Abstract
Aim:
Crossed fused renal ectopia is a rare congenital malformation, which is reported to be usually asymptomatic but may have varied presentations. This survey was conducted to study the clinical profile and the challenges posed in the management of this entity.
Materials and Methods:
Retrospective analysis of 6 patients diagnosed to have crossed fused renal ectopia during 1997-2010. The diagnosis was confirmed during surgical exploration in one patient. In one patient it was detected on antenatal ultrasonography and in the other 4 patients it was detected during investigations for abdominal pain, abdominal mass, anorectal malformation and urinary tract infection.
Results:
The left moiety was crossed and fused with the right moiety in 4 cases. Ultrasonography was found to be a good screening investigation with useful diagnostic contributions from CT scans, radionuclide scintigraphy and magnetic resonance urography. Micturating cystourethrography revealed presence of VUR in 4 cases, 3 of whom have undergone ureteric reimplantation. Two patients required pyeloplasty for pelviureteric junction obstruction; in one of these patients the upper ureter was entrapped in the isthmus. In one patient, a non-functioning moiety resulted in nephrectomy. All children were asymptomatic at last follow-up with stable renal functions.
Conclusions:
Crossed fused renal ectopia was detected in most patients during investigation for other problems. It was found more commonly in boys. The left moiety was crossed to the right in the majority of cases. Associated urological problems were found in most cases and required the appropriate surgical management.
Keywords: Congenital anomalies of kidney, crossed fused renal ectopia, fusion anomalies of kidney
INTRODUCTION
When a kidney is located on the side opposite from which its ureter inserts into the bladder, it is defined as crossed renal ectopia and if it is fused with the opposite kidney then it is defined as crossed fused renal ectopia. Ninety percent of crossed ectopic kidneys are fused to their contralateral mate.
MATERIALS AND METHODS
This is a descriptive study based on the retrospective analysis of 6 patients diagnosed with crossed fused renal ectopia during the period 1997-2010.
CASE REPORTS
Case 1
A 10-year-old boy had previously undergone staged treatment for anorectal malformation. During this treatment, ultrasonography revealed an empty left renal fossa with a crossed left renal ectopia fused to the right kidney. Both kidneys were normal. He now presented with recurrent urinary tract infection for the past one year. The imaging modalities used for investigation included ultrasonography, intravenous pyelography [Figure 1a], radionuclide cortical and renal dynamic scan, and micturating cystourethrogram. The left crossed fused renal ectopia was confirmed and he was diagnosed to have left-sided grade II vesicoureteral reflux with a paraureteral diverticulum. Both kidneys were normal. He underwent left-sided cross trigonal (Cohen's) ureteric reimplantation. Postoperatively, he remained asymptomatic.
Figure 1.
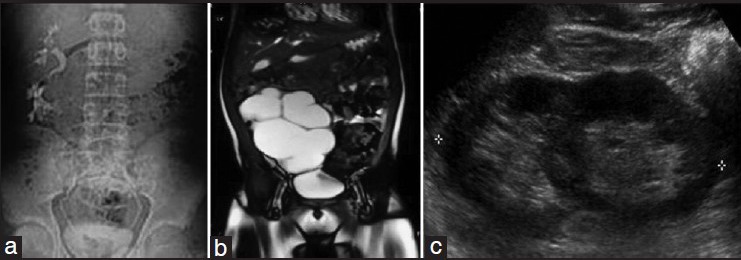
Representative imaging findings in crossed fused renal ectopia. (a) Intravenous pyelography showing crossed fused renal ectopia. (b) Magnetic resonance imaging scan showing crossed fused renal ectopia with hydronephrosis. (c) Ultrasonography showing both renal moieties fused with each other
Case 2
A 2-year-old boy, while being investigated with ultrasonography, radionuclide renal dynamic scan and micturating cystourethrography for an abdominal mass, was shown to have an empty right renal fossa. There was crossed fused right renal ectopia with negligible function, a grossly enlarged but normally functioning left kidney and bilateral grade III vesicoureteral reflux. The right renal moiety and ureter was excised and the left ureter was reimplanted by the cross trigonal (Cohen's) technique. He remained well on follow-up.
Case 3
This boy was diagnosed with crossed fused renal ectopia on antenatal ultrasonography but presented at the age of 2.5 years with fever and was found to have pyuria. This was managed with antibiotic therapy. Subsequent evaluation with ultrasonography, micturating cystourethrogram and magnetic resonance urography [Figure 1b] revealed an empty left renal fossa; the crossed ectopic left renal moiety was fused with the right kidney, which was normally located and both kidneys were hydronephrotic. The radionuclide renal dynamic scan revealed a right:left differential function of 73:27 with an obstructive pattern of drainage in the left moiety and a non-obstructive drainage pattern in the right kidney. At exploration, the right ureter was found to be entrapped in the isthmus; the isthmus was divided and the ureter was released. The pelviureteric junction obstruction of the left moiety was treated by a dismembered pyeloplasty. The postoperative scans showed a non-obstructive drainage pattern in both moieties with preserved renal function.
