Abstract
Immune-based therapies that prevent type 1 diabetes or preserve metabolic function remaining at diagnosis have become a major objective for funding agencies and international trial consortia, and receive backing from notable patient advocate groups. The development of immune-based therapeutic strategies in this arena requires a careful balancing of the risks of the therapy against the potential benefits, because many individuals are diagnosed or identified as being at increased risk of disease in early childhood, a period when manipulation of the developing immune system should be undertaken with caution. In addition, a therapy exists (daily insulin injection) that is life-saving in the acute stages of disease and can be used effectively over a lifetime as maintenance. Conversely, the disease is increasing in incidence; is peaking in ever-younger age groups; carries significant risk of increased morbidity and early mortality; and remains difficult to manage effectively in many settings. With these issues in mind, in this article we review progress towards immune-based strategies for this chronic autoimmune disease.
Keywords: autoimmunity, diabetes, immunotherapy
OTHER ARTICLES PUBLISHED IN THIS SERIES
Immunological biomarkers: Catalysts for translational advances in autoimmune diabetes. Clinical and Experimental Immunology 2013, 172: 178–85.
The perspective
With the exception of one or two early attempts at disease modulation, the field of immunotherapy for type 1 diabetes did not develop significant momentum until the 1980s, during which a series of studies were initiated that made use of a drug (cyclosporin) which had, by then, revolutionized immune suppression in the setting of organ transplantation. Some 20 years on from those early successes, in 2007 we reviewed the status of intervention and prevention trials for type 1 diabetes 1. The timing of our commentary was significant; the first major advance since cyclosporin had recently emerged, notably with the publication of two studies using monoclonal antibodies (mAbs) targeting CD3 and engineered to have limited Fc binding, both of which demonstrated clinically relevant efficacy with manageable toxicity 2,3. At that stage we discussed the fact that these drugs (subsequently emerging as teplizumab and otelixizumab) were lead agents at the head of a therapeutic pipeline of immunomodulators. These included several drugs that were emerging from the fields of transplantation immunology and as treatments for other autoimmune and inflammatory diseases, as well as disease-specific, antigen-based therapeutics. In a subsequent, related review paper we highlighted the potential and pitfalls of harnessing these agents into combinations 4, including a proposed ‘designer combo’ of anti-inflammatory + immune modulator + antigen. Moreover, to facilitate the pipeline, during the same period significant infrastructure was emerging in the form of clinical trial networks, within which clinical studies could be conducted to agreed and standardized designs and protocols. The exemplar of this approach is Type 1 Diabetes TrialNet (http://www.diabetestrialnet.org). There was even significant and demonstrable interest in this disease space being displayed by large pharmaceutical concerns. Consequently, as a result of this constellation of events, in 2007 the clinical trial horizon for type 1 diabetes was viewed with the expectation of success and progress. Some 6 years on, several key questions emerge. What has become of the pipeline and the combination approaches? Using the same format as the 2007 paper, we have updated the data tables with new or contemporary information on trials conducted or in progress at that time, and added information on new and ongoing studies. Information-gathering is based largely on the US National Institutes of Health-sponsored website ClinicalTrials.gov (http://www.clinicaltrials.gov) and the European equivalent (EU Clinical Trials Register; https://www.clinicaltrialsregister.eu/index.html), as well as our knowledge of the sector. Our analyses include studies conducted in the predisease setting, before diabetes onset, for both antigen-specific and non-antigen-specific approaches [primary (high genetic risk) and secondary (high risk identified by islet cell autoantibody positivity) prevention studies, Tables 1 and 2, respectively] and trials in which recruitment centres on subjects who have already developed disease (intervention studies; Tables 3 and 4, respectively). There is a further update on trials using combination approaches (Table 5). What have we learned from the clinical trials that have been conducted? Has our general understanding of the disease altered in any respect in the intervening period, such that we might review our therapeutic options?
Table 1.
Completed, ongoing and planned prevention trials in type 1 diabetes (T1D) using antigen-specific approaches
| Agent | Stage of development in 2007 | Details (including ClinicalTrials.gov Identifier) | References and links | |
|---|---|---|---|---|
| Parenteral insulin | Pilot, completed 1993 | Small, pilot study, suggestive of efficacy | 61 | |
| Parenteral insulin | Pilot, completed 1998 | Small, pilot study, suggestive of efficacy | 62 | |
| Parenteral insulin (DPT-1) | Large efficacy study, completed 2002 | No effect seen on disease progression | 63 and http://www.diabetestrialnet.org | |
| Oral insulin (DPT-1) | Large efficacy study, completed 2005 | No effect seen on disease progression; however, strong evidence from subanalysis of significant treatment effect on subjects with strong evidence of insulin autoimmunity. Repeat study planned (NCT00004984) | 15 and http://www.diabetestrialnet.org | |
| Intranasal insulin (INIT I) | Phase I, completed 2004 | No acceleration of loss of beta cell function in individuals at risk for T1D. Immune changes consistent with mucosal tolerance to insulin detected | 64 | |
| Progress to date | ||||
| Intranasal insulin (DIPP) | Ongoing | RPCT trial of daily intranasal short-acting human insulin (1 unit/kg) in at-risk (high-risk HLA, dual autoantibody-positive) (NCT00223613) | 65 and http://research.utu.fi/dipp | Study published in 2008 28; no effect on primary end-point of progression to Type 1 diabetes |
| Intranasal insulin (INIT II) | Phase II, to start 2006/07 | RPCT of intranasal insulin in at-risk (autoantibody positive) relatives (NCT00336674) | https://studies.thegeorgeinstitute.org/init/PMID | Multi-centre Phase II started 2008 in Australia and extended to Munich in 2011. Currently ongoing. Recent data show significant treatment-associated blunting of insulin antibody response 66 |
| Oral insulin | Efficacy study, starts 2006/07 | Repeat of oral arm of DPT-1. Randomized, double blind, placebo controlled trial of oral insulin in at-risk (autoantibody positive) relatives with insulin autoantibodies as inclusion criterion (NCT00419562) | http://www.diabetestrialnet.org | Started recruitment in 2007 with > 250 enrolled by end 2012. Reporting possible in 2014/2015 |
| Oral insulin | Pilot, starts 2006/07 | Pre-POINT study: dose finding in children with high genetic risk for type 1 diabetes | http://www.diabetes-point.org/ | Ongoing 67 |
| EudraCT number: 2005-001621-29 | ||||
| New studies: progress to date | ||||
| Injection (s.c.) GAD-Alum (DiAPREV-IT) | n.a. | Study designated as ongoing but not recruiting on ClinicalTrials.gov. (NCT01122446). Last subject enrolled January 2012. Planned as double-blind, randomized study to determine the safety and the effect of Diamyd® on the progression to type 1 diabetes in children with multiple islet cell autoantibodies | http://www.diamyd.com/docs/trialsDiabetes.aspx?section=trials | Ongoing |
RPCT: randomized placebo-controlled trial; DPT-1: diabetes prevention trial-1; POINT: Primary Oral Insulin Trial; DiAPREV-IT: Diabetes Prevention Immune-Tolerance. HLA: human leucocyte antigen; n.a.: not available; s.c.: subcutaneous;
Table 2.
Completed, ongoing and planned prevention trials in type 1 diabetes (T1D) using non-antigen-specific approaches
| Agent | Stage of development in 2007 | Details (including ClinicalTrials.gov Identifier) | References and links | |
|---|---|---|---|---|
| Ketotifen (histamine antagonist) | Pilot, completed 1994 | No effect | 68 | |
| CyA | Pilot, completed 1996 | Delay but not prevention in high-risk group | 69 | |
| Nicotinamide (Deutsche Nicotinamide Intervention Study; DENIS) | Efficacy study, completed 1998 | No effect | 70 | |
| Nicotinamide European Nicotinamide Diabetes Intervention Trial (ENDIT) | Efficacy study, completed 2004 | No effect | 71 | |
| Various combinations nicotinamide, CyA, insulin, Vit E | Pilots 1994–2005 | No additive effects | 72–74 | |
| BCG | Various pilots | No effect | 75–77 | |
| Gluten-free diet | Pilot, completed 2002 | No effect on autoantibodies or disease | 78 | |
| Progress to date | ||||
| Vitamin D3 | Phase I, ongoing | Pilot two-arm RCT to study feasibility of 2000 IU per day of vitamin D for the primary prevention of Type 1 Diabetes (main objective to compare 2000 IU with 400 IU (standard of care) in terms of safety and vitamin D-related measurements and feasibility) (NCT00141986) | Completed 79; demonstrated feasibility and safety | |
| Hydrolyzed cow's milk (TRIGR) | Phase I, ongoing | Primary Prevention Study for Type 1 Diabetes in Children at Risk. RCT with assignment to hydrolysed versus non-hydrolysed infant formula (NCT00179777, NCT00570102) | http://trigr.epi.usf.edu/.80. | Recruitment completed. Finnish pilot component (NCT00570102) shows reduced emergence of islet cell autoantibodies 81 |
| Docosahexaenoic acid (DHA); omega-3 fatty acids | Pilot, ongoing | NIP study – Nutritional Intervention to Prevent Diabetes. Pilot and feasibility study of DHA supplementation anti-inflammatory effects during late pregnancy or after birth in high-risk infants (NCT00333554) | http://www.diabetestrialnet.org | In follow-up 82,83 |
| New studies: progress to date | ||||
| Removal of Bovine Insulin From Cow's Milk (FINDIA) Pilot Study | Pilot | Finnish Dietary Intervention Trial for the Prevention of Type 1 Diabetes. Primary prevention pilot study of weaning high-risk genotype infants to a bovine insulin-free cow's milk formula (CMF) with islet cell autoantibodies as outcome (NCT01055080) | Completed. Bovine insulin-free CMF reduced the cumulative incidence of autoantibodies by age 3 years in children at genetic risk of type 1 diabetes mellitus 84 | |
| BABYDIET, gluten-free diet in infancy | Randomized open-label primary prevention study of effect of early or late first gluten exposure on islet cell autoantibody development at 3 years of age in FDRs at high genetic risk (NCT01115621) | No significant differences in autoantibody or diabetes development observed 85 | ||
| Anti-CD3 mAb (Teplizumab) | Phase II | RPCT to prevent or delay the onset of type 1 diabetes in FDRs with multiple islet cell autoantibodies and impaired glucose tolerance (NCT01030861) | http://www.diabetestrialnet.org | In recruitment |
| Abatacept (CTLA-4-Ig) | Phase II | RPCT in autoantibody-positive FDRs to prevent development of abnormal glucose tolerance | http://www.diabetestrialnet.org | Opening 2013 |
CyA: cyclosporin A; VitE: vitamin E; BCG: bacille Calmette–Guérin; TRIGR: Trial to Reduce Insulin Dependent Diabetes in the Genetically at Risk; FDR: first-degree relative; RPCT: randomized placebo-controlled trial; mAb: monoclonal antibody; CTLA-4-Ig: cytotoxic T lymphocyte antigen 4-immunoglobulin.
