Abstract
Aims
To determine the relationship between foot ulcers, arterial calcification, and peripheral occlusive disease in patients with type 2 diabetes.
Methods
We performed a cross-sectional study on 162 patients with type 2 diabetes who underwent assessment of tibial artery calcification (TAC) by non-contrasted CT scan. Peripheral artery occlusive disease was assessed by angiography. Foot status including the presence or absence of ulcers was documented at presentation. A multivariable logistic regression model was used to evaluate the association between foot ulcers, arterial calcification, and the extent of peripheral atherosclerotic occlusive disease.
Results
Patients with foot ulcers (n= 31) were more likely to be older and have a history of tobacco use. They were also more likely to have higher TAC scores (median [IQR]: 4324.6 [609.9, 11163.6] vs. 9.4 [0.0, 343.9], P < 0.001) and more advanced peripheral artery occlusive disease (occlusion index 5.5 [4.8, 6.4] vs. 2.2 [1.0, 3.6], P < 0.001. Foot ulcer was strongly associated with elevated TAC scores in a multivariable regression model (Odds ratio [95% CI] =2.76 [1.61, 4.75], P=0.0002).
Conclusions
There is a strong association between arterial calcification and diabetic foot ulcers that persists after adjusting for the extent of atherosclerosis in patients with type 2 diabetes.
Keywords: diabetes, foot ulcer, artery calcification, atherosclerosis
Chronic, non-healing foot ulceration resulting in osteomyelitis or gangrene is the most common cause of amputation in patients with diabetes.(1) The annual incidence of diabetic foot ulcers is 1 to 4%, and lifetime risk is up to 25%.(2, 3) Diabetic foot ulcers significantly increase the cost of care and only aggressive foot ulcer prevention measures have been shown to decrease amputation rates.(4) Multiple factors are known to contribute to foot ulcer development including peripheral neuropathy, foot deformity, and peripheral artery disease.(5) Yet, the pathophysiology of foot ulcer development in patients with diabetes remains incompletely understood.
One aspect of diabetes that has been linked with poor limb outcomes is the presence of calcification in the arterial wall.(6) In the lower extremities, medial calcification predicts poor cardiovascular outcomes and increased risk of amputation. (6–8) Data from clinical investigations suggest that the relationship between arterial calcification and diabetic foot ulcers is mediated by its strong association with neuropathy.(9–11) Another possibility that must be considered, however, is that increased arterial calcification is associated with diabetic foot ulcers because it signifies extensive atherosclerosis in lower extremity arteries.
Peripheral artery occlusion is a component cause in the pathway to foot ulcer development, and it is a strong predictor of major amputation.(2, 12) In the coronary arteries, there is a strong but inexact association between coronary artery calcification and the extent of atherosclerosis.(13) The relationship between calcification in lower extremity arteries, peripheral artery occlusive disease, and diabetic foot ulcers, however, is not known. We therefore sought to determine whether elevated arterial calcification scores in patients with foot ulcers could be explained by concomitant atherosclerosis in patients with type 2 diabetes.
Research Design and Methods
Study population
Between January 2004 and December 2009, a total of 316 patients were recruited to participate in a prospective study on lower extremity arterial calcification from the vascular and diabetes clinics of Vanderbilt Medical Center and the Nashville Veterans’ Affairs Hospital. Clinic patients were asked to participate if they had evidence of peripheral atherosclerosis by exam and non-invasive testing. We also recruited a group of community volunteers without evidence of vascular disease to serve as controls and to obtain baseline measures of arterial calcification. Ethical approval was obtained from the Institutional Review Board and all patients provided written informed consent. Patients with a creatinine over 1.7 mg/dL were excluded in order to prevent possible confounding from CKD-related calciphylaxis as were patients with type 1 diabetes who were not included in the original study. Also excluded were patients who presented with acute limb ischemia, and those who had previously undergone major amputation, or more than two lower extremity revascularization procedures. From the original cohort, we were able to identified 162 patients with a history of diabetes for the present study.
Procedures
At the time of study entry, patients underwent a standard medical history that included specific questions about tobacco use, hyperlipidemia, hypertension, and diabetes. Patients were asked about symptoms of peripheral artery disease (PAD) including claudication, ischemic rest pain, and history of foot ulcers. Patients underwent evaluation of their lower extremity vascular status by pulse exam and non-invasive arterial Doppler testing. Foot examinations included assessment of pedal pulses, deformities, and ulcers. Skin lesions were evaluated according to the Wagner scale and any patient with a Wagner Grade 1 or greater foot ulcer at the time of initial study evaluation was considered positive.
