Photosynthetic characteristics of Euphorbia milii are reported for the first time. The occurrence of CAM-cycling is shown to serve as a mechanism of water conservation. This is a detailed and novel report of such CAM mode in the genus, which is abundant in constitutive CAM and C4 species. Anatomical evidence for the possible operation of a CO2-concentrating mechanism around the vascular bundle, the C2 route, is provided. Findings are important for our understanding of evolution of CAM in the genus.
Keywords: CAM-cycling, citrate, transpiration, water saving, water-use efficiency
Abstract
Crassulacean acid metabolism (CAM) occurs in many Euphorbiaceae, particularly Euphorbia, a genus with C3 and C4 species as well. With the aim of contributing to our knowledge of the evolution of CAM in this genus, this study examined the possible occurrence of CAM in Euphorbia milii, a species with leaf succulence and drought tolerance suggestive of this carbon fixation pathway. Leaf anatomy consisted of a palisade parenchyma, a spongy parenchyma and a bundle sheath with chloroplasts, which indicates the possible functioning of C2 photosynthesis. No evidence of nocturnal CO2 fixation was found in plants of E. milii either watered or under drought; watered plants had a low nocturnal respiration rate (R). After 12 days without watering, the photosynthetic rate (PN) decreased 85 % and nocturnal R was nearly zero. Nocturnal H+ accumulation (ΔH+) in watered plants was 18 ± 2 (corresponding to malate) and 18 ± 4 (citrate) μmol H+ (g fresh mass)−1. Respiratory CO2 recycling through acid synthesis contributed to a night-time water saving of 2 and 86 % in watered plants and plants under drought, respectively. Carbon isotopic composition (δ13C) was −25.2 ± 0.7 ‰ in leaves and −24.7 ± 0.1 ‰ in stems. Evidence was found for the operation of weak CAM in E. milii, with statistically significant ΔH+, no nocturnal CO2 uptake and values of δ13C intermediate between C3 and constitutive CAM plants; ΔH+ was apparently attributable to both malate and citrate. The results suggest that daily malate accumulation results from recycling of part of the nocturnal respiratory CO2, which helps explain the occurrence of an intermediate value of leaf δ13C. Euphorbia milii can be considered as a CAM-cycling species. The significance of the operation of CAM-cycling in E. milii lies in water conservation, rather than carbon acquisition. The possible occurrence of C2 photosynthesis merits research.
Introduction
Crassulacean acid metabolism (CAM) is of frequent occurrence among the Euphorbiaceae and has appeared polyphyletically several times in the family, particularly in the genus Euphorbia. In this genus, C4 species seem to be rare, whereas they are abundant in the genus Chamaesyse (Webster et al. 1975). In Euphorbia, CAM has been reported in 21 species, and values of δ13C suggest its presence in 44 species (Table 1). Several of these species belong to three different clades within the genus (cladograms in Zimmermann et al. 2010). Twenty-four species can be considered constitutive CAM on the basis of having values of δ13C higher than −17 ‰, a criterion established by Mooney et al. (1977). In the remaining species, values of δ13C average −24.7 ‰. A value as low as −28.9 ‰ found in E. aphylla falls into the lower mode of the bimodal frequency distribution of δ13C in CAM plants, designated as low-level (weak) CAM (Winter and Holtum 2002; Silvera et al. 2005).
Table 1.
Carbon isotopic composition of species of Euphorbia with high to intermediate values of δ13C and CAM mode assigned by authors on the basis of leaf gas exchange, acid accumulation, δ13C and enzyme activity.
| Species | δ13C (‰) | Mode | Reference |
|---|---|---|---|
| angusta | −24.9 | NA | Webster et al. (1975) |
| antiquorum | −14.2 | NA | Batanouny et al. (1991) |
| aphylla | ND | Facultative | Mies et al. (1996) |
| avasmontana | −15.1 | Constitutive | Mooney et al. (1977) |
| bothae | −14.6 | Constitutive | Mooney et al. (1977) |
| bubalina | −13.2 | NA | Webster et al. (1975) |
| burmannii | −18.3 | NA | Mooney et al. (1977) |
| caducifolia | ND | Constitutive | Webster et al. (1975), Sayed (2001) |
| caput-medusae | −13.3 | Constitutive | Mooney et al. (1977) |
| cyathophora | −25.8 | NA | Mies et al. (1996) |
| didieroides | −26.6 | NA | Webster et al. (1975) |
| didieroides | −24.3 | NA | Winter (1979) |
| dregeana | ND | Constitutive | Sayed (2001) |
| drupifera | −14.1 | NA | Webster et al. (1975) |
| gariepina | −14.6 | Constitutive | Mooney et al. (1977) |
| genoudiana | −22.7 | NA | Winter (1979) |
| gorgonis | −12.9 | Constitutive | Mooney et al. (1977) |
| gragaria | −11.6 | Constitutive | Mooney et al. (1977) |
| grandidens | ND | Constitutive | Webster et al. (1975), Sayed (2001) |
| inermis | −13.4 | Constitutive | Mooney et al. (1977) |
| ingezalahiana | −23.6 | NA | Winter (1979) |
| inocua | −28.1 | NA | Webster et al. (1975) |
| leucodendron | −13.2 | NA | Winter 1979 |
| macropodoides | −28.3 | NA | Webster et al. (1975) |
| macropus | −28.9 | NA | Webster et al. (1975) |
| mauritanica | −16.0 | Constitutive | Mooney et al. (1977) |
| milii | ND | Non-CAM | Webster et al. (1975) |
| milii | CAM | McWilliams (1970) | |
| nesemannii | −11.6 | Constitutive | Mooney et al. (1977) |
| nivulia | −15.7 | NA | Webster et al. (1975) |
| nubica | −14.5 | NA | Batanouny et al. (1991) |
| pentagona | −14.9 | Constitutive | Mooney et al. (1977) |
| peperomioides | −25.6 | NA | Webster et al. (1975) |
| plagiantha | −13.2 | NA | Winter (1979) |
| polygona | −10.7 | Constitutive | Mooney et al. (1977) |
| pulcherrima | −25.5 | NA | Mies et al. (1996) |
| squarrosa | −12.5 | Constitutive | Mooney et al. (1977) |
| stenoclada | −12.6 | NA | Winter (1979) |
| submamillaris | ND | Constitutive | Webster et al. (1975), Sayed (2001) |
| tetragona | −14.7 | Constitutive | Mooney et al. (1977) |
| thi | −13.2 | NA | Batanouny et al. (1991) |
| tirucalli | −15.3 | Constitutive | Mies et al. (1996) |
| triangularis | −13.6 | Constitutive | Mooney et al. (1977) |
| trigona | −19.4 | NA | Webster et al. (1975) |
| xylophylloides | ND | Constitutive | Webster et al. (1975), Sayed (2001) |
NA, no mode assigned; ND, not determined.
