source of the immunosuppressive agent adenosine [38, 50].
In conclusion, we demonstrate for the first time an experimental system where GIST cells have, like ICCs, a functional intracellular Ca2+ signal transduction pathway. The signal transduction that leads to ATP release is, at least partly, dependent on [Ca2+]i since several modulators of [Ca2+]i affect the release in GIST. Understanding the mechanisms of ATP release in GISTs may be useful for dissecting the signaling network, mapping exocytotic components, and possibly for drug development. Beside a useful marker for cellular response, release of ATP from GISTs may have importance for tumor tissue homeostasis and immune surveillance escape, as described in other cell systems.
Supplementary Material
Highlights.
ATP release from GIST cells is Ca2+-dependent.
GIST cells have a functional intracellular Ca2+ signal transduction pathway.
Several modulators affect [Ca2+]i and ATP release in GIST cells.
The response in GIST cells resembles the response in interstitial cells of Cajal.
Measuring ATP release may be a useful tool to map signal transduction pathways.
Acknowledgments
Grant support
This work was supported by grants from the Swedish Research Council, the Novo Nordisk Foundation, the Swedish Cancer Society, Funds of Karolinska Institutet, the Swedish Society of Medicine (Bengt Ihre grant), the Tore Nilsson Foundation, the Thuring Foundation, the Jeansson Foundations, Magn. Bergvall Foundations, the Stockholm County Council, Stichting af Jochnick Foundation, The Swedish Diabetes Association, Scandia Insurance Company, Ltd, the Knut and Alice Wallenberg Foundation, and the The Erling-Persson Family Foundation.
Abbreviations
- Tg
Thapsigargin
- GIST
Gastrointestinal stromal tumor
- ICC
Interstitial cell of Cajal
- [Ca2+]i
intracellular Ca2+ concentration
- TKI
Tyrosine kinase inhibitors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest
References
- 1.Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 2.Gold JS, van der Zwan SM, Gonen M, Maki RG, Singer S, Brennan MF, Antonescu CR, De Matteo RP. Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Annals of surgical oncology. 2007;14:134–142. doi: 10.1245/s10434-006-9177-7. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 5.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 7.Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med. 2012;63:247–258. doi: 10.1146/annurev-med-043010-091813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377–389. doi: 10.1097/00000478-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 10.West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R, Goldblum JR, Brown PO, Heinrich MC, van de Rijn M. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, Varma S, Corless CL, Heinrich MC, Smith KS, Wang Z, Rubin B, Nielsen TO, Seitz RS, Ross DT, West RB, Cleary ML, van de Rijn M. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- 12.Torihashi S, Fujimoto T, Trost C, Nakayama S. Calcium oscillation linked to pacemaking of interstitial cells of Cajal: requirement of calcium influx and localization of TRP4 in caveolae. J Biol Chem. 2002;277:19191–19197. doi: 10.1074/jbc.M201728200. [DOI] [PubMed] [Google Scholar]
- 13.Furuzono S, Ohya S, Inoue S, Nakao A, Imaizumi Y, Nakayama S. Inherent pacemaker function of duodenal GIST. Eur J Cancer. 2006;42:243–248. doi: 10.1016/j.ejca.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Walker RL, Koh SD, Sergeant GP, Sanders KM, Horowitz B. TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. Am J Physiol Cell Physiol. 2002;283:C1637–C1645. doi: 10.1152/ajpcell.00266.2002. [DOI] [PubMed] [Google Scholar]
- 15.Huizinga JD, Golden CM, Zhu Y, White EJ. Ion channels in interstitial cells of Cajal as targets for neurotransmitter action. Neurogastroenterol Motil. 2004;16(Suppl 1):106–111. doi: 10.1111/j.1743-3150.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Choi SJ, Yeum CH, Yoon PJ, Choi S, Jun JY. Involvement of thromboxane a(2) in the modulation of pacemaker activity of interstitial cells of cajal of mouse intestine. Korean J Physiol Pharmacol. 2008;12:25–30. doi: 10.4196/kjpp.2008.12.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park CG, Kim YD, Kim MY, Koh JW, Jun JY, Yeum CH, So I, Choi S. Effects of prostaglandin F2alpha on small intestinal interstitial cells of Cajal. World J Gastroenterol. 