Abstract
Background
Pharmacologic studies implicate a significant role of genes encoding the serotonin transporter (SLC6A4) and the 5-HT3AB subunits HTR3A and HTR3B in nicotine dependence (ND). However, whether they are involved in ND remains largely unknown.
Methods
Here, we examined the impact of variations in the three genes on ND in 1366 individuals from 402 African American (AA) and 671 individuals from 200 European American (EA) families. The ND of each smoker was assessed with smoking quantity (SQ), Heaviness of Smoking Index (HSI), and Fagerström Test for Nicotine Dependence (FTND).
Results
Association analysis revealed marginal association of rs10160548 in HTR3A with SQ and HSI in AA, 5-HTTLPR in SLC6A4 with FTND in EA, and rs11606194 in HTR3B with SQ and FTND in the pooled sample. Haplotype-based association analysis revealed a few major haplotypes in HTR3A that were significantly associated with ND in the AA, EA, and pooled samples. However, none of these associations remained significant after correcting for multiple testing except for a haplotype GC-C-T-A-T formed by SNPs rs1150226, rs1062613, rs33940208, rs1985242, rs2276302, and rs10160548 in HTR3A for the AA sample. Considering biological functions of the three genes, we examined interactive effects of variants in the three genes, which revealed significant interactions among rs1062613 and rs10160548 in HTR3A, rs1176744 in HTR3B, and 5-HTTLPR and rs1042173 in SLC6A4 in affecting ND in the three samples.
Conclusions
We conclude that SLC6A4, HTR3A and HTR3B play a significant role in ND through genetic interactions.
Keywords: Serotonin transporter, serotonin receptor 3AB, nicotine dependence, African and European American, gene-by-gene interactions, epistatic
1. INTRODUCTION
Tobacco use continues to be a foremost worldwide health concern. In the United States, 46.0 million adults were cigarette smokers in 2008, and the number of deaths annually from smoking-related illnesses was estimated to be around 440,000 in 2008 (CDC, 2009). The annual economic burden of smoking is also substantial with a staggering $193 billion in medical costs and productivity losses (CDC, 2008). It has been estimated by the World Health Organization that tobacco-related deaths would rise from approximately 5.0 million in 2004 to 8.0 million by 2030 (WHO, 2008).
Cigarette smoking is a complex behavior, with both genetic and environmental components (Al Koudsi and Tyndale, 2005; Sullivan and Kendler, 1999). Many family, adoption, and twin studies of smoking addiction have indicated a heritability of 11% - 78%, with an average heritability of 57% for both male and female smokers (Kendler et al., 1999; Li et al., 2003; Maes et al., 2004; Vink et al., 2005).
Nicotine, the primary psychoactive ingredient in cigarette smoke, exerts its effects by readily crossing the blood-brain barrier and binding with nicotinic acetylcholine receptors (nAChRs) located in various brain structures (Wonnacott, 1997). Activation of nAChRs on dopaminergic terminals induces dopamine release in the mesolimbic brain reward system (Kleijn et al., 2011; Wonnacott et al., 2000). In addition to dopamine, nicotine can induce the release of a variety of other neurotransmitters that regulate the complex brain circuitry modulating addictive behaviors (Mihailescu et al., 1998; Ribeiro et al., 1993). Of these neurotransmitter systems, the serotonin (5-HT) system regulates self-administration of nicotine (Levin et al., 2008), nicotine-induced cognitive enhancement (Levin and Rezvani, 2007), memory (Levin et al., 2005), and smoking progression and cessation (Wang and Li, 2010).
The serotonergic system consists of seven major receptor classes; among these, all but the 5-HT3 receptors are G-protein coupled (Barnes et al., 2009; Boess and Martin, 1994; Connolly and Wafford, 2004). The 5-HT3 receptors belong to the Cys-loop super-family of pentameric neurotransmitter-gated ion channels that also includes nicotinic acetylcholine (nACh), GABAA, and glycine receptors (Enoch et al., 2011). Within this super-family, the closest resemblance to 5-HT3 receptors is shown by nAChRs, which share about 30% structural homology (Maricq et al., 1991). There are several other significant similarities between 5-HT3 and nACh receptors. For example, activation of both pre-synaptically located nACh and 5-HT3 receptors results in Ca2+-dependent neurotransmitter release (Yamauchi et al., 2011) and mediates common physiological functions such as blood pressure and pain (Drisdel et al., 2008). Moreover, they are co-localized on nerve terminals in several brain pathways of reward processing, including dopaminergic terminals in the striatum (Nayak et al., 2000). However, the co-localized endogenous 5-HT3 and nAChR receptors do not appear to interact physically; rather, they cross-regulate each other at a down-stream molecular level, possibly through regulation of intracellular Ca2+ concentrations (Dougherty and Nichols, 2009; Nayak et al., 2000). Together, these findings highlight the important role of the serotonergic pathway in the development of ND.
The 5-HT3A subunit exists as a homomeric structure in the central nervous system. When the 5-HT3A subunit combines with the 5-HT3B subunit, they form pharmacologically more potent 5-HT3AB heteropentameric receptor complexes, which are distributed throughout the limbic structures implicated in addiction (Davies et al., 1999; Dubin et al., 1999; Enoch et al., 2011). On the other hand, serotonin transporter (5-HTT) is the only molecule known to regulate synaptic serotonin concentrations through re-uptake into presynaptic nerve terminals. Thus, it self-modulates the availability of serotonin molecules for binding with the 5-HT3AB receptors. For these reasons, in addition to perform association analysis at both the individual SNP and haplotype levels, we also examined gene-gene interactive effects on ND in this study, with the goal of determining whether variants in the genes encoding the serotonin transporter (SLC6A4) and receptor subunits 3A and 3B (HTR3A and HTR3B) play any role in ND.
2. MATERIALS AND METHODS
2.1 Subjects and ND Measures
Subjects of both African American (AA) and European American (EA) origin were recruited primarily from the US mid-South states of Tennessee, Mississippi, and Arkansas during 1999 to 2004. Proband smokers were required to be at least 21 years old, to have smoked for at least the last 5 years, and to have smoked at least 20 cigarettes per day during the last 12 months. Once proband smokers were identified, their biological parents and siblings were invited to participate whenever possible. Together, a total of 1366 individuals from 402 AA families and 671 individuals from 200 EA families were included. Table 1 provides the detailed characteristics of the two ethnic samples. All participants provided informed written consent, and the study was approved by the Institutional Review Boards of the University of Virginia and the University of Mississippi Medical Center.
