A health economic model was constructed to predict the 5-year incidence of coronary heart disease and diabetes and associated costs after treatment in patients using second-generation antipsychotic agents.
Keywords: second-generation antipsychotic agents, Medicaid, schizophrenia, cardiometabolic
Abstract
Objective:
We assessed the potential clinical and economic impact of coronary heart disease (CHD) and diabetes arising after the use of second-generation (“atypical”) antipsychotic agents for the treatment of chronic schizophrenia. We compared the use of these medications in patients with a higher risk of cardiometabolic adverse events (in a higher-risk scenario) and in patients with a lower risk (in a lower-risk scenario). Our U.S.-based analysis estimated the costs of CHD and diabetes arising from antipsychotic medication–related cardiometabolic effects.
Methods:
We constructed a health economic model to predict the 5-year incidence of CHD and diabetes and associated costs after treatment. In this cost-consequence model, we used CHD risk functions derived from the Framingham Heart Study and diabetes risk functions derived from the Atherosclerosis Risk in Communities (ARIC) study. Patient characteristics and treatment effects on cardiometabolic risk factors were estimated from the Clinical Trials of Antipsychotic Treatment Effectiveness (CATIE) study.
We evaluated two cost-consequence scenarios: the incidence of CHD and diabetes predicted for 1,000 patients with chronic schizophrenia in a higher-risk scenario based on data from CATIE associated with olanzapine (Zyprexa) and in a lower-risk scenario with ziprasidone (Geodon). We evaluated rates of adverse outcomes for each scenario and the cost of treatment for CHD and diabetes. All costs were reported in 2011 U.S. dollars. Because Medicaid is often the payer for patients with chronic schizophrenia, all costs in this analysis were derived from the perspective of Medicaid.
Results:
Over a period of 5 years in 1,000 patients with chronic schizophrenia, the higher-risk scenario with olanzapine showed a 9% increased incidence of CHD and a 59% increased incidence of diabetes, compared with no change in treatment from baseline. By contrast, the lower-risk scenario with ziprasidone showed a 9% reduced incidence of CHD and a 10% reduced incidence of diabetes. The higher-risk scenario led to increased CHD-related costs of $83,206 and to increased diabetes-related costs of $456,399.
Conclusion:
Our study underscores the importance of monitoring the established risk factors for CHD and diabetes in patients using second-generation antipsychotic drugs. Lower-risk agents from this class may lead to substantially decreased costs in the management of CHD and diabetes when compared with higher-risk agents.
INTRODUCTION
Schizophrenia is a chronic mental illness with a typical age of onset in late adolescence or early adulthood. Affected individuals may experience delusions and hallucinations as well as impaired cognitive and social functioning. In addition to the impact on each individual’s life and their families, the economic impact on the U.S. is substantial. In 2002, the annual cost was estimated at $62.7 billion, of which $22.7 billion was attributable to direct medical costs.1 The majority of direct medical care costs are paid through state Medicaid programs.2
Although the cause of schizophrenia remains unknown, treatments are available to relieve symptoms, improve quality of life, and maintain productivity. Treatment usually consists of an antipsychotic medication and psychosocial intervention.3,4 Second-generation antipsychotic drugs have become the mainstay of therapy for schizophrenia. However, these agents have been associated with adverse cardiometabolic events such as increased body mass index (BMI), cholesterol levels, and blood pressure, as well as diabetes.5,6
Patients with psychiatric disorders, when compared with the general population, may also be at a higher risk for the development of obesity, dysregulation of glucose homeostasis, and hyperlipidemia.7 Prescribing drugs associated with a lower risk of metabolic disturbances can be expected to result in reduced rates of coronary heart disease (CHD) and diabetes, ultimately having profound economic implications for Medicaid and other payers.
We developed a model to estimate the risk of CHD and diabetes after treatment with second-generation antipsychotic agents and estimated the potential costs of those drugs carrying a higher risk of cardiometabolic adverse events compared with those drugs carrying a lower risk. We explored the potential economic impact of this risk on state Medicaid programs. Ours was the first cost-consequence study to estimate the economic impact of diabetes and CHD outcomes after treatment with second-generation antipsychotic drugs in the U.S.
PATIENTS AND METHODS
Overview
We created a model that predicted 5-year health consequences and costs to Medicaid of prescribing second-generation antipsychotic drugs in patients with chronic schizophrenia who had a higher risk of cardiometabolic adverse events (in a higher-risk scenario) versus patients with a lower risk (in a lower-risk scenario). Health consequences were limited to the development of CHD and diabetes and were predicted using risk equations published from the Framingham Heart Study and the Atherosclerosis Risk in Communities Study (ARIC).8,9 Patient characteristics and changes in cardiometabolic status were based on data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE).10,39,40
The CATIE Study
Our analysis was based on findings from CATIE. Briefly, patients with chronic schizophrenia in 57 sites in the U.S. were randomly assigned to receive olanzapine (Zyprexa, Eli Lilly), perphenazine, risperidone (Risperdal, Janssen), or ziprasidone (Geodon, Pfizer). Follow-up in the CATIE study was divided into three phases. Phase 1 lasted until treatment was stopped or up to a maximum of 18 months after treatment was begun. Patients who stopped treatment received new therapies in phases 2 and 3.