Table 3.
Completed, ongoing and planned intervention trials in type 1 diabetes (T1D) using antigen-specific approaches
| Agent | Stage of development in 2007 | Details (including ClinicalTrials.gov Identifier) | References and links | |
|---|---|---|---|---|
| Injection (s.c.) APL of insulin B chain peptide | Phase I, completed | NBI-6024 (NCT00873561) | 86 | |
| Injection (s.c.) DiaPep277 (hsp60 peptide) | Phase II, completed | Phase II in adults reports preservation of C-peptide at 12–18 months. | 87,88 | |
| Phase II in children reports no treatment effect | ||||
| Injection (s.c.) GAD-Alum | Phase I, completed in LADA | Phase II completed in children with T1D, report awaited (see below) | 89 http://www.diamyd.com | |
| Progress to date | ||||
| Injection (s.c.) insulin B chain in IFA | Phase I, completed | (NCT00057499) | http://www.immunetolerance.org/research/autoimmune/trials/orban1.html | Study drug safe; induces humoral and cellular anti-insulin responses; CD4 T cell have regulatory phenotype 31 |
| Injection (i.d.) PI (C19-A3) peptide | Phase Ia (ongoing) | Open-label safety and dosing study in long-standing Type 1 diabetes | http://www.dvdc.org | Completed; study drug safe; induces anti-peptide IL-10+ CD4 T cells 16 |
| Injection (i.m.) PI- DNA vaccine | Phase I planned | BHT-3021 – randomized double-blind placebo-controlled safety and pharmacodynamic study with open-label cross-over (NCT00453375) | http://www.bayhilltx.com/ | Completed; report pending |
| Injection (s.c.) GAD-Alum | Phase II | RPCT of GAD-alum in children and adolescents with recent onset Type 1 diabetes. (NCT00435981) | http://www.diamyd.com | Completed; fasting C-peptide declined from baseline significantly less in patients treated closest to diagnosis compared with the placebo group 29 |
| New studies: progress to date | ||||
| PI (C19-A3) peptide | Phase Ib | RPCT of safety and dose frequency study in new-onset Type 1 diabetes (NCT01536431) | http://www.dvdc.org | Ongoing |
| GAD-Alum | Phase II completed | RPCT of effects of GAD-alum on disease progression in new onset subjects (NCT00529399) | http://www.diabetestrialnet.org | No effect 30 |
| GAD-Alum | Phase III terminated | RPCT of effects of GAD-alum on disease progression in new onset subjects. EU (NCT00723411) and USA DIAPREVENT (NCT00751842) | http://www.diamyd.com | No effect at 15 months in EU study; both trials terminated 12 |
| DiaPep277 (hsp60 peptide) | Phase III | RPCT of effects of DiaPep277 in newly diagnosed type 1 diabetes (DIA-AID) (NCT00615264) and DIA-AID2 (NCT01103284) with open-label extension also recruiting (NCT01460251) | http://www.andromedabio.com | DIA-AID is completed and has reported preservation of C-peptide in response to a glucagon-stimulated test 90; DIA-AID2 (using MMTT) is ongoing |
APL: altered peptide ligand; IFA: incomplete Freund's adjuvant; PI: proinsulin; MMTT: mixed meal tolerance test; RPCT: randomized placebo-controlled trial; i.m.: intramuscular; i.d.: intradermal; s.c.: subcutaneous; LADA: latent autoimmune diabetes in adult.
Table 4.
Completed, ongoing and planned intervention trials in type 1 diabetes (T1D) using non-antigen-specific approaches
| Agent/study title | Stage of development | Details (including ClinicalTrials.gov Identifier) | References and links | |
|---|---|---|---|---|
| Cyclosporin | Various trials completed 1984–96 | Remission induced successfully in recent-onset patients, but therapy typically suspended due to unacceptable side effects | 91,92 | |
| Nicotinamide | Pilot | No effect | 93 | |
| Anti-thymocyte globulin plus prednisolone | Pilot | Reduced insulin requirements more than 100 days after therapy; complicated by severe, transient thrombocytopenia | 94 | |
| Bacille Calmette–Guérin (BCG) | Pilot | No effect | 95–97 | |
| Diazoxide | Phase II | No effect | 98,99 | |
| IFN-α | Phase I | Ingested; small pilot; possible effect (NCT00005665) | 100 | |
| Anti-CD3 mAb | Phases I/II, completed 2002 | Disease remission out to 18 months | 2 | |
| hOKT3g1(Ala-Ala); drug subsequently known as Teplizumab | ||||
| Anti-CD3 mAb | Phase II, completed 2005 | Reduced insulin requirement out to 18 months | 3 | |
| ChAglyCD3(TRX4); drug subsequently known as Otelixizumab | ||||
| Progress to date | ||||
| PRODIAB (oral protease) | Phase I | No effect on disease or serum cytokines | Data published 2009 101 | |
| Teplizumab (anti-CD3 mAb) treatment of recent-onset type 1 diabetes | Phase II | Participants had type 1 diabetes for 4–12 months before treatment with teplizumab or placebo (NCT00378508) | Decline in insulin secretion reduced but less of an effect than seen in new-onset period. CD8 biomarker in clinical responders 102 | |
| Protégé: Teplizumab in new onset type 1 diabetes | Phase III | RPCT in North America, Europe, Israel and India of standard, low and partial dose of anti-CD3 mAb at baseline and 26 weeks (NCT00385697) | Primary composite outcome (% patients on < 0·5 U/kg per day insulin HbA1C < 6·5% at 1 year not reached, but 5% of teplizumab groups were not taking insulin at 1 year, compared with no patients in the placebo group (P = 0·03) 13. The Protégé study is still in follow-up | |
| Teplizumab in recently diagnosed type 1 diabetes (AbATE) | Phase II | RPCT in new-onset type 1 diabetes; patients on active drug receive drug at study entry and at 12 months (NCT00129259) | http://www.abatetrial.org/ | Enrolment complete; results awaited |
| Otelixizumab (anti-CD3 mAb) for adults with newly diagnosed type 1 diabetes (DEFEND-1) | Phase III | Randomized placebo-controlled study in new-onset T1D; single dose; primary outcome C-peptide release after mixed meal (NCT00451321; NCT00678886). NCT01123083 (DEFEND-2, follow-up Phase III) and NCT01222078 (redosing study) terminated | http://us.gsk.com/html/media-news/pressreleases/2011/2011_pressrelease_10039.htm | Study results not yet posted; press release indicates failure to reach primary end-point |
| Completed extension of Phase II therapeutic trial 3 of Teplizumab | 4-year metabolic outcome study (NCT00627146) | Treatment can suppress the rise in insulin requirements of recent-onset type 1 diabetic patients over 48 months, depending on their age and initial residual beta cell function 9 | ||
| Anti-CD20 mAb (Rituximab) | Phase II | RPCT in newly diagnosed type 1 diabetes of rituximab on days 1, 8, 15 and 22 (NCT00279305) | http://www.diabetestrialnet.org | The primary outcome at 1 year (residual C-peptide after mixed-meal) was significantly higher in the rituximab than in the placebo group; the rituximab group also had significantly lower levels of HbA1c and required less insulin 8 |
| Autologous umbilical cord blood cells | Phase I | Patients with type 1 diabetes received a single intravenous infusion of autologous umbilical cord blood cells (NCT00305344) | There were no infusion-related adverse events and no evidence of C-peptide preservation at 2 years. An increase in Tregs and naive Tregs were observed but the study lacked a control group 103,104 | |
| Polyclonal anti-T-lymphocyte globulin (ATG) in type 1 diabetes | Polyclonal rabbit ATG in patients with type 1 diabetes within 4 weeks of diagnosis as bolus of 9 mg/kg followed by 3 consecutive doses of 3 mg/kg (NCT00190502) | Interim analysis reported on 11 subjects in 2004; at 12 months significant reduction in insulin dose and improved stimulated C-peptide levels in the ATG group 105 | ||
| Study of thymoglobulin to arrest newly diagnosed type 1 diabetes (START) | Phase II | Study completed 2012 (NCT00515099) | http://www.immunetolerance.org/studies/study-thymoglobulin-arrest-type-1-diabetes-start | Full study report awaited; reported abstract suggests no effect on T1D progression 22. |
| Campath 1H® (anti-CD52 antibody; Alemtuzumab) | Phases I/II | Study withdrawn (NCT00214214) | ||
| Autologous dendritic cell (DC) therapy for type 1 diabetes suppression | Phase I | Autologous DCs manipulated using anti-sense oligonucleotides for CD40, CD80 and CD86 and readministered (NCT00445913) | Well tolerated, significant increase in the frequency of B220 + CD11c-B cells observed. Study reported 2011 106 | |
| New studies: progress to date | ||||
| Anakinra (recombinant IL-1 receptor antagonist) in newly diagnosed type 1 diabetes | Phase I/II | Exploratory, open-label study of daily anakinra administered for 28 days to 15 children diagnosed < 1 week; no effect on blood gene expression profile in vivo; reduced insulin requirements at 1 and 4 months; no effect on C-peptide (NCT00645840) | 107 | |
| Anti-interleukin-1 in diabetes action (AIDA) | Phase II | RPCT of anakinra in new onset-type 1 diabetes (NCT00711503) | Study report awaited; reported abstracts suggest no effect on disease progression 108 | |
| Canakinumab (anti IL-1β) in Newly Diagnosed diabetes | Phase II | RPCT in new-onset type 1 diabetes (NCT00947427) | Study report awaited; reported abstracts suggest no effect on disease progression 109 | |
