Abstract
Cell-based therapies using natural or genetically modified regulatory T cells (Tregs) have shown significant promise as immune-based therapies. One of the main difficulties facing the further advancement of these therapies is that the fate and localization of adoptively transferred Tregs is largely unknown. The ability to dissect the migratory pathway of these cells in a non-invasive manner is of vital importance for the further development of in-vivo cell-based immunotherapies, as this technology allows the fate of the therapeutically administered cell to be imaged in real time. In this review we will provide an overview of the current clinical imaging techniques used to track T cells and Tregs in vivo, including magnetic resonance imaging (MRI) and positron emission tomography (PET)/single photon emission computed tomography (SPECT). In addition, we will discuss how the finding of these studies can be used, in the context of transplantation, to define the most appropriate Treg subset required for cellular therapy.
Keywords: regulatory T cells, whole-body imaging
OTHER ARTICLES PUBLISHED IN THIS SERIES
T cell depletion in paediatric stem cell transplantation. Clinical and Experimental Immunology 2013, 172: 139–47.
Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now? Clinical and Experimental Immunology 2013, 172: 148–57.
Promoting transplantation tolerance; adoptive regulatory T cell therapy. Clinical and Experimental Immunology 2013, 172: 158–68.
Introduction
Survival of a transplanted allograft is dependent upon a delicate balance between effector T cells (Teff) and regulatory T cells (Treg) 1–4, whereby the number of Tregs must equal the number of Teffs to promote tolerance to alloantigens. In preclinical mouse transplant models this equates to a 1·1:1·2 ratio of Tregs to Teff cells, or approximately 33–50% of Tregs, to be present at the transplant site to induce tolerance 5. One way to achieve this number is by expanding Tregs in vitro and adoptively transferring them back into the patient 2. We have demonstrated previously that adoptively transferring ex-vivo expanded murine and human Tregs, with specificity for the alloantigens expressed by the graft, results in graft prolongation in animal models 6,7. These data suggest that Treg cell therapy could be a beneficial therapy for transplant patients 8–12. In fact, these cells have been used in the clinic to alleviate graft-versus-host disease in patients following bone marrow or human stem cell transplantation, resulting in clinical improvement 13. With regard to organ transplantation, the ONE study (a multi-centre Phase I/II study) is investigating the efficacy of ex-vivo-expanded adoptive Tregs for the use in kidney transplant patients.
Although this cellular therapy has therapeutic potential, there are several questions regarding (i) lifespan: how long in vivo do Treg cells survive following adoptive transfer; and (ii) localization: where do adoptively transferred Tregs go, do they localize within the transplanted tissue and/or within the draining lymph node or does this alter during the lifespan of the transplant? (iii) do Treg subsets function at defined locations, all remain unanswered. It is vital to understand each of these points so that the optimal Treg for therapeutic purposes can be created.
Conventional imaging techniques have been utilized to address some of these questions. For example, using fluorescent microscopy in an islet allograft model, Zhang et al. observed that Tregs migrate from blood to the inflamed allograft, wherein they become activated, before migrating to the draining lymph nodes. This process was dependent upon multiple chemokine receptors including CCR2, CCR4 and CCR5 14. In-vivo imaging, using two-photon intravital laser-scanning microscopy of adoptively transferred Tregs in non-obese diabetic (NOD) mice with autoimmune diabetes, have indicated that Tregs are present in lymph nodes; these authors also noted that the contact time between Teff and dendritic cells (DCs) is reduced when Tregs are present, suggesting that the latter cells prevent the clonal expansion of Teff cells by limiting the DC–Teff interaction time 15,16.
Although these studies have contributed towards understanding of the anatomical locations where Tregs reside, following adoptive transfer the techniques used do not allow the longitudinal study of cells within the same recipient. Unlike the aforementioned imaging techniques, whole-body imaging of Tregs has the potential to do precisely this.
Whole-body imaging techniques currently being used to track T cells and other immune cells include both nuclear and optical imaging. Nuclear imaging is composed of administration of radioactive probes that take part in a physiological or biological process by an organism. The uptake of the radiotracer then allows imaging, such that the biological processes in vivo and cell recruitment can be measured 17–19. In comparison, optical imaging uses non-ionizing radiation and measures light generated from a probe within the cell. Although optical imaging (fluorescence and bioluminescence) has been used successfully in small animal models, this technology is not feasible, and has not yet been used in human whole-body scans. The imaging techniques relevant to cell tracking in a clinical setting are via nuclear imaging and magnetic resonance imaging (MRI), and these will be the focus of the next sections; however, for an overview on optical imaging please refer to Kircher et al. 20. What whole-body nuclear imaging systems have been used to look at T cells in vivo so far?
MRI
MRI is a non-invasive imaging technique that provides three-dimensional images with high resolution 21. Its popularity is due to high signal-to-noise ratios and soft tissue contrast as well as the availability of safe intracellular contrast agents. The principle of MRI is based on the hydrogen nuclei spin of organic compounds within an individual 22. One of the main limitations of MRI imaging is low sensitivity; others are highlighted in Table 1 23; however, the main benefit of this technique is that it does not require ionizing radiation.
Table 1.