Table 1.
Clinical Characteristics of AA, EA, and Pooled Samples
| Characteristic | African-American | European-American | Pooled |
|---|---|---|---|
| No. of nuclear families | 402 | 200 | 602 |
| Avg. members/family | 3.14±0.75 | 3.17±0.69 | 3.15±0.73 |
| No. of subjects | 1,366 | 671 | 2,037 |
| Female (%) | 66.1 | 69.5 | 67.2 |
| Age (years) | 39.4±14.4 | 40.5±15.5 | 39.7±14.8 |
| No. of smokers | 1,053 | 515 | 1,568 |
| Age of smoking onset | 17.3±4.7 | 15.5±4.4 | 16.7±4.7 |
| Years smoked | 20.4±12.5 | 23.2±13.5 | 21.3±12.9 |
| Smoking quantity/day | 19.4±13.3 | 19.5±13.4 | 19.5±13.3 |
| HSI | 3.7±1.4 | 3.9±1.4 | 3.8±1.4 |
| FTND score | 6.26±2.15 | 6.33±2.22 | 6.29±2.17 |
Notes: HSI = Heaviness of Smoking Index; FTND = Fagerstrom Test for ND.
The ND of each smoker was assessed with the three commonly used measures of Smoking Quantity (SQ; the number of cigarettes smoked per day), the Heaviness of Smoking Index (HSI; 0-6 scale), and the Fagerström Test for ND (FTND; 0-10 scale; Fagerstrom, 1978). Because of the overlap of the contents of the three measures, a fairly robust correlation exists among them in both populations (r = 0.88-0.94).
2.2 DNA Sample Processing, and Selection and Genotyping of Polymorphic Variants
Genomic DNA was extracted from the peripheral venous blood of each participant using a Maxi kit (Qiagen Inc, Valencia, CA) according to the manufacturer's protocol. Eight SNPs in HTR3A and seven SNPs in HTR3B were selected based on the following characteristics: minor allele frequency ≥ 0.05 as provided on NCBI website (http://www.ncbi.nlm.nih.gov/snp/) and known or putative functional importance of the variants. Detailed information on the 15 SNPs is provided in Supplementary Table 11. All 15 SNPs in HTR3A and HTR3B were genotyped using TaqMan assays in the 384-well microplate format (Applied Biosystems Inc., Foster City, CA) as reported previously (Beuten et al., 2005; Li et al., 2005; Sun et al., 2008). Briefly, 15 ng of DNA was amplified in a total volume of 7 μl containing an MGB probe and 2.5 μl of TaqMan universal PCR master mix. Allelic discrimination analyses were performed on the ABI Prism 7900HT Sequence Detection System. To ensure the quality of genotyping, four no-template negative controls and four positive controls were added to each 384-well plate.
We also selected two known functional polymorphisms in SLC6A4 to examine the association of variations of serotonin transporters with ND. They are rs1042173, located in the 3’-UTR region, and the 5’-serotonin transporter-linked polymorphic region (5’-HTTLPR), located in the promoter region of SLC6A4 (Lesch et al., 1999; Seneviratne et al., 2009). The 5’-HTTLPR long (L) and short (S) alleles were genotyped by PCR amplification of genomic DNA (15 ng) in a final volume of 20 μl containing 5× MyTaq Reaction Buffer, Taq DNA polymerase, and 20 μM each of the forward (5’-TCCTCCGCTTTGGCGCCTCTTCC-3’) and reverse (5’-TGGGGGTTGCAGGGGAGATCCTG-3’) primers. The PCR cycling conditions were: initial denaturation at 95°C for 1 min, followed by 30 cycles at 95°C for 15 seconds, annealing at 60°C for 15 seconds, and extension at 72°C for 10 seconds. The PCR products were electrophoresed on a 3.5% agarose gel, and the amplicons were identified using ethidium bromide staining. The fragments at the 484- and 528-bp levels were identified as “S” and “L” alleles, respectively. The T/G alleles of SNP rs1042173 were genotyped using a TaqMan SNP assay with the same procedure used for SNPs in HTR3A and HTR3B as described above.
2.3 Statistical Analysis
To test for genotyping quality, we assessed Mendelian inconsistencies and departure from Hardy-Weinberg Equilibrium (HWE) using Haploview (v. 4.0) software (Barrett et al., 2005). Subjects with any inconsistent SNP data for a given genetic variant were excluded from further analysis.
2.3.1. Individual and haplotype-based association analysis
Associations between the 17 individual polymorphisms in HTR3A, HTR3B, and SLC6A4 and the three ND measures were determined using the Pedigree-Based Association Test (PBAT; v. 3.5) based on the generalized estimating equation approach (Lange et al., 2003). Pair-wise linkage disequilibrium (LD) and haplotype blocks for the 15 SNPs in HTR3A and HTR3B were assessed by Haploview (v. 4.0) software (Barrett et al., 2005; Gabriel et al., 2002). Association analysis for haplotypes located within these LD blocks with the three ND measures was performed using the Family-Based Association Test (FBAT; v.1.7.3; Horvath et al., 2004). Three genetic models (additive, dominant, and recessive) were tested for all PBAT and FBAT association analyses, with sex and age included as covariates in the AA and EA samples and sex, age and ethnicity in the pooled sample. Statistically significant results (p < 0.05) for individual SNPs and major haplotypes (frequency ≥ 5%) were corrected for multiple testing using Bonferroni correction.
