Abstract
Oxazolone-induced colitis in mice has become a recognized model to study the efficacy of therapeutics targeting the immunological response underlying the development of inflammatory bowel disease. However, this model cannot be used when therapeutics designed to address human targets do not interact with the respective murine counterpart. In this study, we examined the induction of oxazolone mediated colitis in non-obese diabetic-severe combined immunodeficiency interleukin-2Rγnull (NOD-SCID IL2Rγnull) mice engrafted with human peripheral blood mononuclear cells (hPBMC) derived from patients suffering from ulcerative colitis (UC), atopic dermatitis (AD) and healthy volunteers. NOD-SCID IL2Rγ null mice were engrafted with hPBMC followed by challenge with oxazolone or ethanol vehicle. Mice developed the same symptoms as observed previously in immunocompetent mice. The clinical activity score increased and the colon architecture was characterized by the development of oedema, fibrosis, crypt loss and dense infiltration of predominantly T cells into the lamina propria. Fluorescence activated cell sorter (FACS) analysis of lymphocytes in the colon identified natural killer (NK) T cells as a major constituent. In contrast to studies with immunocompetent mice, we observed the same phenotype in the group challenged with ethanol vehicle. The phenotype was most pronounced in mice engrafted with PBMC derived from a patient suffering from UC, suggesting that the immunological history of the donors predisposes the engrafted mice to react to ethanol. The model described here has the potential to study the efficacy of therapeutics targeting human lymphocytes in a model which is more reflective of the human disease. In addition, it might be developed to elucidate molecular mechanisms underlying the disease.
Keywords: animal models/studies – mice/rats, CD1-restricted/natural killer T cells (NK T cells), CD4 T cells (T helper, Th0, Th1, Th2, Th3, Th17), inflammatory bowel disease
Introduction
Ulcerative colitis (UC) and atopic dermatitis (AD) belong to the class of relapsing and chronic inflammatory diseases. Although the clinical and pathological manifestations of UC and AD are quite different, they share similar immunological pathways leading to the generation of phenotypic symptoms. Both are driven by a T helper type 2 (Th2) inflammation signified by the respective cytokine profile of interleukins (IL)-4, 5 and 13 1–4. IL-4 and IL-13 have been shown to play an important role in initiating and sustaining AD 5, whereas in UC IL-13 secreted by natural killer (NK) T cells seems to be the key effector cytokine 4,6. Both interleukins activate the type II IL-4 receptor complex consisting of IL-4 receptor α and IL-13 receptor α1, resulting in the generation of phenotypic symptoms such as immunoglobulin (Ig)E secretion, fibrosis, epithelial hyperplasia and barrier dysfunction 7,8. It is well recognized that the development of AD and UC rely on a genetic predisposition; however, the molecular mechanisms underlying these diseases are unclear.
Oxazolone-induced UC and AD mimic the human diseases in many respects. Oxazolone is thought to be a haptenizing agent which penetrates the mucosal barrier and triggers an immunological response. When applied to the skin of hairless mice (hr/hr) in low doses for a period of 3 weeks, mice develop symptoms characteristic of AD to include barrier dysfunction, secretion of IgE, epithelial cell hyperplasia, fibrosis and influx of inflammatory cells into the dermis and epidermis and secretion of Th2-characterized interleukins 9. Similarly, when oxazolone is applied rectally, C57BI/10 or BALB/c mice develop colitis-like symptoms 4,10. The clinical activity score increases and histological inspection of the colon reveals an influx of inflammatory cells into the lamina propria (LP), the destruction of the mucosa, fibrosis and secretion of IL-4 and IL-13 by CD4 cells and NK T cells.
In this study, we report the development of oxazolone-induced colitis in non-obese diabetic-severe combined immunodeficiency interleukin-2Rγnull (NOD-SCID IL-2Rγnull) mice engrafted with human peripheral blood mononuclear cells (hPBMC) derived from patients suffering from UC and AD and from a healthy volunteer. Mice developed the same symptoms as observed previously for immunocompetent mice, including weight loss and diarrhoea. Challenge with oxazolone resulted in a mixed inflammatory cell infiltrate into the LP consisting of human T and B cells and neutrophils and NK T cells. Colon architecture was characterized by the development of oedema, fibrosis and crypt loss. Engraftment alone without further challenge was not sufficient to cause UC; however, in contrast to the results obtained in immunocompetent mice, ethanol vehicle alone induced UC-like symptoms and phenotype. Non-engrafted mice challenged with oxazolone died within several hours, suggesting that oxazolone might have a severe toxic effect which is mitigated by hPBMC.
Material and methods
Ethical considerations
All donors gave informed written consent and the study was approved by the Institutional Review Board (IRB) of the Medical Faculty at the University of Munich.
All animal studies were approved by the Ethics Committee for animal use of the government of Upper Bavaria (55·2-1-54-2532-65-11), Germany, and performed in compliance with German animal welfare laws and policies.
Isolation and engraftment of human PBMC
Peripheral blood was collected from patients suffering from UC (one), AD (three) and one healthy volunteer from the arm vein. Human PBMC were purified by Ficoll density centrifugation. Thirty ml blood in trisodium citrate solution was diluted with 30 ml Hanks's balanced salt solution (HBSS) (Sigma Aldrich, Deisenhofen, Germany) and loaded onto Leucosep tubes (Greiner Bio One, Frickenhausen, Germany). Cells were separated at 800 g for 15 min, according to the manufacturer's instructions. Human PBMC were isolated, washed in HBSS supplemented with 2500 IE heparin natrium (Braun) and resuspended in phosphate-buffered saline (PBS) at a concentration of 20 × 106/ml.
NOD-SCID IL-2Rγnull mice, 6–16 weeks old, were engrafted with 200 μl of the cell suspension by intravenous injection. The animals rested for 7 days prior to first sensitization with oxazolone.
Cell culture
hPBMC (4 × 106) resuspended in 2 ml RPMI-1640, 10% fetal calf serum (FCS), 1 mM sodium pyruvate, 1% (100 U and 100 mg) penicillin/streptomycin and 2% glutamine (Sigma, Deisenhofen, Germany) were incubated for 14 days in a 24-well plates with IL-4 (50 ng/ml) and 1 μl anti-CD 40 at 1 μg/ml (BD Bioscience, Heidelberg, Germany), as described previously 11,12.
Study protocol
BALB/c mice were obtained from Janvier Europe (Saint Berthevin, France). NOD.cg-PrkdcSCID Il2rgtm1Wjl/Szj (abbreviated as NOD IL-2Rγnull) mice were obtained from Charles River Laboratories (Sulzfeld, Germany). The mice were kept under specific pathogen-free conditions in individually ventilated cages. The facility is controlled by Federation for Laboratory Animal Science Association (FELASA) guidelines.
BALB/c mice, 6 weeks to 4 months old, or NOD-SCID IL-2Rγnull mice 7 days post-engraftment, were treated as described previously by Heller et al. 4. Following anaesthesia with isoflurane, a 2 × 2-cm skin area (region lumbalis) was shaved and depilated on day 1. Animals were presensitized by topical application of 20 μl 5% oxazolone (4-ethoxymethylene-2-phenyl-oxazolin-5-one) (Sigma-Aldrich, Deisenhofen, Germany) in 100% ethanol.
On day 8 mice were challenged with 150 μl 1% oxazolone in 50% ethanol/H2O by rectal application with a balloon catheter (Rüsch Brillant Paediatric size 6) under anaesthesia with isoflurane. The control group was treated with ethanol for presensitization or 50% ethanol/H2O for rectal application. An additional control group was treated with isotonic sodium chloride solution. Mice were inspected twice daily and killed on day 16.
