Summary
Autophagy is an evolutionarily conserved membrane trafficking process. Induction of autophagy in response to nutrient limitation or cellular stress occurs by similar mechanisms in organisms from yeast to mammals. Unlike yeast, metazoan cells rely more on growth factor signaling for a wide variety of cellular activities including nutrient uptake. How growth factor availability regulates autophagy is poorly understood. Here we show that, upon growth factor limitation, the p110β catalytic subunit of the Class IA phosphoinositide 3-kinases (PI3Ks) dissociates from growth factor receptor complexes, and increases its interaction with the small GTPase Rab5. This p110β-Rab5 association maintains Rab5 in its GTP-bound state and enhances the Rab5-Vps34 interaction that promotes autophagy. p110β mutants that fail to interact with Rab5 are defective in autophagy promotion. Hence, in mammalian cells, p110β acts as a molecular sensor for growth factor availability and induces autophagy by activating a Rab5-mediated signaling cascade.
Introduction
Autophagy is a membrane trafficking process that delivers intracellular contents destined for degradation into a double membrane structure termed an autophagosome that then fuses with the lysosome (Levine and Kroemer, 2008; Levine and Yuan, 2005; Mizushima et al., 2008). In metazoans, the initiation of autophagy is critically regulated by a group of phospholipids, phosphoinositides, which are produced by phosphoinositide 3-kinases (PI3Ks). PI3Ks are lipid kinases central to numerous signaling pathways (Cantley, 2002; Carpenter et al., 1990; Engelman et al., 2006; Vanhaesebroeck et al., 2012). Based on substrate specificity and sequence homology, PI3Ks are grouped into three classes: Class I, Class II, and Class III (Domin and Waterfield, 1997; Engelman et al., 2006). Class IA PI3Ks are composed of a p85 regulatory subunit and a p110 catalytic subunit that produces phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3], which activates the Akt/mTOR signaling pathway (Franke et al., 1997; Sarbassov et al., 2005). It is believed that Class IA PI3Ks inhibit autophagy by promoting nutrient uptake and metabolic activities through Akt/mTOR (Levine and Kroemer, 2008; Petiot et al., 2000). In contrast, the Class III PI3K catalytic subunit Vps34 is bound to the regulatory subunit Vps15 and converts phosphatidylinositol (PI) to phosphatidylinositol 3-phosphate [PI(3)P], which is essential for autophagy initiation (Jaber et al., 2012; Kihara et al., 2001; Simonsen and Tooze, 2009; Vergne et al., 2009). Hence, it is generally recognized that, in metazoans, Class III PI3K Vps34 activates autophagy while Class IA PI3Ks inhibit it.
We recently published an unexpected finding that the Class IA p110β subunit is a positive regulator of autophagy in cultured cells and in mouse liver and heart (Dou et al., 2010). This autophagy-promoting function of p110β is independent of its catalytic activity. Rather, p110β acts to regulate the catalytic activity of the Vps34 complex to promote PI(3)P production that is essential for autophagy (Dou et al., 2010). However, the molecular mechanism and the physiological relevance of p110β in promoting autophagy remain to be explored.
It has been reported that the small GTPase Rab5, which plays a critical role in endocytic trafficking, also participates in autophagosome formation through its interaction with the Vps34-Beclin 1 complex (Ravikumar et al., 2008). The GTP-bound form of Rab5 is the active form in regulating membrane trafficking (Barbieri et al., 1994; Stenmark et al., 1994; Zerial and McBride, 2001). Rab5 has been found to directly interact with Vps34/Vps15, and this interaction is believed to recruit Vps34 to early endosomes to facilitate its localized activity (Christoforidis et al., 1999b; Murray et al., 2002). Rab5 also interacts with p110β, but not with p110α (Christoforidis et al., 1999b; Kurosu and Katada, 2001). Interestingly, p110β deficiency and Rab5 inactivation cause certain similar alterations in endocytic and autophagic pathways, suggesting that p110β and Rab5 may exert their functions in the same signaling pathway (Ciraolo et al., 2008; Dou et al., 2010). Indeed, binding of GTP-bound Rab5 stimulates the kinase activity of p110β to facilitate the generation of PI(3,4,5)P3 (Shin et al., 2005). On the other hand, the fact that many of the defects in p110β-null cells can be rescued by kinase-dead mutants of p110β suggests a scaffold function of p110β (Ciraolo et al., 2008; Dou et al., 2010; Jia et al., 2008), possibly by regulating the Rab5 complex. It remains unknown whether p110β can modulate Rab5 activity. In the present work, we examine the possibility that p110β modulates Rab5 to regulate Vps34 activity and autophagy, and address the biological significance of the autophagy-promoting function of p110β.
Results
Active Rab5 rescues the autophagy deficiency in p110β−/− cells
We previously reported that p110β associates with the Vps34-Vps15-Beclin 1-Atg14L complex and stimulates Vps34 kinase activity to promote PI(3)P production (Dou et al., 2010). A further examination using purified or in vitro-translated proteins revealed a lack of direct interaction of p110β with Vps34, Vps15, or Beclin 1, raising the possibility that p110β interacts with other proteins to modulate Vps34 catalytic activity. One candidate is the small GTPase Rab5. If p110β promotes autophagy by activating Rab5, one would predict that a constitutively active form of Rab5 will restore autophagy in p110β-deficient cells. To test this hypothesis, we expressed wild-type Rab5 (Rab5-WT), the Rab5-Q79L constitutively active mutant (Rab5-CA), Vps34-Vps15, or Atg14L in p110β control (β+/+) and p110β knockout (β−/−) mouse embryonic fibroblasts (MEFs) (Fig. 1A) and measured autophagy. As expected, expression of Vps34-Vps15 or Atg14L induced autophagy in β+/+ cells, as indicated by an increase in autophagic GFP-LC3 puncta and LC3-II (Fig. 1B, 1C, and Suppl. Fig. S1A). However, in β−/− cells, Vps34-Vps15 or Atg14L failed to induce autophagy under basal conditions (Fig. 1B and 1C) or in cells treated with the mTOR inhibitor rapamycin (Suppl. Fig. S1B). This result is consistent with our finding that p110β induces autophagy via the regulation of Vps34-Vps15-Beclin 1-Atg14L complex activity (Dou et al., 2010). Interestingly, while both Rab5-WT and Rab5-CA promoted the formation of GFP-LC3 puncta in β+/+ cells as previously reported (Ravikumar et al., 2008), only Rab5-CA induced autophagy in β−/− cells (Fig. 1B and 1C). Moreover, Rab5-CA but not Rab5-WT enhanced the accumulation of LC3-II in cells treated with the lysosomal inhibitor bafilomycin A1 (Fig. 1D). Taken together, these data suggest that Rab5-CA can bypass the requirement for p110β in autophagy induction, and that Rab5-WT can be activated in a p110β-dependent manner.