Since values of δ13C alone are not sufficient to distinguish between C3 species and plants that obtain up to one-third of their carbon during the night, which include weak CAM plants (Winter and Holtum 2002), measurements of physiological and biochemical variables are necessary. In order to demonstrate the operation of CAM, routine determinations include, among others, ΔH+, δ13C and nocturnal CO2 fixation. Griffiths et al. (2007) devised an ingenious method of ascertaining the occurrence of nocturnal CO2 fixation by examining the response of the night-time CO2 exchange rate to intercellular CO2 concentration (Ci).
Intermediate values of δ13C can also suggest the occurrence of C3 metabolism with high water-use efficiency, as the data of Farquhar and Richards (1984) on wheat indicate, or of C2 photosynthesis, as in the case of Euphorbia acuta. In wheat and maize, C2 photosynthesis is responsible for an increase of 8–11 % in photosynthetic rate through re-assimilation of photorespired CO2 (Busch et al. 2013).
Plants of Euphorbia milii subgenus Euphorbia, Section Goniostema, common name crown of thorns, originally from Madagascar, are cultivated worldwide for their ornamental value. Plants are perennial armed shrubs as tall as 1 m, with fleshy stem and branches, and partly succulent leaves. According to observations by Mooney et al. (1977), CAM is present in the weak mode in leafy species of the genus. The medicinal and molluscicidal properties of the latex in E. milii have been extensively investigated (e.g. Mwine and Van Damme 2011); in contrast, literature on the physiology of the species is practically non-existent.
In spite of the succulence of its leaves and the various reports of CAM in the genus, E. milii has been reported as non-CAM (Webster et al. 1975). Nevertheless, recalculation of the data of McWilliams (1970) gives a ΔH+ of 100 μmol (g fresh mass)−1 and a dark CO2 fixation rate of 0.1 μmol m−2 s−1, suggesting that CAM in E. milii operates in the cycling mode, i.e. nocturnal H+ accumulation and daytime but nearly no night-time CO2 fixation (for the definition of CAM modes, see Cushman 2001).
With the aim of contributing to our knowledge of the evolution of CAM in Euphorbia, this study re-examined the possible occurrence of CAM in E. milii through daily leaf gas exchange, including PN/Ci and R/Ci curves (where PN is the photosynthetic rate and R is the respiration rate), measurements of dawn and dusk H+ content, and determinations of δ13C.
Methods
Plant material and cultivation
Plants of E. milii were propagated from one plant purchased at a nursery by inserting cuttings into the soil of 2-L pots filled with silty clay loam (Viveros Exotica Raphia, S.R.L., Caracas); plants were fertilized monthly with N : P : K 15 : 15 : 15 and grown in the garden for 1 year before the beginning of experiments. Plants, ∼50 cm tall, were maintained in the greenhouse under natural light, fully watered every other day and fertilized weekly. Day length was 12 h (06:00–18:00 h), mean maximum daily photosynthetic photon flux density (PPFD) between 09:00 and 14:30 h 507 ± 22 μmol m−2 s−1, mean air temperature 32 ± 5/18.4 ± 0.5 °C (day/night) and relative humidity 60 ± 10 %. Water deficit was imposed by withholding watering.
Anatomy
Free-hand cross-sections of stems (average thickness 5 mm) and leaves were observed under the microscope at ×40 (stem) and ×400 (leaf). Leaf sections were stained with toluidine blue.
Succulence
Leaf and stem water content was determined as the difference between the fresh mass (FM) and the mass after drying for 72 h at 60 °C [dry mass (DM)], divided by the area in the case of leaves (FM/A) and by DM in the case of stems. Leaf thickness was measured with precision calipers. Chlorophyll (Chl) content was determined after Bruinsma (1963) in 80 % cold acetone extracts of leaf or stem sections collected at 18:00 h. The mesophyll succulence index was calculated as Sm = g water (mg Chl)−1 after Kluge and Ting (1978).
Stable carbon isotope composition
The δ13C was determined with a precision of 0.15 ‰ using a ThermoFinnigan DeltaPlusXL Isotope Ratio Mass Spectrometer (San Jose, CA, USA) and PDB as the standard.
