Abstract
Toxoplasma gondii is an obligate intracellular parasite and the causative agent of toxoplasmosis. Protein palmitoylation is known to play roles in signal transduction and in enhancing the hydrophobicity of proteins thus contributing to their membrane association. Global inhibition of protein palmitoylation has been shown to affect T. gondii physiology and invasion of the host cell. However, the proteins affected by this modification have been understudied. This paper shows that the small heat shock protein 20 from T. gondii (TgHSP20) is synthesized as a mature protein in the cytosol and is palmitoylated in three cysteine residues. However, its localization at the inner membrane complex (IMC) is dependent only on N-terminal palmitoylation. Absence or incomplete N-terminal palmitoylation causes TgHSP20 to partially accumulate in a membranous structure. Interestingly, TgHSP20 palmitoylation is not responsible for its interaction with the daughter cells IMCs. Together, our data describe the importance of palmitoylation in protein targeting to the IMC in T. gondii.
Keywords: Toxoplasma gondii, small heat shock protein 20, palmitoylation, inner membrane complex, endodyogeny
1. Introduction
Fatty acylation of proteins is a widespread feature of eukaryotic cells. This post-translational modification (PTM) plays a key role in membrane localization of signal-transducing proteins [1, 2] as well as in regulation of mitochondrial metabolism [3, 4]. Fatty acylation includes myristoylation and palmitoylation. Whereas myristoylation refers to the irreversible attachment of 14-carbon myristate in a co- [5, 6] or post- [7–10] translational manner, palmitoylation is the post-translational attachment of the 16-carbon fatty acid palmitate onto cysteine residues via a labile thioester bond. Palmitate is transferred onto proteins enzymatically [11–13] or in a spontaneous manner [4, 14] with both mechanisms being operational in cells.
Apicomplexan parasites are a large group of obligate intracellular parasites that include Eimeria spp., Cryptosporidium spp., Neospora spp., Plasmodium spp., Theileria spp. and T. gondii. Protein palmitoylation has been described to operate in E. tenella [15], P. falciparum [16–19] and T. gondii [15, 20, 21]. There are several proteins known or predicted to be palmitoylated in T. gondii [22]. In general, protein palmitoylation is important for proper membrane localization of the modified protein. In this regard, it has been described that mutation of the predicted palmitoylation sites in T. gondii inner membrane complex sub-compartment protein 4 (ISP4) and calcium-dependent protein kinase 3 (CDPK3), alters their localization from the pellicle to the cytosol [23–25]. We recently described the functional significance of this modification in T. gondii, where inhibition of protein palmitoylation was able to interfere with the parasite’s physiology and host-cell invasion [20]. Interestingly, the host-cell invasion process depends on an actin- and myosin-based complex located at the pellicle, between the plasma membrane (PM) and inner membrane complex (IMC)[26]. The same effect on host-cell invasion was observed in P. falciparum [27]. In general, these results underscore an important role for palmitoylation of IMC-associated proteins.
T. gondii small heat shock protein 20 (TgHSP20) displays interesting characteristics which could be used to study the role and dynamics of protein palmitoylation. TgHSP20 localizes to the parasite IMC in a set of discontinuous stripes [28]. Recombinant TgHSP20 forms multimers [29], binds phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5 biphosphate phospholipids [28] and has chaperone activity in vitro [29]. It has been reported that antibodies against HSP20 reduce host-cell invasion by B. divergens, T. gondii and N. caninum [30, 31]. In Plasmodium, deletion of the hsp20 gene results in altered sporozoite speed and substrate adhesion leading to an impaired natural malaria transmission in the host [32, 33]. Although HSP20 is predicted to be synthesized in the cytosol, it localizes to the IMC in T. gondii [28], P. falciparum [32] and to the pellicle in Babesia [34]. Moreover, in T. gondii HSP20 is incorporated to the IMC at intermediate stages of daughter cell formation [28]. Here, we confirmed that all the TgHSP20 cysteine residues are palmitoylated in vivo but only N-terminal palmitoylation is necessary for localization at the IMC. Interestingly, palmitoylation was not necessary for the interaction with the IMC of daughter cells during budding, as a non-palmitoylable version of TgHSP20 localized to the IMC along the daughter cell development. Finally, we discuss a possible model of palmitoylation events and sub-cellular localization of T. gondii HSP20 to the IMC.
2. Materials and methods
2.1 Antibodies and reagents
Specialized and common reagents were from Sigma, unless specified. [9,10-3H]-palmitic acid and [35S]-methionine/[35S]-cysteine were from PerkinElmer Life and Analytical Sciences. ECL Plus was from GE Biosciences. Alexa-conjugated secondary antibodies were from Molecular Probes. Tissue culture reagents were from Invitrogen. The serum anti-Ty was kindly provided by Dr Dubremetz (Université de Montpellier, France), anti-IMC1 antibody was generously provided by Dr Ward (University of Vermont, USA) and anti-SAG1 antibody was kindly provided by Dr. Marina Clemente (Universidad de San Martin, Argentina).
2.2 Toxoplasma and host-cell cultures
T. gondii tachyzoites of the RH Δhxgprt strain [35] were used throughout the study. Parasites were maintained by serial passage on confluent monolayers of human foreskin fibroblasts (HFFs) in Dulbecco’s Modified Eagle Medium (DMEM; Gibco BRL) supplemented with 10% v/v bovine serum albumin (BSA), 100 i.u. (international units)/ml penicillin and 100 μg/ml streptomycin (Gibco BRL).
2.3 Metabolic labeling with [3H]-palmitic acid and immunoprecipitation
Freshly lysed (109) tachyzoites were purified using a 3 μm polycarbonate filter and incubated for 4 h with 100 μCi of [9,10-3H]-palmitic acid previously conjugated to BSA fatty acid free (1:1, mol: mol ratio). Then, the cells were washed twice with PBS and finally resuspended in 2 ml of immunoprecipitation buffer (IP buffer; 20 mM Tris-HCl, 150 mM NaCl, 1% TX-100 and Complete protease inhibitor cocktail-Roche, pH 7.4). After incubation for 2 h with rotation at 4°C, the lysate was centrifuged at 10,000 x g for 10 min at 4°C, the pellet was discarded and 5 μl of the appropriate antiserum was added to the supernatant (rabbit anti-HSP20, rabbit preimmune or mouse anti-Ty, mouse preimmune). Protein A/G Plus sepharose (Santa Cruz Biotechnology) was then added (50 μl of slurry previously equilibrated in IP buffer) and incubated 16–18 h with rotation at 4°C. The immunocomplexes were washed three times with IP buffer and finally resuspended in SDS-PAGE loading buffer without any reducing agent. Each sample was divided in three: one gel was transferred to a PVDF membrane and western blotted for the proteins (loading control). The other two gels were subjected to hydroxylamine treatment and its control.