| Beta cell rescue in new onset type 1 diabetes with Efalizumab (anti-CD11a; BRiTE) | Phase II | Drug withdrawn due to safety concerns (NCT00737763) | Terminated | |
| Alpha-1 antitrypsin (AAT, Aralast) in recent-onset type 1 diabetes | Phase II | Open-label; recent-onset patients (< 5 years with residual C-peptide in recruitment) (NCT01319331) | In recruitment | |
| Alpha-1 antitrypsin in new onset T1D (Glassia®) | Phases I/II | Open-label, < 6 months of T1D dose-ranging (NCT01304537) with extension (NCT01661192) | http://www.kamada.com/press_item.php?ID=29 | Study report awaited |
| Intravenous CTLA-4-lg in recent onset type 1 diabetes mellitus | Phase II | RPCT shows beneficial effect on C-peptide preservation in new-onset T1D (NCT00505375) | 14 | |
| Etanercept (anti-TNF-α) in new-onset type 1 diabetes | Phases I/II | RPCT in 18 new-onset T1D patients age 3–18 years (NCT00730392) | Significant retention of C-peptide and lower HbA1C and insulin dose at week 24 in treated group; drug well tolerated 24; extension studies suspended after FDA warning on safety (increased risk of lymphoma) in children | |
| Calcitriol in new-onset type 1 diabetes | RPCT (NCT00960635) | No beneficial effect 110 | ||
| Dose–effect relationship of low-dose IL-2 in type 1 diabetes (DF-IL2) | Phases I/II | RPCT in recent-onset T1D (< 2 years) studying effect of repeated administration of low-dose IL-2 on the kinetics of Tregs (NCT01353833) | Study report awaited | |
| Autologous haematopoietic stem cell transplantation (AHSCT) for early-onset type 1 diabetes | Phases I/II | Open-label; patients with T1D received AHSCT after pretreatment with cyclophosphamide and ATG (NCT00807651) | Complete remission, defined as insulin independence, was observed in 15/28 patients and especially in non-DKA patients 111 | |
| Diabetes intervention with Atorvastatin (DIATOR) | Phase II | Multi-centre RPCT in 89 patients with newly diagnosed T1D of 80 mg/day atorvastatin for 18 months (NCT00974740) | Fasting and stimulated C-peptide levels were not significantly different between groups at 18 months 112,113 | |
| Interferon-α for diabetes mellitus type 1 | Phase II | RPCT in new-onset T1D (< 6 weeks) who received ingested hrIFN-α at 5000 or 30 000 units or placebo once daily for 1 year (NCT00024518) | Treatment was safe; low-dose group maintained more β cell function 1 year after study enrolment than placebo group 114 | |
| Prevention of Diabetes progression trial (PDPT) | Open-label, single-dose anti-CD25 mAb in new-onset (< 3 months) T1D (NCT00198146) | Completed; data not reported | ||
| BCG administration to alter T-lymphocyte profiles in type 1 diabetics condition | Phase II | RPCT in adults with long-term T1D (mean 15 years) (NCT00607230) | C-peptide levels rose transiently in two BCG-treated subjects 115 | |
| New studies (planned and recruiting) | ||||
| Research trial of Aralast in new-onset diabetes (RETAIN-I) | Phases I/II | Open-label, safety and dose level study in adults and children with new onset T1D (NCT01183468) | http://www.immunetolerance.org/studies/research-trial-aralast-new-onset-diabetes-retain | Recruited, in follow-up |
| RETAIN-II | Planned RPCT of aralast in new onset T1D (NCT01183455) | In planning | ||
| Rituximab in early onset type 1 diabetes | Phase II | Open-label, Nanjing Medical University, China (NCT01280682) | In recruitment | |
| Vitamin D in the honeymoon period in children and adolescents with type 1 diabetes | Phase II | RPCT examining effect of vitamin D supplementation (3000 IU cholecalciferol daily for 9 months) on rate of partial clinical remission [assessed by insulin dose adjusted HbA1c (IDAA1c)] (NCT01724190) | In planning | |
| Autologous mesenchymal stem cells in new onset T1D | Phases I/II | Open-label safety and efficacy (NCT01068951) | In recruitment | |
| Atorvastatin in New Onset Type 1 Diabetes | Phase II | RPCT (NCT00529191) | Ongoing, not recruiting | |
| Alefacept (soluble leucocyte function-associated antigen (LFA)-3-Ig fusion) in new-onset T1D (T1DAL) | Phase II | RPCT (NCT00965458) | Ongoing, not recruiting | |
| Neulasta (GCSF) in type 1 diabetes | Phases I/II | RPCT (NCT00662519) | Ongoing, not recruiting | |
| Rilonacept (IL-1 trap) in diabetes mellitus type 1 (RID-A) | Phase I | Open-label (NCT00962026) | In recruitment | |
| Immune therapy using CD4+CD127lo/–CD25+ polyclonal Tregs | Phase I | Open-label, dose escalation of Treg infusion (NCT01210664) | In recruitment | |
| Autologous umbilical cord blood transfusion: pilot study | Phases I/II | EudraCT number: 2007-007694-23 | In recruitment |
RPCT: randomized placebo-controlled trial; DKA: diabetic ketoacidosis; FDA: US Food and Drug Administration; AHSCT: autologous haematopoietic stem cell transplantation; ATG: anti-thymocyte globulin; GCSF: granulocyte colony stimulating factor; IFN: interferon; TNF: tumour necrosis factor; IL: interleukin; mAb: monoclonal antibody; Treg: regulatory T cell.
Table 5.
Completed, ongoing and planned prevention/intervention trials in type 1 diabetes (T1D) using combination approaches
| Agent | Stage of development | Details (including ClinicalTrials.gov Identifier) | References and links | |
|---|---|---|---|---|
| Low-dose cyclosporin and methotrexate | Phases I/II | Open-label; cyclosporin 7·5 mg/kg/day for 6 weeks and then 4 mg/kg/day for 1 year and methotrexate 5 mg/kg/day for 1 year (NCT00905073) | Study completed; no report | |
| Exenatide and Gastrin | Preclinical | http://www.transitiontherapeutics.com | No further information | |
| Progress to date | ||||
| Mycophenolate mofetil (MMF) and anti-CD25 mAb (Daclizumab; DZB) | Phase II | Multi-centre RPCT, new-onset T1D (n = 1267 < 3 months) randomized to either MMF alone, MMF plus DZB or placebo. Mean C-peptide AUC at 2 years was unaffected by MMF alone or MMF plus DZB versus placebo (NCT00100178) | Study reported 116 | |
| Anti-CD3 and intranasal insulin | Phase II | Planned, recent-onset T1D | No further progress; drug access difficulties | |
| Anti-CD3 and Exenatide | Phase II | Various trials planned in recent onset T1D and in at-risk individuals (prevention) | No further progress; drug access difficulties | |
| Proleukin and Rapamune in type 1 diabetes | Phase I | Open-label; 9 recent-onset (6–48 months) T1D subjects treated with 2–4 mg/day rapamycin orally for 3 months and 4·5 × 106 IU IL-2 s.c. three times per week for 1 month showed transient treatment-induced β cell dysfunction (NCT00525889 | Study reported 20 | |
| New studies | ||||
| Autologous stem cell transplantation for early-onset type 1 diabetes mellitus | Phases I/II | Open-label study of high-dose immunosuppression followed by autologous non-myeloablative haematopoietic stem cell transplantation (AHSCT) in newly diagnosed T1D (< 6 weeks; n = 15 patients aged 14–31 years) after conditioning with cyclophosphamide and rabbit ATG. During a 7–36-month follow-up (mean 18·8), 14 patients became insulin-free. At 6 months mean C-peptide response was significantly greater than the pretreatment values. Extension and follow-up shows a majority of patients became insulin-free and C-peptide levels increased significantly at 24 months. Two patients developed bilateral nosocomial pneumonia, 3 patients developed late endocrine dysfunction, and 9 patients developed oligospermia (NCT00315133) | 117,–120 | |
| Haematopoietic stem cell transplantation in T1D | Phase II | Open label; AHSCT in new-onset T1D (NCT01121029) | Study report awaited | |
| Cord blood plus vitamin D and omega 3 s in T1D condition | Phase II | Open label randomized 2:1 (active : placebo); active is single intravenous infusion of autologous cord blood cells followed by 1 year of daily vitamin D and omega 3 fatty acid supplementation in 15 subjects; placebo untreated ( NCT00873925) | Study report awaited | |
| Efficacy and safety study of autologous haematopoietic stem cell transplantation to treat new onset type 1 diabetes | Phase I | Open label, non-myeloablative stem cell transplantation after conditioning with cyclophosphamide and rabbit ATG, Nanjing Medical University, China (NCT01341899) | In recruitment | |
| High-dose immunosuppression and AHSCT in early onset T1D | Phases I/II | Open label, < 5 months from diagnosis; protocol as for NCT00315133 to include rituximab conditioning and quality of life questionnaire (NCT01285934) | In recruitment | |
| Reversing type 1 diabetes after it is established | Phases I/II | Single-blind (participant) pilot safety and feasibility study of ATG and GCSF (Neulasta®) in established T1D (4–24 months) (NCT01106157) | Ongoing |
RCPT: randomized placebo-controlled trial; autologous haematopoietic stem cell transplantation; ATG: anti-thymocyte globulin; s.c.: subcutaneous; mAb: monoclonal antibody.
