Summary
Autophagy constitutes a major cell protective mechanism eliminating damaged components and maintaining energy homoeostasis via recycling nutrients under normal/stressed conditions. Although the core components of autophagy have been well studied, regulation of autophagy at the transcriptional level is poorly understood. Herein, we establish ZKSCAN3, a zinc-finger family DNA-binding protein, as a transcriptional repressor of autophagy. Silencing of ZKSCAN3 induced autophagy and increased lysosome biogenesis. Importantly, we show that ZKSCAN3 represses transcription of a large gene set (>60) integral to, or regulatory for, autophagy and lysosome biogenesis/function and a subset of these genes, including Map1lC3b and Wipi2 represent direct targets. Interestingly, ZKSCAN3 and TFEB are oppositely regulated by starvation and in turn oppositely regulate lysosomal biogenesis and autophagy, suggesting that they act in conjunction. Altogether, our study uncovers an autophagy master-switch regulating the expression of a transcriptional network of genes integral to autophagy and lysosome biogenesis/function.
Introduction
Macroautophagy (hereafter referred to as autophagy) is a highly conserved catabolic process that plays a role in cell homeostasis by degrading long-lived proteins and damaged organelles. Under physiological conditions autophagy occurs at a basal level but can be strongly induced under environmental stresses e.g. starvation, hypoxia, endoplasmic reticulum stress (Levine and Kroemer, 2008). Defects in autophagy have been linked to a myriad of diseases including neurodegenerative disorders, cancer, diabetes, cardiovascular disorders and immune diseases (Levine and Kroemer, 2008).
Autophagy begins with sequestering of cytoplasmic materials into an expanding membrane sac, the phagophore, which subsequently matures into a double-membrane vesicle, the autophagosome (Longatti and Tooze, 2009). At the molecular level, the formation of autophagosomes requires concerted actions of core autophagy machinery proteins. Maturing autophagosomes and lysosomes migrate along microtubules utilizing molecular motors (Kinesin/Dynein) ultimately fusing into a single membrane structure, the autophagolysosome where the cargo is degraded (Kochl et al., 2006; Ravikumar et al., 2005; Yang et al., 2011b). In addition to the above aspects, active lysosome biogenesis and function (e.g. lysosome acidification) is integral to the proper functioning of the autophagosomal pathway (Lee et al., 2010; Settembre et al., 2011).
In recent years, marked efforts have been made to understand the molecular events integral to autophagy (initiation, formation, and expansion of autophagosomes, autophagosome-lysosome fusion) (Levine and Kroemer, 2008). However, the regulatory network that controls the program of autophagy gene transcription remains poorly understood. Recent identification of transcription factors involved in autophagy gene regulation has shed some light on the transcriptional regulation of autophagy genes. The transcription factor FoxO3 upregulates the expression of autophagy genes including map1lc3 in response to Akt phosphorylation (Mammucari et al., 2007). Similarly, HIF-1 (hypoxia inducible factor-1), p53, E2F1 and NFκB also induce autophagy genes in response to different environmental signals (Bellot et al., 2009; Copetti et al., 2009; Crighton et al., 2006; Polager et al., 2008). Recently, a bHLH-leucine zipper transcription factor TFEB has been described as a master positive regulator of a network of genes involved in lysosome biogenesis and autophagy (Sardiello et al., 2009; Settembre et al., 2011).
ZKSCAN3 (ZNF306) belongs to a family of zinc-finger transcription factors harboring KRAB and SCAN domains. This transcriptional repressor protein family was shown to play an important role in several cellular functions including cell proliferation, apoptosis, maintenance of the nucleolus and neoplastic transformation (Urrutia, 2003). ZKSCAN3 was previously identified as a ‘driver’ of colon cancer cell proliferation both in vitro and in vivo (Yang et al., 2008a). Herein, we show that the growth defect associated with ZKSCAN3 silencing reflects in part, increased autophagy leading to cellular senescence. More importantly, we show that ZKSCAN3 transcriptionally modulates the expression of >60 genes encoding proteins involved in the various steps of autophagy and lysosome biogenesis/function.
Results
Silencing ZKSCAN3 promotes senescence
The original report on ZKSCAN3 emerged from a genetic screen for drivers of cell proliferation (Ma et al., 2007) with our own study demonstrating similar findings in colon cancer cells (Yang et al., 2008a). Toward determining the generality of the effect of ZKSCAN3 on proliferation and to gain an understanding of the mechanism, we stably transduced bladder cancer cells (UC13) with lentiviral particles encoding 3 independent shRNAs targeting this transcription factor or a non-targeting shRNA (hereafter referred to as NTLV). Figure S1A demonstrates the efficacy of one of these shRNAs (#3) (hereafter referred to as ZKSCAN3-shRNA) in repressing the expression of this gene as assessed by qRT-PCR and Western blotting. Silencing of ZKSCAN3 dramatically inhibited UC13 cell growth as evident in MTT and clonogenic assays (Figure 1A and 1B). Similarly, attenuated cell numbers were observed on transiently knocking down ZKSCAN3 in HeLa (cervical cancer) and BE2 (neuroblastoma) cell lines (Figure S1B) indicating that this effect was not organ system-specific.
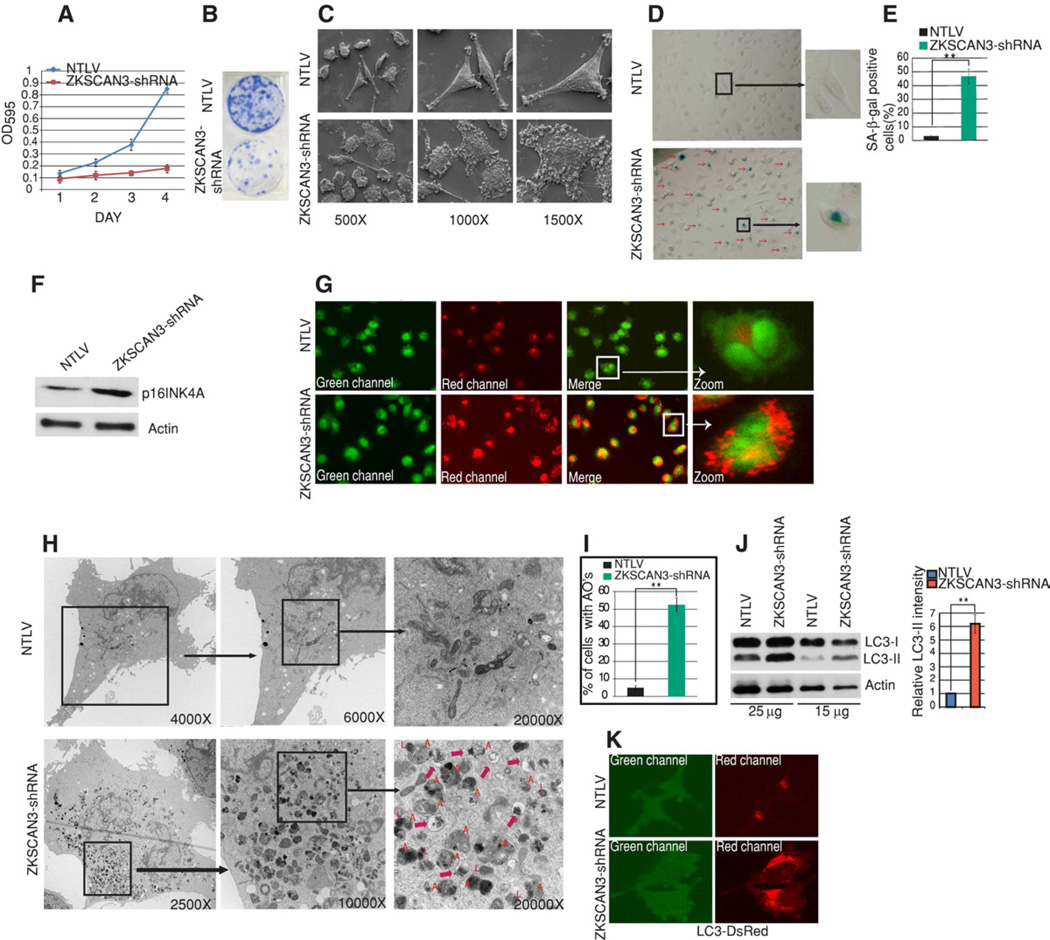
Figure 1. Silencing ZKSCAN3 promotes membrane blebbing, represses cell growth and induces senescence and autophagy.
