Abstract
Antigen presentation by intestinal epithelial cells (IEC) is crucial for intestinal homeostasis. Disturbances of major histocompatibility complex class I (MHC I)- and II-related presentation pathways in IEC appear to be involved in an altered activation of CD4+ and CD8+ T cells in inflammatory bowel disease. However, a comprehensive analysis of MHC I- and II-enriched compartments in IEC of the small and large bowel in the healthy state as opposed to inflammatory bowel diseases is lacking. The aim of this study was to characterize the subcellular expression of MHC I and II in the endocytic pathway of IEC throughout all parts of the intestinal tract, and to identify differences between the healthy state and inflammatory bowel diseases. Biopsies were taken by endoscopy from the duodenum, jejunum, ileum and colon in healthy individuals (n = 20). In Crohn's disease (CD), biopsies were obtained from the ileum and colon and within the colon from ulcerative colitis (UC) patients (n = 15). Analysis of IEC was performed by immunoelectron microscopy. MHC I and II were identified in early endosomes and multi-vesicular, multi-lamellar, electrondense and vacuolar late endosomes. Both molecules were enriched in multi-vesicular bodies. No differences were found between the distinct parts of the gut axis. In CD and UC the expression of MHC I and II showed a shift from multi-vesicular bodies towards the basolateral membranes. Within the multi-vesicular bodies, MHC I and II moved from internal vesicles to the limiting membranes upon inflammation in CD and UC. MHC I- and II-enriched compartments in IEC were identical in all parts of the small and large bowel. CD and UC appear to modulate the MHC I- and II-related presentation pathways of exogenous antigens in IEC.
Keywords: inflammatory bowel disease, intestinal epithelial cells, MHC I, MHC II
Introduction
As the main component of the intestinal barrier, the intestinal epithelial layer is exposed continuously to a high concentration of luminal antigens such as commensal microbiota or nutrient components. The development of immunological tolerance against these non-pathogenic antigens is essential for gut homeostasis. Loss of tolerance has been demonstrated to be crucial for the development of a variety of intestinal pathologies; among these, inflammatory bowel diseases (IBD). The inflammatory process in Crohn's disease (CD) and ulcerative colitis (UC) is driven by an inappropriate activation of CD4+ and CD8+ effector T cells 1–3.
Intestinal epithelial cells (IEC) are recognized increasingly as non-professional antigen-presenting cells (APC). Recent data point to an important role of IEC in sampling luminal antigens and regulating immunological tolerance in the gut. In a transgenic mouse model, Westendorf et al. found a major histocompatibility complex class II (MHC II)-dependent stimulation of CD4+ T cells with a regulatory phenotype by IEC 4. In contrast, IEC were shown to be capable of activating CD4+ and CD8+ effector T cells under inflammatory conditions 2,3. This proinflammatory antigen presentation by IEC might be involved in the perpetuation or aggravation of mucosal inflammation in CD and UC. The underlying mechanisms of antigen processing and presentation that finally determine the outcome of T cell activation by IEC are largely unknown.
Pathways of exogenous antigens in processing and presentation have been analysed in detail in professional APC, such as dendritic cells (DC). Processing of internalized antigens and MHC II peptide loading was reported to take place in specialized endosomes called MIIC (MHC II-enriched compartments) 5. MIIC represent structures in the final stages of the endocytic pathway, which share common features such as an acid pH, proteases and specific marker proteins, e.g. the lysosome-associated membrane protein (LAMP). Distinct subsets of MIIC can be characterized by electron microscopy. This distinct ultrastructural morphology corresponds to multi-vesicular bodies (MVB), multi-lamellar bodies (MLB) and electron-dense bodies (EDB). Some authors have further described MIIC with a vacuolar component (VLE). Although data are sparse, these distinct MIIC are assumed to differ functionally with respect to MHC II-related processing and peptide loading.
Presentation of exogenous antigens via the endocytic pathway is not restricted to MHC II, but also occurs as ‘cross-presentation’ to CD8+ T cells via MHC I 6. Several intracellular pathways of processing exogenous antigens accompanied by binding to MHC I have been identified in professional APC. Among these, both early endosomes (EE) and MIIC might be responsible for MHC I-peptide loading.
Similar to professional APC, IEC express MHC I and II, along with co-stimulatory molecules such as CD40, CD58 or CD86 7. In particular, MHC II and CD40/CD86 were shown to be up-regulated strongly in IBD. In previous in-vivo experiments using mice and human tissue we studied the endocytic uptake of luminally applied ovalbumin in IEC 8–12. Internalized ovalbumin was found to be directed efficiently into MIIC and exposed to MHC I and II therein. In contrast to the increase of epithelial MHC II expression, our data revealed no influence of mucosal inflammation in CD on the endocytic route of ovalbumin in IEC.
Immunological properties and pathophysiologies in the gut are remarkably spatially segregated. ‘Oral’ tolerance towards luminal antigens develops in the upper small bowel, as opposed to the colon 13. UC is confined regularly to the colon, whereas CD often resembles a strictly separated, segmental inflammation, which might affect the entire gut axis. However, comprehensive characterization of epithelial MIIC alongside the gut axis, which might detect regional differences, is lacking. In this study we provide a detailed ultrastructural analysis comprising all segments of the gut. Using biopsies taken endoscopically from healthy controls and IBD patients, our data allow an insight into human pathophysiology.
