Abstract
Purpose of the Study:
Standardized uptake value (SUV) is the most commonly used semi-quantitative PET parameter. Various response assessment criteria grade the tumor uptake relative to liver or mediastinal uptake. However various factors can affect the background SUV values. This prospective study was carried out to assess the variability of liver and mediastinal SUVs normalized to lean body mass (SUL-L, SUL-M), body surface area (SUB-L, SUB-M), and body weight (SUW-L, SUW-M) and their dependence on various factors which can affect SUV values.
Materials and Methods:
Eighty-eight patients who underwent F-18 FDG PET/CT for various oncological indications were prospectively included in this study. SUVs of liver and mediastinum were calculated by ROIs drawn as suggested by Wahl, et al., in PERCIST 1.0 criteria. Multivariate linear regression analysis was done to assess for the various factors influencing the SUVs of liver and mediastinum. Factors assessed were age, sex, weight, blood glucose level, diabetic status, and uptake period. A P value less than 0.01 was considered significant.
Results:
SUL-L, SUL-M, SUB-L, SUB-M, SUW-L, SUW-M were not affected significantly by age, sex, blood glucose levels, diabetic status. The uptake period had a statistically significant effect on SUL-L (P = 0.007) and SUW-L (P = 0.008) with a progressive decrease with increasing uptake time. Body weight showed a statistically significant effect on SUW-L (P = 0.001) while SUL-L and SUB-L were not dependent on weight. SUB-L was least dependent on weight (P = 0.851) when compared with SUL-L (P = 0.425). However SUL-L was also not affected statistically significantly by variations in body weight (P = 0.425). Mediastinal SUVs were not significantly affected by any of the factors.
Conclusions:
As mediastinal SUVs are not affected significantly by any of the factors, it can be considered as background when wide variations occur in uptake times or weight of the patient when comparing two PET/CT studies to evaluate response.
Keywords: F-18 FDG PET/CT, liver SUV, mediastinal SUV, standardized uptake value (SUV)
INTRODUCTION
The interpretation of PET images includes qualitative analysis and quantitative analysis. Visual interpretation is susceptible to inter- and intraobserver variabilities[1] and defining thresholds to predict the outcomes on the basis of visual analysis only may not be possible. Quantitative analysis can overcome these limitations. Quantitative methods include absolute quantitation and semiquantitative methods. Absolute quantitative methods use Patlak analysis, nonlinear regression analysis, etc.,[2–4] However these require dynamic imaging and are cumbersome and not feasible to be practiced in day-to-day practice. Standardized uptake value (SUV), a semiquantitative parameter, most commonly employed in clinical practice is easy to be obtained but depends on various factors.[5] Determination of background SUV of liver and mediastinal blood pool is important as the response assessment criteria like PERCIST 1.0,[6] International Harmonisation Project (IHP),[7] and London criteria[8,9] grade the tumor uptake relative to liver or mediastinal blood pool activity. Recent studies have also shown the importance of liver background SUV values in determining the threshold to define the positive interim PET study to predict progression.[10] With an aim toward standardizing methods of assessing the response, Wahl, et al.,[6] have proposed PERCIST criteria as a standardized method of defining the region of interest (ROI) for measuring liver SUV and have suggested the usage of SUV of liver normalized to lean body mass as a measure to define tumor uptake threshold above which it can be defined as measurable disease. However no prospective study has evaluated further the reliability of determining the background liver uptake according to recommendations suggested by PERCIST criteria. In light of the above, this prospective study was carried to assess the variability of liver and mediastinal SUVs normalized to lean body mass (SUL-L, SUL-M), body surface area (SUB-L, SUB-M), and body weight (SUW-L, SUW-M) and their dependence on various factors which can affect SUV values.
MATERIALS AND METHODS
Eighty-eight patients who had undergone F-18 FDG PET/CT for various oncological indications were prospectively included in this study. Patients with obvious or suspicious involvement of liver by tumor on PET/CT studies were excluded from the study. Liver SUV values were determined by drawing a 3 cm-diameter three-dimensional ROI placed on the normal right lobe of liver as recommended in PERCIST 1.0 criteria.[6] Mediastinal SUV values were determined by drawing an ROI over contiguous slices on descending aorta carefully excluding the walls from the ROI. Factors assessed were age, sex, weight, blood glucose level at the time of injection of F-18 FDG, diabetic status and uptake period. Multivariate linear regression analysis was applied to assess the significance of various factors influencing the SUVs of liver and mediastinum according to the normalization factors applied. A P value less than 0.01 was considered statistically significant.