(A) MTT assays performed at different time points with NTLV cells and ZKSCAN3-shRNA cells. Data shown represent mean ± s.d. from triplicate experiments (B) Clonogenic assays performed with NTLV and ZKSCAN3-shRNA cells. 1,000 cells were seeded in 6-well plates and grown for 21 days. (C) Scanning electron microscopy with NTLV and ZKSCAN3-shRNA cells at the indicated magnifications. (D) Senescence-associated β-gal (SA-β-gal) staining. (E) The graph shows the percentage of SA-β-gal stained cells as measured from 10 different fields from two independent experiments (mean ± s.d.). (F) Western blotting with an anti-p16INK4A (senescence marker) antibody. (G) Live cell imaging of NTLV and ZKSCAN3-shRNA cells stained with acridine orange. Acridine orange stains both nucleus (Green) and acidic vesicles (Red) (H) A typical ultrastructure of a NTLV and ZKSCAN3-shRNA cell visualized using transmission electron microscopy. ZKSCAN3-shRNA cells showed increased autophagic vesicles of different stages of maturation (also see Figure S2C and S3). Autophagosomes are indicated by ‘A’, lysosomes are indicated by ‘L’ and autophagolysosome are indicated by a red arrow. (I) NTLV and ZKSCAN3-shRNA cells (40 cells each) were visualized and cells with more than 10 autophagosome-like structures were considered positive. Graph shows the percentage of cells with autophagic organelles (AOs) from three different experiments (mean ± s.d.). (J) Western blotting using anti-LC3 and Actin antibodies was performed with varying protein inputs. The graph shows quantification (mean ± s.d.) of LC3-II band intensity relative to Actin from three different blots. (K) Representative live cell images of NTLV and ZKSCAN3-shRNA cells stably expressing the DsRed-LC3 fusion protein. Student's unpaired t-test was used to test for statistical significance: **P<0.05. See also Figure S1, S2, S3, S4 and S5.
Visual inspection of ZKSCAN3-shRNA and NTLV UC13 cells by light and scanning electron microscopy highlighted dramatic morphological differences. ZKSCAN3-repressed cells (both stable and transient knock down) showed pronounced membrane blebbing and intracellular vesiculation (Figure 1C and Figure S1C and S1D). Since such morphological changes accompanied with growth reduction have been associated with apoptosis we performed SubG1 analysis (Figure S1F) and also Western blotting for standard apoptosis protein markers (Caspase 3, 8, Parp and Bcl-2) (Figure S1E). The amounts of these apoptosis markers were unaltered between control and ZKSCAN3-shRNA cells and FACS analysis indicated an unchanged SubG1 fraction. In fact no difference in cell death was observed between control and ZKSCAN3-shRNA cells in propidium iodide staining of live cells (Figure S1G). However, cell cycle analysis showed an increased number of cells in G1 in ZKSCAN3-shRNA cells (54%) compared to control cells (41%) pointing towards a partial block in the G1/S transition (Figure S1H). G1/S cell cycle arrest is often associated with replicative senescence (Dulic et al., 2000). Indeed, we noted a strong senescence (~50% cell positivity) as evidenced by increased SA-β-gal staining (Figure 1D–E) and induction of the senescence marker protein p16ink4A (Baker et al., 2011) in ZKSCAN3-repressed cells (Figure 1F). Taken together the data suggests that the proliferation defect in ZKSCAN3-shRNA cells was at least, in part, due to induction of senescence.
ZKSCAN3 regulates autophagy
Recent reports identified autophagy as a new effector of senescence (Singh et al., 2012; Young et al., 2009). In addition, our observations of intracellular vesiculation and growth inhibition were also consistent with autophagy. So, we next undertook several experiments to determine if autophagy was induced by ZKSCAN3 repression. We first employed acridine orange a hydrophobic green fluorescent molecule which, when trapped in acidic vesicles, becomes protonated and emits bright red fluorescence. NTLV cells showed little red fluorescence and the signal was diffuse in nature (Fig 1G). In marked contrast, the cells repressed for ZKSCAN3 showed pronounced punctate red fluorescence in the majority of the cells (Figure 1G). Similar results were achieved in another bladder cancer cell line UC3 (Figure S2A). Note that the low intensity green background in all cells (here and after) is due to a GFP cassette present in the shRNA/NTLV lentiviral vectors. Autophagosomes are double-membrane vesicles and the presence of these organelles identified by transmission electron microscopy is considered a gold standard of autophagy. Indeed, an abundance of these organelles was evident in ZKSCAN3-shRNA cells (Figure 1H and 1I, Figure S2C and Figure S3) but not in cells expressing the non-targeting vector (NTLV). ZKSCAN3-shRNA cells showed an increase not only in autophagosomes (indicated with an "A") but also autophagolysosomes (marked with a red arrow) and lysosome number (marked with an "L") (Figure 1H, Figure S2C and Figure S3). To further corroborate these morphological data, we undertook Western blotting for LC3-II a widely accepted marker of autophagy (Klionsky et al., 2008). During autophagy, the cytosolic LC3 (LC-I) is conjugated to phosphatidylethanolamine to generate LC-II which specifically localizes to autophagic structures from phagophore formation through lysosomal degradation. In Western blotting using an anti-LC3 antibody, ZKSCAN3-shRNA cells showed a marked increase in the amount of LC3-II using varying protein inputs (Figure 1J) consistent with increased autophagy. Similar results (Figure S2B) were evident with an independent cell line (UC3) stably repressed for ZKSCAN3 expression (40% by qRT-PCR). Next, we stably expressed an expression construct encoding a LC3-DsRed fusion protein in NTLV and ZKSCAN3-shRNA cells. Consistent with our prior results, an abundant increase in autophagic vacuoles labeled with the LC3-DsRed fusion protein was observed in ZKSCAN3-shRNA cells compared to NTLV cells (Figure 1K). Thus, these results clearly suggest a role for ZKSCAN3 in regulating autophagy.
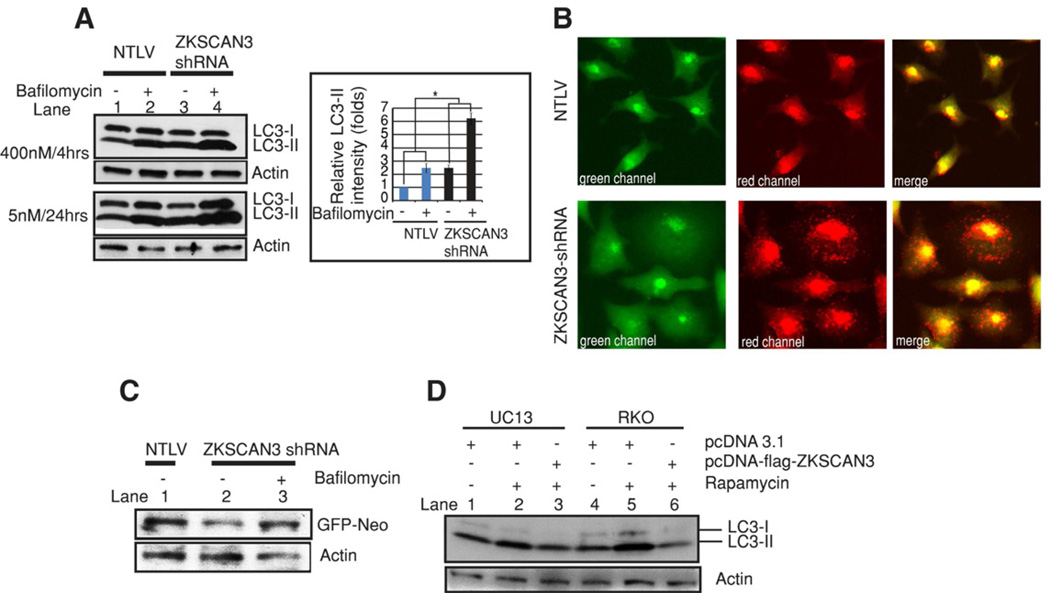
One of the limitations of the abovementioned experiments is that they fail to distinguish between the possibilities that the augmentation in autophagosome markers is due to enhanced autophagosome biogenesis or decreased clearance. The latter could reflect impaired fusion between autophagosomes and lysosomes (Klionsky et al., 2008). To discriminate between these two possibilities we inhibited autophagosome-lysosome fusion using bafilomycin (Klionsky et al., 2008). Expectedly, LC3 II levels increased in the untreated ZKSCAN3-shRNA cells when compared with NTLV cells (compare lane 1 and 3, Figure 2A). More importantly, the elevated level of LC3 II in the ZKSCAN3-shRNA cells was further increased in the presence of bafilomycin (compare lane 3 and 4, Figure 2A) arguing strongly for an augmentation of autophagosome formation rather than reduced autophagosome-lysosome fusion. To further corroborate these findings we made use of an expression vector (ptfLC3) encoding LC3 fused to monomeric red-fluorescence protein and GFP in tandem (Kimura et al., 2007). By way of explanation, in autophagosomes, both red and green fluorescence signals are apparent whereas under acidification (in autophagolysosomes) the monomeric-red fluorescent protein is stable yielding persistent red fluorescence. In contrast, green fluorescence is diminished due to a labile EGFP protein under acidic conditions. We stably transfected NTLV and ZKSCAN3-shRNA cells with ptfLC3 vector (Figure 2B). ZKSCAN3 shRNA cells showed a high density of red puncta in the red and merged channels much more so than the NTLV cells suggestive of lysosomal fusion with the autophagosomes and a strong autophagic flux. Lastly, as a final measure of increased autophagic flux, we evaluated the degradation of a long-lived protein as assessed by monitoring the expression of a GFP-Neo fusion protein (Klionsky et al., 2008; Yang et al., 2011a). Indeed levels of this protein were reduced in ZKSCAN3-shRNA cells compared to NTLV cells (Figure 2C). Moreover reduced level of this protein was partly inhibited by bafilomycin, further arguing for a role of ZKSCAN3 repression in augmenting autophagy flux.
