Abstract
Background
There are no approved pharmacotherapies for preventing psychomotor stimulant relapse. The operant reinstatement model has been suggested as a screen for identifying candidate medications. The present study examined if the anxiolytic buspirone could attenuate reinstatement of extinguished responding in Long-Evans rats that previously self-administered intravenous cocaine or methamphetamine.
Methods
Rats were trained in 2-h daily sessions to self-administer 0.5 mg/kg cocaine or 0.1 mg/kg methamphetamine infusions followed by 12 days of instrumental extinction. Reinstatement was evoked by 17 mg/kg i.p. cocaine primes or response-contingent cocaine-paired cues in cocaine-reinforced rats, and by 1 mg/kg i.p. methamphetamine primes or response-contingent methamphetamine-paired cues in methamphetamine-reinforced rats.
Results
Buspirone (1 and 3 mg/kg) significantly (p<0.05) attenuated cocaine cue but not cocaine prime reinstatement. Buspirone (1 and 3 mg/kg) also significantly attenuated methamphetamine cue reinstatement. Buspirone (3 mg/kg) significantly attenuated methamphetamine prime reinstatement. During all reinstatement tests, 3 mg/kg buspirone reduced levels of inactive lever pressing relative to those of vehicle, significantly so during the cocaine cue-induced reinstatement tests.
Conclusions
Given the complexity of buspirone's neuropharmacology consisting of serotonin 5-HT1A receptor partial agonist activity, and dopamine D2, D3 and D4 receptor antagonist effects, it is uncertain which of these activities or their combination is responsible for the present results. Overall, these results suggest that buspirone may reduce the likelihood of relapse to cocaine and methamphetamine use under some conditions, although this speculation must be interpreted with caution given buspirone's similar potency to attenuate inactive-lever responding.
Keywords: reinstatement, cocaine, methamphetamine, buspirone, rats, self-administration
1. INTRODUCTION
Over the course of their addiction, drug abusers often engage in periods of voluntary or imposed abstinence (Hser et al., 2008). Unfortunately, the majority fails to remain abstinent. Even those that succeed in long-term abstinence often relapse repeatedly before reaching this goal (Price et al., 2001). At least three major classes of events are believed play a role in promoting relapse: re-exposure to the abused drug; re-exposure to external stimuli previously associated with the abused drug; and stressful events (Epstein et al., 2009; Mahoney et al., 2007; Preston and Epstein, 2011; Shiffman et al., 1996). Given the central role of sustained abstinence in successfully treating drug abuse, the development of pharmacological interventions, which can reduce the likelihood of these external events that trigger relapse are critical.
Potential relapse pharmacotherapies are often evaluated preclinically using the operant reinstatement paradigm (Shaham et al., 2003). Operant reinstatement involves training animal subjects to emit a response for self-infusions of a drug of abuse. A period of forced abstinence ensues, induced by instrumental extinction in which responding no longer results in drug administration and response rates generally decline. Subsequently, a reinstating event occurs which precipitates renewed responding analogous to clinical relapse. The operant reinstatement model of a clinical “slip” lapsing into renewed drug seeking is modeled by a response-independent administration of the previously self-administered drug. The animal model of human relapse due to exposure to cues associated with the abused drug is generally achieved by response-contingent presentation of tones and/or lights, which had previously been paired with self-administered drug infusion. In many respects, these animal reinstatement procedures are thought to model events, which provoke craving and relapse in humans (Epstein and Preston, 2003; Epstein et al., 2006).
Although the reinforcing effects of psychomotor stimulants are believed to result primarily from their dopaminergic actions, to varying degrees cocaine, amphetamine and methamphetamine additionally attenuate serotonin reuptake thereby increasing extracellular concentrations of serotonin (Ritz et al., 1988; Rothman et al., 2001). Serotonin (5-HT) is also involved in a variety of pathological neurobehavioral processes, which have been linked to addiction including aggression, anxiety and depression (Kirby et al., 2011). For these reasons, as well as the important function of 5-HT receptors in modulating release of dopamine, the serotoninergic system has been recognized as a target for psychostimulant abuse medication development (Filip et al., 2010; Hayes and Greenshaw, 2011). Clinically available selective serotonin reuptake inhibitor antidepressants (SSRI’s) attenuate cocaine cue-induced but not cocaine prime-induced reinstatement in rats (Baker et al., 2001; Burmeister et al., 2003). The SSRI’s fluoxetine and citalopram also attenuate cocaine-priming reinstatement in squirrel monkeys, although in this study non-extinguished contingent cues were presented along with the cocaine priming dose (Ruedi-Bettschen et al., 2010). Though preclinical reinstatement data with SSRI’s have been promising, clinical trials of selective serotonin reuptake inhibitors for the treatment of cocaine abuse have proven disappointing (Winstanley et al., 2011).
The selective modulation of specific serotonergic receptor subtypes offers another potential treatment approach (Bubar and Cunningham, 2006; Miszkiel et al., 2011; Muller et al., 2007). Several subtypes of 5-HT receptors have been implicated in the abuse-related effects of psychostimulants (Filip et al., 2010; Miszkiel et al., 2011). The 5-HT1A receptor has been the focus of numerous preclinical investigations. The 5-HT1A agonist 8-OH-DPAT alters self-administration of cocaine in rats (Homberg et al., 2004; Peltier and Schenk, 1993) and cynomologus monkeys (Czoty et al., 2005). 8-OH-DPAT also attenuates the locomotor activating effects of both cocaine and amphetamine (Przegalinski and Filip, 1997). Conversely, the 5-HT1A antagonist, WAY 100635, attenuates cocaine priming-induced reinstatement in rats (Schenk, 2000). While neither a full 5-HT1A agonist or antagonist is approved for human use, the partial 5-HT1A agonist buspirone is clinically approved for the short term treatment of anxiety. Buspirone attenuates cocaine self-administration in rhesus monkeys (Bergman et al., 2012; Gold and Balster, 1992), and can either increase or attenuate cocaine self-administration in rats, depending upon the self-administration protocol (Homberg et al., 2004). To our knowledge, however, buspirone has not been examined for its ability to attenuate reinstatement. The present study therefore sought to determine the efficacy of buspirone for preventing cocaine and methamphetamine reinstatement resulting from either drug prime or response-contingent cues.
2. METHODS
2.1 Subjects
Subjects were 144 adult male Long-Evans rats (Harlan Sprague Dawley, Indianapolis, IN) obtained at 275–300 g body weight. The rats were housed in standard plastic rodent cages on wood chip bedding in a 22° C temperature-controlled vivarium on a 12/12 h reversed light/dark cycle. Behavioral testing occurred during the dark phase of the cycle. The rats had continuous access to water except during experimental sessions. The rats’ weights were maintained at 320 g for the duration of the experiment by daily regulated post-session feeding of standard laboratory rodent diet. Studies were reviewed and approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University in accordance with the Guide for the Care and Use of Animals (Committee for the Update of the Guide for the Care and Use of Laboratory and National Research).