2.4 Western blot analysis
A SDS-PAGE gel was used as a loading control of the immunoprecipitation. The gel was electrophoresed at 100 V for 1 h onto a PVDF membrane and blocked in 5% w/v skim milk in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) for 1 h. Then rabbit anti-HSP20 (1/1,000) or mouse anti-Ty (1/500) serum was added and incubated for another hour followed by washes in PBS, PBS-T (PBS with 0.1% v/v Tween-20) and PBS. Secondary antibodies conjugated to HRP were added (1/10,000), incubated for 1 h followed by washes in PBS, PBS-T and PBS. Finally proteins were immunodetected by ECL Plus (GE healthcare).
2.5 Hydroxylamine treatment
Gels were treated with either 1M Tris-HCl pH 7.0 (control) or with 1M NH2OH-NaOH pH 7.0 for 48 h, changing for fresh solutions every 12 h. Then the gels were stained with Coomassie Brillant Blue, destained and incubated for 30 min in ddH2O. Finally the gels were incubated for 30 min in 1M sodium salicylate pH 6.5, dried for 2 h at 80°C using a gel dryer machine and exposed to autoradiographic film typically for a month.
2.6 In silico analyses
Analysis of HSP20 amino acid sequence in Apicomplexa was performed using Toxoplasma gondii (TgHSP20; NCBI accession number AAT66039), Neospora caninum (NcHSP20; NCBI accession number CD537778), Eimeria tenella (EtHSP20; NCBI accession number AI758032), Plasmodium falciparum (PfHSP20; NCBI accession number CAD51208), Plasmodium berghei (PbHSP20; NCBI accession number AAL07529), Plasmodium vivax (PvHSP20; NCBI accession number XP_001615006), Plasmodium yoelii yoelii (PyyHsp20; NCBI accession number EAA16935), Babesia bigemina (BbiHS20; NCBI accession number AAK11630), Babesia bovis (BbiHSP20; NCBI accession number AAK11624) and Theileria parva (TpHSP20; NCBI accession number XP_763804). BioEdit software version 4.0 (Ibis Biosciences) was used for multiple alignment and sequence analysis.
2.7 Treatment with 2-bromopalmitate
A monolayer of HFF cells was infected with T. gondii in a 24-well dish. After 10 min on ice, the parasites were allowed to invade for 1 h at 37°C. Then, the cells were washed twice with PBS and the media was changed with a mix of 1% v/v of 100 μM BSA fatty acid free and 2-bromopalmitate respectively (final concentrations). The infected cells were incubated for 16–18 h at 37°C then were subjected to indirect immunofluorescence studies.
2.8 Indirect immunofluorescence studies
All the steps were carried out at room temperature. Media was discarded and the cells were washed twice with PBS. Cells were fixed with formaldehyde 4% v/v in PBS for 30 min followed by a 1 min wash in PBS. Then the cells were permeabilized with 0.3% v/v Triton X-100 in PBS for 20 min and blocked with 3% w/v BSA in PBS for 30 min. After this, appropriate primary antibodies were added diluted in 3% w/v BSA in PBS for 60 min followed by extensive washes. Anti-species secondary antibodies were added and incubated for another 60 min followed by extensive washes. Finally the samples were mounted with Fluoromont (Abcam). The primary antibodies used were: mouse monoclonal anti-Ty (1/200), rabbit anti-IMC1 (1/1,000), mouse anti-IMC1 (1/2,000), rabbit anti-HSP20 (1/1,000). Parasites were imaged using a Delta Vision deconvolution microscope (Applied Precision).
2.9 Generation of TgHSP20 mutants
To generate the TgHSP20-FL-Ty mutant version, the TgHSP20 ORF was amplified as previously described [28]. To change cysteine in position 3 to serine in TgHSP20, we used the following forward primer: 5′-AGATCTATGAGTTCCTGTGGCGGTAC -3′ (altered nucleotide underlined), to change cysteine 4 to serine: 5′-AGATCTATGAGTTGCTCTGGCGGTAC -3′ and to change cysteines 3 and 4 to serines: 5′-AGATCTATGAGTTCCTCTGGCGGTAC-3′. In order to replace cysteine in position 160 to serine we used the QuikChange® II XL site-directed mutagenesis kit (Agilent Technologies), using as a forward primer 5′-GTTGGAGGTTAAAATCTCCTCCATTCAAACC-3′ and the reverse primer 5′-AGGAGATTTTAACCTCCAACAATCCATTTG-3′. The plasmid ptubHSP20FL-Ty was used as template to generate ptubHSP20C160S-Ty. The plasmid ptubHSP20C3,4s-Ty was used as template to generate TgHSP20C3,4,160S-Ty. Mutations were verified by sequencing and 50 μg of the wild type and each mutagenized vector was transfected into RHΔhpt parasites. The transfected populations were selected under xanthine and mycophenolic acid pressure and cloned by limiting dilution, as previously described [36].
2.10 Transmission electron microscopy analysis
Infected monolayers were rinsed with 2% glutaraldehyde in 0.1 M Na-phosphate buffer pH 7.4. Intracellular parasites were then pelleted for 2 min at full speed and floated in fresh fixative for 90 min. After two washes in phosphate buffer, cells were fixed using 1% osmium tetroxide in phosphate buffer during 1 h at RT. After washing in ddH2O twice, the sample was dehydrated in ethanol and embedded in epon resin containing propylene oxide. Thin sections were obtained with a Leica ultramicrotome and collected in formvar-coated single hole grids.
3. Results
3.1 HSP20 is palmitoylated in T. gondii and the predicted palmitoylation sites are conserved across the phylum
TgHSP20 localizes to the IMC (Figure S1A; [28]). However, its amino acid sequence neither contains a signal peptide (http://www.cbs.dtu.dk/services/SignalP), nor a predicted hydrophobic region or a transmembrane domain (http://www.vivo.colostate.edu/molkit/hydropathy). In addition, there is no evidence for maturation (Figure S1B) that could explain its IMC localization. Hence, we decided to study whether post-translational modifications could account for its localization. In silico analysis shows that TgHSP20 could be subjected to several post-translational modifications (PTM) including phosphorylation, glycosylation with O-linked N-acetylglucosamine and palmitoylation (Figure S1C). Of all these PTM, palmitoylation is the only modification that could facilitate TgHSP20 localization to the IMC. T. gondii HSP20 contains three cysteine residues at positions 3, 4 and 160 (C3, C4 and C160) all of which are predicted to be palmitoylated (Figure S1C).