Clinical activity score
Analyses of the colitis clinical severity score were performed daily according to a modified scoring system, as described previously 13. The loss of body weight was scored as follows: (0) 0%, (1) 1–5%, (2) 6–10%, (3) 11–15% and (4) > 15%. Assessment of diarrhoea (stool consistency) was: (0) normally formed pellets, (1) pasty and semi-formed pellets and (4), liquid stools; bleeding: (0) haemoccult-negative, (4) haemoccult-positive; behaviour: (0) normal, (1) reduced activity, (3) apathy, (1) ruffled fur and (5) spontaneous death. IgE serum levels (ng/ml) were: (0) 0, (1) < 100, (2) < 500 and (3) > 500. The resulting score parameters were added in a total clinical activity score ranging from 0 (healthy) to 24 (maximal ill/activity of colitis). Animals who suffered from weight loss > 15% and animals with a clinical severity score of 8 were euthanized immediately and counted as dead.
Histological score
Distal parts of the colon were fixed for 24 h in formalin, followed by 70% ethanol prior to paraffin embedding. Sections were stained with haematoxylin and eosin (H&E) and Masson's trichrome. Inflammation was scored by a trained histopathologist as follows: (1) infiltration of few inflammatory cells into the LP, (2) major infiltration of inflammatory cells into the LP, (3) confluent infiltration of inflammatory cells into the LP and infiltration of the submucosa and (4) transmural infiltration.
Epithelial erosion was scored as follows: (0) no alteration, (1) discrete lesions, (2) superficial erosions and (3) major damage of the epithelia. The resulting score parameters were added in a total histological score ranging from 0 (healthy) to 6 (maximal histological damage).
Serum IgG and IgE levels
Human serum IgG levels were measured turbidimetrically with Cobas Integra 800 (Roche, Penzberg, Germany). Human serum IgE levels were determined by the Elecsys 2010 Immunoassay (Roche, Penzberg, Germany). Murine IgE levels were analysed by enzyme-linked immunosorbent assay (ELISA) (BD Bioscience).
Immunohistochemistry
Colon tissue samples were fixed for 24 h in 4% neutral buffered formalin and paraffin-embedded. Antigen retrieval was performed by incubating slides for 30 min at 95–100°C in Tris ethylenediamine tetraacetic acid (EDTA) buffer (10 mM Tris, 0·5 mM EDTA, pH 9). Non-specific binding was blocked with normal goat serum (1:10; MP Biomedicals, Solon, OH, USA). Sections were incubated with rabbit anti-human CD3 (1:100; Dako, Hamburg, Germany) for 1 h in Tris-buffered saline (TBS) (50 mM Tris-HCl, 150 mM NaCl, pH 7·6) and rabbit anti-human CD45 (1:400 in TBS; Antibody Online, Aachen, Germany) for 1 h. A biotin-conjugated goat anti-rabbit IgG was used as secondary antibody (1:100 TBS; Dako) and diaminobenzidine (DAB) served as chromogen. Sections were analysed by a Leica microscope (×10) and micrographs were taken with a Leica camera DFC 295 using Leica FireCam software (Leica Microsystems, Wetzlar, Germany).
Flow cytometry analysis
Surface phenotyping of human lymphocytes
Labelling of the human lymphocytes, in human and mouse, was performed using the following monoclonal antibodies (mAbs): anti-human CD3-fluorescein isothiocyanate (FITC) (clone SK7), anti-human CD4-phycoerythrin (PE) (clone SK3), anti-human CD8- allophycocyanin (APC) (clone SK1), anti-human CD19-APC (clone SJ25C1), anti-human CD38-PE (clone HIT2), anti-human CD45-APC-H7 (clone 2D1), CD56-peridinin–chlorophyll–protein complex cyanine dye 5·5 (PerCP-Cy™5·5) anti-human (clone B159) and anti-human CD138-FITC (clone MI15). Anti-mouse CD45 PE-Cy™7 (clone30-F11) was also performed to exclude all murine host cells from the analysis to check the non-cross-reactivity with human cells. All mAbs were purchased from BD Biosciences.
At time of death peripheral mouse blood was collected in heparin tubes from the anaesthetized mouse by cardiocentesis. Single-cell suspensions were prepared from the spleen by mincing with a metal mesh followed by a 100 μm cell strainer (BD Biosciences), as described by Daniel et al. 14. The lymphocytes were stained after erythrocyte lysis step, using ×1 BD Pharm Lyse (BD Biosciences).
Isolation and purification of LP T cells
LP T cells were isolated from colonic specimens using a modification of the technique described by van der Heijden and Stock 15. Following washing several times with HBSS CA/Mg to remove stool and cutting into 5-mm segments, the colonic samples were incubated twice for 15 min at 37°C in 5 ml HBSS containing 2·5 mM EDTA and 2 mM dithiothreitol (DTT). The tissues were digested further by incubation in 5 ml 10% RPMI-1640 medium containing collagenase type IV (4000 U/ml; Worthington, Lakewood, NJ, USA) and DNase (1 mg/ml; Worthington) for 90–120 min at 37°C. The tube was vortexed manually every 5 min. LP T cells released from the tissue were isolated by centrifugation at 600 g for 7 min and resuspended twice with 2% RPMI-1640 medium followed by centrifugation at 600 g for 7 min. The cell pellet was resuspended in 2 ml ice-cold 100% Percoll, overlayed with 40% Percoll gradient and spun at 850 g for 20 min at 4°C. The lymphocytes isolated from the interphase were resuspended in 2 ml 2% RPMI-1640 medium, followed by centrifugation at 850 g for 7 min. The cell pellet was resuspended in 100 μl 10% RPMI-1640. All chemicals were purchased from Sigma-Aldrich, except when noted otherwise. The harvested T cells were analysed by flow cytometry.
Intracellular phenotyping of human lymphocytes
Th1 and Th2 cells were identified based on their cytokine secretion using a human Th1/Th2/Th17 phenotyping kit (BD Biosciences). The harvested lymphocytes from blood and spleen were intracellular-stained according, to standard protocol. Briefly, the cells were polarized using phorbol-12-myristate-13-acetate (PMA) 50 ng/ml and ionomycin 1 μg/ml, both purchased from Sigma-Aldrich, in the presence of GolgiStop™ protein transport inhibitor and incubated at 37°C for 4–5 h. After fixation and permeabilization, the cells were stained by anti-human CD4-PerCP-Cy5·5 (clone SK3), human IL-4 APC (clone MP4-25D2) and human interferon (IFN)-glycidylmethacrylate (GMA) FITC (clone B27).
Measurement was performed using a fluorescence activated cell sorter (FACS)Canto (BD Biosciences). Post-acquisition data were analysed using FlowJo version 7·6.5 software (TreeStar, Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using r, a free software environment for statistical computing and graphing. Group means were compared with analysis of variance (anova), followed by Tukey's multiple comparisons. Where assumptions for anova were not fulfilled, the Kruskal–Wallis test followed by multiple comparisons was applied. Difference in survival was assessed by the Mantel–Haenszel test.
Results
Selection of donors
In a previous study, engrafted NOD-SCID IL-2Rγnull mice were challenged topically with oxazolone to induce AD-like features 16. In this AD model it had been shown that elevated levels of hIgE correlated with histological scores and that PBMC from donors imprinted by AD were required. Therefore, we analysed hPBMC in vitro with regard to their capacity to respond to IL-4 prior to engraftment and selected AD patients as donors at the beginning of the experiments. The similarity of the oxazolone-induced AD or UC animal models in immunocompetent mice further supported this approach. When in-vitro analysis revealed that cultured PBMC from patients with UC also responded significantly to IL-4 with secretion of hIgE, and when the studies in mice revealed that the disease background was not crucial in this model, we expanded the experiment, engrafting PBMC from a UC patient. Results were compared to those obtained from mice engrafted with PBMC derived from healthy volunteers. hPBMC were isolated as described in Material and methods and 4 × 106 cells were incubated for 14 days with 50 ng/ml IL-4, as described previously 11. The induction of hIgE and hIgG synthesis was measured in the supernatant by immunoassay and turbidimetric measurement. The response to IL-4 was compared to the responses of hPBMC derived from healthy volunteers. In all groups, UC, AD and healthy volunteers, we observed a high variability with regard to hIgE secretion. From all tested patients and healthy volunteers we identified those as donors who exhibited elevated levels of IgE upon exposure to IL-4. Isolated PBMC were divided and a part was incubated simultaneously in the absence and presence of IL-4 in vitro. Secretion of hIgG scarcely differed in the AD, UC and healthy volunteer groups following exposure to IL-4, whereas hIgE levels increased considerably. Figure 1 displays representative examples of the individual IgE levels in cell culture supernatants of hPBMC in response to IL-4. Circled dots indicate engrafted samples.
