Summary
Background
Recent evidence shows that subjects diagnosed with an autism spectrum disorder (ASD) have significantly lower levels of glutathione than typically developing children. The purpose of this study was to examine the use of two commonly used glutathione supplements in subjects diagnosed with an ASD to determine their efficacy in increasing blood glutathione levels in subjects diagnosed with an ASD.
Material/Methods
The study was an eight-week, open-label trial using oral lipoceutical glutathione (n=13) or transdermal glutathione (n=13) in children, 3–13 years of age, with a diagnosis of an ASD. Subjects underwent pre- and post-treatment lab testing to evaluate plasma reduced glutathione, oxidized glutathione, cysteine, taurine, free and total sulfate, and whole-blood glutathione levels.
Results
The oral treatment group showed significant increases in plasma reduced glutathione, but not whole-blood glutathione levels following supplementation. Both the oral and transdermal treatment groups showed significant increases in plasma sulfate, cysteine, and taurine following supplementation.
Conclusions
The results suggest that oral and transdermal glutathione supplementation may have some benefit in improving some of the transsulfuration metabolites. Future studies among subjects diagnosed with an ASD should further explore the pharmacokinetics of glutathione supplementation and evaluate the potential effects of glutathione supplementation upon clinical symptoms.
Keywords: autism, glutathione, transsulfuration metabolites, oral, transdermal
Background
Glutathione is a small protein made from three amino acids: glycine, cysteine, and glutamic acid. Glutathione is important because it serves several functions in the body. Glutathione is an antioxidant, necessary for the neutralizing of reactive oxygen species (or free radicals), as well as the regeneration of other antioxidants such as vitamins C and E [1,2]. Glutathione is necessary for optimal detoxification or the removal of toxic substances from the body. In addition, glutathione is important for immune function [3,4].
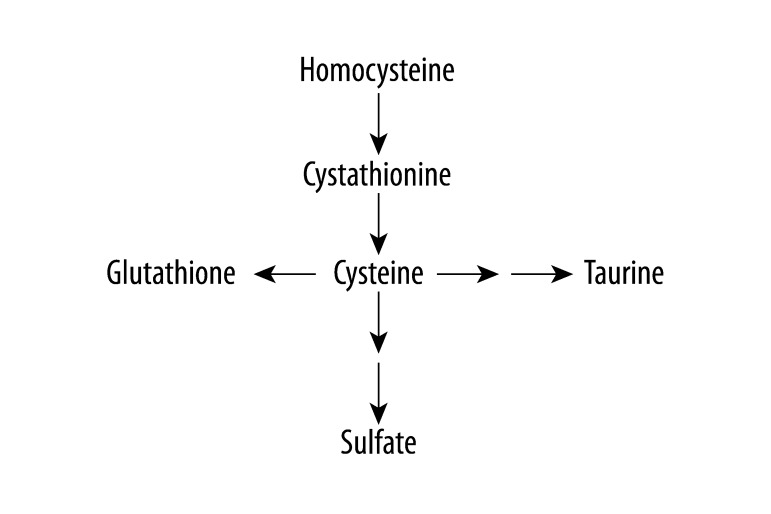
Recent evidence shows that children diagnosed with an ASD have lower levels of plasma reduced glutathione (generally 20–40% lower) than typically developing children and their levels of oxidized glutathione are higher than typically developing children [5–8]. Glutathione is produced in the transsulfuration pathway (Figure 1). Other abnormalities have been found in the transsulfuration metabolites in children diagnosed with an ASD [7], including lower levels of taurine, sulfate, and cysteine [5]. Cysteine is the rate limiting substrate for glutathione production [2].
Figure 1.
Transsulfuration pathway.
Finding ways to safely improve glutathione and other transsulfuration metabolites levels in these children would likely be beneficial to them. Anecdotal reports suggest that oral and transdermal glutathione are currently being used by clinicians to improve glutathione levels in children diagnosed with an ASD. However, empirical evidence in regard to their efficacy is lacking. Some research suggests that glutathione blood levels are not affected by taking glutathione, but that glutathione is made by providing the building blocks or precursors [2].
