INTRODUCTION
In 2005, approximately 285,000 individuals underwent total hip replacement (THR) procedures, and approximately 523,000 individuals had total knee replacement (TKR) procedures in the U.S.1 As the population ages, it is estimated that by 2030 the demand for these surgeries will increase, and the numbers are expected to grow to 572,000 THR procedures and 3,480,000 TKR procedures annually.1
Venous thromboembolism (VTE), which encompasses deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant source of morbidity and mortality following THR and TKR.2 In the absence of thromboprophylaxis, the prevalence of proximal DVT after THR and TKR procedures is 18% to 36% and 5% to 22%, respectively.2 PE occurs in approximately 0.9% to 28% of patients after THR surgery and in 1.5% to 10% of patients after TKR surgery. Rates of fatal PE are estimated to be as high as 2% following these procedures.2
The American College of Chest Physicians (ACCP) and the American Academy of Orthopaedic Surgeons have produced evidence-based clinical practice guidelines for the prevention of VTE following THR or TKR procedures.3,4 The guidelines recommend the use of pharmacological agents, mechanical compressive devices, or both, for thromboprophylaxis.3,4 Recently updated ACCP guidelines recommend the routine use of traditional anticoagulants, such as low-molecular-weight heparins (LMWHs), fondaparinux (Arixtra, GlaxoSmithKline), vitamin K antagonists such as warfarin (Coumadin, Bristol-Myers Squibb), aspirin, or one of the newer oral anticoagulants—rivaroxaban (Xarelto, Janssen), dabigatran (Pradaxa, Boehringer Ingelheim), or apixaban (Eliquis, Pfizer/Bristol-Myers Squibb)—for pharmacological thromboprophylaxis after THR or TKR surgery.3
Despite the availability of clinical practice guidelines for thromboprophylaxis after hip or knee replacement surgery, data from the Global Orthopaedic Registry suggest that compliance with the guideline recommendations is poor in real-world clinical practice, particularly in the U.S.5 Failure to provide optimal thromboprophylaxis after orthopedic surgery clearly puts patients at risk of VTE and has significant cost implications for health care institutions. Since June 2011, the Centers for Medicare & Medicaid Services has required states to implement non-payment policies for provider preventable conditions, namely VTE following THR and TKR.6
The quality of patient care after replacement surgery would be improved by closer adherence to clinical practice guidelines, and ongoing quality monitoring programs may help to address this. The Surgical Care Improvement Project (SCIP) has released a series of National Hospital Quality Measures designed to improve care after surgery by significantly reducing complications.7 Two core measures apply to VTE: SCIP-VTE-1 records the number of patients who have been prescribed VTE prophylaxis, and SCIP-VTE-2 looks at the number of patients who actually receive VTE prophylaxis.7
Even with optimal prophylaxis, some patients remain at risk for VTE, because symptomatic VTE continues to occur in 1.3% to 10% of patients within the 3-month period following THR or TKR surgery.2 There is, therefore, an urgent need for improved prophylactic agents.
The goal of this article is to educate clinicians, including pharmacists, nurses, and physicians, on new developments in VTE prophylaxis following THR or TKR surgery and to present study data, clinical highlights, and pharmacoeconomic analyses of the oral anticoagulant rivaroxaban.
Clinical Trials
ADVANCE: Apixaban Dose Orally vs. Anti-coagulation with Enoxaparin
DURAC: Duration of Anticoagulation
RECORD: Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism
RE-MODEL: Dabigatran Etexilate 150 or 220 mg Once Daily vs. Enoxaparin 40 mg Once Daily in Prevention of Venous Thromboembolism Post Total Knee Replacement
RE-MOBILIZE: Dabigatran Etexilate 220 mg vs. Enoxaparin 30 mg Twice Daily in Prevention of Venous Thromboembolism Post Total Knee Replacement
RE-NOVATE: Dabigatran Etexilate Compared With Enoxaparin in Prevention of Venous Thromboembolism Following Total Hip Arthroplasty
NOVEL ANTICOAGULANTS
Several oral anticoagulants have completed phase 3 trials for VTE prophylaxis following THR or TKR surgery and have now been included in the ACCP guidelines. These anticoagulants include the direct thrombin inhibitor dabigatran and the selective, direct factor Xa inhibitors apixaban and rivaroxaban.3,8–19 These agents have been developed to address some of the limitations associated with current thromboprophylactic options.