Figure 2. Modulated ZKSCAN3 expression regulates autophagic flux.
(A) Protein extracted from bafilomycin-treated or untreated NTLV and ZKSCAN3-shRNA cells was subjected to Western blotting using anti-LC3 and Actin antibodies. The graph shows quantification (mean ± s.d.) of LC3-II band intensity relative to Actin. *P<0.05 (B) Representative live cell images of cells stably expressing a GFP-RFP-LC3 fusion protein. (C) NTLV and ZKSCAN3-shRNA cells were transiently transfected with a plasmid encoding GFP-Neo fusion protein. After 48 h, ZKSCAN3-shRNA cells expressing GFP-Neo were treated or untreated with bafilomycin (5 nM, lane 3) for 24h. Subsequently after total 72 h, protein was extracted and Western blotted using anti-GFP and anti Actin antibodies (D) UC13 and RKO cells were either transiently transfected with an empty vector (pcDNA3.1) or a streptavidin-flag-tagged-ZKSCAN3 expressing vector. After 72h, cells were treated, where indicated, with rapamycin (6h/200 nM), extracted and immunoblotted for LC3 and Actin.
Next, we determined the effect of overexpressing ZKSCAN3 on autophagy. Transient overexpression of ZKSCAN3 in UC13 cells or RKO cells reduced rapamycin-induced autophagy as indicated by LC3-II levels (Figure 2D, compare lanes 3 with 2 and 6 with 5). Interestingly not only LC3-II but also LC3-I levels were diminished on overexpressing ZKSCAN3 (Figure 2D) consistent with the notion that ZKSCAN3 might regulate LC3 synthesis as well. Taken together, the loss- and gain-of-function data demonstrate that ZKSCAN3 is a repressor of autophagy.
To investigate the generality of autophagy induction by ZKSCAN3 repression we determined the effect of ZKSCAN3 knock down on 4 independent cell lines derived from different organ sites. Repression of ZKSCAN3 by two independent siRNAs in HeLa (cervical cancer), RKO (colon cancer), BE2 (neuroblastoma), SKOV3 (ovarian cancer) induced autophagy as evidenced by the increased amount of LC3-II in Western blotting (Figure S4A–B) and an increased number of cells with puncta in acridine orange staining (Figure S4C). The efficiency of ZKSCAN3 knockdown in these cells was confirmed by real time PCR (Figure 4E). These results suggest that ZKSCAN3 represses autophagy across cell types of diverse origin.
Figure 4. ZKSCAN3 regulates autophagy and lysosomal genes mRNA levels.
qRT-PCR validation of selected candidate downstream autophagy targets of ZKSCAN3 identified in expression profiling. RNA isolated from NTLV and ZKSCAN3-shRNA cells were subjected to qRT-PCR with taqman probes against (A, B) autophagy and (C) lysosome genes upregulated and (D) downregulated in ZKSCAN3-repressed and control cells (NTLV). The autophagosome marker gene, Map1cl3b/Atg8f is upregulated in various cell lines (F) on transient repression of ZKSCAN3 (E). Data represent mean ± s.d. of duplicate experiments performed in triplicate (6 values). Statistical significance: *P<0.05; **P<0.02. See also Table S1, S2 and S3.
ZKSCAN3 regulates lysosome biogenesis
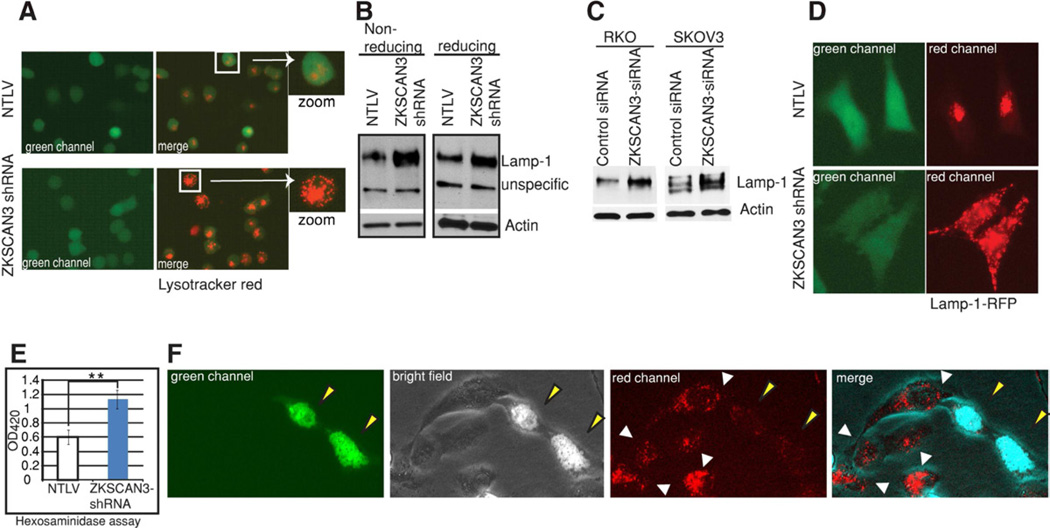
We next determined whether ZKSCAN3 also regulates lysosome biogenesis. Indeed, transmission electron microscopy data already showed an increased number of lysosomes upon ZKSCAN3 repression (Figure 1H, Figure S3). We then employed LysoTracker Red (Invitrogen), a fluorescent acidotropic probe, which labels acidic organelles in live cells, in particular, the lysosomal compartment. While NTLV cells showed little red fluorescence with a diffuse signal (Figure 3A), in contrast, cells repressed for ZKSCAN3 showed distinct punctate structures in the majority of the cells (Figure 3A). Lamp-1 is a frequently used marker for lysosome biogenesis (Settembre et al., 2012). Western blotting showed an increased amount of Lamp-1 in different cell lines stably or transiently repressed for ZKSCAN3 (Figure 3B, 3C). Next, we transfected ZKSCAN3-shRNA and NTLV cells with a construct expressing the Lamp1-RFP fusion protein. Relative to the controls (NTLV), the former cells showed a robust increase in the number of lysosomal organelles as revealed by immunofluorescence (Figure 3D). Consistent with these findings, we observed a significant increase in the activity of the lysosome enzyme β-hexosaminadase in ZKSCAN3-repressed cells (Figure 3E). Next, we investigated the effect of overexpressing ZKSCAN3 on lysosome number. Immunofluorescence of SKOV3 cells transiently transfected with ZKSCAN3-GFP showed that the cells that were positive for ZKSCAN3 (nuclear localized, see green and merged channels, Figure 3F) have reduced number of lysosomes (see red and merged channels Figure 3F) relative to cells not expressing this DNA-binding protein. Altogether, our data show that ZKSCAN3 regulates lysosome biogenesis
Figure 3. Silencing ZKSCAN3 enhances lysosome biogenesis.