Our analysis utilized phase 1 results exclusively. Measurements of cardiometabolic health—systolic blood pressure (BP), BMI, waist circumference, ratio of total cholesterol to high-density lipoprotein-cholesterol (TG/HDL-C), fasting glucose levels, HDL-C levels, and triglyceride levels—from baseline and after the first 3 months of treatment in phase 1 were used as inputs for the risk equations applied to estimate the incidence of CHD and diabetes (Table 1).11 Patients in CATIE had received therapy for schizophrenia prior to enrollment. Changes from baseline cardiometabolic measurements observed in the trial were related to treatment switching and not to the initiation of treatment.
Table 1.
Population demographic input Parameters for Baseline and Risk Scenarios
| Parameter | Baseline Value | Source (Ref. No.) | Higher-Risk Scenario (CI) | Lower-Risk Scenario (CI) | Source (Ref. No.) |
|---|---|---|---|---|---|
| Constant parameter | |||||
| Age (years) | 40.7 | 24 | |||
| Female (%) | 30.0% | 24 | |||
| Height (cm) | 172.7 | 24 | |||
| Family history of diabetes (%) | 10.5% | 11 | |||
| Smoker (%) | 60.0% | 24 | |||
| Black (African-American) (%) | 30.5% | 10 | |||
| Diabetes at baseline (%) | 10.5% | 24 | |||
| Variable parameter | |||||
| Fasting glucose (mg/dL) | 98.6 | 39 | +4.5 (0.0 to 9.0) | 0 (–6.9 to 6.9) | 40 |
| Body mass index (kg/m2) | 29.8 | 39 | +2.3 (–0.5 to 5.1) | −1.1 (–5.7 to 3.5) | 43 |
| Waist size (cm) | 100.1 | 39 | +1.1 (0.4 to 1.9) | −0.2 (–1.0 to 0.6) | 40 |
| Systolic BP (mm Hg) | 124.6 | 39 | +1.4 (–1.6 to 4.4) | +0.5 (–3.8 to 4.8) | 40 |
| TC/HDL | 4.7 | 39 | +0.1 (0.7 to −0.2) | −0.4 (−0.3 to −0.6) | 24 |
| HDL (mg/dL) | 43.5 | 39 | −1.4 (–3.4 to 0.7) | +1.9 (–1.2 to 5.1) | 24 |
| Triglycerides (mg/dL) | 189.2 | 39 | +25.0 (–17.3 to 67.2) | −33.6 (–96.4 to 29.2) | 40 |
BP = blood pressure; CI = confidence interval; TC/HDL = ratio of total cholesterol to high-density lipoprotein.
Population
Baseline characteristics of our simulated patient population were derived from CATIE.11 Mean age, race, sex, smoking status, height, weight, family history of diabetes, prevalence of diabetes, and metabolic parameters are presented in Table 1.10,11,24,39,40,43
Treatment Scenarios
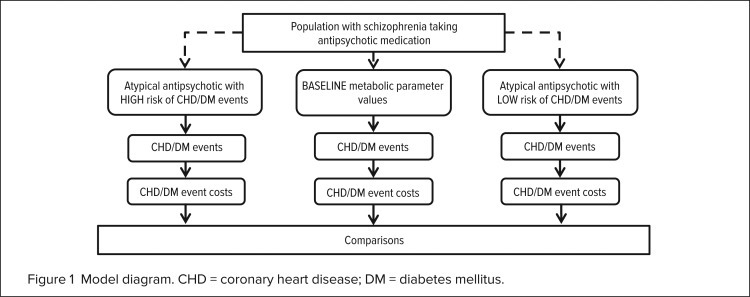
Two treatment scenarios were analyzed: cardiometabolic changes associated with the higher-risk scenario were based on olanzapine, whereas changes in the lower-risk scenario were based on ziprasidone. In the CATIE study, olanzapine induced more adverse changes in cardiometabolic measures; ziprasidone therapy led to no changes or to slightly positive changes. These values were compared with baseline measures (Figure 1). Adherence to the regimen, utilization, and cost were not included. To understand the changes in disease risk with this drug class, we simulated a control. In this scenario, treatment was not given and no changes occurred in patients’ cardiometabolic status.
Figure 1.
Model diagram. CHD = coronary heart disease; DM = diabetes mellitus.
