Summary
Diuretic-resistant congestive heart failure in the form of type 2 cardiorenal syndrome is a problem of growing significance in everyday clinical practice because of high morbidity and mortality. There has been scant progress in the treatment of overhydration, the main cause of symptoms in this group of patients. The aim of our review is to present recent advances in the ultrafiltration therapy of congestive heart failure, with special attention to the new dedicated device for extracorporeal isolated ultrafiltration, as well as modifications of peritoneal dialysis in the form of peritoneal ultrafiltration with icodextrin solution and incremental peritoneal dialysis. Technical and clinical features, costs and potential risks of available devices for isolated ultrafiltration are presented. This method should be reserved for patients with true diuretic resistance as part of a more complex strategy aiming at the adequate control of fluid retention. Peritoneal ultrafiltration is presented as a viable alternative to extracorporeal ultrafiltration because of medical and psychosocial benefits of home-based therapy, lower costs and more effective daily ultrafiltration. In conclusion, large, properly randomized and controlled clinical trials with long-term follow-up will be essential in assessing the logistics and cost-effectiveness of both methods. Most importantly, however, they should be able to evaluate the impact of both methods on preservation of renal function and delaying the progression of heart failure by interrupting the vicious circle of cardiorenal syndrome. Our review is supplemented with the case report of the use of peritoneal ultrafiltration with a single 12-hour nighttime icodextrin exchange as a life-saving procedure in a patient with congestive heart failure resistant to pharmacological treatment.
Keywords: congestive heart failure, isolated ultrafiltration, hemodialysis, peritoneal ultrafiltration, peritoneal dialysis
Background
Diuretic-resistant congestive heart failure (CHF) is a problem of growing significance in everyday clinical practice. It is related closely to type 2 cardiorenal syndrome (CRS), which is characterized by chronic abnormalities in cardiac function, causing progressive chronic kidney disease (CKD) with glomerular filtration rate (GFR) below 60 ml/min/1.73 m2[1]. Data collected from over 200,000 US patients and summarized in the ADHERE Registry indicate that on discharge, nearly 50% of patients gain weight or loose less than 5 lbs (1.25 kg) [2]. The Framingham Study found that 80% of men and 70% of women under age 65 years diagnosed with CHF die within 8 years from the diagnosis [3]. In France, deaths from HF increased by 35.3% between 1992 and 2002, while during the same period the overall death rate increased by only 7.7% [4]. Worldwide prevalence of CHF is estimated at 23 million people and affects mostly elderly individuals. Every year in the US 550,000 new CHF cases are diagnosed, with 1 million hospitalizations/per year and overall costs of treatment exceeding 37 billion USD in 2009 [5]. According to the European Society of Cardiology, the incidence of heart failure (HF) in Europe can be estimated at 0.4–2.0% of the total population, which translates into 500,000–750,000 patients in Poland [6].
Depending on the CHF stage, as well as presence or absence of end-stage renal disease (ESRD), methods complimentary to conservative treatment of overhydration include isolated ultrafiltration (iUF) or hemodiafiltration (HDF) with extracorporeal devices or peritoneal UF or peritoneal dialysis (PD). The aim of our review is to present recent advances in the ultrafiltration treatment of congestive heart failure patients, with special attention given to peritoneal ultrafiltration.
Search Strategy
Studies were identified searching MEDLINE Registry from January 1970 to July 2011, combining the terms “heart failure and ultrafiltration” and ”heart failure and peritoneal ultrafiltration or peritoneal dialysis”. The search strategy was limited to English-language articles on adults. We retrieved all full-text non-duplicated articles documenting clinical studies of UF in heart failure and describing patient characteristics, UF procedures, renal outcome and adverse effects. We analyzed 103 articles found for HF and iUF and 45 found for HF and peritoneal dialysis or peritoneal ultrafiltration.
Definition and Classification of Heart Failure
Since the traditional view of HF – as a myriad of symptoms caused by inadequate performance of the heart – is no longer adequate, 2 new classifications of HF have been introduced [7,8]. The American College of Cardiology/American Heart Association (ACC/AHA) classification is based on the type of structural damage to cardiac muscle (stages A-D), while the functional NYHA classification is based on symptoms and loss of physical activity [7]. According to the ACC/AHA classification, only stages C and D reflect the traditional clinical diagnosis of HF. It should be emphasized that the ACC/AHA classification does not replace the NYHA functional classification, but it highlights some important factors in understanding heart failure:
HF is the common pathway of disorders affecting all parts of the heart, having common pathogenesis beyond stage in the disease;
a decrease in ejection fraction (EF) is not a prerequisite for a diagnosis of heart failure;
heart failure is a progressive disorder;
treatment should be tailored to the stage of disease (according to ACC/AHA classification) rather than to the patient’s symptomatology or NYHA class;
the necessity to increase the dosage of loop diuretics or patients’ resistance to these drugs are usually indicative of CHF – both are also markers of severity of concomitant renal failure.
Congestive Heart Failure – Pathophysiology
Traditionally, congestive heart failure is defined as the state in which an abnormal cardiac function is responsible for the inability of the heart to pump an adequate volume of blood to meet the requirements of the peripheral tissues [9]. However, many patients do have structural cardiac alterations that impair systolic or diastolic function, but do not have clinical signs of heart failure. Experimental and clinical studies showed that HF is characterized by increased neuro-humoral activation of the sympathetic nervous system (SNS), the renin-angiotensin-aldosterone system (RAAS) and increased activity of vasopressin and endothelin, as well as various cytokines, which all contribute to the deteriorating renal function and progression of the disease [9]. Lack of counterbalance by the endogenous vasodilatators such as brain natriuretic peptide (BNP) might be responsible for sodium and water retention. Low cardiac output leads to low tissue perfusion, systemic vasoconstriction, activation of the RAAS and SNS systems, alteration in nitric oxide balance and release of antidiuretic hormone, and, subsequently, sodium retention. Renal vasoconstriction with increased proximal tubular and reduced distal tubular delivery of sodium and water is responsible for resistance to atrial natriuretic peptide (ANP) and increased sensitivity of distal nephrons to aldosterone. Gradual progression of HF to renal hypoperfusion with resulting fluid overload leads to the subsequent development of CRS and the accompanying resistance to diuretics [1,8,9].
Standard Therapy of CHF
The treatment of CHF patients aims to relieve symptoms, to prolong survival and to prevent or delay progression to more severe cardiac dysfunction. It is widely accepted that it should not rely entirely on inotropic drugs, but rather attempt to limit ventricular remodeling and to inhibit neuro-humoral activation [10]. Lifestyle and diet issues are of crucial importance here, especially with regard to salt and fluid restrictions. Pharmacological therapy has evolved greatly during the past 2 decades. All patients with chronic HF and treated on an out-patient basis should be considered for treatment with ACE inhibitors and beta blockers, since both of these prolong survival and prevent progressive dysfunction and remodeling, particularly in systolic HF [11]. The standard therapy of CHF should also include conventional diuretics – mainly loop diuretics combined with spironolactone in patients with GFR >30 ml/min/1.73 m2, as well as sodium-blocking agents exerting their activity in other parts of the nephrons. The proper control of sodium and water balance is of vital importance because up to 80% of hospitalizations from CHF are due to acute overhydration and only 5% are due to low cardiac output [12]. Recent HF therapy guidelines, which underscore the widely accepted safety and efficacy of diuretics, recommend the use of intravenous diuretics in the case of acute decompensating heart failure (ADHF) [8,10].