Figure 1. Rab5 plays a critical role in p110β-mediated autophagy.
β+/+ and β−/− MEFs were transfected with GFP-LC3 together with indicated expression constructs. (A) Western blots of cell lysates were probed with the indicated antibodies. Ponceau S staining shows equal protein loading. (B) Cells were imaged under a deconvolution microscope. Cells with more than 10 cytosolic puncta and diminished nuclear GFP were considered as autophagic. Data are averages of at least 4 blind countings with over 200 cells. Error bars: SD. * p<0.005. (C) Representative images of GFP-LC3 fluorescence in MEFs. Scale bar: 20 μm. (D) β+/+ and β−/− MEFs were transfected with vector (control), GFP-Rab5-WT, or GFP-Rab5-CA. 48 h later, cells were left untreated or treated with 50 nM bafilomycin A1 for 6 h. Relative levels of LC3-II against β-tubulin from three independent experiments are shown on the right. Data were normalized against that of β+/+. Error bars: SEM; * p<0.05; N.S., non-significant.
p110β is required for Rab5 activity
The differential effects of Rab5-WT and Rab5-CA in β−/− MEFs suggest that p110β may act upstream of Rab5 to increase its autophagy-promoting activity. Like other G proteins, Rab5 cycles between an inactive GDP-bound state and an active GTP-bound form that binds and activates its effectors (Christoforidis et al., 1999a; Zerial and McBride, 2001). To determine whether p110β can promote Rab5 activation, we performed pull-down assays using the GST-tagged Rab5-binding domain of Rabaptin5 (residues 739-862, R5BD), which specifically binds to Rab5-GTP (Liu et al., 2007). The specificity of GST-R5BD binding to active Rab5 was verified by its more efficient binding to Rab5-CA compared to Rab5-WT and lack of binding to a dominant-negative Rab5 S43N mutant (Rab5-DN) (Fig. 2A). Importantly, GST-R5BD pulled down less active endogenous Rab5 from β−/− cells than from β+/+ cells, while no difference was detected between p110α control (α+/+) and p110α knockout MEFs (Fig. 2B). Stable (Fig. 2C) or transient (Suppl. Fig. S2A) expression of either p110β-WT or the kinase-dead mutant p110β K805R in β−/− cells increased the amount of Rab5-GTP. Consistent with the lower amount of activated Rab5 in β−/− versus β+/+ MEFs, coimmunoprecipitation of Rab5 with two of its effectors related to autophagy and endocytosis, Vps34 and EEA1, was reduced in β−/− cells (Fig. 2D). Re-expression of p110β restored Rab5 binding to Vps34 and EEA1 (Fig. 2D). Consistent with the effect seen in MEFs, shRNA silencing of p110β in HEK293T cells resulted in a large decrease in Rab5-GTP (Suppl. Fig. S2B, compare lanes 1 and 3). Taken together, these data suggest that p110β plays a critical role in promoting the activation of Rab5.
Figure 2. p110β promotes Rab5 activation.
(A) HEK293T cells were transfected with GFP-tagged Rab5-WT, Rab5-DN, or Rab5-CA. 48 h post transfection, cell lysates were subjected to pull-down with GST (control) or GST-R5BD beads. Western blots of precipitates and total cell lysates were probed with GFP antibody. (B) Lysates of MEFs with the indicated genotypes were subjected to GST or GST-R5BD pull-down, and western blotting was used to analyze endogenous Rab5-GTP levels. Relative levels of Rab5-GTP (expressed as normalized Rab5-GTP/total Rab5 ratios) are shown on the right. Data are means of three independent experiments ± SEM; * p<0.05; N.S., non-significant. (C) β−/− MEFs and those stably reconstituted with wild-type p110β or the kinase-dead K805R mutant were analyzed for Rab5-GTP by the GST-R5BD pull-down assay. Relative levels of Rab5-GTP are shown on the right. Data are average values of three independent experiments ± SEM; * p<0.05; N.S., non-significant. (D) MEFs with the indicated genotypes were subjected to immunoprecipitation with control IgG or Rab5 antibody and analyzed for endogenous Vps34 and EEA1. Immunoblots of the precipitates and the input were probed with the indicated antibodies.
p110β antagonizes the Rab5 GTPase-activating protein (GAP) activity of p85α
We next explored the mechanisms by which p110β regulates Rab5 activity. Sequence and structure analysis of p110β did not implicate it as a putative guanine nucleotide exchange factor (GEF) that activates monomeric GTPases by stimulating the release of GDP to allow binding of GTP. In addition, as p110β binds specifically to Rab5-GTP (Christoforidis et al., 1999b), it is unlikely that p110β acts as a Rab5 GEF or guanine nucleotide dissociation inhibitor (GDI) because these functions require binding to Rab5-GDP. Interestingly, the Class IA PI3K regulatory subunit p85α has been reported to bind directly to Rab5 and to function as a Rab5 GAP (Chamberlain et al., 2004). The Bcr homology (BH) domain of p85α possesses Rab5 GAP activity, and disruption of this activity was reported to affect receptor trafficking (Chamberlain et al., 2004; Chamberlain et al., 2010). A working model can be postulated that while p110-free p85α can function as a Rab5 GAP, the presence of p110β in the Rab5 complex can sequester Rab5 from the p85α GAP function. This hypothesis would suggest that loss of p85α will lead to an increase in Rab5-GTP levels and enhanced autophagy. Indeed, stable silencing of p85α (Fig. 3A) led to increased amounts of Rab5-GTP (Fig. 3B) and increased GFP-LC3 puncta formation (Fig. 3C) in both β+/+ and β−/− cells. The lower level of Rab5-GTP in β−/− versus β+/+ cells upon p85α silencing could be due to the incomplete knockdown of p85α (Fig. 3A and 3B). To test the effect of p85α silencing on autophagosome maturation, LC3 with the tandem conjugation of mCherry and GFP was expressed in MEFs. Due to the more stable nature of mCherry fluorescence in the acidic environment, the appearance of red puncta indicates autolysosomes while the formation of yellow puncta (owing to merged green and red signals) represents autophagosomes (Klionsky et al., 2008; Mizushima et al., 2010). While the number of both red and yellow puncta was higher in β+/+ than in β−/− cells, consistent with defective autophagy in β−/− cells, silencing of p85α led to a marked increase in the number of autophagosomes and autolysosomes in both cell lines (Fig. 3D). These results indicate that downregulation of p85α relieves the inhibition of Rab5 and thus bypasses the requirement of p110β for autophagy induction. It is possible that the increased autophagy upon p85α knockdown could result from reduced expression of p110α (Fruman et al., 2000) and consequent inhibition of Akt/mTOR. Although as expected p85α knockdown led to a slight decrease in the steady-state level of p110α, loss of p85α also increased phosphorylation of Akt and S6K in both β+/+ and β−/− cells (Fig. 3A). A similar effect was reported in the p85α knock-out liver (Taniguchi et al., 2010), and is consistent with the notion that p85α plays a role in activating PTEN (Chagpar et al., 2010). Therefore, downregulation of p85α in these MEFs leads to an increase in autophagy which is not mediated by Akt/mTOR inhibition, but rather by relief of Rab5 inhibition as shown above.