Summary of preclinical imaging methods, their labels and advantages/disadvantages
| Imaging | Isotopes | Labelling | Advantages | Disadvantages | Ref |
|---|---|---|---|---|---|
| PET (high-energy gamma rays) | Carbon-11 | Direct/indirect | Detects picomolar concentrations | Ionizing radiation | 25,26,30,33–35 |
| Fluorine-18 | High running cost | ||||
| Oxygen-15 | Non-invasive | Cyclotron required on site (limited availability) | |||
| Copper-64 | High sensitivity | ||||
| Iodine-124 | Shows organ function | Resolution dependent on isotope used and its positron range | |||
| Nitrogen-13 | |||||
| Zirconium-89 | Limited half-life of the isotopes used | ||||
| SPECT (lower-energy gamma rays) | Iodine-131 | Direct/indirect | Detects picomolar concentrations | Ionizing radiation | 25,26,30,31,33–35,73 |
| Iodine-125 | High running cost | ||||
| Indium-111 | Isotopes widely available | Limited spatial resolution | |||
| Technetium-99 m | Non-invasive | Quantitation | |||
| High sensitivity | |||||
| Long half-life of isotopes used | |||||
| Simultaneous imaging of multiple radiolabels | |||||
| Ability to measure slow kinetic processes | |||||
| MRI (radio waves) | Contrast agents: | Direct/indirect | No ionizing radiation | Low sensitivity | 20,22 |
| SPIO | High resolution | Require higher levels of labelling | |||
| Gadolinium | High soft tissue contrast | Long scan time | |||
| Non-invasive | Very expensive |
PET: positron emission tomography; SPECT: single photon emission computed tomography; MRI: magnetic resonance imaging.
Single photon emission computed tomography (SPECT) and positron emission tomography (PET)
SPECT and PET are the two main techniques used in nuclear imaging 24–26, in conjunction with computed tomography (CT). The sensitivity of PET is typically higher than SPECT, and both are more sensitive than the previously described MRI-based technology. They possess the ability to detect picomolar, or low concentrations, of radioisotopes, making them an attractive prospect for clinical and preclinical small-animal imaging 27,28. SPECT functions by detecting a single gamma ray emitted from radioisotopes. Emitted gamma rays are collected by sensitive ‘gamma camera’ detectors, which rotate around the object. Single or multiple detectors can be used for SPECT to generate multiple single two-dimensional images from various angles which, once reconstructed, generate a three-dimensional tomographic image 26,29,30. In contrast, PET imaging relies on the simultaneous detection of two photons (of 511 KeV) emitted in opposite directions during the annihilation of a positron colliding with an electron in tissue 31,32.
Many different SPECT and PET isotopes are used in nuclear medicine, as summarized in Table 1. Some PET radioisotopes require an on-site cyclotron for their production 25,30,33. The half-lives of these isotopes are typically short, ranging from 2 to 110 min. Although most of the PET isotopes have a short half-life, isotopes such as Copper-64 (t1/2 = 12·7 h) or Iodine-124 (t1/2 = 4·18 days), which have a longer half-life, can be used for imaging over extended periods of time 34. Unlike PET, SPECT radioisotopes are readily available, i.e. they do not require an on-site cyclotron. In a preclinical setting SPECT offers better spatial resolution than PET. Importantly, SPECT offers the unique advantage of imaging multiple isotopes simultaneously, based on the detection of the different energies of the emitted photons, and hence the potential for tracking two or more cell populations at the same time 35. With appropriate image reconstruction techniques PET, and more recently SPECT, can offer quantitative data such as in-vivo concentration of the isotope in a particular region of interest. However, both are not without their limitations, such as sensitivity, specificity resolution and quantitation, as highlighted in Tables 1 and 2.
Table 2.
Summary of spatial resolution, sensitivity and depth of preclinical imaging techniques in further detail
| Imaging technique | Spatial resolution | Sensitivity | Depth | Preclinical use | Clinical use | Cost |
|---|---|---|---|---|---|---|
| MRI | < 1 mm | Low | No limit | Yes | Yes | High |
| PET | 1–2 mm | High | No limit | Yes | Yes | High |
| SPECT | < 1 mm | High | No limit | Yes | Yes | High |
PET: positron emission tomography; SPECT: single photon emission computed tomography; MRI: magnetic resonance imaging.
Combinational techniques (SPECT-computed tomography (CT), PET-MRI and PET-CT)
Combinational approaches are now furthering the advancement of imaging. Functional imaging with SPECT and PET is combined with X-ray CT to provide anatomical and functional information from a single study 23. The main advantage associated with this hybrid imaging approach is the increased accuracy of anatomical localization of the radiotracer uptake, enhanced by the typically high-resolution structural images, which is not achieved using SPECT or PET alone. Additional advantages include the ability to use the structural images as a basis for attenuation correction of the emission data and the use of structural images for more accurate definition of regions of interest for quantification of radiotracer uptake in various organs. There are many advantages associated with this hybrid system, including shorter acquisition time, better attenuation correction, increased specificity and a more accurate image of cell activity. Hybrid imaging modalities such as PET-CT, SPECT-CT and PET-MRI can be used.
The recent introduction of systems that combine PET and MRI opens new horizons for multi-modality molecular imaging. These systems offer simultaneous morphological, functional and molecular information of a living system. In the near future, PET-MRI may emerge as a new powerful multi-modality technique offering considerable potential for imaging applications beyond correlation of functional and anatomical images.