2.3.2. Gene-by-gene interaction analysis of HTR3A, HTR3B, and SLC6A4 variants
Gene-by-gene interactions among polymorphisms in HTR3A, HTR3B, and SLC6A4 were analyzed using Pedigree-Based Generalized Multifactor Dimensionality Reduction (PGMDR) program (Lou et al., 2008). As with the association analysis described above, the three ND measures were covariant-adjusted for age and sex in the models for the AA and EA samples and for age, sex, and ethnicity in the models for the pooled sample. We performed an exhaustive search for all two- to five-locus polymorphic combinations within and among the three genes with interactive effects on ND measures. The best statistical gene-by-gene interaction model for a given order of interaction was determined by three factors: (1) its empirical P value for prediction accuracy (PA) evaluated by a permutation test based on 106 shuffles of the adjusted phenotypic values; (2) the P-value and cross-validation consistency obtained by the sign-test for PA implemented in MDR software (Hahn et al., 2003); and (3) association of the polymorphic combination with all three ND measures examined.
3. RESULTS
3.1 Quality control and pair-wise LD tests
Prior to performing association and genetic interaction analysis, we conducted Hardy-Weinberg Equilibrium tests on all 17 polymorphisms in HTR3A, HTR3B, and SLC6A4, which showed significant deviations (P < 0.001) for SNPs rs33940208 in the EA and rs2276305 in the AA samples. Thus, rs33940208 and rs2276305 were excluded from the association and interaction analyses within EA and AA samples, respectively. For the remaining polymorphisms, the HWE P values ranged from 0.015 to 1.0 in the AA and 0.293 to 1.0 in the EA sample.
3.2 Individual SNP-Based Association Analysis
Results from the individual SNP-based association analyses in the two ethnic samples and the pooled sample are shown in Table 2. Of the HTR3A polymorphisms, SNP rs10160548 showed marginal associations with SQ and HSI (P = 0.030 and 0.042, respectively) in the AAs under an additive model. Of the HTR3B polymorphisms, the only variant significantly associated with SQ and FTND in the pooled samples was rs11606194, with a P value of 0.039 and 0.028, under the additive model. Further, the polymorphism 5-HTTLPR in SLC6A4 showed a marginal association with FTND (P = 0.030) in the EAs under the additive model. However, none of these associations remained significant after Bonferroni correction for multiple testing for 17 variants in the three genes (the corrected P value at 0.05 is 0.003).
Table 2.
Minor Allele Frequencies and P values for Associations of Individual SNPs/Polymorphism with Three ND measures in the AA, EA and Pooled Samples
| GENE | SNP ID/Polymorphism | African-American sample |
European-American sample |
Pooled sample |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minor Allele (MAF) | SQ | HSI | FTND | Minor Allele (MAF) | SQ | HSI | FTND | Minor Allele (MAF) | SQ | HSI | FTND | ||
| HTR3B | rs3758987 | T(0.371) | 0.170d | 0.214d | 0.305d | T(0.302) | 0.691a | -0.936a | 0.989a | T(0.350) | 0.191d | 0.321d | 0.365d |
| rs11606194 | T(0.016) | -0.149r | -0.151r | -0.153r | T(0.074) | -0.088a | -0.156a | -0.163a | T(0.034) | -0.039a | -0.070a | -0.028a | |
| rs4938056 | T(0.371) | -0.293d | -0.573d | -0.860d | T(0.470) | -0.239d | -0.183d | -0.308d | T(0.420) | 0.292r | 0.266r | 0.431r | |
| rs1176744 | C(0.476) | -0.582r | -0.479r | -0.518r | C(0.326) | 0.496r | 0.721r | 0.787r | C(0.430) | -0.895a | -0.579a | -0.664a | |
| rs2276305 | Excluded from analysis as HWE p<0.001 | G(0.006) | -0.988a | -0.868a | -0.789a | G(0.035) | 0.388a | 0.127a | 0.347a | ||||
| rs1672717 | G(0.083) | -0.220r | -0.181r | -0.141r | G(0.350) | 0.892a | 0.336d | 0.588a | G(0.165) | -0.545a | -0.493r | -0.640r | |
| rs17614942 | C(0.104) | -0.336r | -0.469r | -0.831r | C(0.074) | -0.736a | -0.252a | -0.260a | C(0.095) | -0.338r | -0.396a | -0.492a | |
| HTR3A | rs1150226 | G(0.314) | -0.081r | -0.086r | -0.106r | G(0.089) | -0.550d | -0.235d | -0.221d | G(0.244) | -0.133r | -0.097r | -0.129r |
| rs1062613 | T(0.463) | -0.060a | -0.066a | -0.116a | T(0.244) | 0.681d | -0.967d | -0.798d | T(0.395) | -0.147a | -0.098a | -0.151a | |
| rs33940208 | T(0.146) | 0.685d | -0.717r | -0.732r | Excluded from analysis as HWE p<0.001 | T(0.113) | -0.356r | -0.308r | -0.385r | ||||
| rs1985242 | T(0.333) | 0.109a | 0.161a | 0.283a | T(0.322) | -0.503d | -0.349d | -0.216d | T(0.441) | 0.148a | 0.133a | 0.183a | |
| rs2276302 | G(0.496) | 0.135a | 0.175a | 0.366a | G(0.338) | 0.595r | 0.690r | 0.735r | G(0.452) | -0.259a | -0.234a | -0.292d | |
| rs10160548 | T(0.262) | 0.030a | 0.042a | 0.090a | T(0.309) | 0.724d | 0.595d | 0.521d | T(0.396) | 0.063d | 0.092d | 0.174d | |
| rs1150220 | G(0.132) | -0.390a | -0.678a | -0.840a | G(0.202) | -0.491r | -0.340r | -0.193r | G(0.154) | -0.377r | -0.359r | -0.295r | |
| rs1176713 | G(0.321) | -0.421r | -0.417r | -0.449r | G(0.216) | -0.231r | -0.105r | -0.062r | G(0.288) | -0.201r | -0.161r | -0.154r | |
| SLC6A4 | 5-HTTLPR | S(0.227) | -0.528d | -0.615a | -0.597a | S(0.433) | -0.058a | -0.095a | -0.030a | S(0.290) | -0.132a | -0.190a | -0.105a |
| rs1042173 | T(0.211) | -0.080d | -0.154d | -0.130d | T(0.441) | -0.368a | -0.304a | -0.259a | T(0.281) | -0.065d | -0.118d | -0.087d | |
Notes: 1) MAF: minor allele frequency. 2) Significant associations at the 0.05 level before correction for multiple testing are given in bold. 3) Superscripts indicate genetic model used for analysis: 4) For each ethnic-specific sample, age and sex were used as covariates; for the combined sample, age, sex, and ethnicity were used as covariates. Negative signs indicate protective effect with the model specified in superscripted letters.
additive
dominant
recessive.
