Abstract
Background
Our objective was to quantify the short-term total within-person variability in standard and nontraditional kidney measures using national data.
Study Design
Repeat examination study of serum and urine kidney measures.
Setting & Participants
Participants aged 18 or older in the Third National Health and Nutrition Examination Survey (NHANES III) who had repeat blood and urine samples collected during visits occurring ~18 days apart.
Measurements
Standardized serum creatinine, standardized cystatin C, β-trace protein (BTP), β2-microglobulin (B2M) and urine albumin and creatinine. We calculated the within-person coefficient of variation (CVw), which includes both biological and analytical variability. We also evaluated the impact of variability on estimates of the prevalence of reduced estimated glomerular filtration rate and albuminuria.
Results
Serum cystatin C demonstrated the lowest short-term within-person variability (CVw=6.8%). Serum creatinine and B2M (CVw values of 7.6% and 8.4%, respectively) also had low variability. BTP had the most variability of the serum markers (CVw=11.6%). As expected, urine albumin and urine creatinine concentration measurements exhibited high variability (CVw >30% for both), however the albumin-creatinine ratio performed much better than either measure alone, with a CVw of 11.3%. The effect of this variability on the prevalence of reduced estimated glomerular filtration rate was moderate, with an approximately 20% lower prevalence when defined based on single measurements compared to repeated application of the same test ~18 days apart. Repeat testing for albuminuria had a larger effect, showing a 33% lower prevalence of albuminuria when repeat testing was applied.
Limitations
Only two measurements available. General population with low prevalence of kidney disease.
Conclusions
Our results suggest that creatinine, cystatin C, and B2M have similarly low short-term variability. BTP was more variable compared to the other serum filtration makers. Urine albumin and creatinine were highly variable and may benefit from repeat assessments to reduce the misclassification of albuminuria.
Estimated glomerular filtration rate (eGFR) and urine albumin-creatinine ratio (ACR) are increasingly recognized as essential kidney measures for clinical practice and epidemiologic studies (1–3). Serum creatinine concentration is measured as part of the basic metabolic panel and is used in conjunction with demographic information (age, sex, race) to report eGFR by >80% of clinical laboratories in the U.S. (4). However, creatinine is influenced by a number of non–glomerular filtration rate(GFR) determinants such as muscle mass and diet (5). Serum cystatin C is a nontraditional filtration marker that has been recently recommended for use as a confirmatory test for chronic kidney disease (CKD) (6). Cystatin C seems to be less influenced by muscle mass and diet compared to creatinine and it has been shown to improve estimation of GFR, particularly in persons with normal or mildly reduced kidney function (7).
Single measurements of blood and urine analytes such as serum creatinine, cystatin C, and urine ACR are frequently used to guide treatment decisions; but, these measures can be associated with substantial within-person variability. β-Trace protein (BTP) and β2-microglobulin (B2M) are additional nontraditional filtration markers that are being evaluated as alternatives to serum creatinine for the estimation of GFR (8–10). However, the adoption of these markers for clinical and research use depends on their reliability for the classification of kidney disease in individuals. The short-term variability in both nontraditional and standard measures of kidney disease has not been rigorously characterized. The objective of this study was to quantify and compare the short-term within-person variability of a comprehensive panel of kidney measures in a sample of the general population and evaluate the impact of this short-term variability on reclassification of estimates of the prevalence of decreased GFR and albuminuria.
METHODS
Study Populations
The NHANES is a program of cross-sectional studies of the civilian, non-institutionalized population of the U.S. and one of the most important sources of information on the health of the nation, including chronic kidney disease (11, 12). The present study utilized measurements in serum and urine available from the Second Examination of NHANES III (1988–1994), a sub-study of the NHANES III (13, 14). A nonrandom sample of approximately 5% of the original NHANES III study population was obtained by selecting approximately 400 people from each survey location, for a total of 2596 participants (including 2209 persons aged 18 or older). The NHANES III Second Examination took place approximately 2 weeks after the first examination and was conducted by trained personnel following the same standardized protocols. Each participant repeated the interview and extensive physical examination at a mobile examination center, which included blood and urine collections.