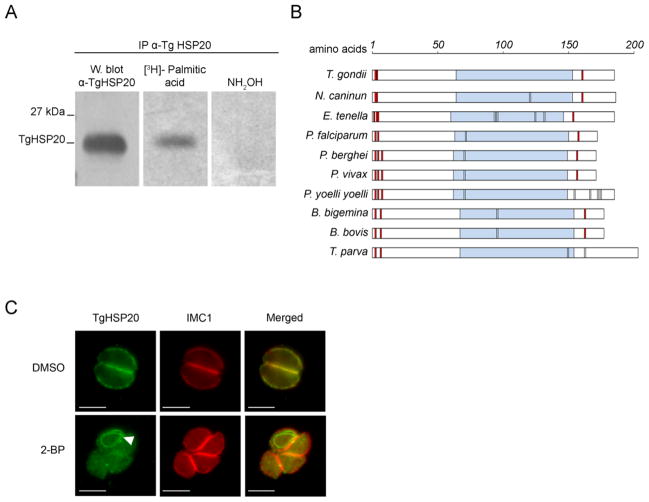
In order to experimentally confirm whether TgHSP20 is palmitoylated, we performed metabolic labeling of tachyzoites with [3H]-palmitic acid followed by immunoprecipitation. Figure 1A shows that this protein is palmitoylated “in vivo”. Furthermore, TgHSP20 radiolabel was removed by neutral hydroxylamine treatment, confirming that the palmitic acid is bound to TgHSP20 through a labile thioester bond. It is important to highlight that the three cysteine residues found in TgHSP20 predicted to be palmitoylated, are conserved in Apicomplexa (Figure 1B). These results could suggest that palmitoylation may be important for TgHSP20 localization and/or function in Apicomplexan parasites.
Figure 1. TgHSP20 is palmitoylated in vivo and this modification is responsible for its IMC localization.
A) TgHSP20 is palmitoylated in vivo. Metabolic labeling of wild type T. gondii parasites using [3H]-palmitic acid followed by specific immunoprecipitation with anti-TgHSP20 antibody. After the immunoprecipitation, the sample was divided in three and run on SDS-PAGE: one sample was used for western blotting as a loading control. The two remaining samples were used to detect the radiolabeled HSP20 as indicated in Materials and Methods. B) Schematic representation of Apicomplexan HSP20 amino acid sequences. The analysis includes the amino acid sequences of HSP20 from Toxoplasma gondii (TgHSP20; NCBI accession number AAT66039), Neospora caninum (NcHSP20; NCBI accession number CD537778), Eimeria tenella (EtHSP20; NCBI accession number AI758032), Plasmodium falciparum (PfHSP20; NCBI accession number CAD51208), Plasmodium berghei (PbHSP20; NCBI accession number AAL07529), Plasmodium vivax (PvHSP20; NCBI accession number XP_001615006), Plasmodium yoelii yoelii (PyyHSP20; NCBI accession number EAA16935), Babesia bigemina (BbiHSP20; NCBI accession number AAK11630), Babesia bovis (BbiHSP20; NCBI accession number AAK11624) and Theileria parva (TpHSP20; NCBI accession number XP_763804). Light-blue boxes indicate α-crystallin domain. Red boxes represent cysteine residues predicted to be palmitoylated. Black lines depict cysteine residues. C) TgHSP20 depends on palmitoylation for IMC localization. Intracellular parasites were treated for 16–18 h with either 100 μM 2-bromopalmitate (2-BP) or DMSO and then TgHSP20 localization was analyzed by indirect immunofluorescence. The localization of the protein IMC1 was unaffected by the treatment. An unknown structure stained with TgHSP20 antibodies is shown in cells treated with 2-BP (arrowhead). Scale bars = 5 μm.
3.2 Palmitoylation of HSP20 is required for proper localization to the IMC
Since palmitoylation has been shown to localize soluble proteins to membranes, we tested whether protein palmitoylation was responsible for TgHSP20 localization to the IMC. In order to do this, intracellular tachyzoites were incubated with the palmitoylation inhibitor 2-bromopalmitate (2-BP; [37]). Figure 1C shows that TgHSP20 changes localization from the IMC to the cytosol and formed an unidentified structure after 2-BP treatment (white arrowhead), suggesting that TgHSP20 is anchored to the IMC by palmitoylation.
3.3 TgHSP20 is palmitoylated in all its cysteine residues
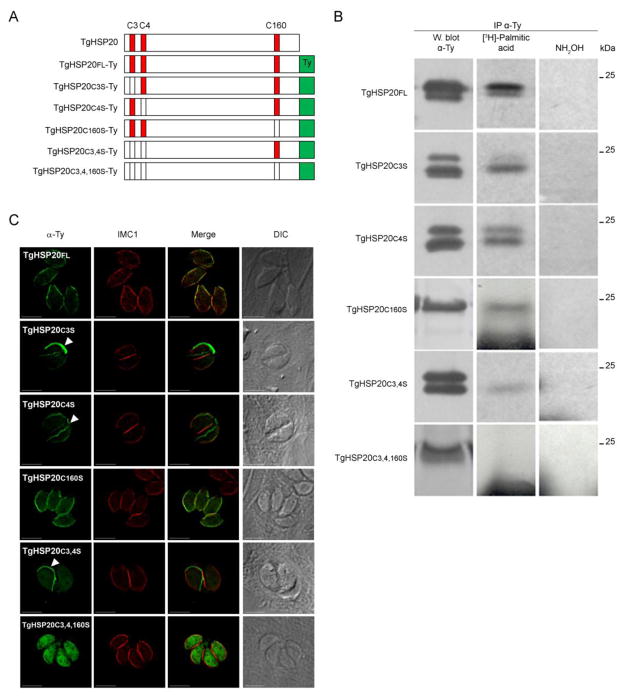
In order to determine which residue/s is/are palmitoylated, different additional copies of HSP20 in which each putative palmitoylated cysteine residue (C3, C4 and C160) were changed to serines as indicated in the scheme depicted in Figure 2A. These mutated genes were cloned into a Toxoplasma expression vector containing a Ty-tag and expressed in tachyzoites. These tachyzoites were metabolically labeled with [3H]-palmitic acid followed by immunoprecipitation with an anti-Ty antibody. Western blot analyses of the immunoprecipitated proteins are shown in Figure 2B. The endogenous TgHSP20 was visualized as a single band in Western blots using anti-TgHSP20 antibody (Figures 1A and S2). However, some of the Ty-constructs appeared as a doublet when using the anti-Ty antibody (Figure 2B and S2) in which the lower band co-migrates with the endogenous TgHSP20 (Figure S2). Mass spectrometry analysis of these bands confirmed that both bands corresponded to TgHSP20 (data not shown). These two bands may correspond to different post-translational modifications or structural differences of the protein. Nevertheless, more studies are required to elucidate the nature of the observed doublet of the TgHSP20-Ty constructs. The expression of all constructs produced proteins with some level of palmitoylation, with the exception of TgHSP20C3,4,160S-Ty (Figure 2B, lower panel). This result shows that TgHSP20 is palmitoylated on all of its cysteine residues. Furthermore, neutral hydroxylamine treatment removed the radiolabel from all the constructs, demonstrating that the lipid attachment was due to a labile thioester bond (Figure 2B).