3.3 Haplotype-Based Association Analysis
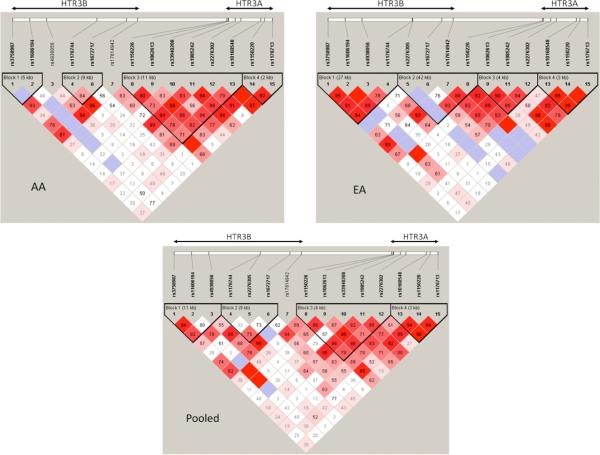
According to the haplotype block definition by Gabriel et al. (2002), we found four blocks within HTR3A and HTR3B in the AA, EA, and pooled samples (Figure 1) and no LD block between the two polymorphisms in SLC6A4.
Figure 1.
LD structures for HTR3B and HTR3A SNPs in the AA, EA, and Pooled samples. Haploview (v. 3.2; Barrett et al., 2005) was used to calculate all D′ values, and haplotype blocks were defined according to Gabriel et al. (2002). The number in each box represents the D′ value for each SNP pair surrounding that box.
We employed the FBAT program to perform haplotype-based association analysis for all different major haplotypes in each abovementioned LD block with the three ND measures in the AA (Table 3), EA (Table 4), and pooled (Table 5) samples. In AAs, there were two major haplotypes located in the 5′ region of HTR3A that were significantly associated with the three ND measures: (1) G-C-C-T-A-T, formed by SNPs rs1150226, rs1062613, rs33940208, rs1985242, rs2276302, and rs10160548 (LD block 3; Figure 1), with a frequency of 19.5%, was associated significantly with SQ (Z = 2.596; P = 0.009), HSI (Z = 3.027; P = 0.002), and FTND (Z = 2.824; P = 0.004) in a dominant model; and (2) G-A, formed by SNPs rs1150220 and rs1176713 (LD block 4; Figure 1), with a frequency of 66.6%, was significantly associated with SQ (Z = 3.041; P = 0.002,), HSI (Z = 3.011; P = 0.003), and FTND (Z = 2.863; P = 0.004). All abovementioned haplotype-based associations remained significant after Bonferroni correction for multiple testing for each LD block. We also detected a nominally significant association of haplotype A-T-C-A-G-G in the LD block 3 of HTR3A with a frequency of 24.1% with SQ under the dominant model (Z = 1.996; P = 0.046).
Table 3.
Haplotypes within HTR3B and HTR3A Associated with Three ND Measures in AA Sample
| rs3758987-rs11606194 (HTR3B) |
SQ |
HSI |
FTND |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | Freq | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global |
| T | T | 0.617 | 197 | 1.164 | 0.244a | 0.072a | 210 | 1.283 | 0.200a | 0.050a | 226 | 1.309 | 0.190a | 0.013a |
| C | T | 0.366 | 79 | -0.665 | 0.506r | 212 | -0.652 | 0.514a | 171 | -0.577 | 0.564d | |||
| rs1176744-rs1672717 (HTR3B) |
SQ |
HSI |
FTND |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | Freq | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global |
| C | A | 0.471 | 104 | -0.605 | 0.545r | 111 | -0.734 | 0.463r | 117 | -0.841 | 0.400r | |||
| A | A | 0.448 | 191 | 1.765 | 0.078a | 0.092a | 205 | 1.814 | 0.070a | 0.112a | 214 | 1.825 | 0.068a | 0.115a |
| A | G | 0.067 | 74 | -1.637 | 0.102a | 79 | -1.485 | 0.138a | 82 | -1.638 | 0.101a | |||
| rs1150226- ..... - rs10160548 (HTR3A) |
SQ |
HSI |
FTND |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 9 | 10 | 11 | 12 | 13 | Freq | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global |
| A | T | C | A | G | G | 0.241 | 102 | 1.996 | 0.046d | 0.006d | 112 | 1.460 | 0.144d | 0.006d | 119 | 1.110 | 0.267d | 0.015d |
| G | C | C | T | A | T | 0.195 | 107 | 1.964 | 0.050a | 117 | 2.542 | 0.011a | 124 | 2.290 | 0.022a | |||
| 103 | 2.596 | 0.009d | 114 | 3.027 | 0.002d | 120 | 2.824 | 0.004d | ||||||||||
| G | T | C | A | G | G | 0.145 | 12 | -1.039 | 0.299r | 14 | -0.812 | 0.417r | 114 | 0.871 | 0.384d | |||
| G | C | T | A | A | G | 0.105 | 75 | -0.696 | 0.486d | 80 | -0.972 | 0.331a | 85 | -1.250 | 0.211d | |||
| G | C | C | T | A | G | 0.079 | 72 | -0.749 | 0.454d | 79 | -0.835 | 0.404d | 83 | -0.726 | 0.468d | |||
| rs1150220-rs1176713 (HTR3A) |
SQ |
HSI |
FTND |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | 15 | Freq | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global |
| G | A | 0.666 | 187 | 2.560 | 0.010a | 202 | 2.663 | 0.008a | 209 | 2.677 | 0.007a | |||
| 110 | 3.041 | 0.002d | 0.037a | 119 | 3.011 | 0.003d | 0.035a | 123 | 2.863 | 0.004d | 0.033a | |||
| G | G | 0.202 | 25 | -1.971 | 0.049r | 0.017d | 26 | -2.025 | 0.043r | 0.024d | 26 | -1.934 | 0.053r | 0.037d |
| A | G | 0.123 | 112 | -1.956 | 0.050a | 121 | -1.695 | 0.090a | 123 | -1.486 | 0.137a | |||
Notes: 1) Only major haplotypes (>5%) are shown; the one with a P value<0.05 is given in bold. 2) Statistically significant P values after Bonferroni correction for individual haplotypes within each haplotype block (given in separate panels) are shown bold italics. 3) Superscripts indicate genetic model used in the analysis: 4) ND measures were corrected for age and sex.
additive
dominant
recessive.