Non-contrast computed tomography (CT) scans of the lower extremities were performed using standardized protocols on a single multi-slice CT scanner. All scoring was performed by investigators who were blinded to the results of the clinical assessment. Tibial artery calcium scoring was performed using standardized calcium scoring software as we have previously reported.(6) Briefly, areas of calcification along the tibial arteries with a cross sectional area greater than 1 mm2 and with a density of > 130 Hounsfield units (HU) were identified automatically. Measurements were started at the bottom of the patella and ended at the widest portion of the ankle malleoli. Calcium scores were determined as described by Agatston et al. (14). This method was chosen because it is currently in widespread use and because it provides a simple transition from calcium scoring in the heart to the leg.
To quantify the extent of atherosclerotic occlusive disease, we calculated a peripheral artery occlusion index using Rutherford’s system.(15) This strategy grades the amount of occlusive disease at each level on a scale of 1–10 where 1 denotes a widely patent artery and 10 denotes an occluded vessel. The method has been validated previously for lower extremity interventions (16–18) and it is independent of the method used to visualize arteries. Patients underwent CT angiography using 100 to 150 ml Visipaque contrast. For each leg, longitudinal and cross sectional images of the superficial femoral, popliteal, and tibial arteries were viewed and graded according to a standard scale(15). Numbers for each level and for both lower extremities were then averaged to give a single value for each patient that ranged from 1 – 10. For angiograms in patients with extensive calcification, contrasted scans were compared with the corresponding non-contrast scans at each level under digital magnification. This allowed confirmation of the patency status of each vessel. Selected scans were reviewed by two independent investigators who were blinded to the patient’s clinical status and each other’s findings to confirm the results.
Statistical analysis
Descriptive statistics were calculated as median with interquartile range (median [IQR]) for continuous variables, and frequency and proportion for categorical variables. Wilcoxon’s rank sum tests were used to compare continuous variables between patients with and without foot ulcer, and Pearson’s chi-square test to compare categorical variables. Spearman’s rank correlation coefficients (rho) were calculated to assess correlation between the tibial artery calcification (TAC) score and continuous clinical variables. Because the TAC score was known to be heavily skewed, relationships between it and clinical factors were further assessed using a proportional odds logistic regression model with age, race, sex, BMI, smoking status, hyperlipidemia and hypertension as covariates.
The effect of the TAC score and peripheral artery occlusion index on the presence of foot ulcer at study entry was examined by applying a binary logistic regression model with foot ulcer being a dependent variable and with adjustment for age, race, gender, BMI, smoking history, hyperlipidemia and hypertension. Because ulcer presence was quantified as a binary variable which has limited statistical power, we used the propensity score adjustment method to avoid over fitting. The propensity score was computed as predicted values of TAC score or occlusion index based on a linear regression as a function of the set of covariates including age, gender, race, BMI, smoking history, hyperlipidemia and hypertension. The final multivariable binary logistic regression was modeled separately for TAC score, and for occlusion index, where each variable was included as a main variable along with the corresponding propensity score. For propensity score computation, and also for inclusion of TAC as a main exposure variable in the final model, TAC scores were naturally logarithm-transformed to improve normality, and 1 unit was added to all patients’ TAC scores before transformation to avoid missing due to transforming zero.
Statistical analyses were performed using R version 2.10.0 (http://www.r-project.org). 2-sided P values less than 0.05 were considered statistically significant.
Results
The median and interquartile age for all patients was 57 (52, 65) years. There were 71 women (44%) and 122 Caucasians (75%). A history of tobacco use was present in 59%, hypertension was present in 77%, and 65% had hyperlipidemia (Table 1). The cohort included 131 patients without and 31 patients with a diabetic foot ulcer. Patients with and without foot ulcers were similar with regard to sex, race, hypertension, and hyperlipidemia. Compared to patients without foot ulcer, patients with foot ulcer were older (P< 0.001) had a lower in BMI (P = 0.01) and were more likely to have a history of tobacco use (P = 0.02). The TAC score was significantly higher among patients with ulcers (median (IQR): 4324.6 [609.9, 11163.6] vs. 9.4 [0.0, 343.9] (P<0.001), as was the amount of occlusive disease in lower extremity arteries as measured by CT angiography (peripheral occlusion index) 5.5 [4.8, 6.4] vs. 2.2 [1.0, 3.6], P < 0.001).