The purpose of this study was to examine the use of two commonly used supplements in subjects diagnosed with an ASD, transdermal glutathione and oral glutathione, to determine their efficacy in raising glutathione levels and the effects of those supplements on other transsulfuration metabolites in children with an ASD diagnosis.
Material and Methods
Overview
The study was an open-label trial of two supplemental programs using two groups of children diagnosed with an ASD (one group for each supplement). The children were 3 to 13 years of age. Each child had a baseline assessment and laboratory measures completed at week 0 and children with a plasma reduced glutathione level below the normal range (3.8–5.5 μmol/L) from Vitamin Diagnostic’s were randomized into one of the two supplemental programs. Participants remained in their respective program for 8 weeks. At Week 8, the laboratory measures were repeated. Side-effect burden and treatment adherence were assessed.
Location and compliance
The study was conducted at the Autism Treatment Center (Dallas, Texas). Phlebotomy took place at Medical Center Plano, Outpatient Phlebotomy (Plano, Texas).
The study protocol received Institutional Review Board (IRB) approval from Liberty IRB, Inc. (Deland, Florida). All parents signed a consent and Health Insurance Portability and Accountability Act (HIPAA) form and all received a copy.
Participants
The present study looked at consecutive qualifying participants (n = 39) who were prospectively recruited from the community of Dallas/Fort Worth, Texas area. All of the children had a diagnosis of an ASD. Children included in the present study were between 3–13 years of age and had an initial Childhood Autism Rating Scale (CARS) score of at least 30 [9,10]. The children could not be on any medications or supplements that have an effect on glutathione. Permissible medications or other supplements that the children were taking were held constant during the course of the study. Though none were identified, this study was designed to exclude children who had a history of Fragile X syndrome, tuberous sclerosis, phenylketonuria (PKU), Lesch-Nyhan syndrome, fetal alcohol syndrome, or any history of maternal illicit drug use.
Of the 39 children who were prospectively recruited, 33 were randomly started on either oral or transdermal glutathione. The children who were excluded either had normal pretest glutathione results (3 children) or underwent a change in treatment (3 children) disqualifying them from continuing. Of the 33 children who started the trial, 26 finished the treatment. Two children were lost to follow-up and five children were withdrawn due to significant adverse side-effects. Demographics of the children who completed the study are shown in Table 1.
Table 1.
This table is a summary of the subjects with an ASD diagnosis finishing the treatment regimen employed in the present study.
| Descriptive information | Trans-dermal glutathione group | Oral glutathione group |
|---|---|---|
| Sex/age | ||
| Male/female (ratio) | 12/1 (12:1) | 10/3 (3.3:1) |
| Mean age in years ±Std (range) | 5.8±1.5 (4–9) | 7.5±2.9 (3–13) |
| Mean birth year ±Std (range) | 2001±1.6 (1998–2003) | 1999±2.8 (1995–2003) |
| Race (n) | ||
| Caucasian | 69.2% (9) | 69.2% (9) |
| Hispanic | 7.7% (1) | 0% (0) |
| Black | 7.7% (1) | 15.4% (2) |
| Asian | 7.7% (1) | 7.7% (1) |
| Mixed | 7.7% (1) | 7.7% (1) |
| Autistic disorder characteristics | ||
| Mean CARS score ± Std (range) | 34.8±6.04 (30–50) | 41.3±5.1 (30–51) |
| Regressive (n)* | 61.5% (8) | 61.5% (8) |
| Non-regressive (n) | 38.5% (5) | 38.5% (5) |
| Autism (n) | 61.5% (8) | 77% (10) |
| Autism spectrum disorders (n)** | 38.5% (5) | 23% (3) |
| Previous treatments | ||
| Supplements (n) | 23.1% (3) | 23.1% (3) |
| Chelation (n) | 0% (0) | 0% (0) |
| Supplements + chelation (n) | 7.7% (1) | 23.1% (3) |
Std – standard deviation. All participants examined in the present study were living in the state of Texas.
Includes participants that had a regressive event in development at any time following birth;
Autism spectrum disorders include participants diagnosed with pervasive developmental disorder – not otherwise specified (PDD-NOS) and Asperger’s disorder.