Fig. 1.
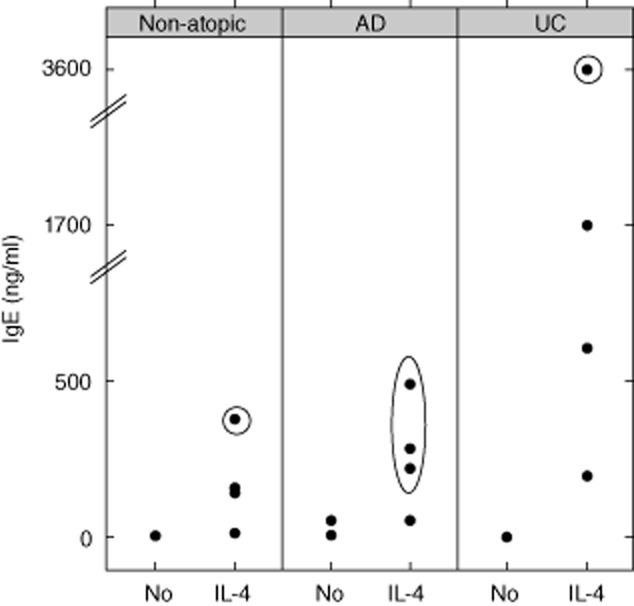
Interleukin (IL)-4-dependent human immunoglobulin (Ig)E secretion from isolated peripheral blood mononuclear cells (PBMC). Donors of PBMC were patients diagnosed with atopic dermatitis (AD, n = 4), ulcerative colitis (UC) n = 4 or healthy control subjects (non-atopic, n = 4). Isolated PBMC were incubated in the presence or absence of IL-4 (50 ng/ml) for 14 days. Circled dots indicate PBMC which had been engrafted simultaneously.
Engraftment of NOD-SCID IL-2Rγnull mice with hPBMC isolated from atopic donors and induction of colitis
NOD-SCID IL-2Rγnull mice were engrafted with 4 × 106 hPBMC from selected donors as described in Material and methods and as shown in Table 1. Seven days post-engraftment, mice were divided into two groups: one was challenged with ethanol vehicle and the other was challenged with oxazolone. The groups were presensitized by ethanol vehicle or 5% oxazolone followed 7 days later by rectal application of ethanol vehicle or 1% oxazolone, respectively, as described by Heller et al. 4. Non-engrafted mice challenged with oxazolone served as control animals. As we had found from our previous studies 16,17 that mice engrafted with PBMCs derived from AD patients did not develop symptoms of UC or AD spontaneously, a control group including unchallenged animals was not required. To exclude, however, that engrafted PBMC derived from a patient with UC have the capacity to induce UC-like symptoms spontaneously, we analysed a group of unchallenged animals engrafted with PBMC from the UC patients (n = 3). None of these mice developed any symptoms of UC spontaneously. The animals did not lose weight, showed no signs of diarrhoea and behaved completely normally. The number of animals per group are indicated in Table 1. To compare the immunological responses to those of immunocompetent mice, a cohort of BALB/c mice challenged with oxazolone and ethanol vehicle according to the same regimen as the engrafted SCID mice was included in this study. The onset of disease was monitored by body weight and visual inspection of the mice and the stools. Symptoms were classified according to a clinical score as described in Material and methods (Fig. 2a). Approximately 12 h after rectal application, mice in the engrafted animals group challenged with oxazolone or ethanol developed symptoms of colitis similar to the results observed in immunocompetent mice 3,4. The stools became soft and the activity of the animals was reduced. Diarrhoea and weight loss peaked at day 2 and 50% recovered, as expected from experiments with immunocompetent mice. When we observed the development of clinical symptoms in the vehicle-challenged group, we included a further control containing engrafted mice challenged with isotonic sodium chloride solution (NaCl, non-atopic, n = 4). We observed a high variability of the clinical score with a median value of 5·5 in the oxazolone-challenged group and of 2·0 in the ethanol vehicle-challenged group. In the group of animals challenged with isotonic sodium chloride solution, the median value was 0·5 (Fig. 2a). Clinical severity scores in the three groups was significantly different. anova (F = 8·9, P = 0·0003), and Tukey's multiple comparisons revealed that the oxazolone group was significantly different from the NaCl group (P = 0·008) and the ethanol group (P = 0·002). Both the oxazolone- and the ethanol vehicle-challenged groups contained animals displaying almost no symptoms and animals developing a high clinical score. The development of symptoms was not dependent upon the disease background of the engrafted cells. hPBMC derived from patients with AD, UC or non-AD induced the same symptoms. In some animals in the oxazolone-challenged group the symptoms became so severe that they had to be killed. At 6 h post-challenge these animals had significantly reduced activity and lost up to 15% of their body weight on day 2. This immediate response was due most probably to the toxic effect of oxazolone, which we observed in the control group of non-engrafted animals challenged with oxazolone. In this group all animals had to be killed due to weight loss and apathy. The survival curves depicted in Fig. 2b show a difference between engrafted and non-engrafted animals. At the end of the experiment 50% of engrafted animals challenged with oxazolone had survived, while all the non-engrafted animals had died. None of the engrafted animals challenged with isotonic sodium chloride solution died, and only two animals died in the ethanol-challenged group, indicating that the observed fatalities were not related to the application procedure. Survival by Mantel–Haenszel test was significantly different when all four groups were compared and when oxazolone + PBMC and oxazolone – PBMC were contrasted (P = 0·03).
Table 1.
Number of animals in the study
| Donor no./challenge | No engraftment | Healthy (1) | AD (3) | UC (1) | Total |
|---|---|---|---|---|---|
| No treatment | 12 | 3 | 15 | ||
| NaCl | 4 | 4 | |||
| Ethanol | 8 | 11 | 4 | 23 | |
| Oxazolone | 8 | 9 | 11 | 6 | 34 |
| Total | 8 | 33 | 22 | 13 | 76 |
AD: atopic dermatitis; UC: ulcerative colitits.
Fig. 2.