Figure 3. p110β suppresses the Rab5 GAP activity of p85 α.
(A) β+/+ and β−/− MEFs were stably infected with lentivirus encoding a non-targeting control shRNA or shRNA against p85α. (B) MEFs generated as in (A) were subjected to GST-R5BD pull-down assays to detect Rab5-GTP. Note that p85α silencing leads to increased levels of Rab5-GTP. Relative levels of Rab5-GTP (expressed as normalized ratios of Rab5-GTP/total Rab5) from three independent experiments are shown on the right. Error bars: SEM; * p<0.05; ** p<0.01. (C) MEFs generated as in (A) were transfected with GFP-LC3 and autophagic cells were quantified. The data are average of at least four blind countings with over 200 cells. Error bars: SD. * p<0.05; ** p<0.001. (D) MEFs generated as in (A) were transfected with mCherry-GFP-LC3. 48 h later, images were taken, and yellow and red puncta were counted. Data are mean values of over 20 cells ± SEM. * p<0.01; ** p<0.001. (E) Purified Rab5 (200 nM) was loaded with GDP or GTP and subjected to pull-down with GST or GST-R5BD beads. The precipitates were analyzed for Rab5-GTP. (F) For in vitro Rab5 GAP assays, 200 nM Rab5-GTP was incubated in the absence of (−), or in the presence of 1 μM (+) or 2 μM (++) of purified p85α, p110β/p85α, or p110α/p85α. Rab5-GTP was then pulled down with GST-R5BD beads and quantified by immunoblotting. The relative amounts of Rab5-GTP are shown. The quantification of Rab5-GTP levels in control and 1 μM of purified p85α, p110β/p85α is shown. Data presented are the mean values from 3 independent experiments. Error bars: SEM. * p<0.05. (G) 200 nM Rab5-GTP was incubated with 2 μM p85α in the absence or presence of 1 μM (+) or 2 μM (++) of p110β/p85α or p110α/p85α. Rab5 GAP activity was assessed as in (F). The relative amounts of Rab5-GTP are shown. (H) HEK293T cells were transfected with Flag-p85α expressing construct. 48 h later, cell lysates were divided into 4 aliquots and mixed with HEK293T cell lysates that overexpressed untagged-p85α alone or together with p110β wild-type or p110β Q596C. The mixed lysates were subjected to pull-down with GST (control) or GST-Rab5 loaded with GTPγS. The precipitates and the input were analyzed as indicated. The relative amount of Flag-p85α against Rab5 is shown.
To test the effect of p85α and p110β on Rab5 activation in a more direct fashion, we assayed Rab5 GAP activity in vitro using purified Rab5, p85α, and the p110β/p85α and p110α/p85α PI3K complexes. Free p110 proteins were not tested because they are thermally unstable and always form heterodimers with p85 in vivo (Geering et al., 2007; Yu et al., 1998). Control experiments showed that GST-R5BD specifically pulled down Rab5 loaded with GTP but not GDP under these in vitro assay conditions (Fig. 3E). Incubation of Rab5-GTP with free p85α led to a decrease in the amount of Rab5-GTP in a dose-dependent manner (Fig. 3F), consistent with the ability of p85α to act as a Rab5 GAP in vitro (Chamberlain et al., 2004). p85α complexed with p110β showed no Rab5 GAP activity, whereas p85α complexed with p110α still possessed GAP activity toward Rab5 (Fig. 3F). These results strongly indicate that p110β, but not p110α, has a negative effect on the Rab5 GAP activity of p85α. It was speculated that the binding affinity of Rab5 is higher for p110β than for p85α (Chamberlain et al., 2004; Christoforidis et al., 1999b). Addition of the p110β/p85α complex antagonized the Rab5 GAP activity of free p85α, whereas the p110α/p85α complex failed to do so (Fig. 3G). In line with this, the p110β/p85α complex competed with Flag-tagged p85α for binding to Rab5-GTP, while the Rab5-binding deficient p110β mutant (Q596C, discussed later) showed impaired ability to do so (Fig. 3H). Taken together, these data suggest that p110β/p85α sequesters Rab5 from p85 GAP activity to maintain a higher level of Rab5-GTP. In addition to p85α, p110β may also protect Rab5-GTP from other Rab5 GAPs, as the p110β/p85α complex was able to reduce RabGAP5 binding to Rab5 (Suppl. Fig S3).
The p110β-Rab5 interaction plays an essential role in Rab5 activation and autophagy
Despite high sequence homology with other p110 isoforms, p110β is the only Class IA isoform known to date to associate with Rab5 (Christoforidis et al., 1999b; Vanhaesebroeck et al., 2012). Since our data indicate that p110β may play an essential role in Rab5 activation, we went on to determine whether the p110β and Rab5 interaction is critical for Rab5 activation. Using a structure based scanning mutagenesis strategy, we have identified mutations in p110β that have no effect on p110β activity but block interactions with Rab5 (Salamon et al, manuscript in preparation). Two point mutants of p110β, namely Q596C and I597S, showed loss of interaction with Rab5 (Fig. 4A). Examination of the crystal structure of p110β (Zhang et al., 2011) revealed that Q596 and I597 are exposed on the outer surface of the helical domain of p110β (Suppl. Fig. S4 A-D). The two mutants possess intact catalytic activity (Fig. 4B) and p85α binding (Fig. 4C) and induced normal phosphorylation of Akt and S6K in cells treated with lysophosphatidic acid (LPA) (Suppl. Fig S4E).
Figure 4. Association of p110β with Rab5 is required for Rab5 activity and autophagy.