In 2006, we measured BTP and B2M in over 7,000 stored serum samples from a subsample of the NHANES III participants with valid cystatin C and serum creatinine measurements in stored surplus specimens that had previously undergone a single freeze-thaw cycle (15–17). In 2009, we selected 938 participants aged 12 or older who participated in both the NHANES III First and Second Examinations and who were not missing measurements of cystatin C to be part of the present serum markers variability study. We oversampled participants aged 60 and older to ensure a range of values in this variability study. Of these, there were 821 participants 18 or older (88%) for whom valid cystatin C, BTP, and B2M measurements were obtained from both their First and Second Examination stored serum samples. Measurement for BTP and B2M were conducted concurrently in 2009. Cystatin C measurements in first examination samples were conducted in 2006 and second examination samples in 2009 and re-aligned to account for laboratory drift during this time period (18).
We utilized existing data measured as part of the original NHANES III Second Examination protocol for our urine marker variability study. Because a significant proportion of persons in the larger NHANES III Second Examination sample were missing urine, we conducted our variability analyses separately among persons with valid urine and valid serum measurements. After excluding 26 persons with extreme values (with observations >3 SDs from the mean: serum creatinine, 8; cystatin C, 14; BTP, 10; and B2M, 8), the final sample size for the serum marker reliability analysis was 795, and the final sample size for the urine marker reliability analysis was 1294. A Venn diagram showing the selection of our study populations from the larger NHANES III Second Examination study and their overlap is provided in the Figure S1 (provided as online supplementary material).
Assays
Serum creatinine was measured using a kinetic rate Jaffe method as part of the original NHANES III (1988–1994) protocol and recalibrated to standardized creatinine (19) (inter-assay CVs were 2.7% at 1.7 mg/dL, 2.1% at 3.5 mg/dL, and 2.0% at 4.4 mg/dL). In 2006, cystatin C was measured in stored serum samples from the first examination participants using the Dade Behring N Latex Cystatin C assay (Siemens Healthcare Diagnostics [formerly Dade-Behring]), an automated particle-enhanced nephelometric immunoassay run on the Dade Behring BNII Nephelometer (Siemens Healthcare Diagnostics; inter-assay CV of 5.1% at a mean cystatin C concentration of 0.970 mg/dL). In 2009, the second examination specimens were analyzed using the same particle-enhanced nephelometric immunoassay reagents run on a Siemens ProSpec analyzer (Siemens Healthcare Diagnostics; inter-assay CV of 4.0% at a mean cystatin C concentration of 0.689 mg/L). All cystatin C measurements were aligned using established calibration equations and converted to ERM471/IFCC (European Reference Material 471/International Federation of Clinical Chemistry)–traceable cystatin C values (16, 18, 20, 21). BTP was measured in first and second examination stored serum samples using the N Latex β-trace protein assay (Siemens Healthcare Diagnostics) with an inter-assay CV of 5.7% (mean, 0.594 mg/L) (21, 22). B2M was measured in first and second examination stored serum samples using the N Latex β-2 microglobulin assay (Siemens Healthcare Diagnostics) with an inter-assay CV of 2.7% (mean, 1.76 mg/L) (21, 22).
As part of the original NHANES III protocol, a random spot urine sample was obtained from each participant at both the first and second examinations. Urine albumin was measured by solid phase fluorescence immunoassay (inter-assay CVs ranged from 4.8% [mean, 7.11 μg/mL] to 16.1% [mean, 1.67 μg/mL]) and urine creatinine was measured by the modified kinetic Jaffe method (inter-assay CVs ranged from 1.5% [mean, 5.77 mg/dL] to 7.7% [mean, 0.91 mg/dL] using a Synchron AS/Astra Analyzer (Beckman Coulter, Fullerton, California).
