Summary
Non-muscle-invasive urothelial carcinoma of the bladder has a high rate of recurrence, necessitating use of a variety of adjuvant treatments. A single, immediate post-operative administration of chemotherapy is an important measure for reducing the rate of recurrences, but the overall effect is not satisfactory and the treatment is a burden to the patient. Hence, there is a need for a new, more effective and cheaper agent for adjuvant treatment of non-muscle-invasive bladder carcinomas.
Although cationic surfactants such as benzalkonium salts are used clinically and hygienically for the control of bacterial growth, there have been reports that cationic surfactants such as benzethonium chloride induce apoptosis in normal and in cancerous cells. We report our experience with benzalkonium bromide (BB) accidentally administered into a patient‘s bladder as saline. It caused severe hematuria and pain, but after a week of persistent administration in the bladder, the patient was cured, as supported by evidence from cystoscopy, indicating that BB destroys bladder mucosa and suggesting that BB maybe a novel agent for use in a single, immediate post-operative administration. We present preliminary data to support this hypothesis and provide discussion we hope will be useful as the foundation for further experiments.
Keywords: benzalkonium bromide, bladder cancer, intravesical therapy
Background
Bladder cancer incidence ranks ninth of all cancers in the world, but it has the highest lifetime treatment costs per patient [1]. Approximately 70% of newly detected cases are exophytic papillary tumors, which are large found in the mucosa or submucosa as non-muscle invasive disease [2]. Transurethral resection of bladder tumors (TURBT) is the current standard of care defined by both European Association of Urology and American Urological Association guidelines. However, it is common for patients with non-muscle invasive tumor to suffer a recurrence or new occurrence. (1 year: 15–61%; 5 years: 31–78%) [3].
All kinds of adjuvant measurements, which include intravesical chemotherapy and intravesical immunotherapy, photodynamic diagnosis with blue light in TURBT process, second TURBT and so on, are used to reduce incidence rate, but the overall effect is not satisfactory and imposes a burden on the patient [1]. Decreasing the high recurrent rate of this kind of bladder tumor is still a big challenge for urologists and oncologists [4].
One immediate post-operative instillation of chemotherapy should be given in all patients after TUR of presumably non-muscle invasive bladder cancer, but, although it reduced the rate of recurrences, it has a limited role [5]. Hence, there is a need for a new, more effective and cheaper agent for adjuvant treatment of non-muscle invasive bladder tumors [2]. Urothelial carcinomas are well-known to feature multifocal development in the urinary tract, both synchronously and asynchronously, both in early and in late stages [1]. This phenomenon can be explained as either seeding of cancer cells in the urinary tract or field cancerization [6]. If we remove soil of seeding or cancerized field at an early stage, there will be a sharp decrease of non-muscle invasive tumor recurrence [7].
The Hypothesis
Our initial idea that benzalkonium bromide (BB) maybe a novel agent for immediate post-operative instillation came from an accident. BB was once administered into a patient’s bladder as saline by mistake and it caused severe hematuria and pain, but after a week of persistent bladder instillation the patient was cured. This was also supported by cystoscopy, indicating that BB destroys bladder mucosa. Moreover, it is reported that benzalkonium salt has injured human conjunctival cells [8] and nasal mucosa [9], showing the features of cell apoptosis [8,10,11]. Enomato et al report that cationic surfactants such as benzethonium chloride induce apoptosis in cancer cells [12]. Since benzalkonium bromide and benzalkonium salt are derivatives of benzalkonium, we supposed that BB may reversibly damage the urothelium of bladder according to the reasons above.
Evaluation of the Hypothesis
To test this hypothesis, we conducted the pilot study to understand what effect BB has on urothelium in rats.
Material and Methods
Experimental protocol
Experiments were carried out on 60 male Sprague-Dawley rats (Animal Center, The Second Xiangya Hospital, Central South University) aged 7 weeks and weighing 150–170 g at the beginning of the study. They were housed in groups under controlled conditions (12 h light/dark cycle, light on at 07: 00 a.m., 22±2°C) and free access to water and food.. All rats were handled in the colony room once per day for 7 days prior to the experiment. The animal care and experimental protocol was approved by The 2nd Xiangya Hospital, Central South University, Changsha, China.
Study design
In a preliminary experiment we found the tolerant concentration which a rat could survive was below 1‰. As a matter of convenience, we used 0.5‰ concentration. A total number of 60 SD rats was assigned into 2 groups (30 each group). Under anaesthesia, Group A (0.5‰ BB) were administered with 0.5 ml corresponding concentration BB, while Group B were administered with 0.5 ml saline as control. Then after 5 minutes both groups were washed by saline.