The vitamin K antagonist warfarin has been used as an oral anticoagulant for more than 60 years. Results from the DURAC studies have shown that extended vitamin K antagonist therapy is associated with a lower rate of VTE recurrence.20,21 Indeed, chronic administration of warfarin is commonly used when anticoagulation is required to prevent recurrent VTE. Although warfarin is highly effective, it has complex pharmacokinetic and pharmacodynamic properties, with a dose response that is highly susceptible to genetic factors, and numerous drug–drug and food–drug interactions.22,23 Warfarin’s narrow therapeutic window makes routine coagulation monitoring and dose adjustments essential,22 and its delayed onset and offset of action complicate its use in practice.22,24 By contrast, the newer oral anticoagulants have predictable pharmacokinetic and pharmacodynamic properties, display minimal interactions with food and other drugs, and do not require routine coagulation monitoring.25
Unlike warfarin, LMWHs such as enoxaparin (Lovenox, Sanofi) do not need to be routinely monitored and they have minimal drug interactions.26 However, subcutaneous (SQ) administration is required for LMWHs, thereby making routine treatment difficult and costly, particularly in the outpatient setting, and creating a potential barrier to patient adherence.
Oral administration and a predictable response of the novel anticoagulants illustrate their advantages over both warfarin and LMWHs. The new oral anticoagulants are selective, reversible inhibitors of different components of the coagulation cascade. The direct thrombin inhibitor dabigatran is administered as a prodrug (dabigatran etexilate), which is rapidly converted to the active entity dabigatran by unspecified plasma esterases. This entity blocks the activity of free and clot-bound thrombin, preventing the transformation of fibrinogen to fibrin.25
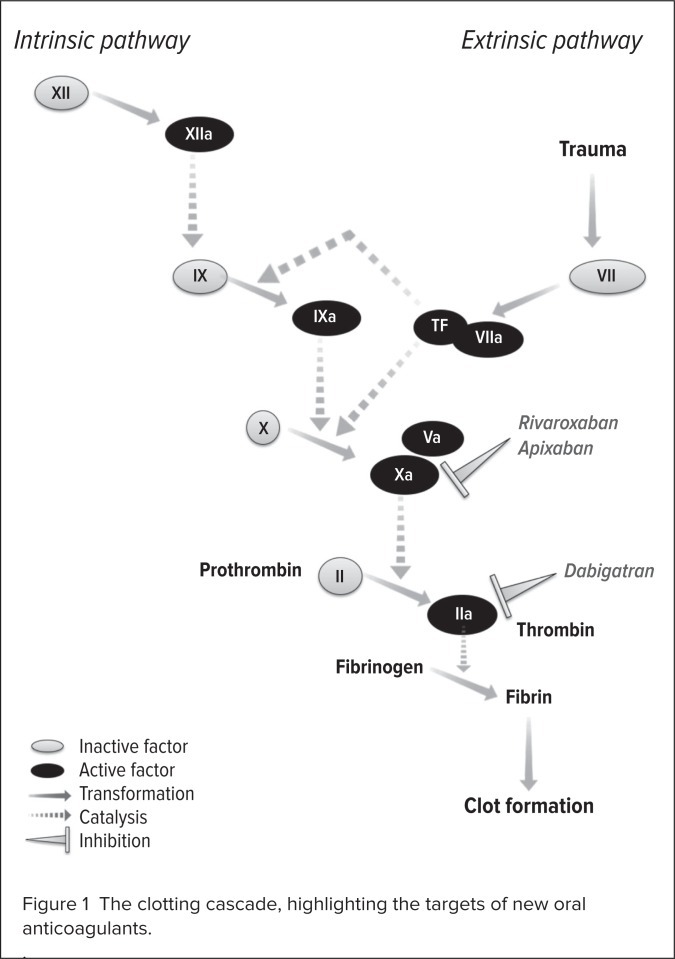
By contrast, the direct factor Xa inhibitors rivaroxaban and apixaban directly block the activity of free and clot-bound factor Xa, preventing the transformation of prothrombin to thrombin.25 Neither rivaroxaban nor apixaban relies on the presence of antithrombin for its activity. With activity higher up the coagulation cascade, at the junction of the extrinsic and intrinsic pathways, factor Xa inhibitors have a theoretical advantage in terms of anticoagulant activity over thrombin inhibitors (Figure 1).