Materials and methods
Specimens
Biopsies were obtained from healthy mucosa in individuals undergoing endoscopy for various reasons; among others, abdominal pain, constipation, screening purposes or occult gastrointestinal bleeding. The latter demanded double-balloon enteroscopy in addition to standardized upper and lower gastrointestinal endoscopy. In total, biopsies were obtained from 20 healthy individuals. Samples of mucosal tissue, 5 mm in diameter, were collected endoscopically from all parts of the human intestinal tract, including the duodenum (n = 5), jejunum (n = 5), ileum (n = 5) and colon (n = 5). In these ‘control’ subjects systemic or intestinal inflammation was excluded by clinical, serological, endoscopic and histopathological means. Control patients did not receive any immunomodulatory medication. To specify the effects of IBD, inflamed mucosal specimens were taken additionally from 10 patients with active CD (ileum n = 5, colon n = 5) and five patients with active UC (colon n = 5), respectively. The particular diagnosis was confirmed by clinical, endoscopic and histopathological criteria. IBD-specific therapy consisted of prednisolone (n = 7), mesalazine (n = 8) and azathioprine (n = 3). An infectious origin was ruled out. The median age of control subjects (10 female, 10 male) was 68 years (range 28–91), and 38 years (range 20–81) in IBD patients (nine female, six male). All patients gave their informed consent. The study was approved by the local Ethics Committee (Medical Faculty, University of Lübeck; 02-073, 03-043). All procedures were performed according to the Declaration of Helsinki.
Immunolabelling of ultrathin frozen sections
Endoscopically obtained specimens were fixed in 5% paraformaldehyde in 50 mmol/l HEPES, cryoprotected in 0·03 M polyvinylpyrrolidone/1·6 M sucrose and finally frozen in liquid nitrogen. For immunoelectron microscopy, labelling of ultrathin frozen sections (60 nm) was performed using the post-embedding technique described by Tokuyasu and Griffiths 14. Ultrathin cryosections (60 nm) were cut at −110°C to −100°C with a Leica Ultracryomicrotome R. For processing, sections were placed on Cu/Pd grids (Plano, Wetzlar, Germany). Prepared sections were incubated with the appropriate primary antibodies followed by immunogold-conjugated secondary antibodies for 45 min each. After staining, the tissue was contrasted with 4% uranyl acetate and embedded in 2% methylcellulose. The ultrastructural morphology and immunogold labelling were visualized and photographed using a Jeol 1011 transmission electron microscope (serial number: EM 18540061; Jeol, Eching, Germany).
Antibodies
Primary antibodies used in this study were affinity-purified polyclonal rabbit antibodies against human MHC I and II (gift from P. J. Peters, National Cancer Institute, Amsterdam, the Netherlands) and a monoclonal mouse anti-human antibody against LAMP2 (clone H4B4; PharMingen, Hamburg, Germany). Binding sites of the primary antibodies were visualized at the electron microscopic level by gold-conjugated goat anti-sera against rabbit and mouse immunoglobulin (Ig)G (6 nm or 12 nm in diameter; Dianova, Hamburg, Germany). To prevent cross-reactivity, double-labelling experiments were performed applying primary antibodies from different species and corresponding immunogold conjugates of different diameters. Possible cross-reactivity of antibodies was tested for each double-labelling experiment. Control incubation of sections with the immunogold-labelled secondary antibodies alone did not produce any staining.
Quantification of MHC I/II and LAMP labelling
The distribution of the subcellular labelling for MHC I, MHC II and LAMP in IEC was determined in tissue specimens of five patients for each localization (controls: duodenum, jejunum, ileum, colon; CD: ileum and colon; UC: colon). Quantification was performed according to Lucocq et al.'s 15 technique by an observer unaware of any information regarding the tissue samples. Labelling was analysed on ultrathin sections of two grids per patient, double-labelled for MHC I/LAMP and MHC II/LAMP, respectively. On each grid, 100 gold particles representing binding sites for MHC I, MHC II or LAMP were counted and assigned to the different subcellular compartments within IEC. The subcellular compartments in IEC were identified by their characteristic ultrastructural morphology and a specific marker protein (LAMP) for the distinction of late endosomes (LE). Gold counts assessed were used to construct a ranking of the labelling distribution. The percentage of gold counts for each compartment was established per antigen and patient. An arithmetic mean of the percentage was calculated for each group and antigen and used to elaborate a ranking of the labelling distribution for MHC I, MHC II and LAMP. Quantification of endosomal compartments was carried out in analogy. Labelling densities for MHC I and II within MVB were determined as gold particles per area (GP/μm2) and on the limiting membranes of these organelles as gold particles per length of membrane (GP/μm). The analysis was carried out according to the method reported by Griffiths 14. For each patient and intestinal region, 15 photographs depicting IEC and MVB were taken randomly from the two grids and used for quantitation. Results were shown as the mean of five patients per region and group. Antibody staining in the cytosol and mitochondria was considered as non-specific background.
Statistics
Results are expressed as means ± standard error of the mean (s.e.m.). P-values were calculated using an independent Student's t-test. Values of P < 0·05 were considered statistically significant.
Results
Endocytic compartments in intestinal epithelial cells alongside the gut axis
Endosomes were identified in IEC due to their ultrastructural morphology and the late endocytic marker protein LAMP (see Table 1).
Table 1.
Characterization of the different endocytic compartments
| Compartment | Morphology | Localization | LAMP2 |
|---|---|---|---|
| EE | Small, particularly vesicle-containing | Below apical membrane or adjacent to upper basolateral membrane | − (+) |
| VLE | Mainly electron-lucent, some electron-dense material (amorphous, vesicular, sheets) | Supranuclear | + |
| MVB | Filled with small vesicles | Supranuclear | + |
| MLB | Filled with membrane sheets | Supranuclear | + |
| EDB | Filled with amorphous electron-dense material | Supranuclear | + |
EE: early endosome; VLE: vacuolar late endosome; MVB: multi-vesicular body; MLB: multi-lamellar body; EDB: electron-dense body; LAMP: lysosome-associated membrane protein.
