Summary
Background
To evaluate the long-term safety and efficacy of etanercept treatment in Polish patients with juvenile idiopathic arthritis (JIA).
Material/Methods
The study involved patients, fulfilling the JIA criteria of the International League of Associations of Rheumatology (ILAR), who were started on etanercept therapy after methotrexate and other synthetic disease-modifying antirheumatic drugs (DMARDs) had proven ineffective. Patient data were collected in an electronic registry. Disease improvement was assessed based on Giannini’s criteria.
Results
The statistical analysis involved 188 patients. Significant improvement was observed in all clinical and laboratory parameters after the first month of therapy and was maintained in the following months. ACR Pediatric 30, 50, 70, 90, and 100 improvement was observed in 81.4%, 65.9%, 27.5%, 16.2%, and 15%, respectively, of patients after 3 months and in 94.7%, 88.4%, 62.1%, 34.7%, and 26.3%, respectively, after 24 months of treatment. Throughout the 72-month safety observation period, 1162 adverse events were reported; the exposure-adjusted AE rate was 2.96 per patient per year.
Conclusions
In patients with various subtypes of JIA resistant to conventional DMARD treatment, etanercept resulted in significant and long-lasting improvements in disease activity. Combination treatment with etanercept and a DMARD was well tolerated.
Keywords: juvenile idiopathic arthritis, etanercept, safety, efficacy, registry
Background
Systematic data on the epidemiology of juvenile idiopathic arthritis (JIA) in Poland are not available. The incidence rate of JIA in Poland is estimated at 5–6 per 100 000 children [1–3]. Treatment of children with JIA mostly relies on nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs (DMARDs). For patients who do not adequately respond to conventional treatment, anti-TNF-α agents are an important alternative. The introduction of anti-TNF drugs into the treatment of rheumatic diseases has created the need for long-term monitoring of patients and, as a result, a number of JIA registries have been established across Europe (ie, in Germany [4–6], the Netherlands [7], the Czech Republic, France, Spain, Sweden, and Great Britain) [8]. In 2003, at the initiative of the Polish Society for Rheumatology, a JIA registry was set up in Poland.
Material and Methods
The registry, which started out with 4 centers and was later extended to 15 centers, is a secured web-based platform collecting data on patients fulfilling the JIA criteria of the International League of Associations of Rheumatology (ILAR) [9] and qualifying for anti-TNF treatment according to the requirements of the Polish Program for JIA funded by the National Health Service. To be eligible for treatment, patients with JIA had to be 4–17 years old and unresponsive or intolerant to methotrexate. Written informed consent regarding the processing and publication of anonymized medical data was obtained from each patient’s parent or legal guardian before inclusion into the registry. Data entry was done by rheumatology specialists at the baseline visit, at 1 and 3 months, and every 6 months thereafter. During each visit, the treating physician completed a 35-item questionnaire. The patients’ disease histories, as well as any previous and current treatments for JIA, were recorded. For rheumatologic follow-up, the following disease activity data were collected [10]:
Physicians’ global assessment of disease activity using a visual analogue scale (VAS) from 0 cm (inactive) to 10 cm (very severe);
Patient or parent global assessment using a VAS from 0 cm (inactive) to 10 cm (very severe);
Physical functioning assessed using the Child Health Assessment Questionnaire (CHAQ) on a scale from 0 to 3;
Number of joints (n=67) with active arthritis, defined as swollen and/or tender joints with limited range of motion (ROM);
Number of joints (n=67) with limited ROM;
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).
Treatment efficacy was assessed using the American College of Rheumatology (ACR) Pediatric 30, 50, 70, 90, and 100 criteria for improvement [10]. Other efficacy parameters included the duration of morning stiffness and the physician and parent or patient evaluation of treatment efficacy on a visual analogue scale (VAS, 0–10). Safety was assessed by documenting any adverse events (AE) reported throughout the study.