Figure 2. TgHSP20 is palmitoylated in all its cysteine residues but only N-terminal palmitoylation determines TgHSP20 localization to the IMC.
A) Schematic representation of all Ty-tagged constructs generated and used in this study. The red boxes indicate the putative palmitoylated cysteine residues. B) T. gondii HSP20 is palmitoylated on all of its cysteine residues. Stable cell-lines over-expressing the different Ty-tagged constructs were metabolically labeled with [3H]-palmitic acid followed by anti-Ty immunoprecipitation. Then, the samples were divided in three and run on SDS-PAGE: one sample was used for Western blot analysis as a loading control. The two remaining samples were used to detect if the radiolabel was incorporated to the protein. C) Palmitoylation of cysteines 3 and 4 is important to confer IMC localization. Intracellular tachyzoites overexpressing the different Ty-tagged constructs were fixed and the localization of the constructs was determined by indirect immunofluorecence assays. Differential interference contrast (DIC) microscopy is shown on the right. White arrowheads indicate a TgHSP20-postive unidentified structure. Scale bars= 5 μm..
3.4 N-terminal palmitoylation determines TgHSP20 localization
In order to establish the role of palmitoylation of each cysteine residue on the localization of TgHSP20, we analyzed the sub-cellular localization of the different Ty-tagged TgHSP20s. Figure 2C shows that the TgHSP20FL-Ty construct localized to the IMC, as previously reported for the endogenous TgHSP20 [28]. Interestingly, stable lines expressing TgHSP20C3S-Ty and TgHSP20C4S-Ty mutants also showed a faint IMC localization and a strong signal in an unidentified structure (Figure 2C, arrowheads). This structure was also observed when tachyzoites were treated with 2-BP (Figure 1C, arrowhead). Of note, this unidentified structure did not react against IMC3 or SAG1 by indirect immunofluorescence (data not shown). TgHSP20C3,4S-Ty localized to the cytosol and displayed a very intense signal in the unidentified structure (indicated with an arrowhead). The non-palmitoylable form of TgHSP20 (TgHSP20C3,4,160S-Ty) displayed a cytosolic localization without the presence of the unidentified structure. Localization of TgHSP20C160S-Ty is similar to that of full length TgHSP20 (Figure 2C).
In order to study the nature of the TgHSP20-positive unidentified structure observed in some mutant lines, a transmission electron microscopy analysis was performed with tachyzoites stably expressing TgHSP20C3,4S-Ty. The image suggests the presence of some accumulation of membranes within the parasite (Figure 3; [38]).
Figure 3. Transmission electron microscopy of the TgHSP20C3,4S-Ty construct suggests the presence membrane accumulation.
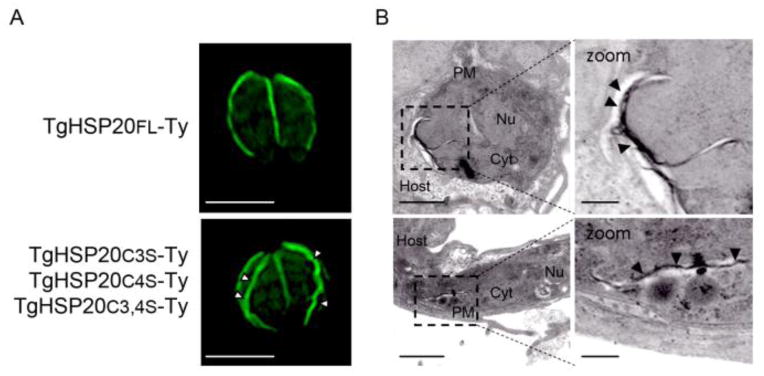
Lack or partial palmitoylation at the N-terminal of TgHSP20 forms an unidentified structure. A) Immunofluorescence assay showing the sub-cellular localization of full-length TgHSP20-Ty (TgHSP20FL-Ty; upper panel) or parasites over-expressing different N-terminal palmitoylable TgHSP20 constructs (same result either with TgHSP20C3S-Ty, TgHSP20C4S-Ty or TgHSP20C3,4S-Ty; lower panel). Scale bars= 5 μm. B) Transmission electron microscopy of the parasites observed in panel A. The white arrowheads show the atypical localization of TgHSP20 with indirect immunofluorescence and black arrowheads in the electron microscopy analysis. PM: plasma membrane; Nu: nucleus; Cyt: cytoplasm. Scale bars= 1 μm and 200 nm for the zoomed images.