Statistical Analysis
We compared the serum creatinine, cystatin C, BTP, B2M, urine albumin, and urine creatinine values obtained during the first NHANES III examination with those obtained from samples collected at the second examination. We estimated GFR from serum creatinine using the isotope-dilution mass spectrometry (IDMS)–traceable 4-variable Modification of Diet in Renal Disease (MDRD) Study equation (23) and the 2009 CKD–Epidemiology Collaboration (CKD-EPI) equation for creatinine (26) and the 2012 CKD-EPI equation for cystatin C (6). We defined decreased GFR as an eGFR <60 ml/min/1.73 m2. We defined albuminuria as an ACR of 30 mg/g or higher. Differences were calculated as Examination 1 minus Examination 2. The within-person coefficients of variation (CVw) were calculated using a standard approach (22). Because urine albumin and urine creatinine are highly skewed, they were analyzed on the log scale. Bland-Altman plots (24) were generated to graphically display the variability in the measurements. Bland-Altman plots of albuminuria and ACR were plotted on a log scale. We evaluated the impact of short-term variability on reclassification of estimates of the prevalence of decreased GFR defined by using one versus two GFR estimates based on the MDRD Study and CKD-EPI creatinine equations and the CKD-EPI cystatin C equation, and albuminuria using the ACR. Reclassification confidence intervals were obtained using bootstrap methods. Because these data are a nonrandom sample of the original NHANES III participants, no weights are used in the analyses of these data and these results should not be interpreted as providing estimates that are nationally representative of the general U.S. population.
RESULTS
Characteristics of the serum and urine variability subsamples are shown in Table 1. The mean number of days between visits was 18. Due to the sampling design, persons in the serum variability sample were older compared to the urine variability subsample (57 years vs 47 years). Both samples had slightly less than half males (47%) and were racially and ethnically diverse.
Table 1.
Selected Characteristics of the Study Population
| Characteristic | Serum Subsample (n = 795) | Urine Subsample (n = 1294) |
|---|---|---|
| Age (y) | 57.7 ± 18.1 | 46.8 ± 18.0 |
| Time between exams 1 and 2 (d) | 17.9 ± 7.8 | 17.2 ± 7.9 |
| Male sex | 47% | 47% |
| Hypertension | 44% | 31% |
| Diabetes | 8% | 6% |
| Race/ethnicity | ||
| Non-Hispanic white | 53% | 42% |
| Non-Hispanic black | 23% | 30% |
| Mexican American | 22% | 25% |
| Other | 3% | 4% |
Note: All estimates are unweighted. Subsamples are from the NHANES III Second Examination, a nonrandom sample of participants from NHANES III (1988–1994). Hypertension was defined as a self-reported physician diagnosis of hypertension, current hypertension medication use, or a mean systolic blood pressure or diastolic blood pressure ≥140 or ≥90 mmHg, respectively. Values for categorical variables are given as percentages; values for continuous variables, as mean +/− standard deviation.
NHANES III, Third National Health and Nutrition Examination Survey;
Table 2 provides the summary statistics for the kidney measurements at each examination, the differences between examinations (Examination 1 minus Examination 2) and the CVw data. Standardized cystatin C and BTP were 3.3% and 6.3% lower, respectively, at Examination 1; such small differences could be due to analytical or biological factors. There were no significant differences between the other serum and urine markers measured at the two examinations. The CVw data indicate that standardized cystatin C exhibited the lowest short-term within-person variability (CVw=6.8%). Standardized creatinine (CVw=7.6%) and B2M (CVw=8.4%) also had low total variability. BTP had the most variability of the serum markers (CVw=11.6%). Among the esGFR equations, the CKD-EPI creatinine and CKD-EPI cystatin C equations both showed lower total variability as compared to the MDRD Study equation, with non-overlapping confidence intervals when comparing these estimates (Table 2). As expected, urine albumin and urine creatinine concentration measurements exhibited very high variability (both CVw >30%), however the ACR performed much better than either measure alone, with a CVw of 11.3%.
Table 2.