2011;17:1143–1151. doi: 10.3748/wjg.v17.i9.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanden Berghe P, Missiaen L, Bellon E, Vanderwinden JM, Janssens J, Tack J. Free cytosolic Ca2+ recordings from myenteric neurones in multilayer intestinal preparations. Neurogastroenterol Motil. 2001;13:493–502. doi: 10.1046/j.1365-2982.2001.00283.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu HN, Ohya S, Nishizawa Y, Sawamura K, Iino S, Syed MM, Goto K, Imaizumi Y, Nakayama S. Serotonin augments gut pacemaker activity via 5-HT3 receptors. PLoS One. 2011;6:e24928. doi: 10.1371/journal.pone.0024928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.So KY, Kim SH, Sohn HM, Choi SJ, Parajuli SP, Choi S, Yeum CH, Yoon PJ, Jun JY. Carbachol regulates pacemaker activities in cultured interstitial cells of Cajal from the mouse small intestine. Mol Cells. 2009;27:525–531. doi: 10.1007/s10059-009-0076-1. [DOI] [PubMed] [Google Scholar]
- 21.Shahi PK, Choi S, Zuo DC, Yeum CH, Yoon PJ, Lee J, Kim YD, Park CG, Kim MY, Shin HR, Oh HJ, Jun JY. 5-hydroxytryptamine generates tonic inward currents on pacemaker activity of interstitial cells of cajal from mouse small intestine. Korean J Physiol Pharmacol. 2011;15:129–135. doi: 10.4196/kjpp.2011.15.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayguinov PO, Hennig GW, Smith TK. Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. J Physiol. 2010;588:4453–4474. doi: 10.1113/jphysiol.2010.196824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jun JY, Choi S, Yeum CH, Chang IY, You HJ, Park CK, Kim MY, Kong ID, Kim MJ, Lee KP, So I, Kim KW. Substance P induces inward current and regulates pacemaker currents through tachykinin NK1 receptor in cultured interstitial cells of Cajal of murine small intestine. Eur J Pharmacol. 2004;495:35–42. doi: 10.1016/j.ejphar.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 24.d'antonio C, Wang B, McKay C, Huizinga JD. Substance P activates a nonselective cation channel in murine pacemaker ICC. Neurogastroenterol Motil. 2009;21:985-e979. doi: 10.1111/j.1365-2982.2009.01318.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi U, Nakayama R, Honda K, Ichikawa H, Hasegawa T, Shitashige M, Ono M, Shoji A, Sakuma T, Kuwabara H, Shimada Y, Sasako M, Shimoda T, Kawai A, Hirohashi S, Yamada T. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26:4100–4108. doi: 10.1200/JCO.2007.14.2331. [DOI] [PubMed] [Google Scholar]
- 26.Suehara Y, Kondo T, Seki K, Shibata T, Fujii K, Gotoh M, Hasegawa T, Shimada Y, Sasako M, Shimoda T, Kurosawa H, Beppu Y, Kawai A, Hirohashi S. Pfetin as a prognostic biomarker of gastrointestinal stromal tumors revealed by proteomics. Clin Cancer Res. 2008;14:1707–1717. doi: 10.1158/1078-0432.CCR-07-1478. [DOI] [PubMed] [Google Scholar]
- 27.Leitner JW, Sussman KE, Vatter AE, Schneider FH. Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology. 1975;96:662–677. doi: 10.1210/endo-96-3-662. [DOI] [PubMed] [Google Scholar]
- 28.Aspinwall CA, Yeung ES. Screening populations of individual cells for secretory heterogeneity. Anal Bioanal Chem. 2005;381:660–666. doi: 10.1007/s00216-004-2981-7. [DOI] [PubMed] [Google Scholar]
- 29.Hellman B, Dansk H, Grapengiesser E. Pancreatic beta-cells communicate via intermittent release of ATP. Am J Physiol Endocrinol Metab. 2004;286:E759–E765. doi: 10.1152/ajpendo.00452.2003. [DOI] [PubMed] [Google Scholar]
- 30.Richards-Williams C, Contreras JL, Berecek KH, Schwiebert EM. Extracellular ATP and zinc are co-secreted with insulin and activate multiple P2X purinergic receptor channels expressed by islet beta-cells to potentiate insulin secretion. Purinergic Signal. 2008;4:393–405. doi: 10.1007/s11302-008-9126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacques-Silva MC, Correa-Medina M, Cabrera O, Rodriguez-Diaz R, Makeeva N, Fachado A, Diez J, Berman DM, Kenyon NS, Ricordi C, Pileggi A, Molano RD, Berggren PO, Caicedo A. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci U S A. 2010;107:6465–6470. doi: 10.1073/pnas.0908935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatof D, Kilic G, Fitz JG. Vesicular exocytosis contributes to volume-sensitive ATP release in biliary cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G538–G546. doi: 10.1152/ajpgi.00355.2003. [DOI] [PubMed] [Google Scholar]