Nocturnal H+ accumulation
Whole leaves were weighed fresh and set to boil in 50 mL distilled water for 10 min in a microwave oven at maximum power; samples were sieved through a plastic colander, leaf segments and the colander were rinsed, and the solution was made up to 100 mL. Samples were titrated to pH 7.0 for the estimation of H+ corresponding to malate according to Nobel (1988), and to pH 8.4 for citrate. Since Franco et al. (1990) noted that there was a strong linear relationship between concentrations of malate and citrate determined enzymatically and by titration, in the absence of an enzymatic method for the determination, titration is an adequate alternative. Latex was collected from cut stems and leaves, suspended in distilled water and titrated likewise. The ΔH+ was calculated as the difference between dawn and dusk H+ contents.
Leaf gas exchange
The PN, R, stomatal conductance (gs) and transpiration rate (E) were measured in the laboratory with a CIRAS 2 IRGA connected to a PLC(B) assimilation chamber (PP Systems, Amesbury, MA, USA) at an incoming CO2 concentration (Ca) of 380 μmol mol−1, a chamber temperature tracking ambient (24 ± 1 °C) and an incident PPFD of 200 (the first morning hours) or 1000 μmol m−2 s−1 (the rest of the daytime). Records were automatically taken every 30 min. Response curves were done in six different leaves of PN and Ci between 10:00 and 11:00 h, and of nocturnal CO2 exchange to Ci between 20:00 and 05:00 h.
Statistics
Values are mean ± SE (n = 6). Statistical significance was assessed where indicated through one- or two-way analysis of variance (ANOVA) (P < 0.05) with the Statistica package.
Results
Leaf cross-sections showed a dorsiventral anatomy, with a compact palisade parenchyma containing many chloroplasts and a spongy parenchyma with large vacuoles and fewer chloroplasts; the spongy parenchyma constituted 40 % of the whole-leaf thickness (Fig. 1). A bundle sheath with large chloroplasts located centrifugally was observed. Cross-sections of the fleshy stems (not shown) have a thick green cortex and colourless pith; the cortex was 54 % of the stem thickness on average.
Figure 1.
Cross-sections of the leaf of E. milii. UE, upper epidermis; PP, palisade parenchyma; VB, vascular bundle; BS, bundle sheath; SP, spongy parenchyma; LE, lower epidermis. Arrowheads point at chloroplasts.
In watered plants, Sm in both leaves and stem green cortex was 2.7 ± 0.2 g water (mg Chl)−1; δ13C was −25.2 ± 0.7 ‰ in leaves and −24.7 ± 0.1 ‰ in stems. In the stem green cortex of watered plants, malate- and citrate-H+ content was 48 ± 9 and 29 ± 5 μmol H+ (g FM)−1, respectively, without daily oscillation. Water suspensions of latex showed no acid content.
Leaves had significant amounts of malate- and citrate-H+ at dawn and dusk, contents increasing with time under drought (Fig. 2A and B). A significant accumulation of malate-H+ took place in watered plants, which remained constant up to 16 days of drought (P < 0.05). A similar trend in dawn and dusk H+ content and ΔH+ for citrate-H+ was found, except that after 16 days of drought ΔH+ became zero. Mean ΔH+ was 18 ± 2 (malate) and 18 ± 4 (citrate) μmol (g FM)−1. Changes in either morning and evening H+ contents or ΔH+ bore no relationship to changes in FM/A, which remained relatively constant for the duration of the experiment, as did Chl content (Fig. 2). The ratio Chl a/b remained unchanged at 3.3 ± 0.2. Stem water content was 11.2 ± 0.5 g water (g DM)−1, twice as high as in leaves, and did not vary with time under drought (P = 0.86).
Figure 2.
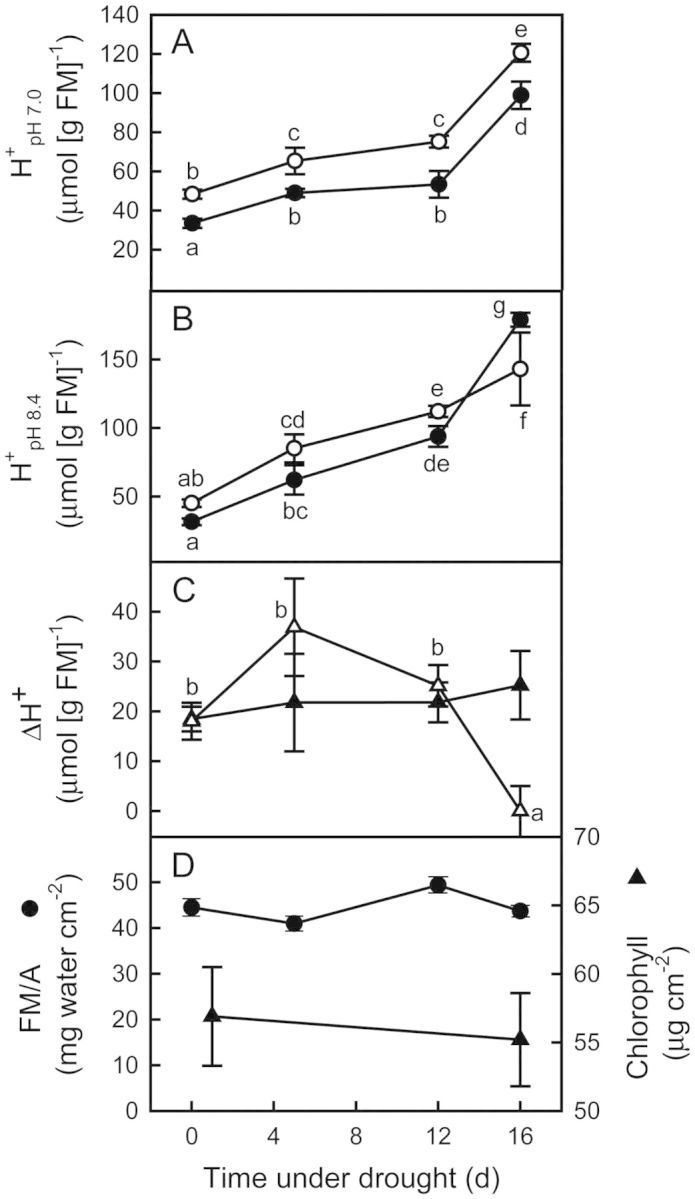
Time course of changes with drought in leaves of E. milii in (A) H+ content titrated to pH 7.0 (empty circles, dawn; filled circles, dusk); (B) H+ content titrated to pH 8.4 (empty circles, dawn; filled circles, dusk); (C) nocturnal H+ accumulation (empty triangles, pH 8.4; filled triangles, pH 7.0), and (D) dawn leaf FM per area (circles) and chlorophyll content (triangles). Values are mean ± SE (n = 12). Different letters indicate significant differences at P < 0.05 after a two-way ANOVA (time under drought × hour of day for each pH in A and B) and a one-way ANOVA (time under drought for each pH in C).