(A) Live cell imaging of NTLV and ZKSCAN3-shRNA cells stained with lysotracker red which fluoresces red in acidic compartments. (B, C) Western blotting of protein extracts from UC13 NTLV and ZKSCAN3-shRNA cells (B) or transiently ZKSCAN3 repressed RKO and SKOV3 cells (C) using anti-Lamp1 and anti-Actin antibodies. (D) Representative live cell images of NTLV and ZKSCAN3-shRNA cells stably expressing a construct encoding the Lamp1-RFP fusion protein. (E) Hexosaminidase assay was performed with total protein extract of the indicated cells. The experiment was performed in triplicate and data are shown as mean ± s.d. **P<0.05. (F) Live cell imaging of lysotracker red-stained SKOV3 cells transiently transfected with a ZKSCAN3-GFP expressing vector. ZKSCAN3-GFP expressing (yellow arrow) and non-expressing (white arrows) are indicated.
Inhibition of autophagy diminishes senescence, restores growth and reverts the morphology achieved by ZKSCAN3 repression
We hypothesized that increased autophagy in UC13 cells repressed for ZKSCAN3, leads to replicative senescence and consequently growth suppression. In this scenario, blocking autophagy would be expected to promote growth and reduce senescence. Indeed, interfering with autophagy with bafilomycin partly restored the growth of ZKSCAN3-shRNA cells without affecting growth of NTLV cells as measured by MTT and clonogenic assays (Figure S5A and S5B). Next, we knocked down the autophagy gene Atg5 in ZKSCAN3-shRNA and NTLV cells which reduced autophagy as expected (data not shown). More importantly, repressing Atg5 expression (Figure S5C) markedly attenuated the intra-cellular vesiculation evident with ZKSCAN3-shRNA cells (Figure S5D). Also consistent with our prior results, cell growth was significantly restored (Figure S5E). Finally, knocking down Atg5 in ZKSCAN3-shRNA cells diminished senescent cell number (Figure S5F and S5G). Together, these results show that increased autophagy contributes to the vesicular phenotype, growth repression and senescence in ZKSCAN3-silenced cells.
ZKSCAN3 regulates autophagy and lysosome biogenesis/function gene expression
We previously described ZKSCAN3, a DNA-binding protein with a SCAN and a KRAB domain and tandem zinc fingers as regulatory for gene expression (Yang et al., 2008b). DNA-binding proteins with a KRAB domain are typically repressive for gene expression (Urrutia, 2003). ZKSCAN3 is mostly localized in the nucleus (Figure S6A) and when over-expressed as a gal4-fusion protein (Gal-ZKSCAN3) it reduced basal transcription from a gal4 promoter-driven luciferase reporter consistent with a repressive function (Figure S6B). To identify the gene targets of ZKSCAN3 we undertook expression profiling with RNA extracted from three independent cultures of NTLV and ZKSCAN3 shRNA #3 cells using the Agilent 4X44k chip that interrogates the entire expressed human genome. Only changed genes in all three experiments were considered putative ZKSCAN3 downstream targets. A false discovery rate of 0.05 was used to assess significantly differentially expressed genes (File S1). Ingenuity pathway analysis software (IPA; www.ingenuity.com) was used to functionally group the differentially expressed genes. Interestingly, autophagy was among the top cellular functions of ZKSCAN3 repression (p=5.51×10−3) in the cellular function and maintenance category (Table S1).
Autophagy involves several steps which include autophagosome biogenesis, maturation and fusion with lysosomes. Additionally, migration of these maturing cell organelles (autophagosomes and lysosomes) takes place along microtubules aided by Dynein and Kinesin molecular motors (Kochl et al., 2006; Yang et al., 2011b). Analysis of microarray data (both using IPA software and a manual literature query) suggested that ZKSCAN3 regulates components of all of these processes to dynamically regulate autophagy (Table S2). More than 60 genes previously implicated in autophagy and lysosomal biogenesis/function (hereafter referred to as lysosome genes) were up-regulated by ZKSCAN3 repression in triplicate expression profiling experiments (Table S2, Table S3). This data is intriguing since it suggests that ZKSCAN3 regulates not only genes involved in the regulation of autophagy (e.g. Diras3, Rela, Tak1, Cdkn2a) but also autophagosome biogenesis (e.g. Map1lc3b, Ulk1, Atg18b, Dfcp1), movement (e.g. protein subunits of Dynein and Kinesin motors, Dynactin) and fusion with lysosomes (e.g. Stx5, Sec22b, Ubqln2). In addition, genes involved in lysosome biogenesis (Bloc1s1, Rilp) and function (e.g. Sgsh, Hexa, Ctsa, Atp6V1a, Arl5b/Arl8) were also induced on ZKSCAN3 repression suggesting a role in this process (Table S2, Table S3). A set of genes, suppressive for autophagy, (Rptor, Akt1, Lamtor2, Ywhaz, Akt1s1/Pras40 and Impa2) was also repressed by ZKSCAN3 silencing (Table S3).
To validate the putative downstream targets of ZKSCAN3, we performed qRT-PCR with a selected set of genes (27 genes) identified in the expression profiling. Figure 4A– 4D shows validation of the genes identified as putative downstream targets of ZKSCAN3. By way of example, expression of Map1lc3b encoding LC3, WIPI2/ATG18B integral to autophagosome biogenesis and maturation (Polson et al., 2010), Syntaxin 5, DYNC2H1 required for autophagosome-lysosome movement and fusion (Ravikumar et al., 2005; Renna et al., 2011; Yang et al., 2011b), ULK1/Atg1 and DIRAS3 regulatory for autophagy (Lu et al., 2008; Mizushima, 2010) and ATP6V1A, CTSA, BLOC1S1 required for biogenesis and function of lysosomes (Dell'Angelica, 2004; Hiraiwa, 1999; Mijaljica et al., 2011) were all significantly increased by ZKSCAN3 repression (Figure 4A–4C). We also verified some of the genes which were downregulated by ZKSCAN3 and are suppressive for autophagy. For example RAPTOR and LAMTOR2 (Figure 4D) are important functional components of the mTORC1 complex (Sancak et al., 2010; Shende et al., 2011) and when suppressed induces autophagy.
To rule out the possibility that regulation of these downstream autophagy targets was unique to UC13 cells, we determined the effect of transiently knocking down ZKSCAN3 in various cell lines of diverse tissue origin on Map1lc3b expression required for autophagosome biogenesis/maturation. ZKSCAN3 repression in SKOV3, Hey8 (ovarian), BE2 (brain), RKO (colon) and HeLa (cervical) (Figure 4E) yielded a significant increase in the level of this transcript (Figure 4F) strongly arguing that ZKSCAN3 regulates these downstream targets across cell types of diverse tissue origin.
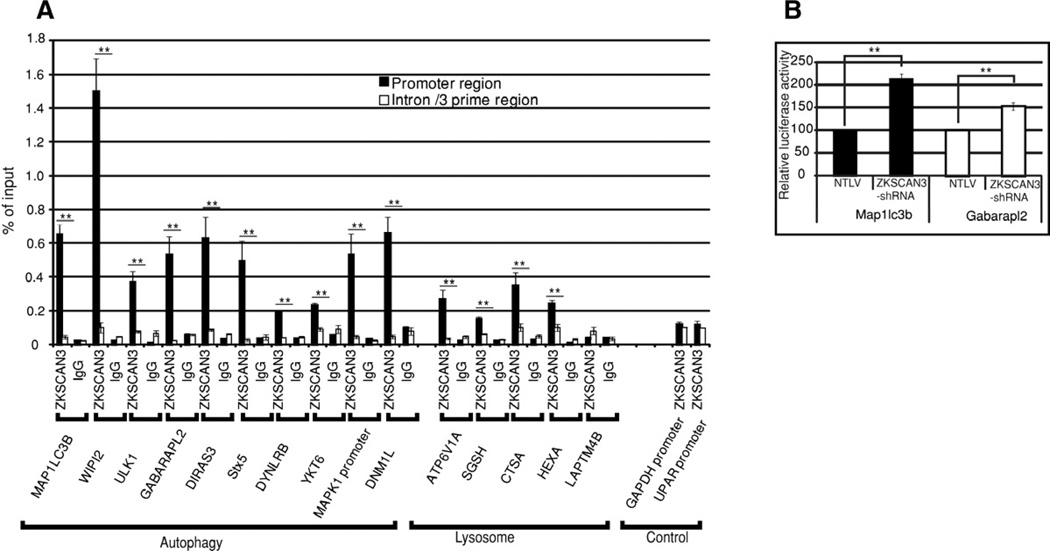
Next, we utilized chromatin immunoprecipitation (ChIP assays) towards determining whether the autophagy and lysosomal genes whose expresssion was changed in the microarray studies represented direct ZKSCAN3 targets. Indeed, autophagy/lysosome gene promoter regions (except LAMPT4B) showed increased enrichment with the ZKSCAN3 antibody relative to the IgG control antibody (Figure 5A). However, there was minimal chromatin enrichment with the ZKSCAN3 antibody using primers corresponding to the 3’ region of each gene or control gene promoter regions (GAPDH, u-PAR) (Figure 5A). We further investigated the presence of the putative ZKSCAN3 binding site (KRDGGG, Yang et al., 2008b) in promoter regions of ZKSCAN3-regulated autophagy genes using the bioinformatic tool “Mobyle” (Supplementary experimental procedures). Multiple ZKSCAN3 binding sites were detected in many of these targeted genes (File S2). These data are consistent with the notion that at least some of the ZKSCAN3-regulated autophagy genes represent direct targets.
