Abstract
Distant soft tissue metastasis and the simultaneous presence of iodine concentrating and nonconcentrating lesions in papillary thyroid cancer are extremely rare. The concerned patient, a histopathologically proven case of papillary thyroid cancer with nodal metastases treated with total thyroidectomy, bilateral cervical nodal dissection, and radioablation, subsequently developed lung, muscle, and liver metastasis. Triggered by increased thyroglobulin, the iodine-131 whole body scan and 200 mci iodine-131 post-therapy scan showed a left gluteus maximus lesion and a liver lesion. Fludeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) scan intended to find additional lesions revealed iodine and FDG nonconcentrating bilateral pulmonary nodules and a single FDG avid hepatic and two muscle metastases. Although FDG concentration in metastatic pulmonary nodules is generally low, the CT characteristics were classical for metastatic lesion. A follow-up FDG PET-CT study six months after 200 mci iodine-131 radioablation showed treatment response in muscle and liver lesions but not lungs.
Keywords: Fludeoxyglucose positron emission tomography-computed tomography, single photon emission tomography-computed tomography, thyroglobulin, whole body scan
INTRODUCTION
Papillary thyroid carcinoma being the most common type of differentiated thyroid malignancy has a predilection for spread through the lymphatic system and thus commonly involves the central and lateral compartmental lymph nodes of the cervical region. Distant metastatic disease is rarely encountered and most commonly involves the lungs and bone. Soft tissue metastasis is even rarer and amenable to radioiodine treatment similar to metastatic lesions in the lung and bones when they concentrate iodine. The concerned case is unique in the sense that the involvement simultaneously of three soft tissue organs is present with different iodine-concentrating abilities, whereas a review of the literature did not find the simultaneous presence of iodine-concentrating metastasis in one organ, iodine-nonconcentrating metastasis in another organ, and multiorgan soft tissue metastasis in the same patient. The case report also emphasizes possible hematogenous route of metastasis which is rare in papillary thyroid malignancy.
CASE REPORT
A 42-year-old male patient with a history of total thyroidectomy and bilateral cervical nodal dissection positive for papillary carcinoma thyroid was referred to our department for a follow positron emission tomography-computed tomography (PET-CT) scan for evaluation of lung nodules and muscle lesions that he developed five years after primary treatment. During the follow-up, prompted by elevated thyroglobulin levels, an iodine-131 whole body scan and single photon emission tomography-computed tomography (SPECT-CT) [Figure 1] showed positive lesions, one in the liver and another in the left gluteal region. The gluteal region was positive for metastatic papillary carcinoma thyroid on biopsy. A fludeoxyglucose (FDG) PET-CT scan [Figure 2] was done to find more lesions and revealed metabolically active lesions in liver segment VI [Figure 3] and the left gluteus and a new paraspinal muscle lesion at the nape of the neck [Figure 4], whereas the lung nodules were metabolically inactive [Figure 5]. A post-therapy scan [Figure 6] at a therapeutic dose of 200 mci of iodine-131 revealed iodine concentration in the liver and a gluteal lesion, whereas there was no concentration in lung nodules and neck lesion. The present FDG PET CT scan [Figure 7] as a response evaluation six month post treatment revealed a metabolic response in the liver and gluteal lesion [Figure 8], but an increasing size of the neck lesion [Figure 9] and lung nodules [Figure 10]. Presently, the patient is put on redifferentiation therapy with sorafenib and suppressive doses of thyroxin.
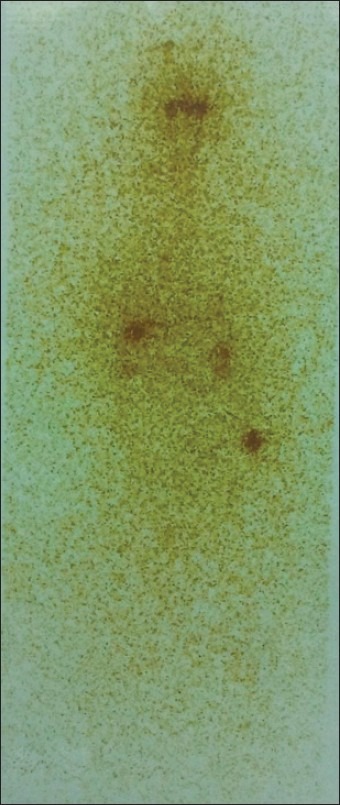
Figure 1.
