Abstract
The human immune system is orchestrated in a complex manner and protects the host against invading organisms and controls adequate immune responses to different antigen challenges in an endo-, auto- and paracrine-regulated fashion. The variety and intensity of immune responses are known to be dependent on stress-sensitive neural, humoral and metabolic pathways. The delayed-type hypersensitivity (DTH) skin test was a validated and standardized measure applied in clinical studies to monitor the integral function of cellular immune responses in vivo. The DTH skin test was, however, phased out in 2002. To obtain insight into the mechanisms of stress-sensitive immune reactions, we have developed an alternative in-vitro assay which allows the evaluation of antigen-dependent cellular immune responses triggered by T lymphocytes. The change in the concentration of proinflammatory cytokines in supernatant of the blood–antigen mixture is of particular interest to mirror the degree and adequacy of cellular immune responses. In this study we report that the proinflammatory cytokines interleukin (IL)-2, interferon (IFN)-γ and tumour necrosis factor (TNF)-α show a time-dependent increase upon ex-vivo bacterial, viral and fungal antigen stimulations. Furthermore, evidence is provided that this assay is sensitive to mirror stress hormone-mediated immune modulation in humans as shown either after hydrocortisone injection or after acute stress exposure during free fall in parabolic flight. This in-vitro test appears to be a suitable assay to sensitively mirror stress hormone-dependent inhibition of cellular immune responses in the human. Because of its standardization and relatively simple technical handling, it may also serve as an appropriate research tool in the field of psychoneuroendocrinology in clinical as in field studies.
Keywords: cytokine, DTH, hydrocortisone, in vitro, stimulation
Introduction
Humans are continuously subjected to environmental challenges which affect the immune function according to the intensity of psychological and physiological stressors. Due to the complex nature of in-vivo immune responses, the delayed-type hypersensitivity (DTH) skin test has served as a standardized tool to monitor the overall status of the immune system by simultaneously placing six antigens and one diluent (as a negative control) intracutaneously into the forearm. With the DTH skin test it was possible to evaluate, to a certain degree, the extent of immunodeficiency, as seen in individuals infected with the human immunodeficiency virus (HIV) 1. In addition to being used as a clinical investigative tool in immune deficiency states, the DTH skin test was also used widely to monitor immune function in states of psychological stress and psychiatric illness. Declines in immune function were found in subjects suffering from severe depression 2,3, in crews wintering in the Antarctic 4,5 and individuals experiencing perceived distress 6–9.
In 2002 this in-vivo skin test (multi-test CMI; Mérieux, Lyon, France) was removed from the market, in part because of the risk of antigen-sensitization when applied repeatedly to the same individual. After the DTH skin test was phased out, no such alternative tests were available to evaluate overall immunity. Standardized in-vitro methods such as the lymphocyte transformation test 10 and in-vitro cytokine induction 11 are used for the measurement of antigen-dependent T cell responses, but these tests are complicated in their performance and may not mirror the immune responses to the pathogenic spectrum that the DTH skin test was able to recall.
Even though the complex skin reaction of the DTH skin test – which includes, e.g. cell migration – cannot be reproduced fully in a whole-blood in-vitro system, DTH reactions also seem possible to be reflected in blood tests 12,13. We have therefore developed a new assay based on a comparable approach to the DTH skin test and its underlying mechanism of action, which are the responses of CD4+ or/ and CD8+ memory T cells to recall antigens 14,15. The new test relies on monitoring immune changes by a profile of proinflammatory cytokines released ex vivo from whole blood in response to specific antigen stimulation and incubation, respectively. However, unlike the DTH skin test, which covered only bacterial and fungal antigens, the in-vitro test presented in this study allows, in addition, the assessment of viral antigen-induced cytokine release. This ability to monitor immune responses to viral antigen challenges is particularly important in humans subjected to highly stressful environments and life events 16–20.
The goals of this study were to characterize this newly developed in-vitro assay and to test if it is suitable and applicable to measure stress hormone-sensitive immune modulation in humans. Therefore, we (1) determined first if there is a cytokine release from human whole blood exposed in vitro to different bacterial, viral and fungal antigens, and evaluated the time-dependent manner of cytokine release as well as the major source of the cell-dependent cytokine production; (2) characterized the immune modulatory effects of hydrocortisone in-vitro at concentrations shown to reflect stress-sensitive responses in humans 20–22; and (3) ascertained whether this test is suitable for monitoring stress hormone-sensitive immune modulation in humans by (i) injecting volunteers with a stress-dose of hydrocortisone (100 mg) or (ii) by subjecting volunteers to the acute stress model of free fall during parabolic flight.
Materials and methods
Blood samples
After ethical approval by the local ethics committee (NR:195/01; 107/11) and informed consent, blood was drawn from fasting healthy male participants (n = 13, age 38 ± 5 years) in the morning (7:30–8:30 a.m.) into a lithium-heparinized tube for the in-vitro test (5 ml) and into a standard serum tube for determination of blood cortisol levels (2 ml), respectively.