Figure 5. ZKSCAN3 directly interacts with the regulatory region of autophagy and lysosome genes.
(A) qRT-PCR followed by chromatin immunoprecipitation assay (ChIP assay) was performed using primers mapping to regulatory or intron regions of autophagy and lysosome genes and DNA precipitated with ZKSCAN3 or IgG antibodies. The graph displays the amount of the immunoprecipitated DNA expressed as a percentage of the total input DNA. Gapdh and uPAR gene promoters were used as control. qRT-PCR with each primer set was performed in triplicate in two separate experiments. (B) Expression of Map1lc3b and Gabarapl2 promoter-driven luciferase reporters was induced in ZKSCAN3-shRNA compared to control NTLV cells in dual luciferase assays. The data represent mean ± s.d. of two experiments performed in triplicate (6 values). **P < 0.05.
To further corroborate our findings, we determined if luciferase reporters driven by the regulatory regions of two of the putative targets (Map1lc3b and Gabarapl2) were regulated by ZKSCAN3. Map1lc3b and Gabarapl2 promoter reporter constructs were transiently transfected into ZKSCAN3-shRNA and control NTLV cells and dual luciferase assays performed. Promoter activity of both genes was significantly increased in ZKSCAN3-shRNA cells compared to NTLV cells (Figure 5B). Taken together the data presented here suggest that ZKSCAN3 regulates autophagy by directly modulating expression of, at least, some of the genes involved in this function.
Starvation induces cytoplasmic accumulation of ZKSCAN3
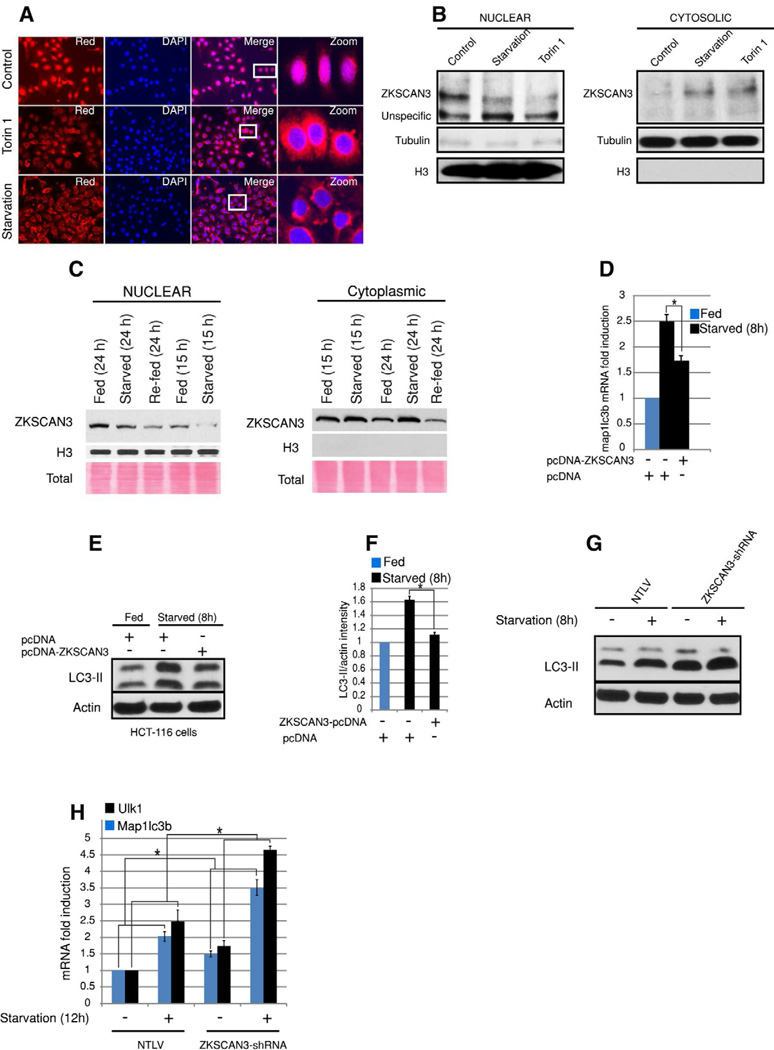
Multiple stresses (starvation, hypoxia and endoplasmic reticulum (ER) stress) induce autophagy (Levine and Kroemer, 2008). Most of these signals regulate autophagy through an mTOR-dependent pathway (Levine and Kroemer, 2008). So next to identify ZKSCAN3-responsive environmental cues we investigated the effect of starvation, hypoxia, a chemical inducer of ER stress (Tunicamycin), a calcium mobilizing agent (A23187) and a specific mTOR inhibitor (torin 1) on ZKSCAN3 expression. With the exception of starvation, ZKSCAN3 mRNA levels were largely unchanged (Figure S7A). We then considered the possibility that stresses invoked nuclear-cytoplasmic shuttling of ZKSCAN3. Interestingly, starvation and torin 1 induced ZKSCAN3 cytoplasmic localization in both SKOV3 and HeLa cells (Figure 6A and 6B and Figure S7B–S7D) in contrast to its nuclear localization under non-stressed conditions. None of the other conditions (Hypoxia) and various signaling pathway inhibitors (Tunicamycin, A23187, AKT, MAPK, JNK, p38MAPK) effected ZKSCAN3 subcellular localization (Figure S7E–G).
Figure 6. Nutritional starvation and mTOR regulates subcellular re-localization of ZKSCAN3.
(A) Immunofluorescence of SKOV3 cells using an anti-ZKSCAN3 antibody (Origene) starved for 12 h or treated with torin 1 (500 nM) for 12h (B) Western blot analysis of nuclear and cytoplasmic subcellular fractions from HeLa cells subjected to starvation or treated with torin1 (500 nM) for 12h using an anti-ZKSCAN3 antibody. (C) Liver tissues from fed/starved/refed mice were subjected to nuclear-cytoplasmic fractionation and Western blotting was carried out using anti-ZKSCAN3 antibody (Sigma). (D) RNA isolated from starved and fed; control and ZKSCAN3-overexpressing cells were subjected to qRT-PCR with taqman probes against map1lc3b. (E) Starved and fed, control and ZKSCAN3-overexpressing cells were western blotted with LC3 and actin antibody. (F) Densitometric analysis of Western blots was done using ImageJ software. (G) Starved and fed NTLV and ZKSCAN3-shRNA cells were western blotted with LC3 and actin antibody. (H) RNA isolated from starved and fed NTLV and ZKSCAN3-shRNA cells were subjected to qRT-PCR with taqman probes against map1lc3b and ulk1. For western blotting quantification, the data represent mean ± s.d. of three experiments performed. For qRT-PCR the data represent mean ± s.d. of two experiments performed in triplicate (6 values). *P < 0.05. See also Figure S7
We then starved HeLa cells (EBSS medium) and then either refed with normal medium or with starvation medium supplemented with serum (10%), aminoacids, or two growth factors (insulin and EGF) or a cytokine (LIF). Only on re-feeding with normal media and in the presence of serum or aminoacids was nuclear re-localization observed indicating that ZKSCAN3 nuclear-shuttling is sensitive to nutrients but not to the two growth factors, insulin and EGF or the cytokine LIF (Figure S7H).