As shown in Fig. 3A, PN of watered plants became saturated at 700 μmol m−2 s−1 PPFD; apparent quantum yield was 0.047 and light-compensation point 48 μmol m−2 s−1. The PN/Ci curves (Fig. 3B) show that PN did not become saturated by Ci, increasing 47 % with an increase in Ci to 800 μmol mol−1 (Ca = 1240 μmol mol−1). This lack of saturation could have been due to very low gs, which remained unchanged by Ci. The CO2 compensation concentration was 32 μmol mol−1.
Figure 3.
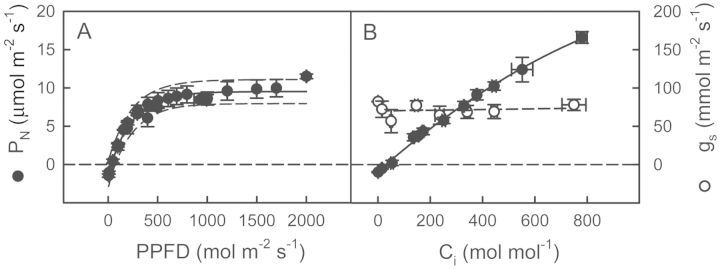
Response curve of the leaf photosynthetic rate to (A) photosynthetic photon flux density and (B) leaf intercellular CO2 concentration in watered plants of E. milii. Values are mean ± SE (n = 6). Filled circles, PN; empty symbols, gs. Measurements were made at a CO2 concentration of 380 μmol mol−1 in (A) and a PPFD of 1000 μmol m−2 s−1 in (B).
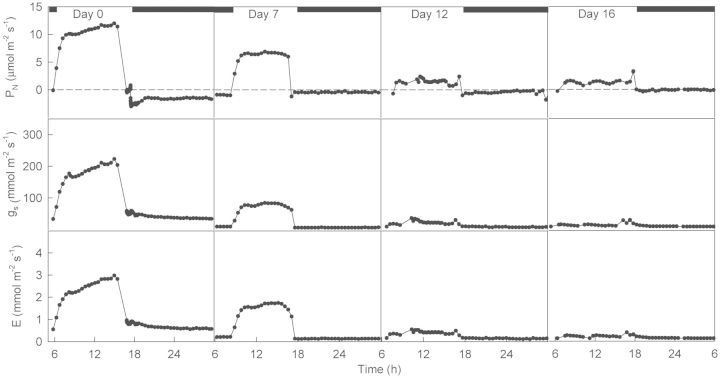
Daily courses of leaf gas exchange done in plants progressively under drought showed a decrease in PN of 85 % with drought; R became nearly zero after 12 and up to 16 days without watering (Fig. 4). Mean daytime water-use efficiency calculated from these courses of leaf gas exchange was relatively high, decreasing significantly with drought only 18 %, from 4.2 ± 0.1 to 3.5 ± 0.1 mmol mol−1 (P = 0.00).
Figure 4.
Daily course of the leaf photosynthetic rate, stomatal conductance and transpiration rate in plants of E. milii under drought for 0, 7, 12 and 16 days. Measurements were made at a CO2 concentration of 380 μmol mol−1, leaf temperature of 24.0 ± 1.0 °C and PPFD of 200 (06:00–10:00 h) and 1000 μmol m−2 s−1 (10:00–18:00 h). The dark bar on the abscissa indicates the length of the dark period.
Stem cross-sections of 1.5 cm2 average area from watered plants introduced in the assimilation chamber showed daytime CO2 assimilation at rates similar to those determined in leaves on a Chl basis (Table 2). Stem sections, as opposed to leaves, showed dark CO2 uptake.
Table 2.
Photosynthetic and respiration rate of stem cross-sections inserted into the IRGA assimilation chamber.
| Organ | PPFD (μmol m−2 s−1) |
PN |
|
|---|---|---|---|
| (μmol m−2 s−1) | (μmol (g Chl)−1 s−1) | ||
| Leaf | 1500 | 9.9 ± 1.2 | 15.1 ± 0.3 |
| Stem | 1500 | 10.0 ± 0.2 | 9.1 ± 1.9 |
| Leaf | 0 | −1.5 ± 0.2 | −2.3 ± 0.3 |
| Stem | 0 | 0.5 ± 0.2 | 4.7 ± 1.3 |
Values are mean ± SE (n = 6). Incident photosynthetic photon flux density is indicated.