The adequate diuretic treatment of CHF requires access to all fluid compartments: intravascular, interstitial and intracellular, with a rate of removal of excess salt and water that avoids depletion of intravascular volume, decrease in renal perfusion and glomerular filtration rate (GFR) as well as activation of the neuro-humoral axis. Importantly, loop diuretics induce salt and water removal in a way that results in hypotonic urine, a temporary reduction of hydrostatic pressure and natriuresis. Some reports suggest that long-term treatment with loop diuretics might result in electrolyte wasting, renal dysfunction and the progression of HF [13].
Among HF patients, older individuals remain particularly resistant to conventional therapies, with typical geriatric issues such as hypoalbuminemia, anemia and renal insufficiency usually to blame, as well as dementia-related forgetfulness leading to patient’s noncompliance with the sodium and fluid intake requirements. Consequently, decrease of renal perfusion and renal failure occurs, posing a significant challenge to modern-day cardiology [14]. New therapies aim at enhancing myocardial contractility and inducing resynchronization, as well as offering mechanical circulatory support. Recently developed drugs such as calcium sensitizers and endothelin antagonists, among others, did not show significant benefit in this high risk population, with the possible exception of eplerenone (a selective aldosterone antagonist) and levosimendan (a calcium sensitizer) [15]. However, the results of several clinical trials of the new devices, including the implantable cardioverter-defibrillators (ICD), cardiac resynchronization and biventricular pacing, are quite encouraging [16]. Recently, the left ventricular assist devices (LVAD) have been used not only as a temporary “bridge” to cardiac transplantation, but also as an “end of the life” or even long-term treatment option [17].
Although enormous progress has been made in the treatment of patients with CHF, mortality rates remain high and the affected population continues to grow. From the nephrologist’s point of view, the underlying cause for lack of success is that little if any progress has been made in the treatment of overhydration, the principal cause of symptoms in this group of patients. Therefore, development of new sodium- and water-removing strategies in close cooperation between nephrologists and cardiologists will be necessary for optimization of existing treatment modalities.
Alternative Methods of Fluid Removal in CHF
Advantages of alternative methods of fluid removal in CHF include: an improvement in cardiac output due to the Frank-Starling mechanism, an increase in the left ventricular diastolic inflow, and improvement in lung compliance after removal of the excess fluid. Extracorporeal therapies are more frequently used for the acute decompensated HF (ADHF) and short-term management of refractory congestive HF [13]. Peritoneal ultrafiltration (pUF) and peritoneal dialysis (PD) as home based procedures have been proposed by some authors for the long treatment of chronic CHF that is resistant to diuretics [18–20].
Extracorporeal Ultrafiltration
The terms “ultrafiltration” and “hemofiltration”, although signifying 2 quite different processes, are often used interchangeably, especially in cardiology. Ultrafiltration (UF) refers to either the removal of water during renal replacement therapy (RRT) or isolated fluid removal from blood (known as isolated UF [iUF]). It involves the mechanical removal of fluid by generating a convective gradient across the filter membrane. Ultrafiltration removes water and electrolytes without affecting plasma electrolytes and without increasing the risk of metabolic disturbances. The electrolyte concentration of the ultrafiltrate is equal to that of plasma, which is in striking contrast with the excretion of sodium (90 mEq/L) and potassium (30 mEq/L) in the urine achieved with diuretics. The generation of isotonic filtrate is associated with a sustained reduction of hydrostatic pressure and avoids the stimulation of the RAAS and SNS systems. In contrast with iUF, hemofiltration is based on the convective removal of plasma water, but also requires the partial or total replacement of the plasma water by a clean solution of known electrolyte concentrations (usually 2–3 L/h) in the continuous type of hemofiltration, or up to 6–8 L/h in high-volume hemofiltration/hemodiafiltration procedures [20].
Isolated Ultrafiltration
Extracorporeal ultrafiltration for fluid removal proposed by Silverstein in 1974 as a modification of the standard HD system has been employed in overhydrated ESRD patients as well as patients with CHF resistant to diuretics [20,21]. Recently, more interest for this method was generated due to the development of a portable device for isolated UF (iUF). This device, which was designed for both hospitalized and ambulatory patients with diuretic-resistant CHF, was approved by U.S. Food and Drug Administration [22–25]. In the next 2 sections of this review, we will describe the principles of this method, as well as clinical results in patients with diuretic-resistant CHF in the context of its potential to be applied on an out-patient basis, similarly to the peritoneal ultrafiltration method, described in the second part of our review.
Available Machines for iUF: Types, Principles and Cost-Effectiveness
Three different types of machines are available for iUF, each corresponding to different logistic and clinical conditions and costs [20]. The machines available are the following:
simplified machines like: Aquadex Flexflow Fluid Removal System100 (CHF Solutions Inc, Brooklyn Park, MN, USA) and Dedyca (Bellco, Mirandola, Italy); both devices are intended mostly for cardiology wards, but are also recommended by manufacturer for ambulatory therapy in so-called Aquapheresis Outpatient Clinics;
machines for continuous renal replacement therapy (CVVHF/HDF) like PrismafleX with AN 69 ST and oXiris filters and the new PrismafleX XEED System with SepteX for removal of cytokines, especially in septic shock (Gambro) used in the ICUs;
machines for standard hemodialysis/hemofiltration, like the AK 200 Ultra S (Gambro) routinely used in nephrology wards.
The treatment specifications for Aquapheresis with the Aquadex Flexflow Fluid Removal System are as follow: central or peripheral venous access; outpatient or inpatient treatment; surface area 0.12 m2–0.25 m2; fluid removal rate from 0 to 500 mL/h; with 10 mL/h increment; BFR 20–40 mL/min; BV 33 mL/circuit; standard anticoagulation; duration of procedure usually 8–12 h. The kinetic characteristics of the UF 500 Blood Circuit Set used with the Aquadex Flexflow 100 device are the following: filter molecular weight cut-off point 65 kD; filter sieving coefficient for urea, creatinine, vitamin B12: 0.98; albumin sieving <0.02; KUF for BFR 40 mL/min and UFR 8.3 mL/h equal to 5.6 mL/h/mmHg [26].
The disposable filter is a truly a central part of the device, since it determines the amount and velocity of fluid removal and the permeability to particles of different molecular weight. In comparison, filters used for HDF with PrismafleX and the AN69 or oXiris membranes are characterized by high rate of absorption of low molecular weight proteins like cytokines, which is especially enhanced through the greater surface area of the latter. In experimental conditions the adsorption on the oXiris membrane, according to initial concentration near to zero, rises up to 750 times in the case of IL6. It can result in improvement of patient’s hemodynamic status [27].
SepteX filters used in the treatment of sepsis enable the removal of molecules of weight up to 45 kDa, such as cytokines. Controlled trials demonstrated the effective elimination and significant reduction of plasma levels of proinflammatory mediators [28–30]. The SepteX membrane effectively removes mediators in diffusive modality. The mean cytokine clearances in human plasma with QD=42 ml/min, QB=200 ml/min for IL-6 and IL-1 are 28 and 45 ml/min, respectively. However, these clearances can be enhanced by increasing dialyzate flow. According to Morgera et al., an increase of QD from 1.0 to 2.5 l/h raises the IL-6 clearance more than 2-fold [28]. The SepteX therapy is operated in CVVHD modality. A non-convective therapy (when QUF=0 ml/min) allows limited albumin looses due to the large pore size of the SepteX membrane, and it improves patient safety. The SepteX can also remove mediators in convective modality (when QUF>0 ml/min). Its membrane pore size is 2 to 3 times larger than standard high-flux membranes. It results in higher values of sieving coefficients of proinflammatory cytokines and higher removal of these molecules. Adsorption in the AN69 ST and oXiris membranes is the mechanism that removes up to 53% of cytokines, the remaining are removed by diffusion and convection.