Trial design for intervention studies
With the premise that type 1 diabetes is an immune-mediated disorder, most efforts to intervene in disease pathogenesis involve immune-based therapy. Without exception, primary study end-points tend to focus on preservation of β cell function, as measured by stimulated C-peptide production after a standardized food challenge (oral glucose tolerance test, OGTT) or glucagon injection. This is a justifiable criterion that is accepted by regulatory agencies such as the US Food and Drug Administration and European Medicines Agency. Several clinical trials assessing immune interventions (teplizumab, otelixizumab, rituximab, abatacept; see Table 4) show a temporary delay in the loss of β cell function as defined by OGTT, while injection of a heat shock protein-derived peptide (DiaPep277; Table 2) only showed a beneficial effect based on glucagon-stimulated measurement of β cell function, but not on OGTT; the reasons for this intriguing finding are not yet known. Improved glycaemic control, as measured by reduction in glycated haemoglobin levels (HbA1c), should not be considered a useful end-point going forward, even though it was used (albeit unsuccessfully) in the Phase III teplizumab (anti-CD3) trial. Patients enrolled into intervention trials should be treated to prespecified HbA1c target levels using standard clinical care, and thus any differences between treatment and placebo groups raise concerns about study design and conduct. In general, therefore, changes in immune correlates of the autoimmune process 5 have not been selected as study end-points, even though the disease process is immune-mediated. Given that defining changes in disease progression by C-peptide measurement imposes long-term study follow-up, and new insights which suggest that β cell function does not necessarily equate with β cell mass 6, there is a strong argument to be made that the field should shift towards alternative, immune-based end-points that can deliver more rapidly and potentially in smaller-sized treatment groups, at least at a ‘proof-of-concept’ stage 5,7.
As the unmet medical needs and potential benefits of successful immunotherapy are greatest in children, it is evident that the inclusion of children in clinical trials is highly desirable, provided that there is adequate risk assessment. Indeed, the inclusion of younger patients in the rituximab trial secured short-term efficacy that would have remained unnoticed if subjects only beyond 18 years of age had been recruited 8. Effects of otelixizumab in older patients became apparent only upon extended follow-up 9. In addition to age, the timing of inclusion and window of opportunity for success in relation to disease progression remain poorly defined. Depending on the type of intervention, it may prove difficult to treat during the medical emergency of newly manifested disease, although early enrolment (typically 3 months after diagnosis) has become the common inclusion criterion for intervention trials. As β cells survive up to decades after diagnosis, together with insulitic lesions 10,11, there is in reality no reason to exclude patients beyond 3–6 months after diagnosis who have measurable C-peptide, other than the slower slope in decline of stimulated β cell function and associated reduced statistical power to define treatment-induced changes. This, again, argues for alternative (surrogate) end-points of therapeutic efficacy 5. Intervention studies beyond the first year after onset would also avoid the confounding effect of the natural remission and temporarily reduced insulin needs (known as the ‘honeymoon’) that often occurs shortly after initiation of insulin replacement therapy. In terms of staging of patients during stratification in trial enrolment, we may need to take lessons from new insights emerging from studies on disease tissue (via the Network for Pancreatic Organ Donors with Diabetes; nPOD 10) and Phase III clinical trials failing to reach end-points 12,13. Both of these imply that type 1 diabetes may be a very heterogeneous disease, manifesting differently in different patient groups and geographical locations. An intriguing example is that of abatacept, which appeared to worsen clinical outcome in African American subjects 14. In addition, the average age at disease onset of patients enrolled on the Indian subcontinent into the teplizumab Phase III study was 44 years 13, an age of disease onset that would usually be considered at the very upper limit. With the exception of oral insulin 15 and proinsulin peptide immunotherapy 16, immunological parameters have not generally been used in selection or randomization of patients in clinical trials. Lessons from the islet transplantation setting, in which baseline immune correlates determine clinical outcome 17–19, may be of use here and it is conceivable that incorporating immune correlates into trial design may improve the chance of detecting therapeutic efficacy and indicate subpopulations of patients with particular benefit, lack of efficacy or even adverse responses to certain immune intervention strategies 7. While common beliefs advocate a combination of drugs for intervention (Table 5), it is important to scrutinize potential adverse interference, as may have played a role in the recent trial combining low-dose interleukin (IL)-2 and rapamycin, in which each of the separate constituents could have yielded clinical benefit 20. Preclinical studies should be used carefully to identify those showing the desired synergy or any concerns in relation to the single components of combinations (i.e. accelerated disease, see below).
Systemic immune modulators
Biological agents have proved to be immensely valuable in the treatment of autoimmune disease, and type 1 diabetes is no exception to this therapeutic track. Biologics targeting lymphocytes or co-stimulation events generally invoke immune suppression rather than modulation. This was perhaps most evident in case of the rituximab intervention study, in which patients were vaccinated under the treatment umbrella in a rare attempt to understand the mechanism of action of anti-CD20 immunotherapy. Indeed, rituximab blunted the induction of immune responses against a neoantigen, whereas after revaccination 1 year later (3 months after cessation of rituximab therapy) vigorous responses to the same neoantigen were established that did not differ from placebo-treated patients 21. This observation underscores the fact that anti-CD20 therapy suppression possibly inhibits new immune responses but, as we know from the intervention study, does not instil or restore tolerance 8. The term ‘biologic cyclosporin’ has been coined in this context. The recently reported failure of anti-thymocyte globulin to preserve C-peptide in a Phase II setting is a further wake-up call in this respect, emphasizing at the same time the complexity of human cellular autoimmune responsiveness and the bluntness of some of the tools at our disposal 22.
While biologics may prevent priming or spreading of the immune response, for most there is little evidence that they affect existing adaptive immunity. Indeed, abatacept [cytotoxic T lymphocyte antigen 4-immunoglobulin (CTLA4-Ig)] is effective at preventing priming alloreactivity, but appears to have little impact in reversing primed islet autoimmunity 14. The reduced requirement for co-stimulation of autoreactive memory T cells 23 probably explains the limited clinical efficacy observed in the established disease process of chronic islet autoimmunity 14. None the less, dimming immune reactivity with abatacept proved successful in delaying the progressive loss of stimulated C-peptide capacity in some patients in this study. The fact that the effect waned, even during continued treatment, again hints at disease heterogeneity, for example in the degree to which autoreactive T cell responses are co-stimulation-dependent.
With the exception of a small study using tumour necrosis factor (TNF)-α blockade 24, which showed potential clinical efficacy (which cannot currently be explored further due to safety concerns; see Table 4), interference in the activity of effector cytokines has not yet delivered in type 1 diabetes, as underlined by two recent failed studies of IL-1 blockade 25 (Table 4). This is in striking contrast with rheumatoid arthritis (e.g. benefits of blockade of TNF-α, IL-6 receptor, IL-1) and psoriasis (TNF-α, IL-23 and IL-17 pathways, IL-1). A central role for these cytokines in the immunopathogenesis may therefore be worthy of greater scrutiny and reconsideration, in spite of their clear role in some preclinical models of autoimmune diabetes and other autoimmune diseases. It remains plausible, of course, that cytokine inhibition will be highly effective and synergistic in combinations with other immune intervention strategies, as preclinical models imply 26.
Antigen-specific approaches
Viewed by many as the best chance to restore immunological self-tolerance in autoimmune diseases, antigen-specific immunotherapy (ASI) faces many challenges in its development and deployment, which is perhaps reflected in the more limited pipelines and activity in this arena (Tables 1 and 3; Fig. 1). Many of the relevant issues have been discussed elsewhere 27, but to put this modality into perspective several of the notable challenges are highlighted in Table 6. Perhaps in reflection of these, there has been limited new activity in this arena since 2007. Notably, a large primary prevention study of daily intranasal insulin reported failure to halt progression to type 1 diabetes 28, while the repeat oral insulin study conducted by Type 1 Diabetes TrialNet will not be able to report results until 2015, at the earliest. In the intervention setting, follow-up studies of alum-conjugated glutamic acid decarboxylase immunization (GAD-Alum), after initial successful pilot data 29, have been disappointing at Phase II 30 and Phase III stages 12; a secondary prevention study is in progress (Table 1).
Fig. 1.
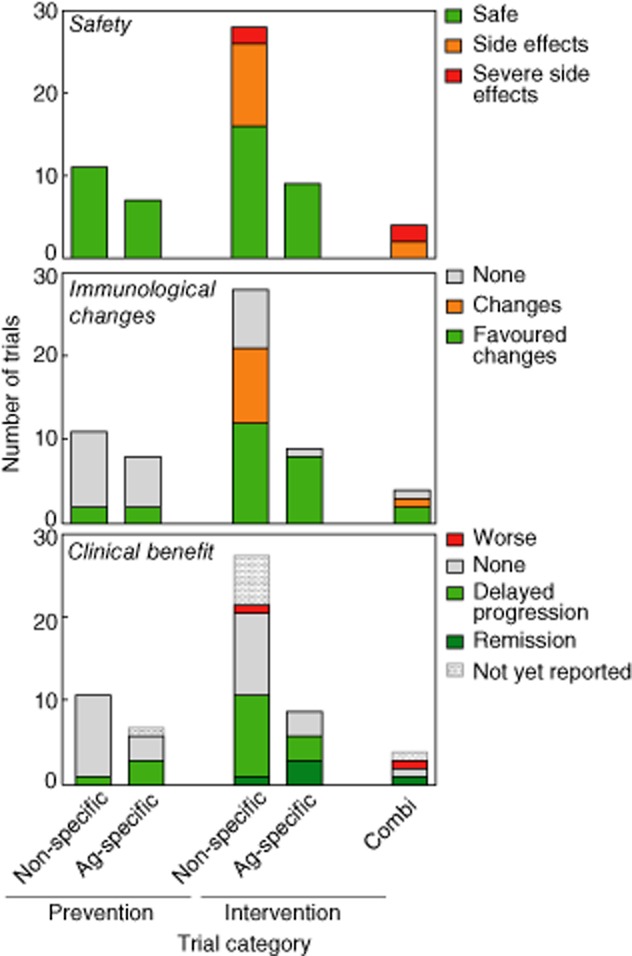
Bar charts show the number of clinical trials in different categories according to the natural history of type 1 diabetes (prevention, intervention) and treatment modality (none- or antigen-specific).