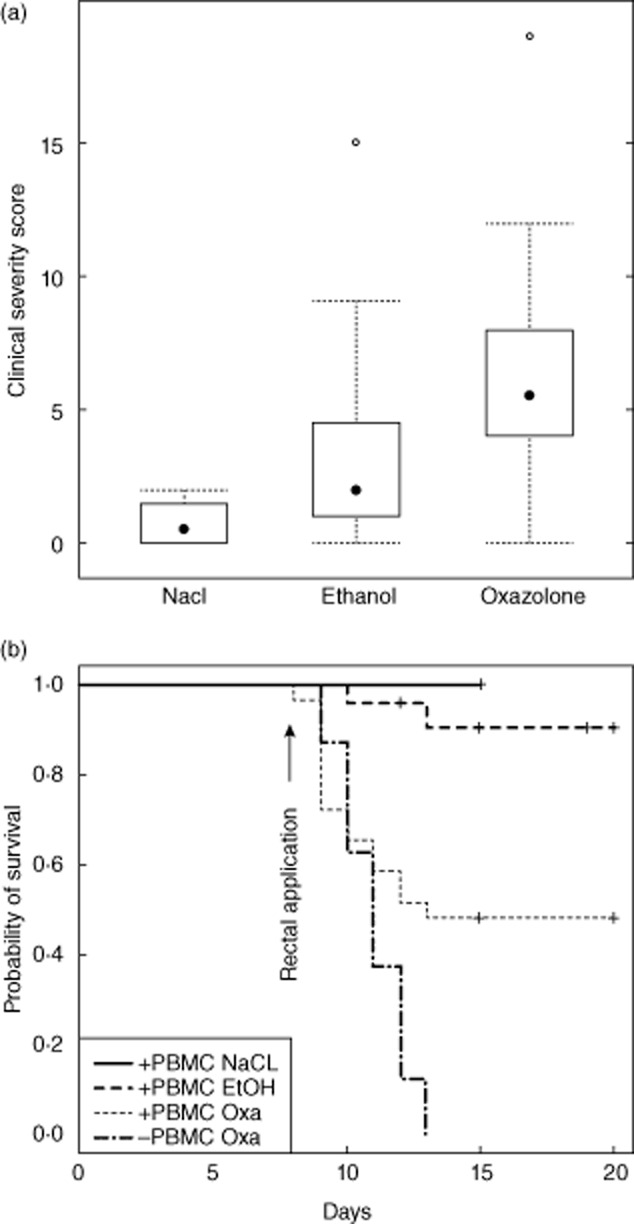
Challenge with oxazolone resulted in the development of clinical symptoms and reduced probability of survival in engrafted and non-engrafted non-obese diabetic-severe combined immunodeficiency interleukin-2Rγnull (NOD-SCID IL-2Rγnull) mice challenged with oxazolone. (a) Symptoms were classified according to clinical severity score and depicted in a box-plot diagram. Animals were treated with isotonic sodium chloride solution (NaCl, n = 4), ethanol (n = 22) or oxazolone (n = 21) (P = 0·008 for comparison of oxazolone versus NaCl, P = 0·3 for ethanol versus NaCl, P = 0·002 for oxazolone versus ethanol, analysis of variance (anova) followed by Tukey's multiple comparisons). (b) Kaplan–Meier plot of engrafted mice challenged with NaCl (+ PBMC NaCl, n = 4), ethanol (+ PBMC ethanol, n = 23) or oxazolone (+ PBMC oxazolone, n = 29) and of non-engrafted mice challenged with oxazolone (– PBMC oxazolone, n = 8). Survival by Mantel–Haenszel test was significantly different when all four groups were compared and when oxazolone + PBMC and oxazolone-PBMC were contrasted (P = 0·03).
We observed hardly any symptoms in the cohort of BALB/c mice.
All animals were killed on days 16–20 if their condition did not require earlier euthanasia. These animals and the animals which had died during the study were counted as survivors.
Macroscopic inspection of the colon
On the day of euthanasia the colon was removed and inspected with regard to appearance of stool and colon length (Fig. 3a). Non-challenged mice had a colon length of approximately 8–9·5 cm (n = 8), depending on the age of the animals, and stool was evenly dispersed and formed in even pellets. In the group of engrafted mice challenged with oxazolone (n = 16) or ethanol (n = 22) we observed high variability. While some colons did not display any alteration, some colons exhibited a moderate reduction in length of approximately 1 cm and the pellets had lost their typical shape. The pellets were enlarged and did not form solid, single pellets. The appearance of the colon was dependent upon the histological score. When histological scores were elevated (Fig. 3b,d), the changes were more pronounced. There was no significant difference between oxazolone- and ethanol-challenged animals. Colons of non-engrafted mice (n = 8), all of which died during the study or had to be killed prematurely, displayed extreme reduction of the colon length of more than 2 cm. Of note, these animals did not develop diarrhoea: on the contrary, on inspection the colons were constipated, indicating that oxazolone itself had severe toxic effects in the absence of hPBMC. Figure 3b depicts the effect of type of challenge and engraftment on changes of colon length. Treatment with oxazolone or ethanol did not have a significant effect on colon length. Colon lengths in the four groups were significantly different (anova, F = 7·1, P = 0·0005), and Tukey's multiple comparisons revealed that the oxazolone – PBMC group was significantly different from all other groups; P = 0. Thus, the presence of hPBMC significantly modulated the response to oxazolone.
Fig. 3.
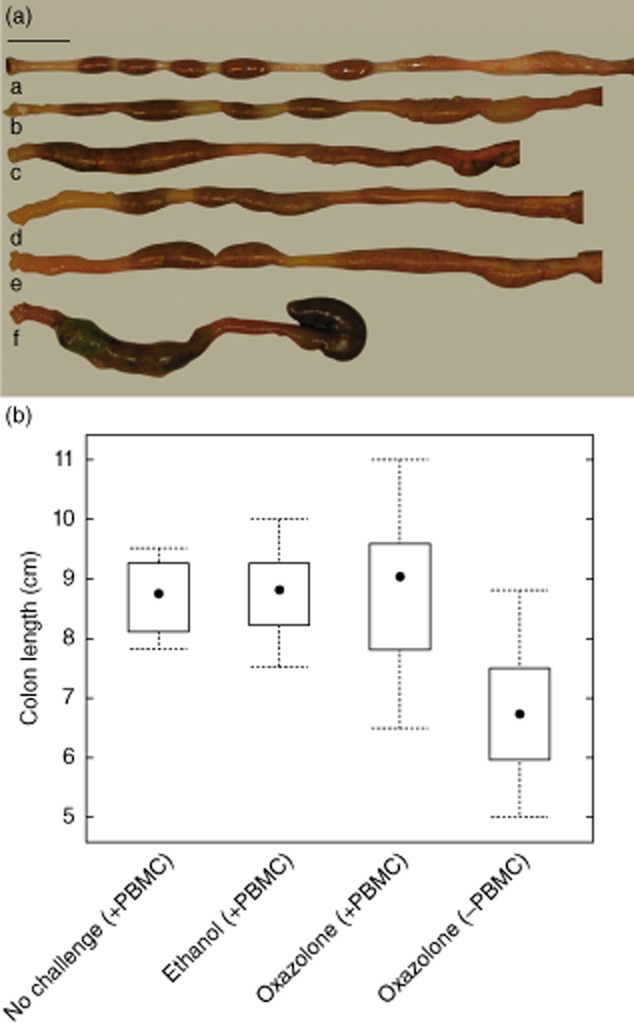
Macroscopic inspection of colon at autopsy reveals shortening of the colon in oxazolone-challenged mice. (a) Macrophotographs of colon of (a) non-obese diabetic-severe combined immunodeficiency interleukin-2Rγnull (NOD-SCID IL-2Rγnull) mouse engrafted and challenged with isotonic sodium chloride solution; (b) engrafted mouse challenged with ethanol (histological score = 1); (c) engrafted mouse challenged with ethanol (histological score = 3); (d) engrafted mouse challenged with oxazolone (histological score = 4); (e) engrafted mouse challenged with oxazolone (histological score = 6); (f) non-engrafted, challenged with oxazolone. Bar = 1 cm. (b) Box-plot diagram depicting colon length in non-challenged engrafted mice [no challenge (+ peripheral blood mononuclear cells: PBMC), n = 8]; engrafted mice challenged with ethanol [ethanol (+ PBMC), n = 22]; engrafted mice challenged with oxazolone [oxazolone (+ PBMC), n = 16]; non-engrafted mice challenged with oxazolone [oxazolone (– PBMC), n = 8] (P = 0·004 for comparison of oxazolone (+ PBMC) versus oxazolone (– PBMC)]; analysis of variance (anova) followed by Tukey's multiple comparisons.
Histological examination of the colon
In order to examine the histological changes induced by oxazolone, sections from the distal part of the colon were stained with H&E and Masson's trichrome. As shown in Fig. 4a, challenge with oxazolone resulted in severe pathomorphological changes of the colon architecture in both mice strains, BALB/c mice [Fig. 4a(b)] and in engrafted NOD-SCID IL-2Rγnull mice [Fig. 4a(d,e)]. The colons exhibited similar alterations to include disrupted epithelial layers and oedema of the LP and of the mucosa, accompanied by dense infiltration of inflammatory cells into the mucosa and LP. Serial sections indicated continuous inflammation and the absence of microabcesses.
Fig. 4.