Corresponding to these criteria, we detected EE, VLE, MVB and MLB as well as EDB in IEC (Fig. 1a,b). EE were found predominantly underlying the apical membrane or near to the apical part of the basolateral membrane (BLM). The above-mentioned subsets of LE were localized mainly in the supranuclear region of the cells. Small transport vesicles were seen throughout IEC. Among endosomal compartments, MVB were identified in the highest numbers followed by EE and VLE. In contrast, EDB and particularly MLB were rarely observed. Labelling for LAMP was detected within all endocytic organelles, including EE. However, staining in EE was faint, whereas strong labelling was seen in VLE, MVB, MLB and EDB. The majority of LAMP epitopes were localized in MVB. A relevant proportion of LAMP was expressed at the apical membrane (APM) and BLM, while labelling of nuclei and mitochondria was negligible. Of note, a similar pattern of endosomes and LAMP expression was found in all parts of the intestinal tract from the duodenum to the colon. Furthermore, mucosal inflammation in CD (ileum and colon) and UC did not cause any changes in this respect (Fig. 2).
Fig. 1.
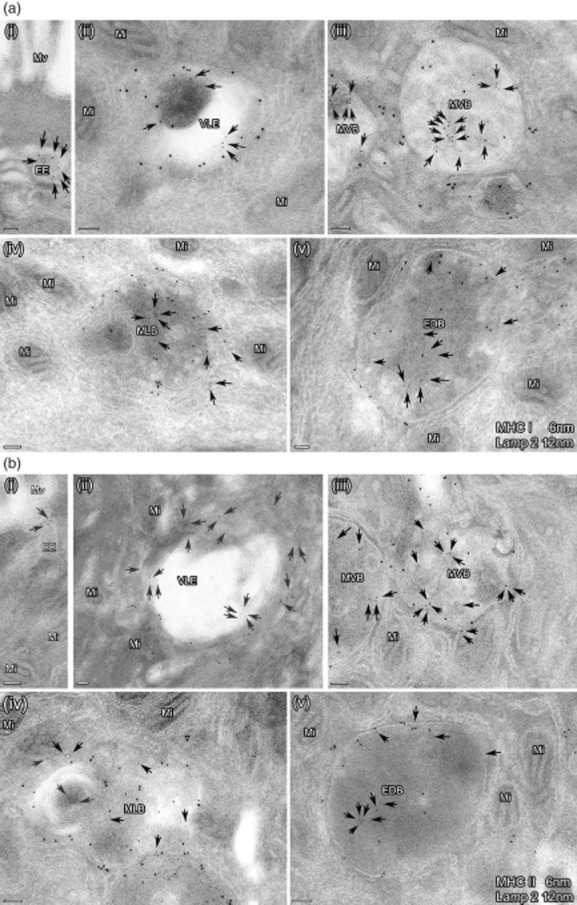
(a,b) Subcellular expression of major histocompatibility complex class I (MHC I) and II in the endocytic pathway of intestinal epithelial cells in one representative picture. Ultrathin cryosections were immunolabelled for MHC I (6 nm) and lysosome-associated membrane protein (LAMP)2 (12 nm) (a) as well as MHC II (6 nm) and LAMP2 (12 nm) (b). The photographs depict the distinct MIIC. EE: early endosome; VLE: vacuolar late endosome; MVB: multi-vesicular body; MLB: multi-lamellar body; EDB: electron-dense body. Bars = 100 nm. The arrows indicate small gold particles (6 nm) representing MHC I (a) and II (b), respectively. Labelling for MHC I and II was found on limiting membranes and in the inner part of all these organelles. LAMP labelling was not seen in EE, in contrast to the other compartments.
Fig. 2.
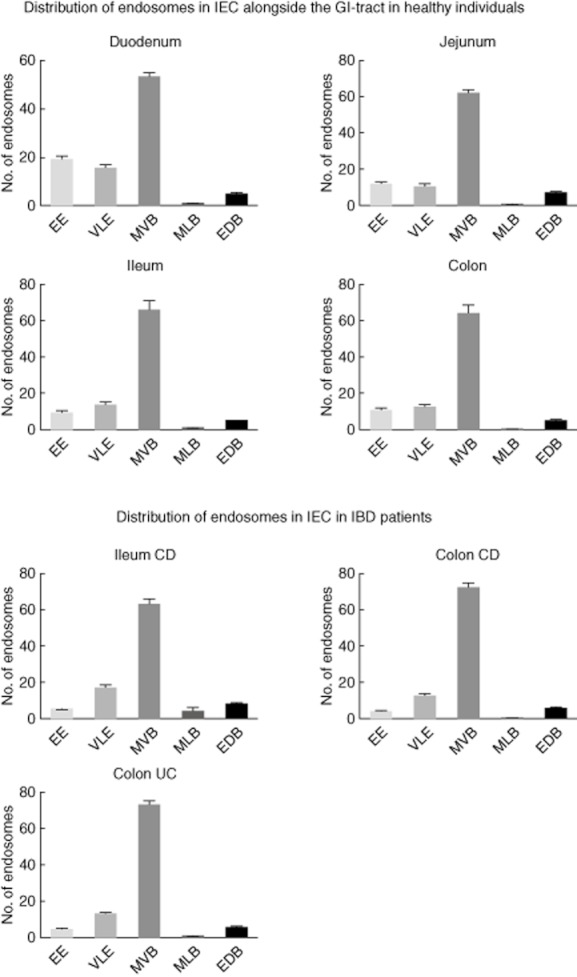
The appearance of endocytic compartments within intestinal epithelial cells (IEC) throughout the gut axis in healthy controls and patients with active IBD. EE: early endosome; VLE: vacuolar late endosome; MVB: multi-vesicular body; MLB: multi-lamellar body, EDB: electron-dense body (error bars represent the standard error of the mean).