Case 4
A 5-year-old boy presented after having undergone a right transverse colostomy at birth, in another hospital, for supralevator anorectal malformation. The anorectal malformation was treated with posterior sagittal anorectoplasty and subsequent colostomy closure. However, investigations for associated problems in anorectal malformation, the ultrasonography, micturating cystourethrography, radionuclide renal dynamic scan and magnetic resonance urography revealed an empty right renal fossa, right crossed renal ectopia fused with left kidney, bilateral grade IV vesicoureteral reflux with normally functioning kidneys and bilateral ectopic vas deferens opening in the urinary bladder in respective paraureteric location. Both the ectopic vas deferens were disconnected from the bladder and ligated. The ureters were reimplanted by the cross trigonal (Cohen's) technique. Postoperatively, the patient is continent for stools, the kidneys show normal function and the renal tracts show normal drainage.
Case 5
A 14-month-old girl, during investigation for vomiting and presumed abdominal pain, was found to have an empty left renal fossa and right hydronephrosis on ultrasonography. Further investigations with radionuclide cortical and renal dynamic scans revealed a normally functioning crossed ectopic left kidney fused with a normally located right kidney, which showed poor function and obstruction at the pelviureteric junction. A right dismembered pyeloplasty was performed. Postoperatively both kidneys show stable function and normal drainage pattern on radionuclide renal dynamic scan.
Case 6
A 1.5-year-boy presented with recurrent episodes of urinary tract infection. Investigations, [Figure 1c], crossed left ectopic kidney fused with the right kidney and left-sided grade III vesicoureteral reflux. The urinary tract infection was treated with appropriate antibiotics and the child is asymptomatic with chemoprophylaxis.
RESULTS
The crossed fused renal ectopia was detected on antenatal ultrasonography in one patient (case 2). In all other cases it was detected during investigations for abdominal pain, abdominal mass, anorectal malformation and urinary tract infection.
Post-natal ultrasonography was found to be a good screening investigation; it revealed an empty renal fossa in all cases. However, further imaging investigations included radionuclide cortical (DMSA) (cases 1-6) and renal dynamic scan (IIEC) (all cases), micturating cystourethrogram (cases 1-6) and magnetic resonance imaging (cases 3 and 4). The radionuclide cortical scan provided information on the fusion of the kidneys, differential function and cortical scarring in cases of vesicoureteral reflux. The radionuclide dynamic scan provided information on the differential function and drainage pattern (obstructive or non-obstructive). Micturating cystourethrography showed the presence and grade of vesicoureteral reflux. Magnetic resonance imaging provided exact anatomical details in two patients (cases 3 and 4).
The salient features of the crossed fused renal ectopia in 6 patients have been summarized in Table 1.
Table 1.
Summary of renal status and treatment in 6 cases of crossed fused renal ectopia
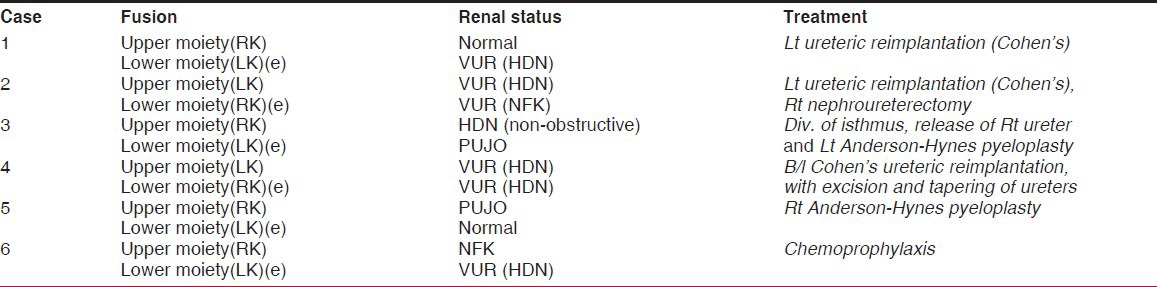
The left moiety was crossed and fused with the right moiety in 4 patients (cases 1, 3, 5 and 6); in the other 2 patients the right moiety was crossed and fused to the left moiety (cases 2,4). The ectopic moiety was normal in only one patient (case 5); in the other 5 patients the ectopic moiety had vesicoureteral reflux in 4 patients (cases 1, 2, 4 and 6) (in case 2 the kidney was non-functioning) and a pelviureteric junction obstruction in one patient (case 3).