3.5 Palmitoylation is not required for localization of TgHSP20 in the IMC of daughter cells
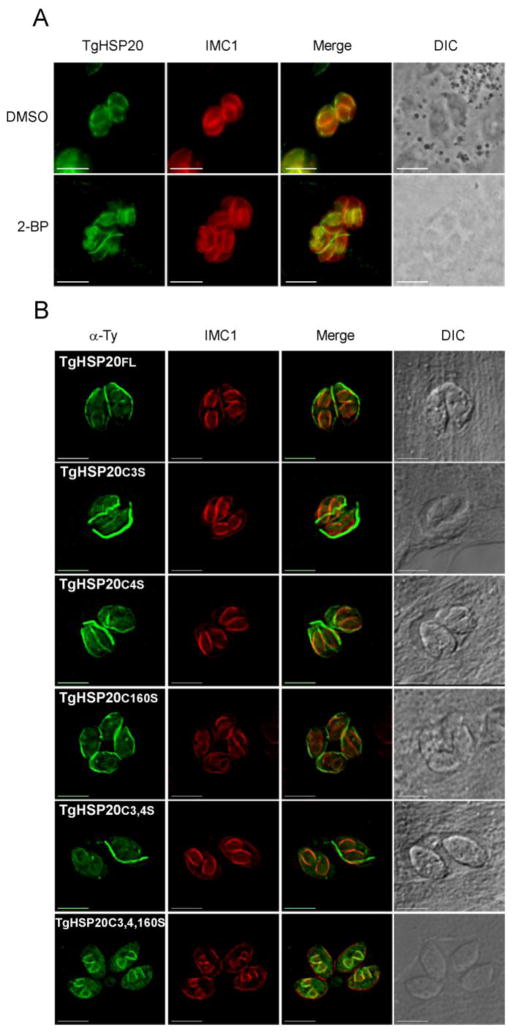
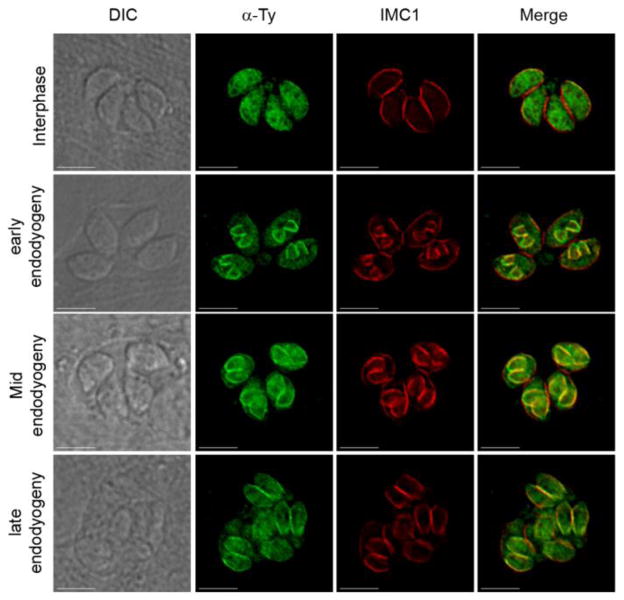
Apicomplexan parasites divide by internal budding into a variable number of daughter cells. T. gondii divides by the simplest mode in the phylum, endodyogeny, and assembles two daughter cells every round of replication. Prior to cytokinesis, daughter cells are dotted with a fully formed pellicle, which includes the IMC [39]. In order to determine how palmitoylation modulates TgHSP20 targeting to the IMC of daughter cells, intracellular replicating parasites were treated with 2-BP and analyzed by indirect immunofluorescence microscopy with anti-HSP20 antibodies. Interestingly, palmitoylation seems not to play a role on TgHSP20 attachment to the IMC of daughter cells (Figure 4A). To confirm this result, stable lines expressing different palmitoylable versions of TgHSP20 were analyzed for their sub-cellular localization in replicating parasites. All the mutant lines lysed the infected monolayer at a similar time than the parental cell-line, not showing any evident phenotype associated to parasite growth (data not shown). All the Ty-tagged TgHSP20s localized to the IMC of daughter cells regardless of the cysteine that was modified (Figure 4B). This indicates that the initial attachment of TgHSP20 to the IMC of daughter cells is a process independent of palmitoylation. Furthermore, the stable line expressing a non-palmitoylable form of TgHSP20 (TgHSP20C3,4,160S-Ty) localized to the IMC of daughter cells but to the cytosol of mature parasites. As such, we decided to investigate at which point of the endodyogeny process TgHSP20C3,4,160S-Ty changed its localization. Figure 5 shows that TgHSP20C3,4,160S-Ty localizes to the IMC of daughter cells until late endodyogeny and moves to the cytosol only when parasites are mature. As such, palmitoylation seems to be required to retain TgHSP20 at the mother IMC in intracellular parasites.
Figure 4. Palmitoylation is not required for TgHSP20 attachment to the IMC of daughter cells.
A) TgHSP20 localization at daughter’ IMC is not affected by 2-BP treatment. Replicating parasites were treated with 2-BP (100 μM) or DMSO for 16–18 h, then fixed and TgHSP20 localization was assessed by indirect immunofluorescence microscopy. Differential interference contrast (DIC) images are shown in the right panels. Scale bars= 5 μm. B) IMC localization at the daughter cell is maintained regardless of the TgHSP20 palmitoylation status. Intracellular replicating parasites overexpressing different Ty-tagged constructs were fixed and TgHSP20 localization was assessed by indirect immunofluorescence microscopy. DIC pictures are in the right panels. The anti-IMC1 antibody labels the IMC from both the mother and daughter cells. Scale bars= 5 μm.
Figure 5. TgHSP20 interacts with IMC throughout endodyogeny in a palmitoylation-independent manner.
The non-palmitoylable TgHSP20C3,4,160S-Ty construct is able to interact with the daughter IMC until the late steps of endodyogeny. Samples of replicating parasites overexpressing the TgHSP20C3,4,160S-Ty construct were obtained at different stages of the endodyogeny process and the Ty localization was assessed by indirect immunofluorescence assays. DIC pictures are in the right panels. Scale bars: 5 μm.
4. Discussion
Our data shows that TgHSP20 is synthesized as a mature protein in the cytosol and that its initial localization to the parasite IMC is independent of palmitoylation, but this PTM of TgHSP20 is required to maintain such localization in the mature parasite. Interestingly, these results indicate that a differential regulation of IMC protein targeting may occur in daughter and mature parasites. Although palmitoylation of T. gondii proteins has been predicted for numerous proteins, mainly for proteins localized to the IMC, there are little experimental evidences of such modification. One interesting finding of this investigation is that TgHSP20 is palmitoylated at the three cysteines present in its amino acid sequence. Up to date, the Apicomplexa phylum presents two IMC proteins where their palmitoylation status determines their localization to the pellicle [19, 22, 40]. The glideosome association protein GAP 45 localizes at the space between the plasma membrane and the IMC and is part of the motor complex of this parasite. However, this protein does not localize to the IMC of daughter cells and it seems to be associated to the plasma membrane from where it interacts with the IMC of mature parasites [41]. The T. gondii enzyme hypoxanthine-xanthine-guanine phosphoribosyltransferase (HXGPRT) presents two isoforms [40]. Whereas the HXGPRT–I localizes to the cytosol, the isoform-II presents an extension at the N-terminal region, which is palmitoylated and that determines its IMC localization [40]. Other interesting examples of T. gondii proteins that localize to the IMC are the IMC Sub-compartment Proteins (ISP1/2/3/4) [42] and the calcium-dependent protein kinase 3 (TgCDPK3) [24, 25]. ISPs 1–3 and TgCDPK3 present predicted myristoylation and palmitoylation sites at the N-terminal region [24, 25, 42], whereas ISP4 presents two predicted palmitoylation sites [23]. Mutation of the predicted N-terminal acylation sites delocalizes the proteins from the pellicle to the cytosol [23–25, 42]. However, the palmitoylation status of all these proteins remains to be demonstrated biochemically. In addition, T. gondii presents several IMC-associated proteins predicted to be palmitoylated such as IMC 1,4,6,11,12,13,14,15, MCL1 and PKG1 [15, 22, 43]. Taken together, it seems that palmitoylation of at least a subset of IMC-associated proteins could be important to retain these proteins in this compartment, being TgHSP20 an example of them.