MHC I and II expression in intestinal epithelial cells of the healthy gut
The subcellular expression of MHC I and II in IEC was analysed on the ultrastructural level by immunolabelling for MHC I/LAMP and MHC II/LAMP, respectively. MHC I and II were expressed constitutively by IEC of the entire small bowel and the colon. Cell surface expression of both molecules was highly polarized, and found predominantly at the BLM. Nevertheless, weak labelling was detected at the APM. MHC I and II were observed throughout all compartments of the endocytic route, including EE, VLE, MVB, MLB and EDB. Labelling for MHC I and II within IEC showed a similar pattern. The majority of both antigens were localized in LAMP+ MVB in the supranuclear region, according to the labelling for LAMP, followed by EE and VLE. Small amounts were seen within MLB and EBD (Fig. 3). The distribution of MHC I and II expression in IEC did not change with respect to the different parts of the small bowel or the colon. Background staining in nuclei and mitochondria was negligible (data not shown).
Fig. 3.
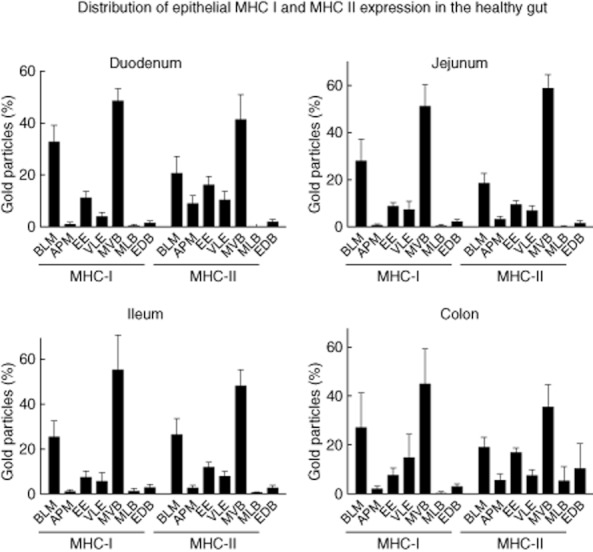
Patterns of labelling for major histocompatibility complex class I (MHC I) and II in intestinal epithelial cells (IEC) of healthy control patients. The proportion of the subcellular expression for MHC I and II in IEC was assessed as described in Materials and methods in five patients per localization. The results are presented as a percentage of total counts. BLM: basolateral membrane; APM: apical membrane; EE: early endosome; VLE: late endosome; MVB: multi-vesicular body; MLB: multi-lamellar body; EDB: electron-dense body (error bars represent the standard error of the mean).
MHC I and II expression in intestinal epithelial cells in Crohn's disease and ulcerative colitis
The epithelial expression of MHC I and II in inflamed samples from CD and UC was analysed as stated for healthy gut. The subcellular localization of both antigens was characterized on the ultrastructural level using immunolabelling for MHC I/LAMP and MHC II/LAMP. According to the most common pattern of inflammation in IBD, CD patients were examined in the terminal ileum and colon. In UC patients, biopsies of the colon were used. Corresponding to the normal mucosa, the bulk of MHC I and II was found at the BLM and within LAMP+ MVB in CD and UC. Consequently, the majority of cell surface expression was detected basolaterally, with only faint labelling seen at the APM (Fig. 4). MHC I and II were localized in all endocytic compartments, and predominantly in MVB, with no differences regarding CD of the ileum/colon or UC. Similar to the healthy gut, the background staining for the antibodies against MHC I and II on nuclei or mitochondria was irrelevant (data not shown). In CD, the percentage of epithelial MHC I and II expression found at the BLM increased significantly compared to non-inflamed mucosa in the ileum. This increase was associated with a significant decrease of the proportion of MHC I and II in MVB.
Fig. 4.
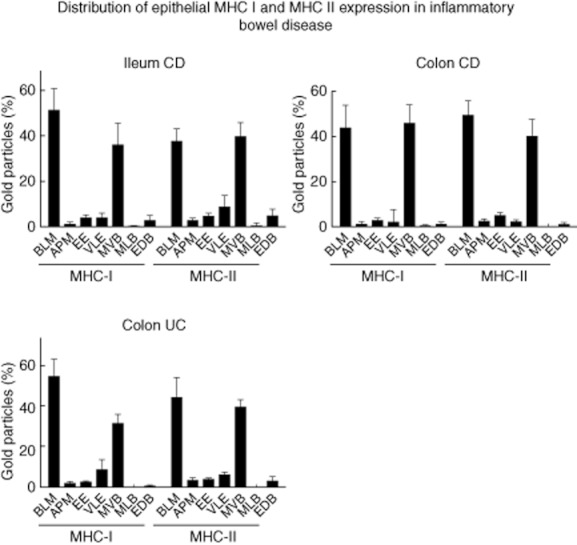
Patterns of labelling for major histocompatibility complex class I (MHC I)/MHC II in intestinal epithelial cells (IEC) of patients with active inflammatory bowel disease (IBD). The proportion of the subcellular expression for MHC I and II in IEC was assessed as described in Materials and methods in five patients per localization. The results are presented as a percentage of total counts. BLM: basolateral membrane; APM: apical membrane; EE: early endosome; VLE: late endosome; MVB: multi-vesicular body; MLB: multi-lamellar body; EDB: electron-dense body (error bars represent the standard error of the mean).