Clinical evaluation
As a baseline, the researchers obtained information regarding demographics, formal diagnosis, age at diagnosis, age of apparent onset, information regarding delay or regression, any current medical issues, medications, and allergies on each child. A baseline CARS evaluation was also performed by Dr. Kern, who was trained in the use of CARS, and has 12 years experience in using the CARS to evaluate hundreds of children with an ASD diagnosis. Dr. Kern interviewed the parents and observed each child.
Lab evaluation
Following the intake evaluation, each participant in the study had blood samples collected. The laboratory specimens were all collected in the morning following an overnight fast. Specimens were immediately taken to and processed at LabCorp in Medical City Hospital (Dallas, Texas) and then shipped overnight to Vitamin Diagnostics, Inc. (Cliffwood Beach, New Jersey) (CLIA-approved) and Genova Diagnostics (Ashville, North Carolina) (CLIA-approved). Tests completed at Vitamin Diagnostics, Inc. were: plasma glutathione (reduced and oxidized); plasma SO4 (free); plasma SO4 (total); plasma cysteine; and plasma taurine. The test completed at Genova Diagnostics was: whole-blood glutathione. The laboratories used in the present study were blinded and received no information regarding the clinical status of any of the participants examined.
Supplementation programs
The following is a description of the two supplementation programs, oral glutathione and transdermal glutathione. In both, dosing began at one-fourth dose for 5 days, then one-half dose for 5 days, then three-fourths dose for 5 days, and then full dose by approximately the third week, depending on how well the treatment was tolerated. These glutathione products were chosen because they are commonly used in treating those with an ASD diagnosis.
Group 1: Lipoceutical GSH (oral)
Lipoceutical GSH is made by Your Energy Systems in Palo Alto, California. Because glutathione taken orally is not well-absorbed and utilized, glutathione, in this product, is placed in tiny nanosize spheres called liposomes, which are absorbed into the body. The liposomes are derived from lecithin. The ingredients in this product were reduced L-glutathione, lecithin, glycerin, and potassium chlorate. The dosing recommended for children was: 50 mg (1/8th teaspoon) for every 30 lbs twice a day for 5 days; 100 mg (1/4th teaspoon) for every 30 lbs twice a day for 5 days; 150 mg (3/8th teaspoon) for every 30 lbs twice a day for 5 days; 200 mg (1/2 teaspoon) for every 30 lbs twice a day as tolerated thereafter. Participants were instructed to take it on an empty stomach.
Group 2: Transdermal GSH
Kirkman’s Glutathione Lotion is made by Kirkman in Lake Oswego, Oregon. The product is oily in nature since stable glutathione products cannot contain water because water promotes glutathione oxidation to oxidized glutathione. The ingredients in this product were isopropyl myristate, mineral oil, caprylic/capric triglyceride, vitamin E acetate (soy free), and L-glutathione. Kirkman’s glutathione lotion is generally dosed at one external application of one gram, two to three times daily. Each gram (one level scoop) of Kirkman Glutathione Lotion contains 180 mg of reduced L-glutathione. The product comes with a one-gram measuring scoop. Product is to be shaken well before each use to ensure uniformity. Because of its oily nature, it is recommended the lotion be applied on areas of the body with a large surface area, such as shoulders, back, upper thighs, etc. The dosing recommended for children was: one/fourth external application of one gram, three times daily for 5 days; one/half external application of one gram, three times daily for 5 days; three/fourths external application of one gram, three times daily for 5 days; one external application of one gram, two to three times daily as tolerated thereafter.
Measures
Childhood Autism Rating Scale (CARS)
The CARS was completed by the investigator (JKK) by observing the subjects and interviewing parent(s). The CARS is a recognized 15-item behavioral rating scale developed to identify autism as well as to quantitatively describe the severity of the disorder [9]. A total score of 15–29.5 is considered nonautistic; a score of 30–36.5 is considered mild to moderate autism; a score from 37–60 is considered moderate to severe autism. The CARS is a well-established measure. The internal consistency reliability alpha coefficient is 0.94; the inter-rater reliability correlation coefficient is 0.71; and the test-retest correlation coefficient is 0.88. CARS scores have high criterion-related validity when compared to clinical ratings during the same diagnostic sessions, with a significant correlation of 0.84 [9].