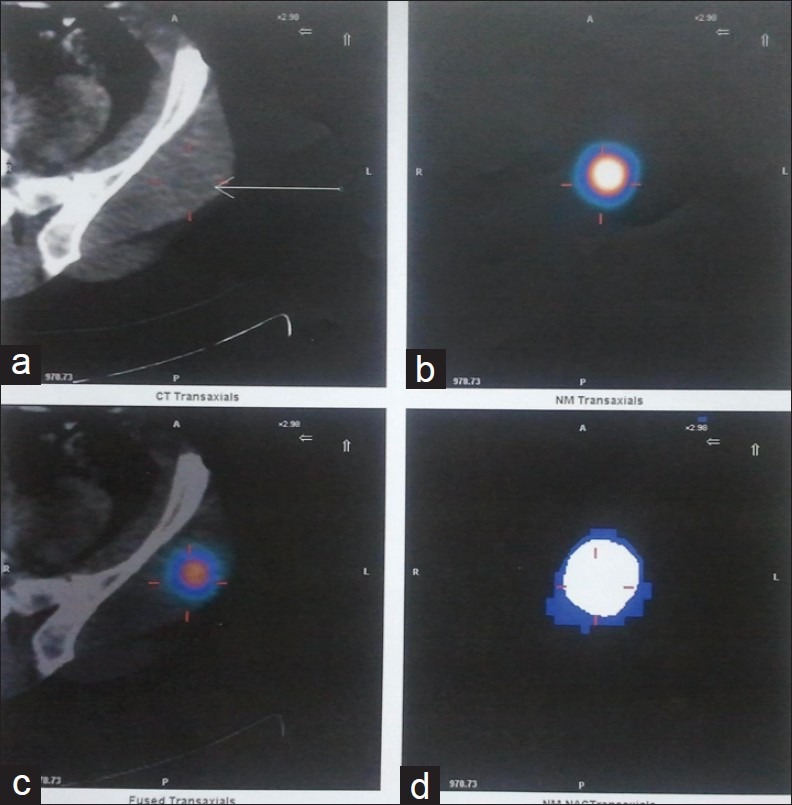
The single photon emission tomography-computed tomography (SPECT–CT) scan shows iodine-concentrating lesion in left gluteal muscle
Figure 2.
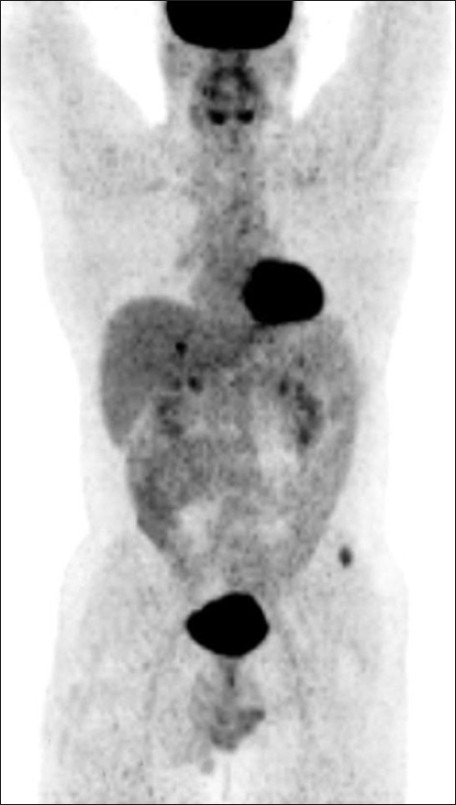
Left gluteal lesions were metabolically active in pretherapy positron emission tomography-computed tomography (PET–CT) study
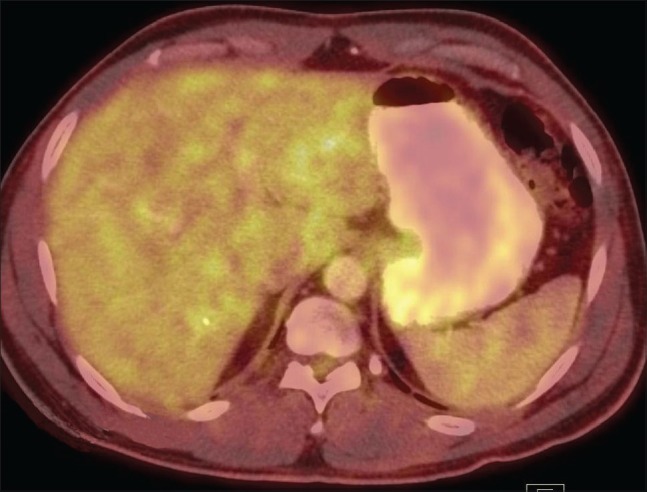
Figure 3.