Risk Equations
To estimate CHD risk, we used risk equations developed from an analysis of the Framingham Heart Study and from populations in the Framingham Offspring Heart Study. Factors influencing the time to the first CHD event were BMI, sex, smoking status, diabetes status, systolic BP, age, and the TC-to-HDL ratio (Table 2).9
Table 2.
Risk Equation Parameters
| Coronary heart disease9 | |
| x = | 14.9756 – 0.0159 * BMI – 0.0571 * Age – 0.4959 * Smoking – 0.007044 * SBP – 0.1432 * TC/HDL – 0.3421* Diabetes + 0.5139 * Female |
| Pr(CHD) = | 1 – exp(–((time* exp(-x)) ^ (1 / 0.7303))) |
| Diabetes8 | |
| x = | −9.9808 + 0.0173* Age + 0.4433 * Black + 0.4981 * Family_History_DM + 0.088 * FG + 0.0111* SBP + 0.0273 * Waist_Circ – 0.0326 * Height – 0.0122 * HDL + 0.00271 * TG |
| Pr(DM) = | 1 – exp(1/9 * log(1–1/(1 + exp(x))) * time) |
BMI = body mass index; CHD = coronary heart disease; Black = African-American; DM = diabetes mellitus; exp = exponential function; FG = fasting glucose; HDL = high-density lipoprotein; Height = height (cm); Pr = probability; SBP = systolic blood pressure; TC/HDL = ratio of total cholesterol to high-density lipoprotein; TG = triglycerides; Waist_Circ = waist circumference (cm).
Data from Schmidt MI, et al. Diabetes Care 2005;28(8):2013–2018;8 and Wilson PW, et al. Circulation 2008;118(2):124–130.9
To calculate costs associated with CHD treatment, patients who were predicted to develop CHD were distributed into outcome categories based on an earlier published analysis: myocardial infarction (MI), 47.0%; coronary insufficiency, 2.7%; angina pectoris, 45.8%; and death from CHD, 4.5%.12 The Framingham Heart Study included men and women residing in the U.S., baseline age 30 to 62 years, with no history of CHD. These patients had been observed for the development of CHD since 1948.
Wilson et al. developed risk equations based on data from the Framingham Offspring Study, which comprised the offspring of participants from the original Framingham Heart Study.9 Risk functions were based on the initial baseline examination in 1971 and on surveillance of CHD events through 24 years of follow-up. D’Agostino et al. based their study on participants from both the Framingham Heart Study and the Offspring Study, who had been evaluated since the 1970s.12
The model estimated the incidence of diabetes among patients who did not yet have the disease and used equations published in the Atherosclerosis Risk in Communities (ARIC) Study (see Table 2).8 Inputs to the risk equation in ARIC were age, race, height, and a family history of diabetes. Factors that varied according to treatment scenario were fasting glucose level, systolic BP, waist circumference, HDL-C, and triglycerides. This U.S. cohort study included men and women (median age, 54 years) with no diabetes at baseline who were observed for the development of diabetes from 1987 to 1989 and from 1996 to 1998. The incidence of diabetes was defined by an oral glucose tolerance test (fasting glucose, 7.0 mmol/L or higher or a 2-hour glucose value of 11.1 mmol/L or greater) at the end of follow-up or as a report of a clinical diagnosis or as a therapy for diabetes during the follow-up period.
Cost
Coronary heart disease (CHD) and diabetes events, as predicted by the risk equations, were assigned to cost categories of acute or follow-up care (Table 3).13–15,17,19 Acute costs occurred during the month of the incident event; follow-up costs were applied to every subsequent month until death or at the end of the time horizon. Medicaid is frequently the payer for patients with chronic schizophrenia; therefore, the costs were estimated from this payer perspective.
Table 3.
Cost inputs for the Treatment of Coronary heart disease and diabetes*
| Health Event | Acute Cost | Follow-up Cost |
|---|---|---|
| Myocardial infarction13,17 | $21,859 | $1,250 |
| Death from coronary heart disease13 | $17,264 | $0 |
| Angina pectoris13,14,19 | $5,438 | $30 |
| Coronary insufficiency13,15,19 | $15,934 | $125 |
| Diabetes17 | $368 | $368 |
In 2011 U.S. dollars. Acute costs are included in model once per new diagnosis; follow-up costs are counted monthly from diagnosis through death or end of time horizon.