Results
Between January 2003 and March 2010, data on 226 patients with JIA treated at 15 specialized pediatric rheumatology centers were collected. Of the 15 centers, 7 included more than 30 patients. Thirty-eight patients were excluded from the statistical analysis due to incomplete data (no data on treatment (n=27), unclear reports (n=4), and treatment with another TNF-α inhibitor (n=7).
The demographic and clinical characteristics of the JIA patients enrolled in the registry are summarized in Table 1. A total of 188 patients received etanercept for up to 72 months. The mean age of the patients was 10 years (range 3.5–18 years). The mean duration of JIA was 52 months (range, 2–183 months). The presented efficacy and safety data cover a period of 48 and 72 months of treatment, respectively; whereas efficacy data beyond 4 years of treatment are not included in the current analysis because of the low patient numbers, the safety analysis includes data covering the entire 72-month treatment period.
Table 1.
Demographic and clinical characteristics of the 188 patients by type of onset of juvenile idiopathic arthritis (JIA).
| Characteristic | Value |
|---|---|
| No. of patients, n (%) | 188 (100%) |
| Age, years | |
| Mean (SD) | 10 (3.9) |
| Range | 3.5–18.0 |
| Sex, n (%) | |
| Female | 123 (65%) |
| Male | 65 (35%) |
| Weight, kg | |
| Mean (SD) | 35 (15.7) |
| Range | 10–99 |
| Height, cm | |
| Mean (SD) | 134 (21.9) |
| Range | 85–185 |
| Duration of JIA from symptom onset to initiation of treatment anti-TNF agent, months | |
| Mean (SD) | 52 (41.7) |
| Range | 2–183 |
| Type of JIA onset and course, n (%) | |
| Systemic onset | 28 (15%) |
| Polyarticular onset | 92 (49%) |
| Rheumatoid factor negative | 79 (42%) |
| Rheumatoid factor positive | 13 (7%) |
| Oligoarticular onset | |
| Extended course | 30 (16%) |
| Persistent course | 27 (14%) |
| Psoriatic arthritis | 2 (1%) |
| Enthesitis | 1 (0.5%) |
| Unclassified course | 8 (4%) |
The development of disease activity based on the 6 core set criteria is summarized in Table 2. A statistically significant improvement in all endpoints was observed as early as 1 month after the initiation of etanercept. Further improvements were observed throughout the first 12 months, followed by stabilization of the disease. For example, the physicians’ mean global assessment of disease activity decreased from 7.1 (SD 1.7) at baseline to 5.1 (2.1) at 1 month and 2.9 (1.8) at 12 months, paralleled by a similar decrease in the parent/patient global assessment. The mean number of active joints decreased from 10 (9.9) at baseline to 6.4 (8.9) at 1 month and 2.5 (4.1) at 12 months. Similar improvements were achieved in the severity of other disease activity parameters (Table 2). The mean duration of etanercept treatment was 25.1 (18) months (range, 1–71 months).
Table 2.
Disease activity indices at baseline and after the initiation of anti-TNF treatment [mean (SD)].