2.2 Catheterization surgery
Following a minimum of 5 days of acclimation to the vivarium each rat’s right jugular vein was implanted under 50 mg/kg i.p. ketamine and 8.7 mg/kg i.p. xylazine anesthesia with a chronic indwelling tapered catheter constructed from 3.5 french polyurethane tubing (Access Technologies, Skokie IL). The catheter was tunneled subcutaneously to the back of the animal where it was connected to a plastic and Dacron mesh back mount pedestal (Plastics One, Roanoke VA). The pedestal was implanted subcutaneously through a 3 cm incision on the back, which was approximately 1 cm lateral to a smaller 0.5 cm incision where the connection post of the pedestal exited in the midscapular region. The incisions on both the neck and back were treated with gentamicin/betamethasone (Betagen, Med-Pharmex, Pomona, CA) and closed with 7.5 mm wound clips. Post-surgical analgesia of 5 mg/kg/day carprofen was administered for 2 days. Catheters were flushed once daily prior to self-administration training, and then post-session thereafter with 0.1 ml of a mixture of 25% glycerol/75% sterile saline (by volume) to which 500 units/ml heparin, 250 mg/ml ticarcillin and 9 mg/ml clavulanic acid (Timentin, SmithKline Beacham) was added. The rats were allowed a minimum of 5 days of postoperative recovery before beginning self-administration training. If during the course of self-administration training the initial catheter was found to be in-patent, as determined by the failure to lose consciousness following a bolus intravenous infusion of 5 mg/kg ketamine, a second catheter was implanted in the left jugular vein and the animal was returned to training. If both catheters failed prior to acquisition of self-administration the rat was removed from the study and replaced.
2.3 Drugs
Cocaine HCl, (±) methamphetamine HCl and buspirone HCl were supplied by the National Institute on Drug Abuse (Rockville, MD). Cocaine and methamphetamine self-administration solutions were prepared in 0.9% sterile saline containing 5 units/ml heparin. Buspirone HCl was prepared in 0.9% sterile saline and administered in a volume of 1 ml/kg i.p. 30 min prior to the start of the reinstatement test sessions. Cocaine and methamphetamine for priming-induced reinstatement were prepared in 0.9% sterile saline. All drug doses are expressed as the salt weight.
2.4 Apparatus
Self-administration, extinction and reinstatement test sessions were conducted in standard rat operant conditioning chambers housed inside individual sound attenuating cubicles (Med-Associates, Saint Albans, VT). The operant chambers were equipped with two retractable levers on the right and left side of the front chamber wall, respectively. Above each lever was a single, white, 3000 milli-candela LED stimulus lamp. The rear wall of the chamber contained an adjustable Sonalert tone generator and a 5 w incandescent house light. Above and extending through a central hole in the roof of each operant chamber was an intravenous infusion tether composed of polyethylene tubing incased in a protective steel spring sheath (Plastics One, Roanoke VA). The tether was suspended from a counterbalanced arm/plastic fluid swivel assembly (Lomir Biomedical, Malone, NY). Polyethylene infusion tubing exited the isolation cubicle where it was connected to a 20 ml syringe fitted into a computer-controlled syringe pump (Med-Associates, Saint Albans, VT). Stimulus events were controlled and lever pressing recorded via Med-Associates SmartCtrl interfacing and Med-PC version 4 operant control software (Med-Associates, Saint Albans, VT) running on Windows XP computers.
2.5 Self-administration training and instrumental extinction
Rats were trained M–F to respond for either 0.5 mg/kg/infusion cocaine or 0.1 mg/kg/infusion methamphetamine during daily, 2-h self-administration training sessions. Cocaine and methamphetamine self-administration doses were chosen based on prior studies from the laboratory indicating that they produced rapid and reliable acquisition of self-administration (Beardsley et al., 2005; Shelton and Beardsley, 2005, 2008; Shelton et al., 2004). During cocaine self-administration training, each response on the right lever (Fixed-ratio 1; "FR1") resulted in an infusion component consisting of a 6 s timeout during which a 0.2 ml infusion of cocaine was administered accompanied by the offset of the house light, 3 Hz flashing of the stimulus lights above both levers and 60 dB sounding of the Sonalert. During methamphetamine self-administration training, the conditions were similar, except that to avoid the potential of inadvertent overdose, an additional 14 s-timeout in the darkened chamber followed the 6 s infusion component. In all groups, responses on the inactive left lever and responses on the right lever during the timeout were recorded but had no scheduled consequences.
Self-administration training continued (M–F) until a rat met acquisition criteria in which it had: 1) completed a minimum of 12 self-administration sessions; 2) received at least 15 drug deliveries per session for 4 consecutive training sessions; and, 3) received a minimum of 125 lifetime drug infusions, before commencing instrumental extinction sessions. Extinction consisted of 2-h daily (Mon-Sun) experimental sessions conducted for 12 consecutive days. Extinction sessions were differentiated according to the type of reinstatement test to be performed. In rats used for drug priming-induced reinstatement, the extinction stimulus conditions were identical to those during drug self-administration except that presses of the previously reinforced lever did not result in activation of the infusion pump. For cue-induced reinstatement studies, depression of the previously reinforced lever did not result in scheduled consequences during each 2-hr extinction session in order to preserve the conditioned effects of the drug-paired stimuli. To habituate the rats to the injection procedure, all rats received a 1 ml/kg injection of saline 30 min prior to the start of each of the last 4 extinction sessions.
2.6 Reinstatement testing
A single two hour reinstatement test session was conducted on the day following the 12th extinction session. Rats were administered either vehicle (saline), 1 or 3 mg/kg i.p. buspirone 30 min prior to the start of the reinstatement test session. Animals were assigned to buspirone treatment doses within a given study after the 12th extinction session in order to achieve, to the degree possible, similar levels of self-administration and extinction responding across groups prior to testing. During drug priming reinstatement studies, separate groups of rats received either an injection of 17 mg/kg i.p. cocaine 10 min before the start of the reinstatement test session or 1 mg/kg i.p. methamphetamine 30 min prior to the start of the reinstatement test session. Cocaine and methamphetamine priming dose was chosen based on prior studies from the laboratory showing they produced robust and generally comparable reinstatement magnitudes (Shelton and Beardsley, 2008; Shelton et al., 2004). A total of 36 rats were used for each of the 4 individual studies, 12 in each buspirone dose or vehicle condition. Conditions during the 2-h prime-induced reinstatement test sessions were identical to those used during extinction. During cue-induced reinstatement test sessions, conditions were identical to those during self-administration, except infusions were not given.
2.7 Data analysis
Number of days to reach acquisition criteria (±SEM) were calculated for each of the four experiments. Lever presses of the active and inactive levers were collected for each animal. Mean lever presses for each group (±SEM) were calculated for the final day of self-administration, extinction day 1, extinction day 12 and the reinstatement test session. The effect of buspirone or vehicle on reinstatement responding was examined by separate, one-way between subject analysis of variance (ANOVA’s) tests for active and inactive lever presses on the test day. Significant (p< 0.05) main effects of buspirone treatment dose were followed by Dunnett’s post-hoc tests comparing the vehicle pretreatment condition to each buspirone dose. The effect of changes in response contingencies (self-administration, extinction day 1, extinction day 12, reinstatement test) on number of lever presses was analyzed using separate two-way ANOVA’s on active and inactive lever presses followed by Tukey post-hoc tests comparing the effect of changes in response contingencies within individual dose assignment groups. Statistical analyses were conducted using microcomputer software (Prism 6 for Macintosh, GraphPad Software Inc., San Diego CA) and all comparisons were considered significant if p<0.05.