In-vitro test set-up
Whole blood, 500 μl, was transferred under aseptic conditions into each tube prefilled with an equal volume (500 μl) of Dulbecco's modified Eagle's medium (DMEM) nutrient mixture (F-12 HAM; Sigma-Aldrich, Steinheim, Germany) and the different stimulants (1000 μl total assay volume). The assay tubes contained DMEM only; DMEM and a bacterial antigen mixture containing diphterie-, tetanus- and pertussis-toxoid (all three combined in 1% Boostrix®; GlaxoSmithKline, Munich, Germany); DMEM and a viral antigen mixture containing cytomegalovirus (CMV) lysate (10 μg/ml; ABI, Columbia, SC, USA) and Epstein–Barr virus (EBV) lysate (10 μg/ml; ABI) and influenza antigens (1% Influvac®; Solvay, Hannover, Germany); DMEM and a fungal antigen mixture containing Candida lysate (10 μg/ml; Allergopharma, Reinbeck, Germany) and trichophyton lysate (10 μg/ml; Allergopharma, Reinbeck, Germany); DMEM and concanavalin A (ConA, 10 μg/ml; Sigma-Aldrich); or DMEM and pokeweed mitogen (PWM) (5 μg/ml; Sigma-Aldrich) as positive controls. While ConA is a T cell mitogen, PWM acts as a strong ‘polyclonal’ activator, inducing mitosis in lymphocytes in a non-receptor-specific manner. PWM was used in this study as a positive control.
The assay tubes were incubated for 48 h at 37°C. At 12-, 24- and 48-h time-points, 50 μl of the supernatant was transferred into Eppendorf tubes and frozen immediately at −80°C for future cytokine analyses. By rarefying these small supernatant volumes, significant dilution effects could be minimized.
Assessment of proinflammatory cytokine production
Frozen supernatants were measured in a blinded fashion after thawing. Concentrations of the prototypic T helper type 1 (Th1) cytokines IL-2, IFN-γ and TNF-α were analysed by LuminexxMAP® technology (Bioplex®) with commercially available reagents from BioRad Laboratories Inc. (Hercules, CA, USA), according to the manufacturer's guidelines. Data were analysed using Bioplex software; the sensitivity threshold was at 2 pg/ml for the analysed cytokines.
Cell-depletion assay
Biotinylated antibodies against CD3 (BioLegend Europe, Uithoorn, the Netherlands) were applied to lithium-heparinized blood. After an incubation period of 10 min anti-biotin MACSiBead™ particles (Miltenyi Biotec, Bergisch Gladbach, Germany) were added for 10 min. Mechanical cell separation took place in a cell separation magnet. Cell-depleted blood was transferred and added to the new cytokine release in-vitro test. Supernatant samples were taken after 24 and 48 h for further cytokine determination. To monitor and control the success of the T cell depletion, anti-CD3 fluorescein isothiocyanate (FITC)-marked antibodies were used subsequently to verify the T cell elimination by flow cytometry.
Intracellular cytokines
Immunostaining of cell surface antigens and intracellular cytokines in T cells were performed according to the manufacturer's guidelines. First, whole blood cultures with 1 ml total volume were treated for 6 h with 20 μl brefeldin A [1:10 dilution, BD Cat. no. 347688; Becton Dickinson Immunocytometry Systems, Palo Alto, CA, USA]. One ml of 1:10-diluted fluorescence activated cell sorter (FACS) lysing solution (BD Cat. no. 349202) was added to 200 μl whole blood from in-vitro stimulation. After 10 min incubation, samples were centrifuged (500 g for 5 min) and the supernatant decanted; 500 μl ×1 FACS permeabilizing solution 2 (BD Cat. no. 340973) was added after ‘vortexing’ for 10 min incubation at room temperature. After washing with phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin (BSA) and 0·1% NaN3 and 5 min centrifugation, 10 μl monoclonal antibodies were added and incubated for 30 min in the dark. Additional washing and resuspension of stained cells in PBS with 1% paraformaldehyde was performed. The following monoclonal antibodies (MAbs) directed against human leucocyte surface markers were used: FastImmune anti-interleukin (IL)-2/CD69/CD4/CD3 (BD Cat. no. 337188), CD4 peridinin chlorophyll (PerCP) (BD Cat. no. 345770) and CD3 allophycocyanin (APC) (BD Cat. no. 345767).
Samples were analysed in a FACSCalibur flow cytometer using CellQuest version 3·1 software (Becton Dickinson).
Cortisol measurement
Free serum or saliva cortisol was measured at the Institute of Clinical Chemistry, Hospital of the University of Munich, Germany by an electrochemiluminescence immunoassay (Elecsys 2010; Roche, Mannheim, Germany).
Testing for the effects of increasing in-vitro concentrations of hydrocortisone
Using the same test set-up as described above, whole blood (n = 7) was incubated in the presence of antigens together with increasing concentrations of hydrocortisone (HC). For each of the antigen compositions, three different hydrocortisone concentrations (20, 40 and 60 μg/dl) were added and the assay was incubated at 37°C. After 48 h of incubation the supernatants were harvested as described.
Testing for the effects of hydrocortisone injected into humans
After basal blood-drawing, 100 mg hydrocortisone was injected intravenously (n = 7). Blood was collected 1 h and 24 h thereafter. At each time-point whole blood was drawn for serum cortisol measurement and incubation with the new in-vitro test. Supernatant was collected after 48 h of incubation.