The costs of acute CHD events were derived from 2008 Healthcare Cost and Utilization Project (HCUP) data, which list national average inpatient care costs for Medicaid.13 For follow-up care, patients who experienced angina were assumed to have been prescribed propranolol, nitroglycerin, aspirin, and statins.14,19 Patients who experienced coronary insufficiency were assumed to have been prescribed the same treatment for angina plus verapamil (Calan, Pfizer) and captopril (Capoten, Apothecon).15,19
We estimated the costs of these drugs to a Medicaid program by lowering average wholesale prices of generic versions by 16.4%.16 The cost of follow-up care for MI was taken from the 2008 Medical Expenditure Panel Survey (MEPS)17 for patients with heart conditions and consisted of outpatient care and prescribed medications. It was assumed that MEPS cost data approximated those of Medicaid. The costs of acute and follow-up care for diabetes were also taken from the MEPS, including the average costs of diabetes care (i.e., glucose monitoring, preventive care, and routine screenings).17 Costs were adjusted to 2011 U.S. dollars using the appropriate medical care component of the Consumer Price Index.18
Sensitivity Analysis
We performed a sensitivity analysis on the higher-risk scenario (with olanzapine) to assess the impact of uncertainty in specific model parameters by varying selected model parameters (i.e., the confidence interval) and observing how they changed total costs. The degree of change indicated the level of influence of these parameters in the model and indirectly demonstrated the uncertainty of model results.
The sensitivity analysis was conducted to evaluate cardio-metabolic risk factors, such as changes in systolic BP and lipid profile. When possible, we estimated ranges of uncertainty from the CATIE trial. The analysis examined the relative sensitivity of the model to each variable metabolic parameter. Input parameters were varied, and the effect on total cost of diabetes and CHD was reported.
RESULTS
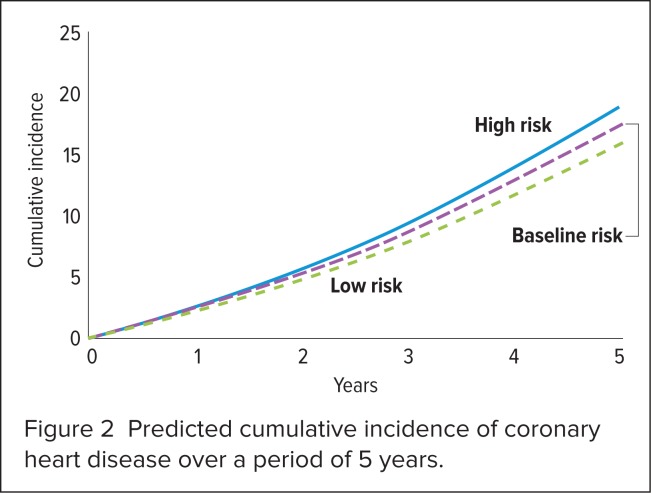
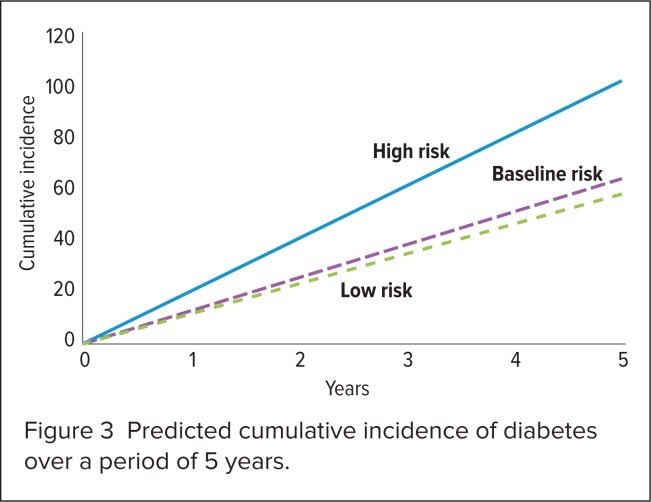
Findings from the 5-year analysis are shown in Table 4, Figure 2, and Figure 3. In a population of 1,000 patients with schizophrenia who received second-generation antipsychotic agents, the lower-risk scenario (with ziprasidone) reduced CHD events by 9% and diabetes events by 10% when compared with baseline. The higher-risk scenario (with olanzapine) increased CHD events by 9% and increased the development of diabetes by 59%. The predicted CHD-related and diabetes-related cost for the cohort using baseline metabolic values was $1,129,380.
Table 4.
Predicted incidence of disease and Expected Costs: Results over a 5-Year Period for 1,000 Patients*
| Higher-Risk Scenario (Olanzapine) | Baseline Value | Lower-Risk Scenario (Ziprasidone) | |
|---|---|---|---|
| Event incidence | |||
| Myocardial infarction | 9 | 8 | 8 |
| Death from coronary heart disease | 1 | 1 | 1 |
| Angina pectoris | 9 | 8 | 7 |
| Coronary insufficiency | 1 | 0 | 0 |
| Diabetes | 102 | 64 | 58 |
| Costs (in 2011 U.S. dollars) | |||
| Myocardial infarction | $444,135 | $408,686 | $372,817 |
| Death from coronary heart disease | $14,068 | $12,947 | $11,813 |
| Angina pectoris | $50,819 | $46,769 | $42,669 |
| Coronary insufficiency | $9,250 | $8,513 | $7,767 |
| Diabetes | $1,043,653 | $652,465 | $587,254 |
| Total | $1,561,925 | $1,129,380 | $1,022,320 |
Results are reported for 1,000 patients with chronic schizophrenia. Costs are from the perspective of Medicaid. Baseline scenario shows incidence and costs predicted for the population before administration of the higher-risk or lower-risk antipsychotic agent.
