Abstract
Neocortical neurons can be classified in four major electrophysiological types according to their pattern of discharge: Regular-Spiking (RS), Intrinsically-Bursting (IB), Fast-Rhythmic-Bursting (FRB), and Fast-Spiking (FS). Previously, we have shown that these firing patterns are not fixed and can change as a function of membrane potential and states of vigilance. Other studies have reported that extracellular calcium concentration ([Ca2+]o) fluctuates as a function of the phase of the cortical slow oscillation. In the present study we investigated how spontaneous and induced changes in [Ca2+]o affect the properties of action potentials (APs) and firing patterns in cortical neurons in vivo. Intracellular recordings were performed in cats anesthetized with ketamine-xylazine during spontaneous [Ca2+]o fluctuation and while changing [Ca2+]o with reverse microdialysis. When [Ca2+]o fluctuated spontaneously according to the phase of the slow oscillation, we found an increase of the firing threshold and a decrease of the afterhyperpolarization (AHP) amplitude during the depolarizing (active, up) phase of the slow oscillation and some neurons also changed their firing pattern as compared with the hyperpolarizing (silent, down) phase. Induced changes in [Ca2+]o significantly affected the AP properties in all neurons. The AHP amplitude was increased in high calcium conditions and decreased in low calcium conditions, in particular the earliest components. Modulation of spike AHP resulted in notable modulation of intrinsic firing pattern and some RS neurons revealed burst firing when [Ca2+]o was decreased. We also found an increase in AHP amplitude in high [Ca2+]o with in vitro preparation. We suggest that during spontaneous network oscillations in vivo, the dynamic changes of firing patterns depend partially on fluctuations of the [Ca2+]o.
Introduction
In both animals and humans, slow-wave sleep is characterized by a cyclic alternation of positive and negative EEG waves referred as slow oscillation (<1 Hz) (Achermann and Borbely, 1997, Steriade, et al., 1993, Steriade, et al., 1993, Steriade, et al., 1993). During sleep and anesthesia, all cortical neurons are silent and hyperpolarized during the depth-positive EEG waves, whereas during the depth-negative waves, cortical neurons are depolarized and usually fire spikes (Chauvette, et al., 2010, Contreras and Steriade, 1995, Steriade, et al., 2001, Timofeev, et al., 2001). Cortical neurons can generate various firing patterns depending on their morphology, passive properties, and active conductances. On the basis of their firing pattern, cortical neurons are classified in four major electrophysiological types: regular-spiking (RS), intrinsically-bursting (IB), fast-rhythmic-bursting (FRB), and fast-spiking (FS) (Gray and McCormick, 1996, McCormick, et al., 1985, Steriade, et al., 1998). However, the intrinsic firing patterns expressed by cortical neurons are affected by the presence of network activities or neuromodulators (Steriade, 2004, Steriade, et al., 1998, Steriade, et al., 2001, Timofeev, et al., 2000, Wang and McCormick, 1993). Active network states are associated with increased extracellular potassium concentration ([K+]o) and decreased [Ca2+]o (Heinemann, et al., 1977). During the cortical slow oscillation, [Ca2+]o reaches its maximum (about 1.2 mM) during silent network states (depth-positive EEG wave) and decreases by approximately 20% during active network states (depth-negative EEG wave) (Crochet, et al., 2005, Massimini and Amzica, 2001). Such ionic changes affect synaptic responses(Crochet, et al., 2005, Jones and Heinemann, 1987, Rausche, et al., 1988, Seigneur and Timofeev, 2010, Somjen, 2002).
Neuronal firing is associated with Ca2+ influx (Abel, et al., 2004, Markram, et al., 1995). A rise of intracellular Ca2+ concentration ([Ca2+]i) activates Ca2+-activated K+ currents (IK(Ca)) that are responsible for the afterhyperpolarizing potential (AHP) following action potentials (APs) (Sah and Faber, 2002, Storm, 1987). In neocortical and other neurons, one can distinguish three components in the AHP: the fast, the medium, and the slow AHP (fAHP, mAHP, and sAHP) (Sah and Faber, 2002). The fAHP is activated immediately during the AP and constitute the largest negative excursion of the membrane potential that follows the spike (Schwindt, et al., 1988); it contributes to the repolarization of APs (Kang, et al., 2000, Storm, 1987). Some studies (Kang, et al., 2000, Schwindt, et al., 1988) suggested that IK(Ca) might not be essential in the generation of fast AHPs. However a large body of literature indicates that fast AHP is mediated by high-conductance voltage- and Ca2+-activated K+ (BK) channels and mediated by IC current (reviewed in (Sah and Faber, 2002)). Two recent studies demonstrated the presence of calcium binding sites on BK channels (Wu, et al., 2010, Yuan, et al., 2010). The mAHP is also activated rapidly following the action potential (<5 ms) but decays with a time course of several hundred milliseconds (Sah and Faber, 2002). The mAHP is mediated by small conductance Ca2+-activated potassium (SK) channels and mediated by IAHP (to insert Sah and Faber, 2002). Although the sAHP is more commonly seen following a train (4–10) of spikes (Faber, et al., 2001, Lancaster and Nicoll, 1987, Schwindt, et al., 1988), it can follow a single AP in some neurons (Hirst, et al., 1985, Sah and McLachlan, 1991). The AHP is mainly mediated by calcium-activated potassium channels, but IB neurons also display a sAHP mediated by sodium-activated potassium current (Franceschetti, et al., 2003). The AHP plays a key role in regulating the cell firing: (a) it limits the firing frequency of the neuron and (b) it is responsible for generating the phenomenon of spike-frequency adaptation (McCormick, 1999).
The presence of extracellular Ca2+ dynamics and the contribution of IK(Ca) to neuronal output, led us to the hypothesis that slow oscillation-dependent fluctuation of [Ca2+]o in the neocortex could contribute to a dynamic control of intrinsic firing patterns and AP properties. To test this hypothesis, we performed intracellular recordings from cortical neurons in vivo in cats anesthetized with ketamine-xylazine; under this condition, cortical activities are dominated by the slow oscillation, similar to slow-wave sleep. We also combined intracellular recordings with the reverse-microdialysis technique to change [Ca2+]o. Finally, we tested this hypothesis in vitro by changing the [Ca2+]o in the ACSF.
Materials and methods
All experimental procedures were performed according to national guidelines and were approved by the committee for animal care of Laval University.