(A) HEK293T cells were transfected with Myc-tagged p110α or p110β constructs together with Flag-p85α. 48 h later, cell lysates were subjected to pull-down with GST or GST-Rab5 loaded with GTPγS. (B) p110β-Q596C and p110β-I597S possess intact kinase activity. The lipid kinase activity assay was performed as previously described (Dbouk et al., 2010). Data presented are the means of three independent experiments, normalized against that of p110β protein levels. Error bars: SEM. (C) HEK293T cells were transfected with indicated constructs. 48 h later, cell lysates were subjected to immunoprecipitation with Myc-antibody conjugated to agarose. Blots of the precipitates and the input were probed with Myc and Flag antibodies. (D) β−/− MEFs were stably reconstituted with indicated constructs and were analyzed for indicated proteins. (E) Lysates of stably reconstituted MEFs were subjected to GST-R5BD pull-down assays to determine the amount of Rab5-GTP. The relative amount of Rab5-GTP against total Rab5 is shown. Error bars: SEM; n=3; * p<0.05. (F) MEFs were analyzed for p62. The relative amount of p62 is shown. Error bars: SEM; n=3; * p<0.05. (G) MEFs as indicated were incubated with [14C]valine for 24 h. The cells were left untreated or serum-starved for 4 h. Degradation of long-lived proteins was measured. Error bars: SEM; n=3; * p<0.05. (H) MEFs generated as in (D) were cultured in complete or serum-free medium for 6 h and treated with or without bafilomycin A1 (50 nM). Western blots of cell lysates were probed for LC3 and β-tubulin. Quantification of LC3-II against β-tubulin is shown. Error bars: SEM; n=3; * p<0.05. (I) MEFs generated as in (D) were transfected with GFP-LC3. 48 h later, cells were cultured in complete or serum-free medium for 4 h, in the absence or presence of bafilomycin A1 (50 nM). Representative images of cells without bafilomycin A1 treatment are shown. Scale bar: 20 μm. Quantification of autophagic cells is shown on the right. Data are average values of at least 4 blind countings with over 200 cells. Error bars: SD; * p<0.05; ** p<0.01.
To test the ability of these mutants to activate Rab5 and autophagy, the p110β-Q596C and p110β- I597S proteins were stably expressed in β−/− MEFs to levels similar to those seen in cells reconstituted with p110β-WT (Fig. 4D). The steady-state levels of p110α, p85α, and phosphorylated Akt and S6K showed little or no change (Fig. 4D). GST-R5BD pull-down assays revealed that p110β-WT, but not the Q596C or I597S mutants, increased the level of Rab5-GTP (Fig. 4E). Consistent with the autophagy-promoting role of p110β-mediated Rab5 activation, cells expressing the mutants showed impaired ability to degrade the autophagy substrate p62 (Fig. 4F) and long-lived proteins (Fig. 4G). In addition, cells expressing the two mutants showed reduced accumulation of LC3-II (Fig. 4H) and GFP-LC3 autophagic puncta (Fig. 4I) in both untreated and serum-deprived conditions. Our data further indicate that the p110β-Rab5 association, but not the kinase activity of p110β, is important for autophagy. While the kinase-deficient p110β K805R mutant was able to rescue Rab5 activation (Fig. 2C and Suppl. Fig. S2A) and autophagy (Dou et al., 2010), the K805R/I597S double mutant failed to interact with Rab5 and to induce autophagy (Suppl. Fig. S4F and S4G). Taken together, these results demonstrate that residues Q596 and I597 in the p110β helical domain are essential for the p110β-Rab5 interaction and that the physical interaction of p110β with Rab5 is required for Rab5 activity and its autophagy-promoting function.
p110β-mediated Rab5 activation is selectively regulated by growth factor availability, but not by nutrient signaling
Our results indicate that p110β can function to activate Rab5 and autophagy (Fig. 1-4). On the other hand, Class IA PI3Ks are well known to be recruited to growth factor receptor signaling complexes where they produce PI(3,4,5)P3 to stimulate downstream signaling cascades, such as the Akt/mTOR pathway (Cantley, 2002; Engelman et al., 2006; Vanhaesebroeck et al., 2012). These two distinct activities of p110β suggest that metazoan cells may be able to respond to growth factor limitation to activate autophagy in a manner that can be directly transduced from the growth factor receptors. To examine this hypothesis, we performed Rab5 immunoprecipitations under various conditions in our genetically modified MEFs. The appropriate effect of these treatments on cell signaling pathways was verified by monitoring the phosphorylation of Akt, S6 (a downstream effector of mTOR), and AMPK (Fig. 5A). Coimmunoprecipitation experiments showed that only serum deprivation enhanced the p110β-Rab5 association (Fig. 5B). Vps34 binding to Rab5, which has been reported to promote Vps34 activity and autophagy (Ravikumar et al., 2008; Shin et al., 2005), was also selectively enhanced by serum deprivation (Fig. 5B).
Figure 5. Withdrawal of growth factors, but not nutrients, induces p110β-Rab5 binding and p110β-dependent Rab5 activation.
(A) β−/− MEFs stably reconstituted with human p110β (hp110β) were left untreated, deprived of serum, glucose, or amino acids for 6 h, or treated with the Akt inhibitor (Akti, 10 μM) or rapamycin (Ra, 50 nM) overnight. (B) Lysates of cells treated as in (A) were subjected to immunoprecipitation with control IgG or Rab5 antibody. The precipitates were analyzed for Rab5, hp110β, and Vps34. (C) β+/+ and β−/− MEFs were left untreated or serum-deprived for 6 h. GST-R5BD pull-down assays were done to determine the amount of Rab5-GTP in cell lysates. The mean values of relative Rab5-GTP against that of total Rab5 from three independent experiments with SEM is shown. * p<0.05; N.S., non-significant. (D) β+/+, β−/−, and the human p110β-reconstituted β−/− MEFs were cultured in complete or serum-free medium for 6 h. Cell lysates were subjected to immunoprecipitation with IgG or Rab5 antibody and analyzed for human p110β and endogenous Vps34 and EEA1. (E) MEFs as indicated expressing GFP-FYVE were untreated or serum-starved, and stained for endogenous Rab5. Around 20 cells were randomly selected and imaged. The numbers of GFP-FYVE puncta and colocalized GFP-FYVE and Rab5 puncta per cell were quantified. Error bars: SEM; * p<0.005; ** p<0.0005. The representative images are shown in Suppl. Figure S5B.