Analysis of the dynamics of the incorporation of TgHSP20 into the IMC shows some interesting traits. We found that HSP20 was targeted to the IMC at an intermediate stage of budding. Once at the IMC, TgHSP20 is retained in this sub-compartment until the late steps of parasite replication, apparently without requirement for palmitoylation as observed for the non-palmitoylable form of TgHSP20 (TgHSP20C3,4,160S-Ty). We expected to observe contact between TgHSP20 and the IMC because all palmitoyl acyl-tranferases (PATs) are transmembrane proteins [44]. In fact, data mining of the T. gondii database (www.toxodb.org) reveals that putative TgPATs contain a signal peptide and several transmembrane domains [22]. However, in mature tachyzoites, after cytokinesis, the presence of TgHSP20 at the IMC requires at least cyteine 3 or 4 to be palmitoylated. Similarly, in higher eukaryotes a well characterized example of protein palmitoylation-dependent sorting is the H- and N-Ras. After translation, these proteins are farnesylated allowing them to associate with ER and Golgi membranes [45]. This single lipidation step produces a transient and weak attachment to the membrane, in this case with the ER. Subsequent palmitoylation steps generate the trapping necessary to keep the stable membrane association, particularly at the PM and Golgi. Palmitoylation and de-palmitoylation of Ras regulates diffusional exchange between the cytosol and endo-membranes [46, 47]. In a similar manner, our data suggest that TgHSP20 palmitoylation at cysteines 3 and 4 traps the chaperone at the IMC.
The fact that TgHSP20 is targeted to the IMC without being palmitoylated indicates that it could have an interacting molecule. Although these parasites express the endogenous TgHSP20 and the mutants versions could tag along with its localization, similar results were obtained when TgHSP20C3,4,160S-Ty was expressed in a TgHSP20-knock out parasite (Figure S3). Up to now, we were unable to detect any protein partner either as interactor and/or substrate [28] but one of them could be the specific PAT that palmitoylates TgHSP20, a speculation that needs to be further explored. Another possibility is that TgHSP20 binds IMC lipids as it has been shown for the recombinant TgHSP20 [28]. This intrinsic property could account for the initial binding of TgHSP20 to the IMC of nascent cells, whereas palmitoylation, as mentioned above, is required to maintain the IMC localization in mature intracellular parasites. In this case, a change in membrane composition or fluidity at the final steps of parasite replication [48, 49] could account for the generation of an unstable environment for non- palmitoylated TgHSP20. In fact, the mature IMC present a different topology along the parasite with palmitoylated proteins locating at three different regions [42]. This suggests that once TgHSP20 is at the IMC, palmitoylation could confer properties to stably interact or bind with specific regions along this sub-compartment. Actually, TgHSP20 presents a distribution in short stripes from the basal to the polar region of the parasite. It is interesting to note that hsp20 expression at the mRNA level is clearly associated to cell cycle, peaking at the mitosis phase (Figure S4), as described by other IMC proteins and suggesting that it occurs because they accompany the building of the new daughter cell [50]. This is in agreement with the possibility that TgHSP20 mature together with the IMC during parasite replication. Further studies would be necessary to address this point.
After treatment of tachyzoites with 2-BP, TgHSP20 showed a localization similar to that observed for the TgHSP20C3,4S-Ty, demonstrating that palmitoylation on cysteine 160 does not play a role in TgHSP20 trapping at the IMC. Considering that cysteine 160 is conserved, the role of its palmitoylation remains to be elucidated. On the other hand, lack of palmitoylation at cysteine 160 (TgHSP20C160S-Ty) did not produce the TgHSP20-positive unidentified structure, suggesting that this structure depends on palmitoylation at this residue. We speculate that this structure may contain misfolded products of TgHSP20. Interestingly, this structure seems to contain membrane structures that are reminiscent of the membranous myelin fibers observed when the apicoplast biogenesis was disturbed [38]. The significance of this finding needs to be further studied. The fact that TgHSP20C3,4,160S-Ty did not produce any unusual structure supports our hypothesis of the idea of a previous requirement of TgHSP20 association to the IMC to generate such structure. Even though the role of cysteine 160 is unclear, palmitoyaltion at this site may stabilize the anomalous attachment of TgHSP20 to the IMC. Of note, these unidentified structures are not naturally observed in the parasite.
In conclusion, we observed that the T. gondii IMC protein HSP20 is palmitoylated at three sites and that these palmitoylated sites have different roles at least in the targeting the chaperone to the IMC. We also observed that TgHSP20 is translated in the cytosol and does not require rapid palmitoylation to bind to the nascent IMC. However, a fine modulation of protein localization can occur at later steps of parasite replication, including palmitoylation as one of such process.
Supplementary Material
Highlights.