Figure 1.
The clotting cascade, highlighting the targets of new oral anticoagulants.
The RE-NOVATE, RE-MODEL, and RE-MOBILIZE Trials
The utility of dabigatran for VTE prophylaxis after hip or knee replacement has been investigated in four phase 3 studies. Dabigatran (150 mg or 220 mg once daily) demonstrated non-inferior efficacy and a similar safety profile to enoxaparin (40 mg once daily) for 28 to 35 days after THR surgery in RE-NOVATE and RE-NOVATE II and for 6 to 10 days after TKR surgery in RE-MODEL.8–10 There was no significant difference in the rates of major bleeding between the treatment groups in either study for dabigatran 150 mg once daily, dabigatran 220 mg once daily, and enoxaparin 40 mg once daily, respectively:8,9
RE-MODEL: 1.3%, 1.5%, and 1.3%
RE-NOVATE: 1.3%, 2.0%, and 1.6%
In RE-MOBILIZE, dabigatran failed to meet the non-inferiority criteria for efficacy after TKR surgery, compared with enoxaparin (30 mg twice daily) for 12 to 15 days.11 Enoxaparin 30 mg twice daily is the regimen most commonly used in North America.11
The ADVANCE Trials
Three phase 3 trials investigated apixaban in patients who had THR or TKR surgery. In the ADVANCE-2 and ADVANCE-3 trials, apixaban (2.5 mg twice daily) demonstrated superiority in reducing VTE compared with enoxaparin (40 mg once daily), in patients who had undergone TKR and THR surgery, respectively.12,13 As with dabigatran in RE-MOBILIZE, however, apixaban (2.5 mg twice daily) failed to demonstrate non-inferiority to enoxaparin (30 mg twice daily) in ADVANCE-1.14
Apixaban was not associated with increased rates of major bleeding events, compared with enoxaparin, in any of the three studies:12–14
ADVANCE-1, 0.7% vs. 1.4%; P = 0.053
ADVANCE-2, 0.6% vs. 0.9%; P = 0.30
ADVANCE-3, 0.8% vs. 0.7%; P = 0.54
Although included in ACCP guidelines, dabigatran and apixaban have not been approved by the FDA for VTE prophylaxis following THR and TKR surgery.3 On the basis of data from three phase 3 trials (RECORD1, 2, and 3), rivaroxaban is the only oral anticoagulant to have received FDA approval for this indication since warfarin in the 1950s.27 In November, rivaroxaban was also approved for the treatment of DVT and PE and for the reduction in the risk of recurrence of DVT and of PE.28
The RECORD Trials
RECORD was a series of large phase 3 clinical trials that compared oral rivaroxaban and enoxaparin in patients undergoing THR (RECORD1 and 2) or TKR (RECORD3 and 4) surgery. In each of these studies, rivaroxaban (10 mg once daily) was administered orally from 6 to 8 hours after wound closure and compared with enoxaparin (40 mg once daily) (RECORD1, 2, and 3) or enoxaparin 30 mg twice daily (RECORD4).