Summary Statistics for NHANES III First and Second Examination Data
| Exam 1 | Exam 2 | Difference | 95% CI for difference | CVw (%)a | |
|---|---|---|---|---|---|
| Serum measurements (N=795) | |||||
| Creatinine (mg/dL)b | 0.88 ± 0.23 | 0.87 (0.22) | 0.01 (0.09) | −0.00 to 0.01 | 7.6 (7.2 to 8.0) |
| Cystatin C (mg/L)b | 0.91 (0.24) | 0.94 (0.26) | −0.03 (0.08) | −0.04 to −0.03* | 6.8 (6.4 to 7.3) |
| BTP (mg/L) | 0.64 (0.24) | 0.68 (0.26) | −0.04 (0.10) | −0.04 to −0.03* | 11.6 (10.9 to 12.3) |
| B2M (mg/L) | 2.19 (0.76) | 2.19 (0.78) | −0.00 (0.25) | −0.02 to 0.02 | 8.4 (7.6 to 9.2) |
| eGFRcr(MDRD) | 87.16 (27.45) | 87.25 (26.78) | −0.09 (11.84) | −0.92 to 0.73 | 9.6 (8.7 to 10.4) |
| eGFRcr(CKD-EPI) | 89.30 (25.14) | 89.53 (24.37) | −0.23 (8.39) | −0.81 to 0.36 | 6.6 (6.1 to 7.1) |
| eGFRcys(CKD-EPI) | 89.53 (24.13) | 86.47 (24.54) | 3.05 (7.77) | 2.51 to 3.59* | 6.7 (6.3 to 7.2) |
| Urine Measurements (N=1,294) | |||||
| Log(Urine Albumin) | 0.88 (0.66) | 0.87 (0.66) | 0.01 (0.53) | −0.02 to 0.04 | 37.3 (34.4 to 40.1) |
| Log(Urine Creatinine) | 2.03 (0.33) | 2.03 (0.33) | 0.00 (0.32) | −0.01 to 0.02 | 43.0 (39.8 to 46.3) |
| Log(ACR) | 0.85 (0.62) | 0.85 (0.64) | 0.00 (0.45) | −0.02 to 0.03 | 11.3 (10.7 to 11.9) |
NOTE: Data for paired visits, an average of 18 days apart. Unless otherwise noted, values are given as mean +/− standard deviation. Conversion factor for serum creatinine in mg/dL to μmol/L, x88.4.
p-value<0.05.
Range is shown in parentheses.
Standardized
Abbreviations and definitions: BTP, β-trace protein; B2M, β2-microglobulin; eGFR, estimated glomerular filtration rate; CVw, the within-person coefficient of variation, is the total with-person variability, which includes biological and analytical variability. NHANES III, Third National Health and Nutrition Examiniation Survey; eGFRcr(CKD-EPI) estimated glomerular filtration rate based on serum creatinine and calculated using the Chronic Kidney Disease Epidemiology Collaboration eGFRcys(CKD-EPI) estimated glomerular filtration rate based on serum cystatin C and calculated using the Chronic Kidney Disease Epidemiology Collaboration equation; eGFRcr(MDRD), estimated glomerular filtration rate based on serum creatinine and calculated using the Modification of Diet in Renal Disease Study equation.
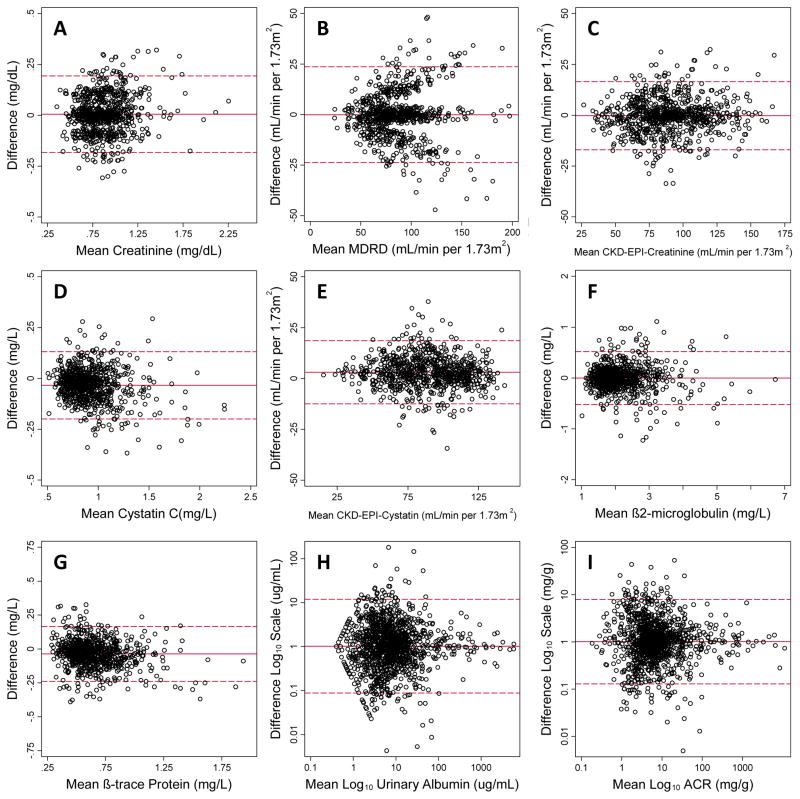
The Bland-Altman plots (Figure 1) visually show the differences in within-person variability and suggest that short-term variability in serum creatinine, cystatin C, BTP, and B2M were normally distributed and relatively consistent across the range of values. For all GFR estimating equations, variability was higher at higher levels of GFR. Compared with the MDRD Study equation, the CKD-EPI creatinine equation exhibited less variability and remained much more constant across the range of values. On the log scale, ACR varied substantially less (CVw = 11.3%) than urine albumin (CVw = 37.3%) or urine creatinine (CVw = 43.0%).