Ten rats from each group (30 rats) were sacrificed on the 1st day, 3rd day, and 7th day of instillation. At the same time, blood of sacrificed rats was analyzed for creatinine (Cr), alanine transaminase (ALT) and aspartate transaminase (AST) concentrations. The bladders were removed from sacrificed rats and placed in 10% formalin, paraffin embedding. Sections were stained with hematoxylin and eosin (H and E) and examined under light microscope. Result was assessed by the grade score (Table 1).
Table 1.
Grade score criterion in histology.
| Description | Score | Description |
|---|---|---|
| Less than 25% musoca disppear | 4 | Non or minimum inflammatory infiltrate |
| 25% to 50% musoca disppear | 3 | Submucosa inflammatory infiltrate |
| 50% to 75% mucosa disppear | 2 | Superficial detrusor inflammatory infiltrate |
| More than 75% mucosaobiteration | 1 | Deep detrusor inflammatory infiltrate |
Data were expressed as the mean SD for renal and live function, and mean value for histology description. Differences in means were examined by Mann-Whitney U-test and P<0.05 was considered statistically significant. Statistical analysis was done using SPSS, version 13.0 for Windows (SPSS, Chicago, IL).
Results
After 1 instillation of 0.5‰ BB or saline, all rats survived well. After instillation, all rats in Group A were found to have hematuria, hypergasia and loss of appetite, but not coma. The condition of the control group (Group B) did not change. On the 1st day of instillation in Group A, we found there were some histological changes containing a small portion of mucosa disappear, edema of wall, congested vessels, a few red cells and serous fluid in the lumen (Figure 1). On the 3rd day of instillation in Group A, we found histologically a majority of rats had mucosa epithelium necrosis, slough, edema of laminae propria, and moderate inflammatory infiltration of wall (Figure 2). On the 7th day of instillation in Group A, the mucosal epitheliums were intact, and there was mild inflammatory infiltration of wall (Figure 3). However, Group B didn’t have evident histological changes.
Figure 1.
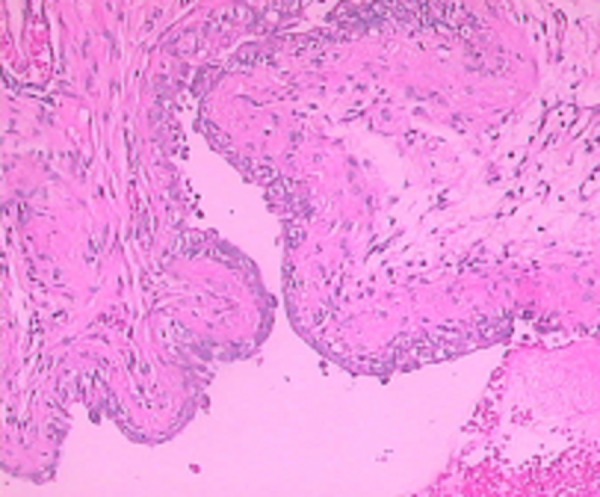
On the 1st day, histological changes in Group A.
Figure 2.
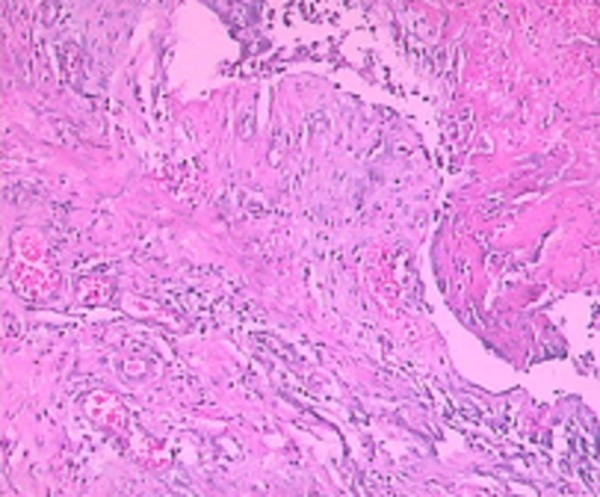
On the 3rd day, histological changes in Group A.
Figure 3.