Next we assessed the effect of starvation on ZKSCAN3 subcellular localization in the liver of 15 h or 24 h starved mice by Western blotting. Starvation induced depletion of ZKSCAN3 from the nuclear fraction with a concomitant increase in the cytoplasmic fraction (Figure 6C). However, unexpectedly ZKSCAN3 nuclear localization was not regained after re-feeding the mice for 24 h (Figure 6C) although this might be due to its cytoplasmic degradation. Taken together, these results suggest that nutrient availability controls subcellular localization of ZKSCAN3 possibly through the mTOR signaling pathway.
We next assessed whether ZKSCAN3 is important for the starvation-induced autophagy response. ZKSCAN3 overexpression was able to blunt the starvation-induced autophagy response as measured by map1lc3b mRNA and LC3B protein levels (Figure 6 D–F, Figure S7I). Conversely, as assessed by qRT-PCR of two autophagy genes (map1lc3b, ulk1) and Western blotting with an anti-LC3 antibody, the starvation-induced autophagy response was further enhanced by ZKSCAN3 knockdown (Figure 6G and 6H).
In ChIP experiments, starvation diminished the interaction of ZKSCAN3 with the promoter regions of map1lc3b and wipi2 (Figure S7J). Taken together the data shows that ZKSCAN3 is an important component of the starvation-induced autophagy response.
TFEB and ZKSCAN3 regulate autophagy oppositely
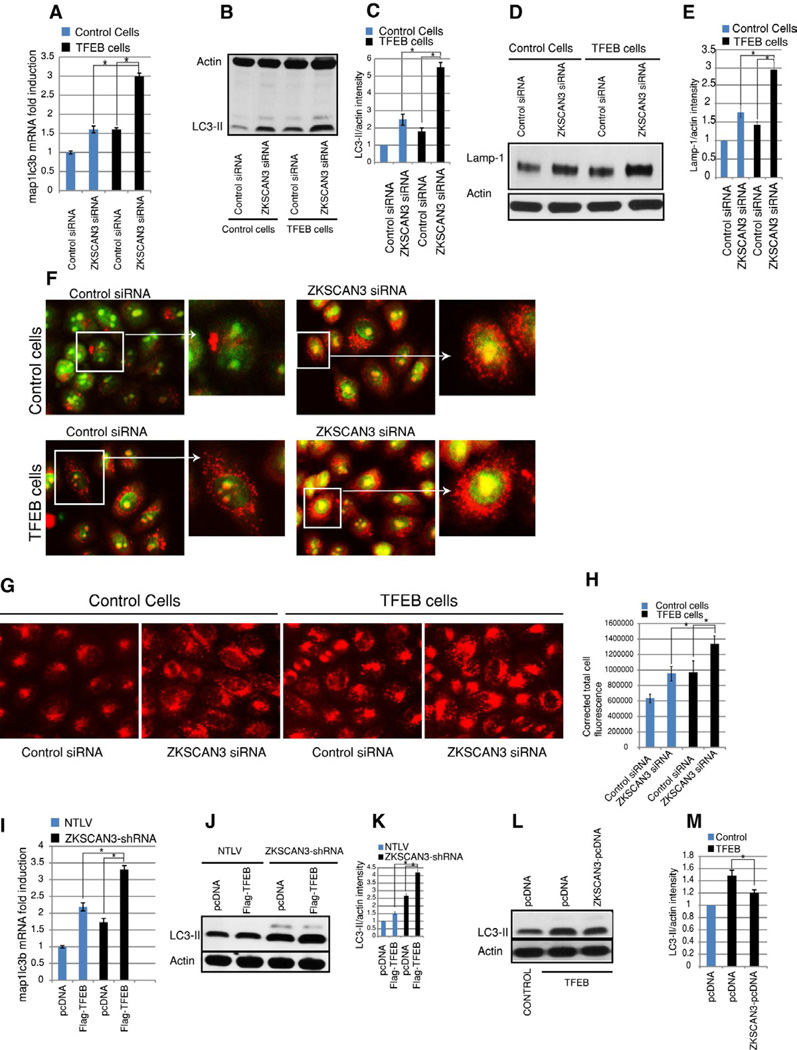
TFEB has been recently described as a master activator of autophagy and lysosome biogenesis (Settembre et al., 2011, Sardiello et al., 2009) and we here show that ZKSCAN3 is a master repressor of these processes. Analysis of our data and that of the TFEB studies suggests that at least a subset of autophagy and lysosomal genes (sgsh, hexa, ctsa, atp6v1e1, stomatin, granulin, map1lc3b) are oppositely regulated by ZKSCAN3 and TFEB. So we next determined whether these two transcription factors work oppositely to regulate autophagy. First, we knocked down ZKSCAN3 in TFEB overexpressing HeLa cells (hereafter referred to as TFEB cells). We observed a robust induction (p<0.05) in map1lc3b mRNA level (Figure 7A) in excess of that achieved by TFEB overexpression alone. Parallel results were evident at the protein level (Figure 7B–C). Additionally, lysosome biogenesis was also further induced on silencing ZKSCAN3 in TFEB cells as assessed by LAMP-1 Western blotting, acridine orange staining and lysotracker staining (Figure 7D–H). Further, transient overexpression of Flag-tagged TFEB in ZKSCAN3-shRNA cells further augmented the amount of the autophagy marker LC3-II at the mRNA and protein levels (Figure 7I–K). In contrast, overexpression of ZKSCAN3 in TFEB cells suppressed autophagy compared to parental cells (Figure 7L–M). Taken together these results suggest that these two transcription factors regulate autophagy in an opposite direction suggesting there conjunction in this process.
Figure 7. TFEB and ZKSCAN3 regulate autophagy oppositely.
(A) RNA isolated from control HeLa cells and stable TFEB-expressing Hela cells (TFEB cells) transfected with control siRNA and ZKSCAN3-siRNA, were subjected to qRT-PCR with taqman probes against map1lc3b. (B–E) Cell lysate from control and TFEB cells transfected with control siRNA and ZKSCAN3-siRNA, were subjected to Western blotting using (B) LC3 antibody (D) and Lamp-1 antibody. (C, E) Densitometric analysis of Western blots using ImageJ software. (F–H) Live cell imaging of (F) acridine orange stained and (G) Lysotracker stained control and TFEB cells transfected with control siRNA and ZKSCAN3-siRNA. (H) Graph represent corrected total cell fluorescence of lysotracker stained cells measured using Image J. (I) RNA isolated from NTLV and ZKSCAN3-shRNA cells which were transiently transfected with pcDNA 3.1 plasmid or Flag-TFEB expressing plasmid were subjected to qRT-PCR with taqman probes against map1lc3b. (J) Cell lysate from NTLV and ZKSCAN3-shRNA cells, transiently transfected with pcDNA 3.1 vector or Flag-TFEB expressing vector were subjected to Western blotting using LC3 and actin antibody.(K) Densitometric analysis of Western blots. (L, M) Cell lysate from control and TFEB cells, transiently transfected with pcDNA 3.1 vector or Flag-ZKSCAN3 expressing vector were subjected to Western blotting using LC3 and actin antibody. (M) Densitometric analysis of Western blots. For all western blotting quantification, the data represent mean ± s.d. of three experiments performed. For qRT-PCR the data represent mean ± s.d. of two experiments performed in triplicate (6 values). *P < 0.05.
Discussion
In this study we have identified ZKSCAN3 as a master transcriptional repressor of autophagy. Our data indicate that ZKSCAN3 directly targets a network of genes spanning the sequential steps of the autophagic process including autophagosome/lysosome biogenesis, their transport and fusion.