Preparation
In vivo experiments were conducted on 36 adult cats anesthetized with ketamine-xylazine anesthesia (10-15 and 2-3 mg/kg i.m., respectively). The animals were paralyzed with gallamine triethiodide (20 mg/kg) after the EEG showed typical signs of deep general anesthesia, essentially consisting of a slow oscillation (0.5-1 Hz). Supplementary doses of the same anesthetics (5 and 1 mg/kg i.m.) were administered at the slightest changes toward the diminished amplitudes of slow waves. All pressure points and the tissues to be incised were infiltrated with lidocaine (0.5%). The cats were ventilated artificially with the control of end-tidal CO2 at 3.5-3.7% (Carbon dioxide analyzer (CD-3A) and carbon dioxide sensor (Model P61B), both from (Applied electrochemistry inc., Sunny Vale, California)). The body temperature was maintained at 37-38°C and the heart rate was ~90-100 beats/min. For intracellular recordings, the stability was ensured by the drainage of cisterna magna, hip suspension, bilateral pneumothorax, and by filling the hole made for recordings with a solution of 4% agar. At the end of experiments, the cats were given a lethal dose of intravenous sodium pentobarbital (50 mg/kg).
In vivo recordings, microdialysis
The intracellular recordings were obtained using glass micropipettes filled with 3 M potassium acetate (direct current resistance, 30-70 MΩ). A high-impedance amplifier (bandpass, 10 kHz) with an active bridge circuitry was used to record and inject currents into the cells. Stepping microdrive with minimal steps 0.5 μm (David Kopf Instruments, California, USA) was used to advance the intracellular micropipettes. Parallel recordings of local field potential were obtained by means of tungsten electrodes inserted at cortical depths with a distance of 3-5 mm from the recording pipettes. All electrical signals were sampled at 20 kHz and digitally stored on Vision (Nicolet, Wisconsin, USA). Offline computer analysis of electrographic recordings was done with IgorPro software (Lake Oswego, Oregon, USA). Statistical analysis was conducted with JMP software (Cary, North Carolina, USA). All numerical values are expressed as a mean ± standard deviation.
The modulation of [Ca2+]o in the neocortex was achieved using reverse microdialysis method. The membrane of the microdialysis probe (2 mm length, 0.22 mm diameter from EICOM, Kyoto, Japan) was inserted in the cortex and the recording micropipettes were placed at 0.2-0.3 mm apart from the membrane. The microdialysis probe was perfused with the following solutions (concentration in mM): Control (NaCl 124, KCl, 2.5, NaHCO3 26, NaH2PO4 1.25, MgSO4 2, MgCl2 1, CaCl2 1), High calcium (NaCl 124, KCl, 2.5, NaHCO3 26, NaH2PO4 1.25, MgSO4 2, MgCl2 0, CaCl2 5), Calcium free (NaCl 125, KCl, 2.5, NaHCO3 26, NaH2PO4 0, MgSO4 2, MgCl2 1, CaCl2 0, MnCl2 1). The osmolarity was adjusted to 300 mOsm. Each dialyzing solution was administrated for 15-30 min. The perfusion velocity was 5 μl/min and the total volume of tubing from the liquid switch to the probe was 12 μl. For other details concerning the microdialysis technique see (Crochet, et al., 2005). Ca2+-sensitive electrodes (Diamond General, Ann Arbor, MI, USA) were used to measure the [Ca2+]o change actually occurring at the intracortical recording site (about 0.2-0, 3 mm from the microdialysis probe, 1 mm depth) using the three different solutions as described previously (Crochet, et al., 2005, Crochet, et al., 2004).
Analysis
The initial step of the analysis consisted in averaging the APs of neurons. Averages were obtained from at least 20 to 50 APs. From averaged APs we estimated the firing threshold as the membrane potential at the time point at which the first derivative of the intracellular signal reaches the threshold of 10 V/s (Sachdev, et al., 2004). This threshold value was chosen because EPSPs have a rising slope slower than 10 V/s and faster rising slopes are reach only by regenerative APs (Crochet, et al., 2004) (Fig. 1). The exact value of the firing threshold was corrected for the pipette offset by subtracting the offset measured when the pipette was withdrawn from the neuron. The spike amplitude was measured as the difference in voltage between the firing threshold and the peak of the AP. The spike-width was measured as the time difference between the ascending and descending fluctuations of membrane potential at spikes’ half-amplitude. The maximal rising and decaying slopes of spikes were taken, respectively, as the maximum and minimum of the first derivative of the AP. The spike-related AHP amplitude was calculated as the difference in voltage between the firing threshold and the maximal hyperpolarization that followed the spike as done by others (Haghdoost-Yazdi, et al., 2008, Hou, et al., 2012, Lin, et al., 2010). In addition we measured the mean firing rates from periods of stable recordings that exceeded 5 min and we noted the depth of recorded neurons from micromanipulator readings.
Fig. 1. Parameters of action potentials measured in the present study.
Upper trace – averaged spike, lower trace - first derivative of the spike. The measured parameters are indicated.
Slice preparation
Sprague Dawley P21-P30, Charles River Laboratories) rats were first anesthetized with ketamine (0.1 mg/g) and then with pentobarbital (0.04mg/g) to be decapitated. The brain was quickly dissected and maintained in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 124, KCl 2.8, CaCl2 2.0, MgSO4 2, NaH2PO4 1.25, NaHCO3 26 and D-glucose 10 (Sigma-Aldrich Canada, Canada), pH 7.4, aerated with 95% O2, 5% CO2. Osmolarity was 300 ± 5 mOsm. Coronal slices (400 μm) were cut with the use of a vibraslice to obtain complete sections containing the somatosensory cortex. Slices were transferred to a holding chamber where they were kept submerged in the same ACSF aerated with 95% O2, 5% CO2 for a period of one hour.
In vitro recordings and stimulation
Brain slices were transferred to a perfusion chamber maintained at room temperature containing the ACSF and the perfusion rate was 4 ml/min. Pyramidal neurons in layers II/III were preselected using an infrared differential interference contrast camera-microscopy on an upright microscope. Somatic whole-cell current-clamp recordings (10-20 MΩ access resistances) were obtained. Recordings were performed using patch pipettes (resistance 3-7 MOhm) containing (in mM): Potassium D-gluconate 130, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)10, KCl 10, MgCl2 2, ATP 2 and GTP 2 (Sigma-Aldrich Canada, Canada) at pH 7.2, and 280 mOsm. We also used modified ACSF that contained lower Ca2+ concentrations (1.2 mM and 0.6 mM) to see the effect on neuronal excitability and AHP amplitude. When a cell was recorded, step pulses of 40 pA from −120 pA to 120 pA were injected to the neuron for the firing pattern identification. The number of spikes induced for each current pulse intensity was counted and compared between every [Ca2+]o. AHP amplitude was measured for all spikes and was plotted for every condition.