| Visit (no. of patients evaluated) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n=186) | Month 1 (n=186) | Month 3 (n=172) | Month 6 (n=158) | Month 12 (n=146) | Month 24 (n=97) | Month 36 (n=58) | Month 48 (n=39) | |
| Core set criteria | ||||||||
| Physicians’ global assessment of disease activity* | 7.1 (1.7) | 5.1 (2.1)** | 4.2 (2.1) | 3.6 (2.0) | 2.9 (1.8) | 2.5 (1.8) | 3.0 (2.2) | 2.4 (1.6) |
| Patient/parent global assessment of disease activity* | 7.3 (1.9) | 4.7 (2.3)** | 3.8 (2.2) | 3.3 (2.0) | 2.7 (2.0) | 2.3 (1.8) | 2.8 (2.2) | 2.0 (1.6) |
| Number of joints with active arthritis | 10.0 (9.9) | 6.4 (8.9)** | 4.9 (8.6) | 3.2 (5.2) | 2.5 (4.1) | 2.1 (4.0) | 2.8 (3.9) | 2.6 (3.8) |
| Number of joints with limited ROM | 7.9 (9.0) | 5.5 (8.3)** | 4.6 (8.1) | 3.7 (5.6) | 3.1 (4.8) | 2.6 (4.0) | 3.3 (4.7) | 3.2 (4.7) |
| CHAQ score | 1.4 (0.7) | 0.9 (0.7)** | 0.7 (0.7) | 0.6 (0.7) | 0.5 (0.6) | 0.4 (0.5) | 0.5 (0.5) | 0.3 (0.4) |
| ESR (mm/h) | 38 (25.5) | 16 (15.4)** | 13 (12.3) | 15 (17.1) | 12 (9.4) | 12 (12.2) | 13 (12.2) | 10 (6.6) |
| CRP (mg/L) | 32 (41.0) | 11 (25.5)** | 8 (19.0) | 6 (13.3) | 4 (11.3) | 6 (19.4) | 6 (13.5) | 4 (6.9) |
| Additional criteria | ||||||||
| Duration of morning stiffness (min) | 84 (71.0) | 32 (42.3)** | 19 (31.0) | 14 (27.0) | 9 (20.5) | 7 (16.4) | 8 (18.8) | 9 (30.3) |
| Physician assessment of treatment efficacy* | 2.2 (2.1) | 6.3 (2.0)** | 7.0 (1.9) | 7.5 (1.7) | 7.9 (1.8) | 8.2 (1.6) | 7.8 (1.9) | 8.3 (1.5) |
| Parent assessment of treatment efficacy* | 2.3 (2.3) | 6.7 (2.0)** | 7.4 (1.9) | 7.9 (1.8) | 8.1 (1.8) | 8.5 (1.5) | 8.3 (1.6) | 8.7 (1.3) |
| Patient assessment of treatment efficacy* | 2.3 (2.3) | 6.8 (2.0)** | 7.5 (1.9) | 8.0 (1.8) | 8.2 (1.8) | 8.6 (1.5) | 8.1 (2.0) | 8.4 (1.9) |
(VAS, 0–10 cm);
p<0.0001 (paired Wilcoxon test) for the difference between baseline and all subsequent visits;
ROM – range of motion; CHAQ – Children Health Assessment Questionnaire; ESR – erythrocyte sedimentation rate; CRP – C-reactive protein.
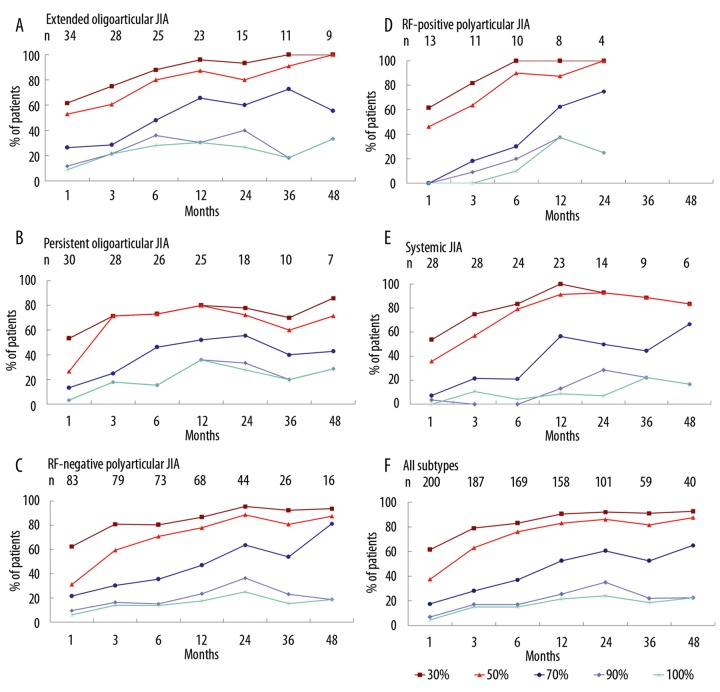
At 1 month, ACR 30, 50, 70, 90, and 100 improvements were achieved in 61.5%, 35.8%, 16.8%, 5.6%, and 3.9% of patients, respectively (Table 3). By 12 months, approximately 90% of the patients had achieved a 30% improvement, a proportion that was sustained through 48 months. No improvement of at least ACR 30 at 1, 3, and 12 months was observed in 38.5%, 18.6%, and 9.5% of patients, respectively. Figure 1 illustrates ACR improvements by JIA subtype. The ACR improvement was accompanied by significant improvements in the duration of morning stiffness and in the physician, parent, and patient assessments of treatment efficacy (Table 2).