In contrast to CVVHF/HDF performed with SepteX membrane in PrismaFlex, Aquapheresis with Aquadex monitor is not an effective method for removal cytokines and other pro-inflammatory molecules. Polysulfone filter used in the Aquadex device has no adsorption capability and, despite high molecular weight cut-off point of maximum 65 kD, the small flows applied do not allow effective cytokines removal. In Aquapheresis with Aquadex, blood flow of 40 ml/min and ultrafiltration typically below 2 l per procedure are applied, which is in contrast with blood flow of 150 ml/min and ultrafiltration of at least 35ml/h/kg in CVVHF/HDF. This is insufficient to remove significant amounts of cytokines. There is no data available of the actual sieving coefficient for cytokines with the Aquadex FlexFlow system. The percentage of cytokines theoretically removed by convection with Aquadex (with UF of 1l) was calculated by Daniel Baczyński from our group, using sieving coefficients according to Bouman et al. [27]. The results for cytokines removed from blood (patient of 70 kg) at UF=1 l are as follows TNF a=0%, IL-6 <3%; IL-8 <3%.
There is no data about rebound effect transport of cytokines from intracellular compartment to blood compartment directly after the Aquapheresis procedure. There is no data confirming that the amount of cytokines removed with Aquapheresis is clinically significant. Thus, CVVHF/HDF with SepteX and oXiris filters remains the only documented method of cytokines removal [27–30].
According to Wertman et al. the cost of the Aquadex device is around $25,000 USD [13]. Fiaccadori et al. estimated the costs of disposables (filters and entire circuit) as: 900 € for Aquadex; 150–250 € for continuous RRT; and 20–50 € for standard HD/hemofiltration [20]. It must be mentioned that the actual costs of iUF are influenced not only by the type of machine and disposable material used, but also by the number of treatments needed, as well as organization and equipment at the ward where the treatment is performed (cardiology/nephrology wards/ICU or special outpatients clinics with separately trained personnel). The high costs of this procedure also result from the high rate of readmissions due to the exacerbations of HF (eg, at least twice a year in the U.S.).
General Recommendations for iUF
The main indication for iUF in recent guidelines (class II a, level B recommendation) is fluid overload in patients with true resistance to diuretics [13,15]. Such patients maintain positive fluid balance despite fluid/salt dietary restrictions and optimal diuretic therapy. Constanzo et al. define optimal diuretic therapy as maximally tolerated doses of intravenous loop diuretics supported by sequential nephron blockade with other drugs [31]. In practice, there is no agreement on the definition of “true diuretic resistance”. The Aquadex Flex Flow device manufacturer characterizes it as: fluid overload >10 lbs (4.5 kg); diuretic dose >80 mg furosemide per day; or inadequate diuretic response; or less than 1.0 L of urine output in 8 hrs and <2.5 L <24 hrs with serum creatinine increase >0.3 mg/dl; or frequent readmissions due to overhydration [26].
Other authors determine the threshold for true resistance to diuretics at the level of 240–320 mg of furosemide per day, supported by agents acting on the other parts of nephrons [25,32]. In 2006 Eshagian et al. published data showing that higher doses of furosemide or its equivalent (≥160 mg/day) were associated with higher mortality rates, and patients were 3 times more likely to receive dialysis in comparison with the group of patients treated with lower doses of furosemide. Moreover, it was shown that patients with CHF exacerbations treated with high doses of oral diuretics on an out-patient basis often showed progressively weaker responses to loop diuretics administered in the hospital [33].
Isolated UF presents as a reasonable option for patients with renal dysfunction associated with potentially reversible fluid overload such as systemic and renal congestion, but not with structural changes of the kidney. In the case of advanced renal failure with metabolic alterations and symptoms of uremic syndrome, other methods such as standard hemodialysis/hemofiltration or peritoneal dialysis should be considered [20,25].
Clinical Effectiveness Trials and Safety Issues of iUF
Since the first report of UF used in the treatment of CHF in 1978, several case reports followed, presenting convincing results of successful removal of fluid overload and improvement of symptoms in therapy-resistant CHF patients treated with UF [13,20,22–25].
The report by Jaski et al. describes one of the first attempts to use the portable Aquadex Flexflow device for safe and effective removal of salt and water in patients with fluid overload (SAFE STUDY) [22]. Recently published data by Fiaccadori et al. discusses technical issues, mechanisms, efficacy, safety, costs and indications for iUF in heart failure, and summarizes the most prominent clinical trials [20]. There as follows: RAPID-CHF trial (Bart et al.), EUPHORIA trial (Constanzo et al., 2005), and UNLOAD trial (Constanzo et al., 2007) [24,34,35]. The first 2 trials were conducted in small groups of patients (up to 20) and within an inconsistent experimental set-up: various doses of diuretics, either peripheral or central vascular access, with or without control groups. On the other hand, the results of the largest-to date randomized, prospective and controlled trial (Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure – UNLOAD) published by Constanzo et al. in 2007, allows for drawing reliable conclusions about the efficacy of the treatment in this group of patients. The above mentioned study involved 200 acutely decompensated patients with 2 or more signs of hypervolemia, who were randomized into 2 groups: those treated with iUF (Aquadex System 100, CHF Solutions, Minneapolis, MN) and those treated with intravenous diuretics administered either continuously or as a bolus. After 48 hours of hospitalization, the iUF group demonstrated 38% greater weight loss and 28% greater net fluid loss as compared to controls (p=0.001) but had equal improvement in dyspnea score (p=0.35); 90 days post-discharge the iUF group demonstrated a 50% reduction in re-hospitalizations for HF (p=0.022) and 63% reduction in total days of re-hospitalizations for HF (p=0.022) [35].
The first long-term study of the results of ultrafiltration in CHF patients comes from Jaski et al., 2008. It followed 100 patients (aged 65±14) predominantly diagnosed with systolic heart dysfunction and treated with iUF for the 43 months and a total of 130 hospitalizations. An average of 7.0±3.9 L (median 6.3 L) of fluid was removed by the UF treatment during 2.1±1.2 iUF sessions per hospitalization, resulting in the average weight loss of 6 kg. At discharge, baseline creatinine level did not change. Although there was no control group, the authors compared their results with the ADHERE database; despite the fact that the study included severely ill patients with significant volume overload, there was no difference in survival rate between UF-treated HF patients and the ADHERE data [36].
Little is known about the effects of iUF on renal function in HF. Small-scale observational studies showed no changes in renal function (assessed by measuring serum creatinine) when iUF was compared to diuretics in stable patients with HF [24]. Rogers et al. found that when glomerular filtration rate (GFR) and renal plasma flow were directly measured, no significant difference was found between groups of patients treated with iUF and diuretics [37].
A cost-effectiveness analysis of the UNLOAD trial data suggests that the actual financial burden on the Health Insurance System might be smaller in the case of iUF as compared to traditional treatments – although generally iUF is more expensive; however, it is associated with shorter and less frequent hospitalizations [20,25].