Table 1.
Characteristics of Study Subjects
| Characteristic | Total Population (N=162) | No Foot Ulcer (N=131) | Foot Ulcer (N=31) | P value* |
|---|---|---|---|---|
| Age, years | 57 (52, 65) | 56 (52, 64) | 66 (61, 77) | <0.001 |
| Sex, no. (%) female | 71 (44) | 62 (47) | 9 (29) | 0.065 |
| Race, no. (%) Caucasian | 122 (75) | 100 (76) | 22 (71) | 0.53 |
| BMI, kg/m2 | 30.5 (27.2, 34.7) | 31.1 (27.6, 35.0) | 27.4 (23.6, 31.7) | 0.01 |
| Tobacco use, no. (%) | 95 (59) | 71 (54) | 24 (77) | 0.02 |
| Hypertension, no. (%) | 124 (77) | 98 (75) | 26 (84) | 0.28 |
| Hyperlipidemia, no. (%) | 105 (65) | 85 (65) | 20 (65) | 0.97 |
| TAC score (Agatston units) | 58 (0, 1018) | 9.4 (0, 343.9) | 4324 (609, 11163) | <0.001 |
| Peripheral occlusion index | 2.9 (1.2, 5.0) | 2.2 (1.0, 3.6) | 5.5 (4.8, 6.4) | <0.001 |
Values are shown as median and interquartile ranges for continuous variables and percentages (%) for categorical variables. TAC = tibial artery calcification, BMI= Body Mass Index.
Wilcoxon’s rank sum test was used for comparing continuous variables, and percentages were compared using Pearson chi-square test in patients with and without foot ulcers.
The relationships between tibial artery calcification and clinical factors among patients with type 2 diabetes are presented in Table 2. The TAC score was positively correlated with age (rho=0.53, P < 0.001) and the peripheral artery occlusion index (rho=0.740, P < 0.001) while it was negatively correlated with BMI (rho=−0.33, P < 0.001). Elevated TAC scores were also observed among male patients (P<0.001) and those with a history of tobacco use (P<0.001). Age, gender, non-Caucasian race, and the occlusion index yielded significant associations after adjusting in a multivariable proportional odds model with TAC as the outcome.
Table 2.
Relationships between tibial artery calcification scores and clinical factors among patients with diabetes
| Characteristic | N | Corr Coef* | Median (IQR) | Unadjusted P value | Adj OR (95%CI) | Adj P value |
|---|---|---|---|---|---|---|
| Age, 10 years | 162 | 0.534 | <0.001 | 2.19 (1.51, 3.17) | <0.001 | |
| Gender | ||||||
| Male | 91 | 243 (10,3140) | <0.001 | 3.32 (1.66, 6.67) | <0.001 | |
| Female | 71 | 0 (0, 171.6) | ref: female | |||
| Race | ||||||
| Non-Caucasian | 40 | 233 (0, 4770) | 0.07 | 2.43 (1.15, 5.16 | 0.02 | |
| Caucasian | 122 | 30 (0, 728) | ref: Caucasian | |||
| Tobacco use | ||||||
| Yes | 95 | 212 (3, 3174) | <0.001 | 1.22 (0.63, 2.38) | 0.56 | |
| No | 67 | 0 (0, 216) | ref: non-smoker | |||
| Hypertension | ||||||
| Yes | 124 | 88 (0, 1194) | 0.28 | 1.28 (0.61, 2.71) | 0.51 | |
| No | 38 | 13 (0, 728) | ref: no HTN | |||
| Hyperlipidemia | ||||||
| Yes | 105 | 93 (0, 1150) | 0.20 | 1.71 (0.89, 3.28) | 0.11 | |
| No | 57 | 16 (0, 481) | ref: no hyperlip | |||
| BMI, 5 kg/m2 | 162 | −0.333 | <0.001 | 0.83 (0.65, 1.05) | 0.13 | |
| Periph Occl Index | 162 | 0.740 | <0.001 | 9.27 (4.68, 18.37) | <0.001 | |
Spearman rho is listed for continuous variables. For binary variables, TAC scores for each group are displayed as median (Interquartile range). Unadjusted P value from Wilcoxon rank sum test or Spearman’s correlation test. Adjusted P value from a multivariable proportional odds regression model with tibial artery calcification scores as outcome and age, BMI, race, gender, smoking history, hypertension, race, hyperlipidemia, and peripheral occlusion index as covariates.