Table 3.
Assessment of ACR Paediatric 30, 50, 70, 90, and 100 improvement during anti-TNF treatment in JIA patients (n=188).
| Months of treatment | N | <30% | 30% | 50% | 70% | 90% | 100% | P value* |
|---|---|---|---|---|---|---|---|---|
| 1 | 179 | 38.5% | 61.5% | 35.8% | 16.8% | 5.6% | 3.9% | – |
| 3 | 167 | 18.6% | 81.4% | 65.9% | 27.5% | 16.2% | 15.0% | <0.0000 |
| 6 | 153 | 13.7% | 86.3% | 78.4% | 35.9% | 16.3% | 14.4% | <0.0018 |
| 12 | 141 | 9.5% | 90.5% | 86.5% | 53.9% | 22.7% | 19.1% | <0.0000 |
| 24 | 95 | 5.3% | 94.7% | 88.4% | 62.1% | 34.7% | 26.3% | <0.0003 |
| 36 | 56 | 10.7% | 89.3% | 82.1% | 51.8% | 21.4% | 17.9% | <0.21 |
| 48 | 38 | 2.6% | 97.4% | 92.1% | 68.4% | 23.7% | 23.7% | <0.09 |
Paired Wilcoxon test versus previous assessment.
Figure 1.
Incidence of 30%, 50%, 70%, 90%, and 100% ACR improvement in patients with juvenile idiopathic arthritis (JIA) receiving etanercept, by JIA subtype.
Complete remission, equivalent to ACR 100 improvement, was observed in 15%, 19.1%, and 23.7% of the patients at 3, 12, and 48 months, respectively. The complete remission resulted in discontinuation of etanercept treatment in 23 (12%) patients, with 4 patients subsequently relapsing. In 9 patients, etanercept was discontinued due to a lack of efficacy, and in 7 patients it was discontinued for other reasons (eg, family reasons, organizational reasons, or lack of approval for the continuation of treatment by the Polish National Health Service). Eight patients (4.3%) were withdrawn from the study upon reaching adult age. Overall, the reasons for discontinuation were documented in 51/188 patients (27.1%) (Table 4).
Table 4.
Reasons for discontinuation of etanercept treatment by JIA subtype.
| JIA subtype | N (%) | Remission | Adverse event | Lack of efficacy | Attainment of adult age | Other reasons | Total |
|---|---|---|---|---|---|---|---|
| Systemic onset | 28 | 1 | 0 | 4 | 3 | 1 | 9 |
| Polyarticular | 92 | 11 | 2 | 4 | 3 | 4 | 24 |
| Oligoarthritis | 57 | 8 | 2 | 1 | 1 | 1 | 13 |
| Psoriatic arthritis | 2 | 1 | 0 | 0 | 0 | 0 | 1 |
| Enthesitis | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Unclassified course | 8 | 2 | 0 | 0 | 0 | 1 | 3 |
| Total | 188 | 23 | 4 | 9 | 8 | 7 | 51 |
Before the initiation of anti-TNF treatment, patients had received 1 (2%), 2 (4%), 3 (22%), or more than 3 (72%) DMARDs. Methotrexate had been received by 178 of the 188 patients (95%), 177 patients (94%) received oral glucocorticosteroids, 137 patients (73%) NSAIDs, 103 patients (55%) sulfasalazine, 84 patients (45%) cyclosporine, 41 patients (22%) hydroxychloroquine, 36 patients (19%) intravenous glucocorticosteroids, 15 patients (8%) azathioprine, and 12 patients (6%) intravenous immunoglobulins. After 4 years of treatment, 95% of the 39 patients remaining in the registry were receiving methotrexate, 92% oral glucocorticosteroids, 74% NSAIDs, 51% sulfasalazine, 33% cyclosporine, 23% intravenous glucocorticosteroids, 18% hydroxychloroquine, 8% azathioprine, and 5% intravenous immunoglobulins.