Limitations of Clinical Trials
Although providing invaluable insights into the new treatment modalities, the above described clinical trials are burdened by several limitations:
exclusion of patients with hypotension/hemodynamic instability and/or treated with vasoactive inotropes;
no data on compliance with low-salt diet;
lack of fluid overload assessment;
too many variations of diuretic therapy – suboptimal administration of intravenous loop diuretics; mean loop diuretics doses not compliant with the current guidelines;
the dose of diuretics in the standard therapy group was at the discretion of attending physicians;
iUF duration, rate, and weight loss of the patients were at the discretion of attending physicians.
Advantages and Disadvantages of Isolated Ultrafiltration Procedure
Listed below are some of the positive and negative aspects of iUF that came to light in the course of the above described clinical trials.
The benefits:
hemodynamic: reduced right atrial and pulmonary artery systolic pressure (PASP), reduced or unchanged pulmonary vascular resistances – factors especially important in patients being prepared for heart transplantation; unchanged or increased cardiac index, unchanged heart rate, and mean arterial pressure, unchanged or reduced peripheral vascular resistance; improved peak exercise capacity;
clinically in comparison with diuretics: more efficient sodium removal and more rapid relief of systemic and pulmonary congestion; no clinically significant impact on heart rate or blood pressure; if iUF and refilling rates are well-balanced the hemodynamic status of the patient is stable;
logistics/costs: in the future possible ambulatory treatment option, associated with possible lower rate of readmissions.
A recent post-hoc analysis of the UNLOAD trial, comparing the readmissions rates of the subgroup of patients treated with iUF with those on IV diuretics, found that although the general differences in the amount of fluid removed by iUF and IV diuretics were not significant (with the only significant differences observed between iUF and IV bolus diuretics groups), fewer readmissions and unscheduled emergency department or office visits were observed with iUF [38].
The shortcomings:
the simple iUF device does not provide correction of the concentration of serum electrolytes like potassium, as well as of BUN and acid/base disturbances because of specific properties of filters with sieving effect for convective transport of sodium, and no diffusive gradient for other particles;
lack of evidence supporting effective removal of proinflammatory cytokines and myocardial depressant factor (MDF) by simple UF devices like Aquadex, in contrast to highly specialized types of membranes used in hemodiafiltration method [25];
conflicting data on decreased activation of SNS, NA and RAAS systems as a result of intensive but intermittent form of ultrafiltration therapy;
limited evidence on restoring sensitivity to diuretics.
The main downside of iUF is the individual hemodynamic status of patient, which depends on several factors influencing vascular refilling rate (VRR). If the intended ultrafiltration is higher than VRR, the risk of hypovolemia and hypotension followed by sharp decrease in renal perfusion might be significant. Apart from renal and hemodynamic complications, iUF has a fair share of problems typical of any other renal replacement therapy (RRT) technique based on an extracorporeal circulation; the most commonly observed are problems linked to venous access (peripheral or central). Additionally, since the typical iUF session if usually longer than the standard HD session (8–12 hours vs. 4 hours, respectively), proper control of anti-coagulation might pose a considerable challenge.
Proposals for the Future
There certainly is a long way to go before introducing the portable iUF devices into everyday out-patient-based practice. According to Kazory et al. [39], several clinical issues still need to be resolved:
the protective influence of iUF on renal function in comparison to diuretics still needs to be reassessed and confirmed, especially because residual renal function (RRF) is a strong predictor of mortality in CHF;
improvement in long-term survival in CHF patients should be confirmed by long-term, controlled clinical trials;
superiority of peripheral vs. central venous access should be confirmed;
because the duration of UF session usually is longer than standard HD the problems of anticoagulation as well as hemodynamic control of velocity of ultrafiltration should be resolved;
the logistics of this method should be improved – at present, iUF is being carried out in various departments (nephrology, cardiology, ICU) or on outpatient basis in Aquapheresis Outpatient Clinics by personnel who are not necessarily well-trained in RRT;
there is a need for studies to confirm the cost-effectiveness of this method, based on fewer readmissions and lower costs of disposables as compared to the traditional treatments.
In conclusion, the iUF clearly has the potential to change the traditional therapeutic approach to patients with proven refractory ADHF and chronic CHF. Until the aforementioned shortcomings of this method are resolved, it should be reserved for selected patients with advanced heart failure and true diuretic resistance, as part of a more complex strategy aimed at adequate control of fluid retention.
Peritoneal Ultrafiltration in Chronic Congestive Heart Failure – A Viable Option
Peritoneal dialysis in its continuous or automatic form (CAPD or APD) is a widely accepted alternative dialysis modality to hemodialysis in patients with end-stage renal disease (ESRD). It provides the medical and psychosocial benefits of home dialysis to a large number of patients, and is associated with lower costs, and has clinical outcomes comparable to that of HD. Patients with type 2 cardiorenal syndrome developed in the course of CHF certainly represent a new and unique niche within the kidney disease spectrum. Depending on the degree of their GFR reduction, this group of patients may be treated with continuous peritoneal ultrafiltration or one of the forms of PD – continuous or automatic (CAPD/APD) – as an alternative to different modifications of HD [18,19,40–42].
Several reports underscore the efficacy of PD as an approach providing daily, continuous, slow ultrafiltration in patients with CHF [19,43–49]. These promising results, together with over 30 years of our own experience in using CAPD/APD as the only available method of home dialysis in Poland, prompted us to introduce our own program of different modifications of PD in CHF resistant to diuretics [49–52].
Kinetics of Peritoneal Ultrafiltration
PD removes excess water and sodium mainly by means of osmotic ultrafiltration. The commonly used dialysis solutions contain glucose at different concentrations as an osmotic gradient resulting in the transfer of water from the peritoneal vascular bed into the dialysate, which then flows into the dialysate container. Unfortunately, as the time of dialysis exchange extends beyond 4 hours, its efficiency is reduced due to a decrease of the osmotic gradient. This decrease is caused by the absorption of glucose into the circulation and can by itself lead to hyperglycemia, hyperinsulinemia, and obesity. Icodextrin, a glucose polymer, has been shown to provide long and efficient ultrafiltration and, as opposed to glucose, to permeate into the bloodstream only to a small extent [18,52].
PD results in the removal of water as well as sodium ions, as illustrated by the sodium sieving phenomenon. The peritoneal membrane contains 3 types of pores: ultra-small, small, and large. Initially, water permeates through ultra-small and small pores as a result of high osmotic pressure due to the presence of a high concentration of glucose in the dialysis solution. At the same time, water molecules carrying sodium ions travel exclusively through small pores. This initial stage of PD is described as convective transport of sodium. As the peritoneal exchange progresses and the glucose osmotic gradient disappears, sodium transport occurs through diffusion. The sodium concentration gradient (blood – hypernatremia/fluid – hyponatremia) forces sodium permeation from the blood into the dialysate. Disappearance of glucose gradient in the first stage is a direct reason why shorter and more frequent peritoneal exchanges are preferred when glucose solutions are used. However, the procedure should be long enough to allow the second phase (sodium diffusion) to take place. As normal blood volume and arterial pressure are achieved, the cardiac load decreases and sympathetic nervous system activity returns to baseline levels. At the same time, responsiveness to diuretic treatment is restored.