RESULTS
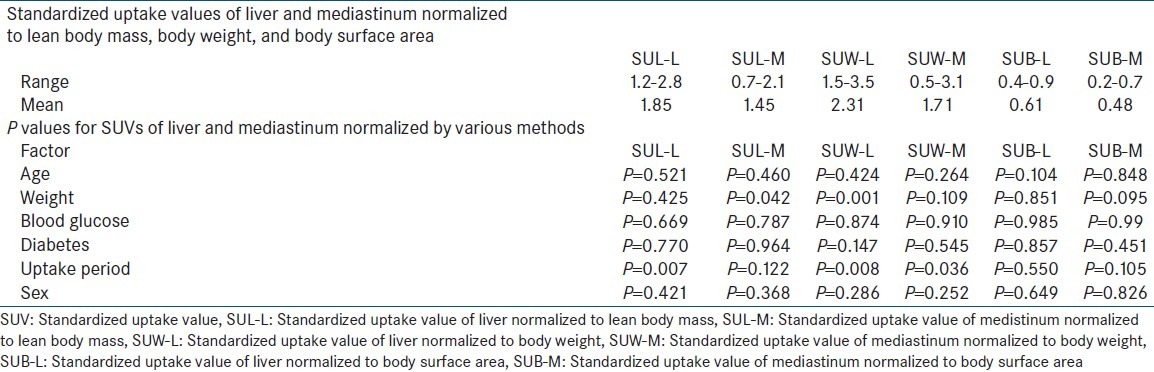
Of the 88 patients included in this study 48 were females and 40 males. Mean age was 50.28 ± 17.43 years (range 14-87 years). Mean weight was 58.5 ± 13.26 kg (range = 33-93 kg). Blood glucose levels at the time of injection of F-18 FDG were 115.8 ± 26.43 (range = 68-177 mg/dl). Twelve of the 88 patients included were diabetic and the blood glucose levels in diabetics ranged from 88-177 with a mean of 126 mg/dl. The mean uptake period was 75.92 ± 19.75 (range = 51-160 minutes) with a median of 77 minutes. SUL-L, SUW-L, SUB-L, SUL-M, SUW-M, SUB-M ranged from 1.2 to 2.8 (mean 1.85), 1.5 to 3.5 (mean 2.31), 0.4 to 0.9 (mean 0.61), 0.7 to 2.1 (mean 1.45), 0.5 to 3.1 (mean 171), 0.2 to 0.7 (mean 0.48) respectively. SUL-L, SUL-M, SUB-L, SUB-M, SUW-L, SUW-M were not affected significantly by age, sex, blood glucose levels, diabetic status. The uptake period had a statistically significant effect on SUL-L (P = 0.007; range = 1.2-2.8) and SUW-L (P = 0.008; range = 1.5-3.5) with progressive decrease with increasing uptake time. Body weight showed a statistically significant effect on SUW-L (P = 0.001; range = 1.5-3.5) while SUL-L and SUB-L were not dependent on weight. SUB-L was least dependent on weight (P = 0.851, range = 0.5-0.9) when compared with SULL (P = 0.425). However SUL-L also was not significantly affected by variations in body weight (P = 0.425). Mediastinal SUVs were not significantly affected by bodyweight when normalized to bodyweight, body surface area of lean body mass. All the results are depicted in Table 1.
Table 1.
The significance (P values) of various factors affecting standardized uptake values normalized by different methods
DISCUSSION
Two types of interpretations namely visual and quantitative analyses are often used in clinical practice to interpret response to therapy with PET or PET/CT. Visual analysis is more commonly used and is relatively easy. However, visual analysis alone may be inadequate to interpret PET/CT during response assessment and has a major disadvantage of high interobserver variability. To observe and estimate the response of tumors to various chemotherapy and radiotherapy regimens it is essential to quantify or establish thresholds using semiquantitative parameters. Thresholds can also be obtained by comparing with background tissues like mediastinum and liver as suggested by London criteria.[8,9] Also to define measurable disease on PET studies determination of background SUV values is necessary.[6] Thus it is essential that these background SUV values do not vary significantly between patients and also with various factors to obtain meaningful assessment of response assessment according to various criteria.