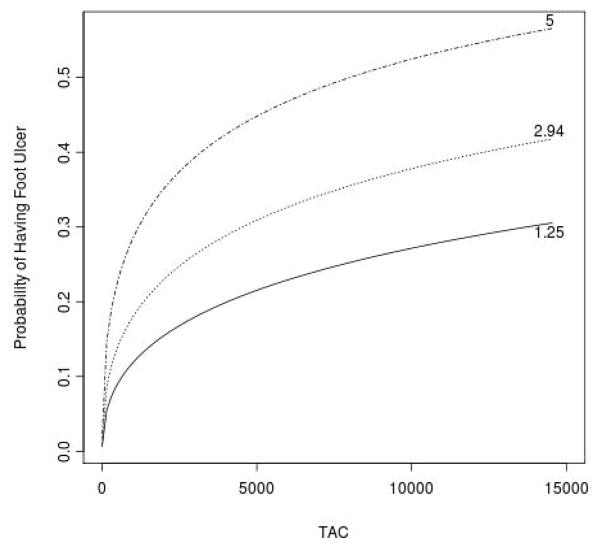
The effect of the TAC score and occlusion index on foot ulcer was examined by applying a binary logistic regression model with foot ulcer being the dependent variable and adjustment for age, race, gender, BMI, smoking history, hyperlipidemia and hypertension (Table 3). TAC scores were significantly elevated in foot ulcer patients after adjusting for cardiovascular risk factors and the peripheral artery occlusion index (odds ratio [95%CI]: 2.76 [1.61 to 4.75], P < 0.0002). The peripheral artery occlusion index was also significantly, but less strongly higher among patients with foot ulcer when included in a model with the TAC score (2.88 [1.21 to 6.82], P = 0.02). For a given amount of atherosclerotic occlusive disease (peripheral occlusion index 25th percentile = 1.25, median = 2.94, 75th percentile = 5.0) the probability of presenting with a foot ulcer increased as the TAC score increased. (Figure 1)
Table 3.
The adjusted effect of tibial artery calcification scores and peripheral occlusion index on foot ulcer.
| Characteristic | Odds Ratio (95%CI)* | P value |
|---|---|---|
| TAC score | 2.76(1.61, 4.75) | 0.0002 |
| Peripheral Occlusion Index | 2.88 (1.21, 6.88) | 0.02 |
Odds ratio for 1 unit increase. Age, sex, race, BMI, smoking history, hyperlipidemia and hypertension were adjusted through a propensity score method.
Figure 1.
(A). Box and whiskers plot (whiskers denote min and max) of TAC scores in patients without and with chronic foot ulcers at presentation. (B). Relationship between TAC score and the probability of having foot ulcer among patients in the twenty-fifth (1.25), median (2.94), and seventy-fifth (5.0) percentile of peripheral occlusion index.
Discussion
The main finding of this study is that there is an association between arterial calcification and diabetic foot ulcer that is independent of peripheral artery occlusion. This suggests that foot ulcers may be more common in patients with increased calcium scores because they have a higher incidence of neuropathy or through other yet unknown mechanism. Multiple factors are known to contribute to diabetic foot ulcer development and progression. Neuropathy, trauma, foot deformity, edema, and PAD have been shown to have contributive effects.(5) It is thought that neuropathy, though its ability to decrease protective sensation, and arterial occlusion, though its resultant decrease in pedal perfusion, allow for the development and progression of ulcers to a point where complete healing is not possible.
Another possibility that has been widely proposed is that calcification in lower extremity arteries increases ulcer risk through its association with atherosclerotic occlusive disease. (8, 19–23) From this point of view, when arterial calcification is seen on x-rays, it can be viewed as a marker of extensive vascular occlusion. If this is correct, we would expect the association between calcification and foot ulcers to weaken by including atherosclerosis in the model. Our multivariable analysis, however, shows both the TAC score and occlusion index continue to maintain a strong and independent association with foot ulcers after adjusting for each other and cardiovascular risk markers. This suggests that calcification does not contribute to foot ulcer development through associated atherosclerotic occlusive disease.