A total of 1162 adverse events (AEs) were reported in 188 patients managed for up to 72 months (Table 5). Overall exposure to etanercept was 393 patient-years, corresponding to an AE rate of 2.96 per patient year. The most common AEs were related to the respiratory system (2.49 per patient year). The most common nonrespiratory AEs were herpes infections (0.1 per patient year), gastroenteritis (0.08 per patient year), common cold (0.08 per patient year), urinary tract infections (0.05 per patient year), and varicella (0.02 per patient year). Sixteen cases of severe respiratory tract infections were reported (0.04 per patient year); 5 patients had a total of 6 serious adverse events (SAEs) (ie, leukopenia, macrophage activation syndrome, tuberculosis plus cytomegalovirus infection, and a neurological disorder [optic disk edema], corresponding to an SAE rate of 0.02 per patient year. Anti-TNF treatment was discontinued due to AEs in 4 patients (Table 4) due to leukopenia, tuberculosis, lower limb paraesthesia, and recurrent respiratory infection. No cases of cancer, lupus, or demyelinization and no deaths were reported.
Table 5.
Adverse events (AEs): numbers and rates per patient year over up to 6 years of anti-TNF treatment in combination with DMARDs (n=188).
| AE by system | Number of AEs | AE rate (per patient/year) |
|---|---|---|
| Respiratory system | 977 | 2.486 |
| Upper respiratory tract infection | 656 | 1.669 |
| Pharyngitis | 161 | 0.410 |
| Bronchitis | 67 | 0.170 |
| Tonsillitis | 30 | 0.076 |
| Optic disc oedema* | 17 | 0.043 |
| Sinusitis | 14 | 0.036 |
| Rhinitis | 17 | 0.043 |
| Lower respiratory/respiratory tract infection | 10 | 0.026 |
| Laryngitis | 3 | 0.008 |
| Asthma | 2 | 0.005 |
| Systemic infection | 82 | 0.209 |
| Herpes infection | 38 | 0.097 |
| Common cold | 31 | 0.079 |
| Varicella | 8 | 0.020 |
| Meningitis | 2 | 0.005 |
| Influenza and parainfluenza | 3 | 0.008 |
| Gastrointestinal system | 39 | 0.099 |
| Gastroenteritis | 32 | 0.081 |
| Diarrhoea | 7 | 0.018 |
| Miscellaneous | 64 | 0.163 |
| Urinary tract infection | 20 | 0.051 |
| Otitis media | 7 | 0.018 |
| Dermatitis | 3 | 0.008 |
| Local reaction | 4 | 0.010 |
| Zoster | 3 | 0.008 |
| Foot inflammation | 3 | 0.008 |
| Leukopenia* | 2 | 0.005 |
| Transaminase increase | 2 | 0.005 |
| Uveitis | 2 | 0.005 |
| Not defined | 2 | 0.005 |
| Gram-negative sepsis, macrophage activation syndrome*, tuberculosis*, scarlet fever, lymphangitis, urticaria, stomatitis, paronychia, cytomegalovirus infection*, steroid osteoporosis fracture*,**, ascariasis, peridental abscess, peridentitis, scabies, skin disorder, Yersinia enterocolica infection, (n=16) | 1 each | 0.003 |
| Total | 1162 | 2.957 |
Serious AE (n=6);
occurred before the initiation of anti-TNF treatment.
