Summary
Background
Purpose of study was evaluation of regional metabolic disorders using 1H MRS in patients with MCI, as a predictor of clinical conversion to dementia based on clinical follow-up.
Material/Methods
The study group consisted of 31 subjects with diagnosis of MCI based on criteria the Mayo Clinic Group. 1H MRS was performed with a single-voxel method using PRESS sequence. The volume of interest (VOI) was located in the hippocampal formation and posterior part of the cingulated gyrus.
Results
Patients had annual clinical control at least twice. At the beginning, 9 had amnestic MCI and the others had multidomain MCI. During follow-up (median 3 yrs) 8 subjects had stable disease (SD), 13 had disease progression (DP) and 10 develop Alzheimer disease (AD). Baseline metabolic ratios (1H MRS) between 3 groups indicated significant difference (P<0.05) in left frontal lobe in mI/H20 ratio, between patients with SD (0.27) and DP. In comparing the groups with DP and AD, a significant difference in NAA/Cr (1.77 vs. 1.43) was found. A significant difference within left temporal external lobes was found between SD and DP in NAA/H2O ratio (0.55 vs. 0.51). An additional significant difference within medial temporal lobe was found between DP and AD in Glx/H2O ratio (0.44 vs. 0.34) on the right side.
Conclusions
1H MRS seems to be sensitive method allows prediction of which patients are liable to progress from MCI to AD. Combined with other biomarkers of disease staging, it is an important approach in the preclinical AD diagnosis, as well as the assessment of dementia progression.
Keywords: mild cognitive impairment (MCI), Alzheimer’s disease (AD), proton magnetic resonance spectroscopy (1H MRS)
Background
Aging leads to senile diseases, including Alzheimer disease (AD), which has become a serious medical and socioeconomic problem. Therefore, progress in early diagnosis and effective treatment of AD is necessary, especially in the preliminary or early stages of the disease [1,2]. Most elderly people experience physiological memory disorders; these disorders require a careful clinical assessment, as it is extremely difficult to delineate the line between physiology and pathology. Thus, when disorders of memory and other cognitive functions go beyond the standards of age and educational level, but have not yet reached the standards of dementia, we diagnose mild cognitive impairment (MCI), which includes several states that either match the standard in its wide meaning, for example Age-Associated Memory Impairment (AAMI), Age-Related Cognitive Decline (ARCD), or we diagnose pathology like MCI, which, although clinically similar, has a different prognosis than AAMI and ARCD [1–4]. Currently, base on criteria established by the Mayo Clinic Group, MCI is define as a transitional state between normal aging and Alzheimer’s disease, in which memory impairment is greater than expected for age, but general cognitive function and daily living activities are preserved [3,4].
Clinical symptoms of MCI are heterogenic. People suffering from MCI, especially amnestic form (aMCI), are at risk for developing dementia, particularly Alzheimer disease (AD) [3–5]. According to various reports, the percentage of conversion from aMCI into AD ranges from 1% to 25% per year, while the mean time of dementia progression from the MCI diagnosis is 4.4 years [4,5].
Obviously, the early identification of patients at risk for Alzheimer disease from among those with MCI would facilitate proper treatment help to delay the symptoms of dementia. Therefore, searching for methods that would help us detect a higher risk of dementia in those patients, as well as looking for objective methods of early diagnosis of patients with MCI, seems reasonable. Many reports confirm a typical repeatable topographical location of neuropathological lesions at various stages of AD, as described by Braak et al and Pantel et al. [6,7], while emphasizing early involvement of the limbic system, including the hippocampus and entorhinal cortex. Earlier pathology in these strategic regions in patients with a higher risk of Alzheimer-type dementia, as well as in certain patients with amnestic MCI, seems likely. Atrophy of the medial temporal lobe in MCI patients with a positive correlation of atrophy level and a risk of conversion to AD, inadequate for age and observed in imaging examinations (CT, MRI), has been clearly demonstrated [8–13]. The level of atrophy is judged by descriptive, volumetric and planimetric methods, or indirectly with the use of linear measurements of particular fluid spaces, such as fissures or certain parts of the ventricular system. Despite many years of experience in structural diagnosis of the dementia diseases, the use of functional methods seems to be very helpful.
In SPECT and PET examinations we observe the lowering of perfusion and metabolism of glucose and oxygen in temporal and parietal areas in patients with possible AD, as well as in those with MCI [14,15]. However, these methods have certain limitations such as relatively low linear resolution, which hinders precise determination of the topography of the measuring (this problem relates to the middle temporal structures and not to the hemispheres’ cortex).