3. RESULTS
3.1 Cocaine priming reinstatement
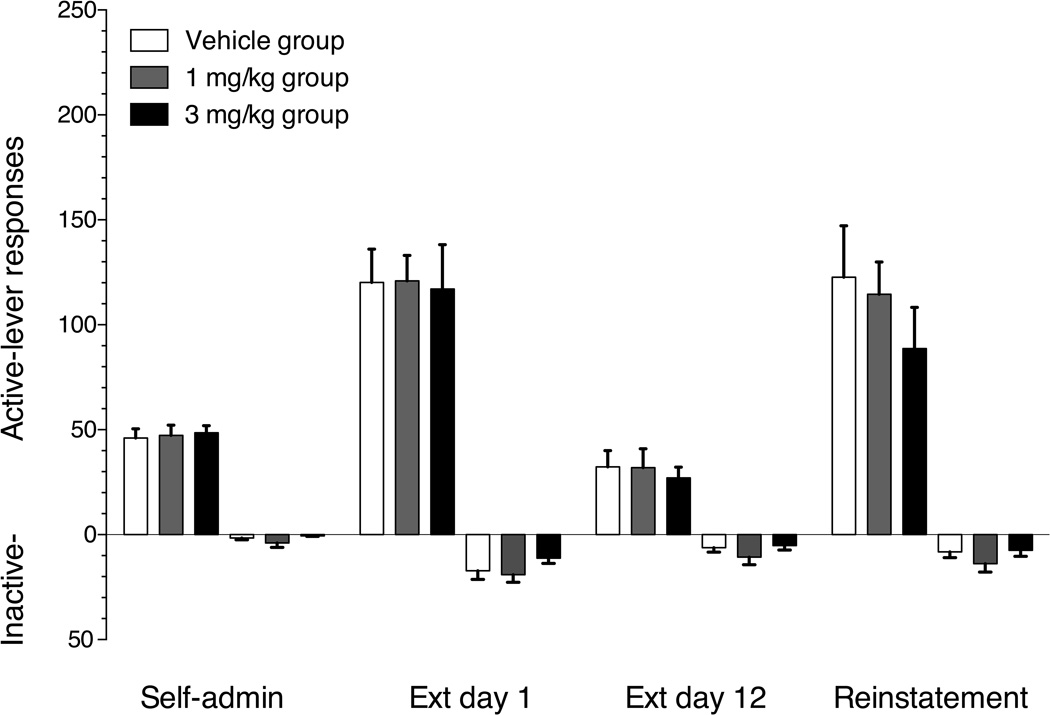
The 36 rats tested required a mean of 14 (±0.6) sessions to reach the self-administration acquisition criteria. Group mean (±SEM) active- and inactive-lever responses on the final day of cocaine self-administration, the first and last day of extinction and the test day in which vehicle, 1 mg/kg or 3 mg/kg buspirone was administered prior to cocaine priming-induced reinstatement are shown in figure 1. The upward rising bars represent active-lever responses and the downward descending bars represent inactive-lever responses. The rats across dose groups emitted 46–49 mean active-lever responses on the final day of self-administration. There was a significant main effect of response contingencies on both active (F(3,99)=42.16, p<0.001) and inactive-lever (F(3,99)=17.50, p<0.001) responses. Active- and inactive-lever responding significantly increased in all three groups on the first day of extinction relative to the last day of self-administration. Active-lever responding on the 12th and final extinction session decreased significantly compared to the first extinction session in all three groups. Pretreatment with 17 mg/kg i.p. cocaine significantly increased active-lever responding in all three groups relative to the final day of extinction. There was no significant main effect of buspirone treatment on cocaine priming-induced reinstatement (F(2,35)=0.77, p=0.47). Likewise, there were no significant effects of buspirone treatment on inactive-lever responding (F(2,35)=1.15, p=0.33) in the priming-induced reinstatement test session.
Figure 1.
Mean (±SEM) active lever (upward bars) and inactive lever (downward bars) responses on the final day of cocaine self-administration, 1st day of extinction, 12th day of extinction and the cocaine-priming-induced reinstatement test session. Open bars represent the group receiving vehicle pretreatment 30 min prior to the 17 mg/kg i.p. cocaine priming reinstatement test session. Gray bars represent the group receiving 1 mg/kg i.p. buspirone pretreatment. Black bars represent the group receiving 3 mg/kg i.p. buspirone pretreatment. * indicates significant reductions in responding on the reinstatement test session (p<0.05) relative to the vehicle control group. n=12/group.
3.2 Cocaine cue reinstatement
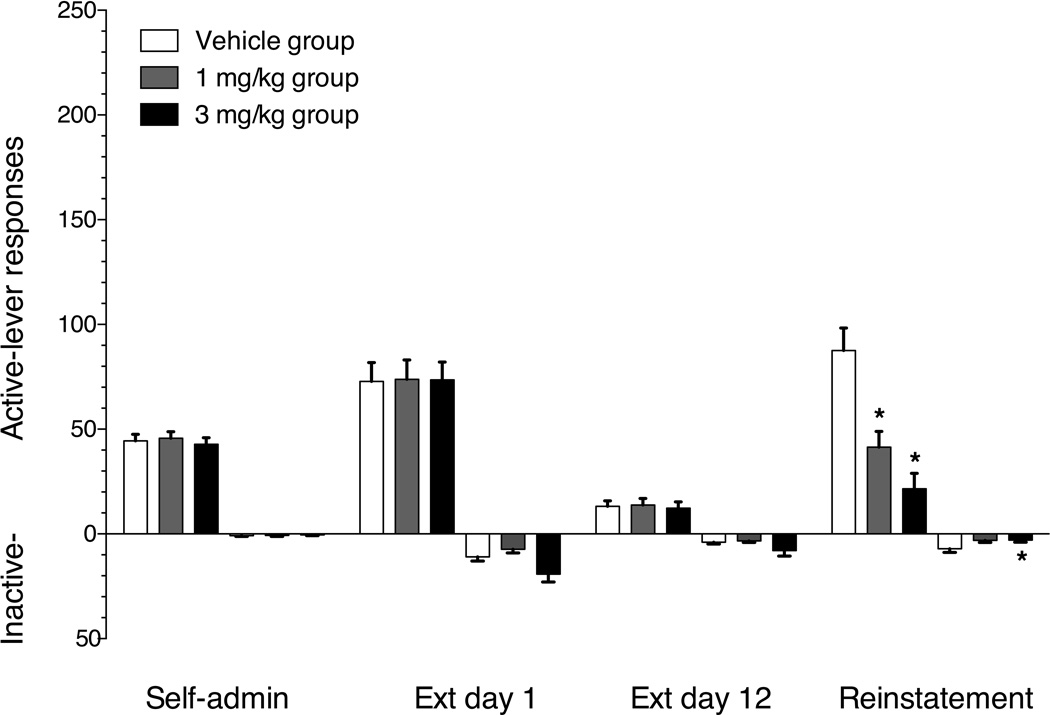
The 36 rats tested required a mean of 14 (±0.5) sessions to reach the self-administration acquisition criteria. Group mean (±SEM) active- and inactive-lever responses on the final day of cocaine self-administration, the first and last day of extinction and the test day in which vehicle, 1 mg/kg or 3 mg/kg buspirone was administered prior to cocaine cue-induced reinstatement are shown in figure 2. The rats across dose groups emitted 43–46 mean active-lever responses on the final day of self-administration. There was a significant main effect of response contingencies on both active (F(3,99)=53.85, p<0.001) and inactive-lever (F(3,99)=30.88, p<0.001) responses. Active- and inactive-lever responding significantly increased in all three test groups on the first day of extinction relative to the last day of self-administration. Active- and inactive-lever responding on the 12th extinction session decreased significantly compared to the first extinction session in all three test groups. Renewed response-contingent presentation of the light+tone cues increased active-lever responding in the vehicle and 1 mg/kg buspirone pretreatment conditions relative to the final day of extinction. There was a significant main effect of buspirone treatment on cocaine cue-induced reinstatement (F(2,35)=15.03, p<0.0001). Active-lever responses decreased from 88 in the vehicle treatment condition to 41 and 21 in the 1 and 3 mg/kg buspirone treatment groups, respectively. Post hoc analysis indicated that the rats treated with 1 and 3 mg/kg buspirone emitted significantly (p<0.05) fewer active-lever responses than the vehicle treated rats. There was also a significant main effect of buspirone treatment on inactive-lever responses (F(2,35)=3.61, p<0.05). Post-hoc analysis indicated that inactive-lever responses were significantly (p<0.05) suppressed in the 3 mg/kg buspirone treatment group relative to vehicle control.