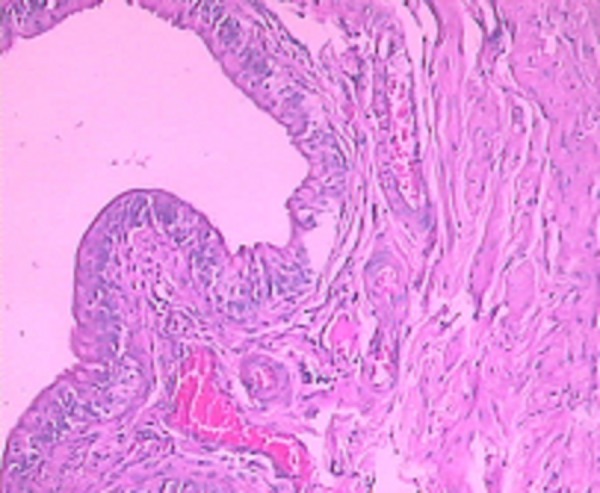
On the 7th day, histological changes in Group A.
The scores of Group A were significant lower than those of Group B (P<0.05) at each time-point measured. In Group A, the score significantly declined from the 1st day to the 3rd day (P<0.05), while on the 7th day it was significantly increased (P<0.05), which indicated very quick healing of the bladder wound. In Group B, there were no statistical differences between the 1st, 3rd and 7th days (P>0.05) (Table 2).
Table 2.
Mean histological score in both groups.
| Group | Mean score | ||
|---|---|---|---|
| 1 day | 3 day | 7 day | |
| A | 6.4 | 5.2 | 6.7 |
| B | 7.7 | 7.7 | 7.9 |
There were no significant differences in liver and renal function between the 2 groups (P>0.05) (Table 3).
Table 3.
Mean liver and renal function between the 2 groups.
| Time | Group | ALT (IU/L) | AST (IU/L) | BUN (mmol/L) | Cr (umol/l) |
|---|---|---|---|---|---|
| 1 day | A | 64.34±3.57 | 275.56±37.73 | 10.36±0.23 | 69.92±5.68 |
| B | 58.56±4.24 | 265.94±25.32 | 9.04±0.46 | 65.58±5.74 | |
| 3 day | A | 59.04±2.53 | 255.54±49.64 | 9.75±0.52 | 70.46±4.74 |
| B | 58.54±9.64 | 262.68±32.74 | 8.79±0.84 | 62.70±4.73 | |
| 7 day | A | 59.99±5.00 | 269.67±31.87 | 9.42±0.17 | 69.42±3.11 |
| B | 61.21±3.33 | 273.05±44.61 | 9.82±0.51 | 71.16±4.21 |
Discussion
The primary treatment of non-muscle invasive bladder cancer is transurethral resection of the bladder, but up to 70% of the tumors will recur, thus intravesical chemotherapy and immunotherapy are used as adjuvant treatments after transurethral resection. Both therapies can reduce the high rate of recurrence, but the effects are not satisfactory. The unquestionable success of a single intravesical instillation of chemotherapy immediately after transurethral resection in preventing tumor recurrences would seem to indicate that clonal implantation is a major mechanism of recurrence in the early stage [13]. However, in many series, well fewer than 50% of recurrences are “prevented”, implying that if the chemotherapy was very effective against implantation, many recurrences are due to field effect mechanisms in early and late stages [14]. It is likely that both mechanisms take place [15].
Cationic surfactants such as benzalkonium salts are clinically and hygienically used for the control of bacterial growth [16]. Benzalkonium salts comprise a group of positively charged surface-active alkylamine biocides with the general formula alkyldimethyl benzylammonium chloride or bromide. They interact with guanine nucleotide triphosphate-binding proteins (G proteins), thereby affecting signal transduction in a variety of cell types and processes [17]. Therefore, benzalkonium salts not only appear to be effective as disinfectants and spermicides, but may also prove useful in the prevention and treatment of neoplasias and other disease, particularly those linked to viruses and originating on the skin or mucosal surface [18]. BB, which is similar to benzalkonium salt as a derivative of benzalkonium, in our study indicated that a certain concentration range of BB can reversibly damage the urothelium in rats, who needed about 7 days to recover.
The morphological findings described in our study indicate that BB can not only induce necrosis in the normal bladder mucosa, but also does not hamper mucosal regeneration. Because of similarity of urothelial cells between humans and rats, and according to Enomato’s study [12], we speculate that BB also could destroy pathological bladder mucosa such as invisible tumor cells (undetected tumor or shedding cells from tumor), thereby decreasing bladder tumor recurrence; however, this needs further validation. Our study shows that firstly BB acts on bladder mucosa definitely by single use; secondly, instillation of BB has obviously no adverse effect on the liver and kidney function, implying that it could act as a new intravesical instillation drug for immediate management after TURBT if it is approved for the intravesical treatment of non-muscle invasive bladder cancer. Furthermore, BB is attractive because of it is extensively used clinically and is very cheap. In view of the direct destruction of urothelial cells and the pain induced by BB, it is suitable as an intravesical instillation agent after TURBT, which plays a role as chemical resection or destroy. Thereafter, the postoperative pain and hematuria can be resolved via routine therapy.