A significant decrease of 65 % in R with Ci was observed without significant changes in gs (Fig. 5).
Figure 5.
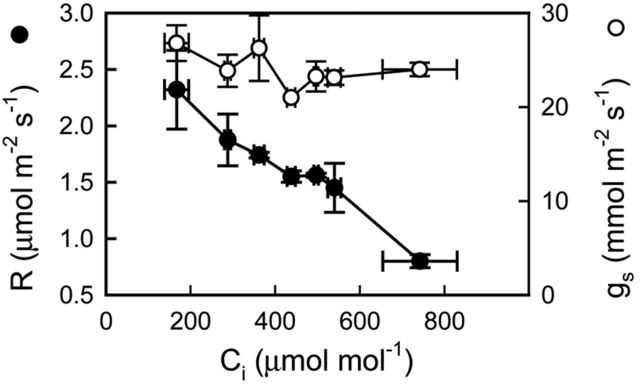
Response curves to leaf intercellular CO2 concentration of nocturnal respiration rate and stomatal conductance in watered plants of E. milii. Filled symbols, R; empty symbols, gs. Values are mean ± SE (n = 6).
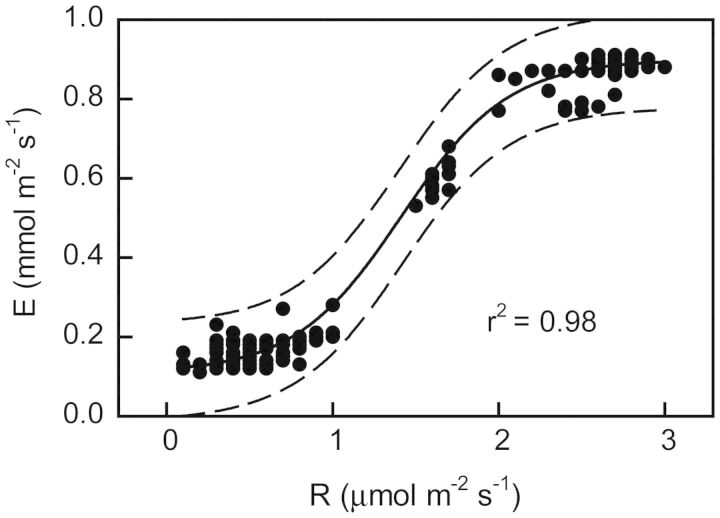
The regression of E vs. R is shown in Fig. 6. Assuming that the accumulated acids were the products of the recycling of respiratory CO2, the absolute recycling, i.e. the amount of CO2 contained in acids, was calculated. Together with the E vs. R regression, it was found that recycling recovered 10 and 37 % of nocturnal CO2 loss in watered plants and after 12 days of drought, respectively, and helped in saving water during the night by 15 % in watered plants and 2 % in plants under drought. Daytime water saving, calculated as the ratio of the absolute amount of CO2 in acid equivalents to integrated PN (after Fetene and Lüttge 1991), was 2 and 86 % in watered plants and plants under drought, respectively.
Figure 6.
Change in nocturnal leaf transpiration rate of plants of E. milii watered and under different degrees of drought as a function of nocturnal respiration rate. Values are data points. The regression line (solid), 95 % confidence intervals (broken lines) and determination coefficient (P < 0.05) are shown.
Discussion
Evidence was found for the operation of weak CAM in E. milii, with statistically significant ΔH+ in watered plants and plants under drought, low δ13C and no nocturnal CO2 uptake; ΔH+ was apparently attributable to both malate and citrate. Results suggest that daily malate and citrate accumulation results from recycling of part (watered plants) or all (plants under drought) of the nocturnal respiratory CO2. Recycling of CO2 through malate synthesis, together with the absence of nocturnal CO2 uptake, helps explain the occurrence of values of leaf δ13C intermediate between C3 and constitutive CAM plants.
Values of ΔH+ determined at pH 7.0 were low compared with CAM species such as Kalanchoe tubiflora and Clusia minor, comparable with the cycling species T. parviflorum and T. mengessi and not as low as in Talinum teretifolium or Zamioculcas zamiifolia (Table 3).
Table 3.
Values of nocturnal acid accumulation and carbon isotopic composition reported for CAM species.
| CAM mode | Species | ΔH+ | δ13C | Reference |
|---|---|---|---|---|
| (μmol (g FM)−1) | (‰) | |||
| Facultative | Clusia minor | 1400 | −24.6 | Borland et al. (1992, 1994) |
| Constitutive | Kalanchoe daigremontiana | 152 | −16.7 | Holtum et al. (1983) |
| Cycling | Talinum calycinum | 39 | −25.2 | Martin and Zee (1983) |
| Cycling | Sedum nuttalianum | 37 | −27.2 | Gravatt and Martin (1992) |
| Cycling | Talinum calcaricum | 29 | −26.0 | Harris and Martin (1991) |
| Cycling | Sedum telephioides | 23 | −26.2 | Gravatt and Martin (1992) |
| Cycling | Euphorbia milii | 18 | −25.2 | This study |
| Cycling | Talinum teretifolium | 14 | −25.4 | Harris and Martin (1991) |
| Cycling | Talinum parviflorum | 11 | −25.8 | Harris and Martin (1991) |
| Cycling | Talinum mengessi | 6 | −24.3 | Harris and Martin (1991) |
| Facultative | Zamioculcas zamiifolia | 5 | ND | Holtum et al. (2007) |
Some values were re-calculated from the data in references. ND, not determined.