Table 6.
Challenges faced in the development of antigen-specific immunotherapy (ASI) for type 1 diabetes
| Challenge | Discussion of issues |
|---|---|
| Setting for clinical trials | Traditionally, new therapies are trialled in the intervention setting (i.e. at disease onset). Disease reversal using antigen alone at this stage will most probably be difficult. Prevention studies are long duration and expensive; but without hints of efficacy as an intervention, will prevention studies be undertaken? |
| Dose | Both high- and low-dose immunological tolerance has been described, probably equating to predominantly deletional and regulatory mechanisms; which is better, and whether both effects could be harnessed, is not known, however |
| Regime | Frequent (daily) dosing has been the norm until now (e.g. for intranasal and oral insulin), but again this may favour deletion over regulation 27 |
| Adjuvants and enhancing combinations | A poorly explored area in general, despite encouraging data in preclinical models (e.g. anti-CD3 plus antigen; see Table 5) |
| Agent | It has yet to be determined whether whole antigens or fragments are superior; similarly, whether protein or DNA-based delivery is better; free peptide or complexed to peptide–human leucocyte antigen multimers or nanoparticles |
| Route of administration | Parenteral or oral/nasal routes predominate, but the relative advantages of either have not been explored head-to-head |
| Staging and stratification | Oral insulin appears effective in the subgroup of patients with high titres of insulin autoantibodies; is this a general principle for ASI? |
| Preclinical models | As a generalization, ASI works well if given early enough in disease models; but trialling the human antigens in humanized models is an under-developed area |
| Role of industry and biotech | Antigens face the dual challenges of being difficult to develop with robust intellectual property and having a clear route to market and have therefore been less favoured for commercial development than biologics and other immune modulators |
New modalities of ASI have emerged, however, including peptide and DNA-based deliveries, in some cases associated with positive biomarker data 16,31 and in the case of Diapep277, with evidence of clinical effectiveness (see discussion above and Table 3). Full reporting of the proinsulin-DNA vaccine and Diapep277 Phase III studies are eagerly awaited. In terms of development, however, it is notable that, for example, in the intervention setting, there has been no attempt as yet to combine antigen with any other treatment modality (Fig. 2), despite encouraging preclinical data 32,33.
Fig. 2.

Venn diagram shows the number of types of therapies used in different modalities (mono- and combination; antigen and non-antigen-specific) and the overlap between them.
The role of preclinical models in trial design
With the somewhat high number of failed clinical trials in type 1 diabetes in the past few years, it has become increasingly tempting to attribute some of the blame to animal models. One often hears remarks such as ‘animal models have misled us’ and the near-ubiquitous comment ‘mice are not humans’. Clearly, we are all aware that diabetes in various rodent models may only model in part how type 1 diabetes develops in humans. However, we would like to argue here that animal models have a key place in the clinical translation for therapeutic approaches in autoimmune disease overall, as long as they are used correctly, not over-interpreted and analysed carefully. It should be helpful, therefore, to first take a closer look at the extent to which animal studies diverge from human trials.
Several ASI trials in man have reported negative (or positive substudy) results (GAD-Alum, oral insulin and intravenous insulin); have shown marginal effects (BayHill DNA vaccine, Diapep277); or were not powered to demonstrate efficacy, yet have not shown any strong clinical effects in established diabetes (adjuvanted insulin B-chain peptide, proinsulin peptide). Each trial is distinctly different and it is therefore worthwhile to look at the facts one by one.
Subcutaneous administration of GAD-Alum was developed on the basis of earlier studies by several teams, which had all used GAD peptides to prevent diabetes in the non-obese diabetic (NOD) mouse spontaneous disease model 34,35. Others have since prevented type 1 diabetes successfully with oral GAD and in some cases GAD DNA vaccines also using other diabetes models 36. A crucial difference between the human trial and all the preclinical studies is that immunization with GAD always worked to prevent diabetes, yet never after diabetes onset. As discussed, this is a universal truth for all ASI, which has not shown disease-reverting effects in animal models after clinical signs of diabetes have developed. Thus, it would be unreasonable to expect a stronger effect (in other words, after onset of diabetes) in humans. Secondly, no preclinical study ever tested the clinical GAD-Alum preparation, and no efficacy was noted in our recent studies in NOD and B6 diabetes models (Pagni, Boettler and von Herrath, unpublished). Again, it is probably unreasonable to expect an antigenic formulation to work in humans when it does not even prevent diabetes in otherwise permissive animal models. Several other theories have been proposed to account for the failure of GAD-Alum in humans, including the lack of GAD expression in β cells; this is a controversial area, as many studies have demonstrated expression of GAD-65 and 67 proteins in murine and human β cells 36. Lastly, one could ask whether the dose of GAD-Alum was sufficient – as most patients mounted a clearly detectable immune response, this appears less likely. However, alum might have been a suboptimal adjuvant for an ASI, as the resulting mixed but T helper type 2 (Th2)-dominated cytokine response of induced GAD-reactive T cells (Arif, Roep and Peakman, unpublished) did not result in protective cell populations. In the absence of a functional mouse model of GAD-Alum preventing diabetes, it will be difficult at this point to clarify these issues.
The question of the antigenic dose might have more bearing on the issue of efficacy with oral insulin 15. As predicted from animal models 37, prophylactic oral insulin given at a daily dose of 7·5 mg had a very marginal effect in preventing diabetes in individuals at high risk (exhibiting multiple autoantibodies 38–41), but not in any other patient groups. However, as has been evident from multiple studies in different mouse models, oral insulin dosages have to be comparatively much higher to induce optimal disease preventive effects, which are seen at a dose of 1 mg given twice per week 42. This dose would equate to approximately 1 g of oral insulin twice per week in humans. In addition, it is likely to be necessary to provide the drug in enteric-coated capsules, without which > 99·99% of the insulin is lost through digestion in the stomach and only minimal amounts of intact antigen or some peptides will reach the lower gut and the Peyer's patches, the location at which oral insulin has been shown to induce its desired immune-regulatory response. Therefore, more precise dose calculations should have probably preceded the oral insulin trial and its current follow-up study.
A further human/mouse mismatch relates to the overall management of expectations when devising trials for ASI. In rodent studies most, if not all, ASI is effective only for early and, at best, late prevention of disease, but never after onset of hyperglycaemia. Thus, we should not expect antigens to reverse human diabetes or even preserve C-peptide after onset (at least with effects detectable in reasonably sized studies); and this has indeed been the case. Rather, these types of studies are potentially invaluable for optimizing dose and administration schemes and biomarker development, if immunological parameters are used as an outcome. Here there will need to be ‘reverse translation’, because immune parameters are analysed rarely on peripheral blood and correlated with successful prevention (or lack thereof) of diabetes on an individual basis in murine studies.
Surprisingly, two recent trials (Andromeda's heat shock protein peptide p277 and Bayhill's proinsulin expressing DNA vaccine BHT3021; Table 4) reported positive outcomes, even in the more stringent recent-onset diabetes setting, by preserving C-peptide at certain dosing regimens. These observations exceeded expectations based on animal studies, where both strategies were only effective in preventing diabetes but not in reversing hyperglycaemia. It will be important to explore whether, in either trial, immunological outcomes were associated with better preservation of C-peptide and thus could perhaps pave the way in future for using such immunological end-points in staging as entry criteria, or to optimize dosing in larger trials, prior to embarking on the more arduous, expensive and time-consuming prevention trials.
What we do better now or know better now?
Recent, seminal lessons from studies on pancreatic tissue of type 1 diabetic donors provide compelling proof of the autoimmune nature of type 1 diabetes; in particular, the demonstration of β cell autoantigen-specific CD8 T cells in destructive insulitic lesions has highlighted a link that had not emerged in 2007. The persistence of β cells and insulin production as well as inflammatory insulitic lesions many years after clinical manifestations of hyperglycaemia are also arresting, providing an apparent disconnect between β cell mass and function. These studies also emphasize differences in immunopathology between men and mice; provide evidence of pathological and aetiological heterogeneity 43–49; and provide potential new biomarkers and therapeutic targets centred on CD8 T cell biology 50–53 that were not envisaged at the time of our last review(Fig. 3). Importantly, the ‘biomarker concept’ that has become a critical piece of new drug development in the pharma industry has also begun to feature strongly in current thinking about type 1 diabetes therapies 5; the term was not even used in the previous paper 1.
Fig. 3.

Frozen pancreas tissue section from an organ donor who was diagnosed with type 1 (T1D) diabetes 1 year earlier. The section was stained for insulin (green, top) and human leucocyte antigen (HLA)-ABC (red, bottom). Individual images were captured by confocal microscopy and automatically combined in-silico into a section-wide overview figure. Note the distinct region in the upper right corner where some insulin-producing beta cells are still present. These remaining insulin-positive islets also hyperexpress major histocompatibility complex (MHC) class I, as evident in the lower panel. Thus, this is a prime example of the lobular distribution of both beta cell loss and immune pathology in T1D. Samples courtesy of Network for Pancreatic Organ Donors with Diabetes (nPOD) (case 6052) and kindly provided by Dr Ken Coppieters.
There is probably more new insight to be gained from studying the diabetic pancreas in settings such as nPOD. For example, the observation that the remaining β cell mass at clinical manifestation of disease may be substantial (as much as 50%, rather than 10–20% cited in most textbooks) disproves a common assumption that the disease process has always reached an end-stage at this point.