A wider clinical application of magnetic resonance spectroscopy (MRS), described by Bootomley as “a window for metabolism” [16] enabled an intravital assessment of regional metabolic disorders in certain structures of the brain. There have been several reports on such dysfunctions in the limbic system in patients with Alzheimer disease [17,18]. A relatively small number of spectroscopic examinations have been carried out on patients with MCI. Some of these were presented by Catani et al., who located voxels in the white matter at the ventricular triangle level [19] and Kantarci et al. [20,21] who examined the posterior part of the cingulate gyrus in patients with MCI and AD. However, these are the locations where anatomopathologic changes, typical for AD, appear later than in the medial temporal lobe (including hippocampal formation). Many authors give up on spectroscopic examinations of hippocampal structures due to their technical limitations, such as the size of examined structures and topographical relations of the surrounding area. The problems mentioned most often are: difficulties with field homogeneity, artifacts from cranial base area, a disqualifying weakening of the signal-to-noise ratio, and the need to reduce the volume of interest (VOI). There have been reports on the technical capacities of such examinations in various brain diseases [19–23]. According to available the available literature, in the majority of papers H1MRS in MCI and AD metabolites were measured in the posterior cingulated gyrus [20,21,24], but parts of papers evaluated the concentration of metabolites in the bilateral hippocampi of MCI patients.
Aim of the study
This study attempted to evaluate the regional metabolic disorders using 1H MRS within the frontal, external and medial temporal lobes in patients with MCI, as a predictor of clinical deterioration to dementia based on clinical follow-up.
Material and Methods
Inclusion criteria for patients and demographic characteristics of examined groups
The examination was performed on a group of 31 randomly selected subjects (19 females and 12 males) with MCI diagnosed under the care of the Department of Neurodegenerative Disorders, Medical Research Centre, Polish Academy of Science. All subjects underwent neurological and psychiatric tests, routine laboratory investigations and standard neuropsychological examinations. Patients with serious CNS injuries, alcoholic abuse, diabetes, and serious hepatic and renal dysfunctions were excluded. MCI diagnosis was established by a team of specialists after analyzing all available information and test results. Diagnostic criteria matched those of the Mayo Clinic Group [1–3].
MR and 1H MRS examination
MRI and 1H MRS examination was performed on a 1.5T Eclipse (Marconi Medical Systems, USA) scanner. Morphological MRI examination of the brain was carried out in transverse planes, parallel to the longitudinal axis of the temporal lobe in SE, FSE and FLAIR sequences, in T1- and T2-weighted images, and in the frontal plane perpendicular to the longitudinal axis of the temporal lobe in FLAIR sequence. We investigated the extent of cerebral atrophy, especially in medial and external temporal lobes and in frontal structures, as well as the presence of cortical-subcortical hyperintensive focuses (which equal angiogenic lesions) in T2-weighted images and FLAIR sequence. Morphological examination enabled us to exclude other pathologies such as extensive ischemic lesions, tumors, paracerebral hematomas, and hydrocephalus, which might lead to cognitive disorders. Changes like leukoaraiosis and/or small single angiogenic focuses of gliosis were not exclusion criteria for the study.
1H MRS was performed with a single-voxel method. The VOI (volume of interest) was located in the frontal, medial and external temporal lobe regions, separately on each side. In case of medial temporal lobe covering the hippocampal formation, it was divided into 2 areas – the anterior (the topographical point of reference in the coronal plane/layer was the level of dens) and the posterior (the point of reference was the output of the middle cerebellar peduncles from the pons). Both areas were then added, and final average data sets were used in further analysis. In cases of reduced volume of the hippocampal formation, the adjacent fluid spaces (eg, the temporal horn of the lateral ventricle and the lateral part of the transverse fissure) were also included into the VOI. Necessary corrections of VOI location were applied to particular frontal cross-sections, as well as to the axial plane, and care was taken not to cover the osseous structures of the temporal bone pyramids. For every localization, the size of VOI was 8 cm3. Additionally, each subject had frontal measurement taken.
1H MRS examination was carried out with a single-voxel method using PRESS sequence. Routine 3-impulse sequences of 90, 180, 180 degrees and double crusher impulse were used. The examination was performed with an automated standardization of the field in the entire encephalon/brain (total shimming) and in the examined sample (local shimming). For water suppression, the MOIST technique was used. Spectra were recorded within the following parameters: TE=35 ms, TR=1500 ms, thickness =15 mm, signal averages =192.
Assignment of resonance lines of particular metabolites was based on N-acetylaspartate signal with chemical shift set to 2.0 ppm. The spectra were analyzed using the manufacturer-supplied software package for the MRS (Marconi). In some cases, it was necessary to correct the phase manually in order to obtain a maximum of symmetrical signal of residual water and to maintain a proper baseline.
Relative concentration ratios of particular metabolites – N-acetylaspartate (NAA), choline (Cho), myoinositol (mI), glutamine and glutamate (Glx) – were analyzed in reference to the signal of unsuppressed water signal, and also to the signal of creatine, considering its level as an inner standard.
Statistical analysis
Results analysis of clinical and biochemical data – age, mini-mental score (MM-score), folic acid, vitamin B12 and homocysteine level – was performed including initial data of all subjects enrolled into the study. Evaluation of relative concentration ratios of metabolites from all VOIs, localized within frontal, medial and external temporal lobes, symmetrical on both sides, were performed in each case.