Discussion
The Polish registry was set up to collect data on patients with JIA treated with anti-TNF drugs and to establish a consistent system for the evaluation of JIA patients cared for by pediatric rheumatologists. Inclusion of patients into the registry was not obligatory, which therefore covered approximately 85% of the Polish JIA population treated with anti-TNF agents. All Polish regions are represented in a balanced manner. The first patients treated with anti-TNF treatment were included in 2003, the year when etanercept was registered.
The results of this analysis were compared with the German registry, because it is the one most similar to the Polish registry in terms of geographic location and patient characteristics. The number of patients is lower than in the registry reported by Horneff et al. [4–6], consistent with the size of the populations of the countries investigated. In both registries, efficacy was measured by ACR Pediatric and showed consistent improvements after 1, 3, and 6 months. The results are comparable except ACR 70, with the number of patients achieving ACR 70 after 1, 3, and 6 months being lower in the Polish registry (17%, 28%, and 36%, respectively) than in the German registry (30%, 38%, and 52%, respectively). This may be due to the longer duration between the onset of JIA symptoms and the initiation of treatment with etanercept in the Polish than in the German study.
The proportion of patients with non-systemic JIA withdrawn due to a lack of efficacy was comparable in both observational studies (4% in the German and 3.1% in the Polish registry). The proportion of patients with systemic JIA withdrawn due to a lack of efficacy differed between studies, being 50% lower in the Polish (14.3%) than among the German patients (26%). This difference may be due to the fact that the Polish patients with systemic JIA were treated for longer durations, resulting in improvements later in the course of treatment; these patients perceived even small symptom improvements as a benefit and therefore continued treatment.
Overall, the results of our study are consistent with those published by Horneff et al. [5,6], the authors of the German and Austrian registry. Horneff followed a group of 604 patients with any form of JIA managed with etanercept, 504 of whom received combination treatment with methotrexate and etanercept and 100 patients who received etanercept monotherapy. Patients who additionally received other DMARDs were excluded from the analysis. Most patients had polyarticular JIA (27%), enthesitis-related JIA (27%), and oligoarticular JIA (25%). The authors found a similar efficacy and tolerability of etanercept in both groups of patients. The disease activity parameters decreased considerably during treatment, both in the etanercept plus methotrexate and in the etanercept monotherapy groups. ACR 30, 50, and 70 improvement at 12 months was achieved in 81%, 74%, and 62%, respectively, of the patients receiving etanercept plus methotrexate and in 70%, 63%, and 45%, respectively, of the patients receiving etanercept alone [6]. In the entire group of 604 patients, there were 25 SAEs related to infection and 23 SAEs unrelated to infection. In the group of patients receiving combination treatment with etanercept and methotrexate, 3 cases of cancer were reported. In the etanercept monotherapy group, 1 infectious and 3 non-infectious SAEs were reported. No cases of cancer were observed. The risk of SAE was low in the etanercept plus methotrexate combination treatment group (0.05 per patient year) and was even lower in the etanercept monotherapy group (0.01 per patient year). According to the authors, the tolerability of both treatment regimens was comparable [5,6].
Our results are also consistent with those published by Lovell et al. [11–13], Prince et al. [7] and others [14,15], both in terms of the degree and duration of the statistically significant improvement in the clinical and laboratory manifestations of the disease. The longest (8-year) observation related to JIA treatment with etanercept has been presented in several reports by Lovell et al. [11–13]. Their study initially enrolled 69 patients with a diagnosis of polyarticular JIA and insufficient response to methotrexate. The efficacy of treatment was assessed using ACR 30, 50, 70, 90, and 100 criteria. Safety was assessed by recording serious adverse events (SAEs) and serious infections. Patients received etanercept at the dose of 0.4 mg/kg twice a week. In the first phase of the study, which was open-label, 74% of the patients achieved an ACR 30 improvement. In the second phase of the study, good responders were randomized to etanercept or placebo. A total of 58 of the 69 initially enrolled patients (84%) entered the third phase of the study, which was again open-label. Of these, 42 patients (72%) continued treatment with etanercept for 4 years and 26 patients (45%) started the 8th year of treatment with etanercept.