New small-animal micro-CT instruments have the ability to generate high-resolution anatomical three-dimensional images. Advancements in micro-CT technology, such as smaller detector elements and more powerful X-ray tubes, allow improved spatial resolution and faster scan times of an entire mouse (0·8 s). Iodinated contrast agents have a short half-life (10 min) in circulation; however, because of faster scan times and the use of clinical contrast agents, perfusion studies may be performed. Gold nanoparticles have now been used as a better contrast agent. The higher the atomic number and electron density, the higher the attenuation coefficients leads to greater the differentiation between tissues for the CT imaging. The atomic number and electron density of gold (79 and 19·32 g/cm3, respectively) are much higher than those of the currently used iodine (53 and 4·9 g/cm3), making it a more effective contrast agent. The major disadvantage of the technique is exposure to ionizing radiation 36–38.
PET-MRI is a relatively new hybrid technology offering both the sensitivity of PET alongside the anatomical and soft tissue information provided by MRI. This hybrid technology has been used to study cells in a cancer setting 17. Functional and morphological information gathered using PET-MRI, in conjunction with the radiolabelled glucose analogue [2-flourine-18]-flouro-2-deoxy-D-glucose ([18F]-FDG), has identified areas of cancerous growth and differentiates between benign and malignant tumours, depending on metabolic uptake 18,19,39.
Direct versus indirect cell labelling
To visualize cells by MRI/SPECT/PET they must first be labelled. This is achieved by either direct or indirect labelling. Direct labelling is a process in which the cells remain free of any genetic alteration or manipulation (Fig. 1a), and can be achieved simply by incubating cells with the radiolabel or contrast agent of choice in vitro 20,40.
Fig. 1.

Outline of direct and indirect cell labelling. (a) Direct cell labelling involves the introduction of a radioactive isotope into cells. Cells isolated from the donor mice can be incubated with the radiolabel. Once labelled, they can be transferred adoptively into the recipient before single photon emission computed tomography (SPECT) scanning. With direct labelling, as the cells divide and the label effluxes, the period of time in which the cells can be tracked is limited. (b) Indirect cell labelling involves genetic modification of the cell. Cells are transduced with a vector encoding a reporter gene, for example human sodium/iodide symporter (hNIS). Reporter genes can also encode for fluorescent proteins or enzymes. Transduced cells are incubated and expanded in vitro prior to adoptive transfer. With the exclusion of fluorescent reporter proteins imaging requires radiolabel injection. The transduced cells can be visualized longitudinally by direct in-vivo injection of the radioisotope. As the reporter gene is passed to newly divided cells, long-term imaging is possible.
MRI
Cells are labelled directly with various magnetic resonance (MR) contrast agents derived from paramagnetic metal cations, e.g. gadolinium, or supermagnetic nanoparticles (ranging from 50 nm to greater than 1 μM in size) such as (supermagnetic iron oxide particles (SPIOs) and ultra-small supermagnetic iron oxide particles (USPIOs) 41. In general, non-phagocytic cells such as T cells have a low labelling efficiency and poor contrast agent incorporation 42–44; however, many methods have been used to improve this, including the use of transfection agents (e.g. poly-L-lysine, lipofectamine, SuperFect and protamine sulphate), electroporation, use of the HIV-TAT peptide, which enables cross-linked USPIOs to translocate across the cell membrane and into the nucleus 45–47, micrometer-sized paramagnetic iron-oxide particles and antibodies 48. The use of the aforementioned reagents is not without problems, including toxicity and immune modulation. Recently, however, Liu et al. developed MRI-fluorescent cellular imaging agents, nano-sized iron-oxide particles coated with polyethylene glycol and conjugated to fluorescent dyes (IOPC-NH2), which labelled more than 90% of both rat and human T cells (Jurkat T cells) in vitro without the requirement of transfection, electroporation, antibodies or the HIV-TAT peptide. No toxic effect, or loss of T cell function, was reported using these particles 49.
SPECT/PET
To date, and as mentioned earlier, only a few imaging agents have been approved clinically for tracking cells by SPECT and PET, including [111In] oxiquinolon ([111In]) 20 and [99m]Tc-hexamethylpropyleneamine oxime ([99mTc]-HMPAO) 20,30,50. [99mTc]-HMPAO is thought to enter the cell via passive diffusion across the plasma membrane, followed by the intracellular conversion of a lipophilic complex to a hydrophilic complex by reducing agents 51. Several disadvantages are associated with the use of direct labelling. These include loss of the radiolabel: the short half-life of the radiolabel, 6 h for [99mTc]-HMPAO, make longitudinal studies difficult using this method 40,51. The viability of the cells that are being imaged, live cells cannot be distinguished from dead cells and, lastly, phagocytic cells in the body, may engulf the administered labelled dead cells, leading to images that are difficult to interpret 21,52.
An alternative method to investigate the long-term migration of viable cells is to label cells indirectly. Indirect cell labelling avoids the previously described limitations of direct labeling, as the cells to be imaged are modified genetically to express a reporter gene, which is translated into either an enzyme or cell surface transporter 20,53. Within the cells, stable clonal expression of the reporter gene allows the labelled cell to be observed longitudinally over their entire lifetime (Fig. 1b). Additionally, the reporter genes are incorporated into progeny daughter cells following division 40. Expression of these reporter genes is limited to living cells, thus eliminating background signals from dead cells. In spite of these advantages, this labelling method may result in epigenetic gene silencing, due to DNA methylation 20, however, this can be prevented by treating cells in vitro in a dose- and time-dependent manner with a DNA methyltransferase inhibitor 54.