A comparison of some metabolic ratios of MRS in patients with MCI, who on follow-up has stable disease (SD), disease progression (DP) and conversion to AD, was performed.
A mean value of measurement parameters were assessed in each group of patients. An analysis of normality was performed using Kolmogorov-Smirnov test. The differences between groups were measured using the Mann-Whitney U test. The threshold value of statistical significance was P<0.05. The statistical analysis was carried out using the Statistica for Windows 7.0 (StatSoft, OK, USA) software package.
Results
All patients had annual clinical follow-up at least twice. At the beginning of the study, subjects were divided into 2 groups – 9 subjects who had amnestic MCI, and the others who had multidomain MCI. There were no statistical differences between groups of patients with multidomain MCI and amnestic MCI, including age, M-M score, level of vitamin B12, folic acid and homocysteine (P>0.05). Comparison of mean value in some selected parameters including both groups is presented in Table 1.
Table 1.
A comparison of mean value of some clinical parameters in 2 group of patients with MCI those amnestic and multidomine.
| Amnestic MCI N=9 (mean and SD) | Multidomine MCI N=22 (mean and SD) | |
|---|---|---|
| Age (SD) | 62.8 (10.7) | 70.55 (6.4) |
| M-M score (SD) | 27.6 (1.4) | 27.5 (1.6) |
| Vitamin B12 (SD) | 334.8 (144.5) | 304.5 (134.5) |
| Folic acid (SD) | 7.3 (2.2) | 8.1 (3.4) |
| H-cysteine (SD) | 13.9 (3.5) | 15.5 (4.5) |
During clinical follow-up (median 3 years) 8 subjects had stable disease (SD), 13 had progression of disease (DP) and 10 developed AD. There was no significant difference between age, M-M score, vitamin B12, folic acid and homocysteine level among these 3 groups of subjects (P>0.05). Mean values of some clinical and biochemical parameters are presented in Table 2.
Table 2.
A comparison of mean value of some clinical parameters in 3 group of patients those with stable disease, progression and conversion to AD (including standard deviation =SD).
| SD N=8 | DP N=13 | AD N=10 | |
|---|---|---|---|
| Age (years) | 72.9 (8.1) | 67.5 (7.4) | 65.4 (9.1) |
| M-M score | 27.4 (1.4) | 27.2 (1.9) | 28.2 (2.3) |
| Vit. B12 | 369.3 (152.1) | 309.7 (107.3) | 278.1 (158.3) |
| Folic acid | 7.6 (3.1) | 7.6 (3.2) | 8.2 (2.5) |
| H-cysteine | 14.5 (4.4) | 16.1 (4.7) | 13.8 (3.4) |
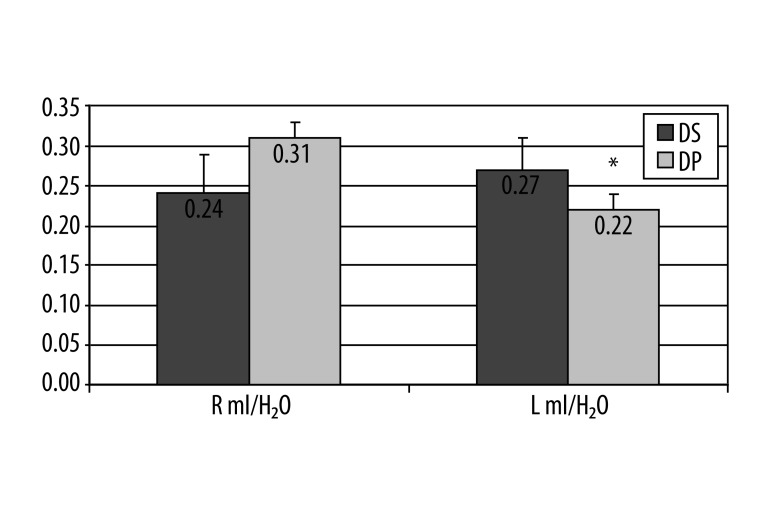
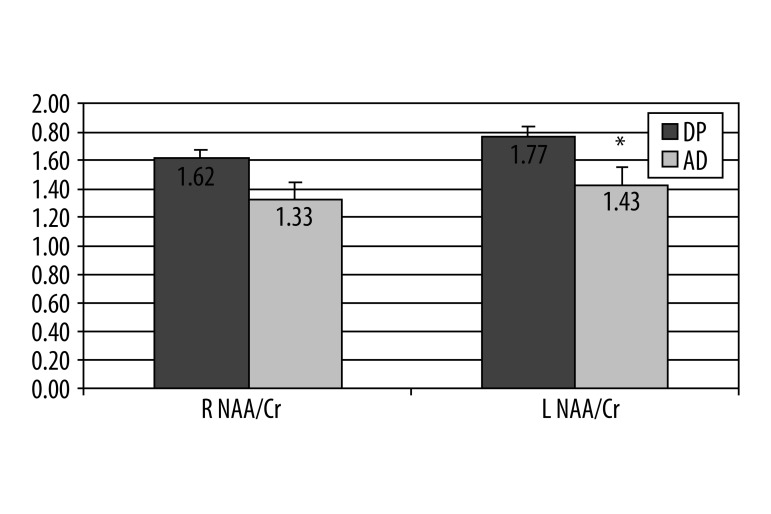
Statistical analyses of baseline metabolic ratios measurement using 1H MRS between the final 3 groups of patients, (SD, DP and AD) found significant difference in frontal lobes in mI/H20 ratio on left, between patients with stable disease (0.27) and those with progression (0.22) (P=0.03) (Figure 1). In the groups of patients with DP and those with conversion to AD, there was a significant difference on the left side in ratio NAA/Cr (1.77 vs. 1.43), (P=0.02) (Figure 2).