Many studies have previously evaluated the factors affecting the SUV values one of which is the method of ROI selection.[11–16] Almost all of the above studies have evaluated the variability of tumoral SUV values. Only few studies have evaluated the variability and factors influencing background SUV values.[17–22] PERCIST criteria proposed by Wahl, et al.,[6] have standardized the method to draw ROIs to determine the background liver SUL. In our study the same method was used and only SUWL was found to be significantly affected by weight with increasing values with increasing weight and this is in corroboration with previous studies.[22,23] However SUL-L and SUB-L were not significantly affected by weight. This is again in concordance with previous studies.[22,23] Scan time had significant correlation with SUW-L, SUB-L, SUL-L with decreasing values with time. This might be explained by the fact that liver has abundance of enzyme glucose-6-phoshphatase causing continuous glycolysis and decrease in FDG retention.[24] It is necessary to obtain the follow-up scans during the same period of uptake time to minimize overdiagnosing of the residual disease. In comparison mediastinal SUV values did not vary with any of the factors affecting SUV values of liver, i.e., weight and scan time. This is an important observation and mediastinal SUV values can be used as background in preference over liver background in overweight patients or when the scan times varies from pretherapy to post therapy scan. Other factors like age, sex, diabetic status, and blood glucose levels at the time of injection of the tracer did not have significant effect on SUV values of liver and blood pool. Though it can be argued that variations of liver SUV can be minimal, this can be significant when calculating the measurable tumor uptake by formula of 1.5 × liver SUV with values reaching up to 2-4 thereby affecting the reliability of response assessment by PET/CT.
When compared by different normalization methods SUB-L values were least affected by all the factors studied but the values were small ranging from 0.5 to 0.8 which might make it difficult to be used for response assessment as small fluctuations of 30%, i.e., 0.2 can occur with minimal variations in acquisition parameters and other technical factors. SUL values appear to be close to SUV-W values and reasonably less affected by various factors except for scan time in case of liver. So it is essential that all the scan times should be within same time limits between pretherapy and follow-up scans.
CONCLUSION
To conclude though SUB-L appears to be least dependent on weight, SUL-L is also not significantly affected by weight and has higher values close to SUW-L. However the uptake period appears to be the only factor which affects SUL-L significantly and this calls for acquisition of scans with standard uptake period with minimal variability in uptake times even when image interpretation does not require quantitation. In contrast mediastinal SUVs were not affected significantly by any of the factors and when normalized by any of the methods and can be used for definition of background when wide variations occur in scan times or in heavily obese patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Zijlstra JM, Comans EF, van Lingen A, Hoekstra OS, Gundy CM, Willem Coebergh J, et al. FDG PET in lymphoma: The need for standardization of interpretation. An observer variation study. Nucl Med Commun. 2007;28:798–803. doi: 10.1097/MNM.0b013e3282eff2d5. [DOI] [PubMed] [Google Scholar]
- 2.Zaidi H, editor. Quantitative Analysis in Nuclear Medicine Imaging. New York: Springer; 2006. [Google Scholar]
- 3.Gjedde A. Calculation of cerebral glucose phosphorylation from brain uptake of glucose analogs in vivo: A re-examination. Brain Res Rev. 1982;4:237–74. doi: 10.1016/0165-0173(82)90018-2. [DOI] [PubMed] [Google Scholar]
- 4.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 5.Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50:11S–20. doi: 10.2967/jnumed.108.057182. [DOI] [PubMed] [Google Scholar]