- 33.Verderio C, Cagnoli C, Bergami M, Francolini M, Schenk U, Colombo A, Riganti L, Frassoni C, Zuccaro E, Danglot L, Wilhelm C, Galli T, Canossa M, Matteoli M. TI-VAMP/VAMP7 is the SNARE of secretory lysosomes contributing to ATP secretion from astrocytes. Biol Cell. 2012;104:213–228. doi: 10.1111/boc.201100070. [DOI] [PubMed] [Google Scholar]
- 34.Akopova I, Tatur S, Grygorczyk M, Luchowski R, Gryczynski I, Gryczynski Z, Borejdo J, Grygorczyk R. Imaging exocytosis of ATP-containing vesicles with TIRF microscopy in lung epithelial A549 cells. Purinergic Signal. 2012;8:59–70. doi: 10.1007/s11302-011-9259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutierrez-Martin Y, Bustillo D, Gomez-Villafuertes R, Sanchez-Nogueiro J, Torregrosa-Hetland C, Binz T, Gutierrez LM, Miras-Portugal MT, Artalejo AR. P2X7 receptors trigger ATP exocytosis and modify secretory vesicle dynamics in neuroblastoma cells. J Biol Chem. 2011;286:11370–11381. doi: 10.1074/jbc.M110.139410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luna C, Li G, Qiu J, Challa P, Epstein DL, Gonzalez P. Extracellular release of ATP mediated by cyclic mechanical stress leads to mobilization of AA in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2009;50:5805–5810. doi: 10.1167/iovs.09-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biswas P, Zanello LP. 1alpha,25(OH)(2) vitamin D(3) induction of ATP secretion in osteoblasts. J Bone Miner Res. 2009;24:1450–1460. doi: 10.1359/JBMR.090306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bumming P, Nilsson O, Ahlman H, Welbencer A, Andersson MK, Sjolund K, Nilsson B. Gastrointestinal stromal tumors regularly express synaptic vesicle proteins: evidence of a neuroendocrine phenotype. Endocr Relat Cancer. 2007;14:853–863. doi: 10.1677/ERC-06-0014. [DOI] [PubMed] [Google Scholar]
- 40.Erlandson RA, Klimstra DS, Woodruff JM. Subclassification of gastrointestinal stromal tumors based on evaluation by electron microscopy and immunohistochemistry. Ultrastruct Pathol. 1996;20:373–393. doi: 10.3109/01913129609016340. [DOI] [PubMed] [Google Scholar]
- 41.Komuro T. Comparative morphology of interstitial cells of Cajal: ultrastructural characterization. Microsc Res Tech. 1999;47:267–285. doi: 10.1002/(SICI)1097-0029(19991115)47:4<267::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 42.Tuveson DA, Willis NA, Jacks T, Griffin JD, Singer S, Fletcher CD, Fletcher JA, Demetri GD. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 43.Minami K, Yano H, Miki T, Nagashima K, Wang CZ, Tanaka H, Miyazaki JI, Seino S. Insulin secretion and differential gene expression in glucose-responsive and - unresponsive MIN6 sublines. Am J Physiol Endocrinol Metab. 2000;279:E773–E781. doi: 10.1152/ajpendo.2000.279.4.E773. [DOI] [PubMed] [Google Scholar]
- 44.Sihto H, Sarlomo-Rikala M, Tynninen O, Tanner M, Andersson LC, Franssila K, Nupponen NN, Joensuu H. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23:49–57. doi: 10.1200/JCO.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 45.Leibiger B, Moede T, Uhles S, Barker CJ, Creveaux M, Domin J, Berggren PO, Leibiger IB. Insulin-feedback via PI3K-C2alpha activated PKBalpha/Akt1 is required for glucose-stimulated insulin secretion. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:1824–1837. doi: 10.1096/fj.09-148072. [DOI] [PubMed] [Google Scholar]
- 46.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rorsman P, Braun M, Zhang Q. Regulation of calcium in pancreatic alpha- and beta-cells in health and disease. Cell Calcium. 2012;51:300–308. doi: 10.1016/j.ceca.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerasimenko JV, Sherwood M, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP, cADPR and IP3 all release Ca2+ from the endoplasmic reticulum and an acidic store in the secretory granule area. J Cell Sci. 2006;119:226–238. doi: 10.1242/jcs.02721. [DOI] [PubMed] [Google Scholar]
- 49.Han S, Kim JS, Jung BK, Han SE, Nam JH, Kwon YK, Nah SY, Kim BJ. Effects of ginsenoside on pacemaker potentials of cultured interstitial cells of Cajal clusters from the small intestine of mice. Mol Cells. 2012;33:243–249. doi: 10.1007/s10059-012-2204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnstock G, Lavin S. Interstitial cells of Cajal and purinergic signalling. Auton Neurosci. 2002;97:68–72. doi: 10.1016/s1566-0702(02)00005-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.