Figure 1.
A graphical illustration of 1H MRS ratio for mI/H20 between patients with DS vs. DP in right and left frontal lobe Mann-Whitney U-test, * P=0.034 on the left side, no significant difference on right.
Figure 2.
A graphical illustration of 1H MRS ratio for NAA/Cr between patients with DP vs. AD in right and left frontal lobe, Mann-Whitney U-test, * P=0.018 on the left side, no significant difference on right.
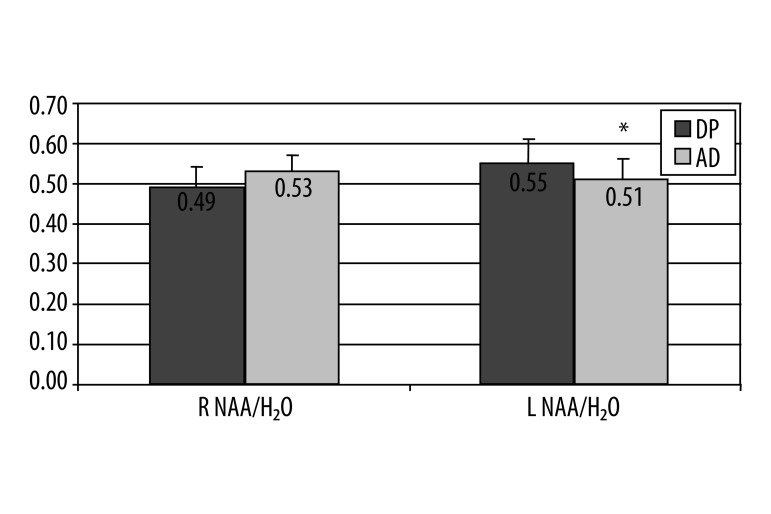
Significant difference within temporal external lobes were found between patients with SD and DP in NAA/H2O ratio on the left side (0.55 vs. 0.51), (P=0.04) (Figure 3).
Figure 3.
A graphical illustration of selected 1H MRS ratio NAA/H2O in patients with DS vs. DP in temporal external lobes, significant difference in the left side (Mann-Whitney U-test, * P=0.04), no difference in right.
Additionally, there were significant differences between patients with SD and AD in Chol/Cr ratio (0.99 vs. 0.88) on the right side (P=0.04) and in mI/Cr (1.09 vs. 0.62) on the left side (P=0.03).
A significant difference within the medial temporal lobe was found between patients with DP and AD in Glx/H2O ratio (0.44 vs. 0.34) on the right side (M-W U-test, p=0.02). There were no other significant differences within 1H MRS metabolic ratios.
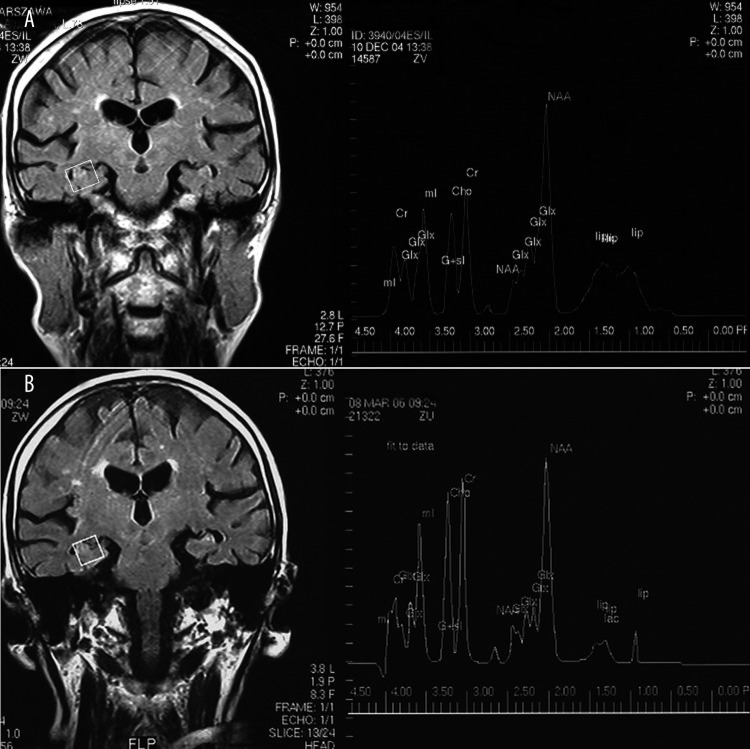
An example of 1H MRS study in patient with initial diagnosis of MCI and clinical deterioration during 2 years of follow-up with final diagnosis of AD is presented in (Figure 4) (A – initial 1H MRS, and after final diagnosis, control 1H MRS study – B).