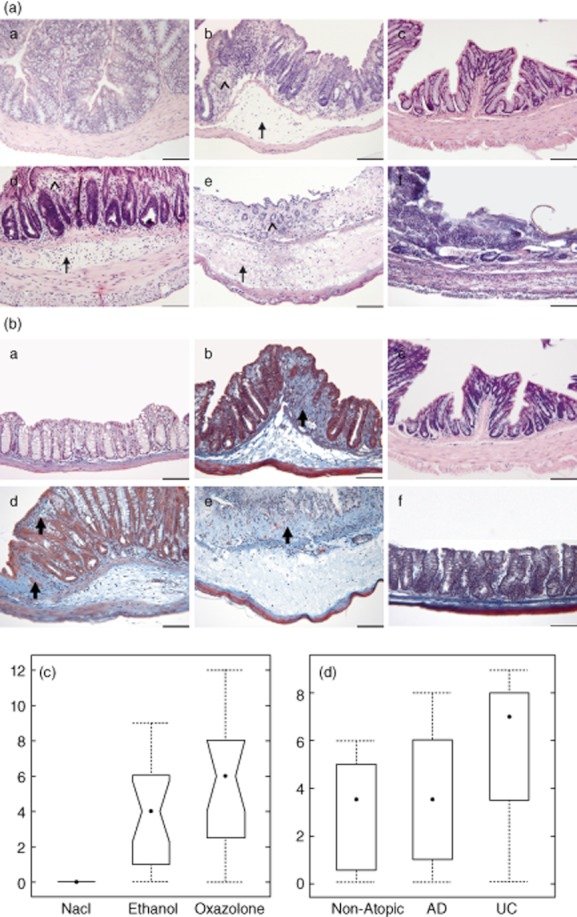
Challenge with oxazolone results in disrupted epithelial layers, oedema and infiltration of inflammatory cells into the mucosa and lamina propria. (a,b) Photomicrographs of stained paraffin sections of distal parts of the colon. Haematoxylin and eosin (H&E) (a) and Masson's trichrome (b); BALB/c challenged with (a) ethanol; (b) oxazolone; (c) non-obese diabetic-severe combined immunodeficiency interleukin-2Rγnull (NOD-SCID IL-2Rγnull) mice non-engrafted no challenge; (d) engrafted NOD-SCID IL-2Rγnull mice challenged with oxazolone, histological score of 4; (e) engrafted NOD-SCID IL-2Rγnull mice challenged with oxazolone, histological score of 8; (f) non-engrafted NOD-SCID IL-2Rγnull mice challenged with oxazolone. Arrows indicate oedema, arrowheads disrupted crypts and bold arrows fibrosis. Bar = 100 μm. (c,d) Histological alterations were classified according to a histological score and depicted in a box-plot diagram. (c) Engrafted NOD-SCID IL-2Rγnull mice treated with isotonic sodium chloride solution (NaCl, n = 4), ethanol (n = 17) or oxazolone (n = 16) (P = 0·01 for comparison of oxazolone versus NaCl, Kruskal–Wallis rank sum test followed by multiple comparison. (d) Disease-dependent development of histological scores in response to challenge with ethanol [non-atopic n = 8, atopic dermatitis (AD) n = 11, ulcerative colitis (UC) n = 4].
The degree of destruction was variable. Figure 4a(d,e) depicts two examples of mice with a histological score of 4 and 8, respectively. Cell infiltrates consisted of a mixture of lymphocytes and neutrophilic granulocytes.
There was, however, a significant difference between BALB/c mice and engrafted NOD-SCID IL-2Rγnull mice with regard to the challenge with ethanol vehicle. While BALB/c mice did not respond to the rectal challenge with ethanol vehicle, we observed the same histopathological changes in mice challenged with ethanol vehicle as in the oxazolone-challenged cohort in those animals which had an elevated clinical severity score.
As expected from the visual inspection of the colon, the colons of non-engrafted NOD-SCID IL-2Rγnull mice were severely damaged. In most cases the epithelial layer was completely erased [Fig. 4a(f)]. As expected from the clinical activity score, colonic sections from unchallenged mice engrafted with PBMC from a UC patient displayed healthy colon architecture (data not shown).
As fibrosis is considered to be a hallmark of colitis, colon sections of challenged and control animals were also stained with Masson's trichrome. As shown in Fig. 4, oxazolone induced fibrosis in oxazolone-challenged BALB/c and engrafted NOD-SCID IL-2Rγnull mice [Fig. 4b(b,d,e)]. Collagen fibres could be detected in the extracellular oedematous fluid and the epithelial layers. In contrast, no fibrosis was observed in non-engrafted NOD-SCID IL-2Rγnull mice challenged with oxazolone [Fig. 4b(f)].
The histological scores of the colons from engrafted NOD-SCID IL-2Rγnull mice are shown in Fig. 4c. Animals challenged with oxazolone or ethanol vehicle displayed changes to various degrees with median score values of 6 and 4, respectively, while isotonic sodium chloride solution did not induce any changes. Histological scores in three groups were significantly different; anova followed by Tukey's HSD multiple comparisons revealed a significant difference between the oxazolone group and the NaCl group (P = 0·01). The difference between the ethanol group and the NaCl group was not significant (P = 0·1). There was a correlation between the clinical severity score and the histological score (P = 0·001, Pearson's product–moment correlation). The response to ethanol was most pronounced in the mice engrafted with PBMC from the UC patient (Fig. 4d).
Immunohistochemical analysis of the cellular infiltrate revealed that the inflammatory cells were of human origin, with T cells being the main constituent (Fig. 5a). An antibody directed against human CD45-positive leucocytes in the LP and mucosa in engrafted NOD-SCID IL-2Rγnull mice exhibited pathomorphological alterations (Fig. 5d,e), while inflammatory cells were not stained in sections of BALB/c mice with this antibody (Fig. 5b). An antibody directed against CD3 identified the majority of the infiltrated cells as T cells (Fig. 5d,e). As this antibody also recognizes murine T cells, colons from BALB/c mice challenged with oxazolone also displayed stained cells (Fig. 5b).
Fig. 5.
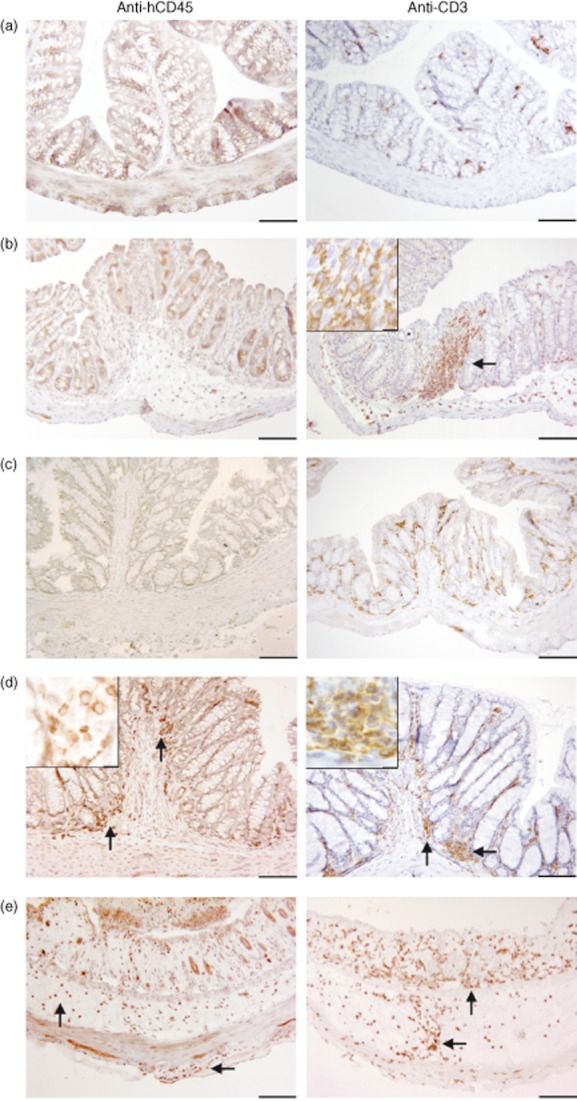
Upon challenge with oxazolone human leucocytes – consisting mainly of T cells – infiltrate the mucosa and lamina propria. Photomicrographs of immunochemical staining of paraffin sections of the colon with an anti-hCD45 and anti-CD3 antibody. BALB/c mice challenged with (a) ethanol; (b) oxazolone; (c) non-engrafted non-obese diabetic-severe combined immunodeficiency interleukin-2Rγnull (NOD-SCID IL-2Rγnull) mice; engrafted NOD-SCID IL-2Rγnull mice challenged with oxazolone (d) and a histological score = of 4, and (e), and a histological score of 8. Bar = 100 μm, insert bar = 10 μm.