Table 4.
Haplotypes within HTR3A Associated with Three ND Measures in EA Sample
| rs10160548-.... -rs1176713 | SQ | HSI | FTND | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 14 | 15 | Freq | No. of families | Z score | P haplotype | P global | No. of families | Z Score | P haplotype | P global | No. of families | Z score | P haplotype | P global |
| T | G | A | 0.680 | 22 | 1.494 | 0.135d | 25 | 1.626 | 0.104d | 25 | 1.472 | 0.141d | |||
| G | A | G | 0.190 | 56 | 0.612 | 0.541a | 0.035a | 60 | 0.560 | 0.576a | 0.036a | 59 | 0.264 | 0.792a | 0.111a |
| G | G | A | 0.111 | 38 | -0.924 | 0.355a | 37 | -0.966 | 0.334d | 42 | -0.682 | 0.495d | |||
Notes: 1) Only major haplotypes with a frequency of >5% are shown; those with a P value <0.05 are given in bold. 2) Statistically significant P values after Bonferroni correction for individual haplotypes within each haplotype block (given in separate panels) are shown bold italics. 3) Superscripts indicate genetic model used in the analysis: r = recessive. 4) ND measures were corrected for age and sex.
additive
dominant
Table 5.
Haplotypes within HTR3A Associated with Three ND Measures in Pooled Sample
| rs1150226-.... -rs2276302 |
SQ |
HSI |
FTND |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 9 | 10 | 11 | 12 | Freq | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global |
| G | C | C | T | A | 0.408 | 182 | 1.823 | 0.068d | 200 | 1.853 | 0.064d | 202 | 2.209 | 0.027d | |||
| A | T | C | A | G | 0.187 | 41 | -1.153 | 0.249r | 42 | -1.008 | 0.314r | 43 | -0.907 | 0.365r | |||
| G | T | C | A | G | 0.142 | 20 | -1.420 | 0.156r | 0.080d | 22 | -1.372 | 0.170r | 0.113a | 180 | 1.631 | 0.103d | 0.049d |
| G | C | C | A | A | 0.075 | 87 | -0.817 | 0.414a | 93 | -1.164 | 0.244a | 99 | -1.262 | 0.207a | |||
| G | C | C | A | G | 0.060 | 73 | 0.572 | 0.568a | 85 | 0.675 | 0.500a | 98 | 0.650 | 0.516a | |||
| rs10160548- .... -rs1176713 |
SQ |
HSI |
FTND |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 14 | 15 | Freq | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global | No. of families | Z score | P haplotype | P global |
| T | G | A | 0.390 | 231 | 1.464 | 0.143a | 0.011a | 199 | 1.386 | 0.166d | 0.028a | 208 | 1.235 | 0.217d | 0.023a |
| G | G | A | 0.318 | 219 | 1.411 | 0.158a | 190 | 2.240 | 0.025a | 201 | 2.244 | 0.025a | |||
| G | A | G | 0.141 | 154 | -0.637 | 0.524a | 106 | -1.313 | 0.189a | 109 | -1.108 | 0.268a | |||
| G | G | G | 0.135 | 22 | -2.377 | 0.017r | 23 | -2.310 | 0.021r | 167 | -2.190 | 0.029a | |||
Notes: 1) Only major haplotypes with a frequency of >5% are shown; those with a P value <0.05 are given in bold. 2) Superscripts indicate genetic model used in the analysis: 3) ND measures were corrected for age, sex, and ethnicity.
additive
dominant
recessive.
For the EA sample, we found no haplotypes in HTR3A or HTR3B showing significant association with ND (Table 4). In the pooled sample (Table 5), we found one haplotype, G-G-G formed by SNPs rs10160548, rs1150220, and rs1176713 of HTR3A, with a frequency of 13.5%, significantly associated with SQ (Z = -2.377; P = 0.017), HSI (Z = -2.310; P = 0.021) and FTND (Z = -2.190; P = 0.029). However, none of them remained significant after Bonferroni correction.
3.4 Gene-by-Gene Interaction Analysis of HTR3A, HTR3B, and SLC6A4
Considering the biological and pharmacological functions of the three genes in regulating serotonin signaling, we performed an exhaustive search of all possible two- to five-locus interaction models among the 17 polymorphisms in HTR3A, HTR3B, and SLC6A4 for their epistatic effect on the three ND measures in the AA, EA, and pooled samples. As shown in Table 6, the detected best interaction model for each sample shows a significant genetic interaction effect on all three ND measures, with an empirical P < 0.01, cross-validation consistency (CVC) of at least 7 of 10, and test accuracies (TA) > 50% based on 106 permutation tests except for the model on FTND in the AA sample, where the empirical P value is 0.057. Of the three samples, the epistatic effect of the detected best interaction model on the three ND measures in the pooled sample appeared to be the strongest, with an empirical P value of 0.00025 to 0.00085.
Table 6.