Therapeutic Benefits of Peritoneal Ultrafiltration
Therapeutic benefits of increased ultrafiltration volume in patients with HF include limiting the pathophysiological process by inhibition of inflammatory cytokines release, normalization of neurohormonal pathways, and restoration of diuretic responsiveness. Unfortunately, since the ultrafiltration-mediated convective clearance of cytokines is non-specific, beneficial cytokines are lost along with the pro-inflammatory ones [53–56]. On the other hand, although the mass clearance of cytokine compounds during PD seems to be low, PD can remove myocardial depressant factors such as e.g.: TNF-a, ranging between 0.5 and 20 to 30 kDa [54]. Some studies demonstrated PD-related decrease in BNP levels (3.5 kDa). Chung et al. studied the relationship between serum N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, left ventricular (LV) dysfunction (measured by M-mode, 2-dimensional cardiac sonograph) and extracellular water (measured by a multifrequency bioimpedance analyzer) in 30 CAPD patients with a mean age of 47±12 y. Serum NT-proBNP levels correlated positively with LV mass index (r=0.768, p=0.01) and extracellular water (r=0.866, p=0.01) but negatively with LV ejection fraction (r=−0.808, p=0.01). The authors suggest that serum NT-proBNP levels can be a clinical predictive marker for LV hypertrophy and dysfunction, as well as a marker of volume status in CAPD patients [57].
There are a limited number of publications addressing the issue of removing cardiac injury markers into the dialysate [54–56]. These markers include medium molecular mass molecules (between 0.5 and 20–30 kDa) such as atrial natriuretic peptide (ANP), tumor necrosis factor-a (TNF-a) and myocardial depressant factor (MDF), as well as interleukin (IL)-1 and IL-6. It has been suggested that removal of these molecules inhibits myocardial remodelling and myocyte apoptosis [54,55]. A study conducted by Zemel et al. on a group of 20 stable patients on CAPD demonstrated that TNF-a and its receptors TNF-a I and TNF-a II diffuse into dialysate from blood. Removal of these molecules is independent of their local production, for example in dialysis-associated peritonitis, and their transport directly correlates with their molecular mass – the smaller it is, the larger the ratio of their dialysate level to serum level (D/S) [54]. Fincher et al. conducted a study on 19 stable CAPD patients, in which D/S for ANP was measured in the 90th minute of a standard dialysis exchange. They demonstrated significantly higher baseline ANP values in patients on CAPD as compared to healthy controls. In the 90th minute of the exchange, ANP could still be detected in the dialysate, and its level correlated with its baseline serum level [55].
Long Dwell PD with Icodextrin Solution – How Does It Work?
In the early 1990s, an alternative to glucose dialysis fluid was introduced in PD, which contained glucose polymer (ie, icodextrin [ICO]), as an osmotic agent [58]. ICO is a starch-derived, water-soluble glucose polymer linked by alpha (1–4) and less than 10% alpha (1–6) glucosidic bonds with an average molecular weight of 16800 Daltons, osmolality of 282 mOsm/L, and pH of 5.3. ICO allows for transcapillary ultrafiltration by means of colloid osmosis. The dialysis fluid containing icodextrin (Extraneal) has been used in long-term dialysis exchanges in patients on CAPD and in long-term daily exchanges in patients on APD. Recently published reports describe the use of this fluid in the treatment of patients with refractory congestive heart failure (CHF) with or without coexisting ESRD as well as in high (H) and high-average (HA) transport diabetic patients [45,59,60]. Moreover, Anna Olszowska from our group showed that dialysis solution containing icodextrin as an osmotic agent is particularly efficient in transperitoneal water transport. The study involved a 16-hours dwell with exchange of 7.5% icodextrin solution in 11 clinically stable patients with average age of 50.4±18.3 years and average CAPD duration of 26.9±22.4 months. The study was performed using the hemodynamic model of diffusive and convective peritoneal transport with 125I-human serum albumin as a volume marker. A significant increase in intraperitoneal volume of dialysate at 16 hours of the exchange in comparison to the initial values was observed in the whole group (p=0.002). This increase was maintained until the end of the study, with minimal and maximal intraperitoneal volume of 2.372 ml and 3.621 ml, respectively. The total amount of removed sodium was 105.13±50.30 mmoles. There were no adverse effects of long dwells with icodextrin in our study [52]. On the basis of our results we proposed icodextrin solution for long dwells in peritoneal ultrafiltration or peritoneal dialysis in patients with CHF resistant to pharmacological treatment. Additional rationale for this therapeutic option was provided by the case report of a 37-year-old patient with terminal CHF who had been considered ineligible for heart transplantation and previously insufficiently treated with hemodialysis [51].
Advantages of icodextrin long dwells are as follows:
more physiological ultrafiltration;
maintenance of euvolemia without additional dextrose exchanges;
lifestyle benefits and reduced risk of touch contamination (and hence of peritonitis) because of a single daily exchange;
possibly less peritoneal inflammation because of avoidance of dextrose solutions.
Disadvantages of icodextrin long dwells are the following:
lack of studies comparing the effects of concentrated glucose solutions and icodextrin in patients with CHF;
no predictable loss of UF due to membrane failure in the long-term observation.
Incremental Peritoneal Dialysis in CHF
Incremental PD first proposed by Mehrotra et al. constitutes one of the variations of PD, in which the prescribed PD dose depends on the level of residual renal function, starting with at least 1 bag and increasing PD dose in proportion to the declining urinary excretion of solutes and fluid [61]. Nakayama et al. introduced the incremental PD method in 12 elderly CKD patients (stages 3–5) with refractory HF (NYHA class III, n=9; IV, n=3, mean age 81±6 years) and more than 3 hospitalizations in the previous year. The patients were initially treated with approximately 19 sessions of sequential hemofiltration, followed by incremental PD, with 3 PD sessions/week (8 hours each) at the start, increasing in frequency and dwelling time as clinically indicated. During follow-up (median, 26.5 months), PD was well tolerated by all patients, and there was no need for HF-related hospitalization. Three patients died due to non-HF-related events. All patients showed improvement in NYHA functional class (class I, n=9; class II, n=3) and significant decrease in the dosages of diuretics prescribed (p<0.05). Kidney function stabilized, while significant improvements in end-diastolic left ventricular diameter (−5%, p<0.05) and hemoglobin count (+15, p<0.05) were achieved. Levels of BNP and aldosterone showed a significant decrease (−46% and −13%, respectively). The authors concluded that incremental PD should represent a new therapeutic option for elderly patients with refractory HF [47].
Peritoneal Ultrafiltration in CHF – Clinical Experience
Peritoneal UF (pUF) in patients with CHF was described for the first time by Schneierson in a 1949 paper entitled “Continuous peritoneal irrigation in the treatment of intractable edema of cardiac origin”. From the 1960s through the 1980s intermittent PD was used as a salvage therapy in severe heart failure resistant to diuretics [18,49]. Since then, more than 300 patients with CHF (NYHA class III to IV) with or without chronic kidney disease have been treated with different forms of peritoneal dialysis, with pUF employed in not more than 100 cases [43,51]. Variations in pUF range from 1 daily long-term exchange with hypertonic glucose or icodextrin solution, through intermittent or incremental PD procedure in patients with GFR above 15.0 mL/min/1.73 m2, to regular CAPD or APD program in patients with CHF and GFR below 15.0 mL/min/1.73 m2[43–49,60].