Field application of the new in-vitro test
In the acute stress model of free fall during parabolic flight, pre- and post-flight saliva was collected in Salivettes® (Sarstedt, Nümbrecht, Germany) for cortisol measurement, and blood was drawn for the in-vitro test at the same time-points. Parabolic flights were performed with an Airbus A300 ZeroG (Novespace, Paris, France). One parabolic flight manoeuvre results in approximately 22 s of free fall. In total, 31 parabolas were flown in 1 flight day.
Statistics
Normal distribution of sample data was tested using the Kolmogorov–Smirnov test. All normal distributed data were tested with a paired t-test. Concerning multiple comparisons, Bonferroni's correction was applied. For repeated measurements within groups, repeated-measures analysis of variance (one-way RM-anova) was calculated followed by Fisher's post-hoc least significant difference (LSD) test. Results were statistically significant if P < 0·05. Results are expressed as mean ± standard error of the mean (s.e.m.) (spss version 15·0; SPSS, Inc., Chicago, IL, USA).
Results
Cytokine release (IL-2, IFN-γ, TNF-α) as a function of antigen stimuli and time of incubation
The proinflammatory cytokine IL-2 showed a significant increase over time when challenged with bacterial, viral and fungal antigens as well as with the positive control PWM and peaked after 24–48 h of incubation. IFN-γ concentrations doubled after 24 h with bacterial and viral antigen stimulation, but remained low following stimulation with fungal antigens. In contrast, TNF-α peaked earlier and significantly at 12 h after viral and 24 h after bacterial antigen challenges (see Table 1). The antigen-free negative control showed only minor cytokine release at all time-points, whereas the positive selective control with ConA and the overall control with PWM resulted in an appropriate cytokine release, with peak concentrations for IL-2 and IFN-γ at 48 h and for TNF-α at 12 h. The T cell mitogen, ConA, followed the kinetic and dynamic of cytokines compared to the stimulations of bacterial, viral and fungal antigens (ConA IL-2 (pg/ml): 12 h: 15·87 ± 0·055; 24 h: 125·00 ± 18·58; 48 h: 141·00 ± 30·90/TNF-α 12 h: 101·01 ± 32·57; 24 h: 220·61 ± 97·40; 48 h: 244·64 ± 49·86/IFN-γ 12 h: 195·64 ± 28·48; 24 h: 670·84 ± 76·44; 48 h: 2274·01 ± 19·11).
Table 1.
Concentrations of proinflammatory cytokines after bacterial, viral and fungal antigen incubation. In coherence with the former delayed-type hypersensitivity (DTH) skin test also a negative control (media + blood without stimuli) is included. To quantify maximal cytokine release from the blood tested, pokeweed mitogen (PWM) is used as positive control in a separate assay. Additionally, baseline plasma cytokine levels are presented at the 0 h time-point
| Time | IL-2 | IFN-γ | TNF-α | |
|---|---|---|---|---|
| Plasma cytokines | 0 h | 0·18 ± 0·17 | 5·98 ± 1·23 | 5·51 ± 0·95 |
| Bacteria | 12 h | 19·50 ± 9·11* | 233·92 ± 113·38* | 4 082·35 ± 2 387·13 |
| 24 h | 106·86 ± 36·17* | 508·11 ± 250·06* | 913·80 ± 427·66* | |
| 48 h | 177·69 ± 53·00* | 825·45 ± 352·89* | 124·19 ± 47·44* | |
| Virus | 12 h | 12·13 ± 4·30* | 70·70 ± 35·37 | 500·46 ± 193·24* |
| 24 h | 49·68 ± 14·15* | 141·38 ± 87·87 | 531·08 ± 269·28* | |
| 48 h | 47·58 ± 12·63* | 165·83 ± 108·43 | 55·96 ± 14·63* | |
| Fungi | 12 h | 3·05 ± 0·99* | 19·53 ± 10·68 | 425·19 ± 288·38 |
| 24 h | 13·53 ± 2·92* | 18·48 ± 11·81* | 273·62 ± 171·55 | |
| 48 h | 61·65 ± 14·90* | 15·10 ± 6·54 | 45·61 ± 36·21 | |
| Positive control | 12 h | 167·58 ± 39·99* | 1680·23 ± 344·65* | 10 714·27 ± 2 105·79* |
| 24 h | 499·07 ± 78·51* | 3894·66 ± 1395·11* | 7 296·07 ± 1 741·89* | |
| 48 h | 3719·38 ± 2005·80* | 6093·11 ± 1910·24* | 4 123·07 ± 1 472·80* | |
| Negative control | 12 h | 0·42 ± 0·31 | 9·85 ± 6·53 | 6·07 ± 2·46 |
| 24 h | 0·65 ± 0·55 | 7·56 ± 6·03 | 2·94 ± 1·21 | |
| 48 h | 0·06 ± 0·04 | 3·24 ± 1·46 | 1·79 ± 0·93 |
Values are given as mean ± standard error of the mean, unit (pg/ml); n = 13.