Figure 1.
Figure Bland-Altman plots of the difference between first and second exam values plotted against the mean of (A) measured creatinine, (B) estimated glomerular filtration rate using the MDRD Study equation, (C) estimated glomerular filtration rate using the CKD-EPI creatinine 2009 equation, (D) measured cystatin C, (E) estimated glomerular filtration rate using the CKD-EPI cystatin 2012 equation, (F) measured β2-microglobulin, (G) measured β-trace protein, (H) log-transformed urinary albumin, and (I) log10-transformed albumin-to-creatinine ratio (ACR). The solid line represents the mean difference while the dashed lines represent the mean ± 2 standard deviations.
Table 3 shows the impact of the observed short-term variability in the kidney measures on estimates of albuminuria and the prevalence of decreased GFR in this population as defined according to the different GFR estimating equations. The prevalence estimate of decreased GFR as defined by the CKD-EPI creatinine equation was 17.6% lower when defined using two assessments as compared to a single measurement. The prevalence of decreased GFR as defined by the CKD-EPI cystatin C equation was 21.2% lower when two assessments were used. Repeat testing for albuminuria had the largest effect, showing the biggest reduction in prevalence when testing was repeated, with a prevalence estimate that was lowered by 32.7%. Conversely, when using “either” of the assessments to define prevalence (i.e., positive on either test), the prevalence estimates for decreased GFR and albuminuria were correspondingly higher. The mean of the measurements at the two visits performed well and resulted in prevalence estimates that were intermediate between using a single measurement or requiring two measurements to define decreased GFR or albuminuria. Nonetheless, the estimates in Table 3 are imprecise due to the small number of cases in this sample of the general population, as indicated by the wide confidence intervals.
Table 3.
Prevalence Estimates of Decreased GFR
| Prevalence Based on 2 Exams vs Prevalence Estimated from 1 Exam** | |||||
|---|---|---|---|---|---|
| Decreased GFR Criteria† | Cases/Total | Prevalence | Absolute Difference* | Percentage Difference* | |
| CKD-EPI Creatinine Equation | |||||
| Exam 1 only | 82/795 | 10.3 | Reference = 10.4** | Reference = 0.0 | |
| Exam 2 only | 83/795 | 10.4 | |||
| Both exams 1 and 2 | 68/795 | 8.6 | −1.8 (−2.5 to −1.1) | −17.6 (−24.3 to −11.6) | |
| Either exam 1 or 2 | 97/795 | 12.2 | +1.8 (1.3 to 2.6) | +17.6 (11.7 to 24.3) | |
| Mean*** | 79/795 | 9.9 | −0.4 (−1.0 to 0.3) | −4.2 (−10.8 to 2.7) | |
| CKD-EPI Cystatin C Equation | |||||
| Exam 1 only | 90/795 | 11.3 | Reference = 13.1** | Reference = 0.0 | |
| Exam 2 only | 118/795 | 14.8 | |||
| Both exams 1 and 2 | 82/795 | 10.3 | −2.8 (−3.5 to −1.9) | −21.2 (−27.3 to −15.5) | |
| Either exam 1 or 2 | 126/795 | 15.8 | +2.8 (2.1 to 3.7) | +21.2 (15.5 to 27.4) | |
| Mean*** | 107/795 | 13.5 | +0.4 (−0.3 to 1.3) | +2.9 (−3.0 to 9.5) | |
| Albuminuria Criteria ‡ | |||||
| Exam 1 only | 151/1294 | 11.7 | Reference = 12.2** | Reference = 0.0 | |
| Exam 2 only | 164/1294 | 12.7 | |||
| Both exams 1 and 2 | 106/1294 | 8.2 | −4.0 (−4.7 to −3.2) | −32.7 (−39.3 to −26.4) | |
| Either exam 1 or 2 | 209/1294 | 16.2 | +4.0 (3.3 to 4.8) | +32.7 (26.4 to 39.4) | |
| Mean*** | 162/1294 | 12.5 | +0.3 (−0.5 to 1.1) | +2.9 (−4.2 to 8.9) | |
Note: prevalence values expressed in percentage.