The normally located renal moiety was normal in only one patient (case 1). It was non-functioning in one patient, possibly due to vesicoureteral reflux (case 6), hydronephrotic with a non-obstructive pattern due to entrapped ureter in one patient (case 3), hydronephrotic due to pelvi-ureteric junction obstruction in one patient (case 5) and hydronephrotic due to vesicoureteral reflux in two patients (cases 2 and 4).
The surgical management depended on the status of the kidney regardless of the ectopic or normal location. One patient (1.5 years of age and normal renal function tests) is well maintained on chemoprophylaxis for vesicoureteral reflux (case 6). Some form of surgical intervention was required in the other 5 patients. One non-functioning ectopic moiety was excised (case 2). Division of isthmus to release an entrapped ureter from a normally located moiety was carried out in one patient (case 3). A dismembered pyeloplasty (Anderson-Hynes technique) was performed in 2 patients; in case 3 it was for the ectopic moiety whereas in case 5 it was for the normally located moiety. Ureteric reimplantation, by the cross trigonal Cohen's technique, was carried out in 4 moieties; 2 normally located moieties (cases 2 and 4) and 2 ectopic moieties (cases 1 and 4).
All children are asymptomatic at last follow-up with stable renal function and non-obstructed drainage.
DISCUSSION
Crossed fused renal ectopia is thought to result from the abnormal development of the ureteric bud and metanephric blastema during the fourth to eighth weeks of gestation. After horse shoe kidney, crossed fused ectopia of the kidneys is the most frequent fusion abnormality of the urinary tract with a male predominance of 3:2. Wilmer, in 1938, first categorized the fusion anomalies of the kidney and McDonald and McClellan, in 1957, included crossed ectopia with fusion, crossed ectopia without fusion, solitary crossed ectopia and bilateral crossed ectopia in a modified classification. The currently accepted classification, which helps in understanding the embryology, renal ascent and rotation, is depicted in Figure 2. These abnormalities are clinically significant because approximately half the patients manifest complications e.g., hydronephrosis, infections and nephrolithiasis. Left to right crossover occurs more frequently and the upper pole of the crossed ectopic kidney is fused to the lower pole of the normally located kidney in most instances. In the present study, this pattern of fusion was seen in all cases although the left to right crossover was seen in 4 of the 6 cases.[1–4]
Figure 2.
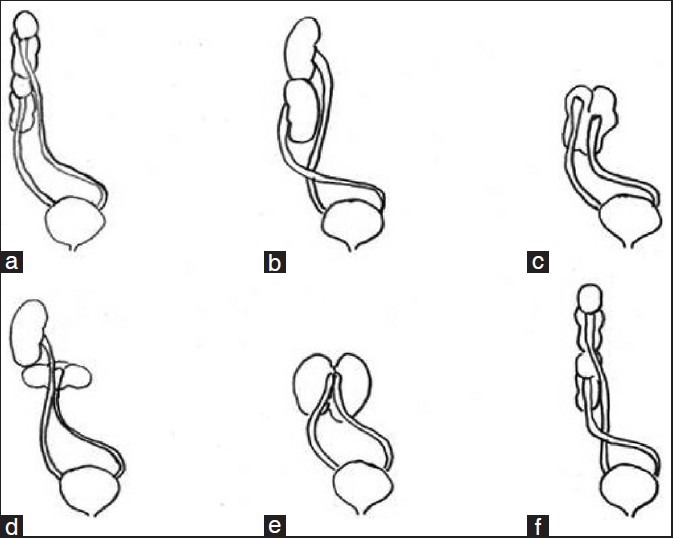
Diagrammatic representation of classification of crossed fused renal ectopia. (a) Unilateral fused kidney (inferior ectopia). (b) Sigmoid or S-shaped kidney. (c) Lump kidney. (d) L-shaped kidney. (e) Disc kidney. (f) Unilateral fused kidney (superior ectopia)
The characteristic ultrasonographic findings in crossed fused renal ectopia include an anterior and/or posterior notch with difference in orientation of the 2 collecting systems in the fused kidneys.[5,6] In addition, ultrasonography can give vital information on the arterial supply and venous drainage, which can be grossly abnormal. Calyceal dilatation and distortion, hydronephrosis and urolithisasis can also be diagnosed with ultrasonography.[7,8] The other imaging modalities that can be used for anatomical imaging include intravenous pyelography and contrast-enhanced CT scans; the magnetic resonance imaging.