P < 0·05 versus negative control for bacteria, virus, fungi or positive control (t-test). IL: interleukin; IFN: interferon; TNF: tumour necrosis factor.
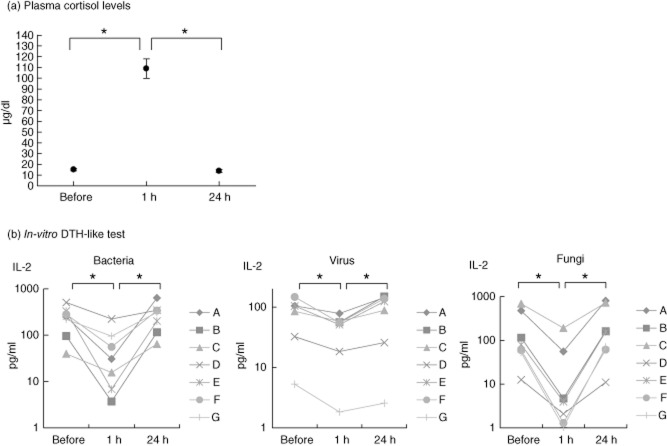
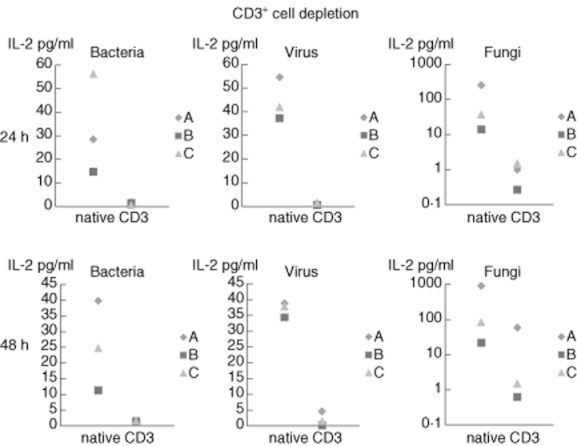
Testing for the primary source of IL-2 production when challenged by the different antigens showed that depletion from CD3+ cells resulted in a blunted IL-2 cytokine response (Fig. 1). Confirmatively, intracellular cytokine measurement in non-cell-depleted whole blood identified CD4+ cells as the primary source for IL-2 after stimulation with antigens from bacteria, virus and fungi (Fig. 2).
Fig. 1.
CD3+ cell depletions, concentrations of interleukin-2 in individual blood preparations; for better visibility figures for ‘Fungi’ are presented on a logarithmic scale.
Fig. 2.
Intracellular interleukin (IL)-2 staining in CD4+ and CD8+ T cells (representative sample).
Characterization of immunomodulatory effects of hydrocortisone in stress-relevant concentrations on the new cytokine release assay
Co-incubation of the test assay (whole blood taken from healthy and unstressed volunteers) with increasing concentrations of hydrocortisone (20, 40, 60 μg/dl) resulted in a significant reduction in IL-2 levels in all three stimulation assays with bacterial, viral and fungal antigen stimulation. The level of statistical significance for hydrocortisone to reduce IL-2 release was reached in all groups at 48 h (Fig. 3).
Fig. 3.

In-vitro Hydrocortisone effects on interleukin-2; (HC: hydrocortisone), *P < 0·05 different HC concentrations versus basal at 48 h, one-way repeated-measures (RM) analysis of variance (anova) followed by Fisher's least significant difference (LSD) post-hoc test, n = 7.
Assessment of stress hormone-sensitive immune modulation in humans as tested by the new cytokine release assay
Effects of hydrocortisone injection at a stress dose of 100 mg
After intravenous (i.v.) injection of hydrocortisone (100 mg) the blood cortisol levels increased significantly (1 h). At the same time, blood was taken and the new test was performed. The concentrations of IL-2 decreased irrespective of the antigen stimulus in all subjects by 50–90% (bacterial antigens: 76·45 ± 6·99; viral antigens: 46·51 ± 6·57; fungal antigens: 90·10 ± 3·63; pg/ml, mean ± s.e.m., Fig. 4). At 24 h after hydrocortisone injection, both blood cortisol concentrations as well as the in-vitro immune test responses returned to normal values.
Fig. 4.
In-vivo application of hydrocortisone (100 mg); (a) plasma cortisol levels, (b) individual responses to delayed-type hypersensitivity (DTH)-resembling in-vitro test, logarithmic scale; *P < 0·05; one-way repeated-measures (RM) analysis of variance (anova) followed by Fisher's least significant difference (LSD) test.