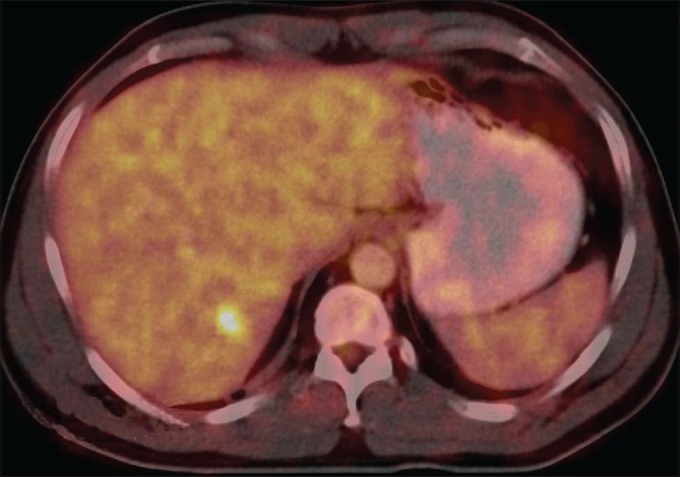
The liver
Figure 4.
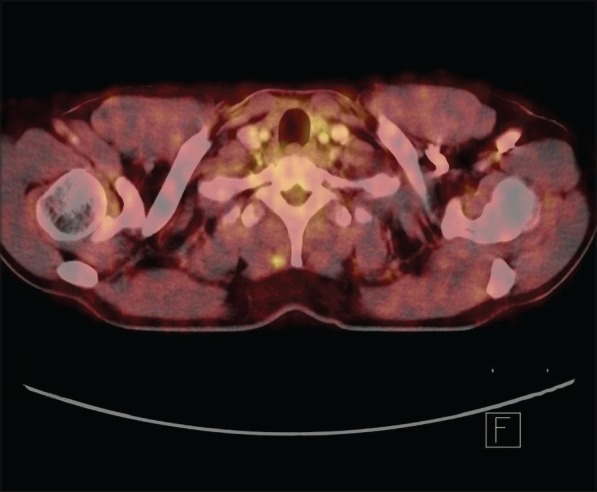
The new lesion at nape of neck
Figure 5.