Figure 2.
Predicted cumulative incidence of coronary heart disease over a period of 5 years.
Figure 3.
Predicted cumulative incidence of diabetes over a period of 5 years.
The higher-risk scenario resulted in an increase of $432,535; the lower-risk scenario resulted in a decrease of $107,060. The higher-risk scenario also resulted in $83,206 more CHD-related costs compared with the lower-risk scenario. Diabetes-related costs were higher by $456,399. Switching to the lower-risk product (ziprasidone) was equivalent to an average, over 5 years, of $539 less per patient for the management of CHD and diabetes. Although the accuracy of this estimate was limited by frequent discontinuations and switches during long-term therapy with this class of medications, we obtained a general understanding of the potential costs when high-risk treatments were continued.
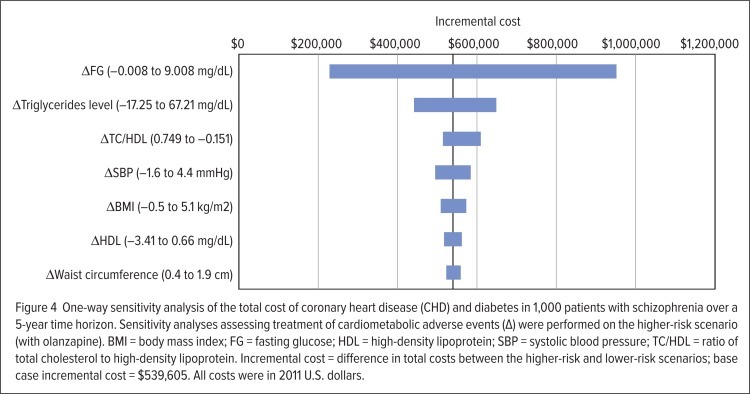
Results of the one-way sensitivity analysis are shown in Figure 4. Cost outputs of the model were most sensitive to changes in levels of fasting glucose and triglycerides, and both risk factors associated with diabetes development. With sensitivity reflecting the uncertainty in the parameter’s value and its influence on model results, the high cost of diabetes care consequently contributes to the degree of sensitivity to fasting glucose and triglycerides.
Figure 4.
One-way sensitivity analysis of the total cost of coronary heart disease (CHD) and diabetes in 1,000 patients with schizophrenia over a 5-year time horizon. Sensitivity analyses assessing treatment of cardiometabolic adverse events (Δ) were performed on the higher-risk scenario (with olanzapine). BMI = body mass index; FG = fasting glucose; HDL = high-density lipoprotein; SBP = systolic blood pressure; TC/HDL = ratio of total cholesterol to high-density lipoprotein. Incremental cost = difference in total costs between the higher-risk and lower-risk scenarios; base case incremental cost = $539,605. All costs were in 2011 U.S. dollars.
DISCUSSION
A lower-risk second-generation antipsychotic agent (ziprasidone) was associated with lower medical costs to Medicaid for managing incident CHD and diabetes compared with a higher-risk agent (olanzapine). Our study underscored the importance of monitoring cardiometabolic parameters in patients treated with these drugs and the potential health gains when the appropriate agent is selected after metabolic risk factors are considered.
However, in a study of three Medicaid state claims databases, Morrato et al. found low rates of laboratory monitoring even after FDA warnings and joint publication of monitoring recommendations by the American Psychiatric Association and the American Diabetes Association.20 This omission suggests that metabolic complications, including diabetes and CHD, may remain undetected, and therefore untreated, until severe and more costly complications arise.