Effects of free fall (parabolic flights)
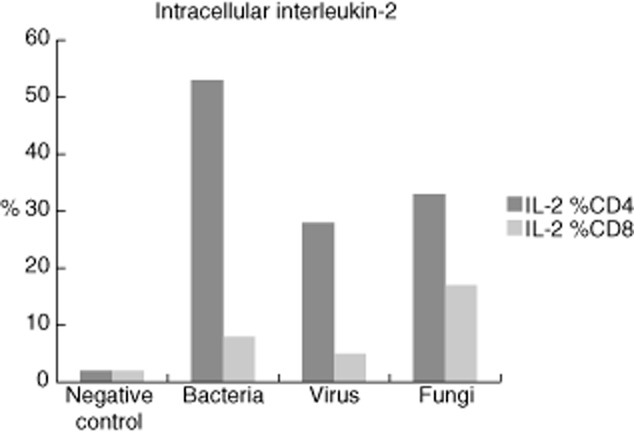
The cytokine plasma responses were analysed in volunteers completing a parabolic flight campaign. Data were distinguished by a median split in participants who showed either high or low saliva cortisol levels after parabolic flight [high cortisol = 0·56 ± 0·087 μg/dl, n = 4; low cortisol = 0·21 ± 0·090 μg/dl, n = 8; P < 0·01; mean ± standard deviation (s.d.)]. The individual data from the participants with high cortisol levels after parabolic flight showed decreased IL-2 concentrations in the new test compared to pre-flight values (Fig. 5). In contrast, lower cortisol values were associated with higher in-vitro cytokine release responses.
Fig. 5.
Interleukin-2 levels in the new in-vitro test pre- and post-parabolic flight in subjects with low (n = 8) or high post-flight cortisol (n = 4).
Discussion
To the best of our knowledge, since the removal of Merieux's multi-test DTH from the market no such standardized alternative test has been available to measure the overall immune response from whole blood. This study presents a new in-vitro cytokine release immune test, monitoring overall cell-mediated immune reactions to recall antigens in a highly standardized fashion using a three-step process: (i) blood collection; (ii) ex-vivo incubation; and (iii) cytokine determination from the assay supernatant. The selected antigens include some of the ‘classic’ antigens which had been used in the DTH skin test, such as bacterial and fungal antigens, but extended the scope of the test by including viral antigens for EBV, CMV and influenza virus. The rationale is that EBV and CMV are endemic, with a high prevalence of latent infection 23,24, conditions that make it a suitable target antigen to mirror stress-related immune impairment. Influenza was included in the viral antigen mix, as it is known to initiate the adaptive immune response, provoking a multi-step process with a sudden ‘cytokine storm’ at 48 h 25.
General mechanisms of immune defence
In general, the immune defence of the human body is a multi-step process triggered and executed by different cell defence lines. The major sources of cell-mediated immune response are leucocytes, whereby B and T cells and their release of cytokines play the most important role. The test presented in this study reflects reactions of both cell types and also of other defence lines as represented, e.g. by macrophages. As the human immune system is a complex organ, the in-vitro test in this study is testing for the overall response. The two important mechanisms are the B cells and their capacity to produce antibodies, and more importantly the T cell activation followed by the T cell-dependent and -independent B cell activation 26. Cytokines play a key role in these activation processes. Recent investigations found that the cytokine release is not only limited to T cells but that B cells also have the potency and capacity to produce cytokines 27. For this reason, the test introduced in this study uses the cytokine responses as a read-out parameter, reflecting both cell lines.
Cytokine patterns as a function of antigen stimuli and time of incubation
Testing for the most suitable and representative read-out parameters to mirror a DTH-like immune response, we focused on three representative cytokines which are involved in T cell-mediated immune responses: IL-2, IFN-γ and TNF-α.
IL-2 is one of the key cytokines involved in T cell activation and proliferation 28. After incubation with the different antigens of either a bacterial, viral or fungal nature, the concentrations of IL-2 in the culture supernatants increased significantly at 24 h and even more significantly at 48 h after onset of incubation, reflecting a strong and time-dependent Th1 response. Moreover, IL-2 is known to be a potent inductor of IFN-γ during Th1 and Th2 differentiation 29. In addition, IFN-γ has been also identified previously as one of the important cytokines involved in mediating skin DTH reactions 30. Accordingly, the time kinetic of IFN-γ followed mainly the IL-2 slope, and showed high concentrations at 24 and 48 h.
TNF-α secreted by macrophages as well as by T cells is a potent initiator, enhancer and primer of T cell signalling and activation 31 in the inflammatory cascade. It is known to be released very early in the inflammation process 32. This was confirmed by our findings showing peak levels of TNF-α for bacterial, viral and fungal antigen stimulation as early as after 12 h after onset of incubation. This is in further accordance with previous results from virus-induced TNF-α secretion, which also occurs very early in the inflammatory process 33,34.
Incubation for 48 h appears to be an adequate duration for monitoring the late cell-mediated immune reaction equivalent, as has been best mirrored by IL-2 due to the lower statistical spread after each of the respective antigen stimulations, e.g. when compared to IFN-γ. The statistical spread as seen in Table 1, especially for TNF-α and IFN-γ, can be explained by the interindividual variability of immune response to different antigens. After depletion of CD3+ cells, IL-2 production was suppressed entirely, thereby identifying the major source of IL-2 production in this test. For further verification and to confirm the depletion experiments the intracellular sources of IL-2 production were determined to be, in particular, CD3+/CD4+ cells. This confirms the results from Ladel et al. on the role of CD4+ cells in DTH reactions 35.