Results
Database
We recorded intracellular activities of 103 cortical neurons in vivo from areas 5 and 7 of suprasylvian gyrus of 36 cats anesthetized with ketamine-xylazine. Out of these we analyzed electrographic activities from 29 cats from our previous database (Crochet, et al., 2005) and 7 experiments were specifically done for this study. All recorded neurons were identified by electrophysiological criteria (Gray and McCormick, 1996, McCormick, et al., 1985, Steriade, et al., 1998). The intracellular activity of 81 neurons was recorded in control conditions (without [Ca2+]o change) to study AP and AHP parameters during spontaneous slow oscillation. The activity of the remaining 22 neurons was recorded in 2 or 3 different conditions of [Ca2+] to investigate the role of Ca2+o influx in the modulation of spike parameters and intrinsic firing pattern. We also recorded the responses to depolarizing current pulses of 10 cortical neurons in vitro in slices prepared from rat brains.
Activity-dependent changes in AP properties
In agreement with a previous study (Chauvette, et al., 2011) in ketamine-xylazine anesthetized cats, cortical neurons from suprasylvian gyrus revealed typically prolonged (>200 ms) hyperpolarizing (silent) states followed by long-lasting (>500 ms) depolarizing (active) states. Most of neocortical neurons fire multiple spikes throughout active states, with apparently different shape (Fig. 2). In control conditions, 56 intracellular recordings were used to compare the parameters of the first spike with those of the last spike during the depolarizing phase of the slow oscillation. The first spontaneous AP occurs when the network activity just switched from silent to active phase, while the last spike occurs at the end of active phase. We found that all measured parameters of APs were affected by network activities (Fig. 2, Table 1). Following the period of network activity the amplitude of the spike, the amplitude of the AHP, the rising and decaying slopes decreased, the spike width increased, and the firing threshold was more depolarized (p<0.0001, paired t-test for each parameter). The amplitude of the AHP of the first spike that fired at the onset of the depolarizing phase was highly correlated with the spike width, maximal rising and decaying slopes, and marginally, but significantly correlated with the firing threshold (Table 2). Out of these parameters, the amplitude of AHP of the last spike did not correlate with the firing threshold. There was no significant correlation between AHP and spike amplitude. The fact that the spike duration, as well as related parameters (maximal rising and in particular decaying slopes), was correlated with AHP amplitude (Fig. 2 E-G) suggests that neurons with thinner spikes have ampler AHP and thus that the intrinsic current mediating fAHP plays a role in controlling spike duration. The decrease in the amplitude of AHP toward the end of active network phase positively correlated with the amplitude of AHP of the first spike (r=0.54, p<0.0001). The reason for the decrease in AHP amplitude toward the end of the active phase was further investigated.
Fig. 2. Modulation of spike parameters in neocortical neurons by spontaneous network activities during slow oscillation.
A and C – periods of spontaneous field potential and intracellular activity from two different neurons. A, regular-spiking (RS) neuron, C, fast-spiking (FS) neuron. B and D, averages of first and last spikes spontaneously occurring during active periods from the same neurons. Scatter plots of AHP amplitude vs. spike duration at half amplitude (E), maximal rising slope (F), and maximal decay slope (G).
Table 1.
Parameters of first and last spikes during active periods.
| Amplitude (mV) |
Firing threshold (mV) |
AHP amplitude (mV) |
Spike width (ms) |
Rising slope (v/s) |
Decay slope (v/s) |
|
|---|---|---|---|---|---|---|
| First spike | 61.7±8.8 | −56.7±5.7 | 4.67±2.87 | 0.80±0.28 | 219.1±65.6 | −99.1±38.2 |
| Last spike | 56.4±8.7 | −53.9±5.7 | 3.32±2.50 | 0.88±0.32 | 181.1±60.3 | −85.236.1 |
| Difference | 5.3±3.0 | 2.8±1.5 | 1.33±0.98 | 0.08±0.06 | 38.0±21.8 | 13.9±8.7 |
Table 2.
Correlation (r) and its significance (p) of first and last spike AHPs with other spike parameters during active periods
| First spike AHP (r) |
First spike AHP (p) |
Last spike AHP (r) |
Last spike AHP (p) |
|
|---|---|---|---|---|
| First spike amplitude |
0.139 | 0.3067 | 0.090 | 0.5079 |
| Last spike amplitude |
0.139 | 0.3059 | 0.090 | 0.5073 |
| First spike AHP |
1 | 0.946 | <0.0001 | |
| Last spike AHP |
0.946 | <0.0001 | 1 | |
| First spike width |
−0.470 | 0.0003 | −0.432 | 0.0009 |
| Last spike width |
−0.503 | <0.0001 | −0.471 | 0.0003 |
| First spike threshold |
0.328 | 0.0135 | 0.263 | 0.0502 |
| Last spike threshold |
0.290 | 0.0299 | 0.228 | 0.0916 |
| First spike raising slope |
0.484 | 0.0002 | 0.430 | 0.0010 |
| Last spike raising slope |
0.531 | <0.0001 | 0.469 | 0.0003 |
| First spike decay slope |
−0.499 | <0.0001 | −0.451 | 0.0005 |
| Last spike decay slope |
−0.553 | <0.0001 | −0.511 | <0.0001 |
To characterize the activity-dependent modulation of spike AHP we analyzed the parameters of spikes elicited by depolarizing current pulses during silent vs. active network states (n=25, Fig. 3). In this set of experiments, a slight negative holding current (-0.2 nA) was injected into neurons to abolish spontaneous firing. Averages of spikes elicited during the active or the silent periods showed that all the studied spike parameters except the AHP amplitude and the firing threshold were not statistically different (p>0.1). The firing threshold for spikes elicited during silent states was −49.9 ±4.6 mV and it was −52.7±5.0 mV during active states (p<0.0001, Paired t-test). The AHP amplitude of APs elicited during silent network states was 5.73±2.79 mV and decreased to 3.24±2.70 mV during active states. This difference was also highly significant (p<0.0001, paired t-test). Changes in the firing patterns of some neurons correlated with a change in the AHP amplitude. All the three FRB neurons recorded in this set of experiments revealed a typical FRB firing pattern during active states, consisting in high frequency spike-bursts repeated at gamma frequencies, and fired single spikes with occasional spike doublets during silent states (see example in Figure 3C).
Fig. 3. Modulation of spike parameters of cortical neurons during active versus silent network states.
To prevent extensive spontaneous firing, a −0.2 nA holding current was applied to both neurons shown in panels A and B, and C and D. A, depolarizing current pulses of 0.5 nA were applied to a RS neuron during active and silent network states. B, averages of spikes elicited during active (thick line) and silent (thin line) network states. C, depolarizing current pulses of 1.0 nA were applied to the FRB neuron during active and silent network states. Note that FRB pattern of firing was seen only during active network states. D, averages of spikes elicited during active (thick line) and silent (thin line) network states. Note in panels B and D that the spike amplitude, duration, rising, and decaying phases was similar, but the AHP of spike elicited during active network states was reduced in amplitude. Note that in B and D, the averaged spikes were aligned to spike thresholds to better illustrate the difference in AHP amplitude.