The primary efficacy endpoint for each trial was a composite of DVT, nonfatal PE, and all-cause mortality. The main secondary efficacy endpoint was the incidence of major VTE (proximal DVT, nonfatal PE, and death from VTE), and the primary safety endpoint was the incidence of major bleeding.15–18 Major bleeding was defined as fatal bleeding; bleeding that involved a critical organ; bleeding that required reoperation; clinically overt bleeding outside the surgical site that was associated with a decrease in hemoglobin of 2 g/dL or more; or clinically overt bleeding requiring an infusion of 2 units of blood or more.
RECORD1 and 2
RECORD1 and 2 were conducted to compare the efficacy and safety of rivaroxaban and enoxaparin in patients undergoing THR surgery.15,16 In RECORD1, rivaroxaban (10 mg once daily) was more effective than enoxaparin (40 mg once daily) for 30 to 35 days in 4,541 patients;15 however, RECORD2 demonstrated superior efficacy of long-term thromboprophylaxis with rivaroxaban (10 mg once daily) for 31 to 39 days compared with short-term thromboprophylaxis with enoxaparin (40 mg once daily) for 10 to 14 days, in 2,509 patients.16
Outcomes for the primary and main secondary efficacy endpoints were significantly better with rivaroxaban than with enoxaparin in both RECORD1 (P < 0.001) and RECORD2 (P < 0.001). Rivaroxaban also displayed a bleeding-event profile similar to that of enoxaparin in both studies (Table 1).15,16
Table 1.
Summary of Safety and Efficacy Data from the RECORD Trials
| Study Name | Indication | R Regimen | E Regimen | Duration of Therapy (Days) |
Primary Efficacy Outcomea R vs. E |
Secondary Efficacy Outcomeb R vs. E |
Primary Safety Endpointc R vs. E |
Clinically Relevant Non-Major Bleeding R vs. E |
|---|---|---|---|---|---|---|---|---|
| RECORD1 | THR | 10 mg p.o. q.d. | 40 mg SQ q.d. | 31–39 | 1.1% vs. 3.7% (ARR 2.6%; 95% CI, 1.5–3.7; P < 0.001) | 0.2% vs. 2.0% (ARR 1.7%; 95% CI, 1.0–2.5; P < 0.001) | 0.3% vs. 0.1% (P = 0.18) | 2.9% vs. 2.4%d |
| RECORD2 | THR | 10 mg p.o. q.d. | 40 mg SQ q.d. | R: 31–39 E: 10–14 | 2.0% vs. 9.3% (ARR 7.3%; 95% CI, 5.2–9.4; P < 0.0001) | 0.6% vs. 5.1% (ARR 4.5%; 95% CI, 3.0–6.0; P < 0.0001) | <0.1% vs. <0.1% | 3.3% vs. 2.7%d |
| RECORD3 | TKR | 10 mg p.o. q.d. | 40 mg SQ q.d. | 10–14 | 9.6% vs. 18.9% (ARR 9.2%; 95% CI, 5.9–12.4; P < 0.001) | 1.0% vs. 2.6% (ARR 1.6%; 95% CI, 0.4–2.8; P = 0.01) | 0.6% vs. 0.5% (P = 0.77) | 2.7% vs. 2.3%d |
| RECORD4 | TKR | 10 mg p.o. q.d. | 30 mg SQ b.i.d. | 10–14 | 6.9% vs. 10.1% (ARR 3.19%; 95% CI, 0.71–5.67; P = 0.0118) | 1.2% vs. 2.0% (ARR 0.8%; 95% CI, −0.22–1.82; P = 0.1237) | 0.7% vs. 0.3% (P = 0.1096) | 2.6% vs. 2.0%d |
Composite of deep vein thrombosis, nonfatal pulmonary embolism, or death from any cause.
Major venous thromboembolism.
Major bleeding.
No statistical difference between groups.
ARR = absolute risk reduction; b.i.d. = twice daily; CI = confidence interval; E = enoxaparin; q.d. = once daily; p.o. = orally; R = rivaroxaban; SQ = subcutaneously; THR = total hip replacement; TKR = total knee replacement.