In 2009, Archives of Internal Medicine published our comprehensive literature review supported by own clinical case report in which we proposed PD as an alternative to HD, able to provide continuous slow ultrafiltration and to improve quality of life in diuretic-resistant CHF patients. This claim was strongly encouraged by very promising results of pUF obtained with 1 daily dwell with icodextrin solution as an osmotic agent [51,52].
The 2010 report by Sanchez et al. assessed the efficacy of PD treatment in 17 refractory CHF patients (64±9 years old) observed for 15±9 months. Thirteen patients underwent only 1 nocturnal icodextrin exchange (2 L), while others were treated with different modifications of PD, depending on the degree of renal failure. All patients improved their NYHA functional status (65% by 2 classes; the rest, by 1; p<0.001), with an important improvement in their pulmonary artery systolic pressure-PASP (44±12 vs. 27±9 mmHg; p=0.007), but with no changes in left ventricular ejection fraction. Hospitalization rates underwent a significant reduction (from 62±16 to 11±5 days/patient/year; p=0.003) before and after PD treatment. PD treatment raised life expectancy to 82% after 12 months of treatment, and 70% and 56% after 18 and 24 months, respectively. The authors also concluded that PD was cost-effective when compared to the conservative therapy [19].
The dramatic decrease in PASP found by Sanchez et al. could potentially be associated with a better clinical outcome. The important prognostic value of an elevated PASP was addressed in a prospective study of Cappola et al. in a group of 1134 patients who underwent right heart catheterization and endomyocardial biopsy, and were followed for 4.4 years. In this study, elevated PASP was found to be the most important hemodynamic predictor of death [62]. Decrease of PASP observed in patients with CHF treated with pUF or different forms of PD may prove helpful in qualifying CHF patients for a heart transplantation procedure [19].
Case Report
The case of successful peritoneal ultrafiltration treatment in a 60-year old patient diagnosed with diuretic-resistant congestive heart failure fulfilling the criteria for type 2 cardio-renal syndrome (CRS) is presented.
A 60-year-old patient admitted to our Institute in December 2010 had been diagnosed with advanced biventricular class IV NYHA heart failure in the course of dilated cardiomyopathy, established atrial fibrillation, secondary pulmonary hypertension, post-pulmonary embolism state, and type 2 diabetes treated with insulin. In September 2010, the patient was provided with an implantable cardioverter-defibrillator device (ICD) with the cardiac resynchronization function.
At admission, overhydration was estimated at 30 kg and daily diuresis did not exceed 500 ml, in spite of administration of intravenous diuretics. Echocardiography detected cardiomegaly, dilated pulmonary artery with decreased pulmonary flow acceleration time (66 seconds), normal thickness of the left ventricular wall, generalized disturbances in left ventricular contractility reflected by the decrease in ejection fraction value (EF, 25%), as well as significant tricuspid valve insufficiency and features of pulmonary hypertension with RVSP of 55 mmHg. At that time, impedance cardiography (ICG) was performed in order to properly assess the patient’s hemodynamic status. Hemodynamic parameters were as follows: low initial stroke index (SI) at 32 ml/m2 ml, high resting heart rate (HR) 91/min, low Heather index (HI), the parameter characterizing cardiac inotropism: 4.5 Ohm/s2, high thoracic fluid content (TFC): 49.7 1/kOhm and moderate systemic vascular resistance index (SVRI): 2052 dyns·cm·−5m2 (Figure 1A).
Figure 1.
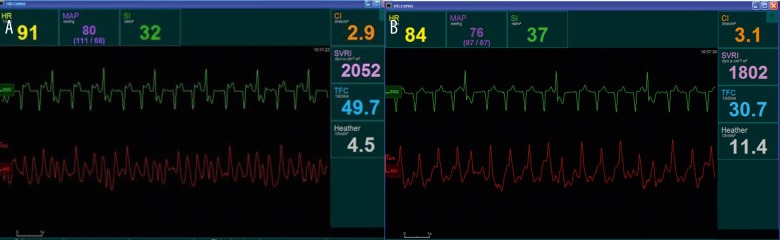
Impedance cardiography at the beginning of the ultrafiltration treatment (A) and 6 months after the initiation of peritoneal ultrafiltration protocol (B).
At that point, as the failure of all non-invasive treatment options was becoming more and more evident, the patient was enrolled in the peritoneal ultrafiltration program. Two weeks after the implantation of a Tenckhoff catheter into the peritoneal cavity, the patient began the ultrafiltration program. Due to the advanced degree of kidney damage (3rd stage of chronic kidney disease and eGFR 39 ml/min/1.73 m2, according to the MDRD formula), he was scheduled for 1 12-hour nighttime dialysis exchange with 2.0 l of glucose polymer – icodextrin as an osmotic agent. The mean ultrafiltration rate was 1000±500 ml and diuresis was 1000 ml/24 hours.
Six months after the initiation of pUF protocol, a significant improvement in the patient’s well-being and standard laboratory test results were observed. His exercise tolerance improved from NYHA class IV to class II/III. Physical examination revealed no ascites, a mild degree of pulmonary hemostasis and slightly pronounced peripheral edema. A reduction in body weight occurred as compared to the December 2010 value of 30 kg. Importantly, the treatment was able to restore the patient’s sensitivity to oral diuretics. Administration of 320 mg of furosemide resulted in a diuresis of 1400–2000 ml per 24 hours.
On echocardiography, increase in ejection fraction from 25% to 32% and decrease in the size of the left atrium from 5.4 to 4.7 cm were observed. RVSP decreased from 55 to 45 mmHg. The serum NT-proBNP level, which is related to the state of overhydration, significantly decreased from 12,853 to 8411 after 6 months of ambulatory pUF (normal values <194).
A follow-up impedance cardiography test was performed after 3 and 6 months and revealed an increase in SI (after 3 months: 33 ml/m2, after 6 months: 37 ml/m2) and HI (after 3 months: 5.5 Ohm/s2, after 6 months: 11.4 Ohm/s2) with concurrent significant progressive decrease in TFC (after 3 months: 35.7 1/kOhm, after 6 months: 30.7 1/kOhm). The increase in SVRI after 3 months to 2182 dyns·cm·−5m2 compelled us to add a low dosage of inhibitor of angiotensin converting enzyme as a precaution (ramipril, 1.25 mg) that resulted in acceptable decrease of SVRI after 6 months: 1802 dyns·cm·−5m2). Figure 1B present the results of ICG after 6 months of pUF.
Importantly, no technical or clinical complications, such as dialysis-related peritonitis, occurred during the above described treatment.
In conclusion, our case report underlines the diagnostic value of impedance cardiography in CHF patients treated with pUF. In this method, in contrast to standard bioimpedance, it is possible to estimate thoracic fluid content as well as other hemodynamic parameters characterizing cardiac systolic function and vascular resistance. Starting with low SI(<35 ml/m2) and high TFC (>35 l/kOhm), after 6 months the patient improved in both parameters. Referring to the study of Packer et al., such improvement decreases the short-term risk of worsening heart failure by almost 7-fold [63]. Importantly, pUF offered a chance for a relatively normal quality of life by giving the patient the option of home dialysis.