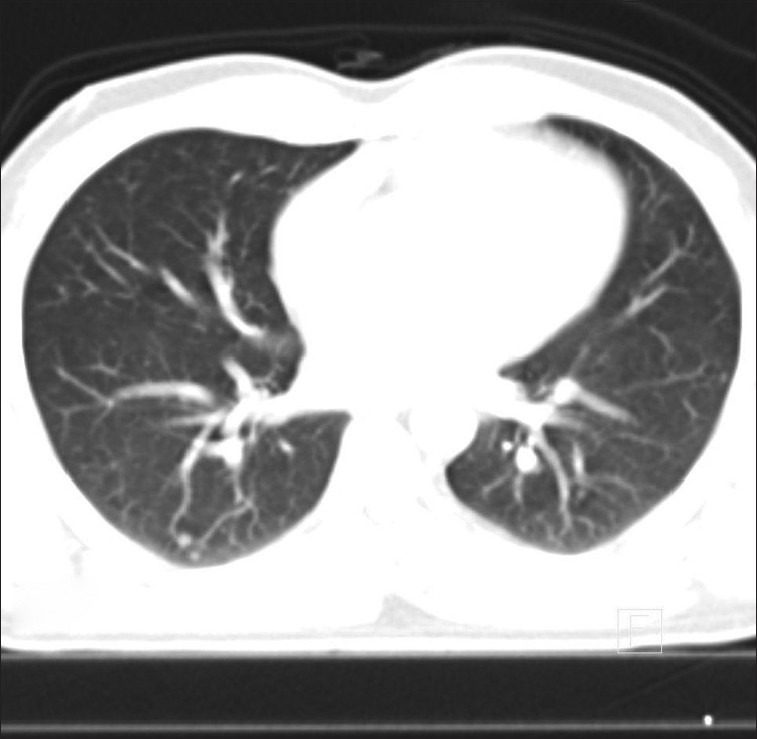
Fludeoxyglucose (FDG) activity but not lung nodules
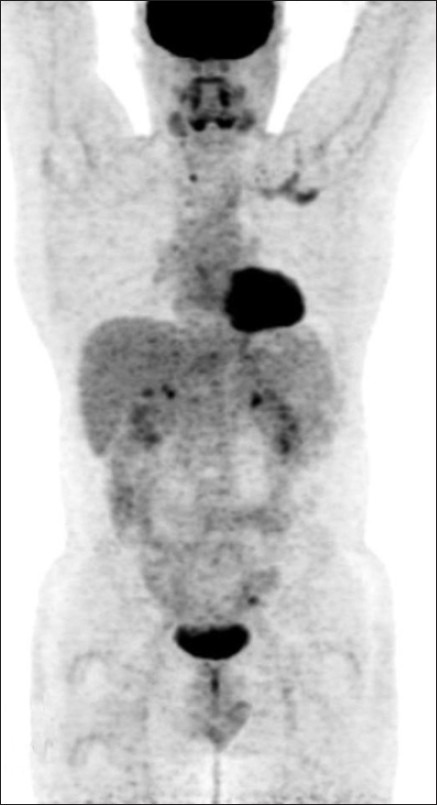
Figure 6.
The 200 mci post-therapy scan
Figure 7.
Concentration in liver and gluteal lesion but no concentration in neck lesion and lung nodules. The positron emission tomography-computed tomography (PET–CT) scan after six months
Figure 8.
Iodine therapy shows treatment response in liver
Figure 9.
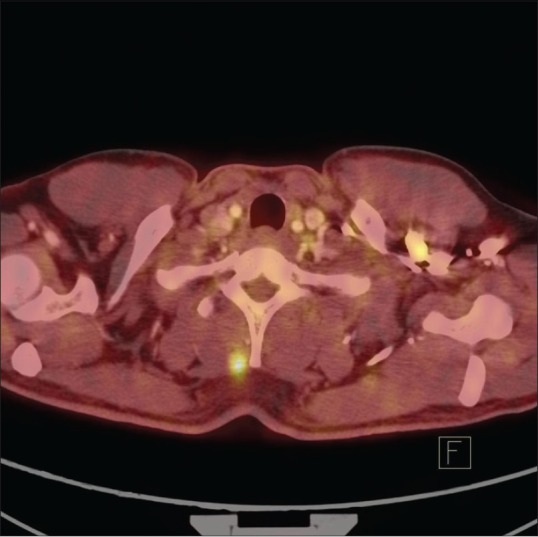
Soft tissue deposit in the neck demonstrating increase in size and FDG uptake (Disease Progression)
Figure 10.