RECORD3
RECORD3 was conducted to compare rivaroxaban with enoxaparin for efficacy and safety for 10 to 14 days after TKR surgery.17 Rivaroxaban (10 mg once daily) demonstrated superior efficacy to enoxaparin (40 mg once daily) in 2,531 patients. In addition, rivaroxaban was superior to enoxaparin for the secondary efficacy endpoint (the incidence of major VTE).17 Bleeding events were similar between the groups (Table 1).17
RECORD4
Rivaroxaban (10 mg once daily) was compared with a higher dosage of enoxaparin (30 mg twice daily) for up to 2 weeks after elective TKR.18 Rivaroxaban significantly reduced the incidence of the composite primary efficacy endpoint compared with enoxaparin (6.9% vs. 10.1%, respectively; P < 0.012).18 The incidence of major VTE (the secondary efficacy endpoint) was also lower with rivaroxaban.18 Rates of major bleeding were higher with rivaroxaban, but they did not reach statistical significance.18
CLINICAL HIGHLIGHTS
Rivaroxaban is administered orally. In orthopedic patients, it is given as a once-daily 10-mg dose and may be taken with or without food. The initial dose should be taken at least 6 to 10 hours after surgery and after hemostasis has been established. A treatment duration of 35 days is recommended for patients undergoing THR surgery, and a duration of 12 days is recommended for patients undergoing TKR surgery.28
The RECORD series of trials excluded individuals younger than 18 years of age, pregnant or breast-feeding women, and patients with clinically significant liver disease or severe renal impairment, defined as a creatinine clearance (CrCl) below 30 mL/minute. Recommendations regarding rivaroxaban use in these special patient populations thus reflect the lack of clinical experience.
Because rivaroxaban undergoes partial renal excretion, it should be avoided in patients with severe renal impairment (CrCl below 30 mL/minute, based on the Cockcroft–Gault formula) because of an expected increase in rivaroxaban exposure and pharmacodynamic effects in these patients. Patients with moderate renal impairment (CrCl, 30–50 mL/minute) should be observed closely and promptly evaluated if there are any signs or symptoms of blood loss.
Patients who experience acute renal failure while receiving rivaroxaban should discontinue treatment. Rivaroxaban should also be avoided in patients with moderate-to-severe hepatic impairment (Child–Pugh scores B/C) because of the potential for an increase in rivaroxaban exposure, leading to a heightened risk of bleeding.28
As with most anticoagulants, rivaroxaban carries an increased risk of bleeding and epidural or spinal hematomas in patients receiving neuraxial anesthesia or undergoing spinal puncture.28 Hematomas may result in long-term or permanent paralysis; it is therefore important to weigh the benefits and the risks before neuraxial intervention is performed or in patients at increased risk of bleeding, as this procedure may result in serious or fatal bleeding.
In light of this risk, epidural catheters should not be removed until at least 18 hours after the last dose of rivaroxaban is given. The next dose of rivaroxaban should be withheld for at least 6 hours after catheter removal. In the event of a traumatic puncture, the administration of rivaroxaban should be delayed for 24 hours.28 Because appropriate timing of rivaroxaban administration may present practical challenges in “real-world” clinical practice, patients must be frequently monitored for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, and bowel or bladder dysfunction).29
In deciding whether to prescribe rivaroxaban to patients who have an increased risk of bleeding, the clinician should weigh the risk of thrombotic events against the risk of bleeding. The concomitant use of rivaroxaban with strong, combined P-glycoprotein and cytochrome P450 (CYP) 3A4 inhibitors such as ketoconazole (Nizoral, Janssen) and ritonavir (Norvir, Abbott) may increase the risk of bleeding and should be avoided. Drugs that increase rivaroxaban exposure and affect hemostasis (e.g., aspirin, P2Y12 platelet inhibitors, other antithrombotic agents, fibrinolytic therapy, and NSAIDs), may also increase the risk of bleeding and should also be avoided when rivaroxaban is prescribed.28
The most common adverse event reported in clinical trials of rivaroxaban was bleeding.28 Unlike dabigatran, rivaroxaban was not associated with dyspepsia.28,30
The value of monitoring prothrombin time (PT) in patients receiving rivaroxaban has not been established.31 Although rivaroxaban prolongs PT, only small increases in PT are observed at clinically relevant rivaroxaban plasma concentrations, and the effect of rivaroxaban on the PT test is short-lived.31 Furthermore, several studies have shown that the relationship between rivaroxaban concentration and PT varies, depending on the thromboplastin reagent used.32,33 In contrast to vitamin K antagonists, this variation is not eliminated by conversion of the PT values to International Normalized Ratio (INR) values.