Peritoneal Versus Extracorporeal Ultrafiltration in CHF – Concluding Remarks
Both peritoneal and extracorporeal ultrafiltration methods can prove beneficial in HF – extracorporeal iUF should be considered as an option for patients with ADHF, while pUF seems to be advantageous in patients with chronic CHF. Potential drawbacks of pUF in ADHF as compared with extracorporeal UF include the possibility of early leaks and a difficulty in predicting fluid removal in the long-term [18]. Peritoneal ultrafiltration should be considered in chronic HF, particularly in elderly patients due to:
better quality of life (home-based therapy);
more effective control of hypervolemia achieved by slow, daily ultrafiltration;
improvement of circulatory parameters, especially PASP – important in the management of CHF patients qualified for heart transplantation;
possibly more efficient removal of cytokines and myocardial depressant factors involved in the pathogenesis of cardiorenal syndrome in CHF patients, in comparison with extracorporeal iUF with dedicated UF devices;
no need for central venous access and therefore no problems associated with anticoagulation;
lower hospitalization rates for HF in PD than for HD patients still needs further confirmation.
In spite of all the above-mentioned advantages, several unresolved issues remain regarding the use of peritoneal ultrafiltration in HF patients; these need to be addressed in the near future. They include preservation of peritoneum as an ultrafiltration membrane, the question of the possible advantage of long-dwell icodextrin solutions over standard glucose solutions, as well as reduction in hospitalization rates.
Large, properly randomized and controlled clinical trials with long-term follow-up will be essential in assessing the logistics and cost-effectiveness of both methods. Most importantly, however, such trials will be able to evaluate the impact of both methods on preservation of renal function and delaying the progression of heart failure by interrupting the vicious circle of cardiorenal syndrome.
Abbreviations
- HR
heart rate
- MAP
mean arterial pressure
- SI
stroke index
- CI
cardiac index
- SVRI
systolic vascular resistance index
- TFC
thoracic fluid component
- Heather/HI
low Heather index
Footnotes
Source of support: Departmental sources
References
- 1.Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC. ADHERE Scientific Advisory Committee, The Acute Decompensated Heart Failure National Registry (ADHERE): opportunites to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4(Suppl 7):S21–30. [PubMed] [Google Scholar]
- 3.American Heart Association. Heart disease and stroke statistics: 2005 update. Dallas, TX: American Heart Association; 2005. [Google Scholar]
- 4.United Nations Economic Commissions for Europe (UNECE) Trends in Europe and North America. The statistical yearbook of the Economic Commission for Europe (Web page) Geneva: UNECE; 2003. [assesed May 25, 2007]. [Available at: www.unece.org/stats/trend/trend_h.htm. [Google Scholar]
- 5.Lloyd-Jones D, Adams R, Camethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–86. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 6.Korewicki J, Browarek A, Zembala M, et al. Polish registry of patients with severe heart failure scheduled for heart transplant POLKARD-HF 2003–2007. Folia Cardiologica Excerpta. 2008;3:403–21. [Google Scholar]
- 7.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 8.Jessup M, Brozena S. Heart Failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365(9474):1877–89. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- 10.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29( 19):2388–442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN. Structural basis for heart failure. Ventricular remodeling and its pharmacological inhibition. Circulation. 1995;91(10):2504–7. doi: 10.1161/01.cir.91.10.2504. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Zannad F, Sopko G, et al. Acute heart failure syndromes; current state and framework for future research. Circulation. 2005;112:3958–68. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 13.Wertman BM, Gura V, Swarz ER. Ultrafiltration for the Management of Acute Decompensated Heart Failure. J Card Fail. 2008;9(14):754–55. doi: 10.1016/j.cardfail.2008.07.230. [DOI] [PubMed] [Google Scholar]
- 14.Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–39. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 15.Jessup M, et al. 2009 Focused Update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 16.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 17.Park SJ, Tector A, Piccioni W, et al. Left ventricular assist devices as destination therapy: a new look at survival. J Thorac Cardiovasc Surg. 2005;129(1):9–17. doi: 10.1016/j.jtcvs.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan A, Oreopoulos DG. Peritoneal dialysis in congestive heart failure. Adv Perit Dial. 2007;23:82–89. [PubMed] [Google Scholar]
- 19.Sánchez JE, Ortega T, Rodríguez C, et al. Efficacy of peritoneal ultrafiltration in the treatment of refractory congestive heart failure. Nephrol Dial Transplant. 2010;25:605–10. doi: 10.1093/ndt/gfp484. [DOI] [PubMed] [Google Scholar]
- 20.Fiaccadori E, Regolisti G, Maggiore U, et al. Ultrafiltration in heart failure. Am Heart J. 2011;161:439–49. doi: 10.1016/j.ahj.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Asaba H, Bergstrom J, Furst P, et al. Treatment of diuretic-resistant fluid retention with ultrafiltration. Acta Med Scand. 1978;204:145–49. doi: 10.1111/j.0954-6820.1978.tb08416.x. [DOI] [PubMed] [Google Scholar]
- 22.Jaski BE, Ha J, Denys BG, et al. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail. 2003;9:227–31. doi: 10.1054/jcaf.2003.28. [DOI] [PubMed] [Google Scholar]
- 23.Liang KV, Hiniker AR, Williams AW, et al. Use of a novel ultrafiltration device as a treatment strategy for diuretic resistant, refractory heart failure: initial clinical experience in a single center. J Card Fail. 2006;12:707–14. doi: 10.1016/j.cardfail.2006.08.210. [DOI] [PubMed] [Google Scholar]
- 24.Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol. 2005;46:2043–46. doi: 10.1016/j.jacc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 25.Kazory A, Ross EA. Contemporary trends in the pharmacological and extracorporeal management of heart failure: a nephrologic perspective. Circulation. 2008;117:975–83. doi: 10.1161/CIRCULATIONAHA.107.742270. [DOI] [PubMed] [Google Scholar]
- 26.Bouman CSC, Van Olden RW, Stoutenbeek CP. Cytokine filtration and adsorption during pre- and postdilution hemofiltration in four different membranes. Blood Purif. 1998;16(5):261–68. doi: 10.1159/000014343. [DOI] [PubMed] [Google Scholar]
- 27.Morgera S, Slowinski T, Melzer C, et al. Renal replacement therapy with high-cut-off hemofilters: Impact of convection and diffusion on cytokine clearances and protein status. Am J Kidney Dis. 2004;43(3):444–453. doi: 10.1053/j.ajkd.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Gambro Internal data- 2572-SepteX clearances
- 29.Haase M, Bellomo R, Baldwin I, et al. Hemodialysis Membrane With a High-Molecular-Weight Cutoff and Cytokine levels In Sepsis Complicated by Acute Renal Failure: A Phase 1 Randomized Trial. Am J Kidney Dis. 2007;50(2):296–304. doi: 10.1053/j.ajkd.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Costanzo MR, Jessup M. Treatment of congestion in heart failure with diuretics and extracorporeal therapies: effects on symptoms, renal function, and prognosis. Heart Fail Rev. 2011 doi: 10.1007/s10741-011-9248-0. [DOI] [PubMed] [Google Scholar]