A value of Sm higher than 1 g water (mg Chl)−1 was also suggestive of CAM, as proposed by Kluge and Ting (1978). The succulent nature of leaves was corroborated by the microscopic observation of cross-sections, in which cells with a large volume and few chloroplasts are present, as in many leaf-succulent CAM plants (Kluge and Ting 1978). In facultative CAM species, values of Sm are intermediate (Table 4) but, given that low as well as high values of Sm can be found in constitutive CAM species (Table 4), it becomes apparent that for a leaf to perform full CAM a large proportion of vacuole volume to chloroplasts is not required. The lack of significant differences in FM/A between five strong CAM and three weak CAM species (Nelson and Sage 2008) lends support to this hypothesis.
Table 4.
Values of mesophyll succulence index reported for constitutive, facultative- and cycling-CAM species.
| Species | Sm g water (mg Chl)−1 | CAM mode | Reference |
|---|---|---|---|
| Kalanchoe daigremontiana | 1.3 | Constitutive | Kluge and Ting (1978) |
| Euphorbia milii | 2.7 | Cycling | This study |
| Talinum paniculatum | 3.4 | Facultative | Güerere et al. (1996) |
| Talinum triangulare | 6.0 | Facultative | Herrera et al. (1991) |
| Puya floccosa | 6.2 | Facultative | Herrera et al. (2010) |
| Sedum morganianum | 13.0 | Constitutive | Kluge and Ting (1978) |
The presence in E. milii of bundle sheath cells with chloroplasts is indicative of the possible operation of C2 photosynthesis, as reported in E. acuta. This species shares with E. milii low, C3-like values of δ13C (−25.5 ‰ according to Webster et al. 1975, and −28.5 ‰ according to Sage et al. 2011) and an intermediate value of CO2 compensation concentration (32 mmol mol−1; Sage et al. 2011). The occurrence of CAM, Kranz anatomy and C4 photosynthesis in the same leaf has been reported in Portulacaceae (Guralnick and Jackson 2001), but to date no report on CAM together with C2 photosynthesis has been published. Given that the C2 route of carbon fixation has been proposed as an intermediate evolutionary step from C3 to C4 (Sage et al. 2011), investigating the functioning of C2 photosynthesis in E. milii would bring interesting viewpoints on the evolution of CAM and C4 plants, particularly in Euphorbia.
A significant oscillation in H+ content corresponding to citrate was found, equivalent to 12 μmol citrate (g FM)−1 after 12 days of drought, comparable with the lower end, ∼22 μmol (g FM)−1, of the range in species of Clusia, a genus abundant in CAM species performing different modes (Lüttge 2007). The role of citrate accumulation in carbon or water balance during CAM remains unclear (Lüttge 2007); citrate does not provide net CO2 gain, as does malate, but should prove more effective than malate in increasing Ci during the day because its breakdown produces three molecules of CO2 as opposed to one in the case of malate (Lüttge 2007). Increased Ci during the day is a photoprotective mechanism well recognized in CAM plants, as shown in plants of the facultative CAM species Talinum triangulare under drought (Pieters et al. 2003).
Photosynthetic characteristics in E. milii were consistent with those of a sun plant: high apparent quantum yield, saturating PPFD and Chl a/b ratio (Pearcy and Franceschi 1986).
The absence of net dark CO2 fixation was consistent with the proportion of dark CO2 uptake calculated using the regression equations of the proportion of CO2 fixed during the night against δ13C found by Winter and Holtum (2002) and Pierce et al. (2002). In many facultative CAM species, δ13C tends towards low values. In a review of 23 facultative CAM species (Herrera 2009), the mean, maximum and minimum δ13C were −23.9, −14.0 and −30.0 ‰, respectively, indicating that the variability in δ13C values may lead researchers to classify a species as a C3, facultative or constitutive CAM plant. In Euphorbia aphylla, δ13C ranged from −27.1 ‰ for the youngest cladode in the dry season during summer to −24.5 ‰ for the oldest cladode in the rainy season during winter (Mies et al. 1996), reflecting the effects on δ13C of day/night temperatures, water availability and developmental stage.
Values of δ13C higher in stems than in leaves suggest the occurrence of nocturnal CO2 fixation, although this could not be demonstrated in intact plants. The occurrence of an assimilation rate in the dark amounting to a third of PPFD-saturated leaf PN strongly suggested that stems are capable of nocturnal CO2 fixation. Stem internal CO2 re-fixation in young twigs and branches possessing a green cortex may compensate for 60–90 % of the potential respiratory carbon loss (Pfanz et al. 2002). If stem recycling in E. milii occurred through phosphoenolpyruvate carboxylase (PEPC) activity, that would explain the apparent 13C enrichment. There are two alternative explanations for higher δ13C in the stems of E. milii. One explanation is that this variable was determined in sections comprising all tissues, green as well as non-autotrophic; non-autotrophic organs of C3 plants, such as stems, have been found to be enriched in δ13C by ∼1–3 ‰ relative to leaves (Cernusak et al. 2009). Another, simpler, explanation is that barriers to CO2 diffusion into the stem are larger than into leaves, hindering entrance of the heavier 13CO2. Actual nocturnal CO2 fixation by stems of E. milii remains to be determined accurately by methods such as carbon labelling, involving all stem tissues.
The response of leaf dark respiration to Ci suggests the operation of a carboxylation system, most likely PEPC, which makes recycling of respiratory CO2 possible. In the constitutive CAM plant Kalanchoe daigremontiana, a PN/Ci curve during phase I of the CAM cycle showed a pronounced increase at low Ci and saturation at a Ci of ∼250 μmol mol−1 (Griffiths et al. 2007). Our results show that the response of dark respiration to Ci was not an artefact caused by changes in gs, because gs remained constant in spite of increasing Ci.