Delivery of reporter genes to cells can be accomplished by either viral or non-viral methods. Viral methods, e.g. lentiviral and retroviral vectors, integrate the gene of choice into the genome 20, while non-viral gene transfer includes the use of polymer, nanoparticles or chemical vectors 55. The herpes simplex virus thymidine kinase type 1 (HSV1-tk) and its variants are commonly used reporter genes which mediate the uptake of radiolabels, including [18F] 56, and is one of the most common reporter genes for PET imaging. This receptor functions by phosphorylating exogenously administered substrates, which are then retained in the cell due to a negative charge. Despite being used in patients, HSV1-tk and its derivatives are not expressed endogenously in humans, presenting a major immunological concern in that their use risks generating an immune response against cells and tissues transduced with this gene 57. However, the use of the human mitochondrial thymidine kinase 2 (hTK2) gene may help to resolve this issue.
Several other human reporter genes have been developed. The human noradrenaline transporter gene (hNET) encodes a transmembrane protein that mediates the transport of noradrenaline analogues across the cell membrane 56. Its exclusive localization to the central and peripheral sympathetic nervous systems and clinically approved radiolabelled probe make this gene an attractive reporter. [123I]-Metaiodobenzylguanidine ([123I]-MIBG) is a clinically approved radiolabel probe for imaging tissues expressing high levels of hNET. MIBG can be radiolabelled with 123I or 131I for SPECT and γ-camera imaging and with 124I for PET imaging 58.
The human sodium/iodide symporter (hNIS) is a transmembrane glycoprotein ion channel that allows the receptor-mediated uptake of a broad range of radiolabels, including radioiodine ([123I], [124I] and [131I]) and [99mTc]-pertechnetate ([99mTcO4−]), into cells. This receptor is expressed naturally in a few organs, including thyroid gland, stomach and salivary gland, and under physiological conditions, transports iodine into the cells for sodium exchange 56. It has the advantage that several radiotracers, including [99mTcO4], have been clinically approved for PET and SPECT imaging. Like hNET, hNIS is a human gene (with the same sequence as NIS expressed in the human thyroid), therefore it is thought that it will not elicit an immune response if applied clinically 24,56,59,60.
Whole-body imaging of T cell therapies in vivo
MRI
Both MRI and SPECT/PET-CT whole-body imaging of CD4+ and CD8+ murine and human T cells have been reported for cancer, autoimmune and transplant studies 61. For example, Srinivas et al. investigated the migration of antigen-specific T cells using Flourine-19 MRI contrast agents in a murine model of inflammation. These authors observed that over a period of 21 days antigen-specific T cells migrated to the draining lymph node 62. In a mouse ovalbumin (OVA)-specific melanoma model, this technique was used to track tumour-specific cytotoxic T cells (CTLs) labelled with highly derivatized cross-linked iron oxide nanoparticles in vivo 21. These authors observed not only the recruitment of cells to the tumour, but also reported that serial injections of tumour-specific CTLs resulted in recruitment to different tumour anatomical locations with each administration. Although this study reported that as few as three cells/voxel within the tumour could be detected, Smirnov et al. were able to image individual anionic maghemite nanoparticle-labelled tumour-specific T cells in a similar model by modifying the signal-to-noise ratio by reducing the noise of the detection magnetic coil 63.
Liu et al. tracked adoptively transferred IOPC-NH2 nanoparticle-labelled rat T cells in a rat and lung transplant model. These authors showed that localized infiltration of IOPC-NH2-labelled T cells into the allograft myocardium as well as the lungs within 24 h using MRI 49. MRI has also been used to track murine CD8+ T cells infiltrating the pancreas. This was achieved by co-culturing CD8+ T cells from non-obese diabetic (NOD) mice or by injecting NOD mice with supermagnetic nanoparticles coated with multiple copies of a high-affinity β cell peptide/major histocompatibility complex (MHC) ligand. This leads to labelling through endocytosis of these beads, of 90% of CD8+ T cells in/from diabetic NOD animals and 2% in/from prediabetic NOD mice. MRI in-vivo tracking of adoptively transferred labelled CD8+ T cells from diabetic animals was performed on the day of transfer and up to 16 days later. T cells were found in the pancreatic tissue and increased in numbers over time, reaching maximum numbers by day 9 and decreasing by day 16. Infiltration was confirmed histologically.
SPECT/PET-CT
SPECT/PET-CT technology is now used preclinically, employing both indirect and direct methods of radiolabelling to visualize the localization of T cells in vivo in animal models. CD4+ T cells labelled directly; using anti-CD4 antibodies coupled to [111In] before injection and SPECT imaging, were visualized in the colon of mice with colitis 64. Direct labelling of in-vitro-expanded influenza haemagglutinin (HA)-specific CD8+ T cells with [111In]-oxine was used to assess the efficacy of these cells as an adoptive therapy for cancer. Using SPECT-CT to track these antigen-specific cells 2, 24, 48 and 120 h post-injection into mice expressing HA+ and HA− tumours, Pittet et al. observed that HA-specific T cells localized centrally in the HA+ tumours as early as 2 h after transfer, but remained in the periphery of HA− tumours 52. They also correlated tumour growth with increased density of CTLs at the tumour site 52. Direct labelling has also been extended to human T cells. Tracking of [111In] directly labelled human T cells, engineered to express a chimeric antigen receptor recognizing a breast cancer antigen in a preclinical breast cancer model, Parente-Pereira et al. observed that human T cells migrate to the lungs after intravenous injections, then to the liver, spleen and lymph nodes, although not to tumour mass located within the peritoneal cavity, after 3 h. This was achieved only when the human T cells were administered at the tumour site via intraperitoneal injection 65.