Immune monitoring in clinical islet transplantation has shown that the current immune suppressive regimes that include thymoglobulin are ineffective for the control of memory T cells; this concept is supported further by the lack of efficacy observed in the recent anti-thymocyte globulin (ATG) trial (Table 4) 54. Thus the autoreactive memory T cell, and the nature of its biology and control, emerge as important research questions, built on knowledge gained in recent years. As discussed already, the disappointing outcome of trials targeting the proinflammatory cytokine IL-1 25 may require a revision of thinking in relation to the importance of this immune pathway. Finally, a relatively new paradigm has come to prominence, namely that the biology of β cells can contribute to the cell's own demise through active participation at key points of the interface with the immune system, from immune recognition to immune cell recruitment and killing 55–57. A better understanding of these processes could be useful in devising better combination-based candidate strategies of immune intervention and prevention in type 1 diabetes.
The future pipeline
We would like to argue that animal models, when employed correctly, can be extremely useful for testing and optimizing new interventions for human type 1 diabetes. In addition, the new knowledge being accrued must be assimilated. We suggest the following strategic guidelines for pipeline development.
Defining the optimal dose for an antigen or biologic. Treating with the correct dose is of paramount importance, for ASI treatment with incorrect doses may result in loss of efficacy (see above) or may even be accelerating. For biologics, treating at an incorrect dose may not only mean loss of effect (as with otelixizumab in Phase III), but also increased side effects, if too much drug is given. Assumptions may be made that, for example, a monoclonal antibody targeting T cells will be effective as long as there is target molecule internalization; however, studies in mice show that there may be an approximate log-fold difference in dose between internalization and full efficacy. Thus, careful dosing studies in models, coupled with appropriate biomarkers, will be critical in attaining good efficacy in humans.
Preclinical testing of combinations. Despite the logic of this approach, it is becoming clear that not all combinations exhibit additive effects, let alone synergies. Thus, careful optimization of combinations prior to clinical trials is needed. As a case in point, for example, not all antigens synergize with anti-CD3 therapy 32. To accelerate translation in this arena, the Immune Tolerance Network (ITN; http://www.immunetolerance.org) has established a combination therapy testing consortium, in which four independent laboratories evaluate combinations of biologics and antigens in recent-onset diabetes in NOD mice. Such studies have so far demonstrated limited additive effects when examining potentially new combinations of biologics and antigens in recent-onset diabetes. Clearly, it will be important to establish which combinations work, and how.
Assessing patient heterogeneity. Is all type 1 diabetes the same? Our knowledge to date indicates that this is unlikely to be the case, and this should caution us to anticipate subgroup effects. For example, the rate of β cell loss varies between individuals, being most rapid in younger individuals aged 20 or less 58. The fact that Diapep277 only had its effects in older patients and in those with lower-risk major histocompatibility complex (MHC) illustrates this 59. To date, we are not certain whether the underlying immune pathology varies between different forms of type 1 diabetes (for example, a more IFN-dependent versus a more IL-dependent diabetes, or T cell-dependent versus NK cell-dependent islet destruction 60), but nPOD studies may elucidate this. In that case, stratifying patients by immune phenotypes may become an increasingly important feature of trial design.
Defining the optimal disease stage for a given therapy. One paradigm that may emerge from ongoing diabetes trials is that the more aggressive the immune CD8 reactivity to islets, the more advanced β cell loss is, the less likely it is that any treatment will be effective (various studies, unpublished). Monoclonal anti-CD3 antibodies do not appear to preserve C-peptide in patients with advanced β cell loss (lower C-peptide at trial entry).
Managing expectations. Taking the above issues at face value, not over-interpreting the data from animal models or being excessively optimistic (‘this has to work’) and refraining from conducting trials simply because drugs are available and effective in other immune disorders is an important message set to help avoidance of disappointments with future diabetes trials.
Acknowledgments
M.P. acknowledges support from the UK Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London. M.P. and B.O.R. receive funding via the EU FP7 Framework 7 Large-scale Focused Collaborative Research Project on Natural Immunomodulators as Novel Immunotherapies for Type 1 diabetes (NAIMIT) and EE-ASI (Beta cell preservation via antigen-specific immunotherapy in Type 1 Diabetes: Enhanced Epidermal Antigen Delivery Systems); M.P. is also funded via the EU FP7 PEVNET programme (Persistent virus infection as a cause of pathogenic inflammation in type 1 diabetes – an innovative research program of biobanks and expertise) and as part of the Juvenile Diabetes Research Foundation Autiommunity Centers Consortium (1-2007-1803). We are grateful to Dr Ken Coppieters for providing the image used in Fig. 3. This image was generated with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/our-partners.php.
References
- 1.Staeva-Vieira T, Peakman M, von Herrath M. Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol. 2007;148:17–31. doi: 10.1111/j.1365-2249.2007.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 3.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 4.Matthews JB, Staeva TP, Bernstein PL, Peakman M, von Herrath M. Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN–JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol. 2010;160:176–184. doi: 10.1111/j.1365-2249.2010.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roep BO, Peakman M. Surrogate end points in the design of immunotherapy trials: emerging lessons from type 1 diabetes. Nat Rev Immunol. 2010;10:145–152. doi: 10.1038/nri2705. [DOI] [PubMed] [Google Scholar]
- 6.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed S, Akirav E, Bradshaw EM, et al. Immunologic biomarkers: catalysts for translational advances in autoimmune diabetes. Clin Exp Immunol. 2013;172:178–185. doi: 10.1111/cei.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53:614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 10.Campbell-Thompson M, Wasserfall C, Kaddis J, et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28:608–617. doi: 10.1002/dmrr.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48:2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 12.Ludvigsson J, Krisky D, Casas R, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 13.Sherry N, Hagopian W, Ludvigsson J, et al. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial – Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 16.Thrower SL, James L, Hall W, et al. Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study. Clin Exp Immunol. 2009;155:156–165. doi: 10.1111/j.1365-2249.2008.03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huurman VA, van der Torren CR, Gillard P, et al. Immune responses against islet allografts during tapering of immunosuppression – a pilot study in five subjects. Clin Exp Immunol. 2011;169:190–198. doi: 10.1111/j.1365-2249.2012.04605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilbrands R, Huurman VA, Gillard P, et al. Differences in baseline lymphocyte counts and autoreactivity are associated with differences in outcome of islet cell transplantation in type 1 diabetic patients. Diabetes. 2009;58:2267–2276. doi: 10.2337/db09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huurman VA, Hilbrands R, Pinkse GG, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS ONE. 2008;3:e2435. doi: 10.1371/journal.pone.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long SA, Rieck M, Sanda S, et al. Diabetes TrialNet and the Immune Tolerance Network Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta-cell function. Diabetes. 2012;61:2340–2348. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pescovitz MD, Torgerson TR, Ochs HD, et al. Type 1 Diabetes TrialNet Study G. Effect of rituximab on human in vivo antibody immune responses. J Allergy Clin Immunol. 2011;128:1295–1302. doi: 10.1016/j.jaci.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gitelman SE, Fisher LK, Gottlieb PA, et al. START Study Team Effect of anti-thymocyte globulin (ATG) on preserving beta cell function in new-onset type 1 diabetes. Poster Presentation 454, European Association for the Study of Diabetes. 2012.
- 23.Viglietta V, Kent SC, Orban T, Hafler DA. GAD65-reactive T cells are activated in patients with autoimmune type 1a diabetes. J Clin Invest. 2002;109:895–903. doi: 10.1172/JCI14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastrandrea L, Yu J, Behrens T, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244–1249. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran A, Bundy B, Becker DJ, et al. Group AS. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicenter, randomized double-masked, placebo-controlled trials. Lancet. 2013 doi: 10.1016/S0140-6736(13)60023-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ablamunits V, Henegariu O, Hansen JB, et al. Synergistic reversal of type 1 diabetes in NOD mice with anti-CD3 and interleukin-1 blockade: evidence of improved immune regulation. Diabetes. 2012;61:145–154. doi: 10.2337/db11-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tree TI, Lawson J, Edwards H, et al. Naturally arising human CD4 T-cells that recognize islet autoantigens and secrete interleukin-10 regulate proinflammatory T-cell responses via linked suppression. Diabetes. 2010;59:1451–1460. doi: 10.2337/db09-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanto-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372:1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 29.Ludvigsson J, Faresjo M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359:1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 30.Wherrett DK, Bundy B, Becker DJ, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319–327. doi: 10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orban T, Farkas K, Jalahej H, et al. Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J Autoimmun. 2010;34:408–415. doi: 10.1016/j.jaut.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bresson D, Togher L, Rodrigo E, et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takiishi T, Korf H, Van Belle TL, et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest. 2012;122:1717–1725. doi: 10.1172/JCI60530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufman DL, Clare-Salzler M, Tian J, et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993;366:69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 36.Bresson D, Fradkin M, Manenkova Y, Rottembourg D, von Herrath M. Genetic-induced variations in the GAD65 T-cell repertoire governs efficacy of anti-CD3/GAD65 combination therapy in new-onset type 1 diabetes. Mol Ther. 2010;18:307–316. doi: 10.1038/mt.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fousteri G, Chan JR, Zheng Y, et al. Virtual optimization of nasal insulin therapy predicts immunization frequency to be crucial for diabetes protection. Diabetes. 2010;59:3148–3158. doi: 10.2337/db10-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorus FK, Balti EV, Vermeulen I, et al. Screening for insulinoma antigen 2 and zinc transporter 8 autoantibodies: a cost-effective and age-independent strategy to identify rapid progressors to clinical onset among relatives of type 1 diabetic patients. Clin Exp Immunol. 2013;171:82–90. doi: 10.1111/j.1365-2249.2012.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabbay MA, Sato MN, Duarte AJ, Dib SA. Serum titres of anti-glutamic acid decarboxylase-65 and anti-IA-2 autoantibodies are associated with different immunoregulatory milieu in newly diagnosed type 1 diabetes patients. Clin Exp Immunol. 2012;168:60–67. doi: 10.1111/j.1365-2249.2011.04538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curnock RM, Reed CR, Rokni S, Broadhurst JW, Bingley PJ, Williams AJ. Insulin autoantibody affinity measurement using a single concentration of unlabelled insulin competitor discriminates risk in relatives of patients with type 1 diabetes. Clin Exp Immunol. 2012;167:67–72. doi: 10.1111/j.1365-2249.2011.04495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiberti C, Yu L, Lucantoni F, et al. Detection of four diabetes specific autoantibodies in a single radioimmunoassay: an innovative high-throughput approach for autoimmune diabetes screening. Clin Exp Immunol. 2011;166:317–324. doi: 10.1111/j.1365-2249.2011.04479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peakman M, von Herrath M. Antigen-specific immunotherapy for type 1 diabetes: maximizing the potential. Diabetes. 2010;59:2087–2093. doi: 10.2337/db10-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hober D, Sane F, Jaidane H, Riedweg K, Goffard A, Desailloud R. Immunology in the Clinic Review Series; focus on type 1 diabetes and viruses: role of antibodies enhancing the infection with Coxsackievirus-B in the pathogenesis of type 1 diabetes. Clin Exp Immunol. 2012;168:47–51. doi: 10.1111/j.1365-2249.2011.04559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaidane H, Sane F, Hiar R, et al. Immunology in the Clinic Review Series; focus on type 1 diabetes and viruses: enterovirus, thymus and type 1 diabetes pathogenesis. Clin Exp Immunol. 2012;168:39–46. doi: 10.1111/j.1365-2249.2011.04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lind K, Huhn MH, Flodstrom-Tullberg M. Immunology in the Clinic Review Series; focus on type 1 diabetes and viruses: the innate immune response to enteroviruses and its possible role in regulating type 1 diabetes. Clin Exp Immunol. 2012;168:30–38. doi: 10.1111/j.1365-2249.2011.04557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grieco FA, Sebastiani G, Spagnuolo I, Patti A, Dotta F. Immunology in the Clinic Review Series; focus on type 1 diabetes and viruses: how viral infections modulate beta cell function. Clin Exp Immunol. 2012;168:24–29. doi: 10.1111/j.1365-2249.2011.04556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stene LC, Rewers M. Immunology in the Clinic Review Series; focus on type 1 diabetes and viruses: the enterovirus link to type 1 diabetes: critical review of human studies. Clin Exp Immunol. 2012;168:12–23. doi: 10.1111/j.1365-2249.2011.04555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coppieters KT, Wiberg A, Tracy SM, von Herrath MG. Immunology in the Clinic Review Series: focus on type 1 diabetes and viruses: the role of viruses in type 1 diabetes: a difficult dilemma. Clin Exp Immunol. 2012;168:5–11. doi: 10.1111/j.1365-2249.2011.04554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gale EA. Viruses and type 1 diabetes: ignorance acquires a better vocabulary. Clin Exp Immunol. 2012;168:1–4. doi: 10.1111/j.1365-2249.2011.04553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han D, Cai X, Wen J, et al. Innate and adaptive immune gene expression profiles as biomarkers in human type 1 diabetes. Clin Exp Immunol. 2012;170:131–138. doi: 10.1111/j.1365-2249.2012.04650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abreu JR, Martina S, Verrijn Stuart AA, et al. CD8 T cell autoreactivity to preproinsulin epitopes with very low human leucocyte antigen class I binding affinity. Clin Exp Immunol. 2012;170:57–65. doi: 10.1111/j.1365-2249.2012.04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coppieters KT, Amirian N, von Herrath MG. Incidental CD8 T cell reactivity against caspase-cleaved apoptotic self-antigens from ubiquitously expressed proteins in islets from prediabetic human leucocyte antigen-A2 transgenic non-obese diabetic mice. Clin Exp Immunol. 2011;165:155–162. doi: 10.1111/j.1365-2249.2011.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mallone R, Mannering SI, Brooks-Worrell BM, et al. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin Exp Immunol. 2011;163:33–49. doi: 10.1111/j.1365-2249.2010.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bresson D, von Herrath MG. Anti-thymoglobulin (ATG) treatment does not reverse type 1 diabetes in the acute virally induced rat insulin promoter–lymphocytic choriomeningitis virus (RIP–LCMV) model. Clin Exp Immunol. 2011;163:375–380. doi: 10.1111/j.1365-2249.2010.04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skowera A, Ellis RJ, Varela-Calvino R, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knight RR, Kronenberg D, Zhao M, et al. Human beta-cell killing by autoreactive preproinsulin-specific CD8 T cells is predominantly granule-mediated with the potency dependent upon T-cell receptor avidity. Diabetes. 2013;62:205–213. doi: 10.2337/db12-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kronenberg D, Knight RR, Estorninho M, et al. Circulating preproinsulin signal peptide-specific CD8 T cells restricted by the susceptibility molecule HLA-A24 are expanded at onset of type 1 diabetes and kill beta-cells. Diabetes. 2012;61:1752–1759. doi: 10.2337/db11-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenbaum CJ, Beam CA, Boulware D, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61:2066–2073. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buzzetti R, Cernea S, Petrone A, et al. C-peptide response and HLA genotypes in subjects with recent-onset type 1 diabetes after immunotherapy with DiaPep277: an exploratory study. Diabetes. 2011;60:3067–3072. doi: 10.2337/db10-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simoni Y, Diana J, Ghazarian L, Beaudoin L, Lehuen A. Therapeutic manipulation of natural killer (NK) T cells in autoimmunity: are we close to reality? Clin Exp Immunol. 2013;171:8–19. doi: 10.1111/j.1365-2249.2012.04625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keller RJ, Eisenbarth GS, Jackson RA. Insulin prophylaxis in individuals at high risk of type I diabetes. Lancet. 1993;341:927–928. doi: 10.1016/0140-6736(93)91215-8. [DOI] [PubMed] [Google Scholar]
- 62.Fuchtenbusch M, Rabl W, Grassl B, Bachmann W, Standl E, Ziegler AG. Delay of type I diabetes in high risk, first degree relatives by parenteral antigen administration: the Schwabing Insulin Prophylaxis Pilot Trial. Diabetologia. 1998;41:536–541. doi: 10.1007/s001250050943. [DOI] [PubMed] [Google Scholar]
- 63.Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 64.Harrison LC, Honeyman MC, Steele CE, et al. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004;27:2348–2355. doi: 10.2337/diacare.27.10.2348. [DOI] [PubMed] [Google Scholar]
- 65.Kupila A, Sipila J, Keskinen P, et al. Intranasally administered insulin intended for prevention of type 1 diabetes – a safety study in healthy adults. Diabetes Metab Res Rev. 2003;19:415–420. doi: 10.1002/dmrr.397. [DOI] [PubMed] [Google Scholar]
- 66.Fourlanos S, Perry C, Gellert SA, et al. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes. 2011;60:1237–1245. doi: 10.2337/db10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Achenbach P, Barker J, Bonifacio E. Modulating the natural history of type 1 diabetes in children at high genetic risk by mucosal insulin immunization. Curr Diab Rep. 2008;8:87–93. doi: 10.1007/s11892-008-0017-y. [DOI] [PubMed] [Google Scholar]
- 68.Bohmer KP, Kolb H, Kuglin B, et al. Linear loss of insulin secretory capacity during the last six months preceding IDDM. No effect of antiedematous therapy with ketotifen. Diabetes Care. 1994;17:138–141. doi: 10.2337/diacare.17.2.138. [DOI] [PubMed] [Google Scholar]
- 69.Carel JC, Boitard C, Eisenbarth G, Bach JF, Bougneres PF. Cyclosporine delays but does not prevent clinical onset in glucose intolerant pre-type 1 diabetic children. J Autoimmun. 1996;9:739–745. doi: 10.1006/jaut.1996.0096. [DOI] [PubMed] [Google Scholar]
- 70.Lampeter EF, Klinghammer A, Scherbaum WA, et al. The Deutsche Nicotinamide Intervention Study: an attempt to prevent type 1 diabetes. DENIS Group. Diabetes. 1998;47:980–984. doi: 10.2337/diabetes.47.6.980. [DOI] [PubMed] [Google Scholar]
- 71.Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363:925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 72.Crino A, Schiaffini R, Ciampalini P, et al. A two year observational study of nicotinamide and intensive insulin therapy in patients with recent onset type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18:749–754. doi: 10.1515/jpem.2005.18.8.749. [DOI] [PubMed] [Google Scholar]
- 73.Crino A, Schiaffini R, Manfrini S, et al. A randomized trial of nicotinamide and vitamin E in children with recent onset type 1 diabetes (IMDIAB IX) Eur J Endocrinol. 2004;150:719–724. doi: 10.1530/eje.0.1500719. [DOI] [PubMed] [Google Scholar]