Hydrocortisone in stress-relevant concentrations suppressed the new in-vitro test responses
Both acute and chronic stress induce neuro-humoral and metabolic responses. A hallmark of stress responses is the activation of the autonomic nervous system and the hypothalamo–pituitary axis affecting cardiovascular, metabolic and cognitive pathways as well as the regulation of immune responses 36,37. Inadequate neuro-humoral regulation secondary to chronic stress, for example, can result in an impaired host-immune response when challenged with infectious agents. Because the alteration in immune homeostasis can impair health, the assessment of overall immune responsiveness is an attractive and necessary clinical and research strategy in the field of stress-immune physiology. To test whether the new in-vitro test is suitable to monitor immune responses affected by stress hormones, we co-incubated whole blood with increasing hydrocortisone concentrations, representing incrementing physiological stress cortisol levels. The lowest concentration of hydrocortisone added was 20 μg/dl, reflecting normal cortisol plasma levels 21,22, while 40 μg/dl is a concentration seen comparably in highly stressed people, and 60 μg/dl is comparable to patients taking oral or continuous intravenous cortisone supplementation 21, respectively. We demonstrated that hydrocortisone concentrations resulted in significant and proportional immune suppression, as quantified by a reduction in IL-2 concentrations up to 60%.
This in-vitro test can monitor stress hormone-sensitive immune modulation in humans after pharmacological (hydrocortisone) or real (free fall in parabolic flight) stress challenges
Injection of a single therapeutic dose of hydrocortisone showed a clear and highly significant suppressive effect on IL-2 concentrations in response to the antigen stimulation. Because this effect was reverted the next day, this demonstrates the role of in-vivo hydrocortisone concentrations to be related inversely to the new in-vitro test responses upon stimulation.
The question of whether or not these iatrogenic-provoked, pharmacological effects of corticoids on the new in-vitro immune test results can – at least partially – be also reflected by intrinsic elevation of cortisol concentrations, has been tested in another set-up: blood was drawn from healthy volunteers undergoing a parabolic flight mission and tested for the in-vitro test responses. The volunteers had not been subjected previously to such a condition of stress provoked by a repeated free fall experience in a specially designed plane that followed the slope of a parabola 38. In accordance to the results seen when hydrocortisone was injected, the immune test responses were linked inversely to the cortisol responses: participants with low to normal post-flight cortisol values showed higher IL-2 responses in the in-vitro assay, while participants with elevated cortisol levels had, inversely, less pronounced IL-2 responses. This reflects the properties of this new assay to mirror the consequences of stress-mediated cortisol release on the cellular immune functions when challenged to recall antigens.
Commonalities and differences of the classical DTH skin test
The test described in this report includes some key elements of the former skin DTH reaction and also shows relevant similarities with respect to read-out time-points and the modulation through hormones released under stressful conditions. However, it cannot claim to mirror entirely, and hence replace, the classical skin DTH first, and most importantly, because a one-to-one comparison of both tests is no longer realizable, as the DTH skin test was phased-out 10 years ago. Secondly, this whole blood test seems limited in mirroring the reactions of tissue immune cells in the skin in triggering DTH immune reactions upon intracutaneously placed antigens while, conversely, some evidence exists that DTH reactions are considered to be not only limited to the skin, and skin DTH reactions with antigen-specific T cells such as nickel-contact eczema are also detectable in blood 12,13.
Therefore, the assay presented indicates a more ‘universal’ in-vitro test for demonstrating antigen-dependent memory and effector cell reactions with additional aspects to those implemented into the former Merieux test, i.e. by addressing challenges to viral antigens.
Outlook
Based on the questions addressed in this series of investigations, this in-vitro test could offer an effective system for monitoring changes in the overall immune response. Moreover, this test aims to be a more universal in-vitro system for demonstrating antigen-dependent memory and effector cell reactions to viral antigens, which was not addressed in the previous Merieux DTH, and in addition seems to be an adequate tool for monitoring the effects of stress-permissive hormones on overall immune responses.
Longitudinal studies are needed to investigate the use of this in-vitro immune test under similar clinical and research conditions to those used with the DTH skin test 7,30,39, e.g. in patients with HIV 40, in heart-transplanted 41 and intensive care patients 42, respectively.
In summary, the evaluation of this new in-vitro cytokine release immune assay shows that the release of a panel of physiologically relevant proinflammatory cytokines can be induced gradually by standard sets of bacterial, viral and fungal recall antigen compositions, thus giving an indication of cellular immune responses in whole blood taken from healthy adults. The immune modulatory effects of stress-relevant hydrocortisone concentrations on IL-2 cytokine production were also mirrored directly from stressed subjects under field conditions. Even though testing for DTH response cascades in-vitro is limited by default, the use of some key elements of the former DTH skin test in this new cytokine release assay might help to fill the gap left following the discontinuation of the classical DTH skin test. Also, because of its standardization and simplicity, it may be a particularly suitable research tool in the field of psychoneuroendocrinology in clinical, as well as under extreme field conditions, such as in space flight experiments.