To test whether Rab5 activation induced by serum deprivation is dependent on p110β, we performed GST-R5BD pull-down assays on lysates of β+/+ and β−/− MEFs. Indeed, serum withdrawal increased the level of Rab5-GTP in β+/+ but not in β−/− cells (Fig. 5C). A similar effect was also seen in HEK293T cells with shRNA silencing of p110β (Suppl. Fig. S2B). As a correlative measure of Rab5 activation, we observed enhanced coimmunoprecipitation of Rab5 with its effectors Vps34 and EEA1 in response to serum deprivation only in β+/+ MEFs (Fig. 5D). Reconstitution of β−/− MEFs with wild-type p110β restored Vps34 and EEA1 association with Rab5 that are induced by serum deprivation (Fig. 5D), whereas the Rab5-binding deficient p110β mutants failed to rescue the defects (Suppl. Fig. S5A). Vps34 activity leads to production of PI(3)P that can be visualized by the GFP-conjugated FYVE domain. Expression of GFP-FYVE revealed an increase in puncta formation upon serum deprivation in β−/− MEFs reconstituted with wild-type p110β (Fig. 5E and Suppl. Fig. S5B). This observation is consistent with a previous report showing that serum withdrawal but not mTOR inhibition leads to enhanced GFP-FYVE puncta formation (Lipinski et al., 2010). In addition, we noticed an enhanced colocalization between GFP-FYVE and Rab5 upon serum deprivation in p110β-reconstituted cells (Fig. 5E and Suppl. Fig. S5B), consistent with a role of Rab5 in stimulating Vps34 activity. By contrast, the increase in GFP-FYVE puncta and the colocalization with Rab5 were drastically impaired in β−/− MEFs and in β−/− MEFs reconstituted with p110β I597S (Fig. 5E and Suppl. Fig. S5B), which is correlated with the lack of Vps34 association to Rab5 in these cells (Fig. 5D and Suppl. Fig. S5A). In addition, expression of the dominant-negative Rab5 S43N mutant (Rab5-DN) led to impaired GFP-LC3 puncta and LC3-II formation (Suppl. Fig. S5C and S5D), supporting the role of Rab5 in mediating autophagy induced by serum deprivation. Taken together, these results indicate that growth factor limitation selectively enhances the p110β-Rab5 interaction, which plays an essential role in Rab5 activation and autophagy.
p110β dissociates from growth factor signaling complexes and increases its interaction with Rab5 upon growth factor limitation
The above results show that serum withdrawal selectively induces p110β-mediated Rab5 activation, which may be responsible for autophagy induction under these conditions. Although serum is well regarded as a source of growth factors, it is a relatively undefined mixture that mediates complex changes in signaling and contains various nutrients. Therefore, to further pursue the possible selective role of p110β in Rab5 activation and autophagy in response to growth factor limitation, we used the immortalized breast epithelial MCF10A cell line, whose growth is dependent on the presence of defined growth factors (insulin, EGF, cholera toxin, and hydrocortisone). Removal of the growth factors has been shown to induce autophagy in MCF10A cells (Fung et al., 2008). To detect changes in p110β partitioning between subcellular pools, we immunoprecipitated insulin receptor signaling complexes and found that the association of p110β with insulin receptor substrate (IRS)-1 and IRS-2 was diminished upon deprivation of the growth factors (Fig. 6A, left panel). Similarly, pull-down with phosphotyrosine antibodies showed reduced binding of p110β to tyrosine-phosphorylated proteins (Fig. 6A, left panel). By contrast, p110β and Rab5 coprecipitation increased upon withdrawal of the growth factors (Fig. 6A, middle panel). The Rab5-binding deficient mutant p110β I597S showed abrogated binding to Rab5 even upon growth factor deprivation (Suppl. Fig. S6A). To visualize the change in subcellular localization of p110β, we performed immunofluorescence microscopy imaging of MCF10A cells. The specificity of the p110β antibody used for immunofluorescence was verified using cells treated with shRNA to knock down p110β (Suppl. Fig. S6B). Staining of endogenous p110β and Rab5 in cells cultured in complete medium showed a portion of p110β on the plasma membrane and Rab5 mostly in the cytoplasm (Fig. 6B). In the absence of the hormonal factors, the plasma membrane p110β signal was greatly reduced, and enhanced colocalization of p110β and Rab5 was observed (Fig. 6B). The p110β I597S mutant showed loss of colocalization with Rab5 (Suppl. Fig. S6C). These data indicate that growth factor limitation leads to p110β dissociation from growth factor receptor signaling complexes and increased p110β-Rab5 interaction.
Figure 6. p110β dissociates from growth factor receptor complexes and interacts with Rab5 upon growth factor deprivation.
(A and B) MCF10A cells were grown in complete or basal (without growth factors) medium for 24 h. Cell lysates were subjected to immunoprecipitation with the indicated antibodies or with phosphotyrosine antibody conjugated to agarose. Western blotting of the precipitates and the input is shown. Quantification of each association is shown (A). (B) Immunofluorescence confocal microscopy was used to visualize endogenous p110β and Rab5 in fixed cells. Representative images are shown. Scale bar: 10 μm. The percentage of Rab5 colocalizing with p110β was quantified in over 50 cells and is shown on the right. Error bars: SEM. * p<0.01. (C) MCF10A cells were stably infected with lentiviruses encoding a tetracycline-inducible non-targeting control shRNA or shRNA against p110β. The two cell lines were treated in complete medium with doxycycline (Dox, 1 μg/ml) for the indicated times and then harvested for western blotting. (D) MCF10A cells generated as in (C) were treated with Dox for 3 d to silence p110β, then cultured in complete or basal medium for 24 h in the presence of Dox. Cell lysates were analyzed for the amount of Rab5-GTP using GST-R5BD pull-down assays. The input of Rab5 and p110β are shown. n.s., non-specific band. (E) Cells generated as in (C) stably expressing GFP-LC3 were left untreated or starved in basal medium for 24 or 48 h in the absence or presence of bafilomycin A1 (20 nM). Representative images of cells not treated with bafilomycin A1 are shown. Scale bar: 20 μm. Autophagic cells from the indicated culture conditions were quantified and shown in (F). Data are averages of at least 4 blind countings with over 500 cells. Error bars: SD. (G) MCF10A cells were stably infected with tetracycline-inducible control shRNA or shRNA against p110β. Cells were treated with Dox for 3 d to allow knock-down of p110β. Cells were then left untreated or cultured in basal medium for 36 h in the absence or presence of bafilomycin A1 (20 nM). Short and long exposures of the LC3 immunoblots are shown. Relative amount of LC3-II normalized against β-tubulin was quantified from three independent experiments. Error bars: SEM; * p<0.05; N.S., non-significant.