Treatment Adherence Measure (TAM)
The TAM is a ten-item self-report on treatment adherence that asks specific questions regarding the dose and frequency of use. The TAM was completed by the parent at the end of the study and used to calculate the level of adherence to the treatment. It is a Morisky-type self-report adherence measure, designed to measure treatment adherence. Morisky-type adherence measures have been used widely and shown good reliability as a self-report measure [11].
Frequency and Intensity of Side-effect Rating (FISER)/Global Rating of Side-effect Burden (GRSEB)/ Patient Report of Incidence of Side-Effects (PRISE)
The FISER/GRSEB/PRISE include global measures, each using a 7-point Likert-type scale rated 0–6, one rating anchored for frequency, another rating the intensity of side-effects encountered in the prior week that the caregiver believes were due to the treatment, and the third asking caregivers to estimate the overall burden or degree of interference in day-to-day activities and function due to the side-effects attributable specifically to the treatment [12]. Frequency of side-effects is rated as a percent time present: 0 = no side-effects; 1 = present 10% of the time; 2 = 25% of the time; 3 = 50% of the time; 4 = 75%; 5 = 90%; and 6 = present all of the time. Intensity of side-effects ranges from 0 = no side-effects to 6 = intolerable side-effects. Impairment due to side-effects ranges from 0 = no side-effects to 6 = unable to function at all due to side-effects. The PRISE lists a variety of possible side-effects to choose from and a scale to rate the specific side-effect. The list of side-effects also includes gastrointestinal side-effects. The measure also has a place to list any side-effects not previously listed.
Statistical analyses
The statistical package contained in StatsDirect (StatsDirect Ltd., Cheshire, UK; Version 2.6.3) was utilized in the present study. In order to evaluate the pre-versus post-test results obtained in the present study, the non-parametric Wilcoxon matched-pairs signed-ranks test statistic was utilized. The null hypothesis was that there would be no difference in the data distributions pre- versus post-treatment. A two-tailed p-value ≤0.05 was considered to be statistically significant.
Results
A summary of the effects of treatment on transsulfuration metabolites including plasma and whole-blood glutathione is in Table 2. The oral treatment group showed significant increases in plasma reduced glutathione. The whole-blood glutathione level slightly increased in both treatment groups (9–12%), but the increase was not statistically significant. Both groups showed significant increases in plasma sulfate, cysteine, and taurine. There was little change in plasma oxidized glutathione in either group. Plasma free sulfate rose slightly in both groups, but the increase was not statistically significant.
Table 2.
This table is a summary of the effects of treatment on transsulfuration metabolites.
| Laboratory test | Treatment group | Pre-treatment* | Post-treatment* | % change | p-value** |
|---|---|---|---|---|---|
| Trans-dermal glutathione (n) | |||||
| Plasma cysteine (μmol/L) | 12 | 19.4±11.2 | 24.7±12.9 | 21 | <0.05 |
| Plasma taurine (μmol/L) | 13 | 51.4±21 | 68.1±32.2 | 25 | <0.05 |
| Plasma reduced glutathione (μmol/L) | 13 | 3.06±0.57 | 3.43±0.4 | 11 | NS |
| Plasma oxidized gluathione (μmol/L) | 13 | 0.47±0.17 | 0.45±0.16 | −4 | NS |
| Plasma sulfate (μmol/g P) | 13 | 950±186 | 1,095±231 | 13 | <0.01 |
| Plasma free sulfate (μmol/g P) | 13 | 1.23±0.37 | 1.47±0.45 | 16 | NS |
| Whole-blood glutathione (μmol/L) | 11 | 977±275 | 1.075±336 | 9 | NS |
| Oral glutathione (n) | |||||
| Plasma cysteine (μmol/L) | 13 | 17.6±8.5 | 24±10.6 | 27 | <0.05 |
| Plasma taurine (μmol/L) | 13 | 45.8±12.7 | 56.6±13.6 | 19 | <0.05 |
| Plasma reduced glutathione (μmol/L) | 13 | 3.22±0.54 | 3.83±0.63 | 16 | <0.05 |
| Plasma oxidized glutathione (μmol/L) | 13 | 0.53±0.15 | 0.57±0.13 | 7 | NS |
| Plasma sulfate (μmol/g P) | 13 | 863±291 | 1,087±188 | 21 | <0.01 |
| Plasma free sulfate (μmol/g P) | 13 | 1.41±0.52 | 1.6±0.5 | 12 | NS |
| Whole-blood glutathione (μmol/L) | 13 | 984±371 | 1,106±317 | 12 | NS |
Mean ± Standard Deviation;
Wilcoxon Matched-Pairs Signed-Ranks Test Statistic.