Detected Best SNP Combination of SLC6A4, HTR3A, and HTR3B Associated with ND Measures in EA, AA, and Pooled Sample on Basis of Test Accuracy and Empirical P value from 106 Permutations
| Sample | SNP Combination | ND Measure | Test Accuracy | Cross-Validation Consistency (CVC) | Permutated P value |
|---|---|---|---|---|---|
| EA | HTR3A: rs1062613, rs1150220; | SQ | 0.5678 | 7 | 0.003 |
| HTR3B: rs1176744; | HSI | 0.5699 | 9 | 0.002 | |
| SLC6A4: 5-HTTLPR, rs1042173 | FTND | 0.5703 | 10 | 0.002 | |
| AA | HTR3A: rs10160548; | SQ | 0.5500 | 10 | 0.005 |
| SLC6A4: 5-HTTLPR, rs1042173 | HSI | 0.5458 | 10 | 0.009 | |
| FTND | 0.5317 | 8 | 0.057 | ||
| Pooled | HTR3A: rs1062613, rs10160548; | SQ | 0.5516 | 8 | 0.00051 |
| HTR3B: rs1176744; | HSI | 0.5547 | 8 | 0.00025 | |
| SLC6A4: 5-HTTLPR, rs1042173 | FTND | 0.5479 | 10 | 0.00085 | |
4. DISCUSSION
By employing a series of association analyses at both the individual polymorphic and haplotype levels and genetic interaction analyses among 17 variants in HTR3A, HTR3B, and SLC6A4, we revealed significant interactions among the variants of these genes in affecting ND in the AA, EA, and pooled samples. This finding is highly significant and novel, as it highlights the significance of variations in genes encoding the serotonin transporter and receptors involved in governing trans-synaptic serotonergic signaling underlying the pathophysiology of ND.
In the pooled sample, an interaction model consisting of five loci in HTR3A, HTR3B, and SLC6A4 showed significant epistatic effects on all the three ND measures examined. These five loci were rs1062613 and rs10160548 in HTR3A, rs1176744 in HTR3B, and 5-HTTLPR and rs1042173 in SLC6A4. Interestingly, the minor allele frequencies of these five polymorphisms are considerably high, with the lowest frequency being 0.211 for rs1042173 in SLC6A4 in the AA sample and 0.244 for rs1062613 in HTR3A in the EA sample. Of them, three polymorphisms have been demonstrated to alter the expression of the RNA and/or protein encoded by the respective genes (Niesler et al., 2001). For example, the rs1062613 is a translation regulatory variant located in an open reading frame upstream of the translation initiation site of HTR3A mRNA (Niesler et al., 2001). The two polymorphisms in SLC6A4 were shown to alter 5-HTT expression through transcription regulation for 5-HTTLPR and degradation of mRNA transcripts for rs1042173 (Heils et al., 1997, 1996; Seneviratne et al., 2009; Vallender et al., 2008). Of the remaining SNPs, rs10160548 is located in intron 6 near an intron-exon boundary. It is thus reasonable to speculate that it alters the expression of functional HTR3A transcripts through alternative splicing. The rs1176744 in HTR3B does not alter expression, but it was shown to substantially change serotonergic signaling through altered gating kinetics of the 5-HT3AB receptor complex (Krzywkowski et al., 2008).
By analyzing the AA and EA samples independently, we revealed slightly different interaction models for each ethnic sample. In the AA sample, there was a significant interactive effect of SNPs rs10160548 in HTR3A and 5-HTTLPR and rs1042173 in SLC6A4 on all three ND measures. Although the two SLC6A4 polymorphisms were also included in the best interaction model detected in the EA sample, the model contained three additional loci: rs1062613 and rs1150220 in HTR3A and rs1176744 in HTR3B. In previous studies by other research groups, SNP rs1062613 in HTR3A has been associated with several psychiatric disorders in individuals of European descent (Gatt et al., 2010; Walstab et al., 2010). Yet, whether rs1062613 has ethnicity-specific cis-acting effects on the differential translation levels of HTR3A in AAs and EAs remains to be characterized. However, inclusion of rs1062613 in the best interaction model in the pooled samples, with even stronger interaction effects than were seen in EAs only, argues against this possibility. The other HTR3A SNP, detected only in the EA sample, was rs1150220, which is moderately correlated with rs10160548 in both EAs and AAs (r2 = 0.42 in AAs and r2 = 0.51 in EAs) in an LD block located at the 3’-end of the HTR3A gene. The second main difference between AA and EA samples was the absence of HTR3B rs1176744 in the best model for AAs. Although SNPs rs1176744 and 5-HTTLPR in SLC6A4 have been found to be significantly associated with alcohol dependence in AAs (Enoch et al., 2011), we found no significant association of these two polymorphisms with ND in our AA, EA, or pooled samples, except for 5-HTTLPR, which showed a marginal association with FTND in the EA sample. However, our genetic interaction analysis demonstrated the two polymorphisms in SLC6A4 play an important role in ND through interactions with other SNPs in HTR3A and HTR3B in the AA, EA, and pooled samples.
Another noteworthy consideration of our results is that none of the polymorphisms included in the above-mentioned epistatic models was significant at the individual locus level. Significant epistatic effects of variants without major genetic effect have become an increasingly identified phenomenon in studies of complex disorders (Li et al., 2008; Steen, 2012; Zuk et al., 2012). For example, several other studies and the present study have shown marginal or no association of the polymorphism 5-HTTLPR in SLC6A4 with ND (Gerra et al., 2005; Trummer et al., 2006). Yet, as clearly demonstrated by our gene-by-gene interaction analysis, the effect of 5-HTTLPR on ND is highly significant when its epistatic effect is taken into consideration. Another unique strength of our findings is that the interaction models detected in AAs, EAs, and the pooled samples were highly significant across multiple ND measures, providing further support for their role in smoking-related behaviors.
The biological basis for the genetic interaction effect we detected can be explained by the effects of nicotine on serotonergic signaling. Nicotine competes with its natural ligand serotonin for 5-HT3 receptors (Breitinger et al., 2001). Depending on whether the 5-HT3 receptors are located pre- or post-synaptically, nicotine binding either can result in the release of various neurotransmitters or affect the propagation of fast-acting serotonergic signal along the post-synaptic neuron. The availability of synaptic serotonin for binding to the 5-HT3 receptors is modulated by the presynaptic 5-HTTs. Prior studies have reported mixed effects of chronic nicotine exposure on the density of 5-HTTs, thus regulating the amount of synaptic serotonin available for 5-HT3 receptors. For example, Semba and Wakuta (2008) reported a reduction in the density of 5-HTTs in the rat brain whilst two other studies reported an elevation in 5-HTTs (Awtry and Werling, 2003; Slotkin and Seidler, 2010). On the other hand, Staley et al. (2001) reported an elevation 5-HTTs in the human brain, and in human platelets they were reported to be reduced (Patkar et al., 2003). Serotonin plays a crucial role mediating cognitive behavioral functions, stress response, mood, appetite, and motor functions (Jasinska et al., 2012). Thus, the interactions among the three genes may represent the interacting biological effects of nicotine on fast-acting serotonergic signaling in ND.