Acknowledgments
The authors are grateful for the intramural, institutional support of the Department of Anaesthesiology. The experimental part of the study using the model of parabolic flights was supported generously by a grant from the German National Space Program by the German Space Agency (DLR) on behalf of the Federal Ministry of Economics and Technology (BMWi 50WB0523 and 50WB0719) and was also supported by the European Space Agency (ESA) and the Centre National d'Etudes Spatiales (CNES). The authors thank all the volunteers, who participated with extreme professionalism in this study, and extend their appreciation to the efficient support from DLR (Dr U. Friedrich, Dr H.-U. Hoffmann) and NOVESPACE (F. Gai) during preparation and performance of this investigation. This investigation is part of the MD theses of Markus Gruber and Florian Muckenthaler.
Disclosure
W.M. is affiliated to Immumed Inc., a laboratory for applied immunology offering a testing service for immunological parameters to commercial, medical and research clients.
References
- 1.Kornbluth RS, McCutchan JA. Skin-test responses as predictors of tuberculous infection and of progression in HIV-infected persons. Ann Intern Med. 1993;119:241–243. doi: 10.7326/0003-4819-119-3-199308010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Hickie I, Hickie C, Bennett B, et al. Biochemical correlates of in-vivo cell-mediated immune dysfunction in patients with depression – a preliminary report. Int J Immunopharmacol. 1995;17:685–690. doi: 10.1016/0192-0561(95)00055-7. [DOI] [PubMed] [Google Scholar]
- 3.Hickie I, Hickie C, Lloyd A, Silove D, Wakefield D. Impaired in vivo immune responses in patients with melancholia. Br J Psychiatry. 1993;162:651–657. doi: 10.1192/bjp.162.5.651. [DOI] [PubMed] [Google Scholar]
- 4.Williams DL, Climie A, Muller HK, Lugg DJ. Cell-mediated immunity in healthy adults in Antarctica and the sub-Antarctic. J Clin Lab Immunol. 1986;20:43–49. [PubMed] [Google Scholar]
- 5.Thom O, Lugg DJ. Cell mediated immunity and alcohol intake in Antarctic wintering personnel. Int J Circumpolar Health. 2002;61:208–215. doi: 10.3402/ijch.v61i3.17454. [DOI] [PubMed] [Google Scholar]
- 6.Vedhara K, Nott K. The assessment of the emotional and immunological consequences of examination stress. J Behav Med. 1996;19:467–478. doi: 10.1007/BF01857679. [DOI] [PubMed] [Google Scholar]
- 7.Smith AJ, Vollmer-Conna U, Bennett B, Hickie IB, Lloyd AR. Influences of distress and alcohol consumption on the development of a delayed-type hypersensitivity skin test response. Psychosom Med. 2004;66:614–619. doi: 10.1097/01.psy.0000130962.28801.af. [DOI] [PubMed] [Google Scholar]
- 8.Cogoli A. The effect of space flight on human cellular immunity. Environ Med. 1993;37:107–116. [PubMed] [Google Scholar]
- 9.Turner-Cobb JM, Koopman C, Rabinowitz JD, Terr AI, Sephton SE, Spiegel D. The interaction of social network size and stressful life events predict delayed-type hypersensitivity among women with metastatic breast cancer. Int J Psychophysiol. 2004;54:241–249. doi: 10.1016/j.ijpsycho.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 10.von Baehr V, Mayer W, Liebenthal C, von Baehr R, Bieger W, Volk HD. Improving the in vitro antigen specific T cell proliferation assay: the use of interferon-alpha to elicit antigen specific stimulation and decrease bystander proliferation. J Immunol Methods. 2001;251:63–71. doi: 10.1016/s0022-1759(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 11.He Q, Tran Minh NN, Edelman K, Viljanen MK, Arvilommi H, Mertsola J. Cytokine mRNA expression and proliferative responses induced by pertussis toxin, filamentous hemagglutinin, and pertactin of Bordetella pertussis in the peripheral blood mononuclear cells of infected and immunized schoolchildren and adults. Infect Immun. 1998;66:3796–3801. doi: 10.1128/iai.66.8.3796-3801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minang JT, Arestrom I, Troye-Blomberg M, Lundeberg L, Ahlborg N. Nickel, cobalt, chromium, palladium and gold induce a mixed Th1- and Th2-type cytokine response in vitro in subjects with contact allergy to the respective metals. Clin Exp Immunol. 2006;146:417–426. doi: 10.1111/j.1365-2249.2006.03226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rustemeyer T, von Blomberg BM, van Hoogstraten IM, Bruynzeel DP, Scheper RJ. Analysis of effector and regulatory immune reactivity to nickel. Clin Exp Allergy. 2004;34:1458–1466. doi: 10.1111/j.1365-2222.2004.02045.x. [DOI] [PubMed] [Google Scholar]
- 14.Dannenberg AM., Jr Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 15.Black CA. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol Online J. 1999;5:7. [PubMed] [Google Scholar]
- 16.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–179. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- 17.Coskun O, Sener K, Kilic S, et al. Stress-related Epstein–Barr virus reactivation. Clin Exp Med. 2010;10:15–20. doi: 10.1007/s10238-009-0063-z. [DOI] [PubMed] [Google Scholar]