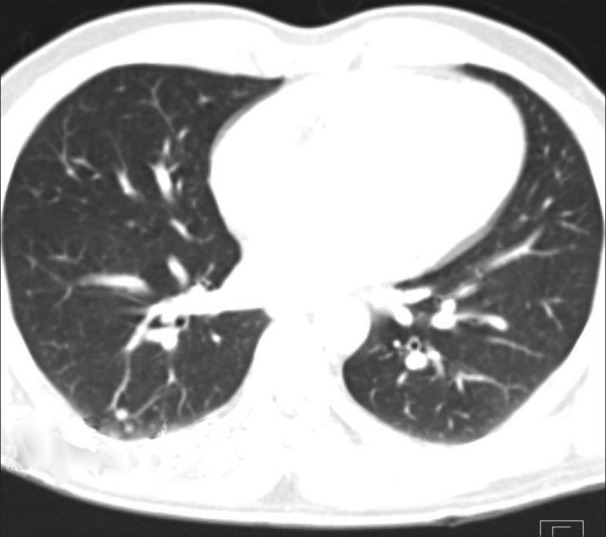
Lung nodules
DISCUSSION
Papillary thyroid carcinoma is the most common type of differentiated thyroid carcinoma accounting for at least 70% of all follicular cell-derived thyroid malignancies[1,2] and is considered to be a relatively indolent tumor in which distant metastasis and death are rare.[3] The five-year survival rate for papillary thyroid cancer according to the stage is 100% for stage I and II, 93% for stage III, and 51% for stage IV. Distant metastasis is the principal cause of death in cases of well-differentiated thyroid carcinomas. About 10% of papillary carcinomas develop distant metastasis, with about 50% of patients having such metastasis at the time of diagnosis. The prognosis of these patients is poor, and over 50% of the patients are likely to die within five years, irrespective of the histology of the tumor.
Distant metastasis, although relatively uncommon, has been known to occur most commonly in the lungs,[4] followed in frequency by bone and brain (0.1 to 5.0%).[5] Isolated cases of soft tissue metastasis have been documented in the orbit, skull, skin, muscles, liver, spleen, pancreas, and adrenal gland.[6–13] Metastatic lesions have also been documented in silent or occult thyroid neoplasms.[14,15]
Radioactive iodine treatment is considered to be the first line of treatment for distant metastasis from thyroid carcinomas that concentrate a significant amount of radioiodine. Unlike our patient who developed metastatic disease and related symptoms five years after treatment of primary disease, for patients who develop distant metastatic disease at the time of initial diagnosis, a positive iodine-131 whole body scan post treatment of primary lesions in the neck makes way for successful radioiodine treatment of metastasis. However, solitary metastatic lesions which do not concentrate radioiodine can be dealt with surgical removal and/or external beam radiation. To add to the confusion, our patient showed radioiodine concentration in one of the muscle lesions and liver but no concentration in another muscle lesion and lung nodules. Consequently, the muscle lesions responded to radioiodine treatment, whereas the neck lesion and lung nodules showed progression over the period of treatment. The patient has been put on a suppressive dose of thyroxin and redifferentiation therapy. In the past, retinoic acid, thalidomide, and rosiglitazone showed efficacy in redifferentiation therapy of iodine-nonconcentrating thyroid malignancy metastatic lesions; however, currently, sorafenib (400 mg twice daily) and sunitinib (50 mg daily for 28 days followed by 14 days of no treatment per cycle), approved for other indications, show promise for thyroid cancer.[16,17]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–11. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 2.Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Prognostic factors in papillary and follicular thyroid carcinoma: Their implications for cancer staging. Ann Surg Oncol. 2007;14:730–8. doi: 10.1245/s10434-006-9207-5. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferri EL, Young RL. Papillary thyroid carcinoma: A 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70:511–8. doi: 10.1016/0002-9343(81)90573-8. [DOI] [PubMed] [Google Scholar]