Figure 4.
An example of 1H MRS study in patient with initial diagnosis of MCI and clinical deterioration during 2 years of follow-up with final diagnosis as AD, (A) initial 1H MRS study; (B) follow-up control 1H MRS study after final diagnosis.
Summary of initial 1H MRS ratios of some metabolites in frontal, external and medial temporal lobes both sides are presented in Table 3.
Table 3.
Mean value of selected metabolic ratios of 1H MRS in frontal, temporal external and temporal medial lobes in patients with MCI.
| Right side | Frontal | Temporal external | Temporal medial | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DS | DP | AD | DS | DP | AD | DS | DP | AD | |
| NAA/Cr | 1.80 | 1.62 | 1.33 | 1.77 | 1.75 | 1.80 | 1.64 | 1.68 | 1.37 |
| NAA/H2O | 0.63 | 0.55 | 0.56 | 0.49 | 0.53 | 0.43 | 0.50 | 0.50 | 0.54 |
| Chol/Cr | 1.12 | 0.80 | 0.63 | 0.99 | 0.93 | 0.88 | 1.12 | 0.91 | 0.81 |
| Chol/H2O | 0.39 | 0.26 | 0.26 | 0.26 | 0.22 | 0.18 | 0.46 | 0.28 | 0.36 |
| mI/Cr | 0.85 | 0.73 | 0.77 | 0.94 | 0.81 | 0.83 | 1.08 | 0.80 | 0.80 |
| mI/H2O | 0.24 | 0.31 | 0.29 | 0.28 | 0.21 | 0.15 | 0.34 | 0.25 | 0.27 |
| Glx/Cr | 2.06 | 1.40 | 0.75 | 1.63 | 1.85 | 1.35 | 1.36 | 1.62 | 1.26 |
| Glx/H2O | 0.74 | 0.46 | 0.27 | 0.56 | 0.48 | 0.30 | 0.44 | 0.45 | 0.34 |
| Left side | |||||||||
| NAA/Cr | 1.82 | 1.77 | 1.43 | 1.95 | 1.76 | 1.67 | 1.60 | 1.42 | 1.20 |
| NAA/H2O | 0.63 | 0.58 | 0.55 | 0.55 | 0.51 | 0.49 | 0.44 | 0.48 | 0.47 |
| Chol/Cr | 1.21 | 1.00 | 0.91 | 0.88 | 0.79 | 0.75 | 0.93 | 0.92 | 0.97 |
| Chol/H2O | 0.41 | 0.32 | 0.28 | 0.20 | 0.14 | 0.11 | 0.51 | 0.36 | 0.38 |
| mI/Cr | 0.69 | 0.93 | 0.65 | 1.09 | 0.81 | 0.62 | 1.05 | 0.96 | 1.04 |
| mI/H2O | 0.27 | 0.22 | 0.14 | 0.19 | 0.14 | 0.12 | 0.40 | 0.36 | 0.42 |
| Glx/Cr | 1.50 | 2.33 | 2.67 | 2.42 | 2.22 | 1.68 | 2.43 | 1.61 | 1.19 |
| Glx/H2O | 0.39 | 0.59 | 0.53 | 0.62 | 0.50 | 0.77 | 0.54 | 0.43 | 0.5 |
Discussion
Proton magnetic resonance spectroscopy – 1H MRS – enables an in vivo noninvasive assessment of the degree of biochemical disorders in a specified VOI of the examined tissue, as well as monitoring the progress of those changes in the course of the disease and its treatment [5,17–25]. The method we used, along with the parameters mentioned above, resulted in satisfactory quality of spectra in examined patients.
Recent studies have demonstrated the value of H1MRS in many types of cognitive disorders, not only in degenerative diseases [26,27].
In MRS diagnosis, normal tissues present a constant proton spectrum, while ratio changes of the metabolites can be considered as reflections of certain biochemical transformations. Several cytological and histochemical investigations have contributed to the study of the relationship between certain chemical substances and precisely defined intracellular structures or biochemical processes – physiological as well as pathological.