Ours was the first study to estimate the potential economic impact from metabolic changes associated with the use of second-generation antipsychotics arising from incident cases of diabetes and CHD from the U.S. payer perspective. Other trials have examined the economic consequences in Europe.21,22 However, changes in CHD risk using the same Framingham risk equation have shown findings that are in line with the current study. For example, Del Valle et al., estimating changes in Framingham risk scores in a post hoc analysis after 6 weeks of treatment, concluded that men receiving ziprasidone experienced a decrease in adverse events and men receiving olanzapine experienced an increase in adverse events.23 Differences among women were not statistically significant. Similarly, Daumit et al. predicted a higher 10-year risk for CHD in patients in CATIE who received olanzapine rather than ziprasidone.24
Our study focused on estimating the potential cost consequences of CHD and diabetes; unlike a cost-effectiveness analysis, however, our analysis did not compare antipsychotic treatment costs or capture benefits such as reduced psychiatric hospitalization rates, which could offset the costs of higher CHD and diabetes events that we observed. This is important to note, because some studies have found second-generation antipsychotic treatment to be associated with economic and clinical benefits (e.g., fewer outpatient visits and psychiatric hospitalizations) compared with older treatments,25 whereas other studies have not supported this finding.26,27 For example, a cost-effectiveness analysis concluded that olanzapine saved costs compared with another second-generation antipsychotic drug, aripiprazole (Abilify, Bristol-Myers Squibb/Otsuka), because of the reduced hospitalization and outpatient visits for relapses.28 Although that short-term trial-based analysis did not consider the long-term incidence of CHD and diabetes, the substantial savings estimated with olanzapine use would certainly have offset the costs of higher CHD and diabetes incidence found in our study. By contrast, a cost-effectiveness analysis capturing diabetes, CHD, and reduced psychiatric hospitalization comparing the cost-effectiveness of several antipsychotic medications in Canada found that ziprasidone was the most cost-effective treatment.29
Although the impact on overall conclusions from specific cost-effectiveness assessments may be uncertain, our study nevertheless highlights the potentially significant costs associated with cardiometabolic adverse events after therapy with higher-risk antipsychotic agents. Our findings, therefore, reinforce the importance of an awareness of these adverse events when selecting treatments and the potential value of carefully monitoring patients.
LIMITATIONS OF THE STUDY
As a cost-consequence study, our study estimated risks and economic outcomes related only to CHD and diabetes. We focused on cardiometabolic adverse events resulting from second-generation antipsychotic agents but did not consider the potential differences in mental health benefits or associated costs among treatments.
Our analysis did not account for switches in treatment; rather, we made simplifying assumptions. Although discontinuation and the likelihood of switching treatments were high in the CATIE trial, our analysis could not account for treatment switching primarily because of an unavailability of data that might have linked different treatment series and cardiometabolic changes.
We had sought to compare higher-risk and lower-risk second-generation antipsychotic medications, not different series of treatments. For that reason, the 5-year risk assessment was based only on findings from CATIE phase 1; we made no assumptions regarding the effect of treatment switches.
Other second-generation antipsychotic agents that could be classified as higher-risk (e.g., as with olanzapine) or lower-risk (e.g., as with ziprasidone) might not be captured entirely by the inputs used in these analyses, which were based on CATIE results. Our analysis also relied on 3-month measurements from CATIE to project a 5-year risk of disease, which might have underestimated actual risk. In an analysis of eight placebo-controlled studies, 2.2% of patients receiving olanzapine experienced high fasting glucose levels at 12 weeks but 12.8% of patients experienced high fasting glucose levels at 48 weeks.30 This change suggests that the risk of cardiometabolic adverse events may increase with prolonged exposure to antipsychotic therapy. Our study also did not consider polypharmacy with antipsychotic medications, although such treatment patterns are common and may also affect cardiometabolic risk factors.31–33
So far, no CHD or diabetes risk equations based on patients with schizophrenia have been published, even though these patients are considered more likely to have inactive lifestyles and poor dietary practices, raising their risks of diabetes and CHD relative to the general population.34–38 Our predictions of diabetes and CHD incidence, therefore, were based on well-known studies of risk from general U.S. populations. Although the parameters in these risk equations accounted for some differences in patient behaviors that affect the pertinent risk factors, additional factors might not have been captured in these equations (e.g., homelessness, difficulty in accessing health care services); this may have raised the risk of these events among patients with schizophrenia. Therefore, we believe that our estimates of risk might be conservative.
The CATIE trial was sponsored by the National Institute of Mental Health and provided most of the inputs necessary to implement these risk functions.39,40 The CATIE population might not be entirely representative of the risk profiles of the Medicaid population. Whether the risk profiles are markedly different is unknown, because CATIE exclusion criteria were intended to be minimal. However, CATIE did exclude patients during their first episode of schizophrenia and any patients with a documented history of failing to respond to one of the therapies in randomized trials.
CONCLUSION
Payers and health care professionals who decide to prescribe second-generation antipsychotic agents for the management of chronic schizophrenia should consider the potential long-term medical consequences as well as the costs associated with cardiometabolic adverse events. Elevated risks of diabetes and coronary heart disease argue for close monitoring of metabolic risk factors in this vulnerable population.41,42 Although the second-generation antipsychotic class has its advantages, tailoring therapy and balancing efficacy, tolerability, and safety with appropriate monitoring help to promote optimal outcomes and minimize the long-term impact of adverse cardiometabolic disturbances.
Footnotes
Disclosure: Funding for this project was provided by Novartis Pharmaceuticals Corp. to United BioSource Corp., who were paid consultants to Novartis at the time of study conduct.