Figure 2.
Mean (±SEM) active lever (upward bars) and inactive lever (downward bars) responses on the final day of cocaine self-administration, 1st day of extinction, 12th day of extinction and the cocaine-cue-induced reinstatement test session. Open bars represent the group receiving vehicle pretreatment 30 min prior to the 2 hr test session in which each response on the active-lever produced the 6 s light+tone stimulus complex, which had previously accompanied each cocaine injection during self-administration training. Gray bars represent the group receiving 1 mg/kg i.p. buspirone pretreatment. Black bars represent the group receiving 3 mg/kg i.p. buspirone pretreatment. * indicates significant reductions in responding on the reinstatement test session (p<0.05) relative to the vehicle control group. n=12/group.
3.3 Methamphetamine priming reinstatement
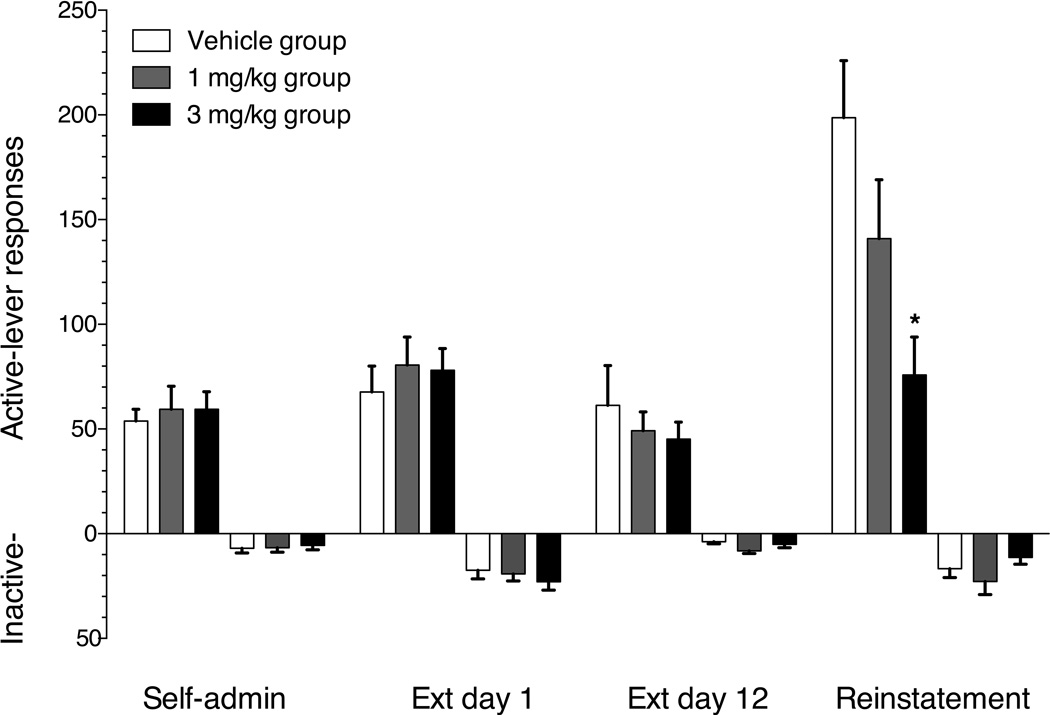
The 36 rats tested required a mean of 13 (±0.4) sessions to reach the self-administration acquisition criteria. Group mean (±SEM) active- and inactive-lever responses on the final day of methamphetamine self-administration, the first and last day of extinction and the test day in which vehicle, 1 mg/kg or 3 mg/kg buspirone was administered prior to methamphetamine priming-induced reinstatement are shown in figure 3. The rats across dose groups emitted 54–59 mean active-lever responses on the final day of self-administration. There was a significant main effect of response contingencies on both active (F(3,99)=27.31, p<0.001) and inactive-lever (F(3,99)=19.09, p<0.001) responses. Neither active- or inactive-lever responding was significantly altered on the first day of extinction relative to the last day of self-administration. There were also no significant differences in active or inactive lever presses between the first and 12th extinction session. Treatment with 1 mg/kg i.p. methamphetamine significantly increased active as well as inactive-lever responding in the vehicle and 1mg/kg buspirone test groups relative to the final day of extinction. There was a significant main effect of buspirone treatment on methamphetamine priming-induced reinstatement (F(2,35)=6.08, p=0.006). Mean active-lever responses decreased from 199 responses in the buspirone vehicle treatment condition, to 141 and 76 responses in the 1 and 3 mg/kg buspirone treatment conditions, respectively. Post hoc analysis indicated that buspirone significantly (p<0.05) suppressed active-lever responding at the 3 mg/kg treatment dose relative to the vehicle control. There was no significant main effect of buspirone treatment on inactive-lever responses as a result of methamphetamine priming (F(2,35)=1.48, p=0.25).
Figure 3.
Mean (±SEM) active lever (upward bars) and inactive lever (downward bars) responses on the final day of methamphetamine self-administration, 1st day of extinction, 12th day of extinction and the methamphetamine-priming-induced reinstatement test session. Open bars represent the group receiving vehicle pretreatment 30 min prior to the 1 mg/kg i.p. methamphetamine priming reinstatement test session. Gray bars represent the group receiving 1 mg/kg i.p. buspirone pretreatment. Black bars represent the group receiving 3 mg/kg i.p. buspirone pretreatment. * indicates significant reductions in responding on the reinstatement test session (p<0.05) relative to the vehicle control group. n=12/group.