Changes of average NAA/Cr and NAA/H2O ratios in subjects with MCI
N-acetylaspartate is commonly considered as a neuronal marker due to its presence only in mature nerve cells. There are several reports of age-dependent lowering of NAA level in certain parts of the encephalon, which is being interpreted not only as an effect of a progressive decrease in number of neurons, but also as their dysfunction. It also seems clear that there is a lowering of the NAA concentration in atrophic structures of the limbic system in patients with AD, and a reverse correlation of the level of this metabolite with the level of dementia [17,18]. However, the 1H MRS examinations of the posterior part of the cingulate gyrus (frontal lobe) in patients with MCI, carried out by the Rochester group, did not prove statistically significant differences of the NAA ratios as compared to the control group [20]. In our study, we found a significant difference between the ratio of NAA/Cr between patients with DS vs. DP in the left frontal lobe. We also found significant difference in NAA/H2O between patients with DS and DP in the left external temporal lobe. We could not find any significant difference between NAA/Cr and NAA/H2O within the medial temporal lobe on any sides. This could due to the frequent technical difficulties mentioned before, as measurement of the medial temporal lobe (hippocampus area) is often inconvenient. The neuronal integrity marker NAA/Cr or NAA/H2O ratio (independent of creatine level) is currently declined in patients with progressive MCI and those with conversion to AD compared to cognitively normal elderly subjects [20,21,25].
Our findings generally agree with others reports that found lower levels of NAA in patients with MCI and deterioration of brain function and who convert to AD. Others reports indicated the left side more often has a drop in NAA-rich neurons compare to the right side [20–26].
Medial temporal lobes (hippocampus structures) are widely considered as the region in which the earliest AD pathologies occur. Therefore it seems that the lowering of NAA concentration in this localization in patients with MCI proves the theory of “successive” metabolic disorders in progression of dementia, according to which a lower concentration of myoinositol precedes the decline in NAA concentration [21–24]. In many reports, NAA depletion seems to be good marker, as in Metastasio et al. recent report of neuronal breakdown and energetic deficiency [25]. Single reports indicated that the significant reduction of NAA has great predictive value within the occipital area in those based on ROC with threshold level <1.61 of NAA/Cr ratio; other regions and others ratios were not significant in this study [5]. Despite this inhomogeneous data, numerous studies have shown that NAA plays an important role in neuronal integrity, with the left side more often affected in patients with progressive MCI and in those with conversion to AD [18–26].
Changes of average mI/Cr and mI/H2O ratios in subjects with MCI
Some studies have found an elevated level of mI/Cr, with good correlation of disease progression and increase of neurofibrillary tangles in patients with conversion into AD [30]. In our study we did not find any of these findings; on the contrary, we detected some depletion of mI/Cr in patients with conversion to AD compared to those with stable disease, with a significant difference on the left external temporal lobe (1.09 vs. 0.62). We found the same tendency in mI/H2O ratio on the left frontal lobe between patients with disease stability and disease progression. Others reported differences between patients with MCI and AD, with significantly higher signal ratio of mI/Cr in AD [28]. Others publications did not report any difference in this metabolite ratio in the group of patients with MCI [25]. The differences in results of various groups of researchers could be explained by selection of the study population. The same research group indicated different results, dependent on study group – for instance, no difference was found in selected population of patients with amnestic MCI [25] compare to a heterogenous population with significant difference in mI signal [19]. The potential role of mI in etiopathogenesis of the MCI and AD remains unclear. Some reports indicated that an elevated level of this metabolite in structures of the limbic system in patients with MCI or dementia is connected with regional gliosis. This theory is supported by the presence of visibly higher mI concentrations in glia cells than in neurocytes. However, thus far the exact role of mI in MCI and AD is unclear. There are many other disorders of the brain with mI disturbance, so specificity of this marker and ratio of mI/Cr or mI/H2O seems to be low. Recent data suggests good discrimination using mI/Cr ratio in different types of dementia using ROC curve [28]. Additional theory posits an extensive cellular capture of mI in patients at risk of dementia as the result of Na/mI osmoregulator dysfunction. The study of adults with Down syndrome, who constitute a clinically exceptional group of subjects with 100% risk of dementia similar to the Alzheimer’s disease, demonstrated a greater that 50% rise of the myoinositol level with age compared to the healthy control group [29]. The authors associated this with an elevated activity of sodium-myoinositol transporter/ transmitter, which results from the existence of the additional 21 chromosome in Down syndrome adults, which includes the gene-encoding protein of the transmitter. In our study, significant differences in mI/Cr and mI/H2O were found in frontal and external temporal lobes, but not in the medial temporal lobe.
Changes of average Chol/Cr and Chol/H2O ratios in subjects with MCI
Our 1H MRS study shows a significant difference between Chol/Cr ratio only in the external temporal lobe on the left side, with a significant drop in the Chol/Cr ratio between patients with stable disease and AD. This result agrees with Metastasio et al. [25], who showed a decreased, but not significant, depletion of Chol/Cr in MCI patients with disease progression compare to those with stable disease in both hemispheres. This could be related to cholinergic neuronal damage, which is indirectly confirmed by increased activity of choline acetyltransferase (ChAT), and which probably represents a compensatory (but insufficient) response in AD [30].
Impairment of mitochondrial activity seen in elderly patients, and more obviously in patients with AD, could be related to increase of mitochondrial membrane damage and evaluated level of membrane phospholipid [31] and decrease in phosphatidyl-choline and phosphatidylethanolamine described previously [32].