Modulation of AHP by [Ca2+]o
As we mentioned in the introduction, the [Ca2+]o decreases during active network states. We asked, could a change in the [Ca2+]o affect the amplitude of AHP mediated by IK(Ca) and therefore modify the firing pattern? To test this hypothesis we modified the [Ca2+]o with reversed microdialysis method and recorded intracellular activities from neurons located at 0.2-0.3 mm from the probe. The mean [Ca2+]o during active periods measured with Ca2+ sensitive electrodes placed at ~ 0.2 mm from the probe (n=3) was 1.0±0.1 mM in control, 2.6±0.2 mM in high, and 0.6±0.1 mM in low [Ca2+]o conditions. The local changes of [Ca2+]o did not influence the slow oscillation general pattern, but within affected area the synaptic noise during active states was higher in high Ca2+ conditions and it was lower in low Ca2+ conditions and the spike threshold showed reverse correlation with Ca2+ concentration (Crochet, et al., 2005, Crochet, et al., 2004). In these conditions the spike amplitude and the maximal rising slope of spikes were not significantly different (Fig. 4, Table 3). In high [Ca2+]o condition, the width of APs at half amplitude was shortened by 0.05±0.02 ms and this difference was significant (Table 3). In the same neurons the decay slope significantly decreased by 8.3±3.3 V/s in low [Ca2+]o condition, additionally indicating that IK(Ca) could contribute to the control of spike duration. However, the most significant changes were observed with spike AHPs (Table 3). The AHP in high [Ca2+]o condition increased from control by 4.34 mV and it decreased by 2.17 mV in low [Ca2+]o condition (Fig. 4). A decrease in AHP amplitude in low [Ca2+]o condition changed the spontaneous firing pattern. Out of 15 neurons identified as RS in which spontaneous firing patterns were investigated in control condition, 4 revealed spontaneous bursts of APs in low [Ca2+]o condition (not shown) or revealed groups of spikes (Fig. 4).
Fig. 4. Impact of [Ca2+]o modulation on spontaneous firing of cortical neurons.
A, The same neuron recorded under high, control, and low [Ca2+]o conditions. Below, superposed spontaneous spikes (n=10) selected during these three different levels of [Ca2+]o. B, Averages of spontaneous spikes during these three different [Ca2+]o conditions. Note the increase of AHP amplitude as [Ca2+]o is high. C, Histograms of mean ± SD AHP amplitude in three different [Ca2+]o conditions (n=22).
Table 3.
Statistical significance of differences (p) in action potential parameters in pairs of different Ca2+ conditions.
| Spike amplitude (mV) |
Spike width (ms) |
Rising slope (v/s) |
Decaying slope (v/s) |
AHP amplitude (mV) |
|
|---|---|---|---|---|---|
| Control-High (n=20) |
0.1112 | 0.0261 | 0.0977 | 0.2550 | <0.0001 |
| Control-Low (n=15) |
0.0902 | 0.1427 | 0.8421 | 0.0226 | <0.0001 |
| High-Low (n=13) |
0.7029 | 0.0141 | 0.6874 | 0.0103 | <0.0001 |
Statistically significant differences are indicated in bold.
[Ca2+]o modulates intrinsic excitability
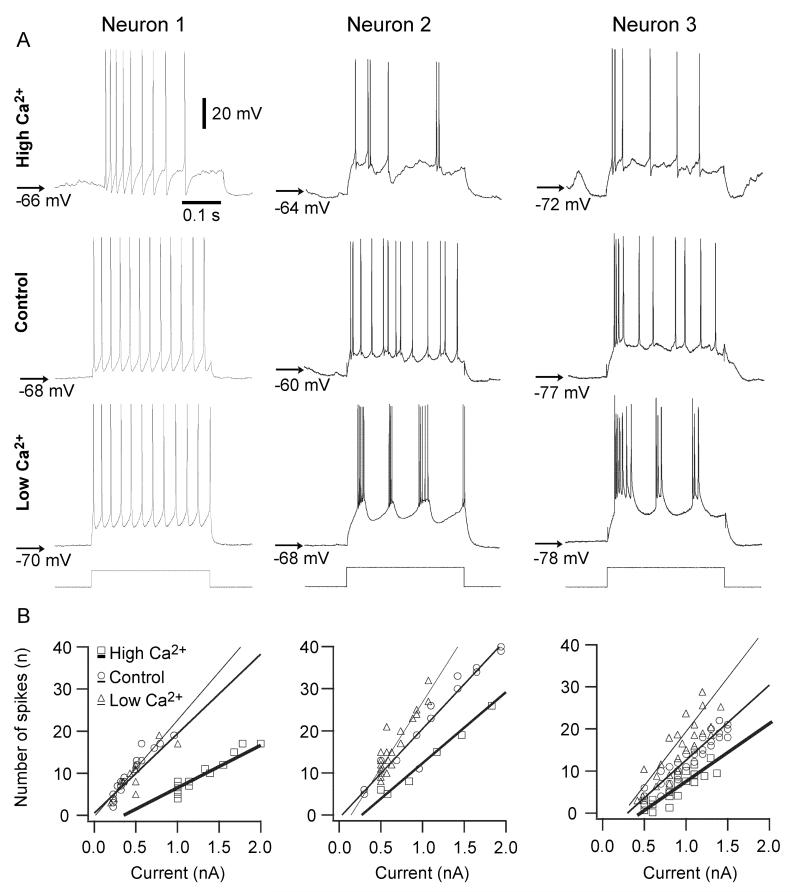
To estimate the changes in intrinsic excitability related to the modulation of [Ca2+]o we applied intracellularly depolarizing current pulses of variable amplitude (range 0.0-2.0 nA, duration 0.3 s) in different Ca2+ conditions and counted the number of spikes elicited per current pulse by neurons demonstrating RS firing pattern (n=22, Fig. 5). In all the levels of tested currents the increase in [Ca2+]o to 2.6 mM significantly (p<0.01 [Tukey, HSD test]) decreased the number of spikes elicited per pulse. In some neurons (7 out of 22 tested) the lowering of [Ca2+]o to 0.6 mM markedly increased the number of spikes elicited by current pulses of the same amplitude (Fig. 5), however, the statistical comparison for the studied population of neurons did not reveal any significant differences in a large range of depolarizing current pulses. The only significant increase in the number of spikes per current pulse (p<0.05) was observed when current pulses of 1.5 nA or higher intensity were used. Decreasing [Ca2+]o had another important effect; in 8 out of 22 neurons in which intracellular current pulses were applied, the RS firing pattern recorded in control and high Ca2+ conditions changed to a bursting pattern in low Ca2+ conditions (Fig. 5, middle and right columns).