Increasing amounts of MHC I and II at the BLM in comparison to the other subcellular compartments in IEC were also detected in CD of the colon and UC. However, the change of basolateral MHC II expression in UC did not reach statistical significance. Compared to the healthy colon, the labelling for MHC I and II in MVB showed a trend towards a reduction in colonic CD and UC, although not statistically significant. Nevertheless, the relation between the expression of MHC I and II at the BLM versus MVB in colonic CD and UC showed a clear shift towards the BLM (Fig. 5).
Fig. 5.
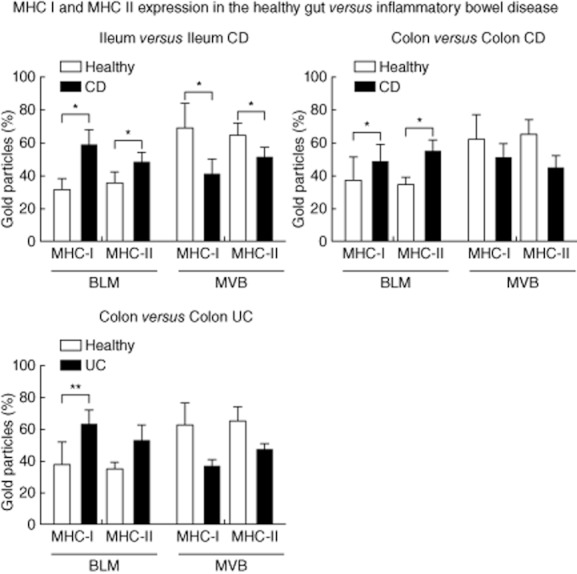
Comparison of labellings for major histocompatibility complex class I (MHC I) and MHC II in intestinal epithelial cells (IEC) of healthy controls and patients with active inflammatory bowel disease (IBD). Proportions of immunogold labelling at the basolateral membrane (BLM) and in multi-vesicular bodies (MVB) were analysed as described in Materials and methods. The results are presented as the percentage of total counts in five patients per localization. Error bars represent the standard error of the mean; *P < 0·05; **P < 0·005.
Influence of inflammatory bowel disease on the localization of MHC I and II molecules in multi-vesicular bodies
Previous data from our group pointed to an accumulation of luminal antigens in MHC I/MHC II+ MVB of IEC after internalization. The results obtained in this study demonstrated that MVB harbour the majority of intracellular MHC I and II antigens in human IEC independent of the inflammatory state and position alongside the gut axis. In addition, the present findings indicate a shift of MHC I and II from MVB to the BLM under inflammatory conditions in IBD. A localization of both molecules at the outer, limiting membrane of MVB might be one prerequisite for their transport towards the BLM. Thus, we were interested to investigate if mucosal inflammation in IBD might influence the localization of MHC I and II in MVB of IEC.
The labelling density of MHC I on the internal vesicles of MVB in the healthy ileum was 8·57 GP/μm2 (mean). In CD, the labelling density decreased significantly to 5·53 GP/μm2 (mean, P < 0·005). In contrast, the expression of MHC I at the limiting membranes increased significantly in response to CD inflammation (1·1 versus 1·84 GP/μm; P = 0·005). A similar shift was seen for MHC II antigens. Under healthy conditions, the labelling density at the limiting membranes was 0·72 GP/μm (mean) opposed to 2·11 GP/μm (mean) in CD (P < 0·000005). The MHC II expression observed on the internal vesicles of MVB showed an increase during CD (1·91 versus 4·47 GP/μm2; mean) that was also statistically significant (P < 0·000005).
Similar results were obtained in the colon of patients with CD. MHC I expression increased on the limiting membranes from 0·73 in healthy individuals to 3·58 GP/μm in CD (mean, P < 0·000005). The labelling on internal vesicles was not modified in CD (8·2 versus 9·0 GP/μm2, mean). The results for MHC II were 0·44 (healthy) versus 2·57 (CD) GP/μm (mean, P < 0·000005) for the limiting membranes and 2·3 (healthy) versus 2·5 (CD) GP/μm2 (mean), respectively.
Results comparable to those shown for CD in the colon were obtained in UC patients. Again, the MHC I expression increased on the limiting membranes [0·73 (healthy) versus 1·87 (UC) GP/μm, mean, P < 0·005] and decreased on the internal vesicles [8·2 (healthy) versus 3·7 (UC) GP/μm2, mean, P < 0·00005]. The results for MHC II were 0·44 (healthy) versus 1·81 (UC) GP/μm [mean, P < 0·0000005) for the limiting membranes and 2·3 (healthy) versus 3·0 (UC] GP/μm2 (mean, not significant), respectively (Fig. 6). Taken together, our results indicate a shift of MHC I and II peptides towards the limiting membrane of the internal vesicles in response to IBD.
Fig. 6.