NS – Not Statistically Significant.
Treatment compliance among the participants who finished the study was good. Frequency and dosing was well adhered to such that most participants did not miss any doses and less than 10% missed a dose once or twice.
Side-effects, as mentioned earlier, were noted. Of the 33 children started on the treatment, none to minimal side-effects were noted in 17 children (51.5%); mild side-effects were noted in 10 children (30.3%); moderate side-effects were noted in 1 child (3.0%); and intolerable side-effects were noted in 5 children (15.1%). Of the five children whose side-effects were considered intolerable, 2 were due to a rash (transdermal glutathione) and 3 were from irritability (2 from transdermal glutathione and 1 from oral and transdermal glutathione).
Discussion
Plasma sulfate, cysteine, and taurine improved significantly in both treatment groups, and plasma reduced glutathione improved significantly in the oral glutathione group. Plasma glutathione may have improved slightly in the transdermal glutathione group (11%), but the small change was not statistically significant and may not be clinically significant. These findings suggest that in children that tolerate the treatment, transdermal and oral glutathione may be used to improve levels of some of the transsulfuration metabolites. However, the increase in the sulfate, cysteine, and taurine may have been due to breakdown of the glutathione and not a true improvement in the transsulfuration pathway. Previous research by Fukagawa et al. suggests it might be due to glutathione breakdown [13]. Fukagawa et al., using a tracer, found that, after infusion of glutathione, blood cysteine increased by 61%, which was essentially equivalent to the rate of exogenous glutathione infusion. The authors stated that the data suggest that glutathione breakdown accounted for all the increase in measured cysteine turnover during exogenous glutathione infusion.
The present study showed an increase in plasma glutathione with oral glutathione use. Although there are no studies on transdermal glutathione, Hagen et al. found that, in rats, direct supplementation of oral glutathione does improve plasma glutathione levels and ultimately improved oxidative stress [14]. We did not see a change in whole-blood glutathione which suggests that increasing intracellular glutathione may require the use of precursors or building blocks for glutathione. The current literature suggests that supplementing the diet with glutathione in order to increase glutathione levels may not be optimal because glutathione is not transported into the cell [15]. For the most part, the human cell cannot absorb the glutathione molecule and it must be synthesized intracellularly [2,15]. The only study the authors found on the use of oral or transdermal glutathione in humans was a study by Witschi et al. that examined the systemic availability of glutathione in seven healthy volunteers after a single oral dose of 3 g of glutathione [16]. These results were not significant; however, sample size and methodology may have been a factor. Aebi et al. evaluated plasma and urinary total glutathione levels following intravenous infusion of 2 g/m2 of glutathione [17]. These investigators observed significant increases in the plasma and urinary total glutathione levels following the infusion, but the overall half-life of glutathione was very short at 14.1±9.2 min.
Conclusions
Several significant improvements in the plasma transsulfuration parameters were seen in this study. The changes in cysteine and glutathione were similar in magnitude to differences observed between autistics and controls reported in other studies [8]; however, the small size of the change observed makes it unclear if these changes are of clinical significance. A higher dose to possibly improve outcomes may not be reasonable considering that side-effects were noted in almost half of the participants, particularly in the transdermal group. Further, the dose used for the oral glutathione represented a high-end dose for this product.
Overall, the results suggest that oral and transdermal glutathioine supplementation may have some benefit on transsulfuration metabolites, but it is unclear whether the metabolites increased due to breakdown of glutathione. Other forms of supplementation should be investigated, especially since whole-blood or intracellular glutathione were not significantly affected. Finding ways to safely improve the glutathione and other transsulfuration metabolites levels in children with an ASD diagnosis with minimal side-effects may prove to be helpful.