Fig. 5. Modulation of [Ca2+]o affects intrinsic excitability and firing patterns.
A, neuronal excitability was tested by injection of depolarizing current pulses of variable intensity. Examples of responses shown for three different neurons during high, control, and low conditions of [Ca2+]o. B, plots showing the number of action potentials elicited by intracellularly applied current pulses of different intensity. Note a decrease in the number of action potentials as [Ca2+]o increases and the bursting response during low [Ca2+]o conditions for the second (middle column) and third neuron (right column).
In all experiments described above the network activity by itself could influence the parameters of AHP. To measure the effect of [Ca2+]o changes without effects of organized network activities, we performed neuronal whole-cell recordings in rat neocortex in vitro at different [Ca2+]o (Fig. 6). Since in these conditions, the network was not active, we injected depolarizing current pulses to elicit spikes. We observed a reduction in the excitability of cells and a large increase in the AHP amplitude in high [Ca2+]o (Fig. 6c). In low calcium conditions, the spike width at half-amplitude was increased, spike amplitude was decreased, and the AHP amplitude was slightly decreased (Fig. 6b, c). These in vitro results support our in vivo observations.
Fig. 6. Excitability in different extracellular calcium concentration in rat slice in vitro.
A. Neuronal depolarization to current pulse (300 pA, 750 ms) in [Ca2+]o of 2.0 mM (black trace), 1.2 mM (red trace), and 0.6 mM (blue trace). B. Superimposed averaged spikes (n=100) for the three conditions. Note the smaller spike amplitude in low calcium conditions and the higher AHP amplitude in high calcium condition. C. Excitability (number of spikes) in function of injected current (pA) for the three different calcium concentration. Note the reduced excitability in high calcium conditions. D. Afterhyperpolarization potential (AHP) amplitude in the three calcium conditions. Note that the AHP amplitude is larger in the 2 mM calcium condition.
Discussion
In this study we demonstrated that network activities influence multiple parameters of AP generated by neocortical neurons. In particular, the spike-related AHP, which was positively correlated with the firing threshold and rising slope, and negatively correlated with the spike duration and decaying slopes, was decreased during active periods. The spike duration was increased toward the end of active network states. Consistently with previous studies in hippocampus (Henze and Buzsaki, 2001), somatosensory cortex (Sachdev, et al., 2004), and spinal cord motoneurons (Krawitz, et al., 2001), APs generated at the end of active network states had a higher firing threshold. Since the AHP is mediated by IK(Ca), the major influence in the reduction of AHP amplitude at the end of active periods was probably due to a decreased Ca2+ entrance, which might be related to the decrease in [Ca2+]o that occurs during active states (Crochet, et al., 2005, Massimini and Amzica, 2001). An increase in the [K+]o during active phases of slow oscillation (Amzica and Steriade, 2000) would also favour a reduction in AHP amplitude. However, the [K+]i is of about 135 mM and the [K+]o is of about 3 mM in normal conditions. The extracellular potassium concentration varies in the range of 0.2-0.8 mM during slow oscillation (Dufour, et al., 2011, Seigneur, et al., 2006). The calcium concentrations are of about 1.2 mM in the extracellular space and of 0.1 μM in the cell, and the [Ca2+]o varies by 0.2-0.3 mM during slow oscillation (Massimini and Amzica 2001; Crochet et al. 2005). Thus inside/outside ratio of concentration varies much more for the Ca2+ than for the K+, which suggests that the Ca2+ ratio is more important in shaping the AHP. In keeping with this idea, we found that an artificial increase in [Ca2+]o either via reverse microdialysis method in vivo or in the bath solution in vitro significantly increased the AHP amplitude and a decrease in [Ca2+]o decreased the AHP amplitude. Interestingly, the reduction of AHP amplitude, either during spontaneous activities or in conditions of artificially reduced [Ca2+]o, turned the RS firing pattern of some neurons into a bursting pattern. Thus, in addition to its effect on passive membrane properties (Destexhe, et al., 2003, Paré, et al., 1998), shunting inhibition (Borg-Graham, et al., 1998, Hirsch, et al., 1998), and reduction of synaptic efficacy (Crochet, et al., 2005, Seigneur and Timofeev, 2010), network activity also modulates the discharge properties of neocortical neurons: it affects spike-related AHP and subsequently, the intrinsic responsiveness and the firing patterns of neocortical neurons.
Dynamics of intrinsic firing patterns
Neocortical neurons reveal at least four distinct firing patterns: (a) RS, (b) IB, (c) FRB, and (d) FS (Gray and McCormick, 1996, McCormick, et al., 1985, Steriade, et al., 1998). The ability of cortical neurons to generate spikes with a particular pattern is not stable and depends on multiple factors. An intracellular injection of a steady depolarizing current could transform an IB firing pattern to a RS one (Timofeev, et al., 2000, Wang and McCormick, 1993). A similar effect was observed after a bath application of acetylcholine to neocortical slices maintained in vitro (Wang and McCormick, 1993), activation induced by stimulation of mesopontine cholinergic nuclei (Steriade, et al., 1993) or during a change in behavioral states, from natural slow-wave sleep to REM sleep (Steriade, et al., 2001). Even in the absence of activity in cholinergic structures, the network activity, likely due to its depolarizing effects, decreases the incidence of IB neurons. The occurrence of cortical IB neurons is much higher (up to 40-60%) in cortical slices (Yang, et al., 1996) or cortical slabs in vivo (Timofeev, et al., 2000) than in the anesthetized animals (10 %) (Nuñez, et al., 1993). The increase in [K+]o induces a conversion of some neurons with a RS firing pattern to an IB one (Jensen, et al., 1994, Jensen and Yaari, 1997). In this study we have shown that the presence of spontaneous activity as well as lowering [Ca2+]o convert some neurons displaying RS firing patterns to FRB neurons (Fig. 3). Fast-rhythmic-bursting neurons are found mainly in vivo (Gray and McCormick, 1996, Steriade, et al., 1998, Steriade, et al., 2001). In slices maintained in vitro, the FRB firing pattern could be induced either by prolonged intracellular stimulation (Kang and Kayano, 1994) or by the use of modified artificial cerebrospinal fluid, which contained physiological levels of [Ca2+]o (Brumberg, et al., 2000). Neuronal burst also appears after presence of high potassium and low calcium in the bath (Seigneur Timofeev 2010). The AHP controls the spike duration as well as the firing pattern of cortical neurons (Chen and Fetz, 2005). We found a significant correlation between the amplitude of spike AHP, the spike duration, and maximal slope of the spike repolarization (Table 2). The stronger the AHP is, the faster is the decay and the shorter is the spike duration. A strong AHP postponed the generation of consecutive spikes, thus preventing the generation of bursts. Since the AHP is mediated by an activation of IK(Ca), the AHP amplitude decrease was caused by a decrease in [Ca2+]o either due to activity-related fluctuations (Crochet, et al., 2005, Massimini and Amzica, 2001) or to artificial changes. Despite the presence of high-threshold Ca2+ spikes in neocortical neurons in vivo (Paré and Lang, 1998), our results support previous reports that transient depolarization mediating bursts in neocortical neurons is not Ca2+-dependent (Brumberg, et al., 2000, Mantegazza, et al., 1998) because an increase in [Ca2+]o never led to a burst firing. By contrast, a lowering in [Ca2+]o diminished AHP mediated by IK(Ca) and induced a burst firing in a subset of neurons.