Secretion of human IgG and human IgE in response to challenge with ethanol and oxazolone
Blood samples were taken from animals at the end of the experiment and analysed for human IgG and IgE concentrations. Engrafted mice that were not challenged served as control. Figure 6 depicts individual hIgG and hIgE expression levels in engrafted mice in response to ethanol or oxazolone. In all groups we observed a high variability. Animals without any challenge (n = 15) expressed mean hIgG levels of 93 ± 215 μg/ml (Fig. 6a). This observation is in agreement with previous studies, which had detected levels of 40·9 ± 11·5 μg/ml hIgG at day 30 18. Challenge of animals with either oxazolone (n = 15) or ethanol vehicle (n = 22) had an impact on the expression levels of hIgG and there was no significant difference between the ethanol- and oxazolone-challenged groups or in the cohorts engrafted with atopic and non-atopic donors. The hIgG levels reached values of up to 2550 μg/ml in the oxazolone-challenged group (mean = 221 ± 647 μg/ml) and 1125 in the ethanol-challenged group (mean = 221 ± 330). The difference in both groups compared to the unchallenged group was not statistically significant.
Fig. 6.
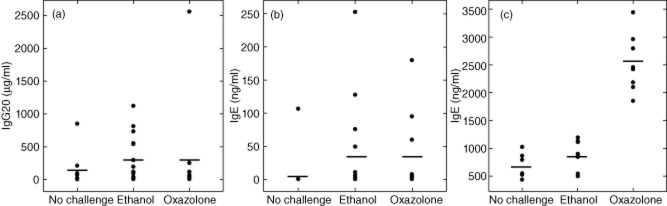
Secretion of immunoglobulin (Ig)G and IgE in response to challenge. (a) hIgG and (b) hIgE in sera of engrafted non-obese diabetic-severe combined immunodeficiency interleukin-2Rγnull (NOD-SCID IL-2Rγnull) mice (no challenge, n = 15, ethanol, n = 22, oxazolone n = 15). (c) BALB/cJ mice. No challenge, n = 9, ethanol, n = 8, oxazolone, n = 8). Bars represent mean values.
Similarly, hIgE secretion expression levels varied considerably (Fig. 6b). In the group of unchallenged animals the mean hIgE value was 7·1 ± 27 ng/ml. Upon challenge with ethanol vehicle or oxazolone hIgE levels increased in some animals, reaching a maximum of 252 ng/ml in the ethanol-challenged group with a mean value of 23·3 ± 58 ng/ml. In the oxazolone-challenged group the mean value was 23·4 ± 51 ng/ml. When both challenged groups were combined and contrasted with the unchallenged group, the difference was statistically significant (P = 0·05; Kruskall–Wallis rank sum test). When each single group was tested the difference was not significant.
The response to ethanol and the high variability are in contrast to results obtained in BALB/c mice (Fig. 6c). Murine IgE expression levels increased from a basal level of 698 ± 227 ng/ml (n = 9) to 2517 ± 521 ng/ml in the oxazolone (n = 8)-challenged group at day 12 post-presensitization, while the control group challenged with ethanol vehicle displayed only a moderate increase up to 865 ± 263 (n = 8). Statistical analysis revealed that the oxazolone-challenged group was significantly different (anova, F = 64·1, P < 10E-5).
Analysis of human T cells in mouse spleen and blood
In order to analyse subsets of T cells in the spleen and blood of mice engrafted with PBMC derived from the patient suffering from UC (Table 1), human lymphocytes isolated from the spleen as described in Material and methods and blood from mice were subjected to FACS analysis, as described in Material and methods. The percentage of human splenic CD45+ cells served to determine the level of engraftment. Engraftment levels varied considerably in all three groups, ranging from 0·15 to 81·5%, regardless of the treatment (median = 6·7%). Engraftment levels below 1% were considered poorly engrafted, and those animals were excluded from the study. This applied to two animals in the ‘no treatment’ and one animal in the ‘ethanol’ group.
The CD4 : CD8 ratio was determined by surface phenotyping using anti-CD4 and anti-CD8 antibodies, as described in Material and methods. The Th2 : Th1 ratio was determined by intracellular phenotyping, as described in Material and methods. Ratios were compared to the whole blood sample of the patient.
As shown in Fig. 7a, in the whole blood sample of the patient the CD4 : CD8 and Th2 : Th1 ratios were extremely high (4 and 0·68, respectively) compared to AD patients and healthy subjects. Here, CD4 : CD8 ratios ranged from 1·5 to 2·4 and Th2 : Th1 from 0·04 to 0·08 (data not shown). The high ratios are conserved in the spleen and blood of engrafted mice and were alike in all three groups. Thus, challenge had no influence on the ratios.
Fig. 7.
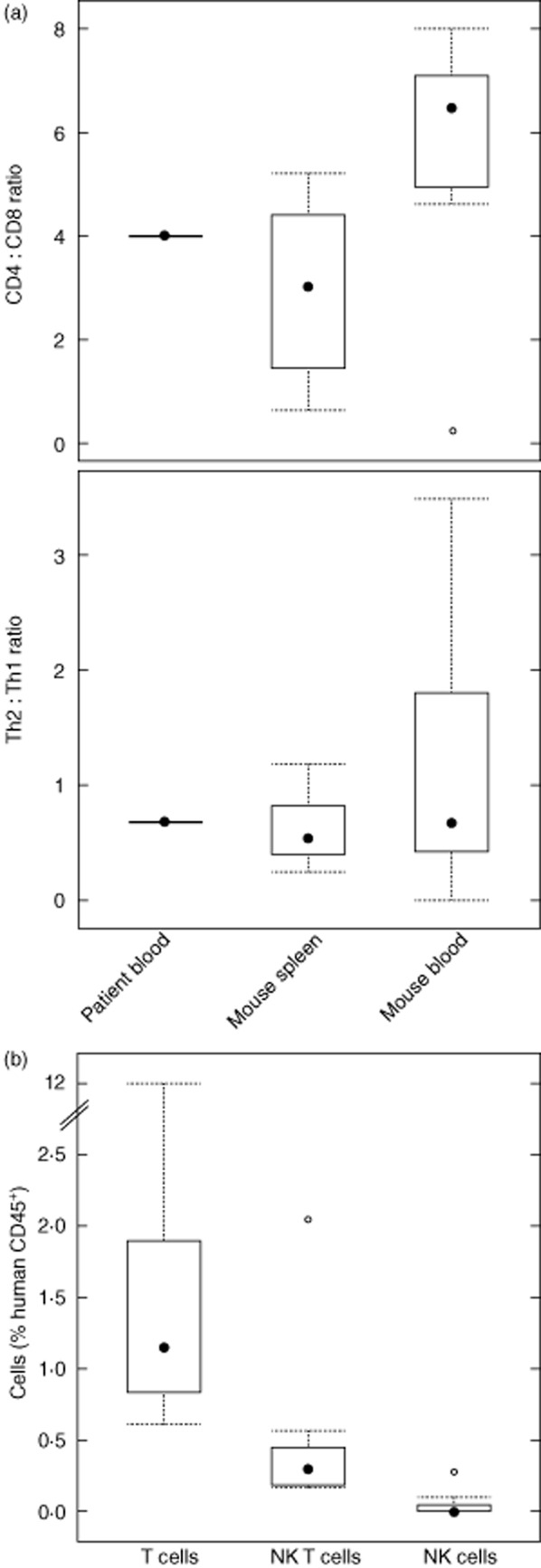
Analysis of human T cell subsets and natural killer (NK) cells. (a) Human splenic and blood lymphocytes isolated from non-obese diabetic-severe combined immunodeficiency interleukin-2Rγnull (NOD-SCID IL-2Rγnull) mice engrafted with peripheral blood mononuclear cells (PBMC) derived from a patient suffering from ulcerative colitis (UC) were analysed for percentage of CD4, CD8, T helper type 2 (Th2) and Th1 T cells by flow cytometry as described in Material and methods and depicted in a box-plot diagram. Median values are compared to the ratio in the whole blood of the donor (patient blood) at time of engraftment. (b) Analyses of human T cells, NK T cells and NK cells in colonic lymphocytes. Human lymphocytes isolated from the colon of NOD-SCID IL-2Rγnull mice engrafted with PBMC derived from a patient suffering from UC (n = 10) were analysed for the percentage of CD3, CD3 + CD56 and CD56 cells as described in Material and methods and depicted in a box-plot diagram.