Acknowledgement
The oral glutathione used in the study was donated by Your Energy Systems in Palo Alto, California and the transdermal glutathione used in the study was purchased at a discounted research rate from Kirkman in Lake Oswego, Oregon.
Footnotes
Potential conflicts of interest
The authors have no conflicts of interest to report.
Source of support: The research was conducted at the Autism Treatment Center, Dallas, Texas, USA. This research was funded by a grant from the Autism Research Institute, non-profit CoMeD, Inc., and by the non-profit Institute of Chronic Illnesses, Inc. through a grant from the Brenen Hornstein Autism Research & Education (BHARE) Foundation
References
- 1.Sen CK. Nutritional biochemistry of cellular glutathione. J Nutr Biochem. 1997;8:660–72. [Google Scholar]
- 2.Gutman J. Glutathione – Your Bodies Most Powerful Protector. 3rd Edition. Montreal: Communications kudo.ca Inc; 2002. [Google Scholar]
- 3.Kipp A, Banning A, Brigelius-Flohe R. Activation of the glutathione peroxidase 2 (GPx2) promoter by beta-catenin. Biologic Chem. 2007;388:1027–33. doi: 10.1515/BC.2007.137. [DOI] [PubMed] [Google Scholar]
- 4.Millman AC, Salman M, Dayaram YK, et al. Natural killer cells, glutathione, cytokines, and innate immunity against Mycobacterium tuberculosis. J Interferon Cytokine Res. 2008;28:153–65. doi: 10.1089/jir.2007.0095. [DOI] [PubMed] [Google Scholar]
- 5.Geier DA, Kern JK, Garver CR, et al. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem Res. 2009;34:386–93. doi: 10.1007/s11064-008-9782-x. [DOI] [PubMed] [Google Scholar]
- 6.Geier DA, Kern JK, Adams JB, Geier MR. Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci. 2009;280:101–8. doi: 10.1016/j.jns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 7.James J, Cutler P, Melnyk S, et al. Metabolic biomarkers of oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–17. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- 8.James SJ, Melnyk S, Jernigan S, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:947–56. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale. Western Psychological Services; 12031 Wilshire Boulevard, Los Angeles, California: 1994. pp. 90025–251. [Google Scholar]
- 10.Schopler E, Reicher RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism rating Scale (CARS) J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 11.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self reported measure of medication adherence. Medical Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH. Self-rated global measure of the frequency, intensity and burden of medication side-effects. J Psychiatr Prac. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Fukagawa NK, Ajami AM, Young VR. Plasma methionine and cysteine kinetics in response to an intravenous glutathione infusion in adult humans. Am J Physiol. 1996;270(2 Pt 1):E209–14. doi: 10.1152/ajpendo.1996.270.2.E209. [DOI] [PubMed] [Google Scholar]
- 14.Hagen TM, Wierzbicka GT, Sillau AH, et al. Bioavailability of dietary glutathione: effect on plasma concentration. Am J Physiol. 1990;259:G524–29. doi: 10.1152/ajpgi.1990.259.4.G524. [DOI] [PubMed] [Google Scholar]
- 15.Baruchel S, Viau G, Oliver R, Bounous G. Nutraceutical modulation with a humanized native milk serum protein isolate, Immunocal®: application in AIDS and cancer. In: Montagnier L, Olivier R, Pasquier C, editors. Oxidative stress in cancer, AIDS and neurodegenerative diseases. New York, NY: Marcel Dekker, Inc; 1998. pp. 447–61. [Google Scholar]
- 16.Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43:667–69. doi: 10.1007/BF02284971. [DOI] [PubMed] [Google Scholar]
- 17.Aebi S, Assereto R, Lauterburg BH. High-dose intravenous glutathione in man. Pharmacokinetics and effects on cyst(e)ine in plasma and urine. Eur J Clin Invest. 1991;21:103–10. doi: 10.1111/j.1365-2362.1991.tb01366.x. [DOI] [PubMed] [Google Scholar]