To study the requirement for p110β in growth factor limitation-induced autophagy in MCF10A cells, we stably expressed an shRNA against p110β or a non-targeting control shRNA under the control of a tetracycline-inducible system in MCF10A cells. Addition of doxycycline led to a progressive reduction in p110β protein levels (Fig. 6C), with little or no effect on the steady-state level of p110α, p85, or phosphorylated Akt and S6K (Fig. 6C). Similar to what we observed in serum-deprived MEFs (Fig. 5C) and HEK293T cells (Suppl. Fig. 2B), growth factor deprivation led to an increased amount of endogenous Rab5-GTP in the control but not in p110β knock-down MCF10A cells with endogenous Rab5 (Fig. 6D). Reconstitution with shRNA-resistant wild-type p110β, but not the I597S mutant, rescued Rab5-GTP levels (Suppl. Fig. S6D). Upon removal of the growth factors, there was a marked increase in GFP-LC3 puncta in control MCF10A cells, whereas this response was greatly impaired in the p110β knock-down cells (Fig. 6E and 6F). In MCF10A cells, the LC3 signal was not readily detectable by immunoblotting (Fig. 6G). In the presence of the lysosomal inhibitor bafilomycin A1, a significant increase in LC3 was observed. p110β knock-down cells displayed a lower level of LC3-II than the control cells (Fig. 6G), consistent with the positive role of p110β in basal level Rab5 activity and autophagy. Noticeably, the amount of LC3-II induced by growth factor withdrawal was greatly reduced in p110β knock-down cells. Reconstitution with shRNA-resistant wild-type p110β rescued the GFP-LC3 puncta and LC3-II formation, whereas the I597S mutant failed to do so (Suppl. Fig. S6E and S6F). In contrast to growth factor deprivation, other autophagic stimuli including rapamycin, the ER stressor tunicamycin, or the proteasome inhibitor MG132 did induce significant LC3-II production in the p110β knock-down cells (Suppl. Fig. S6G). It is noteworthy that the levels of autophagy were lower in p110β-deficient MCF10A cells, similar to what we observed in β−/− MEFs, indicating a general role of p110β in regulating autophagy. Nevertheless, our data here indicate that p110β plays an indispensable and selective role in growth factor limitation-induced autophagy.
Plasma membrane targeted p110β does not activate autophagy upon growth factor limitation
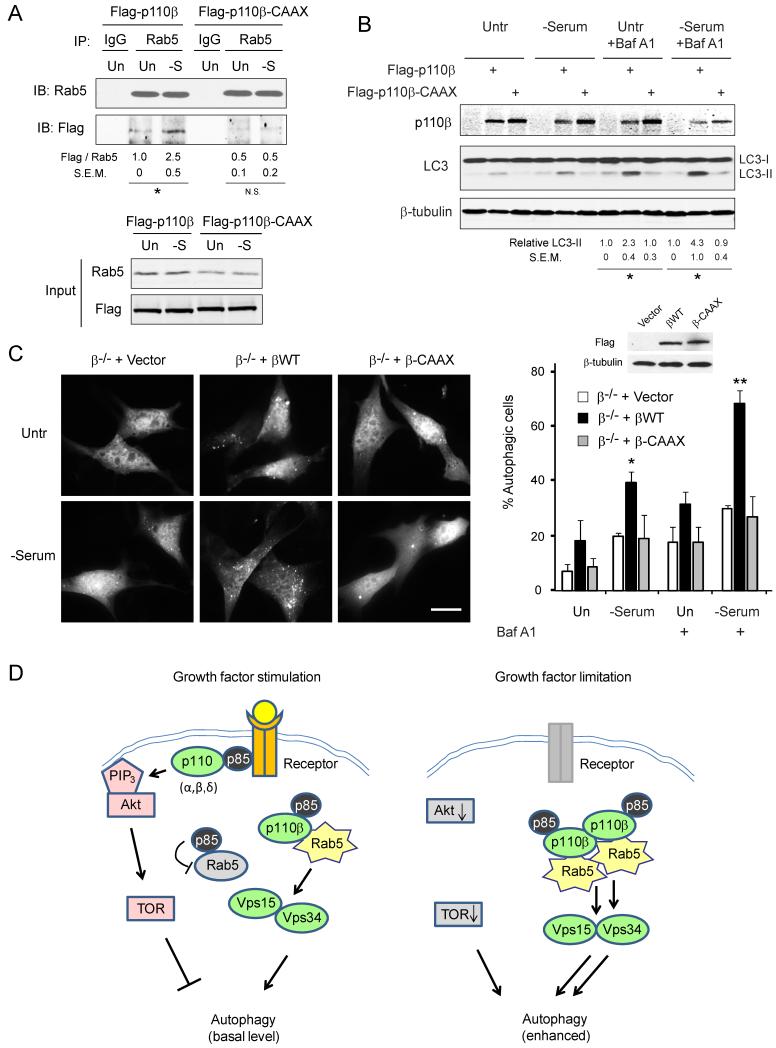
To further investigate the role of p110β translocation in mediating growth factor limitation-induced autophagy, we targeted ectopically expressed p110β to the plasma membrane by tagging it with the carboxy-terminal CAAX sequence of K-Ras, which has been shown to effectively target proteins to the plasma membrane (Hancock et al., 1991). Immunofluorescence imaging of the Flag-p110β-CAAX mutant expressed in Hs578T cells confirmed that it did not dissociate from the plasma membrane upon serum deprivation (Suppl. Fig. S7A). Coimmunoprecipitation experiments showed a reduced interaction between Rab5 and the p110β-CAAX mutant under basal conditions and a lack of enhanced binding upon serum starvation (Fig. 7A). Importantly, while reconstitution of wild-type p110β in β−/− MEFs restored the serum deprivation-induced increases in LC3-II level (Fig. 7B) and GFP-LC3 puncta accumulation (Fig. 7C), expression of the p110β-CAAX mutant failed to do so. The failure of p110β-CAAX to restore autophagy is not due to its possible elevated kinase activity because the p110β-specific kinase inhibitor TGX-221 did not rescue the GFP-LC3 puncta in the β−/− cells expressing p110β-CAAX (Suppl. Fig. S7B). The same concentration of TGX-221inhibited LPA-stimulated Akt phosphorylation (Suppl. Fig. S7C) and abrogated the slightly elevated phosphorylation of Akt and S6K imposed by p110β-CAAX (Suppl. Fig. S7D). Therefore, these results strongly support the theory that cytosolic p110β, through its interaction with Rab5, plays a critical role in promoting autophagy induced by growth factor limitation.
Figure 7. Cells with membrane-targeted p110β-CAAX display impaired Rab5 association and autophagy.