- 6.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–50. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of Positron Emission Tomography for Response Assessment of Lymphoma: Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 8.Horning SJ, Juweid ME, Schöder H, Wiseman G, McMillan A, Swinnen LJ, et al. Interim positron emission tomography (PETscans) in diffuse large B-cell lymphoma: An independent expert nuclear medicine evaluation of the Eastern Cooperative Oncology Group E3404 study. Blood. 2010;115:775–7. doi: 10.1182/blood-2009-08-234351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrington SF, Qian W, Somer EJ, Franceschetto A, Bagni B, Brun E, et al. , Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:1824–33. doi: 10.1007/s00259-010-1490-5. [DOI] [PubMed] [Google Scholar]
- 10.Itti E, Juweid ME, Haioun C, Yeddes I, Hamza-Maaloul F, El Bez I, et al. Improvement of early 18F-FDG PET interpretation in diffuse large B-cell lymphoma: Importance of the reference background. J Nucl Med. 2010;51:1857–62. doi: 10.2967/jnumed.110.080556. [DOI] [PubMed] [Google Scholar]
- 11.Hoekstra CJ, Hoekstra OS, Stroobants SG, Vansteenkiste J, Nuyts J, Smit EF, et al. Methods to monitor response to chemotherapy in non-small cell lung cancer with 18F-FDG PET. J Nucl Med. 2002;43:1304–9. [PubMed] [Google Scholar]
- 12.Hoekstra CJ, Paglianiti I, Hoekstra OS, Smit EF, Postmus PE, Teule GJ, et al. Monitoring response to therapy in cancer using [F-18]-2-fluoro-2-deoxy-D-glucose and positron emission tomography: An overview of different analytical methods. Eur J Nucl Med. 2000;27:731–43. doi: 10.1007/s002590050570. [DOI] [PubMed] [Google Scholar]
- 13.Stahl A, Ott K, Schwaiger M, Weber WA. Comparison of different SUV-based methods for monitoring cytotoxic therapy with FDG PET. Eur J Nucl Med Mol Imaging. 2004;31:1471–9. doi: 10.1007/s00259-004-1626-6. [DOI] [PubMed] [Google Scholar]
- 14.Westerterp M, Pruim J, Oyen W, Hoekstra O, Paans A, Visser E, et al. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: Effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging. 2007;34:392–404. doi: 10.1007/s00259-006-0224-1. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y, Oriuchi N, Otake H, Endo K, Murase K. Variability of lesion detectability and standardized uptake value according to the acquisition procedure and reconstruction among five PET scanners. Ann Nucl Med. 2008;22:543–8. doi: 10.1007/s12149-008-0152-1. [DOI] [PubMed] [Google Scholar]
- 16.Mankoff DA, Muzi M, Krohn KA. Quantitative positron emission tomography imaging to measure tumor response to therapy: What is the best method? Mol Imaging Biol. 2003;5:281–5. doi: 10.1016/j.mibio.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Minn H, Zasadny KR, Quint LE. Lung cancer: Reproducibility of quantitative measurements for evaluating 2-[F-18]-fluoro-2-deoxy-D-glucose uptake at PET. Radiology. 1995;196:167–73. doi: 10.1148/radiology.196.1.7784562. [DOI] [PubMed] [Google Scholar]
- 18.Weber WA, Ziegler SI, Thodtmann R. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med. 1999;40:1771–7. [PubMed] [Google Scholar]
- 19.Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804–8. doi: 10.2967/jnumed.108.054239. [DOI] [PubMed] [Google Scholar]
- 20.Paquet N, Albert A, Foidart J, Hustinx R. Within-patient variability of 18F-FDG: Standardized uptake values in normal tissues. J Nucl Med. 2004;45:784–8. [PubMed] [Google Scholar]
- 21.Kanstrup IL, Klausen TL, Bojsen-Møller J, Magnusson P, Zerahn B. Variability and reproducibility of hepatic FDG uptake measured as SUV as well as tissue-to-blood background ratio using positron emission tomography in healthy humans. Clin Physiol Funct Imaging. 2009;29:108–13. doi: 10.1111/j.1475-097X.2008.00846.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim CK, Gupta NC, Chandramouli B, Alavi A. Stardized uptake values of FDG: Body surface area correction is preferable to body weight correction. J Nucl Med. 1994;35:164–7. [PubMed] [Google Scholar]
- 23.Kim CK, Gupta N. Dependency of standardized uptake values of fluorine-18 fluorodeoxy glucose on body size. Comparison of body surface area correction and lean body mass correction. Nucl Med Commun. 1996;17:890–4. doi: 10.1097/00006231-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Torizuka T, Tamaki N, Inokuma T, Magata Y, Sasayama S, Yonekura Y, et al. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med. 1995;36:1811–7. [PubMed] [Google Scholar]