With rivaroxaban’s relatively short half-life (5–13 hours),28 delaying or discontinuing the drug may be acceptable if bleeding occurs. Strategies to manage bleeding should be tailored to each patient according to the severity and site of the hemorrhage, but standard protocols should be applied as for other anticoagulants (e.g., mechanical compression, surgical hemostasis, fluid replacement, and hemodynamic support with blood products or platelets).29 However, stopping rivaroxaban can increase the risk of thrombotic events. In clinical trials, an increased rate of stroke was observed following rivaroxaban discontinuation in patients with atrial fibrillation.28 If anticoagulation with rivaroxaban must be discontinued for a reason other than pathological bleeding, another anticoagulant should be considered.28
A specific antidote for rivaroxaban is not available. Because of its high plasma protein binding, rivaroxaban is not expected to be dialyzable. Protamine sulfate and vitamin K are not likely to affect the drug’s anticoagulant activity, and there is no experience with antifibrinolytic agents such as tranexamic acid (e.g., Lysteda, Ferring) or aminocaproic acid (Amicar, Xanodyne) in patients receiving rivaroxaban. In addition, there is no scientific rationale for benefit or clinical experience with systemic hemostatic drugs such as desmopressin (DDAVP, Sanofi-Aventis), and aprotinin (Trasylol, Bayer) in rivaroxaban treatment.28
The use of procoagulant reversal agents, such as prothrombin complex concentrate (PCC), activated prothrombin complex concentrate (APCC), and recombinant factor VIIa (rFVIIa) may be considered, but their utility has not been fully evaluated in clinical trials.28 Results from a small randomized, double-blind, placebo-controlled study conducted in 12 healthy male volunteers suggest that the anticoagulant effect of rivaroxaban might be rapidly and completely reversed by a single bolus infusion of PCC 50 IU/kg. By contrast, the same study found that PCC 4 had no influence on the anticoagulant action of dabigatran.34 Further studies using PCC products available in the U.S. will be required to validate these results.
PHARMACOECONOMIC IMPACT
VTE is the most common cause of hospital readmissions following THR2 and can lead to a considerable increase in the overall cost of care per patient. A study of data taken from a large health care claims database suggests that for patients with in-hospital VTE, mean billed charges were $18,834 higher than for matched controls; the increment was $7,351 for TKR surgical patients and $27,034 for THR surgical patients.35
An economic model developed by Kwong followed patients for 1 year after surgery and specifically evaluated the costs associated with symptomatic VTE and major bleeding in the RECORD trials; the model assumed the drug acquisition costs of rivaroxaban to be equivalent to those of enoxaparin 40 mg.36 Enoxaparin represents a valid comparator for rivaroxaban in pharmacoeconomic analyses, because although enoxaparin has higher acquisition costs than warfarin, it has proved to be as cost-effective as warfarin for VTE prophylaxis in several studies.37
After THR surgery, 35 days of rivaroxaban therapy resulted in cost savings of $5,945 per symptomatic event avoided, compared with 14 days of enoxaparin therapy, and a savings of $82 per patient versus 35 days of enoxaparin.36 After TKR surgery, 12 days of rivaroxaban therapy resulted in cost savings of $291 per patient and 16 fewer symptomatic events per 1,000 patients compared with 12 days of enoxaparin (30 mg twice daily).36 There was also a savings of $284 per patient and 18 fewer symptomatic events (per 1,000 patients) compared with enoxaparin (40 mg once daily).36 This economic model conservatively evaluated rivaroxaban cost, as it did not take into account the costs associated with the home administration of enoxaparin by nurses or the costs of training patients to self-inject.36
Duran et al. also conducted a cost-effectiveness analysis for rivaroxaban versus enoxaparin 40 mg once daily, using post-approval pricing data for rivaroxaban in the U.S. and data from RECORD1, 2, and 3.38 In patients who had undergone THR, 35 days of rivaroxaban thromboprophylaxis saved $695 per patient compared with 35 days of enoxaparin. Compared with 10 to 14 days of prophylaxis with enoxaparin, 35 days of rivaroxaban prevented 9.9 additional symptomatic VTE events per 1,000 patients and saved $244 per patient. Among patients who had undergone TKR, 10 to 14 days of rivaroxaban prevented 13.1 additional symptomatic VTE events per 1,000 patients and saved $411 per patient compared with 10 to 14 days of enoxaparin.