In order to corroborate further that the observed pathomorphological changes were caused by oxazolone-induced colitis, human T cells, NK T cells and NK cells were identified by FACS analysis in cell suspensions isolated from colonic tissue, as described in Material and methods, using anti-human CD3 and anti-human CD56 antibodies. Figure 7b shows that T cells represent 1·1% of human CD45+ cells and that 0·3% of human CD45+ cells consist of NK T cells, whereas NK cells are not detectable in most tissue extracts (median = 0).
Expression of periostin in colonic sections
Recently, periostin has been identified as a component of subepithelal fibrosis in asthma and as a gene downstream of IL-4 and IL-13 signals 19. Periostin is encoded by the POSTN gene, which has been shown to be induced by IL-4/IL-13 in bronchial epithelial cells and fibroblasts 20,21. In order to corroborate further that IL-13 is the key effector cytokine in this model, paraffin sections from distal parts of the colon were analysed for the expression of periostin. Figure 8 shows that periostin expression increases significantly in sections displaying a high histological score, indicating that in oxazolone-induced colitis periostin is also an indicator of colon remodelling.
Fig. 8.
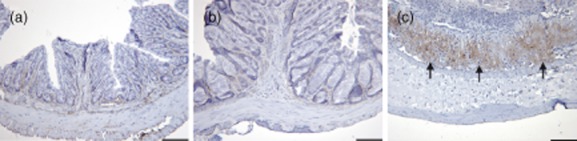
Challenge with oxazolone induces the expression of periostin. Photomicrographs of immunochemical staining of paraffin sections of the colon with an anti-periostin antibody. (a) Non-engrafted non-obese diabetic-severe combined immunodeficiency interleukin-2Rγnull (NOD-SCID IL-2Rγnull); (b) engrafted engrafted NOD-SCID IL-2Rγnull challenged with oxazolone, histological score = 4; (c) engrafted NOD-SCID IL-2Rγnull challenged with oxazolone, histological score = 8.
Discussion
In this study we describe the induction of oxazolone- and ethanol-mediated colitis in an immunocompromised NOD-SCID IL-2Rγnull mouse engrafted with human PBMC. Upon challenge with oxazolone or ethanol, engrafted mice developed the same symptoms and the same phenotype as observed in immunocompetent mice challenged with oxazolone. Clinical severity score, development of oedema, disruption of the epithelial layer, fibrosis and influx of inflammatory cells were similar to the previously described observations obtained in C57BI/10 and BALB/c mice 3,4,10, and to our own results from experiments carried out with BALB/c mice. A high proportion of cellular infiltrate was of human origin, with T cells being the major subset. Upon challenge with oxazolone or ethanol, T cells migrated into the epithelial layer and the LP. In contrast to our previous study 16, development of the phenotype was not dependent upon the disease background of the donor. Mice engrafted with PBMC from healthy donors or from AD and UC patients responded similarly.
FACS analysis identified a large proportion of the T cells in colonic tissue as NK T cells, which have been identified previously as the major source of IL-13 in oxazolone-induced colitis 4,6. The presence of NK T cells and the absence of NK cells suggest that in this model IL-13 is also the mediator of the pathomorphological changes, as observed previously in immunocompetent mice. The phenotype generated by IL-13 includes fibrosis, induction of periostin expression and IgE secretion 7,19. All three phenotypes were detected in this model. Staining of paraffin sections from distal parts of the colon with Masson trichrome revealed that fibrosis is indeed a hallmark of the remodelling of the colonic architecture in this model. In addition, the expression of periostin as a component of subepithelial fibrosis downstream of IL-4 and IL-13 signals was detected in colonic sections. Furthermore, the induced secretion of hIgE is an additional piece of evidence. Upon challenge, elevated levels of hIgE were detected in mouse sera, just as we observed in BALB/c mice.
NOD-SCID IL-2Rγnull mice engrafted with hPBMC have also become a model to study xeno-graft-versus-host disease (GVHD) 18,22. The development of xeno-GVHD requires a cytoreductive conditioning regimen, including radiation therapy or chemotherapy, which elicits a cytokine storm of the proinflammatory cytokines IL-1 and tumour necrosis factor (TNF)-α. Both cytokines cause damage in the gastrointestinal tract and thereby allow the penetration of microbial products such as lipopolysaccharide into the mucosa leading to the activation of T cells. Unlike oxazolone-induced colitis, however, GVHD is characterized by a Th1 cytokine profile and the presence of NK cells. The fact that we did not observe NK cells, but activation of IL-13 response genes, suggests that in our model the generated phenotype is caused by colitis.
In addition, it has been shown that cytokines that polarize donor T cells into Th2 cells reduce the occurrence of GVHD 23–25. The fact that we observed elevated CD4 : CD8 and Th2 : Th1 ratios in the engrafted PBMC further corroborates our data.
There were, however, some important differences to the results obtained in oxazolone-induced colitis in BALB/c mice. First, the immunological response in BALB/c mice was restricted to the oxazolone-treated group. Murine IgE levels increased throughout the experiment and hardly altered in ethanol-challenged mice. Similarly, no alterations of the colon architecture were observed in this control group. In engrafted NOD-SCID IL-2Rγnull mice, however, the ethanol- and oxazolone-challenged animals responded similarly with regard to histological scores and hIgE secretion. One interpretation of this result might be that the hPBMC bearing the memory of the respective donors react to ethanol as a contact allergen. Alternatively, the protein denaturing abilities of ethanol might be sufficient to activate NK T cells present in the engrafted mice. A prospective cohort study with UC patients in remission revealed that the consumption of alcohol increases the likelihood of a relapse 26. Thus, engrafted mice might mimic patients in remission who experience a ‘relapse’ upon challenge, even as mild as ethanol. This hypothesis is corroborated by the fact that the PBMC from the patient suffering from UC reacted to the challenge with ethanol as strongly as to the challenge with oxazolone.
Secondly, the variability of hIgE levels was much higher in engrafted NOD-SCID IL-2Rγnull mice. This variability might be due to poor engraftment in some animals, to insufficient challenge under these conditions, or to delayed response, compared to BALB/c mice. Previous studies from our group [16] have shown that changes in hIgE levels remain relatively unchanged for 23 days post-sensitization in engrafted animals. Thus, significant changes would not be expected to be seen at day 13. The fact that there is no correlation between hIgE levels and phenotype development suggests that in this model hIgE is also no indicator for the disease. This is consistent with human disease, which is not characterized by elevated hIgE levels at time of relapse. This observation, however, is in contrast to results from our previous study 16, where we observed a correlation of the histological score for AD and hIgE levels.