3.4 Methamphetamine cue reinstatement
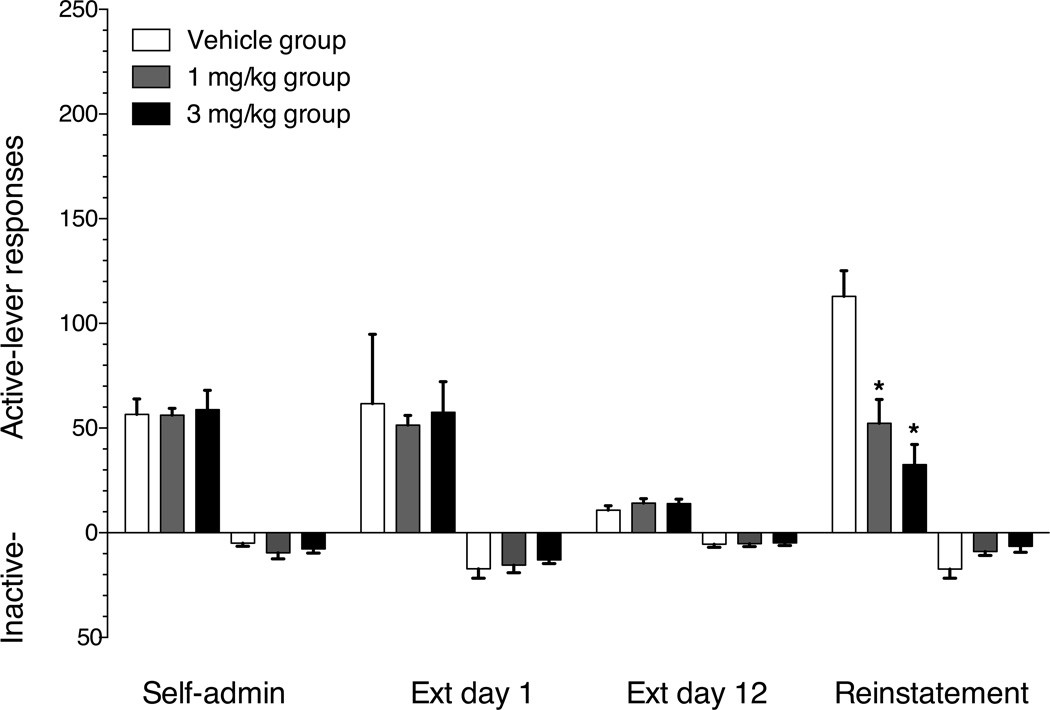
The 36 rats tested required a mean of 13 (±0.2) sessions to reach the self-administration acquisition criteria. Group mean (±SEM) active- and inactive-lever responses on the final day of methamphetamine self-administration, the first and last day of extinction and the test day in which vehicle, 1 mg/kg or 3 mg/kg buspirone was administered prior to methamphetamine cue-induced reinstatement are shown in figure 4. The rats across dose groups emitted 56–59 mean active-lever responses on the final day of methamphetamine self-administration. There was a significant main effect of response contingencies on both active (F(3,99)=13.95, p<0.001) and inactive-lever (F(3,99)=13.6, p<0.001) responses. Neither active- or inactive-lever responding was significantly increased on the first day of extinction relative to the last day of self-administration. Active-lever responding on the 12th and final extinction session decreased significantly compared to the first extinction session in vehicle and 3 mg/kg buspirone groups. Inactive-lever responding on the 12th extinction session decreased significantly compared to the first extinction session in all three groups. Renewed response-contingent presentation of the light+tone cues significantly increased active-as well as inactive-lever responding in the vehicle but not the 1 or 3 mg/kg buspirone groups. There was a significant main effect of buspirone treatment on active-lever (F(2,35)=14.12, p<0.0001) but not inactive-lever (F(2,35)=3.22, p=0.053) responses in the cue-induced reinstatement test session. Specifically, buspirone doses of 1 and 3 mg/kg reduced active-lever responding to 43% and 29% of vehicle control. Post-hoc analysis indicated that buspirone significantly (p<0.05) suppressed active-lever responses at both 1 and 3 mg/kg, relative to the vehicle control condition.
Figure 4.
Mean (±SEM) active lever (upward bars) and inactive lever (downward bars) responses on the final day of methamphetamine self-administration, 1st day of extinction, 12th day of extinction and the methamphetamine-cue-induced reinstatement test session. Open bars represent the group receiving vehicle pretreatment 30 min prior to the 2 hr test session in which each response on the active-lever produced the 6 s light+tone stimulus complex, which had previously accompanied each methamphetamine injection during self-administration training. Gray bars represent the group receiving 1 mg/kg i.p. buspirone pretreatment. Black bars represent the group receiving 3 mg/kg i.p. buspirone pretreatment. * indicates significant reductions in responding on the reinstatement test session (p<0.05) relative to the vehicle control group. n=12/group.
4. DISCUSSION
The present results suggest that buspirone may have some degree of efficacy in attenuating relapse to cocaine use resulting from exposure to drug cues and to methamphetamine use resulting from drug “slips” as well as drug cues. However, the dose-related, and at times, statistically significant reductions of inactive-lever responding imposed by buspirone complicates interpretations. It is plausible that some, or perhaps all, of buspirone’s effects may instead have been due to nonselective suppression of behavior. Nonselective effects of a drug are an important consideration especially as they may be an indicator of side effects, which might limit medications compliance and reduce the efficacy of a pharmacotherapy. Treatment noncompliance due to side effects are a significant problem for medications for the treatment of many psychiatric disorders including schizophrenia, depression and anxiety (Nutt, 2010; Taylor et al., 2012; Young et al., 1986).
In the present study the higher test dose of 3 mg/kg buspirone significantly reduced inactive lever-responding in one of the four experiments. However, inactive lever-responding is not a perfect control given the generally low number of inactive responses relative to those emitted on the active lever. In rat drug discrimination experiments, 1 mg/kg buspirone clearly suppresses and higher doses nearly totally suppress operant responding maintained by food (Ator, 1991; Rijnders and Slangen, 1993). Notably, in the study by Ator (1991), the same strain of rat (Long-Evans hooded) was used as in the present study making that data particularly salient. Other drug discrimination experiments failed to demonstrate response-rate suppression in pigeons or rats by 1 mg/kg (Sanger and Schoemaker, 1992; Wolff and Leander, 1997) or even 3 mg/kg buspirone (Wada and Fukada, 1993). Taken together, these data support the position, with qualification, that effects of buspirone on reinstatement, at least at the 1 mg/kg dose, are unlikely completely attributable to its nonspecific reduction of operant responding.
Among all the conditions tested, cocaine-priming induced reinstatement was the only one buspirone failed to significantly attenuate. Numerous studies have demonstrated that a drug may be effective in attenuating reinstatement produced by only one class of reinstating event be that prime, cues or stress (Alleweireldt et al., 2003; Baker et al., 2001; Burmeister et al., 2003). Specificity based entirely on reinstating event cannot reconcile the lack of an effect of buspirone on cocaine-priming reinstatement given that buspirone was effective in attenuating methamphetamine-priming reinstatement. Instead, the mismatch between the cocaine and methamphetamine priming reinstatement findings are most likely the result of either the pharmacological interaction of buspirone in combination with the cocaine and methamphetamine priming injections, or uncontrolled methodological factors.