In summary, by examining association of variants in HTR3A, HTR3B, and SLC6A4 with three ND measures at both individual SNP and haplotype levels, we only found marginal association of rs10160548 in HTR3A, rs11606194 in HTR3B, and 5-HTTLPR in SLC6A4 with one or two ND measures in one of the three samples. However, when we examined these variants interactively through gene-gene interaction approach, we identified a combination of functional polymorphisms in the three genes with significant genetic interaction effects on ND. This indicates these genetic variants play a significant role in ND through a genetic epistatic effect. This suggests it is important to investigate genetic epistatic effect when one searches susceptibility loci for a complex trait such as ND investigated in this study.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
REFERENCES
- Al Koudsi N, Tyndale RF. Genetic influences on smoking: a brief review. Ther. Drug Monit. 2005;27:704–709. doi: 10.1097/01.ftd.0000179842.63515.c6. [DOI] [PubMed] [Google Scholar]
- Awtry TL, Werling LL. Acute and chronic effects of nicotine on serotonin uptake in prefrontal cortex and hippocampus of rats. Synapse. 2003;50:206–211. doi: 10.1002/syn.10259. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Beuten J, Ma JZ, Payne TJ, Dupont RT, Crews KM, Somes G, Williams NJ, Elston RC, Li MD. Single- and multilocus allelic variants within the GABA(B) receptor subunit 2 (GABAB2) gene are significantly associated with nicotine dependence. Am. J. Hum. Genet. 2005;76:859–864. doi: 10.1086/429839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Breitinger HG, Geetha N, Hess GP. Inhibition of the serotonin 5-HT3 receptor by nicotine, cocaine, and fluoxetine investigated by rapid chemical kinetic techniques. Biochemistry. 2001;40:8419–8429. doi: 10.1021/bi0106890. [DOI] [PubMed] [Google Scholar]
- CDC Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000-2004. MMWR. 2008;57:1226–1228. [PubMed] [Google Scholar]
- CDC Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR. 2009;58:1227–1232. [PubMed] [Google Scholar]
- Connolly CN, Wafford KA. The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function. Biochem. Soc. Trans. 2004;32:529–534. doi: 10.1042/BST0320529. [DOI] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- Dougherty JJ, Nichols RA. Cross-regulation between colocalized nicotinic acetylcholine and 5-HT3 serotonin receptors on presynaptic nerve terminals. Acta Pharmacol. Sin. 2009;30:788–794. doi: 10.1038/aps.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisdel RC, Sharp D, Henderson T, Hales TG, Green WN. High affinity binding of epibatidine to serotonin type 3 receptors. J. Biol. Chem. 2008;283:9659–9665. doi: 10.1074/jbc.M703672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin AE, Huvar R, D'Andrea MR, Pyati J, Zhu JY, Joy KC, Wilson SJ, Galindo JE, Glass CA, Luo L, Jackson MR, Lovenberg TW, Erlander MG. The pharmacological and functional characteristics of the serotonin 5-HT(3A) receptor are specifically modified by a 5-HT(3B) receptor subunit. J. Biol. Chem. 1999;274:30799–30810. doi: 10.1074/jbc.274.43.30799. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Gorodetsky E, Hodgkinson C, Roy A, Goldman D. Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity predict alcohol and drug dependence. Mol. Psychiatry. 2011;16:1139–1146. doi: 10.1038/mp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict. Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Williams LM, Schofield PR, Dobson-Stone C, Paul RH, Grieve SM, Clark CR, Gordon E, Nemeroff CB. Impact of the HTR3A gene with early life trauma on emotional brain networks and depressed mood. Depress. Anxiety. 2010;27:752–759. doi: 10.1002/da.20726. [DOI] [PubMed] [Google Scholar]
- Gerra G, Garofano L, Zaimovic A, Moi G, Branchi B, Bussandri M, Brambilla F, Donnini C. Association of the serotonin transporter promoter polymorphism with smoking behavior among adolescents. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2005;135:73–78. doi: 10.1002/ajmg.b.30173. [DOI] [PubMed] [Google Scholar]
- Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics. 2003;19:376–382. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism--basic research and clinical implications. J. Neural. Transm. 1997;104:1005–1014. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J. Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Horvath S, Xu X, Lake SL, Silverman EK, Weiss ST, Laird NM. Family-based tests for associating haplotypes with general phenotype data: application to asthma genetics. Genet. Epidemiol. 2004;26:61–69. doi: 10.1002/gepi.10295. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Chua HF, Ho SS, Polk TA, Rozek LS, Strecher VJ. Amygdala response to smoking-cessation messages mediates the effects of serotonin transporter gene variation on quitting. Neuroimage. 2012;60:766–773. doi: 10.1016/j.neuroimage.2011.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol. Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kleijn J, Folgering JH, van der Hart MC, Rollema H, Cremers TI, Westerink BH. Direct effect of nicotine on mesolimbic dopamine release in rat nucleus accumbens shell. Neurosci. Lett. 2011;493:55–58. doi: 10.1016/j.neulet.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Krzywkowski K, Davies PA, Feinberg-Zadek PL, Brauner-Osborne H, Jensen AA. High-frequency HTR3B variant associated with major depression dramatically augments the signaling of the human 5-HT3AB receptor. Proc. Natl. Acad. Sci. U. S. A. 2008;105:722–727. doi: 10.1073/pnas.0708454105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Lyon H, DeMeo D, Raby B, Silverman EK, Weiss ST. A new powerful non-parametric two-stage approach for testing multiple phenotypes in family-based association studies. Hum. Hered. 2003;56:10–17. doi: 10.1159/000073728. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Jatzke S, Meyer J, Stober G, Okladnova O, Mossner R, Riederer P. Mosaicism for a serotonin transporter gene promoter-associated deletion: decreased recombination in depression. J. Neural. Transm. 1999;106:1223–1230. doi: 10.1007/s007020050236. [DOI] [PubMed] [Google Scholar]