Unexpectedly, oxazolone applied rectally to non-engrafted animals was highly toxic. None of the animals from that group survived the experiment as opposed to engrafted animals challenged with oxazolone, where some of the animals displayed hardly any symptoms. Analysis of the colon length corroborated this observation. While in engrafted animals the observed reduction of colon length was moderate, non-engrafted animals experienced a significant reduction. As Heller et al. 4 did not report any alterations, colon-length reduction does not seem to be a hallmark in this model. Furthermore, in most non-engrafted animals the mucosa was erased completely and there was no indication of fibrosis. Thus, the presence of hPBMC in engrafted mice seemed to mitigate the toxic effect of oxazolone. Fifty per cent of the animals survived and the colon seemed moderately effected macroscopically. These results suggest that the observed symptoms in engrafted mice are a combination of the toxic effects of oxazolone and immunological responses to this agent. Results obtained in the first study describing oxazolone-mediated colitis 3 suggest that the toxic effects of oxazolone at high concentrations also dominated the immunological effect in immunocompetent mice. Visual inspection of the colon revealed the same histopathological changes that we observed in non-engrafted animals. The optimized treatment regimen, which stipulates presensitization followed by rectal application of oxazolone at a lower concentration, seems to enforce the immunological response.
Thus, the presence of immune cells primed by presensitization seems to mitigate the toxic effect of oxazolone. In the experimental setting described in this study, this protective effect appears to implicate the engrafted hPBMC. The high variability of the clinical severity score, ranging from almost no symptoms to identical symptoms observed in non-engrafted mice, might simply be a reflection of the status of engraftment. Presensitization and the concentration of oxazolone might interplay with the immunological background of a strain of engrafted mouse and might tip the balance towards a toxic outcome or an immunological response counteracting the challenge with oxazolone. The oxazolone-induced toxicity resembles sepsis. Oxazolone is thought to have a dual function, acting as a haptenizing agent in addition to activating signal transducer and activator of transcription-6 (STAT 6)-mediated secretion of chemokines 27. The secreted cytokines might attract and activate mouse neutrophils, which damage the mucus layer and allow colonic bacteria to penetrate the layer. This, in turn, might result in a cytokine storm, leading to the complete destruction of the mucus layer and organ failure. The fibrosis observed in the presence of PBMC might protect the mucus layer and inhibit the penetration of colonic bacteria. The observed balance of toxicity and the ‘healing’ process might be an interesting aspect of the disease in humans. Given that in some patients the genetic predisposition includes defects in barrier integrity 28, and that IL-13 is thought to cause barrier defects 6, one might speculate that in humans the observed inflammation also protects from further damage. In summary, we have shown that in engrafted NOD-SCID IL-2Rγnull mice human lymphocytes perform similar functions to resident lymphocytes in immunocompetent BALB/c and in C57BI/10 mice. Thus, this model might be useful to study the efficacy of therapeutics targeting human lymphocytes in vivo. Furthermore, it may also be a useful surrogate model for non-human primates, which are used when high sequence homology and cross-reactivity of human proteins are necessary. In light of the conserved immunological background of engrafted hPBMC imprinted either by exposure to allergens and/or disease, we feel confident that this promising model reflects more closely the human disease and also enables us to elucidate cellular mechanisms inducing and sustaining flares of the disease in an in-vivo model.
Acknowledgments
We thank Origenis GmbH for support and discussion. We thank Eric Whalley for critically reading the manuscript and Lisa Pichl for excellent technical assistance. This work was supported by a grant from the Bundesministerium für Bildung und Forschung (grant number PTJ 0315466).
Disclosure
None of the authors have a financial interest related to the work presented in this manuscript.
References
- 1.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niebuhr M, Werfel T. Innate immunity, allergy and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:463–468. doi: 10.1097/ACI.0b013e32833e3163. [DOI] [PubMed] [Google Scholar]
- 3.Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 5.Obara W, Kawa Y, Ra C, Nishioka K, Soma Y, Mizoguchi M. T cells and mast cells as a major source of interleukin-13 in atopic dermatitis. Dermatology. 2002;205:11–17. doi: 10.1159/000063145. [DOI] [PubMed] [Google Scholar]
- 6.Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111:291–297. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller TD, Zhang JL, Sebald W, Duschl A. Structure, binding, and antagonists in the IL-4/IL-13 receptor system. Biochim Biophys Acta. 2002;1592:237–250. doi: 10.1016/s0167-4889(02)00318-x. [DOI] [PubMed] [Google Scholar]
- 9.Man MQ, Hatano Y, Lee SH, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima R, Kuroda S, Ohkishi T, Nakamaru K, Hatakeyama S. Oxazolone-induced colitis in BALB/C mice: a new method to evaluate the efficacy of therapeutic agents for ulcerative colitis. J Pharmacol Sci. 2004;96:307–313. doi: 10.1254/jphs.fp0040214. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Haruo N, Sugane K, Ochs HD, Agematsu K. Interleukin-21 stimulates B-cell immunoglobulin E synthesis in human beings concomitantly with activation-induced cytidine deaminase expression and differentiation into plasma cells. Hum Immunol. 2009;70:35–40. doi: 10.1016/j.humimm.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Mayer RJ, Bolognese BJ, Al-Mahdi N, et al. Inhibition of CD23 processing correlates with inhibition of IL-4-stimulated IgE production in human PBL and hu-PBL-reconstituted SCID mice. Clin Exp Allergy. 2000;30:719–727. doi: 10.1046/j.1365-2222.2000.00812.x. [DOI] [PubMed] [Google Scholar]
- 13.Khalil PN, Weiler V, Nelson PJ, et al. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944–954. doi: 10.1053/j.gastro.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 14.Daniel C, Sartory NA, Zahn N, et al. FTY720 ameliorates oxazolone colitis in mice by directly affecting T helper type 2 functions. Mol Immunol. 2007;44:3305–3316. doi: 10.1016/j.molimm.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Van der Heijden PJ, Stok W. Improved procedure for the isolation of functionally active lymphoid cells from the murine intestine. J Immunol Methods. 1987;103:161–167. doi: 10.1016/0022-1759(87)90285-7. [DOI] [PubMed] [Google Scholar]
- 16.Nolte T, Zadeh-Khorasani M, Safarov O, et al. Induction of oxazolone mediated features of atopic dermatitis in NOD-scid IL2R γnull mice engrafted with human peripheral blood mononuclear cells. Dis Model Mech. 2013;6:125–134. doi: 10.1242/dmm.009167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zadeh-Khorasani M, Nolte T, Mueller T, et al. NOD-scid IL2R gnull mice engrafted with human peripheral blood mononuclear cells as a model to test therapeutics targeting human signaling pathways. J Transl Med. 2013;11:4–10. doi: 10.1186/1479-5876-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King M, Pearson T, Shultz LD, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126:303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 20.Masuoka M, Shiraishi H, Ohta S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidhu SS, Yuan S, Innes AL, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shultz LD, Pearson T, King M, et al. Humanized NOD/LtSz-scid IL2 receptor common gamma chain knockout mice in diabetes research. Ann NY Acad Sci. 2007;1103:77–89. doi: 10.1196/annals.1394.002. [DOI] [PubMed] [Google Scholar]
- 23.Fowler DH, Kurasawa K, Smith R, Eckhaus MA, Gress RE. Donor CD4-enriched cells of Th2 cytokine phenotype regulate graft-versus-host disease without impairing allogeneic engraftment in sublethally irradiated mice. Blood. 1994;84:3540–3549. [PubMed] [Google Scholar]
- 24.Pan L, Delmonte J, Jr, Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–4429. [PubMed] [Google Scholar]
- 25.Krenger W, Snyder KM, Byon JC, Falzarano G, Ferrara JL. Polarized type 2 alloreactive CD4+ and CD8+ donor T cells fail to induce experimental acute graft-versus-host disease. J Immunol. 1995;155:585–593. [PubMed] [Google Scholar]
- 26.Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53:1479–1484. doi: 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koeper LM, Schulz A, Ahr HJ, Vohr HW. In vitro differentiation of skin sensitizers by cell signaling pathways. Toxicology. 2007;242:144–152. doi: 10.1016/j.tox.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Franke A, Balschun T, Sina C, et al. Genome-wide association study for ulcerative colitis identifies risk loci at 7q22 and 22q13 (IL17REL) Nat Genet. 2010;42:292–294. doi: 10.1038/ng.553. [DOI] [PubMed] [Google Scholar]