Water saving through respiratory CO2 recycling was significant, as in the case of T. paniculatum, a facultative CAM species, in which the amount of water saved was 5–12 times that lost by transpiration (Güerere et al. 1996). Similarly, in T. calycinum, 5–44 % of water was potentially conserved by CAM-cycling (Martin et al. 1988).
Leaf water balance in E. milii seems to rest on both recycling of respiratory CO2 and strict stomatal control, rather than on water supply from the succulent stem, as leaf FM/A remained unchanged after 16 days of drought and stem water content did not vary significantly during this time.
Conclusions
In view of the observations presented here, E. milii can be considered as a CAM-cycling species that in watered plants shows diurnal, but not nocturnal, CO2 uptake and low ΔH+; plants under drought have very low PN, equally low ΔH+ and no net dark CO2 exchange. The significance of the operation of such a low CAM in E. milii resides in water conservation, rather than carbon acquisition. The occurrence of C2 photosynthesis remains to be demonstrated.
Sources of Funding
Experiments were done with equipment acquired through grant PG-03.00.6524.2006 and technical assistance provided by grant PG 03.7381.2011-1 (CDCH-UCV).
Conflicts of Interest Statement
None declared.
Acknowledgements
Thanks are due to the PaleoLab at the College of Marine Science of the University of South Florida and to Dr Enrique Montes for the determinations of δ13C. Help with leaf section preparation and the provision of photographs by L. Hermoso, M. Escala and A. Menéndez are gratefully acknowledged.
Literature Cited
- Batanouny KH, Stichler W, Ziegler H. Photosynthetic pathways and ecological distribution of Euphorbia species in Egypt. Oecologia. 1991;87:565–569. doi: 10.1007/BF00320421. [DOI] [PubMed] [Google Scholar]
- Borland AM, Griffiths H, Maxwell C, Broadmeadow MSJ, Griffiths NM, Barnes JD. On the ecophysiology of the Clusiaceae in Trinidad: expression of CAM in Clusia minor L. during the transition from wet to dry season and characterization of tree endemic species. New Phytologist. 1992;122:349–357. doi: 10.1111/j.1469-8137.1992.tb04240.x. [DOI] [PubMed] [Google Scholar]
- Borland AM, Griffiths H, Broadmeadow MSJ, Fordham MC, Maxwell C. Carbon-isotope composition of biochemical fractions and the regulation of carbon balance in leaves of the C3-CAM Clusia minor L. growing in Trinidad. Plant Physiology. 1994;106:493–501. doi: 10.1104/pp.106.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma JB. The quantitative analysis of chlorophylls a and b in plant extracts. Photochemistry and Photobiology. 1963;2:241–249. [Google Scholar]
- Busch FA, Sage TL, Cousins AB, Sage RF. C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant, Cell and Environment. 2013;36:200–212. doi: 10.1111/j.1365-3040.2012.02567.x. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Tcherkez G, Keitel C, Cornwell WK, Santiago LS, Knohl A, Barbour MM, Williams DG, Reich PB, Ellsworth DS, Dawson TE, Griffiths HG, Farquhar GD, Wright IJ. Why are non-photosynthetic tissues generally 13C enriched compared with leaves in C3 plants? Review and synthesis of current hypotheses. Functional Plant Biology. 2009;36:199–213. doi: 10.1071/FP08216. [DOI] [PubMed] [Google Scholar]
- Cushman JC. Crassulacean acid metabolism. A plastic photosynthetic adaptation to arid environments. Plant Physiology. 2001;127:1439–1448. [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Richards RA. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology. 1984;11:539–552. [Google Scholar]
- Fetene M, Lüttge U. Environmental influences on carbon recycling in a terrestrial CAM bromeliad, Bromelia humilis Jacq. Journal of Experimental Botany. 1991;42:25–31. [Google Scholar]
- Franco AC, Ball E, Lüttge U. Patterns of gas exchange and organic acid oscillations in tropical tress of the genus Clusia. Oecologia. 1990;85:108–114. doi: 10.1007/BF00317350. [DOI] [PubMed] [Google Scholar]
- Gravatt DA, Martin CE. Comparative ecophysiology of five species of Sedum (Crassulaceae) under well-watered and drought-stressed conditions. Oecologia. 1992;92:532–541. doi: 10.1007/BF00317845. [DOI] [PubMed] [Google Scholar]
- Griffiths H, Cousins AB, Badger MR, Caemmerer S. Discrimination in the dark. Resolving the interplay between metabolic and physical constraints to phosphoenolpyruvate carboxylase activity during the crassulacean acid metabolism cycle. Plant Physiology. 2007;143:1055–1067. doi: 10.1104/pp.106.088302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güerere I, Tezara W, Herrera C, Fernández MD, Herrera A. Recycling of CO2 during induction of CAM by drought in Talinum paniculatum (Portulacaceae) Physiologia Plantarum. 1996;98:471–476. [Google Scholar]
- Guralnick LJ, Jackson MD. The occurrence and phylogenetics of CAM in the Portulacaceae. International Journal of Plant Science. 2001;162:257–262. [Google Scholar]