- Levin E, Icenogle L, Farzad A. Ketanserin attenuates nicotine-induced working memory improvement in rats. Pharmacol. Biochem. Behav. 2005;82:289–292. doi: 10.1016/j.pbb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem. Pharmacol. 2007;74:1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Johnson M, Petro A, Horton K, Williams P, Rezvani AH, Rose JE. Ketanserin, a 5-HT2 receptor antagonist, decreases nicotine self-administration in rats. Eur. J. Pharmacol. 2008;600:93–97. doi: 10.1016/j.ejphar.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Beuten J, Ma JZ, Payne TJ, Lou XY, Garcia V, Duenes AS, Crews KM, Elston RC. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Hum. Mol. Genet. 2005;14:1211–1219. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Li MD, Lou XY, Chen G, Ma JZ, Elston RC. Gene-gene interactions among CHRNA4, CHRNB2, BDNF, and NTRK2 in nicotine dependence. Biol. Psychiatry. 2008;64:951–957. doi: 10.1016/j.biopsych.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou XY, Chen GB, Yan L, Ma JZ, Mangold JE, Zhu J, Elston RC, Li MD. A combinatorial approach to detecting gene-gene and gene-environment interactions in family studies. Am. J. Hum. Genet. 2008;83:457–467. doi: 10.1016/j.ajhg.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, Kendler KS. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol. Med. 2004;34:1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Mihailescu S, Palomero-Rivero M, Meade-Huerta P, Maza-Flores A, Drucker-Colin R. Effects of nicotine and mecamylamine on rat dorsal raphe neurons. Eur. J. Pharmacol. 1998;360:31–36. doi: 10.1016/s0014-2999(98)00658-x. [DOI] [PubMed] [Google Scholar]
- Nayak SV, Ronde P, Spier AD, Lummis SC, Nichols RA. Nicotinic receptors colocalize with 5-HT(3) serotonin receptors on striatal nerve terminals. Neuropharmacology. 2000;39:2681–2690. doi: 10.1016/s0028-3908(00)00109-x. [DOI] [PubMed] [Google Scholar]
- Niesler B, Flohr T, Nothen MM, Fischer C, Rietschel M, Franzek E, Albus M, Propping P, Rappold GA. Association between the 5' UTR variant C178T of the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics. 2001;11:471–475. doi: 10.1097/00008571-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Gopalakrishnan R, Berrettini WH, Weinstein SP, Vergare MJ, Leone FT. Differences in platelet serotonin transporter sites between African-American tobacco smokers and non-smokers. Psychopharmacol. (Berl.) 2003;166:221–227. doi: 10.1007/s00213-002-1353-2. [DOI] [PubMed] [Google Scholar]
- Ribeiro EB, Bettiker RL, Bogdanov M, Wurtman RJ. Effects of systemic nicotine on serotonin release in rat brain. Brain Res. 1993;621:311–318. doi: 10.1016/0006-8993(93)90121-3. [DOI] [PubMed] [Google Scholar]
- Semba J, Wakuta M. Chronic effect of nicotine on serotonin transporter mRNA in the raphe nucleus of rats: reversal by co-administration of bupropion. Psychiatry Clin. Neurosci. 2008;62:435–441. doi: 10.1111/j.1440-1819.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- Seneviratne C, Huang W, Ait-Daoud N, Li MD, Johnson BA. Characterization of a functional polymorphism in the 3' UTR of SLC6A4 and its association with drinking intensity. Alcohol. Clin. Exp. Res. 2009;33:332–339. doi: 10.1111/j.1530-0277.2008.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Mimicking maternal smoking and pharmacotherapy of preterm labor: interactions of fetal nicotine and dexamethasone on serotonin and dopamine synaptic function in adolescence and adulthood. Brain Res. Bull. 2010;82:124–134. doi: 10.1016/j.brainresbull.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O'Malley S, Innis RB. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- Steen KV. Travelling the world of gene-gene interactions. Brief. Bioinform. 2012;13:1–19. doi: 10.1093/bib/bbr012. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob. Res. 1999;1(Suppl 2):S51–57. doi: 10.1080/14622299050011811. discussion S69-70. [DOI] [PubMed] [Google Scholar]
- Sun D, Ma JZ, Payne TJ, Li MD. Beta-arrestins 1 and 2 are associated with nicotine dependence in European American smokers. Mol. Psychiatry. 2008;13:398–406. doi: 10.1038/sj.mp.4002036. [DOI] [PubMed] [Google Scholar]
- Trummer O, Koppel H, Wascher TC, Grunbacher G, Gutjahr M, Stanger O, Ramschak-Schwarzer S, Boehm BO, Winkelmann BR, Marz W, Renner W. The serotonin transporter gene polymorphism is not associated with smoking behavior. Pharmacogenomics J. 2006;6:397–400. doi: 10.1038/sj.tpj.6500389. [DOI] [PubMed] [Google Scholar]
- Vallender EJ, Priddy CM, Hakim S, Yang H, Chen GL, Miller GM. Functional variation in the 3' untranslated region of the serotonin transporter in human and rhesus macaque. Genes Brain Behav. 2008;7:690–697. doi: 10.1111/j.1601-183X.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav. Genet. 2005;35:397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Walstab J, Rappold G, Niesler B. 5-HT(3) receptors: role in disease and target of drugs. Pharmacol. Ther. 2010;128:146–169. doi: 10.1016/j.pharmthera.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Li MD. Common and unique biological pathways associated with smoking initiation/progression, nicotine dependence, and smoking cessation. Neuropsychopharmacology. 2010;35:702–719. doi: 10.1038/npp.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Report on the Global Tobacco Epidemic, 2008: The MPOWER package. World Health Organization; Geneva: 2008. [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur. J. Pharmacol. 2000;393:51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Yamauchi JG, Nemecz A, Nguyen QT, Muller A, Schroeder LF, Talley TT, Lindstrom J, Kleinfeld D, Taylor P. Characterizing ligand-gated ion channel receptors with genetically encoded Ca2++ sensors. PLoS One. 2011;6:e16519. doi: 10.1371/journal.pone.0016519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: Genetic interactions create phantom heritability. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1193–1198. doi: 10.1073/pnas.1119675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.