TgHSP20 is synthesized is palmitoylated in three cysteine residues
N-terminal palmitoylation determines its IMC localization
Incomplete palmitoylation causes TgHSP20 to partially accumulate in a membranous structure
TgHSP20 palmitoylation is not responsible for its interaction with daughter cells IMCs
Acknowledgments
We would like to thank Maria E. Francia and Beth Richardson for technical assistant with EM and the Plant Biology Electron Microscopy Facility at the University of Georgia. This work was supported by: ANPCyT grant BID 1728 OC-AR PICT 2010-1494 (MMC), a PIP grant 2010-0190 (MMC) and a National Institutes of Health-National Institute of Allergy and Infectious Diseases (NIH-NIAID) grant AI083162 (to SOA and MMC). NDM, MMC and SOA are researchers from the National Council of Research (CONICET) and UNSAM. MGDN is a PhD fellow from CONICET and was supported in part by a Fogarty International Center Training Grant (NIH D43TW007888). Work in SNJM laboratory was supported by NIH grant AI096836.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCabe JB, Berthiaume LG. Functional roles for fatty acylated amino-terminal domains in subcellular localization. Mol Biol Cell. 1999;10:3771–3786. doi: 10.1091/mbc.10.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCabe JB, Berthiaume LG. N-terminal protein acylation confers localization to cholesterol, sphingolipid-enriched membranes but not to lipid rafts/caveolae. Mol Biol Cell. 2001;12:3601–3617. doi: 10.1091/mbc.12.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthiaume L, Deichaite I, Peseckis S, Resh MD. Regulation of enzymatic activity by active site fatty acylation. A new role for long chain fatty acid acylation of proteins. J Biol Chem. 1994;269:6498–6505. [PubMed] [Google Scholar]
- 4.Corvi MM, Soltys CL, Berthiaume LG. Regulation of mitochondrial carbamoyl-phosphate synthetase 1 activity by active site fatty acylation. J Biol Chem. 2001;276:45704–45712. doi: 10.1074/jbc.M102766200. [DOI] [PubMed] [Google Scholar]
- 5.Deichaite I, Casson LP, Ling HP, Resh MD. In vitro synthesis of pp60v-src: myristylation in a cell-free system. Mol Cell Biol. 1988;8:4295–4301. doi: 10.1128/mcb.8.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcox C, Hu JS, Olson EN. Acylation of proteins with myristic acid occurs cotranslationally. Science. 1987;238:1275–1278. doi: 10.1126/science.3685978. [DOI] [PubMed] [Google Scholar]
- 7.Utsumi T. Analysis of co- and post-translational modifications of proteins by in vitro and in vivo metabolic labeling. Seikagaku. 2003;75:373–378. [PubMed] [Google Scholar]
- 8.Warden SM, Richardson C, O’Donnell J, Jr, Stapleton D, Kemp BE, Witters LA. Post-translational modifications of the beta-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochem J. 2001;354:275–283. doi: 10.1042/0264-6021:3540275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin DD, Vilas GL, Prescher JA, Rajaiah G, Falck JR, Bertozzi CR, Berthiaume LG. Rapid detection, discovery, and identification of post-translationally myristoylated proteins during apoptosis using a bio-orthogonal azidomyristate analog. FASEB J. 2008;22:797–806. doi: 10.1096/fj.07-9198com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilas GL, Corvi MM, Plummer GJ, Seime AM, Lambkin GR, Berthiaume LG. Posttranslational myristoylation of caspase-activated p21-activated protein kinase 2 (PAK2) potentiates late apoptotic events. Proc Natl Acad Sci U S A. 2006;103:6542–6547. doi: 10.1073/pnas.0600824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Linder ME, Deschenes RJ. New insights into the mechanisms of protein palmitoylation. Biochemistry. 2003;42:4311–4320. doi: 10.1021/bi034159a. [DOI] [PubMed] [Google Scholar]
- 13.Duncan JA, Gilman AG. A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS) J Biol Chem. 1998;273:15830–15837. doi: 10.1074/jbc.273.25.15830. [DOI] [PubMed] [Google Scholar]
- 14.Bano MC, Jackson CS, Magee AI. Pseudo-enzymatic S-acylation of a myristoylated yes protein tyrosine kinase peptide in vitro may reflect non-enzymatic S-acylation in vivo. Biochem J. 1998;330(Pt 2):723–731. doi: 10.1042/bj3300723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donald RG, Liberator PA. Molecular characterization of a coccidian parasite cGMP dependent protein kinase. Mol Biochem Parasitol. 2002;120:165–175. doi: 10.1016/s0166-6851(01)00451-0. [DOI] [PubMed] [Google Scholar]
- 16.Kato N, Sakata T, Breton G, Le Roch KG, Nagle A, Andersen C, Bursulaya B, Henson K, Johnson J, Kumar KA, Marr F, Mason D, McNamara C, Plouffe D, Ramachandran V, Spooner M, Tuntland T, Zhou Y, Peters EC, Chatterjee A, Schultz PG, Ward GE, Gray N, Harper J, Winzeler EA. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat Chem Biol. 2008;4:347–356. doi: 10.1038/nchembio.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moskes C, Burghaus PA, Wernli B, Sauder U, Durrenberger M, Kappes B. Export of Plasmodium falciparum calcium-dependent protein kinase 1 to the parasitophorous vacuole is dependent on three N-terminal membrane anchor motifs. Mol Microbiol. 2004;54:676–691. doi: 10.1111/j.1365-2958.2004.04313.x. [DOI] [PubMed] [Google Scholar]
- 18.Russo I, Oksman A, Goldberg DE. Fatty acid acylation regulates trafficking of the unusual Plasmodium falciparum calpain to the nucleolus. Mol Microbiol. 2009;72:229–245. doi: 10.1111/j.1365-2958.2009.06639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rees-Channer RR, Martin SR, Green JL, Bowyer PW, Grainger M, Molloy JE, Holder AA. Dual acylation of the 45 kDa gliding-associated protein (GAP45) in Plasmodium falciparum merozoites. Mol Biochem Parasitol. 2006;149:113–116. doi: 10.1016/j.molbiopara.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Alonso AM, Coceres VM, De Napoli MG, Nieto Guil AF, Angel SO, Corvi MM. Protein palmitoylation inhibition by 2-bromopalmitate alters gliding, host cell invasion and parasite morphology in Toxoplasma gondii. Mol Biochem Parasitol. 2012 doi: 10.1016/j.molbiopara.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilk SD, Gaskins E, Ward GE, Beckers CJ. GAP45 phosphorylation controls assembly of the Toxoplasma myosin XIV complex. Eukaryot Cell. 2009;8:190–196. doi: 10.1128/EC.00201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corvi MM, Berthiaume LG, Napoli MG. Protein palmitoylation in protozoan parasites. Front Biosci (Schol Ed) 2011;3:1067–1079. doi: 10.2741/211. [DOI] [PubMed] [Google Scholar]
- 23.Fung C, Beck JR, Robertson SD, Gubbels MJ, Bradley PJ. Toxoplasma ISP4 is a central IMC sub-compartment protein whose localization depends on palmitoylation but not myristoylation. Mol Biochem Parasitol. 2012;184:99–108. doi: 10.1016/j.molbiopara.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy JM, Whitehead L, van Dooren GG, Tonkin CJ. TgCDPK3 Regulates Calcium-Dependent Egress of Toxoplasma gondii from Host Cells. PLoS Pathog. 2012;8:e1003066. doi: 10.1371/journal.ppat.1003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrison E, Treeck M, Ehret E, Butz H, Garbuz T, Oswald BP, Settles M, Boothroyd J, Arrizabalaga G. A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog. 2012;8:e1003049. doi: 10.1371/journal.ppat.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opitz C, Soldati D. ‘The glideosome’: a dynamic complex powering gliding motion and host cell invasion by Toxoplasma gondii. Mol Microbiol. 2002;45:597–604. doi: 10.1046/j.1365-2958.2002.03056.x. [DOI] [PubMed] [Google Scholar]