- 74.Pozzilli P, Visalli N, Boccuni ML, et al. Randomized trial comparing nicotinamide and nicotinamide plus cyclosporin in recent onset insulin-dependent diabetes (IMDIAB 1). The IMDIAB Study Group. Diabet Med. 1994;11:98–104. doi: 10.1111/j.1464-5491.1994.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 75.Dahlquist G, Gothefors L. The cumulative incidence of childhood diabetes mellitus in Sweden unaffected by BCG-vaccination. Diabetologia. 1995;38:873–874. doi: 10.1007/BF03035306. [DOI] [PubMed] [Google Scholar]
- 76.Huppmann M, Baumgarten A, Ziegler AG, Bonifacio E. Neonatal bacille Calmette–Guérin vaccination and type 1 diabetes. Diabetes Care. 2005;28:1204–1206. doi: 10.2337/diacare.28.5.1204. [DOI] [PubMed] [Google Scholar]
- 77.Parent ME, Siemiatycki J, Menzies R, Fritschi L, Colle E. Bacille Calmette–Guérin vaccination and incidence of IDDM in Montreal, Canada. Diabetes Care. 1997;20:767–772. doi: 10.2337/diacare.20.5.767. [DOI] [PubMed] [Google Scholar]
- 78.Hummel M, Bonifacio E, Naserke HE, Ziegler AG. Elimination of dietary gluten does not reduce titers of type 1 diabetes-associated autoantibodies in high-risk subjects. Diabetes Care. 2002;25:1111–1116. doi: 10.2337/diacare.25.7.1111. [DOI] [PubMed] [Google Scholar]
- 79.Wicklow BA, Taback SP. Feasibility of a type 1 diabetes primary prevention trial using 2000 IU vitamin D3 in infants from the general population with increased HLA-associated risk. Ann NY Acad Sci. 2006;1079:310–312. doi: 10.1196/annals.1375.047. [DOI] [PubMed] [Google Scholar]
- 80.Akerblom HK, Virtanen SM, Ilonen J, et al. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia. 2005;48:829–837. doi: 10.1007/s00125-005-1733-3. [DOI] [PubMed] [Google Scholar]
- 81.Knip M, Virtanen SM, Seppa K, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363:1900–1908. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller MR, Seifert J, Szabo NJ, Clare-Salzler M, Rewers M, Norris JM. Erythrocyte membrane fatty acid content in infants consuming formulas supplemented with docosahexaenoic acid (DHA) and arachidonic acid (ARA): an observational study. Matern Child Nutr. 2010;6:338–346. doi: 10.1111/j.1740-8709.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller MR, Yin X, Seifert J, et al. Erythrocyte membrane omega-3 fatty acid levels and omega-3 fatty acid intake are not associated with conversion to type 1 diabetes in children with islet autoimmunity: the Diabetes Autoimmunity Study in the Young (DAISY) Pediatr Diabetes. 2011;12:669–675. doi: 10.1111/j.1399-5448.2011.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaarala O, Ilonen J, Ruohtula T, et al. Removal of bovine insulin from cow's milk formula and early initiation of beta-cell autoimmunity in the FINDIA pilot study. Arch Pediatr Adolesc Med. 2012;166:608–614. doi: 10.1001/archpediatrics.2011.1559. [DOI] [PubMed] [Google Scholar]
- 85.Hummel S, Pfluger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011;34:1301–1305. doi: 10.2337/dc10-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alleva DG, Maki RA, Putnam AL, et al. Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope. Scand J Immunol. 2006;63:59–69. doi: 10.1111/j.1365-3083.2005.01705.x. [DOI] [PubMed] [Google Scholar]
- 87.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, Phase II trial. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 88.Lazar L, Ofan R, Weintrob N, et al. Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: a randomised, double-blind Phase II study. Diabetes Metab Res Rev. 2006;23:286–291. doi: 10.1002/dmrr.711. [DOI] [PubMed] [Google Scholar]
- 89.Agardh CD, Cilio CM, Lethagen A, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications. 2005;19:238–246. doi: 10.1016/j.jdiacomp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Peakman M, Stevens EJ, Lohmann T, et al. Naturally processed and presented epitopes of the islet cell autoantigen IA-2 eluted from HLA-DR4. J Clin Invest. 1999;104:1449–1457. doi: 10.1172/JCI7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bougneres PF, Landais P, Boisson C, et al. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes. 1990;39:1264–1272. doi: 10.2337/diab.39.10.1264. [DOI] [PubMed] [Google Scholar]
- 92.Jenner M, Bradish G, Stiller C, Atkison P. Cyclosporin A treatment of young children with newly-diagnosed type 1 (insulin-dependent) diabetes mellitus. London Diabetes Study Group. Diabetologia. 1992;35:884–888. doi: 10.1007/BF00399937. [DOI] [PubMed] [Google Scholar]
- 93.Elliott RB, Chase HP. Prevention or delay of type 1 (insulin-dependent) diabetes mellitus in children using nicotinamide. Diabetologia. 1991;34:362–365. doi: 10.1007/BF00405010. [DOI] [PubMed] [Google Scholar]
- 94.Eisenbarth GS, Srikanta S, Jackson R, et al. Anti-thymocyte globulin and prednisone immunotherapy of recent onset type 1 diabetes mellitus. Diabetes Res. 1985;2:271–276. [PubMed] [Google Scholar]
- 95.Allen HF, Klingensmith GJ, Jensen P, Simoes E, Hayward A, Chase HP. Effect of bacillus Calmette–Guérin vaccination on new-onset type 1 diabetes. A randomized clinical study. Diabetes Care. 1999;22:1703–1707. doi: 10.2337/diacare.22.10.1703. [DOI] [PubMed] [Google Scholar]
- 96.Elliott JF, Marlin KL, Couch RM. Effect of bacille Calmette–Guérin vaccination on C-peptide secretion in children newly diagnosed with IDDM. Diabetes Care. 1998;21:1691–1693. doi: 10.2337/diacare.21.10.1691. [DOI] [PubMed] [Google Scholar]
- 97.Shehadeh N, Calcinaro F, Bradley BJ, Bruchim I, Vardi P, Lafferty KJ. Effect of adjuvant therapy on development of diabetes in mouse and man. Lancet. 1994;343:706–707. doi: 10.1016/s0140-6736(94)91583-0. [DOI] [PubMed] [Google Scholar]
- 98.Bjork E, Berne C, Kampe O, Wibell L, Oskarsson P, Karlsson FA. Diazoxide treatment at onset preserves residual insulin secretion in adults with autoimmune diabetes. Diabetes. 1996;45:1427–1430. doi: 10.2337/diab.45.10.1427. [DOI] [PubMed] [Google Scholar]
- 99.Ortqvist E, Bjork E, Wallensteen M, et al. Temporary preservation of beta-cell function by diazoxide treatment in childhood type 1 diabetes. Diabetes Care. 2004;27:2191–2197. doi: 10.2337/diacare.27.9.2191. [DOI] [PubMed] [Google Scholar]
- 100.Brod SA, Atkinson M, Lavis VR, et al. Ingested IFN-alpha preserves residual beta cell function in type 1 diabetes. J Interferon Cytokine Res. 2001;21:1021–1030. doi: 10.1089/107999001317205141. [DOI] [PubMed] [Google Scholar]
- 101.Kempf K, Manzo G, Hanifi-Moghaddam P, et al. Effect of combined oral proteases and flavonoid treatment in subjects at risk of Type 1 diabetes. Diabet Med. 2009;26:1309–1310. doi: 10.1111/j.1464-5491.2009.02879.x. [DOI] [PubMed] [Google Scholar]
- 102.Herold KC, Gitelman SE, Willi SM, et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391–400. doi: 10.1007/s00125-012-2753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haller MJ, Wasserfall CH, Hulme MA, et al. Autologous umbilical cord blood transfusion in young children with type 1 diabetes fails to preserve C-peptide. Diabetes Care. 2011;34:2567–2569. doi: 10.2337/dc11-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haller MJ, Wasserfall CH, McGrail KM, et al. Autologous umbilical cord blood transfusion in very young children with type 1 diabetes. Diabetes Care. 2009;32:2041–2046. doi: 10.2337/dc09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saudek F, Havrdova T, Boucek P, Karasova L, Novota P, Skibova J. Polyclonal anti-T-cell therapy for type 1 diabetes mellitus of recent onset. Rev Diabet Stud. 2004;1:80–88. doi: 10.1900/RDS.2004.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sumpter KM, Adhikari S, Grishman EK, White PC. Preliminary studies related to anti-interleukin-1beta therapy in children with newly diagnosed type 1 diabetes. Pediatr Diabetes. 2011;12:656–667. doi: 10.1111/j.1399-5448.2011.00761.x. [DOI] [PubMed] [Google Scholar]
- 108.Mandrup-Poulsen T. Philadephia, PA: 2012. IL-1 receptor antagonist in recent onset type 1 diabetes- a multicentre randomized, placedbo controlled trial [IT-SYO1] Presented at: 72nd Scientific Session of the American Diabetes Association; 11 June. [Google Scholar]
- 109.Moran A. Philadelphia, PA: 2012. Canakinumab, an anti-IL-1 monoclonal antibody in recent -onset type 1 diabetes [IT-SY01] Presented at: 72nd Scientific Session of the American Diabetes Association; 11 June. [Google Scholar]
- 110.Walter M, Kaupper T, Adler K, Foersch J, Bonifacio E, Ziegler AG. No effect of the 1alpha,25-dihydroxyvitamin D3 on beta-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care. 2010;33:1443–1448. doi: 10.2337/dc09-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gu W, Hu J, Wang W, et al. Diabetic ketoacidosis at diagnosis influences complete remission after treatment with hematopoietic stem cell transplantation in adolescents with type 1 diabetes. Diabetes Care. 2012;35:1413–1419. doi: 10.2337/dc11-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Strom A, Kolb H, Martin S, et al. Improved preservation of residual beta cell function by atorvastatin in patients with recent onset type 1 diabetes and high CRP levels (DIATOR trial) PLoS ONE. 2012;7:e33108. doi: 10.1371/journal.pone.0033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin S, Herder C, Schloot NC, et al. Residual beta cell function in newly diagnosed type 1 diabetes after treatment with atorvastatin: the Randomized DIATOR Trial. PLoS ONE. 2011;6:e17554. doi: 10.1371/journal.pone.0017554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rother KI, Brown RJ, Morales MM, et al. Effect of ingested interferon-alpha on beta-cell function in children with new-onset type 1 diabetes. Diabetes Care. 2009;32:1250–1255. doi: 10.2337/dc08-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Faustman DL, Wang L, Okubo Y, et al. Proof-of-concept, randomized, controlled clinical trial of bacillus-Calmette–Guérin for treatment of long-term type 1 diabetes. PLoS ONE. 2012;7:e41756. doi: 10.1371/journal.pone.0041756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gottlieb PA, Quinlan S, Krause-Steinrauf H, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care. 2010;33:826–832. doi: 10.2337/dc09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 118.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 119.Barcala Tabarrozzi AE, Castro CN, Dewey RA, Sogayar MC, Labriola L, Perone MJ. Cell-based interventions to halt autoimmunity in type 1 diabetes mellitus. Clin Exp Immunol. 2013;171:135–146. doi: 10.1111/cei.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Oliveira GL, Malmegrim KC, Ferreira AF, et al. Up-regulation of fas and fasL pro-apoptotic genes expression in type 1 diabetes patients after autologous haematopoietic stem cell transplantation. Clin Exp Immunol. 2012;168:291–302. doi: 10.1111/j.1365-2249.2012.04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