Conclusions
Since BB can destroy the normal bladder mucosa and does not hamper its regeneration, we speculate that BB also could destroy pathological bladder mucosa. This drug may prove useful for intravesical instillation if our hypothesis is confirmed. Our study achieved 2 aims: firstly, it proved that BB acted on bladder mucosa of rats definitely and reversibly; secondly, it verified the proper concentration of BB on bladder mucosa of rats. These findings will be the foundation of further study of BB as a novel intravesical medicine.
Footnotes
Source of support: Departmental sources
References
- 1.Sievert KD, Amend B, Nagele U, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009;27(3):295–300. doi: 10.1007/s00345-009-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurtowska N, Kloskowski T, Drewa T. Ciprofloxacin criteria in antimicrobial prophylaxis and bladder cancer recurrence. Med Sci Monit. 2010;16(10):RA218–23. [PubMed] [Google Scholar]
- 3.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–77. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 4.Wirth M, Plattner VE, Gabor F. Strategies to improve drug delivery in bladder cancer therapy. Expert Opin Drug Deliv. 2009;6(7):727–44. doi: 10.1517/17425240903022758. [DOI] [PubMed] [Google Scholar]
- 5.Babjuk M, Oosterlinck W, Sylvester R, et al. EAU Guildelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59(6):997–1008. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Kakizoe T. Development and progression of urothelial carcinoma. Cancer Sci. 2006;97(9):821–28. doi: 10.1111/j.1349-7006.2006.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braakhuis BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63(8):1727–30. [PubMed] [Google Scholar]
- 8.De Saint Jean M, Brignole F, Bringuier AF, et al. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40(3):619–30. [PubMed] [Google Scholar]
- 9.Riechelmann H, Deutschle T, Stuhlmiller A, et al. Nasal toxicity of benzalkonium chloride. Am J Rhinol. 2004;18(5):291–99. [PubMed] [Google Scholar]
- 10.Debbasch C, Brignole F, Pisella PJ, et al. Quaternary ammoniums and other preservatives’ contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest Ophthalmol Vis Sci. 2001;42(3):642–52. [PubMed] [Google Scholar]
- 11.Hamard P, Blondin C, Debbasch C, et al. In vitro effects of preserved and unpreserved antiglaucoma drugs on apoptotic marker expression by human trabecular cells. Graefes Arch Clin Exp Ophthalmol. 2003;241(12):1037–43. doi: 10.1007/s00417-003-0777-7. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto R, Suzuki C, Ohno M, et al. Cationic surfactants induce apoptosis in normal and cancer cells. Ann NY Acad Sci. 2007;1095:1–6. doi: 10.1196/annals.1397.001. [DOI] [PubMed] [Google Scholar]
- 13.Solsona E, Iborra I, Ricós JV, et al. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term followup. J Urol. 1999;161(4):1120–23. [PubMed] [Google Scholar]
- 14.Wein AJ, Kavoussi LR, Novick AC, et al. 2007 Campell-Walsh Urology. In: Dahl DM, Scott McDougal W, editors. Urothelial Tumors of the Bladder. 9th edn. Chapter 75 WB Saunders; Philadelphia Section XV: [Google Scholar]
- 15.Duggan B, Williamson K. Molecular markers for predicting recurrence, progression and outcomes of bladder cancer (do the poster boys need posters?) Curr Opin Urol. 2004;14(5):277–86. doi: 10.1097/00042307-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 16.McBain AJ, Ledder RG, Moore LE, et al. Effects of quaternary-ammonium-based formulations on bacterial community dynamics and antimicrobial susceptibility. Appl Environ Microbiol. 2004;70(6):3449–56. doi: 10.1128/AEM.70.6.3449-3456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patarca R, Fletcher MA. Effects of benzalkonium salts on eukaryotic and microbial G-protein-mediated processes and surface membranes. Crit Rev Oncog. 1995;6(3–6):327–56. doi: 10.1615/critrevoncog.v6.i3-6.80. [DOI] [PubMed] [Google Scholar]
- 18.Patarca R, Rosenzwei JA, Zuniga AA, et al. Benzalkonium salts: effects on G protein-mediated processes and surface membranes. Crit Rev Oncog. 2000;11(3–4):255–305. [PubMed] [Google Scholar]