The potential physiologic mechanisms that may link arterial calcification with limb events are currently under investigation. It is possible if not likely that the association between calcification and ulcers reflects concomitant neuropathy. Calcification in foot arteries was more severe in patients with neuropathy and foot ulcers than in those without.(9, 10) And in a longitudinal study, abnormal motor nerve conduction velocity predicted new foot ulceration and death best over a 6-year follow-up period whereas medial artery calcification was a better predictor of amputation, a consequence of ulceration.(11) A recent report by Moon et al, however, suggests that medial artery calcification and diabetic polyneuropathy may not be as strongly correlated as previously believed.(24) Further efforts to understand this relationship are warranted. Calcification may also contribute to decreased pedal perfusion by increasing arterial stiffness and evidence that decreased compliance can decrease muscle perfusion has been presented recently.(25) It also remains possible that calcification may be related to ulcers because of its association with increased systemic inflammation or skin perfusion defects or via effects on the microcirculation. Further efforts in this regard are needed.
Our study has several limitations. First, we employed a cross sectional analysis of patients with type 2 diabetes comparing those with and without foot ulcers. We cannot, therefore, make predictive statements about calcification and foot ulcer development. We have not included all potential confounding variables in our analysis. In particular, we did not include neuropathy, pedal edema, foot deformity, proteinuria, or previous ulceration. The relationship between arterial calcification and foot ulcer has previously been noted, and a complete assessment of the factors that contribute to their development was not our intent. The patient population studied was derived, in part from a referral population for vascular care and as such, it may represent a subpopulation with more extensive atherosclerosis. However, we did not exclude any patient that met enrollment criteria which were broad. This allowed us to obtain a patient population with more peripheral artery disease and, likely, more calcification.
Based on these data, we believe that the clinical value of tibial artery calcification scoring may be in the identification of a group of high risk, vulnerable patients with type 2 diabetes who deserve more aggressive foot protection efforts. It is also hoped, however, that future clinical efforts to reduce arterial calcification, either through risk factor modification or pharmacologic intervention, may prevent foot ulcer development and its consequences.
Our findings reveal an association between arterial calcification and diabetic foot ulcers that is independent of the extent of peripheral atherosclerosis. Further efforts to determine whether calcification may independently contribute to foot ulcer development are needed, as are attempts to prevent amputation in our patients with diabetes.
Acknowledgments
We would like to acknowledge the following sources of funding: NIH DK067368 and HL105641 (RJG) and P60 AR056116 (CMS). No potential conflicts of interest relevant to this article are reported.
Abbreviations
- TAC
tibial artery calcification
- PAD
peripheral artery disease
- CT
computed tomography
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Contribution Statement
RG designed the study, analyzed the data, and wrote the manuscript. AB and SA performed the statistical analysis and contributed to the writing of the manuscript. CMS reviewed the manuscript and contributed to the design, presentation, and discussion of the data. RG is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 2.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13(5):513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 3.Singh N, Armstrong DG, Lipsky BA. Preventing Foot Ulcers in Patients With Diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 4.Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52(3 Suppl):17S–22S. doi: 10.1016/j.jvs.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Boulton AJM. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes/Metabolism Research and Reviews. 2008;24(S1):S3–S6. doi: 10.1002/dmrr.833. [DOI] [PubMed] [Google Scholar]