REFERENCES
- 1.Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66(9):1122–1129. doi: 10.4088/jcp.v66n0906. [DOI] [PubMed] [Google Scholar]
- 2.Croghan TW, Johnstone BM, Buesching DP, Kessler RC. Information needs for medication coverage decisions in a state Medicaid program. Med Care. 1999;37(4 Suppl):AS24–AS31. doi: 10.1097/00005650-199904001-00005. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Schizophrenia. 2006. Available at: www.nimh.nih.gov/health/publications/index.shtml. Accessed November 1, 2011. [Google Scholar]
- 4.US Public Health Service. Office of the Surgeon General. Mental Health. A Report of the Surgeon General. 1999. Available at: http://profiles.nlm.nih.gov/ps/retrieve/ResourceMetadata/NNBBHS. Accessed November 1, 2011.
- 5.Leslie DL, Rosenheck RA. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am J Psychiatry. 2004;161(9):1709–1711. doi: 10.1176/appi.ajp.161.9.1709. [DOI] [PubMed] [Google Scholar]
- 6.Bell RC, Farmer S, Ries R, Srebnik D. Metabolic risk factors among Medicaid outpatients with schizophrenia receiving second-generation antipsychotics. Psychiatric Serv. 2009;60(12):1686–1689. doi: 10.1176/ps.2009.60.12.1686. [DOI] [PubMed] [Google Scholar]
- 7.Kabinoff GS, Toalson PA, Healey KM, et al. Metabolic issues with atypical antipsychotics in primary care: Dispelling the myths. Prim Care Companion J Clin Psychiatry. 2003;5(1):6–14. doi: 10.4088/pcc.v05n0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt MI, Duncan BB, Bang H, et al. Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28(8):2013–2018. doi: 10.2337/diacare.28.8.2013. [DOI] [PubMed] [Google Scholar]
- 9.Wilson PW, Bozeman SR, Burton TM, et al. Prediction of first events of coronary heart disease and stroke with consideration of adiposity. Circulation. 2008;118(2):124–130. doi: 10.1161/CIRCULATIONAHA.108.772962. [DOI] [PubMed] [Google Scholar]
- 10.Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophrenia Res. 2005;80(1):45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of anti-psychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 12.D’Agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal: New results from the Framingham study. Am Heart J. 2000;139(2 Part 1):272–281. doi: 10.1067/mhj.2000.96469. [DOI] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality (AHRQ) Statistics on Hospital Stays. Nationwide Inpatient Sample. 2008. Available at: http://hcupnet.ahrq.gov. Accessed November 3, 2011.
- 14.Angina pectoris. 2011. Available at: www.emedicinehealth.com/angina_pectoris/page8_em.htm. Accessed April 1, 2011
- 15.Coronary Artery Disease. 2011. Available at: www.emedicine-health.com/coronary_artery_disease-health/page13_em.htm. Accessed April 1, 2011.
- 16.Agency for Health Care Administration. Florida Medicaid, Summary of Services, Fiscal Year 10/11.2011. Available at: www.ahca.myflorida.com/Medicaid/flmedicaid.shtml. Accessed April 1, 2011.
- 17.Agency for Healthcare Research and Quality (AHRQ) Medical Panel Expenditure Survey. 2011. Available at: http://meps.ahrq.gov/mepsweb. Accessed April 4, 2011.