However, for longitudinal analysis of viable cells in-vivo, indirect labelling is more preferable. This approach has been used to visualize human T cells in vivo in a cancer setting. Using Epstein–Barr virus (EBV)-specific human T cells transduced with the HSV1-tk gene, Koehne et al. 66 observed, following administration of [131] and [124] 2′-fluoro-2′-deoxy-1-b-D-arabinofuransyl-5-iodouracil (FIAU), via microPET scanning, that adoptive transferred HSV1-EBV-specific cells locate to a human EBV tumour xenograft in SCID mice. These cells accumulated 1, 8 and 15 days after adoptive transfer at the tumour site. Radiolabelled transduced cells retained cytolytic capacities following exposure to the isotope, suggesting that indirect labelling did not affect T cell function. Yaghoubi et al. expanded this study and published the first human study looking at reporter gene-based imaging of therapeutic T cells expressing the interleukin-13 zetakine gene (which encodes a receptor protein that targets T cells to tumour cells) and the PET imaging reporter gene herpes simplex virus 1 thymidine kinase (HSV1 tk) suicide gene 67. These authors detected genetically engineered CTLs non-invasively, using PET, in a patient with a grade IV glioblastoma with the PET reporter probe, [18F]-radiolabelled 9-[4-fluoro-3-(hydroxymethyl)butyl]guanine ([18F]-FHBG). A PET scan to detect engineered CTLs was performed 3 days after the patient had completed a 5-week course of CTL infusions. These authors showed [18F]-FHBG accumulation in the intact tumour of the CTL-infused patient, suggesting that the engineered CTLs were present at the tumour site; this was confirmed by biopsy.
In addition to the HSV-tk reporter, hNET expressing human EBV-specific T cells have been imaged in vivo using SPECT and PET in NOD/severe combined immunodeficiency (SCID) mice expressing an EBV tumour 56. As few as 104 hNET-expressing antigen-specific T cells could be imaged using either SPECT or PET following direct intratumoral injection, highlighting the sensitivity of these imaging modalities. A selective and progressive accumulation over 28 days of EBV-restricted CD8+ T cells within an EBV-expressing tumour was observed using SPECT and PET, with image intensity correlating closely with the number of cells accumulating at the antigen site 56.
Whole-body imaging of Tregs in vivo
Although whole-body imaging has been used to track CD8+ T cells in tumour and autoimmune disease models and CD4+ T cells in colitis, very little visualization of adoptively transferred Tregs in real time has been reported. In one study Feng et al. observed, using SPECT, that prior to transplantation directly labelled (99mTcO4−) adoptively transferred Treg cells are localized mainly in the spleen, liver and lungs, but following skin transplantation they were found primarily in the allograft and spleen 68. Unfortunately, because the cells were labelled directly, imaging was performed at only one time-point (5 h after transplantation) and not longitudinally. The fate of these cells was not examined over the lifespan of the transplanted tissue and no correlation with graft outcome was assessed. Recently, we also utilized SPECT-CT technology to image Treg cells in vivo in a non-invasive manner 69; however, unlike Feng et al. we used an indirect labelling method. Murine Treg lines were transduced retrovirally with a construct expressing the hNIS glycoprotein ion channel gene 69. We confirmed that NIS-expressing Treg cells maintained their phenotype and suppressive ability following [99mTcO4−] radiolabel exposure both in vitro and in vivo 69. This was an important observation, given that longitudinal studies will require several injections of [99mTcO4−] into the same recipient. Using SPECT-CT, we observed that adoptively transferred NIS-expressing Tregs localized within the lung and spleen of the recipient mouse 24 h after adoptive transfer and [99mTcO4−] injection in the absence of any graft (Fig. 2). We have now extended our earlier study to image antigen-specific Tregs expressing NIS within a murine skin transplant model, and our preliminary data highlight the promising nature of these imaging modalities for assessing the location of transferred cells and graft outcome (unpublished data). Although we have not discussed the use of bioluminescence for Tregs tracking, two recent papers have been published which the reader might find interesting 70,71.
Fig. 2.
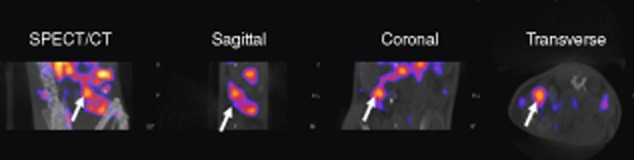
Sodium/iodide symporter NIS-expressing Tregs can be imaged in vivo by nano single photon emission-computed tomography-computed tomography (NanoSPECT-CT) through in-vivo uptake of [99mTcO4−]. C57BL/6 mice were adoptively transferred with 1 × 106 NIS-expressing Tregs via intravenous injection; 24 h later mice received 20 MBq of [99mTcO4−] before being imaged using NanoSPECT-CT under general anaesthesia for 1 h. The white arrow indicates the spleen in different views of the scan 69.