(A) HEK293T cells were transfected with wild-type Flag-p110β or Flag-p110β-CAAX. 48 h later, cells were left untreated or serum-deprived for 24 h. Cell lysates were subjected to immunoprecipitation with IgG or Rab5 antibody. The normalized relative binding of Flag-p110β to Rab5 from three independent experiments with SEM is shown. * p<0.05; N.S., non-significant. (B) β−/− MEFs expressing indicated constructs were left untreated or serum-deprived in the absence or presence of bafilomycin A1 (50 nM) for 6 h. The relative amount of LC3-II normalized against that of vector control from three independent experiments with SEM is shown. * p<0.05, comparing wild-type p110β versus vector and p110β-CAAX mutant. (C) β−/− MEFs were transfected with the indicated constructs together with GFP-LC3. 48 h later, cells were left untreated or serum-deprived for 6 h, in the absence or presence of bafilomycin A1 (50 nM). The pictures shown are of cells in the absence of bafilomycin A1. Scale bar: 20 μm. Expression of the constructs and quantification of autophagic cells are shown on the right. Data are average of 4 blind countings with over 200 cells. Error bars: SD; * p<0.05; ** p<0.005. (D) Schematic representation of the role of p110β in regulating autophagy. See text for explanation.
Discussion
In this study, we show that serum-replete cells contain at least two pools of p110β/p85 (Fig. 7D, left panel). One pool is associated with active growth factor receptor signaling complexes at the plasma membrane and signals via its kinase activity to Akt/mTOR to inhibit autophagy. A second intracellular pool of p110β/p85 promotes basal autophagy in a kinase-independent manner by binding to the small GTPase Rab5. The p110β-Rab5 interaction protects Rab5-GTP from the GAP activity of p110β-free p85α (and perhaps other Rab5 GAPs). This leads to an increase in the amount of activated Rab5, which promotes Rab5 interaction with its effectors including Vps34 to induce basal autophagy. Upon growth factor limitation, p110β/p85 dissociates from growth factor receptor signaling molecules and increases its interaction with Rab5 to enhance autophagy (Fig. 7D, right panel). This study provides a direct molecular connection between growth factor availability and activation of the Rab5-Vps15/Vps34 complex, and implies a mechanism whereby growth factor availability transduces signals to modulate Vps34 independently of nutrient and energy sensing.
Our finding that p110β positively regulates Rab5 activity adds a new twist to the p110β-Rab5 interaction. Among Class IA PI3K p110 catalytic isoforms, p110β contains some unique structural features that confer distinct regulatory properties on the enzyme. Unlike other p110s, whose activities are regulated by Ras (Castellano and Downward, 2011), p110β catalytic activity does not seem to be modulated by Ras (Kurosu and Katada, 2001). Rather, association with Rab5 enhances the kinase activity of p110β (Shin et al., 2005). Here we show that the p110β-Rab5 association also enhances the activation of Rab5. The interaction of p110β with Rab5 protects Rab5-GTP from being hydrolyzed by its GAPs such as RabGAP5 and p85α. This Rab5 interaction and activation function of p110β is independent of its catalytic activity. The kinase-deficient K805R mutant of p110β has been found to rescue numerous phenotypes caused by p110β deficiency, including defects in embryonic development, endocytosis, EEA1 endosomal recruitment, Vps34 activity, and autophagy (Ciraolo et al., 2008; Dou et al., 2010; Jia et al., 2008). Our finding that p110β activates Rab5 independently of its catalytic activity provides a plausible mechanism for p110β scaffold function.
Our study elucidates an important new mechanism of autophagy regulation in mammals. Our data implicate Class IA PI3Ks not only as regulators of growth factor-mediated cell activity, but also as mediators of cell homeostasis by promoting autophagy upon growth factor limitation. Bioenergetics in yeast and other unicellular eukaryotes are largely dependent on the abundance of extracellular nutrients. Upon nutrient or energy shortage, they trigger autophagy in order to increase energy supply and survival (Inoue and Klionsky, 2010). Mammalian cells, by contrast, are often bathed in a nutrient-rich environment, and extrinsic growth factor signals regulate nutrient uptake and metabolism (Lum et al., 2005). Limitation of growth factors triggers autophagy. While autophagy induction in mammals and unicellular organisms shares many common features and is believed to be induced largely by intracellular nutrient shortage, our work indicates that autophagy in mammals can be induced directly by cell surface receptors. This study helps to advance our understanding of the molecular evolution of Class IA PI3K function in metazoans and provides new insight into the regulation of autophagic responses in mammalian cells, which exist in a complex and context-dependent environment.
Experimental Procedures
Cell lines, culture, transfection, and treatments
The SV40-immortalized p110β+/+, p110β−/−, p110α+/+, and p110α−/− MEFs from p110αflox/+ or p110βflox/+ matings were described previously (Dou et al., 2010; Lu et al., 2009). Culture, transfection, infection, and treatment of cells are described in “Supplemental Experimental Procedures”.
Plasmids, antibodies, and other reagents
These reagents are described in “Supplemental Experimental Procedures”.
Immunofluorescence and immunoprecipitation
Please refer to “Supplemental Experimental Procedures” for details.
GST-Rab5 and GST-R5BD pull-down assays
GST-Rab5-GTPγS pull-downs were performed according to a published protocol (Christoforidis et al., 1999a) with slight modifications. The GST-R5BD construct and pull-down assays were described previously (Liu et al., 2007) with slight modification. See details in “Supplemental Experimental Procedures”.
In vitro Rab5 GAP assays
Please refer to “Supplemental Experimental Procedures” for details.
Measurement of long-lived protein degradation
Long-lived protein degradation assay was performed as previously described (Dou et al., 2010).
Statistics
Student’s t test was used to compare the differences between two groups. One-way ANOVA with Tukey’s post-hoc test was used for comparisons between more than two groups. Results were considered significant when p was less than 0.05.
Image processing and densitometry measurements
Images captured by deconvolution and confocal microscopes were viewed and processed by AxioVision LE and Zeiss LSM image browser, respectively. Images were processed in Adobe Photoshop to enhance the brightness and contrast. Densitometry of immunoblot bands was determined by ImageJ software or by the Odyssey Infrared Imaging System.
Supplementary Material
Highlights.
The p110β-Rab5 binding positively regulates Rab5 activity and autophagy.
Rab5 binding deficient p110β mutants fail to promote Rab5 activation and autophagy.
Growth factor limitation stimulates p110β-Rab5 interaction.
p110β is a molecular sensor of growth factor availability to regulate autophagy.