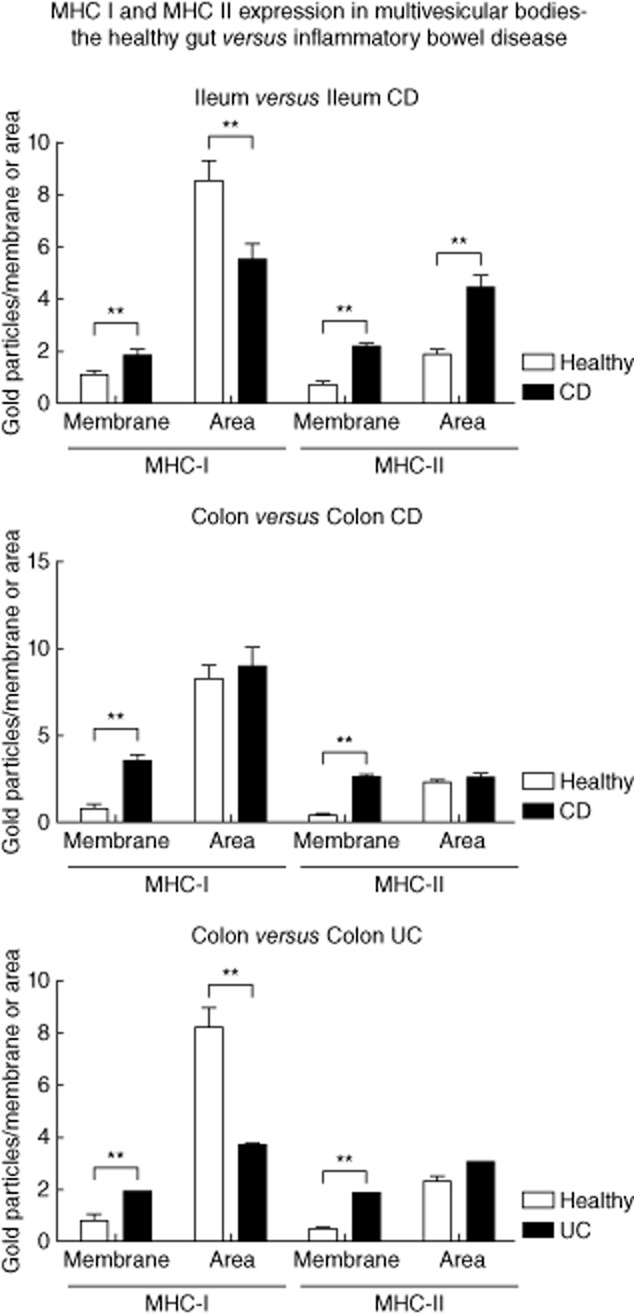
The influence of inflammatory bowel disease (IBD) on the expression of major histocompatibility complex class I (MHC I) and II in multi-vesicular bodies. Labelling for MHC I and II at the limiting membrane (GP/μm) and on internal vesicles (GP/μm2) assessed in multi-vesicular bodies of intestinal epithelial cells (IEC), as described in Materials and methods. Results were compared between healthy subjects and IBD patients. Error bars represent the standard error of the mean; *P < 0·05; **P < 0·005.
Discussion
Our study provides a comprehensive analysis of MIIC in IEC alongside the entire gut axis carried out in humans. We were able to identify all subsets of MIIC described previously in professional APC due to ultrastructural features. IEC harbour these endocytic compartments responsible for MHC I- and II-related antigen processing and presentation independent of the intestinal localization. Mucosal inflammation in CD or UC does not have an impact on the appearance of distinct MIIC in IEC. Of note, the inflammatory process in IBD induces a reallocation of MHC I and II complexes from intraluminal vesicles of MVB to their limiting membranes and further to the basolateral membranes of IEC.
The recent developments in IBD research point clearly to a defect in the mucosal barrier of the gut as the pivotal and primary pathogenic mechanism 16. Subsequently, mucosal tolerance is disturbed and effector T cells are stimulated constantly, perpetuating the inflammatory process. In CD, inflammation is driven by CD4+ T helper type 1 (Th1) and Th17 cells that, among others, secrete interferon (IFN)-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-17, IL-21, IL-22 and IL-26. The immune response in UC appears to be less polarized, but reveals a strong Th2 component with IL-4, IL-5 and IL-13. The role of CD8+ effector T cells is still uncertain. Activated DC are considered to play the major role in antigen presentation and activation of the afore-mentioned effector T cells.
Data regarding T cell activation by IEC are sparse, and result either from transgenic mouse models or the allogenic stimulation of human T cells. According to their experiments using Villin-haemagglutinin (HA) mice, Westendorf et al. suggested a balanced activation of CD4+ effector and regulatory T cells by IEC under healthy conditions 4. In contrast, the stimulation of CD4+ effector T cells predominated under inflammatory conditions 17. Antigen presentation via MHC II was found to be involved essentially in the stimulation of both effector and regulatory T cells. Results from Bisping et al., applying the allogenic stimulation of human T cells by IEC, showed an activation of CD8+ effector T cells during active IBD, a process related to MHC I 3. These data emphasize the importance of the MHC I- and II-associated antigen presentation by IEC for the homeostasis of the gut. Disturbances in both pathways might affect the T cell responses critically, and consequently contribute to mucosal inflammation in IBD.
In professional APCs the editing and binding of exogenous antigens to MHC I, so-called ‘cross-presentation’, or to MHC II may occur in all endosomes of the endocytic route 6. Traditionally, MIIC were attributed to endosomes late in the endocytic pathway. Among the MIIC, MVB were found to represent the majority in professional APC, but also in fibroblasts 5,18. This is in line with our findings showing MVB to predominate in IEC, irrespective of the intestinal region. Contrary to MVB, MLB were reported to be undetectable in several immune and non-immune cells. Although numbers of MLB in IEC were low in our experiments, they were seen constantly in the upper small intestine, the ileum and the colon. As well as MVB and MLB, we further identified VLE and EDB in the entire intestine; their occurrence was not related to the inflammatory state. Thus, IEC in humans harbour all subsets of MIIC described in professional APC. The expression of MIIC within the gut epithelium seems to be highly conserved and stable. This argues against a distinct regional or inflammation-associated expression of MIIC to be responsible for differences in immune tolerance or susceptibility towards IBD. In this study we did not prove the uptake of luminally applied markers into IEC, a key feature for compartments of the endocytic route. Nevertheless, our previous data obtained in mice and humans indicated clearly the uptake of ovalbumin in all the above-mentioned MIIC in the small and large bowel, independent of mucosal inflammation 9–12.