Summary and the future for transplantation studies
In conclusion, whole-body imaging techniques such as MRI and SPET/PET-CT have helped to answer important T cell therapy questions, including the localization of antigen-specific T cells following adoptive transfer in vivo. As all cell therapy needs to be tested for both efficacy and toxicity, preclinical in-vivo studies are important. The finding that, following adoptive transfer into immunodeficient mice, both unmodified and gene-modified human cells migrate to sites of antigen provides validation of these models for preclinical studies of adoptive T cell immunotherapy. This is good news for the transplant field, where clinical trials of human Treg therapy is under way for graft-versus-host disease and solid organ transplants 13. Now that long-term SPECT-CT imaging of modified murine Tregs in vivo is possible, the next step is to achieve this using human Tregs. We have shown recently that adoptive transfer of a human Treg line with direct allospecificity protects against immune-mediated skin allograft injury in a humanized mouse model of xenotransplantation in vivo 7. Extending this study to image human alloantigen-specific Tregs using SPECT-CT through the expression of human reporter genes 69, or using nano-sized iron-oxide particles coated with polyethylene glycol and conjugated to fluorescent dyes and MRI 72, in a humanized mouse model of skin/islet/vessel would help to bring Treg therapy one step closer to clinical use. Using these preclinical models, the effective targeting of cellular therapy to the transplant site as well as the location of multiple administered Tregs should also be addressed. This is important, as tumour antigen-specific T cells given intravenously did not reach a peritoneal grown tumour while T cells administered via the intraperitoneal route did 65. Whether regional administration is essential for Treg function within transplant tolerance has yet to be assessed. In addition, whether or not serially administered Tregs locate to different transplant locations would also be of interest. Because SPECT-CT imaging allows the possibility of tracking two different cell types simultaneously, future studies looking at the localization of defined Treg subsets or the interaction between Tregs and Teffs will be possible.
In conclusion, whole-body imaging will help significantly in assessing the efficacy of Treg-based immunotherapy, helping to map the location and accumulation of adoptively transferred cells within the body during the life of the transplanted tissue.
Acknowledgments
The research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. In addition, the authors acknowledge financial support from the Medical Research Council (MRC). J.M.L. and L.A.S. were funded by a BHF programme grant.
Disclosure
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Authors declare no financial or commercial conflict of interest.
References
- 1.Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108:426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safinia N, Sagoo P, Lechler R, Lombardi G. Adoptive regulatory T cell therapy: challenges in clinical transplantation. Curr Opin Organ Transplant. 2010;15:427–434. doi: 10.1097/MOT.0b013e32833bfadc. [DOI] [PubMed] [Google Scholar]
- 3.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7:1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 5.Tang Q, Lee K. Regulatory T-cell therapy for transplantation: how many cells do we need? Curr Opin Organ Transplant. 2012;17:349–354. doi: 10.1097/MOT.0b013e328355a992. [DOI] [PubMed] [Google Scholar]
- 6.Tsang JY, Tanriver Y, Jiang S, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3:83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6:577–583. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 9.Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells – ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18:103–110. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Waldmann H, Graca L, Cobbold S, Adams E, Tone M, Tone Y. Regulatory T cells and organ transplantation. Semin Immunol. 2004;16:119–126. doi: 10.1016/j.smim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Xia G, Shah M, Luo X. Prevention of allograft rejection by amplification of Foxp3(+)CD4(+)CD25(+) regulatory T cells. Transl Res. 2009;153:60–70. doi: 10.1016/j.trsl.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood KJ, Luo S, Akl A. Regulatory T cells: potential in organ transplantation. Transplantation. 2004;77:S6–8. doi: 10.1097/01.TP.0000106477.70852.29. [DOI] [PubMed] [Google Scholar]
- 13.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12:417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Q, Adams JY, Tooley AJ, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Q, Krummel MF. Imaging the function of regulatory T cells in vivo. Curr Opin Immunol. 2006;18:496–502. doi: 10.1016/j.coi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 17.de Kemp RA, Epstein FH, Catana C, Tsui BM, Ritman EL. Small-animal molecular imaging methods. J Nucl Med. 2010;51(Suppl. 1):18S–32S. doi: 10.2967/jnumed.109.068148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaa J, Rummeny EJ, Seemann MD. Whole-body imaging with PET/MRI. Eur J Med Res. 2004;9:309–312. [PubMed] [Google Scholar]
- 19.Pichler BJ, Kolb A, Nagele T, Schlemmer HP. PET/MRI: paving the way for the next generation of clinical multimodality imaging applications. J Nucl Med. 2010;51:333–336. doi: 10.2967/jnumed.109.061853. [DOI] [PubMed] [Google Scholar]