- 18.Consumer Price Index (CPI). Series cuur0000sam. 2011. Available at: www.bls.gov/cpi. Accessed April 1, 2011
- 19.Red Book. Montvale, N.J.: PDR Network, LLC.; 2011. Available at: www.pdr.net. Accessed January 2, 2013. [Google Scholar]
- 20.Morrato EH, Druss B, Hartung DM, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry. 2010;67(1):17–24. doi: 10.1001/archgenpsychiatry.2009.179. [DOI] [PubMed] [Google Scholar]
- 21.Barnett AH, Millar HL, Loze JY, et al. UK cost-consequence analysis of aripiprazole in schizophrenia: Diabetes and coronary heart disease risk projections (STAR study) Eur Arch Psychiatry Clin Neurosci. 2009;259(4):239–247. doi: 10.1007/s00406-008-0863-2. [DOI] [PubMed] [Google Scholar]
- 22.Neubauer AS, Deuschle M, Marschall D, et al. Cost-consequence of diabetes and coronary heart disease in patients with schizophrenia in Germany: A social and health insurance perspective. Gesundheitsokonomie Qualitatsmanage. 2009;14(2):95–103. [Google Scholar]
- 23.Del Valle MC, Loebel AD, Murray S, et al. Change in Framingham risk score in patients with schizophrenia: A post hoc analysis of a randomized, double-blind, 6-week trial of ziprasidone and olanzapine. Prim Care Companion J Clin Psychiatry. 2006;8(6):329–333. doi: 10.4088/pcc.v08n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daumit GL, Goff DC, Meyer JM, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105(1–3):175–187. doi: 10.1016/j.schres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tunis SL, Faries DE, Nyhuis AW, et al. Cost-effectiveness of olanzapine as first-line treatment for schizophrenia: Results from a randomized, open-label, 1-year trial. Value Health. 2006;9(2):77–89. doi: 10.1111/j.1524-4733.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosenheck RA, Leslie DL, Sindelar J, et al. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry. 2006;163(12):2080–2089. doi: 10.1176/ajp.2006.163.12.2080. [DOI] [PubMed] [Google Scholar]
- 27.Davies LM, Lewis S, Jones PB, et al. Cost-effectiveness of first- v. second-generation antipsychotic drugs: Results from a randomised controlled trial in schizophrenia responding poorly to previous therapy. Br J Psychiatry. 2007;191:14–22. doi: 10.1192/bjp.bp.106.028654. [DOI] [PubMed] [Google Scholar]
- 28.Ascher-Svanum H, Stensland MD, Peng X, et al. Cost-effectiveness of olanzapine vs. aripiprazole in the treatment of schizophrenia. Curr Med Res Opin. 2011;27(1):115–122. doi: 10.1185/03007995.2010.537594. [DOI] [PubMed] [Google Scholar]
- 29.McIntyre RS, Cragin L, Sorensen S, et al. Comparison of the metabolic and economic consequences of long-term treatment of schizophrenia using ziprasidone, olanzapine, quetiapine, and risperidone in Canada: A cost-effectiveness analysis. J Eval Clin Pract. 2010;16(4):744–755. doi: 10.1111/j.1365-2753.2009.01189.x. [DOI] [PubMed] [Google Scholar]
- 30.Zyprexa (olanzapine), package insert/full prescribing information. Indianapolis: Eli Lilly; revised June 2, 2011. Available at: http://pi.lilly.com/us/zyprexa-pi.pdf. Accessed January 2, 2013
- 31.Correll CU, Frederickson AM, Kane JM, Manu P. Does anti-psychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1–3):91–100. doi: 10.1016/j.schres.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilmer TP, Dolder CR, Folsom DP, et al. Antipsychotic polypharmacy trends among Medicaid beneficiaries with schizophrenia in San Diego County, 1999–2004. Psychiatr Serv. 2007;58(7):1007–1010. doi: 10.1176/ps.2007.58.7.1007. [DOI] [PubMed] [Google Scholar]
- 33.Misawa F, Shimizu K, Fujii Y, et al. Is antipsychotic polypharmacy associated with metabolic syndrome even after adjustment for lifestyle effects?: A cross-sectional study. BMC Psychiatry. 2011;11:118. doi: 10.1186/1471-244X-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Hert M, Dekker JM, Wood D, et al. Cardiovascular disease and diabetes in people with severe mental illness. Position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) Eur Psychiatry. 2009;24(6):412–424. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 35.De Hert M, Falissard B, Mauri M, et al. Epidemiological study for the evaluation of metabolic disorders in patients with schizophrenia: The METEOR study. European Neuropsychopharmacol. 2008;18(S4):S444–S445. [Google Scholar]
- 36.De Hert M, Schreurs V, Vancampfort D, Van Winkel R. Metabolic syndrome in people with schizophrenia: A review. World Psychiatry. 2009;8(1):15–22. doi: 10.1002/j.2051-5545.2009.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacob R, Chowdhury AN. Metabolic comorbidity in schizophrenia. Indian J Med Sci. 2008;62(1):23–31. [PubMed] [Google Scholar]
- 38.Kannabiran M, Singh V. Metabolic syndrome and atypical antipsychotics: A selective literature review. German J Psychiatry. 2008;11(3):111–122. [Google Scholar]
- 39.McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: Prospective data from phase 1. Schizophrenia Res. 2008;101(1–3):273–286. doi: 10.1016/j.schres.2007.12.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nulkar A, Watkinson HMO, Waton A, Mackin P. Cardio-metabolic risk profiles of community psychiatric patients in North Tyneside, United Kingdom. J Cancer Educ. 2009;24:S535. [Google Scholar]
- 42.Usher K, Foster K, Park T. The metabolic syndrome and schizophrenia: The latest evidence and nursing guidelines for management. J Psychiatric Mental Health Nurs. 2006;13(6):730–734. doi: 10.1111/j.1365-2850.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 43.van Winkel R, De Hert M, Wampers M, et al. Major changes in glucose metabolism, including new-onset diabetes, within 3 months after initiation of or switch to atypical antipsychotic medication in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2008;69(3):472–479. doi: 10.4088/jcp.v69n0320. [DOI] [PubMed] [Google Scholar]