As in all other cells in the human body, MHC I molecules were expressed constitutively in IEC 7. The constitutive expression of MHC II in the gut epithelium is stimulated mainly by IFN-γ induced via bacterial colonization. We found MHC II in all subsets of MIIC that we predefined due to their ultrastructural morphology. In addition to the late endocytic structures VLE, MVB, MLE and EDB, MHC II was also detected in EE, revealing only small amounts of LAMP. Most endosomal MHC II was localized in MVB. We did not quantify the absolute amount of the MHC II expression within the different organelles, therefore the high amount of MHC II in MVB might be due to the higher frequency of MVB, as well as preferential MVB targeting and accumulation of MHC II as opposed to other MIIC. It is of note that distribution of the endosomal MHC I expression in IEC did not differ from MHC II antigens. MHC I was observed in EE and all MIIC, with the majority confined to MVB. Similar to the morphological characterization of MIIC, the subcellular expression of MHC I and II did not show significant differences between the examined bowel segments. IEC along the gut axis appear to contain a similar amount of MHC I- and MHC II-bearing MIIC. Aware of the lack of any functional analysis, our results point to the fact that antigen binding to MHC I and II in IEC might occur in similar compartments, as demonstrated for professional APC. In our previous studies, ovalbumin exposed to the mucosal surface of the gut accumulated within MVB of IEC 12. Tjelle et al. demonstrated that MVB were responsible for the processing of this particular antigen in macrophages 19. In accordance with this, the somewhat descriptive data of our present study strengthen the postulated central role of MVB in regulating immune responses to luminal antigens via MHC I and II by IEC.
Several mechanisms might contribute theoretically to the proinflammatory capacity of IEC to activate effector T cells in IBD. In mature DC, which are highly efficient in the stimulation of effector T cells, MHC I and II complexes derived from MIIC up-regulate on the cell surface on contact with T cells 20. MHC II–peptide complexes were generated on the internal vesicles of MIIC, particularly within MVB. Upon activation, class II complexes move to the outer membrane of these compartments and were delivered subsequently to the cell surface. It is well known that the inflammatory process in IBD increases the expression of MHC I and II in IEC of the small and large bowel 7,21. We used biopsies of the inflamed terminal ileum and colon, the typical localization of CD, to analyse CD-specific changes in subcellular MHC I and II expression in IEC. A UC-specific influence was investigated in inflamed colonic tissue. Not surprisingly, we consistently found a relative increase of MHC I and II on the BLM of IEC, the counterpart of interacting T cells. Similar to previous studies, this up-regulation was observed in CD and UC in the ileum as well as the colon. Remarkably, the up-regulation was accompanied by a shift of both MHC antigens from the MVB, the predominant MIIC, to the BLM. This argues against a simple increase of the MHC I and II expression induced by mucosal inflammation. Rather, our results point to a defined transport of generated MHC complexes from MIIC to the BLM for T cell presentation, similar to the maturation process in DC. The fact that these changes were seen about equally in CD and UC suggests a disease-unspecific mechanism, which is most probably related secondarily to the inflammatory surrounding.
Similar to DC, we found modulation of the MHC I and II expression within the predominant MIIC, the MVB. Under inflammatory conditions during CD and UC, the quantity of both molecules increased significantly on the outer membrane. This increase seems not to be related exclusively to the increased targeting of MHC I and II antigens to MVB, as the density of MHC I within the compartment on the internal vesicles was found to be down-regulated simultaneously. With respect to MHC II our results did not yield a consistent conclusion, but similarly to MHC I we observed a relative increase of the outer membrane staining as opposed to the internal vesicles. These findings indicate that in IBD, MHC I and II complexes generated inside MVB were recruited to the limiting membranes for sorting to the BLM. However, our experiments do not allow depiction of the route from the MVB to the BLM. In professional APC this transport might occur, e.g. via vesicular intermediates, tubular formation or direct fusion of the MVB 22–24.
In summary, our study demonstrates that IBD impact the MIIC in IEC and the MHC I and II presentation pathway. The changes caused by CD and UC resemble elements of DC maturation, and might point to a similar maturation of IEC to APC. Although our descriptive data do not allow any functional conclusions, they argue in favour of secondary inflammatory changes in IEC during IBD that enable these cells to activate effector T cells and perpetuate the mucosal inflammation. Future studies will need to focus on the functional consequences of alterations in the MHC I and II pathways unravelled here.
Acknowledgments
This research was supported by a postgraduate scholarship from the Land Schleswig-Holstein GS Schl.-H. II, G.I.221-0-2-1 (to G. Hundorfean) and by grants from the Medical Faculty, University of Lübeck, JU04-2004 (to J. Büning).The authors thank Heidi Schlichting, Harry Manfeldt and Christo Örün for excellent technical assistance and Dr P. J. Peters and Dr J. J. Neefjes for the antibodies against MHC I and II.