ACR 70 improvement was achieved by 100% of the patients treated for the full 8 years (11 out of 11), while 61% achieved ACR 70 improvement in the entire group continuing in the study and irrespective of the duration of follow-up. In the entire group of patients (n=69) included in the efficacy analysis, ACR 30, 50, 70, 90 and 100 improvement was observed in 83%, 77%, 61%, 41%, and 18% of the patients, respectively. According to the authors, the improvement did not decrease with the duration of follow-up. Only 7 patients were withdrawn due to a lack of efficacy. In most children, previous glucocorticosteroids could be discontinued, with a low dose of methotrexate. Sixteen subjects developed 39 SAEs, and the risk of an SAE was 0.12 per patient-year. The risk of serious infections was low. No cases of tuberculosis, opportunistic infections, cancer, lupus, or demyelinization were reported. The authors concluded that long-term treatment with etanercept was effective and safe over the 8 years of follow-up, and that the number of SAEs did not increase with the duration of the follow-up.
The Dutch registry on JIA treatment with etanercept included 146 patients with JIA [7]. The mean duration of follow-up (from the diagnosis) was 2.5 years (0.3–7.3 years), and the duration of etanercept treatment was 1.7 years (0.1–6.8 years). The observation included patients with all clinical forms of JIA, with most patients presenting with systemic JIA (27%), polyarticular JIA (46%; rheumatoid factor (RF) negative, 8%; RF-positive, 38%), and extended oligoarticular JIA (19%). Most patients (77%) showed an ACR 30 response as early as 3 months after the start of treatment, and the improvement was maintained in most of them. Patients with systemic disease required longer periods of treatment. Complete remission rates were 38% in the systemic JIA subgroup, 32% in the extended oligoarticular JIA subgroup, 38% in the RF-negative polyarticular JIA subgroup, and 36% in the RF-positive polyarticular JIA subgroup. The risk of SAEs was low (0.029 per patient year). Thirty-nine children (27%) developed adverse reactions, including injection-site reactions, nausea, headache, and concentration problems. Non-serious infections were reported in 17 children, severe gastrointestinal infections in 4, sarcoidosis in 2, immune intestinal diseases in 2, and epilepsy in 1 child. Three patients with systemic JIA died. Treatment in these patients was discontinued due to the lack of efficacy no later than 8 months before death. One of these children died in the course of tuberculosis after another biological agent – interferon – was started, the second child died from generalized infection during immunosuppressant treatment, and the third child died, most probably, from macrophage activation syndrome [7].
It is worth emphasizing that ACR 30, 50, and 70 after 12 months of combination treatment in our study were 90.5%, 86.5%, and 53.9%, respectively; comparing well with the results reported by Horneff et al. [6]. By 3 months, 81.4% of our patients had achieved an ACR 30 response, which is similar to the results obtained in the Dutch registry [7]. A complete remission at 12 and 24 months was seen in 19.1% and 26.3% of our entire patient sample, respectively. In patients with systemic JIA, remission rates at 12 and 24 were only 9% and 7%, respectively. This difference may be explained by the longer duration of the disease before anti-TNF treatment was started (mean, 52 months) and the selection of patients resistant to multiple DMARDs (72% of the patients had received 4 or more DMARDs). The rate of infectious and non-infectious SAEs was 0.02 per patient-year.
This registry has allowed us to collect multi-directional data about our patients treated with anti-TNF agents. These data will enable future analysis of the safety and efficacy of etanercept, as well as an assessment of the quality of the patient’s life. An analysis of the impact of disease and therapy on patient growth will be also possible. Finally, the registry can be of assistance in determining the cost-benefit ratio of treatment with anti-TNF agents. The growing number of national registries will become a future source of reliable data on treatment with biological agents and their long-term effects. The great advantage of registries is their uniformity and fast data collection and transfer, enabling instant data analysis and facilitating comparison of data from different registries.