Changes of average Chol/Cr and Chol/H2O ratios in subjects with MCI
In our study, significant difference in glutamine and glutamate (Glx) ratio was noted only on the medial temporal lobe on the right side, which indicates disturbance of Glx. This finding agrees with data presented by Kantarci et al. [33], who found a trend toward decreased Glu + Gln/Cr ratios from normal to MCI to AD, but there were no statistically significant differences. Others reported less consistent disturbances of glutamine and glutamate (Glx) ratios [34]. Detailed analysis of glutamine and glutamate in MCI and AD could be explored using the new 3T system, which currently is commercially available.
In the medial temporal lobe 1H MRS has several limitations that could influence our results and our interpretation. In view of the complicated spatial shape of the medial temporal-hippocampal formation (which is considered to be the region first changed by the progression of dementia pathology) and its relatively small size, our volume of interest also covered other tissue structures outside the target structure, which could have caused partial falsification of the results as to its volume effect [35,36]
On the other hand, these structures belong to the limbic system, and show pathological changes in the subsequent stages of the disease. According to reports in the literature, including the adjacent fluid spaces (temporal horn of the lateral ventricle, lateral part of the transverse fissure) into the VOI should not affect the results. Some researchers claim that chemical shift imaging (CSI), also known as single voxel spectroscopy (SVS), which allows recording spectra from many neighboring voxels within the examined area, is better for assessment of metabolic disorders within the hippocampus because it enables a more precise measurement. This theory was not corroborated in comparative studies of both of the methods used on a group of patients with temporal lobe epilepsy [37].
Conclusions
1H MRS seems to be a very sensitive method that provides biochemical information using an in vivo approach in patients with initial MCI, who in significant numbers developed disease progression and/or converted to AD. This suggests that 1H MRS can identify subjects with prodromal phase AD.
The significance of our metabolic ratio results needs further prospective study. A potential advantage could be achieved by using 3T systems, which can better discriminate quantization of Glu + Gln/Cr and Gln/Cr ratios.
Footnotes
Source of support: Departmental sources
References
- 1.Petersen RG, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Smith GE, Waring SC, et al. Ageing, memory and mild cognitive impairment. Int Psychogeriatr. 1997;9:65–70. doi: 10.1017/s1041610297004717. [DOI] [PubMed] [Google Scholar]
- 3.Smith GE, Petersen RC, Parisi JE, et al. Definition, course and outcome of mild cognitive impairment. Ageing, Neuropsychl & Cognition. 1996;3:141–47. [Google Scholar]
- 4.Gabryelewicz T, Pawlowska-Detko A, Misko J, et al. Prediction of deterioration of mild cognitive impairment with CT and SPECT. Med Sci Monit. 2007;13(Suppl 1):31–37. [PubMed] [Google Scholar]
- 5.Modrego PJ, Fayed N, Pina MA. Conversion from mild cognitive impairment to probable Alzheimer’s disease predicted by brain MR spectroscopy. Am J Psychiatry. 2005;162:667–75. doi: 10.1176/appi.ajp.162.4.667. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Neuropathological staging of Alzheimer’s disease. Acta Neuropathol (Berl) 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 7.Pantel J, Huger Dr, Kratz B, et al. Structural cerebral changes in subjects with mild cognitive impairment. Nervenarzt. 2002;73:845–50. doi: 10.1007/s001150101154. [DOI] [PubMed] [Google Scholar]
- 8.Chetelat G, Baron JC. Early diagnosis of Alzheimer’s disease: contribution of structural neuroimaging. Neuroimage. 2003;18:525–41. doi: 10.1016/s1053-8119(02)00026-5. [DOI] [PubMed] [Google Scholar]
- 9.Jack CR, Petersen RC, Xu Y, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–94. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kordower JH, Chu Y, Stebbins GT, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–13. [PubMed] [Google Scholar]
- 11.Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;71:441–47. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czarnecka A, Zimny A, Sąsiadek M. Correlation of CT perfusion and CT volumetry in patients with AD. Pol J Radiol. 2010;75:15–21. [PMC free article] [PubMed] [Google Scholar]
- 13.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–36. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 14.Celsis P, Agneil A, Cardebrat D. Age related cognitive decline: a clinical entity? A longitudinal study of cerebral blood flow and memory performance. J Neurol Neurosurg Psychiatry. 1997;62:601–8. doi: 10.1136/jnnp.62.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berent S, Giordani B, Foster N. Neuropsychological function and cerebral glucose utilization in isolated memory impairment and Alzheimer’s disease. J Psychiatr Res. 1999;33:7–16. doi: 10.1016/s0022-3956(98)90048-6. [DOI] [PubMed] [Google Scholar]