Until recently, autophagy was considered a cellular process regulated, largely, at the translational level. However, emerging studies suggest that transcriptional regulation represents an alternate robust molecular mechanism to regulate the autophagic process under divergent stress conditions. Indeed, several transcription factors including FoxO3, HIF-1, p53, TFEB have been shown to activate the expression of autophagy genes in response to various stresses (Bellot et al., 2009; Crighton et al., 2006; Mammucari et al., 2007; Settembre et al., 2011). For example, FoxO3, in response to AKT repression, translocates to the nucleus thereby activating several autophagy genes (Mammucari et al., 2007). Hypoxia inducible factor-1 (HIF-1), in response to hypoxia, induces autophagy by increasing the expression of bnip3 and bnip3l involved in autophagy regulation (Bellot et al., 2009). However, perhaps the most intriguing of autophagy-regulating transcription factors is TFEB which has been recently described as a master activator of autophagy (Settembre et al., 2011). While the aforementioned autophagy transcriptional regulators increase expression of a limited repertoire of genes involved in the early steps of autophagosome formation, TFEB activates transcription of a network of genes encoding proteins affecting the entire process; thus it, not only, induces autophagosome biogenesis but also expedites delivery of these organelles to lysosomes, robustly increases lysosome biogenesis, thereby culminating in accelerated degradation of substrates (Sardiello et al., 2009; Settembre et al., 2011). In this respect ZKSCAN3 is similar to TFEB; however while the former represses, the latter activates the autophagy process. Thus ZKSCAN3 and TFEB are oppositely regulated by starvation and in turn oppositely regulate lysosomal biogenesis and autophagy. Parallel ZKSCAN3 repression and TFEB upregulation has an additive effect on autophagy and lysosome biogenesis. This finding could have broad implication in developing strategies against diseases where modulated autophagy is beneficial.
The strength of the ZKSCAN3-modulated transcription program lies in co-regulation of genes involved in multiple components across the autophagy process (autophagosome/lysosome biogenesis, organelle migration/fusion). ZKSCAN3 regulates Ulk1, Wipi2 and Dfcp1all with well-established roles in initiation of autophagosome biogenesis (Axe et al., 2008; Mizushima, 2010; Polson et al., 2010). Map1lc3b is another ZKSCAN3-regulated gene that encodes cytosolic LC3 (and ultimately, the processed bio-active form, LC3-II) a protein important for the formation and maturation of autophagosomes and present throughout their maturation to autophagolysosomes. Further, two kinesin subunits (Kif1b, Kif21) and several Dynein subunits (Dync2h1, Dynlrb1, Dnal1 and Dnah1) were also upregulated on ZKSCAN3 repression. Since the Dynein and Kinesin molecular motors drive the movement of autophagosomes/lysosome along the microtubules (Ravikumar et al., 2005; Yang et al., 2011b), increased expression of these subunits likely hastens juxta-positioning of these organelles. In regard to autophagosome-lysosome fusion, ZKSCAN3 regulated Stx5, Ubqln2, Sec22b and Bet1 all involved in this process (N'Diaye et al., 2009; Renna et al., 2011). Likewise, lysosome number and lysosomal function, essential to the autophagosomal content degradation, were both augmented by ZKSCAN3 repression likely affected by increased expression of lysosomal hydrolases (e.g. Sgsh, hexa, Ctsa) and several lysosome H+ transporting ATPase subunits (e.g. Atp6v1a, Atp6v1e1, Atp6v1d). In addition to regulating the expression of proteins integral to the autophagic process, ZKSCAN3 also suppressed genes modulatory for autophagy regulation (Diras3, Bad, Sapk1 and RelA). Taken together, we posit that under nutrient rich conditions ZKSCAN3, by virtue of repressing the expression of essential genes for multiple autophagy steps, this cellular process is kept in check. However, under sustained starvation conditions, ZKSCAN3 is translocated from the nucleus thereby de-repressing this network of genes, allowing for an activated autophagy response.
It is becoming increasingly evident that autophagy dysfunction is associated with the pathogenesis of several diseases (cancer, neurodegeneration, cardiac dysfunction, microbial infection) (Levine and Kroemer, 2008). Understanding this process may ultimately allow us to exploit it towards the development of novel treatments. Our work identifies an autophagy master-switch regulating the expression of a transcriptional network of genes integral to autophagy, lysosome biogenesis and function. The ZKSCAN3 mediated repression of autophagy is so vital that merely reducing its expression is enough to induce this process in fully fed cells or in the absence of any stress. Exploitation of this network "node" could potentially represent a novel therapeutic means of modulating autophagy or lysosome function in disease.
Experimental procedures
Cell culture, reagents and Plasmids
Cell lines were maintained in McCoy's 5A media with 10% FBS and antibiotics in a humidified incubator at 37°C. For starvation cells were cultured in either EBSS media (Gibco) supplemented with 1% serum or DMEM medium with no glucose and serum (Gibco). For experiments where cells were treated with Torin 1, both control and treated cells were grown in opti-MEM (Gibco).
Transfection and stable cell line production
Transfections were performed using Lipofectamine-2000 as per the manufacturer's guidelines (Invitrogen). For transient knock down (using siRNA) or over-expression (using plasmids), cells were harvested after 48h (otherwise indicated) and processed for Western blotting, immunofluorescence or qRT-PCR. Stable cell lines for Lamp1-RFP or LC3-DsRed were generated by selecting the transfected cells in 1mg/ml of G418 (Sigma) for 2–3 weeks. Lentiviral transduction to achieve stable knock down of ZKSCAN3 expression was performed as per the manufacture's protocol (Thermo scientific).
Western blotting
Western blotting was performed as described earlier (Chauhan and Boyd, 2012). Nuclear/cytoplasmic fractionation was done using the NE-PER nuclear extraction kit (Pierce).
Immunofluorescence
Cells were seeded on poly-lysine coated chamber slides. The next day, they were subjected to starvation, hypoxia (24 h, 1% O2) or drug treatment as indicated. Cells were then washed (PBS), fixed (methanol or 3.7 % paraformaldehyde), permeablized (0.1% TritonX-100), blocked (1% BSA) and incubated with primary antibody in 1% BSA overnight at 4°C. After rinsing 4 times with PBS, cells were incubated with secondary antibody for 1 h at room temperature. Subsequently, the cells were washed thrice with PBS, incubated with DAPI (as required), air dried, mounted (ProLong gold Anti-Fade, Invitrogen) and visualized using a fluorescence (Olympus 1X71) or confocal microscope (Zeiss LSM 510).
Live cell imaging
Cells were grown in chamber slides. Next day, GFP/RFP tagged proteins were directly visualized by fluorescent microscopy. For acridine orange or lysotracker red staining the cells were washed with PBS and incubated with acridine orange or lysotracker red (50 nM) in culture media. Cells then were washed once with PBS and visualized in media or PBS using fluorescent microscope (Olympus 1X71).
Transmission Electron Microscopy
Samples were fixed with a solution containing 3% glutaraldehyde/ 2% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.3, for 1h. After fixation, the samples were washed and treated with 0.1% Millipore-filtered cacodylate buffered tannic acid, postfixed with 1% buffered osmium tetroxide for 30 min, and stained en bloc with 1% Millipore-filtered uranyl acetate. The samples were dehydrated in increasing concentrations of ethanol, infiltrated, and embedded in LX-112 medium. The samples were polymerized in a 70 °C oven for 2 days. Ultrathin sections were cut in a Leica Ultracut microtome (Leica, Deerfield, IL), stained with uranyl acetate and lead citrate in a Leica EM Stainer, and examined in a JEM 1010 transmission electron microscope (JEOL, USA, Inc., Peabody, MA) at an accelerating voltage of 80 kV. Digital images were obtained using the AMT Imaging System (Advanced Microscopy Techniques Corp, Danvers, MA).
Morphological criteria to distinguish autophagosome, lysosome and autophagolysosome: Autophagosomes are double membrane structure with undigested/undegraded cellular organelles and cytoplasmic material. Autophagolysosome are single membrane structures with partially degraded or fully degraded cytoplasmic organelles. Lysosomes are single membrane bound electron-dense spherical structures.
Chromatin immunoprecipitation assay (ChIP assay) and quantitative real-time PCR (qRT-PCR)
ChIP assays and qRT-PCR were performed as described earlier (Chauhan and Boyd, 2012). The chromatin immunoprecipitation (ChIP) experiments were performed using the ChIP-IT-Express kit from Active Motif (Catalog No. 53009) according to the manufacturer's instructions. Briefly, cells were fixed; cross-linked using 1% formaldehyde at room temperature for 10 min. Fixation was stopped by adding glycine. DNA was extracted from nuclear fraction and was subjected to enzymatic digestion (using a micrococcal nuclease-containing enzymatic cocktail from Active Motif Catalog No. 53009) for 35 min at 37°C to obtain mononucleosomes. The resulting chromatin preparation was incubated at 4°C with 10 µg of either the ZKSCAN3 antibody (Sigma, A33609) or rabbit-IgG antibody (Cell signaling, 2729) and precipitated complexes washed four times. DNA-protein cross-linking was reversed at 65°C (4 h), treated with Proteinase K (2 h at 42°C), purified by ethanol extraction, air dried and re-dissolved in H2O. The retrieved DNA was then subjected to real-time RT–PCR amplification using specific primers (amplicon size 90–110 bp) listed in and a SYBR green qPCR master mix (Applied Biosytem). Input (in each qRT–PCR reaction) was used to normalize the values. All ChIP assays were repeated twice and individual qPCR reactions performed in triplicates with results presented as average values ± SD.