- 31.Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology. 2001;96:132–43. doi: 10.1159/000047397. [DOI] [PubMed] [Google Scholar]
- 32.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–64. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 33.Costanzo MR, Saltzberg M, O’Sullivan J, Sobotka P. Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047–51. doi: 10.1016/j.jacc.2005.05.099. [DOI] [PubMed] [Google Scholar]
- 34.Costanzo MR, Guglin ME, Saltzberg MT, et al. UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–83. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 35.Jaski BE, Romeo A, Ortiz B, et al. Outcomes of volume-overloaded cardiovascular patients treated with ultrafiltration. J Card Fail. 2008;14:515–20. doi: 10.1016/j.cardfail.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Rogers HL, Marshall J, Bock J, et al. A randomized, controlled trial of the renal effects of ultrafiltration as compared to furosemide in patients with acute decompensated heart failure. J Card Fail. 2008;14:1–5. doi: 10.1016/j.cardfail.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Costanzo MR, Agostoni P, Marenzi G. Extracorporeal fluid removal in heart failure patients. Contrib Nephrol. 2010;164:173–98. doi: 10.1159/000313730. [DOI] [PubMed] [Google Scholar]
- 38.Kazory A, Ejaz AA, Ross EA. Ultrafiltration for heart failure: how fast should we move? Am Heart J. 2009;157:205–7. doi: 10.1016/j.ahj.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Thodis E, Passadakis P, Vargemezis, Oreopoulos PG. Peritoneal dialysis: better than, equal to, or worse than hemodialysis? Perit Dial Int. 2001;21:25–35. [PubMed] [Google Scholar]
- 40.Wańkowicz Z. Peritoneal dialysis – the role in the integrated renal care, pitfalls and benefits of therapy. Advances in Clinical and Experimental Medicine. 2003;12:2. http://www.advances.am.wroc.pl. [Google Scholar]
- 41.Wańkowicz Z. Peritoneal dialysis and its role in the demography and epidemiology of chronic kidney disease. Pol Arch Int Med. 2009;119:810–13. [PubMed] [Google Scholar]
- 42.Gotloib L, Fudin R, Yakubovich M, Vienken J. Peritoneal dialysis in refractory end-stage congestive heart failure: a challenge facing a no-win situation. Nephrol Dial Transplant. 2005;20(Suppl 7):vii, 32–36. doi: 10.1093/ndt/gfh1105. [DOI] [PubMed] [Google Scholar]
- 43.Ryckelynck JP, Lobbedez T, Valette B, et al. Peritoneal ultrafiltration and treatment -resistant heart failure. Nephrol Dial Transplant. 1998;13(Suppl 41):56–59. doi: 10.1093/ndt/13.suppl_4.56. [DOI] [PubMed] [Google Scholar]
- 44.Díez Ojea B, Rodríguez Suárez C, Vidau P, et al. Peritoneal dialyisis role in heart failure treatment, experience in our center. Nefrologia. 2007;27:605–11. [PubMed] [Google Scholar]
- 45.Montejo JD, Bajo MA, del Peso G, Selgas R. Role played by peritoneal dialysis in treating refractory heart failure. Nefrologia. 2010;30:21–27. doi: 10.3265/Nefrologia.pre2010.Jan.10204. [DOI] [PubMed] [Google Scholar]
- 46.Cnossen TT, Kooman JP, Konings CJ, et al. Peritoneal dialysis in patients with primary cardiac failure complicated by renal failure. Blood Purif. 2010;30:146–52. doi: 10.1159/000321102. [DOI] [PubMed] [Google Scholar]
- 47.Nakayama M, Nakano H, Nakayama M. Novel therapeutic option for refractory heart failure in elderly patients with chronic kidney disease by incremental peritoneal dialysis. J Cardiol. 2010;55:49–54. doi: 10.1016/j.jjcc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Takane H, Nakamoto H, Arima H, et al. Continuous ambulatory peritoneal dialysis is effective for patients with severe congestive heart failure. Adv Perit Dial. 2006;22:141–46. [PubMed] [Google Scholar]
- 49.Wańkowicz Z. Peritoneal dialysis – 40 years of own experiences. Pol Arch Int Med. 2004;112(Spec):19–24. [PubMed] [Google Scholar]
- 50.Próchnicka A, Olszowska A, Baczyński D, et al. Peritoneal dialysis as a therapeutic approach in congestive heart failure resistant to pharmacological treatment. Pol Arch Int Med. 2009;119:815–18. [PubMed] [Google Scholar]
- 51.Próchnicka A, Olszowska A, Baczyński D, et al. Peritoneal dialysis as a therapeutic approach in congestive heart failure resistant to pharmacological treatment: case report. Pol Arch Int Med. 2009;119:834–37. [PubMed] [Google Scholar]
- 52.Olszowska A, Zelichowski G, Waniewski J, et al. The kinetics of water transperitoneal transport during long-term peritoneal dialysis performed using icodextrin dialysis fluid. Pol Arch Int Med. 2009;119:305–10. [PubMed] [Google Scholar]
- 53.Horl WH. Natriretic peptides in acute and chronic kidney disease and during renal replacement therapy. J Investig Med. 2005;53:366–70. doi: 10.2310/6650.2005.53709. [DOI] [PubMed] [Google Scholar]
- 54.Zemel D, Imholz AL, de Waart DR, et al. Appearance of tumor necrosis factor-alpha and soluble TNF-receptors I and II in peritoneal effluent of CAPD. Kidney Int. 1994;46:1422–30. doi: 10.1038/ki.1994.414. [DOI] [PubMed] [Google Scholar]
- 55.Fincher ME, Campbell HT, Sklar AH, et al. Atrial natriuretic peptide (ANP) is removed by peritoneal dialysis in humans. Adv Perit Dial. 1989;5:16–19. [PubMed] [Google Scholar]
- 56.Zoccali C, Mallamaci F, Benedetto FA, et al. Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001;12:1508–15. doi: 10.1681/ASN.V1271508. [DOI] [PubMed] [Google Scholar]
- 57.Chung JH, Yun NR, Ahn CY, et al. Relationship between Serum N-Terminal Pro-Brain Natriuretic Peptide Level and Left Ventricular Dysfunction and Extracellular Water in Continuous Ambulatory Peritoneal Dialysis Patients. Electrolyte Blood Press. 2008;6(1):15–21. doi: 10.5049/EBP.2008.6.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mistry CD, Gokal R, Peers E, et al. A randomized multicenter clinical trial comparing isosmolar icodextrin with hypersmolar glucose solutions in CAPD. Kidney Int. 1994;46:496–503. doi: 10.1038/ki.1994.300. [DOI] [PubMed] [Google Scholar]
- 59.Basile C, Chimienti D, Bruno A, et al. Efficacy of peritoneal dialysis with icodextrin in the long-term treatment of refractory congestive heart failure. Perit Dial Int. 2009;29:116–18. [PubMed] [Google Scholar]
- 60.Paniaqua R, Orihuela O, Avila-Diaz M, et al. Echocardiographic, electrocardiographic and blood changes induced by icodextrin solution in diabetic patients on peritoneal dialysis. Kidney Int. 2008;73:125–30. doi: 10.1038/sj.ki.5002613. [DOI] [PubMed] [Google Scholar]
- 61.Mehrotra R, Nolph KD, Gotch F. Early initiation of chronic dialysis: role of incremental dialysis. Perit Dial Int. 1997;17:426–30. [PubMed] [Google Scholar]
- 62.Cappola TP, Felker GM, Kao WH, et al. Pulmonary hypertension and risk of death in cardiomyopathy: patients with myocarditis are at higher risk. Circulation. 2002;105(14):1663–68. doi: 10.1161/01.cir.0000013771.30198.82. [DOI] [PubMed] [Google Scholar]
- 63.Packer M, Abraham WT, Mehra MR, et al. Utility of impedance cardiography for the identification of short-term risk of clinical decompensation in stable patients with chronic heart failure. J Am Coll Cardiol. 2006;47(11):2245–52. doi: 10.1016/j.jacc.2005.12.071. [DOI] [PubMed] [Google Scholar]