- 16.Bottomley PA. The trouble with spectroscopy papers. Radiology. 1991;181:344–50. doi: 10.1148/radiology.181.2.1924769. [DOI] [PubMed] [Google Scholar]
- 17.Schuff N, Amend D, Ezekiel F, et al. Changes of hippocampal N-acetyl aspartate and volume in Alzheimer’s disease. A proton MR spectroscopic imaging and MRI study. Neurology. 1997;49:1513–21. doi: 10.1212/wnl.49.6.1513. [DOI] [PubMed] [Google Scholar]
- 18.Block W, Jessen F, et al. Regional N-acetylaspartate reduction in the hippocampus detected with fast proton magnetic resonance spectroscopic imaging in patients with Alzheimer’s disease. Arch Neurol. 2002;59:828–34. doi: 10.1001/archneur.59.5.828. [DOI] [PubMed] [Google Scholar]
- 19.Catani M, Cherubini A, Howard R, et al. 1H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport. 2001;12:2315–17. doi: 10.1097/00001756-200108080-00007. [DOI] [PubMed] [Google Scholar]
- 20.Kantarci K, Jack CR, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: a 1H MRS study. Neurology. 2000;55:210–17. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantarci K, Smith GE, Ivnik RJ, et al. 1H magnetic resonance spectroscopy, cognitive function, and apolipoprotein E genotype in normal aging, mild cognitive impairment and Alzheimer’s disease. J International Neuropsychological Society. 2002;8:934–42. doi: 10.1017/s1355617702870084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazeyras F, Charles HC, Tupler LA, et al. Metabolic brain mapping in Alzheimer’s disease using proton magnetic resonance spectroscopy. Psychiatry Res. 1998;82:95–106. doi: 10.1016/s0925-4927(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 23.Adalsteinsson E, Sullivan EV, Kleinhans N, et al. Longitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer’s disease. Lancet. 2000;355(9216):1696–97. doi: 10.1016/s0140-6736(00)02246-7. [DOI] [PubMed] [Google Scholar]
- 24.Kantarci K, Reynolds G, Petersen RC, et al. Proton MR spectroscopy in mild cognitive impairment and Alzheimer disease: comparison of 1.5 and 3T. Am J Neuroradiol. 2003;24:843–49. [PMC free article] [PubMed] [Google Scholar]
- 25.Metastasio A, Rinaldi P, Tarducci R, et al. Conversion of MCI to dementia: Role of MRS. Neurobiol Aging. 2006;27:926–32. doi: 10.1016/j.neurobiolaging.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Walecki J, Pawłowska A, Gabryelewicz T, et al. 1H-MRS in mild cognitive impairment (MCI) – the role of particular metabolites in prediction of MCI conversion to AD. Med Sci Monit. 2010;16(Suppl 1):11–18. [Google Scholar]
- 27.Tarasow E, Kochanowicz J, Mariak Z, Walecki MR spectroscopy in patients after surgical clipping and endovascular embolisation of intracranial aneurysms. Pol J Radiol. 2010;75(4):25–30. [PMC free article] [PubMed] [Google Scholar]
- 28.Rose SE, de Zubicaray GI, Wang D, et al. A 1H MRS study of probably Alzheimer disease and normal aging: implication for longitudinal monitoring of fementia progression. Magn Reson Imaging. 1999;17:291–99. doi: 10.1016/s0730-725x(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Bisbal MC, Arana E, et al. Cognitive impairement classification of 1h MRS. Eur J Neurol. 2004;11:187–93. doi: 10.1046/j.1468-1331.2003.00746.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang W, Alexander GE, Daly EM, et al. High brain myo-inositol levels in the predementia phase of Alzheimer’s disease in adults with Down’s syndrome: a 1H MRS study. Am J Psychiatry. 1999;156:1879–86. doi: 10.1176/ajp.156.12.1879. [DOI] [PubMed] [Google Scholar]
- 31.DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–55. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 32.Farber SA, Slack BE, Blusztajn JK. Acceleration of phosphatidylcholine synthesis and breakdown by inhibitors of mitochondrial function in neuronal cells: a model of the membrane defect of Alzheimer’s disease. FASEB J. 2000;14:2198–206. doi: 10.1096/fj.99-0853. [DOI] [PubMed] [Google Scholar]
- 33.Nitsch RM, Blusztajn JK, Pittas AG, et al. Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci. 1992;89:1671–75. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantarci k, Reynolds G, Petersen RC, et al. Proton MR spectroscopy in mild cognitive impairment and Alzheimer’s disease: comparison of 1,5 and 3 T. Am J Neuroradiol. 2003;24:843–49. [PMC free article] [PubMed] [Google Scholar]
- 35.Jones RS, Waldman AD. 1H-MRS evaluation of metabolism in Alzheimer’s disease and vascular dementia. Neurol Res. 2004;26:488–95. doi: 10.1179/016164104225017640. [DOI] [PubMed] [Google Scholar]
- 36.Pieniążek P, Sokół M, Walecki J, et al. Metodyka badań 1H MRS pojedynczego voxela ludzkiego mózgu in vivo. Diagnostyka Obrazowa. 2002;1:13–19. [in Polish] [Google Scholar]
- 37.Yuan-Yu H, Chen C, et al. Proton MR spectroscopy in patients with complex partial seizures: single-voxel spectroscopy versus chemical-shift imaging. Am J Neuroradiol. 1999;20:643–51. [PMC free article] [PubMed] [Google Scholar]