Values in parentheses are 95% confidence intervals, which were bias-corrected bootstrap estimates using 1,000 replications
Decreased GFR was defined as estimated GFR < 60 mL/min/1.73 m2.
Albuminuria was defined as an albumin-creatinine ratio ≥30 mg/g.
The reference prevalence was estimated by averaging the prevalence at the two visits
(exam 1 measurement + exam 2 measurement)/2
GFR, glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CI, confidence interval
DISCUSSION
The goal of this study was to compare the short-term within-person variability of kidney measures in a general population sample. We found that the total short-term variability in serum cystatin C (CVw of 6.8%) was comparable to that for serum creatinine (CVw of 7.6%). B2M also had low variability (CVw of 8.4%). BTP had reasonably low total variability (CVw of 11.6%) but was somewhat more variable than the other three serum markers. The CVw data for the filtration markers examined here are comparable to those of other common blood tests used in clinical practice. For example, a previous study of the NHANES III Second Examination data reported short-term within-person “biological” CVs (after subtracting the analytical method component from the total variability) of 8.2%, 8.3%, and 1.5% for total cholesterol, plasma glucose, and glycated hemoglobin, respectively (25). Previous studies have demonstrated that the CKD-EPI creatinine equation performs better than MDRD Study equation at low creatinine values (26) and this was reflected in the lower CVw for CKD-EPI creatinine equation vs CVw for MDRD Study equation in our study. Our results support recommendations that values from the CKD-EPI creatinine equation can be accurately estimated across the range of GFR, while GFR estimated from the MDRD Study equation should be reported as a numeric value only at values <60 mL/min/1.73 m2 (26).
Our results also suggest that if repeat assessments were required to define decreased GFR in epidemiologic studies, prevalence estimates would be lower. For decreased GFR defined using the MDRD Study and CKD-EPI creatinine or cystatin C equations, the decrease in prevalence using a single measurement versus requiring a repeat test by the same measurement would be approximately 20%. This suggests that epidemiologic studies with only single measurements of eGFR may overstate the prevalence by approximately 20% as compared to a setting in which two measurements of decreased GFR (separated by a few weeks) are required. Urine albumin and urine creatinine exhibited poorer reliability than the filtration markers. Requiring repeat testing to define albuminuria would result in a 33% lower prevalence of this condition. This was despite using the ACR, which improved overall reliability compared to urine albumin concentration alone. Our data support the use of the ACR and recommendations for repeat testing in clinical practice (5, 27). The implication for identification of CKD, defined as either decreased GFR or albuminuria, in clinical practice is less certain. Chronicity can be determined using other methods besides a repeat creatinine or ACR value; for example, small or cystic kidneys on ultrasound, or known persistent urine protein or hematuria. With the well-known exception of blood pressure, the mean is rarely used to reduce misclassification of measurements in the clinical setting but may be useful in circumstances where repeat assessments of the same measurement are available weeks apart.
Understanding the variability in cystatin C, BTP, and B2M has important implications for the use of these measures in clinical practice and interpretation of their performance. In particular, the newly developed CKD-EPI equations using serum cystatin C or serum creatinine showed similar high reliability and were superior to the MDRD Study equation (6, 7, 26). This is an expected result of the shallower associations of low serum creatinine with eGFR for the CKD-EPI equation (proportional to [serum creatinine in mg/dL]−0.329 in women and [serum creatinine in mg/dL]−0.411 in men at lower serum creatinine values and [serum creatinine in mg/dL]−1.209 in both women and men at higher serum creatinine values) compared to the steeper associations in the MDRD Study equation ([serum creatinine in mg/dL]−1.154).