Physiological and pathological implications
[Ca2+]o fluctuates during slow oscillation, a dominant rhythm of deep sleep. It is well known that a lowering of [Ca2+]o reduces synaptic efficacy (Katz, 1969). A reduction of synaptic efficacy by itself should reduce network excitability during active phases of slow oscillation. In this study we demonstrated that a lowering of [Ca2+]o increased intrinsic excitability and even induced bursting. A burst firing increases the reliability of synaptic transmission (Lisman, 1997, Timofeev, et al., 2000). Therefore, it is plausible to suggest that such increase in intrinsic excitability in low Ca2+ conditions provides a homeostatic up-regulation of network excitability and in some range of Ca2+ conditions the overall network excitability remains stable. A severe or prolonged changes in [Ca2+]o may lead however to pathological conditions. It is well known that cortically generated seizures arise from sleep slow oscillation (Timofeev and Steriade, 2004).
Calciumopathies result in complex polygenic diseases such as epilepsy, migraine, and autism (Gargus, 2009). The increased intrinsic excitability in conditions of reduced [Ca2+]o could play a significant role in the maintenance of paroxysmal activities. Zero mM [Ca2+] conditions promote epileptiform discharges in hippocampal slices maintained in vitro (Jefferys and Haas, 1982, Taylor and Dudek, 1982). In neocortex too, electrographic seizures are associated with a marked reduction of [Ca2+]o down to a concentration 0.6 mM (Amzica, et al., 2002, Heinemann, et al., 1977). In these conditions the synaptic responsiveness of cortical neurons is largely impaired (Cisse, et al., 2004, Crochet, et al., 2005, Seigneur and Timofeev, 2010, Steriade and Amzica, 1999). As a consequence, the synchronization between different neocortical neurons is loose (Boucetta, et al., 2008, Neckelmann, et al., 1998). Low [Ca2+]o increases the opening of hemichannels (connexons) that mediate electrical coupling of neurons and glial cells (Thimm, et al., 2005), and thus could contribute to the local synchronization. During seizures, neocortical neurons generate paroxysmal depolarizing shifts, which contain an important intrinsic component (de Curtis, et al., 1999, Timofeev and Steriade, 2004). The IK(Ca) plays an important role in the control of paroxysmal depolarizing shifts amplitude and duration (Timofeev, et al., 2004, Timofeev and Steriade, 2004). Thus, we suggest that the enhanced bursting propensity of neurons in conditions of reduced [Ca2+]o might contribute to the generation of paroxysmal activities.
Calciumopathies result in complex polygenic diseases and in addition to seizures they are associated with migraine and autism (Gargus, 2009). Electrophysiological data on migraine and autism are very limited. Our results suggest that if Ca2+ homeostasis is implicated in the genesis of these conditions, overall these diseases could be associated with an increased neuronal firing and increased incidence of bursting.
Highlights.
The AHP is progressively reduced toward the end of each cycle of slow oscillation
The AHP parameters affected the spike parameters and the firing pattern of neurons
[Ca2+]o modulation affected the parameters of AHP and changed the firing pattern of neurons
Spontaneous [Ca2+]o fluctuations contribute to the overall firing exhibited by cortical neurons
Acknowledgements
We appreciate the technical assistance of P. Giguère.
Grants:
This study was supported by Canadian Institutes of Health Research (MOP-37862, MOP-67175), National Science and Engineering Research Council of Canada (grant 298475) and National Institute of Neurological Disorders and Stroke (1R01-NS060870 and 1R01-NS059740). S.Cr. was supported by a Pickwick fellowship from the National Sleep Foundation. S.Ch. was a Canadian Institutes of Health Research fellow. I.T. is Fonds de la Recherche en Santé du Québec Research Scholar.
Abbreviations
- AHP
afterhyperpolarizing potential
- AP
action potential
- IK(Ca)
calcium activated potassium current
- IB
intrinsically-bursting
- FRB
fast-rhythmic-bursting
- FS
fast-spiking
- RS
regular-spiking
- [K+]o
extracellular potassium concentration
- [Ca2+]o
extracellular calcium concentration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
There is no potential conflict of interests.