- 20.Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011;8:677–688. doi: 10.1038/nrclinonc.2011.141. [DOI] [PubMed] [Google Scholar]
- 21.Kircher MF, Allport JR, Graves EE, et al. In vivo high resolution three-dimensional imaging of antigen-specific cytotoxic T-lymphocyte trafficking to tumors. Cancer Res. 2003;63:6838–6846. [PubMed] [Google Scholar]
- 22.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 23.Kircher MF, Willmann JK. Molecular body imaging: MR imaging, CT, and US. Part I. Principles. Radiology. 2012;263:633–643. doi: 10.1148/radiol.12102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002;43:1188–1200. [PubMed] [Google Scholar]
- 25.Mariani G, Bruselli L, Kuwert T, et al. A review on the clinical uses of SPECT/CT. Eur J Nucl Med Mol Imaging. 2010;37:1959–1985. doi: 10.1007/s00259-010-1390-8. [DOI] [PubMed] [Google Scholar]
- 26.Marsee DK, Shen DH, MacDonald LR, et al. Imaging of metastatic pulmonary tumors following NIS gene transfer using single photon emission computed tomography. Cancer Gene Ther. 2004;11:121–127. doi: 10.1038/sj.cgt.7700661. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrandt IJ, Gambhir SS. Molecular imaging applications for immunology. Clin Immunol. 2004;111:210–224. doi: 10.1016/j.clim.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Khalil MM, Tremoleda JL, Bayomy TB, Gsell W. Molecular SPECT imaging: an overview. Int J Mol Imaging. 2011;2011:796025. doi: 10.1155/2011/796025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madsen MT. Recent advances in SPECT imaging. J Nucl Med. 2007;48:661–673. doi: 10.2967/jnumed.106.032680. [DOI] [PubMed] [Google Scholar]
- 30.Signore A, Mather SJ, Piaggio G, Malviya G, Dierckx RA. Molecular imaging of inflammation/infection: nuclear medicine and optical imaging agents and methods. Chem Rev. 2010;110:3112–3145. doi: 10.1021/cr900351r. [DOI] [PubMed] [Google Scholar]
- 31.Buck AK, Nekolla S, Ziegler S, et al. Spect/Ct. J Nucl Med. 2008;49:1305–1319. doi: 10.2967/jnumed.107.050195. [DOI] [PubMed] [Google Scholar]
- 32.Lecomte R. Novel detector technology for clinical PET. Eur J Nucl Med Mol Imaging. 2009;36(Suppl. 1):S69–85. doi: 10.1007/s00259-008-1054-0. [DOI] [PubMed] [Google Scholar]
- 33.Del Guerra A, Belcari N. State-of-the-art of PET, SPECT and CT for small animal imaging. Nucl Instrum Methods Phys Res A. 2007;583:119–124. [Google Scholar]
- 34.Hohn A, Zimmermann K, Schaub E, Hirzel W, Schubiger PA, Schibli R. Production and separation of ‘non-standard’ PET nuclides at a large cyclotron facility: the experiences at the Paul Scherrer Institute in Switzerland. Q J Nucl Med Mol Imaging. 2008;52:145–150. [PubMed] [Google Scholar]
- 35.Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29:193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 36.Popovtzer R, Agrawal A, Kotov NA, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8:4593–4596. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fushiki H, Kanoh-Azuma T, Katoh M, et al. Quantification of mouse pulmonary cancer models by microcomputed tomography imaging. Cancer Sci. 2009;100:1544–1549. doi: 10.1111/j.1349-7006.2009.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grassi R, Cavaliere C, Cozzolino S, et al. Small animal imaging facility: new perspectives for the radiologist. Radiol Med (Torino) 2009;114:152–167. doi: 10.1007/s11547-008-0352-8. [DOI] [PubMed] [Google Scholar]
- 39.Patel CM, Sahdev A, Reznek RH. CT, MRI and PET imaging in peritoneal malignancy. Cancer Imaging. 2011;11:123–139. doi: 10.1102/1470-7330.2011.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ottobrini L, Martelli C, Trabattoni DL, Clerici M, Lucignani G. In vivo imaging of immune cell trafficking in cancer. Eur J Nucl Med Mol Imaging. 2011;38:949–968. doi: 10.1007/s00259-010-1687-7. [DOI] [PubMed] [Google Scholar]
- 41.Tavare R, Sagoo P, Varama G, et al. Monitoring of in vivo function of superparamagnetic iron oxide labelled murine dendritic cells during anti-tumour vaccination. PloS ONE. 2011;6:e19662. doi: 10.1371/journal.pone.0019662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeh TC, Zhang W, Ildstad ST, Ho C. In vivo dynamic MRI tracking of rat T-cells labeled with superparamagnetic iron-oxide particles. Magn Reson Med. 1995;33:200–208. doi: 10.1002/mrm.1910330209. [DOI] [PubMed] [Google Scholar]
- 43.Dodd SJ, Williams M, Suhan JP, Williams DS, Koretsky AP, Ho C. Detection of single mammalian cells by high-resolution magnetic resonance imaging. Biophys J. 1999;76:103–109. doi: 10.1016/S0006-3495(99)77182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeh TC, Zhang W, Ildstad ST, Ho C. Intracellular labeling of T-cells with superparamagnetic contrast agents. Magn Reson Med. 1993;30:617–625. doi: 10.1002/mrm.1910300513. [DOI] [PubMed] [Google Scholar]
- 45.Morawski AM, Winter PM, Crowder KC, et al. Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with MRI. Magn Reson Med. 2004;51:480–486. doi: 10.1002/mrm.20010. [DOI] [PubMed] [Google Scholar]