Conclusions
Etanercept treatment in patients with various subtypes of JIA resistant to conventional DMARD treatment resulted in a significant and long-lasting improvement in disease activity. Combination treatment with etanercept and a DMARD was well tolerated.
Acknowledgements
The authors wish to thank the patients, their parents, and all participating centers for their cooperation. The registry was supported by a grant from Pfizer (formerly Wyeth). Also, we are thankful to the following pediatric rheumatologists for including patients into registry: Wezgraj Jadwiga, Adamczak Karolina, Dzieński Paweł, Sobczyk Małgorzata, Olesińska Edyta, Wierzbowska Malgorzata, Paczkowska Bożena, Kaminiarczyk Dominika, Boćkowska Małgorzata, Teresa Kołcun-Penkowska, Marusak-Banacka Maria, and Gruenpeter Anna.
Footnotes
Source of support: Grant from Pfizer (formerly Wyeth)
References
- 1.Rutkowska-Sak L, Tuszkiewicz-Misztal E, Brózik H, et al. The Paediatric Rheumatology Expert Panel of the National Consultant for Rheumatology standpoint for the biologic therapy of juvenile idiopathic arthritis. Reumatologia. 2009;47:111–15. [Google Scholar]
- 2.Wolny-Niedzielska A. Choroby układu ruchu u dzieci kierowanych do Poradni Reumatologicznej w Kielcach w latach 1999–2003. Reumatologia. 2005;43:265–73. [in Polish] [Google Scholar]
- 3.Zygmunt A, Biernacka-Zielinska M, Brózik H, et al. Choroby reumatyczne w populacji dzieci i młodzieży z regionu łódzkiego. Ped Pol. 2005;80:995–1001. [in Polish] [Google Scholar]
- 4.Horneff G, Schmeling H, Biedermann T, et al. The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63:1638–44. doi: 10.1136/ard.2003.014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horneff G, Ebert A, Fitter S, et al. Safety and efficacy of once weekly etanercept 0.8 mg/kg in a multicentre 12 week trial in active polyarticular course juvenile idiopathic arthritis. Rheumatology. 2009;48:916–19. doi: 10.1093/rheumatology/kep122. [DOI] [PubMed] [Google Scholar]
- 6.Horneff G, De Bock F, Foeldvari I, et al. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis. 2009;68:635–41. doi: 10.1136/ard.2007.087593. [DOI] [PubMed] [Google Scholar]
- 7.Prince FH, Twilt M, Ten Cate R, et al. Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch national register. Ann Rheum Dis. 2009;68:635–41. doi: 10.1136/ard.2007.087411. [DOI] [PubMed] [Google Scholar]
- 8.Summary of report of the 3rd Workshop of European Biologics Registries. Ann Rheum Dis. 2005;64:644. Available from: URL: http://ard.bmj.com/content/suppl/2005/03/21/64.4.644.DC1/644644summary2.pdf. [Google Scholar]
- 9.Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991–94. [PubMed] [Google Scholar]
- 10.Giannini EH, Ruperto N, Ravelli A, et al. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202–9. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 11.Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342:763–69. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 12.Lovell DJ, Reiff A, Jones OY, et al. Long-term safety and efficacy of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2006;54:1987–94. doi: 10.1002/art.21885. [DOI] [PubMed] [Google Scholar]
- 13.Lovell DJ, Reiff A, Ilowite NT, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008;58:1496–504. doi: 10.1002/art.23427. [DOI] [PubMed] [Google Scholar]
- 14.Lahdenne P, Vahasalo P, Honkanen V. Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis. 2003;62:245–47. doi: 10.1136/ard.62.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quartier P, Taupin P, Bourdeaut F, et al. Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum. 2003;48:1093–101. doi: 10.1002/art.10885. [DOI] [PubMed] [Google Scholar]