RNA isolation and quantitative real-time PCR
RNA isolation and qRT-PCR were performed as described prior (Chauhan and Boyd, 2012). For target validation Taqman probes (applied biosystem) were used in qRT-PCR.
Gene expression microarray
Expression profiling was undertaken with RNA extracted from three independent sets of cultured NTLV and ZKSCAN3 shRNA #3 UC13 cells using the Agilent 4X44k chip and described in detail in supplementary experimental procedures section.
Cell growth assays
MTT assay
Unless stated otherwise, 5,000 cells were plated in in triplicate in 25 cm2 flasks and allowed to grow for 5 days (unless indicated) at 37°C. Subsequently, at each time point, cells were treated with MTT solution ((3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide, 4 mg/ml) for 2 h. Next, MTT solution was removed; flasks were air dried in dark and equal volumes of DMSO added to each flask to solubilize intracellular purple formazan dye. Absorbance was measured at 570 nm.
Clonogenic assays
Cells (1,000) were seeded in 6-well plates and allowed to grow for three weeks. Cell colonies were fixed with methanol for 10 minutes at −20°C and stained with crystal violet (0.2%). Pictures were digitally captured.
β-hexosaminadase Assay
Cells (50–60% confluent) were trypsinized and washed with PBS. Equal numbers of cells (NTLV or ZKSCAN3-shRNA cells) were lysed using 200 µl of 0.1 % triton X-100 for 20 minutes and centrifuged at 10000 g and the supernatant harvested. 10 µl of 1mM p-nitrophenyl-N-acetyl-β-D-glucosaminide (p-NAG) was added to 10 µl of each clarified sample and incubated at 37 °C for 1 hr. At the end of incubation, 250 µl of 0.1M Na2CO3/NaHCO3 solution was added and absorbance read at 400 nm.
Senescence-associated β-gal staining
Senescence cells were identified using a senescence-associated B-gal assay kit (Cell signaling) as per the manufacturer’s instructions. Images were acquired by light microscopy (200× magnification).
Luciferase assays
Map1lc3b and Gabarapl2 promoter reporter constructs in pGL3 vector were kind gifts from Dr. Kenichi Yoshida (Meiji University, Japan). These luciferase reporter plasmids along with Renilla luciferase expression vector were transiently transfected in NTLV and ZKSCAN3-shRNA cells and after 48 h luciferase assay were performed using Dual Luciferase Reporter assay System as per the manufacture guidelines (Promega). Normalization for transfection efficiency was performed using Renilla luciferase values.
Supplementary Material
Highlights.
Silencing of ZKSCAN3 induces autophagy and lysosome biogenesis.
ZKSCAN3 transcriptionally regulates an extensive set of genes involved in autophagy.
ZKSCAN3 nuclear localization is regulated by nutrient availability.
ZKSCAN3 and TFEB regulate autophagy in opposite directions.
Acknowledgements
We are grateful to Dr. Ralph Nixon and Dr. Josef Mautner for providing the LC3-DsRed and GFP-Neo plasmids, respectively. We are greatful to Dr. Andrea Ballabio for providing TFEB overexpressing HeLa cells and Flag-TFEB plasmid. We acknowledge Mr. Kenneth Dunner Jr. at High Resolution Electron Microscopy Facility at UTMDACC for performing SEM and TEM. This work was supported by the National Institutes of Health grant (R01CA58311) to DDB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S, Boyd DD. Regulation of u-PAR gene expression by H2A.Z is modulated by the MEK-ERK/AP-1 pathway. Nucleic Acids Res. 2012;40:600–613. doi: 10.1093/nar/gkr725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C. p65/RelA modulates BECN1 transcription and autophagy. Mol Cell Biol. 2009;29:2594–2608. doi: 10.1128/MCB.01396-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC. The building BLOC(k)s of lysosomes and related organelles. Curr Opin Cell Biol. 2004;16:458–464. doi: 10.1016/j.ceb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Dulic V, Beney GE, Frebourg G, Drullinger LF, Stein GH. Uncoupling between phenotypic senescence and cell cycle arrest in aging p21-deficient fibroblasts. Mol Cell Biol. 2000;20:6741–6754. doi: 10.1128/mcb.20.18.6741-6754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa M. Cathepsin A/protective protein: an unusual lysosomal multifunctional protein. Cell Mol Life Sci. 1999;56:894–907. doi: 10.1007/s000180050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochl R, Hu XW, Chan EY, Tooze SA. Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic. 2006;7:129–145. doi: 10.1111/j.1600-0854.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longatti A, Tooze SA. Vesicular trafficking and autophagosome formation. Cell Death Differ. 2009;16:956–965. doi: 10.1038/cdd.2009.39. [DOI] [PubMed] [Google Scholar]
- Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, Kondo S, Kondo Y, Yu Y, Mills GB, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118:3917–3929. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wang X, Gao X, Wang L, Lu Y, Gao P, Deng W, Yu P, Ma J, Guo J, et al. Identification of five human novel genes associated with cell proliferation by cell-based screening from an expressed cDNA ORF library. Life Sci. 2007;81:1141–1151. doi: 10.1016/j.lfs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Mijaljica D, Prescott M, Devenish RJ. V-ATPase engagement in autophagic processes. Autophagy. 2011;7:666–668. doi: 10.4161/auto.7.6.15812. [DOI] [PubMed] [Google Scholar]
- Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- N'Diaye EN, Kajihara KK, Hsieh I, Morisaki H, Debnath J, Brown EJ. PLIC proteins or ubiquilins regulate autophagy-dependent cell survival during nutrient starvation. EMBO Rep. 2009;10:173–179. doi: 10.1038/embor.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TA, Zou L, Xie XJ, Corey DR, Brugarolas J. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 2011;30:3242–3258. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polager S, Ofir M, Ginsberg D. E2F1 regulates autophagy and the transcription of autophagy genes. Oncogene. 2008;27:4860–4864. doi: 10.1038/onc.2008.117. [DOI] [PubMed] [Google Scholar]
- Polson HE, de Lartigue J, Rigden DJ, Reedijk M, Urbe S, Clague MJ, Tooze SA. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy. 2010:6. doi: 10.4161/auto.6.4.11863. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O'Kane CJ, Brown SD, Rubinsztein DC. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet. 2005;37:771–776. doi: 10.1038/ng1591. [DOI] [PubMed] [Google Scholar]
- Renna M, Schaffner C, Winslow AR, Menzies FM, Peden AA, Floto RA, Rubinsztein DC. Autophagic substrate clearance requires activity of the syntaxin-5 SNARE complex. J Cell Sci. 2011;124:469–482. doi: 10.1242/jcs.076489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F, Krishnan J, Lerch R, Hall MN, Ruegg MA, et al. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- Singh K, Matsuyama S, Drazba JA, Almasan A. Autophagy-dependent senescence in response to DNA damage and chronic apoptotic stress. Autophagy. 2012;8:236–251. doi: 10.4161/auto.8.2.18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia R. KRAB-containing zinc-finger repressor proteins. Genome Biol. 2003;4:231. doi: 10.1186/gb-2003-4-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Hamilton SR, Sood A, Kuwai T, Ellis L, Sanguino A, Lopez-Berestein G, Boyd DD. The previously undescribed ZKSCAN3 (ZNF306) is a novel"driver" of colorectal cancer progression. Cancer Res. 2008a;68:4321–4330. doi: 10.1158/0008-5472.CAN-08-0407. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang L, Wu Q, Boyd DD. Unbiased screening for transcriptional targets of ZKSCAN3 identifies integrin beta 4 and vascular endothelial growth factor as downstream targets. J Biol Chem. 2008b;283:35295–35304. doi: 10.1074/jbc.M806965200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell'antonio G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011a;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Feng LQ, Zheng XX. Microtubule and kinesin/dynein-dependent, bidirectional transport of autolysosomes in neurites of PC12 cells. Int J Biochem Cell Biol. 2011b;43:1147–1156. doi: 10.1016/j.biocel.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavare S, Arakawa S, Shimizu S, Watt FM. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.