In regard to the second possibility, there were several uncontrolled factors, which might have played a role. Firstly, we did not explicitly attempt to equate the relative reinstating efficacy of our cocaine and methamphetamine priming doses. However, as a percentage increase from the day 12 extinction baseline in their respective vehicle pretreatment groups, the magnitude of reinstatement produced by 17 mg/kg cocaine and 1 mg/kg methamphetamine primes were similar (376% and 324% increases, respectively). Nor did we attempt to equate the reinforcing efficacy of the unit self-administration doses of the two drugs although acquisition rates were nearly identical, varying by only a single day, between the cocaine and methamphetamine-trained rats. Therefore at least using these metrics, the methamphetamine and cocaine unit doses were equivalent. Given the general trend toward a suppression of cocaine priming reinstatement by buspirone, the failure to significantly attenuate reinstatement may simply be due to lack of sufficient statistical power, despite the group size of 12 subjects per condition is somewhat larger than the sample size normally reported in rat reinstatement studies. Therefore, what can be conservatively concluded is that buspirone was ineffective in significantly attenuating cocaine priming reinstatement under the specific conditions tested, but this does not preclude the possibility it might be effective under other conditions.
The mechanism through which buspirone attenuates reinstatement is unclear. The most well established action of buspirone is as a serotonin 5-HT1A receptor partial agonist which is believed to underlie its anxiolytic activity (Tunnicliff, 1991). When given acutely, low doses of buspirone primarily target autoreceptors and attenuate 5-HT release (VanderMaelen et al., 1986). This would seem an unlikely mechanism given that depletion of 5-HT enhances, rather than decreases cocaine-priming reinstatement (Tran-Nguyen et al., 2001). The 5-HT1A antagonist, WAY 100635, blocked cocaine priming-induced but not cue-induced reinstatement (Burmeister et al., 2004; Schenk, 2000) which is an interesting contrast to our results in which buspirone attenuated cocaine cue-induced but not prime-induced reinstatement. Given the paucity of available data, additional reinstatement studies with more selective 5-HT1A agonists will be necessary to explore this potential mechanism. However, it is important to note that the acute effects of buspirone on 5-HT1A autoreceptors is to attenuate 5-HT release, but chronic buspirone desensitize 5-HT1A autoreceptors in some brain areas leading to enhanced release of 5-HT (Okazawa et al., 1999; Sharp et al., 1993). This oppositional effect of acute versus repeated administration may have substantial implications for the use of the present data as predictors of potential treatment efficacy of 5-HT1A agonists, given that any treatment drug would be given chronically and the present effects were observed following acute administration.
Buspirone has been shown to modulate a number of other behavioral effects of psychomotor stimulants. Buspirone reduces cocaine self-administration in rhesus monkeys (Bergman et al., 2012; Gold and Balster, 1992). Buspirone reduces progressive-ratio responding for cocaine self-administration in high, but not low-grooming rats (Homberg et al., 2004), but does not affect cocaine conditioned place preference in mice (Ali and Kelly, 1997). Buspirone attenuates amphetamine-induced enhancement of locomotor activity (Jackson et al., 1994). In drug discrimination buspirone partially substitute for methamphetamine, but cocaine does not substitute for buspirone (Koetzner et al., 1996; Munzar et al., 1999). Buspirone has been alternatively reported to attenuate (Callahan and Cunningham, 1997) or have no effect on the discriminative stimulus effects of cocaine (Rapoza, 1993). Lastly, buspirone fails to attenuate cocaine-induced anxiety-like behaviors in rats (Paine et al., 2002).
Buspirone binds to dopamine D2 receptors and has a number of D2 antagonist-like effects (Bergman et al., 2012; Peroutka, 1985; Protais et al., 1998). In buspirone-trained rats, the dopamine D2 antagonist, haloperidol, partially or completely generalizes to the buspirone stimulus (Ator, 1991; Rijnders and Slangen, 1993). Buspirone blocks the discriminative stimulus of the D2 agonist, apomorphine (Kamien and Woolverton, 1990), and attenuates apomorphine-induced aggression and sniffing (Protais et al., 1998; Pruus et al., 2000). Dopamine D2 antagonists reliably prevent cocaine cue- and priming-induced reinstatement (Gal and Gyertyan, 2006; Milivojevic et al., 2004; Schenk and Gittings, 2003) as well as priming-induced reinstatement of amphetamine-rewarded runway responding (Ettenberg, 1990). These studies suggest that buspirone could be exerting its effects on reinstatement via a dopamine D2 mechanism, although it has been postulated that the D2 antagonist-like behavioral effects of buspirone may instead be mediated indirectly by 5-HT1A receptor activity (Nader and Woolverton, 1994).
Lastly, buspirone and its metabolites function in vitro as not only D2 but also D3 and D4 antagonists (Bergman et al., 2012; Tallman et al., 1997). Buspirone attenuates fixed-ratio responding for intravenous cocaine infusions in rhesus monkeys, an effect which was speculated to be D3 mediated (Bergman et al., 2012). However most reports that have tested selective D3 antagonists have failed to observe reductions of ongoing cocaine self-administration under FR schedules (for review, see Heidbreder and Newman, 2010). Some effects of buspirone on reinstatement in the present study, however, are consistent with a D3 receptor mechanism. For instance, the dopamine D3 receptor antagonist, SB-277011A, attenuates methamphetamine-priming induced reinstatement (Higley et al., 2011a). The D3 preferring antagonist, PG01037, reduces cocaine-priming reinstatement (Achat-Mendes et al., 2010) and methamphetamine-cue induced reinstatement (Higley et al., 2011b). The D3 preferring antagonists, NGB 2904 and SB-277011-A, attenuate cocaine cue-induced reinstatement, and NGB 2904 also reduces cocaine-priming reinstatement (Cervo et al., 2007; Xi and Gardner, 2007). Based on available data, the hypothesis that buspirone may be acting through D3 and/or D4 receptor mechanisms merits additional study, although the lack of efficacy of buspirone for attenuating cocaine-priming induced reinstatement in the present study appears discrepant with the effects of other D3 receptor antagonists, and its D2-like antagonist pharmacology is likely responsible for its nonspecific rate-reducing effects obscuring its direct effects on reinstatement.
In summary, the mechanism or mechanisms through which buspirone exerts its reinstatement-reducing actions are uncertain. The actions of buspirone on 5-HT1A, dopamine D2, D3 or D4 receptors are all plausible candidates. Additional studies examining buspirone in combination with antagonists or selective agonists of the respective receptors will be necessary to more fully delineate their involvement. Regardless of mechanism, the present results suggest that buspirone may have some degree of efficacy for attenuating relapse to cocaine use triggered by previously cocaine-paired cues. Additionally, buspirone may also be effective in attenuating relapse to methamphetamine use as a consequence of methamphetamine cues or a drug “slip.” Based in part on the present data and other studies (Bergman et al., 2012), it was recently announced that buspirone would be entering human clinical trials for the prevention of cocaine relapse (Winhusen et al., 2012). However, the reinstatement-attenuating effects of buspirone exert themselves at doses that are very near those which also have nonselective response-rate suppressing effects. The predicted clinical utility of buspirone for preventing relapse to psychomotor stimulant abuse based on the present reinstatement data is therefore cautiously optimistic.