- 18.Glaser R, Friedman SB, Smyth J, et al. The differential impact of training stress and final examination stress on herpesvirus latency at the United States Military Academy at West Point. Brain Behav Immun. 1999;13:240–251. doi: 10.1006/brbi.1999.0566. [DOI] [PubMed] [Google Scholar]
- 19.Stowe RP, Pierson DL, Barrett AD. Elevated stress hormone levels relate to Epstein–Barr virus reactivation in astronauts. Psychosom Med. 2001;63:891–895. doi: 10.1097/00006842-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80:1116–1122. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oppert M, Reinicke A, Graf KJ, Barckow D, Frei U, Eckardt KU. Plasma cortisol levels before and during ‘low-dose’ hydrocortisone therapy and their relationship to hemodynamic improvement in patients with septic shock. Intensive Care Med. 2000;26:1747–1755. doi: 10.1007/s001340000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeager MP, Pioli PA, Wardwell K, et al. In vivo exposure to high or low cortisol has biphasic effects on inflammatory response pathways of human monocytes. Anesth Analg. 2008;107:1726–1734. doi: 10.1213/ane.0b013e3181875fb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess RD. Routine Epstein–Barr virus diagnostics from the laboratory perspective: still challenging after 35 years. J Clin Microbiol. 2004;42:3381–3387. doi: 10.1128/JCM.42.8.3381-3387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moltedo B, Lopez CB, Pazos M, Becker MI, Hermesh T, Moran TM. Cutting edge: stealth influenza virus replication precedes the initiation of adaptive immunity. J Immunol. 2009;183:3569–3573. doi: 10.4049/jimmunol.0900091. [DOI] [PubMed] [Google Scholar]
- 26.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 28.Hoyer KK, Dooms H, Barron L, Abbas AK. Interleukin-2 in the development and control of inflammatory disease. Immunol Rev. 2008;226:19–28. doi: 10.1111/j.1600-065X.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 29.Seder RA, Germain RN, Linsley PS, Paul WE. CD28-mediated costimulation of interleukin 2 (IL-2) production plays a critical role in T cell priming for IL-4 and interferon gamma production. J Exp Med. 1994;179:299–304. doi: 10.1084/jem.179.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhabhar FS, Satoskar AR, Bluethmann H, David JR, McEwen BS. Stress-induced enhancement of skin immune function: a role for gamma interferon. Proc Natl Acad Sci USA. 2000;97:2846–2851. doi: 10.1073/pnas.050569397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKarns SC, Schwartz RH. Biphasic regulation of Il2 transcription in CD4+ T cells: roles for TNF-alpha receptor signaling and chromatin structure. J Immunol. 2008;181:1272–1281. doi: 10.4049/jimmunol.181.2.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger D, Dayer JM. Cytokines, acute-phase proteins, and hormones: IL-1 and TNF-alpha production in contact-mediated activation of monocytes by T lymphocytes. Ann NY Acad Sci. 2002;966:464–473. doi: 10.1111/j.1749-6632.2002.tb04248.x. [DOI] [PubMed] [Google Scholar]
- 33.Hui KP, Lee SM, Cheung CY, et al. Induction of proinflammatory cytokines in primary human macrophages by influenza A virus (H5N1) is selectively regulated by IFN regulatory factor 3 and p38 MAPK. J Immunol. 2009;182:1088–1098. doi: 10.4049/jimmunol.182.2.1088. [DOI] [PubMed] [Google Scholar]
- 34.Peiris JS, Poon LM, Nicholls JM, Guan Y. The role of influenza virus gene constellation and viral morphology on cytokine induction, pathogenesis, and viral virulence. Hong Kong Med J. 2009;15:21–23. [PubMed] [Google Scholar]
- 35.Ladel CH, Daugelat S, Kaufmann SH. Immune response to Mycobacterium bovis bacille Calmette Guerin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 36.Bellinger DL, Millar BA, Perez S, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 38.Kaufmann I, Schachtner T, Feuerecker M, Schelling G, Thiel M, Chouker A. Parabolic flight primes cytotoxic capabilities of polymorphonuclear leucocytes in humans. Eur J Clin Invest. 2009;39:723–728. doi: 10.1111/j.1365-2362.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- 39.Altemus M, Dhabhar FS, Yang R. Immune function in PTSD. Ann NY Acad Sci. 2006;1071:167–183. doi: 10.1196/annals.1364.013. [DOI] [PubMed] [Google Scholar]
- 40.Karlsson A, Moberg L, Bratt G, et al. An eleven year follow-up of delayed-type hypersensitivity testing for the identification of HIV-1 infected patients at increased risk of developing AIDS. Scand J Infect Dis. 1996;28:125–130. doi: 10.3109/00365549609049062. [DOI] [PubMed] [Google Scholar]
- 41.Liu AY, Wagner WO, Piedmonte MR, Stewart RW. Anergic response to delayed hypersensitivity skin testing. A predictor of early mortality in heart transplant recipients. Chest. 1993;104:1668–1672. doi: 10.1378/chest.104.6.1668. [DOI] [PubMed] [Google Scholar]
- 42.Christou NV, Meakins JL, Gordon J, et al. The delayed hypersensitivity response and host resistance in surgical patients. 20 years later. Ann Surg. 1995;222:534–546. doi: 10.1097/00000658-199522240-00011. discussion 46–8. [DOI] [PMC free article] [PubMed] [Google Scholar]