Cystatin C standardization efforts make it a viable candidate for use in clinical practice. Nonetheless, in the present study, the cystatin C measurements from stored serum samples in this study were not conducted concurrently. The first examination measurements of cystatin C were conducted in 2006 and the second examination measurements were conducted in 2009. These data have been directly aligned to correct for laboratory drift during this time period (18), but it is possible that recalibration may have contributed to total variability of cystatin C in this study. BTP and B2M reflect physiologic pathways distinct from serum creatinine and cystatin C. Creatinine is a muscle breakdown product, cystatin C is a protein encoded by CST3, a housekeeping gene, BTP is a prostaglandin D synthase (28) that originates from epithelial cells of the choroid plexus of the central nervous system (29), and B2M is a small subunit of the major histocompatibility class I molecule present on all nucleated cells (30). All four markers are freely filtered by the kidney, but different metabolic pathways suggest that their non-GFR determinants would differ from one another.
Possibly, estimating GFR using BTP and B2M can be useful to identify the effect of non-GFR determinants on GFR estimates using serum creatinine (e.g., muscle mass and diet) or cystatin C (e.g., thyroid function). Factors that affect GFR would influence GFR estimates based on all four filtration markers, whereas factors that affect the non-GFR determinants of only one of the filtration markers would affect only the GFR estimate based on that filtration marker.
In summary, these data provide estimates of short-term (18 day) within-person variability of a panel of nontraditional and standard kidney measures and their impact on the prevalence of reduced eGFR and albuminuria. Our study demonstrates that when repeat assessments are used, estimates of the prevalence of reduced eGFR and albuminuria in the population will be lower. We also found that newly developed CKD-EPI equations for estimating GFR have superior reliability to the MDRD Study equation. Finally, the low variability of BTP and B2M support the idea that these markers may be useful as adjunct measurements to serum creatinine and cystatin C in estimating GFR (31).
Supplementary Material
Figure S1: Venn diagram showing selection of repeat serum markers and repeat urine markers study samples.
Acknowledgments
The authors thank Mr. Yang Ning for assistance with data analysis.
Support: Siemens Healthcare Diagnostics provided a grant to University of Minnesota for labor and reagents to conduct the B2M and BTP and some cystatin C assays. This project was partially funded by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases grant U01 DK067651. SPJ was supported by NIH/National Heart, Lung and Blood Institute grant T32 HL007024
Footnotes
Financial Disclosure: JC has consulted for Amgen and Merck and has an investigator-initiated grant from Amgen. ASL and LAI have an investigator-initiated grant from Pharmalink and Gilead Inc. JHE is a consultant for Gentian, a manufacturer of cystatin C reagents. ES and SPJ declare that they have no relevant financial interests.
N SECTION: Because an author of this manuscript is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Joachim H. Ix, MD, MAS) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chronic Kidney Disease Prognosis C. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leoncini G, Viazzi F, Pontremoli R. Overall health assessment: a renal perspective. Lancet. 2010;375(9731):2053–4. doi: 10.1016/S0140-6736(10)60748-9. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 4.Miller G. Current Status Of Reporting Estimated Glomerular Filtration Rate (eGFR) [Last accessed, October 17, 2012];Report of the College of American Pathologists. 2010 Online: http://www.cap.org/apps/docs/committees/chemistry/current_status_of_reporting_eGFR.pdf.