References
- 1.Abel HJ, Lee JC, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol. 2004;91:324–335. doi: 10.1152/jn.00583.2003. [DOI] [PubMed] [Google Scholar]
- 2.Achermann P, Borbely AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- 3.Amzica F, Massimini M, Manfridi A. Spatial buffering during slow and paroxysmal sleep oscillations in cortical networks of glial cells in vivo. J Neurosci. 2002;22:1042–1053. doi: 10.1523/JNEUROSCI.22-03-01042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amzica F, Steriade M. Neuronal and glial membrane potentials during sleep and paroxysmal oscillations in the neocortex. J Neurosci. 2000;20:6648–6665. doi: 10.1523/JNEUROSCI.20-17-06648.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg-Graham LJ, Monier C, Fregnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature. 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- 6.Boucetta S, Chauvette S, Bazhenov M, Timofeev I. Focal generation of paroxysmal fast runs during electrographic seizures. Epilepsia. 2008;49:1925–1940. doi: 10.1111/j.1528-1167.2008.01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brumberg JC, Nowak LG, McCormick DA. Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. J Neurosci. 2000;20:4829–4843. doi: 10.1523/JNEUROSCI.20-13-04829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauvette S, Crochet S, Volgushev M, Timofeev I. Properties of Slow Oscillation during Slow-Wave Sleep and Anesthesia in Cats. J Neurosci. 2011;31:14998–15008. doi: 10.1523/JNEUROSCI.2339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauvette S, Volgushev M, Timofeev I. Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex. 2010;20:2660–2674. doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D, Fetz EE. Characteristic membrane potential trajectories in primate sensorimotor cortex neurons recorded in vivo. J Neurophysiol. 2005;94:2713–2725. doi: 10.1152/jn.00024.2005. [DOI] [PubMed] [Google Scholar]
- 11.Cisse Y, Crochet S, Timofeev I, Steriade M. Synaptic responsiveness of neocortical neurons to callosal volleys during paroxysmal depolarizing shifts. Neuroscience. 2004;124:231–239. doi: 10.1016/j.neuroscience.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crochet S, Chauvette S, Boucetta S, Timofeev I. Modulation of synaptic transmission in neocortex by network activities. Eur J Neurosci. 2005;21:1030–1044. doi: 10.1111/j.1460-9568.2005.03932.x. [DOI] [PubMed] [Google Scholar]
- 14.Crochet S, Fuentealba P, Timofeev I, Steriade M. Selective amplification of neocortical neuronal output by fast prepotentials in vivo. Cereb Cortex. 2004;14:1110–1121. doi: 10.1093/cercor/bhh071. [DOI] [PubMed] [Google Scholar]
- 15.de Curtis M, Radici C, Forti M. Cellular mechanisms underlying spontaneous interictal spikes in an acute model of focal cortical epileptogenesis. Neuroscience. 1999;88:107–117. doi: 10.1016/s0306-4522(98)00201-2. [DOI] [PubMed] [Google Scholar]
- 16.Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- 17.Dufour S, Dufour P, Chever O, Vallee R, Amzica F. In vivo simultaneous intra- and extracellular potassium recordings using a micro-optrode. Journal of neuroscience methods. 2011;194:206–217. doi: 10.1016/j.jneumeth.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Faber ES, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. J Neurophysiol. 2001;85:714–723. doi: 10.1152/jn.2001.85.2.714. [DOI] [PubMed] [Google Scholar]
- 19.Franceschetti S, Lavazza T, Curia G, Aracri P, Panzica F, Sancini G, Avanzini G, Magistretti J. Na+-activated K+ current contributes to postexcitatory hyperpolarization in neocortical intrinsically bursting neurons. J Neurophysiol. 2003;89:2101–2111. doi: 10.1152/jn.00695.2002. [DOI] [PubMed] [Google Scholar]
- 20.Gargus JJ. Genetic calcium signaling abnormalities in the central nervous system: seizures, migraine, and autism. Annals of the New York Academy of Sciences. 2009;1151:133–156. doi: 10.1111/j.1749-6632.2008.03572.x. [DOI] [PubMed] [Google Scholar]
- 21.Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–113. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- 22.Haghdoost-Yazdi H, Janahmadi M, Behzadi G. Iberiotoxin-sensitive large conductance Ca2+ -dependent K+ (BK) channels regulate the spike configuration in the burst firing of cerebellar Purkinje neurons. Brain research. 2008;1212:1–8. doi: 10.1016/j.brainres.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Heinemann U, Lux HD, Gutnick MJ. Extracellular free calcium and potassium during paroxysmal activity in the cerebral cortex of the cat. Exp Brain Res. 1977;27:237–243. doi: 10.1007/BF00235500. [DOI] [PubMed] [Google Scholar]
- 24.Henze DA, Buzsaki G. Action potential threshold of hippocampal pyramidal cells in vivo is increased by recent spiking activity. Neuroscience. 2001;105:121–130. doi: 10.1016/s0306-4522(01)00167-1. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch JA, Alonso JM, Reid RC, Martinez LM. Synaptic integration in striate cortical simple cells. J Neurosci. 1998;18:9517–9528. doi: 10.1523/JNEUROSCI.18-22-09517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirst GD, Johnson SM, van Helden DF. The slow calcium-dependent potassium current in a myenteric neurone of the guinea-pig ileum. J Physiol. 1985;361:315–337. doi: 10.1113/jphysiol.1985.sp015648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou GQ, Pan X, Liao CS, Wang SH, Li DF. SK channels modulate the excitability and firing precision of projection neurons in the robust nucleus of the arcopallium in adult male zebra finches. Neuroscience bulletin. 2012;28:271–281. doi: 10.1007/s12264-012-1241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jefferys JG, Haas HL. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature. 1982;300:448–450. doi: 10.1038/300448a0. [DOI] [PubMed] [Google Scholar]
- 29.Jensen MS, Azouz R, Yaari Y. Variant firing patterns in rat hippocampal pyramidal cells modulated by extracellular potassium. J Neurophysiol. 1994;71:831–839. doi: 10.1152/jn.1994.71.3.831. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MS, Yaari Y. Role of intrinsic burst firing, potassium accumulation, and electrical coupling in the elevated potassium model of hippocampal epilepsy. J Neurophysiol. 1997;77:1224–1233. doi: 10.1152/jn.1997.77.3.1224. [DOI] [PubMed] [Google Scholar]
- 31.Jones RS, Heinemann UH. Differential effects of calcium entry blockers on pre- and postsynaptic influx of calcium in the rat hippocampus in vitro. Brain research. 1987;416:257–266. doi: 10.1016/0006-8993(87)90905-x. [DOI] [PubMed] [Google Scholar]
- 32.Kang J, Huguenard JR, Prince DA. Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons. J Neurophysiol. 2000;83:70–80. doi: 10.1152/jn.2000.83.1.70. [DOI] [PubMed] [Google Scholar]
- 33.Kang Y, Kayano F. Electrophysiological and morphological characteristics of layer VI pyramidal cells in the cat motor cortex. J Neurophysiol. 1994;72:578–591. doi: 10.1152/jn.1994.72.2.578. [DOI] [PubMed] [Google Scholar]
- 34.Katz B. The release of neuronal transmitter substances. Thomas; Springfield, Illinois: 1969. [Google Scholar]