- Harris FS, Martin CE. Correlation between CAM-cycling and photosynthetic gas exchange in five species of Talinum (Portulacaceae) Plant Physiology. 1991;96:1118–1124. doi: 10.1104/pp.96.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A. Crassulacean acid metabolism and fitness under water deficit stress: if not for carbon gain, what is facultative CAM good for? Annals of Botany. 2009;103:645–653. doi: 10.1093/aob/mcn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A, Delgado J, Paraguatey I. Occurrence of facultative crassulacean acid metabolism in leaves of Talinum triangulare (Portulacaceae) Journal of Experimental Botany. 1991;42:493–499. [Google Scholar]
- Herrera A, Martin CE, Tezara W, Ballestrini C, Medina E. Induction by drought of crassulacean acid metabolism in the terrestrial bromeliad, Puya floccosa. Photosynthetica. 2010;48:383–388. [Google Scholar]
- Holtum JAM, O'Leary MH, Osmond CB. Effect of varying CO2 partial pressure on photosynthesis and on carbon isotope composition of carbon-4 of malate from the crassulacean acid metabolism plant Kalanchoe daigremontiana Hamet et Perr. Plant Physiology. 1983;71:602–609. doi: 10.1104/pp.71.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtum JAM, Winter K, Weeks MA, Sexton TR. Crassulacean acid metabolism in the ZZ plant, Zamioculcas zamiifolia (Araceae) American Journal of Botany. 2007;94:1670–1676. doi: 10.3732/ajb.94.10.1670. [DOI] [PubMed] [Google Scholar]
- Kluge M, Ting IP. Crassulacean acid Metabolism. Berlin: Springer; 1978. [Google Scholar]
- Lüttge U. Clusia. A woody neotropical genus of remarkable plasticity and diversity. Berlin: Springer; 2007. pp. 167–169. [Google Scholar]
- Martin CE, Zee AK. C3 photosynthesis and CAM in a Kansas rock outcrop succulent, Talinum calycinum (Portulacaceae) Plant Physiology. 1983;73:718–723. doi: 10.1104/pp.73.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, Higley M, Wang W-Z. Ecophysiological significance of CO2-recycling via CAM in Talinum calycinum Engelm. (Portulacaceae) Plant Physiology. 1988;86:562–568. doi: 10.1104/pp.86.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams EL. Comparative rates of dark CO2 uptake and acidification in the Bromeliaceae, Orchidaceae, and Euphorbiaceae. Botanical Gazette. 1970;131:285–290. [Google Scholar]
- Mies B, Jiménez MS, Morales D. Ecophysiology and distribution of the endemic leafless spurge Euphorbia aphylla and the introduced E. tirucalli (Euphorbiaceae, Euphorbia sect. Tirucalli) in the Canary Islands. Plant Systematics and Evolution. 1996;202:27–36. [Google Scholar]
- Mooney HA, Troughton JH, Berry JA. Carbon isotope ratio measurements of succulent plants in southern Africa. Oecologia. 1977;30:295–305. doi: 10.1007/BF00399762. [DOI] [PubMed] [Google Scholar]
- Mwine JT, Van Damme P. Why do Euphorbiaceae tick as medicinal plants? A review of Euphorbiaceae family and its medicinal features. Journal of Medicinal Plants Research. 2011;5:652–662. [Google Scholar]
- Nelson EA, Sage RF. Functional constraints of CAM leaf anatomy: tight cell packing is associated with increased CAM function across a gradient of CAM expression. Journal of Experimental Botany. 2008;59:1841–1850. doi: 10.1093/jxb/erm346. [DOI] [PubMed] [Google Scholar]
- Nobel PS. Environmental biology of agaves and cacti. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Pearcy RW, Franceschi WR. Photosynthetic characteristics and chloroplast ultrastructure of C3 and C4 tree species grown in high- and low-light environments. Photosynthesis Research. 1986;9:317–331. doi: 10.1007/BF00029797. [DOI] [PubMed] [Google Scholar]
- Pfanz H, Aschan G, Langenfeld-Heyser R, Wittmann C, Loose M. Ecology and ecophysiology of tree stems: corticular and wood photosynthesis. Naturwissenschaften. 2002;89:147–162. doi: 10.1007/s00114-002-0309-z. [DOI] [PubMed] [Google Scholar]
- Pierce S, Winter K, Griffiths H. Carbon isotope ratio and the extent of daily CAM use by Bromeliaceae. New Phytologist. 2002;156:75–83. [Google Scholar]
- Pieters AJ, Tezara W, Herrera A. Operation of the xanthophyll cycle and degradation of D1 protein in the facultative CAM plant, Talinum triangulare, under water deficit. Annals of Botany. 2003;92:393–399. doi: 10.1093/aob/mcg153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage TL, Sage RF, Vogan PJ, Rahman B, Johnson DC, Oakley JC, Heckel MA. The occurrence of C2 photosynthesis in Euphorbia subgenus Chamaesyce (Euphorbiaceae) Journal of Experimental Botany. 2011;62:3183–3195. doi: 10.1093/jxb/err059. [DOI] [PubMed] [Google Scholar]
- Sayed OH. Crassulacean acid metabolism 1975–2000, a check list. Photosynthetica. 2001;39:339–352. [Google Scholar]
- Silvera K, Santiago LS, Winter K. Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Functional Plant Biology. 2005;32:397–407. doi: 10.1071/FP04179. [DOI] [PubMed] [Google Scholar]
- Webster GL, Brown WV, Smith BN. Systematics of photosynthetic carbon fixation pathways in Euphorbia. Taxon. 1975;24:27–33. [Google Scholar]
- Winter K. δ13C values of some succulent plants from Madagascar. Oecologia. 1979;40:103–112. doi: 10.1007/BF00388814. [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM. How closely do the δ13C values of crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiology. 2002;129:1843–1851. doi: 10.1104/pp.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann NFA, Ritz CM, Hellwig FH. Further support for the phylogenetic relationships within Euphorbia L. (Euphorbiaceae) from nrITS and trnL–trnF IGS sequence data. Plant Systematics and Evolution. 2010;286:39–58. [Google Scholar]