- 4.Ilgan S, Karacalioglu AO, Pabuscu Y, Atac GK, Arslan N, Ozturk E, et al. Iodine-131 treatment and high-resolution CT: Results in patients with lung metastases from differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2004;31:825–30. doi: 10.1007/s00259-004-1460-x. [DOI] [PubMed] [Google Scholar]
- 5.Cha ST, Jarrahy R, Mathiesen RA, Suh R, Shahinian HK. Cerebellopontine angle metastasis from papillary carcinoma of the thyroid: Case report and literature review. Surg Neurol. 2000;54:320–6. doi: 10.1016/s0090-3019(00)00306-2. [DOI] [PubMed] [Google Scholar]
- 6.Prietzel T, Macher A, Haferkorn I, Schmitz N, Schmidt F, Aigner T. Soft tissue metastasis of thyroid carcinoma in the knee region mimicking a paraarticular inflammatory lesion. Arch Orthop Trauma Surg. 2010;130:1425–8. doi: 10.1007/s00402-009-1043-1. [DOI] [PubMed] [Google Scholar]
- 7.Khan OA, Roses DF, Peck V. Papillary thyroid carcinoma metastatic to skin may herald aggressive disease. Endocr Pract. 2010;16:446–8. doi: 10.4158/EP09178.CR. [DOI] [PubMed] [Google Scholar]
- 8.Panoussopoulos D, Theodoropoulos G, Vlahos K, Lazaris ACh, Papadimitriou K. Distant solitary skeletal muscle metastasis from papillary thyroid carcinoma. Int Surg. 2007;92:226–9. [PubMed] [Google Scholar]
- 9.Pucci A, Suppo M, Lucchesi G, Celeste A, Viberti L, Pellerito R, et al. Papillary thyroid carcinoma presenting as a solitary soft tissue arm metastasis in an elderly hyperthyroid patient. Case report and review of the literature. Virchows Arch. 2006;448:857–61. doi: 10.1007/s00428-006-0187-4. [DOI] [PubMed] [Google Scholar]
- 10.Lecumberri B, Alvarez-Escolá C, Martín-Vaquero P, Nistal M, Martín V, Riesco-Eizaguirre G, et al. Solitary hemorrhagic cerebellar metastasis from occult papillary thyroid microcarcinoma. Thyroid. 2010;20:563–7. doi: 10.1089/thy.2010.0062. [DOI] [PubMed] [Google Scholar]
- 11.Rocha Filho FD, Lima GG, Ferreira FV, Lima MG, Hissa MN. Orbital metastasis as primary clinical manifestation of thyroid carcinoma-case report and literature review. Arq Bras Endocrinol Metabol. 2008 Dec;52:1497–500. doi: 10.1590/s0004-27302008000900014. [DOI] [PubMed] [Google Scholar]
- 12.Agriantonis DJ, Hall L, Wilson MA. Utility of SPECT/CT as an adjunct to planar whole body I-131 imaging: Liver metastasis from papillary thyroid cancer. Clin Nucl Med. 2009;34:247–8. doi: 10.1097/RLU.0b013e31819a1eb3. [DOI] [PubMed] [Google Scholar]
- 13.Borschitz T, Eichhorn W, Fottner C, Hansen T, Schad A, Schadmand-Fischer S, et al. Diagnosis and treatment of pancreatic metastases of a papillary thyroid carcinoma. Thyroid. 2010;20:93–8. doi: 10.1089/thy.2009.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strate SM, Lee EL, Childers JH. Occult papillary carcinoma of the thyroid with distant metastases. Cancer. 1984;54:1093–100. doi: 10.1002/1097-0142(19840915)54:6<1093::aid-cncr2820540628>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Mccormack KR. Bone metastases from thyroid carcinoma. Cancer. 1966;19:181–4. doi: 10.1002/1097-0142(196602)19:2<181::aid-cncr2820190207>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Sherman SI. Advances in Chemotherapy of Differentiated Epithelial and Medullary Thyroid Cancers. J Clin Endocrinol Metab. 2009;94:1493–9. doi: 10.1210/jc.2008-0923. [DOI] [PubMed] [Google Scholar]
- 17.Mazzaferri EL. Management of solitary thyroid nodule. N Engl J Med. 1993;328:553–9. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]