- 27.Jones ML, Collins MO, Goulding D, Choudhary JS, Rayner JC. Analysis of protein palmitoylation reveals a pervasive role in Plasmodium development and pathogenesis. Cell Host Microbe. 2012;12:246–258. doi: 10.1016/j.chom.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Miguel N, Lebrun M, Heaslip A, Hu K, Beckers CJ, Matrajt M, Dubremetz JF, Angel SO. Toxoplasma gondii Hsp20 is a stripe-arranged chaperone-like protein associated with the outer leaflet of the inner membrane complex. Biol Cell. 2008;100:479–489. doi: 10.1042/BC20080004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Miguel N, Braun N, Bepperling A, Kriehuber T, Kastenmuller A, Buchner J, Angel SO, Haslbeck M. Structural and functional diversity in the family of small heat shock proteins from the parasite Toxoplasma gondii. Biochim Biophys Acta. 2009;1793:1738–1748. doi: 10.1016/j.bbamcr.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Precigout E, Valentin A, Carcy B, Gorenflot A, Nakamura K, Aikawa M, Schrevel J. Babesia divergens: characterization of a 17-kDa merozoite membrane protein. Exp Parasitol. 1993;77:425–434. doi: 10.1006/expr.1993.1102. [DOI] [PubMed] [Google Scholar]
- 31.Coceres VM, Alonso AM, Alomar ML, Corvi MM. Rabbit antibodies against Toxoplasma Hsp20 are able to reduce parasite invasion and gliding motility in Toxoplasma gondii and parasite invasion in Neospora caninum. Exp Parasitol. 2012 doi: 10.1016/j.exppara.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Montagna GN, Buscaglia CA, Munter S, Goosmann C, Frischknecht F, Brinkmann V, Matuschewski K. Critical role for heat shock protein 20 (HSP20) in migration of malarial sporozoites. J Biol Chem. 2012;287:2410–2422. doi: 10.1074/jbc.M111.302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montagna GN, Matuschewski K, Buscaglia CA. Small heat shock proteins in cellular adhesion and migration: Evidence from Plasmodium genetics. Cell Adh Migr. 2012;6:78–84. doi: 10.4161/cam.20101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montero E, Rodriguez M, Gonzalez LM, Lobo CA. Babesia divergens: identification and characterization of BdHSP-20, a small heat shock protein. Exp Parasitol. 2008;119:238–245. doi: 10.1016/j.exppara.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Donald RG, Carter D, Ullman B, Roos DS. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- 36.Cerede O, Dubremetz JF, Soete M, Deslee D, Vial H, Bout D, Lebrun M. Synergistic role of micronemal proteins in Toxoplasma gondii virulence. J Exp Med. 2005;201:453–463. doi: 10.1084/jem.20041672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resh MD. Use of analogs and inhibitors to study the functional significance of protein palmitoylation. Methods. 2006;40:191–197. doi: 10.1016/j.ymeth.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tawk L, Dubremetz JF, Montcourrier P, Chicanne G, Merezegue F, Richard V, Payrastre B, Meissner M, Vial HJ, Roy C, Wengelnik K, Lebrun M. Phosphatidylinositol 3-monophosphate is involved in toxoplasma apicoplast biogenesis. PLoS Pathog. 2011;7:e1001286. doi: 10.1371/journal.ppat.1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Striepen B, Jordan CN, Reiff S, van Dooren GG. Building the perfect parasite: cell division in apicomplexa. PLoS Pathog. 2007;3:e78. doi: 10.1371/journal.ppat.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhary K, Donald RG, Nishi M, Carter D, Ullman B, Roos DS. Differential localization of alternatively spliced hypoxanthine-xanthine-guanine phosphoribosyltransferase isoforms in Toxoplasma gondii. J Biol Chem. 2005;280:22053–22059. doi: 10.1074/jbc.M503178200. [DOI] [PubMed] [Google Scholar]
- 41.Frenal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D. Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe. 2010;8:343–357. doi: 10.1016/j.chom.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Beck JR, Rodriguez-Fernandez IA, Cruz de Leon J, Huynh MH, Carruthers VB, Morrissette NS, Bradley PJ. A Novel Family of Toxoplasma IMC Proteins Displays a Hierarchical Organization and Functions in Coordinating Parasite Division. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson-White BR, Ivey FD, Cheng K, Szatanek T, Lorestani A, Beckers CJ, Ferguson DJ, Sahoo N, Gubbels MJ. A family of intermediate filament-like proteins is sequentially assembled into the cytoskeleton of Toxoplasma gondii. Cell Microbiol. 2011;13:18–31. doi: 10.1111/j.1462-5822.2010.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res. 2006;47:1118–1127. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, Philips MR. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 46.Goodwin JS, Drake KR, Rogers C, Wright L, Lippincott-Schwartz J, Philips MR, Kenworthy AK. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J Cell Biol. 2005;170:261–272. doi: 10.1083/jcb.200502063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PI. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 48.Hu K, Mann T, Striepen B, Beckers CJ, Roos DS, Murray JM. Daughter cell assembly in the protozoan parasite Toxoplasma gondii. Mol Biol Cell. 2002;13:593–606. doi: 10.1091/mbc.01-06-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mann T, Gaskins E, Beckers C. Proteolytic processing of TgIMC1 during maturation of the membrane skeleton of Toxoplasma gondii. J Biol Chem. 2002;277:41240–41246. doi: 10.1074/jbc.M205056200. [DOI] [PubMed] [Google Scholar]
- 50.Behnke MS, Wootton JC, Lehmann MM, Radke JB, Lucas O, Nawas J, Sibley LD, White MW. Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PLoS One. 2010;5:e12354. doi: 10.1371/journal.pone.0012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wichroski MJ, Melton JA, Donahue CG, Tweten RK, Ward GE. Clostridium septicum alpha-toxin is active against the parasitic protozoan Toxoplasma gondii and targets members of the SAG family of glycosylphosphatidylinositol-anchored surface proteins. Infect Immun. 2002;70:4353–4361. doi: 10.1128/IAI.70.8.4353-4361.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Besteiro S, Michelin A, Poncet J, Dubremetz JF, Lebrun M. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog. 2009;5:e1000309. doi: 10.1371/journal.ppat.1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gajria B, Bahl A, Brestelli J, Dommer J, Fischer S, Gao X, Heiges M, Iodice J, Kissinger JC, Mackey AJ, Pinney DF, Roos DS, Stoeckert CJ, Jr, Wang H, Brunk BP. ToxoDB: an integrated Toxoplasma gondii database resource. Nucleic Acids Res. 2008;36:D553–556. doi: 10.1093/nar/gkm981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radke JR, White MW. Expression of herpes simplex virus thymidine kinase in Toxoplasma gondii attenuates tachyzoite virulence in mice. Infect Immun. 1999;67:5292–5297. doi: 10.1128/iai.67.10.5292-5297.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.