Acknowledgements
The authors would like to thank Stephen Carter, Lindsey King, Molly Creighton and Desiree Sedio for their excellent technical assistance. The authors would also like to acknowledge the support of Drs. David McCann and Jane Acri of the NIDAs Medication Discovery and Toxicology Branch for their scientific input on this project.
Role of funding source
Funding for this study was provided by NIH contract N01 DA-09-8889. NIH initially provided buspirone as an unidentified numbered test compound for evaluation as a relapse-prevention medication. The NIH and its employees had no role in the actual collection, analysis and interpretation of data, in the writing of the report or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions
K. L. Shelton, E. S. Hendrick and P. M. Beardsley contributed equally to the research design, data analysis and writing of the manuscript. All authors approved the final manuscript.
Conflict of interest statement
No disclosure: KLS, ESH, PMB
REFERENCES
- Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys. J. Pharmacol. Exp. Ther. 2010;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I, Kelly ME. Buspirone fails to affect cocaine-induced conditioned place preference in the mouse. Pharmacol. Biochem. Behav. 1997;58:311–315. doi: 10.1016/s0091-3057(97)00239-6. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Kirschner KF, Blake CB, Neisewander JL. D1-receptor drugs and cocaine-seeking behavior: investigation of receptor mediation and behavioral disruption in rats. Psychopharmacol. (Berl.) 2003;168:109–117. doi: 10.1007/s00213-002-1305-x. [DOI] [PubMed] [Google Scholar]
- Ator NA. Discriminative stimulus effects of the novel anxiolytic buspirone. Behav. Pharmacol. 1991;2:3–14. [PubMed] [Google Scholar]
- Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacol. (Berl.) 2001;155:18–26. doi: 10.1007/s002130000676. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacol. (Berl.) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Bergman J, Roof RA, Furman CA, Conroy JL, Mello NK, Sibley DR, Skolnick P. Int. J. Neuropsychopharmacol. Epub ahead of print; 2012. Modification of cocaine self-administration by buspirone (Buspar): potential involvement of D(3) and D(4) dopamine receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr. Top. Med. Chem. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2004;29:660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacol. (Berl.) 2003;168:146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. Modulation of the discriminative stimulus properties of cocaine : comparison of the effects of fluoxetine with 5-HT1A and 5-HT1B receptor agonists. Neuropharmacology. 1997;36:373–381. doi: 10.1016/s0028-3908(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int. J. Neuropsychopharmacol. 2007;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Committee for the Update of the Guide for the Care and Use of Laboratory, National Research, Guide for the Care and Use of Laboratory Animals. Eighth Edition. Washington, DC: The National Academies Press; [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Effects of the 5-HT(1A) agonist (+/−)-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on cocaine choice in cynomolgus monkeys. Behav. Pharmacol. 2005;16:187–191. doi: 10.1097/00008877-200505000-00008. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacol. (Berl.) 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacol. (Berl.) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch. Gen. Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A. Haloperidol prevents the reinstatement of amphetamine-rewarded runway responding in rats. Pharmacol. Biochem. Behav. 1990;36:635–638. doi: 10.1016/0091-3057(90)90268-m. [DOI] [PubMed] [Google Scholar]
- Filip M, Alenina N, Bader M, Przegalinski E. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict. Biol. 2010;15:227–249. doi: 10.1111/j.1369-1600.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL. Effects of buspirone and gepirone on i.v. cocaine self-administration in rhesus monkeys. Psychopharmacol. (Berl.) 1992;108:289–294. doi: 10.1007/BF02245114. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Greenshaw AJ. 5-HT receptors and reward-related behaviour: a review. Neurosci. Biobehav. Rev. 2011;35:1419–1449. doi: 10.1016/j.neubiorev.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann. N. Y. Acad. Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Kiefer SW, Li X, Gaal J, Xi ZX, Gardner EL. Dopamine D(3) receptor antagonist SB-277011A inhibits methamphetamine self-administration and methamphetamine-induced reinstatement of drug-seeking in rats. Eur. J. Pharmacol. 2011a;659:187–192. doi: 10.1016/j.ejphar.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J. Psychopharmacol. 2011b;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Arends B, Wardeh G, Raaso HS, Schoffelmeer AN, de Vries TJ. Individual differences in the effects of serotonergic anxiolytic drugs on the motivation to self-administer cocaine. Neuroscience. 2004;128:121–130. doi: 10.1016/j.neuroscience.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Brecht ML, Li L. Comparing the dynamic course of heroin, cocaine, and methamphetamine use over 10 years. Addict. Behav. 2008;33:1581–1589. doi: 10.1016/j.addbeh.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DM, Johansson C, Lindgren LM, Bengtsson A. Dopamine receptor antagonists block amphetamine and phencyclidine-induced motor stimulation in rats. Pharmacol. Biochem. Behav. 1994;48:465–471. doi: 10.1016/0091-3057(94)90554-1. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Woolverton WL. Buspirone blocks the discriminative stimulus effects of apomorphine in monkeys. Pharmacol. Biochem. Behav. 1990;35:117–120. doi: 10.1016/0091-3057(90)90214-3. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koetzner L, Riley AL, Glowa JR. Discriminative stimulus effects of dopaminergic agents in rhesus monkeys. Pharmacol. Biochem. Behav. 1996;54:517–523. doi: 10.1016/0091-3057(95)02282-1. [DOI] [PubMed] [Google Scholar]
- Mahoney JJ, 3rd, Kalechstein AD, De La Garza R, 2nd, Newton TF. A qualitative and quantitative review of cocaine-induced craving: the phenomenon of priming. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:593–599. doi: 10.1016/j.pnpbp.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic N, Krisch I, Sket D, Zivin M. The dopamine D1 receptor agonist and D2 receptor antagonist LEK-8829 attenuates reinstatement of cocaine-seeking in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:576–582. doi: 10.1007/s00210-004-0937-2. [DOI] [PubMed] [Google Scholar]
- Miszkiel J, Filip M, Przegalinski E. Role of serotonin (5-HT)1B receptors in psychostimulant addiction. Pharmacol. Rep. 2011;63:1310–1315. doi: 10.1016/s1734-1140(11)70695-8. [DOI] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog. Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Munzar P, Laufert MD, Kutkat SW, Novakova J, Goldberg SR. Effects of various serotonin agonists, antagonists, and uptake inhibitors on the discriminative stimulus effects of methamphetamine in rats. J. Pharmacol. Exp. Ther. 1999;291:239–250. [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Blockade of the discriminative stimulus effects of d-amphetamine in rhesus monkeys with serotonin 5-HT1A agonists. Behav. Pharmacol. 1994;5:591–598. doi: 10.1097/00008877-199410000-00004. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Rationale for barriers to appropriate medication for the long-term treatment of depression. J. Clin. Psychiatr. 2010;71(Suppl. E1):e02. doi: 10.4088/JCP.9058se1c.02gry. [DOI] [PubMed] [Google Scholar]