Disclosure
Authors have no conflicts of interest.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Dotan I, Allez M, Nakazawa A, Brimnes J, Schulder-Katz M, Mayer L. Intestinal epithelial cells from inflammatory bowel disease patients preferentially stimulate CD4+ T cells to proliferate and secrete interferon-gamma. Am J Physiol Gastrointest Liver Physiol. 2007;292:1630–1640. doi: 10.1152/ajpgi.00294.2006. [DOI] [PubMed] [Google Scholar]
- 3.Bisping G, Lügering N, Lütke-Brintrup S, et al. Patients with inflammatory bowel disease (IBD) reveal increased induction capacity of intracellular interferon-gamma (IFN-gamma) in peripheral CD8+ lymphocytes co-cultured with intestinal epithelial cells. Clin Exp Immunol. 2001;123:15–22. doi: 10.1046/j.1365-2249.2001.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westendorf AM, Fleissner D, Groebe L, et al. CD4+Foxp3+ regulatory T cell expansion induced by antigen-driven interaction with intestinal epithelial cells independent of local dendritic cells. Gut. 2009;58:211–219. doi: 10.1136/gut.2008.151720. [DOI] [PubMed] [Google Scholar]
- 5.Stern LJ, Potolicchio I, Santambrogio L. MHC class II compartment subtypes: structure and function. Curr Opin Immunol. 2006;18:64–69. doi: 10.1016/j.coi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Burgdorf S, Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr Opin Immunol. 2008;20:89–95. doi: 10.1016/j.coi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells – polarity and complexity. Immunol Today. 2000;21:123–128. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 8.Zimmer KP, Büning J, Weber P, Kaiserlian D, Strobel S. Modulation of antigen trafficking to MHC class II-positive late endosomes of enterocytes. Gastroenterology. 2000;118:128–137. doi: 10.1016/s0016-5085(00)70421-5. [DOI] [PubMed] [Google Scholar]
- 9.Büning J, Schmitz M, Repenning B, et al. Interferon-gamma mediates antigen trafficking to MHC class II-positive late endosomes of enterocytes. Eur J Immunol. 2005;35:831–842. doi: 10.1002/eji.200425286. [DOI] [PubMed] [Google Scholar]
- 10.Büning J, Hundorfean G, Schmitz M, et al. Antigen targeting to MHC class II-enriched late endosomes in colonic epithelial cells: trafficking of luminal antigens studied in vivo in Crohn's colitis patients. FASEB J. 2006;20:359–361. doi: 10.1096/fj.05-4807fje. [DOI] [PubMed] [Google Scholar]
- 11.Hundorfean G, Zimmer KP, Strobel S, Gebert A, Ludwig D, Büning J. Luminal antigens access late endosomes of intestinal epithelial cells enriched in MHC I and MHC II molecules: in vivo study in Crohn's ileitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G798–808. doi: 10.1152/ajpgi.00135.2007. [DOI] [PubMed] [Google Scholar]
- 12.Büning J, von Smolinski D, Tafazzoli K, et al. Multivesicular bodies in intestinal epithelial cells: responsible for MHC class II-restricted antigen processing and origin of exosomes. Immunology. 2008;125:510–521. doi: 10.1111/j.1365-2567.2008.02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakabayashi A, Kumagai Y, Watari E, et al. Importance of gastrointestinal ingestion and macromolecular antigens in the vein for oral tolerance induction. Immunology. 2006;119:167–177. doi: 10.1111/j.1365-2567.2006.02418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths G. Fine structure immunocytochemistry. Heidelberg, Germany: Springer; 1993. [Google Scholar]
- 15.Lucocq JM, Habermann A, Watt S, Backer JM, Mayhew TM, Griffiths G. A rapid method for assessing the distribution of gold labeling on thin sections. J Histochem Cytochem. 2004;52:991–1000. doi: 10.1369/jhc.3A6178.2004. [DOI] [PubMed] [Google Scholar]
- 16.Rosenstiel P, Sina C, Franke A, Schreiber S. Towards a molecular risk map – recent advances on the etiology of inflammatory bowel disease. Semin Immunol. 2009;21:334–345. doi: 10.1016/j.smim.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Westendorf AM, Fleissner D, Hansen W, Buer J. T cells, dendritic cells and epithelial cells in intestinal homeostasis. Int J Med Microbiol. 2010;300:11–18. doi: 10.1016/j.ijmm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Kleijmeer MJ, Morkowski S, Griffith JM, Rudensky AY, Geuze HJ. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J Cell Biol. 1997;139:639–649. doi: 10.1083/jcb.139.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tjelle TE, Brech A, Juvet LK, Griffiths G, Berg T. Isolation and characterization of early endosomes, late endosomes and terminal lysosomes: their role in protein degradation. J Cell Sci. 1996;109:2905–2914. doi: 10.1242/jcs.109.12.2905. [DOI] [PubMed] [Google Scholar]
- 20.Barois N, de Saint-Vis B, Lebecque S, Geuze HJ, Kleijmeer MJ. MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic. 2002;3:894–905. doi: 10.1034/j.1600-0854.2002.31205.x. [DOI] [PubMed] [Google Scholar]
- 21.Mayer L, Eisenhardt D, Salomon P, Bauer W, Plous R, Piccinini L. Expression of class II molecules on intestinal epithelial cells in humans. Differences between normal and inflammatory bowel disease. Gastroenterology. 1991;100:3–12. doi: 10.1016/0016-5085(91)90575-6. [DOI] [PubMed] [Google Scholar]
- 22.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleijmeer M, Ramm G, Schuurhuis D, et al. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol. 2001;155:53–63. doi: 10.1083/jcb.200103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turley SJ, Inaba K, Garrett WS, et al. Transport of peptide–MHC class II complexes in developing dendritic cells. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]