The exact incidence of crossed fused renal ectopia is not known because a large majority of patients are asymptomatic; the estimated prevalence in autopsy series is 1:2000.[2] Anecdotal cases have been reported with hypertension.[9] These malformations may be associated with symptoms related to infection (pyelonephritis), obstruction (hydronephrosis due to pelviureteric junction obstruction) and urolithiasis.[7,8] Entrapment of the ureter in the isthmus leading to hydronephrosis, as seen in case 3, has not been reported before. Vesicoureteral reflux is also commonly associated;[10,11] this may be responsible for the high incidence of pyelonephritis. A micturating cystourethrogram is required whenever vesicoureteral reflux is suspected. These associations also place demands on the functional assessment of the upper tracts; we have preferred radionuclide scans for such assessments because of lesser radiation exposure, easy availability, recurrent usage and effective diagnostic accuracy. The cortical scans, using DMSA, help in the detection of scars and the renal dynamic scans, using IIEC, help in the evaluation of differential renal function and the drainage pattern (obstructive or non-obstructive) of the upper tracts. Thus, the investigations need to be tailored to patient's specific needs.
There are no specific guidelines for the management of crossed fused renal ectopia. The fused renal units do not need to be separated. The treatment is guided toward the associated problems that lead to either symptoms or the deterioration of the upper tracts e.g., a pelviureteric junction obstruction would require a pyeloplasty or vesicoureteral reflux would require either injection of a bulking agent or reimplantation of ureter(s).
In conclusion, crossed fused renal ectopia is mostly detected incidentally during investigation for other problems. It is more common in boys. The left moiety crosses over to the right in the majority of cases. And, when urological problems are associated, they require appropriate surgical management.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bauer BS. Anomalies of form and fusion, crossed renal ectopia with and without fusion. In: Alan J, editor. Wein: Campbell-Walsh Urology Book. 9th ed. Philadelphia: WB Saunders; 2007. pp. 3269–304. [Google Scholar]
- 2.Patel TV, Singh AK. Crossed fused ectopia of the kidneys. Kidney Int. 2008;73:662. doi: 10.1038/sj.ki.5002418. [DOI] [PubMed] [Google Scholar]
- 3.Meyers MA, Whalen JP, Evans JA, Viamonte M. Malposition and displacement of the bowel in renal agenesis and ectopia: New observations. Am J Roentgenol Radium Ther Nucl Med. 1973;117:323–33. doi: 10.2214/ajr.117.2.323. [DOI] [PubMed] [Google Scholar]
- 4.Dähnert W. Urogenital tract: Anatomy and function of urogenital tract: Developmental renal anomalies. In: Dähnert W, editor. Dänhert – Radiology Review Manual. 4th ed. Phoenix: Lippincott Williams and Wilkins; 1999. [Last accessed on 2013 Jan 26]. p. 2446. Available from: http://el.trc.gov.om:4000/htmlroot/MEDICAL/tcolon/radiology/General/E-Books/Radiology-Review-Manual.pdf . [Google Scholar]
- 5.Yuksel A, Batukan C. Sonographic findings of fetuses with an empty renal fossa and normal amniotic fluid volume. Fetal Diagn Ther. 2004;19:525–32. doi: 10.1159/000080166. [DOI] [PubMed] [Google Scholar]
- 6.Goodman JD, Norton KI, Carr L, Hsu-Chong Y. Crossed fused renal ectopia: Sonographic diagnosis. Urol Radiol. 1986;8:13–6. doi: 10.1007/BF02924064. [DOI] [PubMed] [Google Scholar]
- 7.Warkany J, Passarge E, Smith LB. Congenital malformations in autosomal trisomy syndromes. Am J Dis Child. 1966;112:502–17. doi: 10.1001/archpedi.1966.02090150046002. [DOI] [PubMed] [Google Scholar]
- 8.Abeshouse BS, Bhisitkul I. Crossed renal ectopia with and without fusion. Urol Int. 1959;9:63–91. doi: 10.1159/000277442. [DOI] [PubMed] [Google Scholar]
- 9.Mininberg DT, Roze S, Pearl M. Hypertension associated with crossed renal ectopia in an infant. Pediatrics. 1971;48:454–7. [PubMed] [Google Scholar]
- 10.Kramer SA, Kelalis PP. Ureteropelvic junction obstruction in children with renal ectopy. J Urol (Paris) 1984;5:331–6. [PubMed] [Google Scholar]
- 11.Guarino N, Tadini B, Camardi P, Silvestro L, Lace R, Bianchi M. The incidence of associated urological abnormalities in 57 children with renal ectopia. J Urol. 2004;172:1757–9. doi: 10.1097/01.ju.0000138376.93343.74. [DOI] [PubMed] [Google Scholar]