CONCLUSION
Novel anticoagulants represent promising alternatives to the traditional anticoagulants for VTE prophylaxis following THR and TKR surgery. Clinical data show that apixaban and rivaroxaban are more effective in this setting than enoxaparin, whereas dabigatran has equivalent efficacy. Preliminary analyses for rivaroxaban suggest that it may be cost-effective and cost-saving in these patients.
Acknowledgments
The author would like to thank Richard Dobson, PhD, who provided editorial support with funding from Janssen Scientific Affairs, LLC, in Raritan, N.J.
REFERENCES
- 1.Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: Preparing for an epidemic. J Bone Joint Surg Am. 2008;90:1598–1605. doi: 10.2106/JBJS.H.00067. [DOI] [PubMed] [Google Scholar]
- 2.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 3.Falck-Ytter Y, Francis CW, Johanson N, et al. Prevention of VTE in Orthopedic Surgery Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl):e278S–e3255S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preventing Venous Thromboembolic Disease in Patients Undergoing Elective Hip and Knee Arthroplasty: Evidence-Based Guidelines and Evidence Report. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2011. [DOI] [PubMed] [Google Scholar]
- 5.Friedman RJ, Gallus A, Gil-Garay E, et al. Practice patterns in the use of venous thromboembolism prophylaxis after total joint arthroplasty: Insights from the Multinational Global Orthopaedic Registry (GLORY) Am J Orthop (Belle Mead NJ) 2010;39:14–21. [PubMed] [Google Scholar]
- 6.Medicare and Medicaid move aggressively to encourage greater patient safety in hospitals and reduce never events. Medical News Today; Aug 1, 2008. Available at: www.medicalnewstoday.com/releases/116957.php. Accessed December 17, 2012. [Google Scholar]
- 7.Drake K. SCIP core measures: Deep impact. Nurs Manage. 2011;42:24–30. doi: 10.1097/01.NUMA.0000396344.54830.0d. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: The RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–2185. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–956. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primar y total hip arthroplasty (RE-NOVATE II): A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105:721–729. doi: 10.1160/TH10-10-0679. [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg JS, Davidson BL, Comp PC, et al. Oral thrombin inhibitor dabigatran etexilate vs. North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1–9. doi: 10.1016/j.arth.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 12.Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 13.Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): A randomised double-blind trial. Lancet. 2010;375:807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 14.Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 16.Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: A double-blind, randomised controlled trial. Lancet. 2008;372:31–39. doi: 10.1016/S0140-6736(08)60880-6. [DOI] [PubMed] [Google Scholar]
- 17.Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–2786. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 18.Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): A randomised trial. Lancet. 2009;373:1673–1680. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]
- 19.Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105:444–453. doi: 10.1160/TH10-09-0601. [DOI] [PubMed] [Google Scholar]