- 46.Skajaa T, Cormode DP, Falk E, Mulder WJ, Fisher EA, Fayad ZA. High-density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:169–176. doi: 10.1161/ATVBAHA.108.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahrens ET, Flores R, Xu H, Morel PA. In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol. 2005;23:983–987. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro EM, Medford-Davis LN, Fahmy TM, Dunbar CE, Koretsky AP. Antibody-mediated cell labeling of peripheral T cells with micron-sized iron oxide particles (MPIOs) allows single cell detection by MRI. Contrast Media Mol Imaging. 2007;2:147–153. doi: 10.1002/cmmi.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L, Ye Q, Wu Y, et al. Tracking T-cells in vivo with a new nano-sized MRI contrast agent. Nanomedicine. 2012;8:1345–1354. doi: 10.1016/j.nano.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roca M, de Vries EF, Jamar F, Israel O, Signore A. Guidelines for the labelling of leucocytes with (111)In-oxine. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur J Nucl Med Mol Imaging. 2010;37:835–841. doi: 10.1007/s00259-010-1393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Vries EF, Roca M, Jamar F, Israel O, Signore A. Guidelines for the labelling of leucocytes with (99m)Tc-HMPAO. Inflammation/ Infection Taskgroup of the European Association of Nuclear Medicine. Eur J Nucl Med Mol Imaging. 2010;37:842–848. doi: 10.1007/s00259-010-1394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pittet MJ, Grimm J, Berger CR, et al. In vivo imaging of T cell delivery to tumors after adoptive transfer therapy. Proc Natl Acad Sci USA. 2007;104:12457–12461. doi: 10.1073/pnas.0704460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gambhir SS, Herschman HR, Cherry SR, et al. Imaging transgene expression with radionuclide imaging technologies. Neoplasia. 2000;2:118–138. doi: 10.1038/sj.neo.7900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gravina GL, Festuccia C, Marampon F, et al. Biological rationale for the use of DNA methyltransferase inhibitors as new strategy for modulation of tumor response to chemotherapy and radiation. Mol Cancer. 2010;9:305. doi: 10.1186/1476-4598-9-305. doi: 10.1186/1476-4598-9-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lam AP, Dean DA. Progress and prospects: nuclear import of nonviral vectors. Gene Ther. 2010;17:439–447. doi: 10.1038/gt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serganova I, Ponomarev V, Blasberg R. Human reporter genes: potential use in clinical studies. Nucl Med Biol. 2007;34:791–807. doi: 10.1016/j.nucmedbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Hooker JM. Modular strategies for PET imaging agents. Curr Opin Chem Biol. 2010;14:105–111. doi: 10.1016/j.cbpa.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doubrovin MM, Doubrovina ES, Zanzonico P, Sadelain M, Larson SM, O'Reilly RJ. In vivo imaging and quantitation of adoptively transferred human antigen-specific T cells transduced to express a human norepinephrine transporter gene. Cancer Res. 2007;67:11959–11969. doi: 10.1158/0008-5472.CAN-07-1250. [DOI] [PubMed] [Google Scholar]
- 59.Lee WW, Moon DH, Park SY, Jin J, Kim SJ, Lee H. Imaging of adenovirus-mediated expression of human sodium iodide symporter gene by 99mTcO4 scintigraphy in mice. Nucl Med Biol. 2004;31:31–40. doi: 10.1016/s0969-8051(03)00100-8. [DOI] [PubMed] [Google Scholar]
- 60.Barton KN, Stricker H, Brown SL, et al. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol Ther. 2008;16:1761–1769. doi: 10.1038/mt.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akins EJ, Dubey P. Noninvasive imaging of cell-mediated therapy for treatment of cancer. J Nucl Med. 2008;49(Suppl. 2):180S–195. doi: 10.2967/jnumed.107.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srinivas M, Turner MS, Janjic JM, Morel PA, Laidlaw DH, Ahrens ET. In vivo cytometry of antigen-specific T cells using 19F MRI. Magn Reson Med. 2009;62:747–753. doi: 10.1002/mrm.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smirnov P, Poirier-Quinot M, Wilhelm C, et al. In vivo single cell detection of tumor-infiltrating lymphocytes with a clinical 1·5 Tesla MRI system. Magn Reson Med. 2008;60:1292–1297. doi: 10.1002/mrm.21812. [DOI] [PubMed] [Google Scholar]
- 64.Kanwar B, Gao DW, Hwang AB, et al. In vivo imaging of mucosal CD4+ T cells using single photon emission computed tomography in a murine model of colitis. J Immunol Methods. 2008;329:21–30. doi: 10.1016/j.jim.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parente-Pereira AC, Burnet J, Ellison D, et al. Trafficking of CAR-engineered human T cells following regional or systemic adoptive transfer in SCID beige mice. J Clin Immunol. 2011;31:710–718. doi: 10.1007/s10875-011-9532-8. [DOI] [PubMed] [Google Scholar]
- 66.Koehne G, Doubrovin M, Doubrovina E, et al. Serial in vivo imaging of the targeted migration of human HSV-TK-transduced antigen-specific lymphocytes. Nat Biotechnol. 2003;21:405–413. doi: 10.1038/nbt805. [DOI] [PubMed] [Google Scholar]
- 67.Yaghoubi SS, Jensen MC, Satyamurthy N, et al. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng NH, Wu HF, Wu J, et al. Transplantation tolerance mediated by regulatory T cells in mice. Chin Med J. 2004;117:1184–1189. [PubMed] [Google Scholar]
- 69.Sharif-Paghaleh E, Sunassee K, Tavare R, et al. In vivo SPECT reporter gene imaging of regulatory T cells. PloS ONE. 2011;6:e25857. doi: 10.1371/journal.pone.0025857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen VH, Zeiser R, Dasilva DL, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 71.Suffner J, Hochweller K, Kuhnle MC, et al. Dendritic cells support homeostatic expansion of Foxp3+ regulatory T cells in Foxp3.LuciDTR mice. J Immunol. 2010;184:1810–1820. doi: 10.4049/jimmunol.0902420. [DOI] [PubMed] [Google Scholar]
- 72.Nadig SN, Wieckiewicz J, Wu DC, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16:809–813. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peterson TE, Shokouhi S. Advances in preclinical SPECT instrumentation. J Nucl Med. 2012;53:841–844. doi: 10.2967/jnumed.111.099853. [DOI] [PMC free article] [PubMed] [Google Scholar]