- Okazawa H, Yamane F, Blier P, Diksic M. Effects of acute and chronic administration of the serotonin1A agonist buspirone on serotonin synthesis in the rat brain. J. Neurochem. 1999;72:2022–2031. doi: 10.1046/j.1471-4159.1999.0722022.x. [DOI] [PubMed] [Google Scholar]
- Paine TA, Jackman SL, Olmstead MC. Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav. Pharmacol. 2002;13:511–523. doi: 10.1097/00008877-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Peltier R, Schenk S. Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacol. (Berl.) 1993;110:390–394. doi: 10.1007/BF02244643. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. Selective interaction of novel anxiolytics with 5-hydroxytryptamine1A receptors. Biol. Psychiatry. 1985;20:971–979. doi: 10.1016/0006-3223(85)90194-5. [DOI] [PubMed] [Google Scholar]
- Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacol. (Berl.) 2011;218:29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RK, Risk NK, Spitznagel EL. Remission from drug abuse over a 25-year period: patterns of remission and treatment use. Am. J. Public Health. 2001;91:1107–1113. doi: 10.2105/ajph.91.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protais P, Lesourd M, Comoy E. Similar pharmacological properties of 8-OH-DPAT and alnespirone (S 20499) at dopamine receptors: comparison with buspirone. Eur. J. Pharmacol. 1998;352:179–187. doi: 10.1016/s0014-2999(98)00361-6. [DOI] [PubMed] [Google Scholar]
- Pruus K, Skrebuhhova-Malmros T, Rudissaar R, Matto V, Allikmets L. 5-HT1A receptor agonists buspirone and gepirone attenuate apomorphine-induced aggressive behaviour in adult male Wistar rats. J. Physiol. Pharmacol. 2000;51:833–846. [PubMed] [Google Scholar]
- Przegalinski E, Filip M. Stimulation of serotonin (5-HT) 1A receptors attenuates the locomotor, but not the discriminative, effects of amphetamine and cocaine in rats. Behav. Pharmacol. 1997;8:699–706. doi: 10.1097/00008877-199712000-00004. [DOI] [PubMed] [Google Scholar]
- Rapoza D. Buspirone fails to affect the discriminative stimulus effects of cocaine. Pharmacol. Biochem. Behav. 1993;45:179–183. doi: 10.1016/0091-3057(93)90102-y. [DOI] [PubMed] [Google Scholar]
- Rijnders HJ, Slangen JL. The discriminative stimulus properties of buspirone involve dopamine-2 receptor antagonist activity. Psychopharmacology. 1993;111:55–61. doi: 10.1007/BF02257407. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine self-administration appears to be mediated by dopamine uptake inhibition. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1988;12:233–239. doi: 10.1016/0278-5846(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ruedi-Bettschen D, Rowlett JK, Spealman RD, Platt DM. Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys: kappa opioid and serotonergic mechanisms. Psychopharmacol. (Berl.) 2010;210:169–177. doi: 10.1007/s00213-009-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ, Schoemaker H. Discriminative stimulus properties of 8-OH-DPAT: relationship to affinity for 5HT1A receptors. Psychopharmacology. 1992;108:85–92. doi: 10.1007/BF02245290. [DOI] [PubMed] [Google Scholar]
- Schenk S. Effects of the serotonin 5-HT(2) antagonist, ritanserin, and the serotonin 5-HT(1A) antagonist, WAY 100635, on cocaine-seeking in rats. Pharmacol Biochem Behav. 2000;67:363–369. doi: 10.1016/s0091-3057(00)00377-4. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gittings D. Effects of SCH 23390 and eticlopride on cocaine-seeking produced by cocaine and WIN 35,428 in rats. Psychopharmacol. (Berl.) 2003;168:118–123. doi: 10.1007/s00213-002-1276-y. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacol. (Berl.) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sharp T, McQuade R, Bramwell S, Hjorth S. Effect of acute and repeated administration of 5-HT1A receptor agonists on 5-HT release in rat brain in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 1993;348:339–346. doi: 10.1007/BF00171331. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Beardsley PM. Interaction of extinguished cocaine-conditioned stimuli and footshock on reinstatement in rats. Int. J. Comp. Psych. 2005;18:154–166. [Google Scholar]
- Shelton KL, Beardsley PM. Effect of drug-paired exteroceptive stimulus presentations on methamphetamine reinstatement in rats. Pharmacol. Biochem. Behav. 2008;90:434–440. doi: 10.1016/j.pbb.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL, Hendrick E, Beardsley PM. Interaction of noncontingent cocaine and contingent drug-paired stimuli on cocaine reinstatement. Eur. J. Pharmacol. 2004;497:35–40. doi: 10.1016/j.ejphar.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J. Consult. Clin. Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Tallman JF, Primus RJ, Brodbeck R, Cornfield L, Meade R, Woodruff K, Ross P, Thurkauf A, Gallager DW. I. NGD 94-1: identification of a novel, high-affinity antagonist at the human dopamine D4 receptor. J. Pharmacol. Exp. Ther. 1997;282:1011–1019. [PubMed] [Google Scholar]
- Taylor S, Abramowitz JS, McKay D. Non-adherence and non-response in the treatment of anxiety disorders. J. Anxiety Disord. 2012;26:583–589. doi: 10.1016/j.janxdis.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Bellew JG, Grote KA, Neisewander JL. Serotonin depletion attenuates cocaine seeking but enhances sucrose seeking and the effects of cocaine priming on reinstatement of cocaine seeking in rats. Psychopharmacol. (Berl.) 2001;157:340–348. doi: 10.1007/s002130100822. [DOI] [PubMed] [Google Scholar]
- Tunnicliff G. Molecular basis of buspirone's anxiolytic action. Pharmacol. Toxicol. 1991;69:149–156. doi: 10.1111/j.1600-0773.1991.tb01289.x. [DOI] [PubMed] [Google Scholar]
- VanderMaelen CP, Matheson GK, Wilderman RC, Patterson LA. Inhibition of serotonergic dorsal raphe neurons by systemic and iontophoretic administration of buspirone, a non-benzodiazepine anxiolytic drug. Eur. J. Pharmacol. 1986;129:123–130. doi: 10.1016/0014-2999(86)90343-2. [DOI] [PubMed] [Google Scholar]
- Wada T, Fukada N. Discriminative stimulus properties of a new anxiolytic, DN-2327, in rats. Psychopharmacology. 1993;110:280–286. doi: 10.1007/BF02251282. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Brady KT, Stitzer M, Woody G, Lindblad R, Kropp F, Brigham G, Liu D, Sparenborg S, Sharma G, Vanveldhuisen P, Adinoff B, Somoza E. Evaluation of buspirone for relapse-prevention in adults with cocaine dependence: an efficacy trial conducted in the real world. Contemp. Clin. Trials. 2012;33:993–1002. doi: 10.1016/j.cct.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley EL, Bigelow GE, Silverman K, Johnson RE, Strain EC. A randomized controlled trial of fluoxetine in the treatment of cocaine dependence among methadone-maintained patients. J. Subst. Abuse Treat. 2011;40:255–264. doi: 10.1016/j.jsat.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. Differentiation of 5-HT1A receptor ligands by drug discrimination. Eur. J. Pharmacol. 1997;333:113–122. doi: 10.1016/s0014-2999(97)01125-4. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Zonana HV, Shepler L. Medication noncompliance in schizophrenia: codification and update. Bull. Am. Acad. Psychiatry Law. 1986;14:105–122. [PubMed] [Google Scholar]