- 35.Krawitz S, Fedirchuk B, Dai Y, Jordan LM, McCrea DA. State-dependent hyperpolarization of voltage threshold enhances motoneurone excitability during fictive locomotion in the cat. J Physiol (Lond) 2001;532:271–281. doi: 10.1111/j.1469-7793.2001.0271g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin M, Hatcher JT, Chen QH, Wurster RD, Cheng ZJ. Small conductance Ca2+-activated K+ channels regulate firing properties and excitability in parasympathetic cardiac motoneurons in the nucleus ambiguus. American journal of physiology. Cell physiology. 2010;299:C1285–1298. doi: 10.1152/ajpcell.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- 39.Mantegazza M, Franceschetti S, Avanzini G. Anemone toxin (ATX II)-induced increase in persistent sodium current: effects on the firing properties of rat neocortical pyramidal neurones. J Physiol. 1998;507:105–116. doi: 10.1111/j.1469-7793.1998.105bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markram H, Helm P, Sakmann B. Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J Physiol. 1995;485:1–20. doi: 10.1113/jphysiol.1995.sp020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massimini M, Amzica F. Extracellular calcium fluctuations and intracellular potentials in the cortex during the slow sleep oscillation. J Neurophysiol. 2001;85:1346–1350. doi: 10.1152/jn.2001.85.3.1346. [DOI] [PubMed] [Google Scholar]
- 42.McCormick DA. Membrane potential and action potential. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental neuroscience. Academic Press; San Diego, London, Boston, New York, Sydney, Tokyo, Toronto: 1999. pp. 129–154. [Google Scholar]
- 43.McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- 44.Neckelmann D, Amzica F, Steriade M. Spike-wave complexes and fast components of cortically generated seizures. III. Synchronizing mechanisms. J Neurophysiol. 1998;80:1480–1494. doi: 10.1152/jn.1998.80.3.1480. [DOI] [PubMed] [Google Scholar]
- 45.Nuñez A, Amzica F, Steriade M. Electrophysiology of cat association cortical cells in vitro: Intrinsic properies and synaptic responses. J Neurophysiol. 1993;70:418–430. doi: 10.1152/jn.1993.70.1.418. [DOI] [PubMed] [Google Scholar]
- 46.Paré D, Lang EJ. Calcium electrogenesis in neocortical pyramidal neurons in vivo. Eur J Neurosci. 1998;10:3164–3170. doi: 10.1046/j.1460-9568.1998.00323.x. [DOI] [PubMed] [Google Scholar]
- 47.Paré D, Shink E, Gaudreau H, Destexhe A, Lang EJ. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons in vivo. J Neurophysiol. 1998;79:1450–1460. doi: 10.1152/jn.1998.79.3.1450. [DOI] [PubMed] [Google Scholar]
- 48.Rausche G, Sarvey JM, Heinemann U. Lowering extracellular calcium reverses paired pulse habituation into facilitation in dentate granule cells and removes a late IPSP. Neuroscience letters. 1988;88:275–280. doi: 10.1016/0304-3940(88)90223-6. [DOI] [PubMed] [Google Scholar]
- 49.Sachdev RNS, Ebner FF, Wilson CJ. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J Neurophysiol. 2004;92:3511–3521. doi: 10.1152/jn.00347.2004. [DOI] [PubMed] [Google Scholar]
- 50.Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 51.Sah P, McLachlan EM. Ca(2+)-activated K+ currents underlying the afterhyperpolarization in guinea pig vagal neurons: a role for Ca(2+)-activated Ca2+ release. Neuron. 1991;7:257–264. doi: 10.1016/0896-6273(91)90264-z. [DOI] [PubMed] [Google Scholar]
- 52.Schwindt PC, Spain WJ, Crill WE. Influence of anomalous rectifier activation on afterhyperpolarization of neurons from cat sensorimotor cortex in vitro. Journal of Neurophysiology. 1988;59:468. doi: 10.1152/jn.1988.59.2.468. [DOI] [PubMed] [Google Scholar]
- 53.Schwindt PC, Spain WJ, Foehring RC, Stafstrom CE, Chubb MC, Crill WE. Multiple potassium conductances and their functions in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1988;59:424–449. doi: 10.1152/jn.1988.59.2.424. [DOI] [PubMed] [Google Scholar]
- 54.Seigneur J, Kroeger D, Nita DA, Amzica F. Cholinergic action on cortical glial cells in vivo. Cereb. Cortex. 2006;16:655–668. doi: 10.1093/cercor/bhj011. [DOI] [PubMed] [Google Scholar]
- 55.Seigneur J, Timofeev I. Synaptic impairment induced by paroxysmal ionic conditions in neocortex. Epilepsia. 2010;52:132–139. doi: 10.1111/j.1528-1167.2010.02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Somjen GG. Ion regulation in the brain: implications for pathophysiology. Neuroscientist. 2002;8:254–267. doi: 10.1177/1073858402008003011. [DOI] [PubMed] [Google Scholar]
- 57.Steriade M. Neocortical cell classes are flexible entities. Nat Rev Neurosci. 2004;5:121–134. doi: 10.1038/nrn1325. [DOI] [PubMed] [Google Scholar]
- 58.Steriade M, Amzica F. Intracellular study of excitability in the seizure-prone neocortex in vivo. J Neurophysiol. 1999;82:3108–3122. doi: 10.1152/jn.1999.82.6.3108. [DOI] [PubMed] [Google Scholar]
- 59.Steriade M, Amzica F, Nuñez A. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J Neurophysiol. 1993;70:1385–1400. doi: 10.1152/jn.1993.70.4.1385. [DOI] [PubMed] [Google Scholar]
- 60.Steriade M, Contreras D, Dossi RC, Nuñez A. The slow (<1 Hz) oscillation in reticular thalamic and thalamo-cortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steriade M, Nuñez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillations and other sleep rhythms of electroencephalogram. J Neurosci. 1993;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steriade M, Nuñez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steriade M, Timofeev I, Dürmüller N, Grenier F. Dynamic properties of corticothalamic neurons and local cortical interneurons generating fast rhythmic (30-40 Hz) spike bursts. J Neurophysiol. 1998;79:483–490. doi: 10.1152/jn.1998.79.1.483. [DOI] [PubMed] [Google Scholar]
- 64.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 65.Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor CP, Dudek FE. Synchronous neural afterdischarges in rat hippocampal slices without active chemical synapses. Science. 1982;218:810–812. doi: 10.1126/science.7134978. [DOI] [PubMed] [Google Scholar]
- 67.Thimm J, Mechler A, Lin H, Rhee S, Lal R. Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J Biol Chem. 2005;280:10646–10654. doi: 10.1074/jbc.M412749200. [DOI] [PubMed] [Google Scholar]
- 68.Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 69.Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: An intracellular study. Proc Natl Acad Sci U S A. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Timofeev I, Grenier F, Steriade M. Contribution of intrinsic neuronal factors in the generation of cortically driven electrographic seizures. J Neurophysiol. 2004;92:1133–1143. doi: 10.1152/jn.00523.2003. [DOI] [PubMed] [Google Scholar]
- 71.Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 72.Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetilcholine, and 1S, 3R-ACPD. J Neurosci. 1993;13:2199–2216. doi: 10.1523/JNEUROSCI.13-05-02199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Y, Yang Y, Ye S, Jiang Y. Structure of the gating ring from the human large-conductance Ca(2+)-gated K(+) channel. Nature. 2010;466:393–397. doi: 10.1038/nature09252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang CR, Seamans JK, Gorelova N. Electrophysiological and morphological properies of layers V-VI principal pyramidal cells in rat prefrontal cortexin vitro. J Neurosci. 1996;16:1904–1921. doi: 10.1523/JNEUROSCI.16-05-01904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan P, Leonetti MD, Pico AR, Hsiung Y, MacKinnon R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 A resolution. Science. 2010;329:182–186. doi: 10.1126/science.1190414. [DOI] [PMC free article] [PubMed] [Google Scholar]