- 6.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RMJ, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;51(20):1967–1974. doi: 10.1016/j.jacc.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayfield JA, Caps MT, Boyko EJ, Ahroni JH, Smith DG. Relationship of medial arterial calcinosis to autonomic neuropathy and adverse outcomes in a diabetic veteran population. J Diabetes Complications. 2002;16(2):165–71. doi: 10.1016/s1056-8727(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 8.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978–83. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 9.Corbin DO, Young RJ, Morrison DC, Hoskins P, McDicken WN, Housley E, Clarke BF. Blood flow in the foot, polyneuropathy and foot ulceration in diabetes mellitus. Diabetologia. 1987;30(7):468–73. doi: 10.1007/BF00279614. [DOI] [PubMed] [Google Scholar]
- 10.Jones EW, Peacock I, McLain S, Fletcher E, Edwards R, Finch RG, Jeffcoate WJ. A clinico-pathological study of diabetic foot ulcers. Diabet Med. 1987;4(5):475–9. doi: 10.1111/j.1464-5491.1987.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 11.Carrington AL, Shaw JE, Van Schie CH, Abbott CA, Vileikyte L, Boulton AJ. Can motor nerve conduction velocity predict foot problems in diabetic subjects over a 6-year outcome period? Diabetes Care. 2002;25(11):2010–5. doi: 10.2337/diacare.25.11.2010. [DOI] [PubMed] [Google Scholar]
- 12.Reiber GE, Vileikyte L, Boyko EJ, del Aguila M, Smith DG, Lavery LA, Boulton AJ. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22(1):157–62. doi: 10.2337/diacare.22.1.157. [DOI] [PubMed] [Google Scholar]
- 13.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31(1):126–33. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 16.Davies MG, Saad WE, Peden EK, Mohiuddin IT, Naoum JJ, Lumsden AB. Impact of runoff on superficial femoral artery endoluminal interventions for rest pain and tissue loss. J Vasc Surg. 2008;48(3):619–25. doi: 10.1016/j.jvs.2008.04.013. discussion 625–6. [DOI] [PubMed] [Google Scholar]
- 17.Biancari F, Alback A, Ihlberg L, Kantonen I, Luther M, Lepantalo M. Angiographic runoff score as a predictor of outcome following femorocrural bypass surgery. Eur J Vasc Endovasc Surg. 1999;17(6):480–5. doi: 10.1053/ejvs.1999.0825. [DOI] [PubMed] [Google Scholar]
- 18.Toursarkissian B, D’Ayala M, Stefanidis D, Shireman PK, Harrison A, Schoolfield J, Sykes MT. Angiographic scoring of vascular occlusive disease in the diabetic foot: relevance to bypass graft patency and limb salvage. J Vasc Surg. 2002;35(3):494–500. doi: 10.1067/mva.2002.120046. [DOI] [PubMed] [Google Scholar]
- 19.An WS, Son YK, Kim SE, Kim KH, Yoon SK, Bae HR, Rha SH. Vascular calcification score on plain radiographs of the feet as a predictor of peripheral arterial disease in patients with chronic kidney disease. Int Urol Nephrol. 2010;42(3):773–80. doi: 10.1007/s11255-009-9697-8. [DOI] [PubMed] [Google Scholar]
- 20.Ohtake T, Oka M, Ikee R, Mochida Y, Ishioka K, Moriya H, Hidaka S, Kobayashi S. Impact of lower limbs’ arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J Vasc Surg. 2011;53(3):676–83. doi: 10.1016/j.jvs.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 21.Parr A, Buttner P, Shahzad A, Golledge J. Relation of infra-renal abdominal aortic calcific deposits and cardiovascular events in patients with peripheral artery disease. Am J Cardiol. 2010;105(6):895–9. doi: 10.1016/j.amjcard.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 22.Chiu YW, Adler S, Budoff M, Takasu J, Ashai J, Mehrotra R. Prevalence and prognostic significance of renal artery calcification in patients with diabetes and proteinuria. Clin J Am Soc Nephrol. 2010;5(11):2093–100. doi: 10.2215/CJN.03730410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monreal M, Alvarez L, Vilaseca B, Coll R, Suarez C, Toril J, Sanclemente C. Clinical outcome in patients with peripheral artery disease. Results from a prospective registry (FRENA) Eur J Intern Med. 2008;19(3):192–7. doi: 10.1016/j.ejim.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Moon J-S, Clark VM, Beabout JW, Swee RG, Dyck PJ. A Controlled Study of Medial Arterial Calcification of Legs: Implications for Diabetic Polyneuropathy. Arch Neurol. 2011;68(10):1290–1294. doi: 10.1001/archneurol.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahimastos AA, Dart AM, Lawler A, Blombery PA, Kingwell BA. Reduced arterial stiffness may contribute to angiotensin-converting enzyme inhibitor induced improvements in walking time in peripheral arterial disease patients. J Hypertens. 2008;26(5):1037–42. doi: 10.1097/HJH.0b013e3282f8e3b6. [DOI] [PubMed] [Google Scholar]