- 5.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67(6):2089–100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 6.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. New England Journal of Medicine. 2012;367(1):20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR Using Serum Cystatin C Alone and in Combination With Serum Creatinine: A Pooled Analysis of 3,418 Individuals With CKD. American Journal of Kidney Diseases. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filler G, Priem F, Lepage N, Sinha P, Vollmer I, Clark H, et al. Beta-trace protein, cystatin C, beta(2)-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem. 2002;48(5):729–36. [PubMed] [Google Scholar]
- 9.Priem F, Althaus H, Birnbaum M, Sinha P, Conradt HS, Jung K. Beta-trace protein in serum: a new marker of glomerular filtration rate in the creatinine-blind range. Clin Chem. 1999;45(4):567–8. [PubMed] [Google Scholar]
- 10.Chen HH. beta-trace protein versus cystatin C: which is a better surrogate marker of renal function versus prognostic indicator in cardiovascular diseases? J Am Coll Cardiol. 2011;57(7):859–60. doi: 10.1016/j.jacc.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 12.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–9. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term Variability in Measures of Glycemia and Implications for the Classification of Diabetes. Archives of Internal Medicine. 2007;167(14):1545–51. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control. [Last accessed, October 17, 2012];NHANES III Second Laboratory Data File Documentation. ftp://ftpcdcgov/pub/Health_Statistics/NCHS/nhanes/nhanes3/2A/lab2-accpdf.
- 15.Kottgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J. Serum Cystatin C in the United States: The Third National Health and Nutrition Examination Survey (NHANES III) American Journal of Kidney Diseases. 2008;51(3):385–94. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. [Last accessed, October 17, 2012];NHANES III Cystatin C Surplus Sera Laboratory Component. ftp://ftp.cdc.gov/pub/health_statistics/nchs/nhanes/nhanes3/27a/SSCYSTAT.pdf.
- 17.Centers for Disease Control and Prevention. [Last accessed, October 17, 2012];NHANES III (1988–1994) Beta trace protein and beta-2 microglobulin (surplus sera) documentation. http://www.cdc.gov/nchs/nhanes/nhanes3/SSNH3BTP.htm.
- 18.Selvin EJS, Eckfeldt JH, Levey AS, Inker LA, Coresh J. Calibration of Cystatin C in the National Health and Nutrition Examination Surveys. American Journal of Kidney Diseases. doi: 10.1053/j.ajkd.2012.09.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selvin E, Manzi J, Stevens LA, Van LF, Lacher DA, Levey AS, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50(6):918–26. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58(4):682–4. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. [Last accessed, October 17, 2012];NHANES III (1988–1994) Second Examination (Surplus Sera) cystatin C, beta-trace protein, and beta-2 microglobulin. http://www.cdc.gov/nchs/nhanes/nhanes3/SSNH3CYS.htm.
- 22.Juraschek SP, Coresh J, Inker LA, Rynders GP, Eckfeldt JH, Selvin E. The effects of freeze-thaw on beta-trace protein and beta 2-microglobulin assays after long-term sample storage. Clin Biochem. 2012;45(9):694–6. doi: 10.1016/j.clinbiochem.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 25.Lacher DA, Hughes JP, Carroll MD. Estimate of Biological Variation of Laboratory Analytes Based on the Third National Health and Nutrition Examination Survey. Clinical Chemistry. 2005;51(2):450–2. doi: 10.1373/clinchem.2004.039354. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2011 doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 28.Melegos DN, Grass L, Pierratos A, Diamandis EP. Highly elevated levels of prostaglandin D synthase in the serum of patients with renal failure. Urology. 1999;53(1):32–7. doi: 10.1016/s0090-4295(98)00453-1. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann A, Nimtz M, Conradt HS. Molecular characterization of beta-trace protein in human serum and urine: a potential diagnostic marker for renal diseases. Glycobiology. 1997;7(4):499–506. doi: 10.1093/glycob/7.4.499. [DOI] [PubMed] [Google Scholar]
- 30.Woitas RP, Stoffel-Wagner B, Poege U, Schiedermaier P, Spengler U, Sauerbruch T. Low-molecular weight proteins as markers for glomerular filtration rate. Clin Chem. 2001;47(12):2179–80. [PubMed] [Google Scholar]
- 31.Bhavsar NA, Appel LJ, Kusek JW, Contreras G, Bakris G, Coresh J, et al. Comparison of Measured GFR, Serum Creatinine, Cystatin C, and Beta-Trace Protein to Predict ESRD in African Americans With Hypertensive CKD. Am J Kidney Dis. 2011;58(6):886–93. doi: 10.1053/j.ajkd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Venn diagram showing selection of repeat serum markers and repeat urine markers study samples.