Acknowledgements
We thank Dr. José A. Esteban for reagents, and Drs. Deborah Brown, Michael Frohman, and Howard Crawford for insightful comments. This work was supported by NIH (CA129536 and GM97355 to WXZ, GM74692 to GL, GM55692 and AG039632 to JMB, DK62722 and CA136754 to RZL) and Department of Veterans Affairs Merit Award (to RZL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbieri MA, Li G, Colombo MI, Stahl PD. Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. J Biol Chem. 1994;269:18720–18722. [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Duckworth BC, Auger KR, Cohen B, Schaffhausen BS, Cantley LC. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990;265:19704–19711. [PubMed] [Google Scholar]
- Castellano E, Downward J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. Genes Cancer. 2011;2:261–274. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagpar RB, Links PH, Pastor MC, Furber LA, Hawrysh AD, Chamberlain MD, Anderson DH. Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 2010;107:5471–5476. doi: 10.1073/pnas.0908899107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain MD, Berry TR, Pastor MC, Anderson DH. The p85alpha subunit of phosphatidylinositol 3′-kinase binds to and stimulates the GTPase activity of Rab proteins. J Biol Chem. 2004;279:48607–48614. doi: 10.1074/jbc.M409769200. [DOI] [PubMed] [Google Scholar]
- Chamberlain MD, Oberg JC, Furber LA, Poland SF, Hawrysh AD, Knafelc SM, McBride HM, Anderson DH. Deregulation of Rab5 and Rab4 proteins in p85R274A-expressing cells alters PDGFR trafficking. Cell Signal. 2010;22:1562–1575. doi: 10.1016/j.cellsig.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999a;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol. 1999b;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, et al. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk HA, Pang H, Fiser A, Backer JM. A biochemical mechanism for the oncogenic potential of the p110beta catalytic subunit of phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2010;107:19897–19902. doi: 10.1073/pnas.1008739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domin J, Waterfield MD. Using structure to define the function of phosphoinositide 3-kinase family members. Febs Letters. 1997;410:91–95. doi: 10.1016/s0014-5793(97)00617-0. [DOI] [PubMed] [Google Scholar]
- Dou Z, Chattopadhyay M, Pan JA, Guerriero JL, Jiang YP, Ballou LM, Yue Z, Lin RZ, Zong WX. The class IA phosphatidylinositol 3-kinase p110-beta subunit is a positive regulator of autophagy. J Cell Biol. 2010;191:827–843. doi: 10.1083/jcb.201006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature Reviews Genetics. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Mauvais-Jarvis F, Pollard DA, Yballe CM, Brazil D, Bronson RT, Kahn CR, Cantley LC. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat Genet. 2000;26:379–382. doi: 10.1038/81715. [DOI] [PubMed] [Google Scholar]
- Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell. 2008;19:797–806. doi: 10.1091/mbc.E07-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc Natl Acad Sci U S A. 2007;104:7809–7814. doi: 10.1073/pnas.0700373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Cadwallader K, Paterson H, Marshall CJ. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991;10:4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Klionsky DJ. Regulation of macroautophagy in Saccharomyces cerevisiae. Semin Cell Dev Biol. 2010;21:664–670. doi: 10.1016/j.semcdb.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia SD, Liu ZN, Zhang S, Liu PX, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, et al. Essential roles of PI(3)K-p110 beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–U102. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Katada T. Association of phosphatidylinositol 3-kinase composed of p110beta-catalytic and p85-regulatory subunits with the small GTPase Rab5. J Biochem. 2001;130:73–78. doi: 10.1093/oxfordjournals.jbchem.a002964. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski MM, Hoffman G, Ng A, Zhou W, Py BF, Hsu E, Liu X, Eisenberg J, Liu J, Blenis J, et al. A genome-wide siRNA screen reveals multiple mTORC1 independent signaling pathways regulating autophagy under normal nutritional conditions. Dev Cell. 2010;18:1041–1052. doi: 10.1016/j.devcel.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lamb D, Chou MM, Liu YJ, Li G. Nerve growth factor-mediated neurite outgrowth via regulation of Rab5. Mol Biol Cell. 2007;18:1375–1384. doi: 10.1091/mbc.E06-08-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZJ, Jiang YP, Wang W, Xu XH, Mathias RT, Entcheva E, Ballou LM, Cohen IS, Lin RZ. Loss of Cardiac Phosphoinositide 3-Kinase p110 alpha Results in Contractile Dysfunction. Circulation. 2009;120:318–325. doi: 10.1161/CIRCULATIONAHA.109.873380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: Cell survival in the land of plenty. Nature Reviews Molecular Cell Biology. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JT, Panaretou C, Stenmark H, Miaczynska M, Backer JM. Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. Traffic. 2002;3:416–427. doi: 10.1034/j.1600-0854.2002.30605.x. [DOI] [PubMed] [Google Scholar]
- Petiot A, Ogier-Denis E, Blommaart EFC, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29cells. Journal of Biological Chemistry. 2000;275:992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Imarisio S, Sarkar S, O’Kane CJ, Rubinsztein DC. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci. 2008;121:1649–1660. doi: 10.1242/jcs.025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Current Opinion in Cell Biology. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol. 2005;170:607–618. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Tooze SA. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. Journal of Cell Biology. 2009;186:773–782. doi: 10.1083/jcb.200907014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Winnay J, Kondo T, Bronson RT, Guimaraes AR, Aleman JO, Luo J, Stephanopoulos G, Weissleder R, Cantley LC, et al. The phosphoinositide 3-kinase regulatory subunit p85alpha can exert tumor suppressor properties through negative regulation of growth factor signaling. Cancer Res. 2010;70:5305–5315. doi: 10.1158/0008-5472.CAN-09-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- Vergne I, Roberts E, Elmaoued RA, Tosch V, Delgado MA, Proikas-Cezanne T, Laporte J, Deretic V. Control of autophagy initiation by phosphoinositide 3-phosphatase Jumpy. EMBO J. 2009;28:2244–2258. doi: 10.1038/emboj.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederschain D, Wee S, Chen L, Loo A, Yang G, Huang A, Chen Y, Caponigro G, Yao YM, Lengauer C, et al. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle. 2009;8:498–504. doi: 10.4161/cc.8.3.7701. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang X, Vadas O, Perisic O, Anderson KE, Clark J, Hawkins PT, Stephens LR, Williams RL. Structure of lipid kinase p110beta/p85beta elucidates an unusual SH2-domain-mediated inhibitory mechanism. Mol Cell. 2011;41:567–578. doi: 10.1016/j.molcel.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.