- 20.Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism: Duration of Anticoagulation Trial Study Group. N Engl J Med. 1995;332:1661–1665. doi: 10.1056/NEJM199506223322501. [DOI] [PubMed] [Google Scholar]
- 21.Schulman S, Granqvist S, Holmström M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med. 1997;336(6):393–398. doi: 10.1056/NEJM199702063360601. [DOI] [PubMed] [Google Scholar]
- 22.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th ed. Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 23.Tufano A, Coppola A, Cerbone AM, et al. Preventing postsurgical venous thromboembolism: Pharmacological approaches. Semin Thromb Hemost. 2011;37:252–266. doi: 10.1055/s-0031-1273089. [DOI] [PubMed] [Google Scholar]
- 24.Baker RI, Coughlin PB, Gallus AS, et al. Warfarin reversal: Consensus guidelines, on behalf of the Australasian Society of Thrombosis and Haemostasis. Med J Aust. 2004;181:492–497. doi: 10.5694/j.1326-5377.2004.tb06407.x. [DOI] [PubMed] [Google Scholar]
- 25.Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and Factor Xa inhibitors in development. Clin Pharmacokinet. 2009;48:1–22. doi: 10.2165/0003088-200948010-00001. [DOI] [PubMed] [Google Scholar]
- 26.Lovenox (enoxaparin sodium injection), prescribing information. Bridgewater, N.J.: Sanofi-Aventis; 2011. [Google Scholar]
- 27.FDA approves Xarelto® (rivaroxaban tablets) to help prevent deep vein thrombosis in patients undergoing knee or hip replacement surgery. Jul 1, 2011. Available at: www.prnewswire.com/news-releases/fda-approves-xarelto-rivaroxaban-tablets-to-help-prevent-deep-vein-thrombosis-in-patients-undergoing-knee-or-hip-replacement-surgery-124872829.html. Accessed December 17, 2012.
- 28.Xarelto (rivaroxaban), prescribing information. Titusville, N.J.: Janssen; 2012. [Google Scholar]
- 29.Xarelto (rivaroxaban). Summary of product characteristics. Janssen; 2011. [Google Scholar]
- 30.Pradaxa (dabigatran etexilate mesylate), prescribing information. Ridgefield, Conn.: Boehringer Ingelheim; 2011. [Google Scholar]
- 31.Samama MM, Guinet C. Laboratory assessment of new anticoagulants. Clin Chem Lab Med. 2011;49:761–772. doi: 10.1515/CCLM.2011.134. [DOI] [PubMed] [Google Scholar]
- 32.Samama MM, Martinoli JL, Leflem L, et al. Assessment of laboratory assays to measure rivaroxaban: An oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103:815–825. doi: 10.1160/TH09-03-0176. [DOI] [PubMed] [Google Scholar]
- 33.Mueck W, Borris LC, Dahl OE, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100:453–461. [PubMed] [Google Scholar]
- 34.Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: A randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–1579. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 35.Oster G, Ollendorf DA, Vera-Llonch M, et al. Economic consequences of venous thromboembolism following major orthopedic surgery. Ann Pharmacother. 2004;38:377–382. doi: 10.1345/aph.1C518. [DOI] [PubMed] [Google Scholar]
- 36.Kwong LM. Cost-effectiveness of rivaroxaban after total hip or total knee arthroplasty. Am J Manag Care. 2011;17:S22–S26. [PubMed] [Google Scholar]
- 37.Friedman RJ, Dunsworth GA. Cost analyses of extended prophylaxis with enoxaparin after hip arthroplasty. Clin Orthop. 2000;370:171–182. doi: 10.1097/00003086-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Duran A, Sengupta N, Diamantopoulos A, et al. Cost and outcomes associated with rivaroxaban vs. enoxaparin for the prevention of postsurgical venous thromboembolism from a U.S. payer’s perspective. J Med Econ. 2011;149(6):824–834. doi: 10.3111/13696998.2011.623203. [DOI] [PubMed] [Google Scholar]