Abstract
Background
Sex differences in epidemiological, clinical and pathological characteristics of colorectal cancer have been under intensive investigation for the last three decades. Given that most of the sex-related differences reported were also age-related, this study sought to determine the potential effect of a sex-age interaction on colorectal cancer development and progression.
Material/Methods
Statistical data on sex- and age-specific colon or rectal cancer incidence, disease stage and survival for white persons were derived from the United States Surveillance, Epidemiology and End Results (SEER) Program. Age-specific incidence rates in 2002–2006 were analyzed by 5-year age groups (45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84 years) in men and women. Sex differences were measured by calculating rate differences (RD) and rate ratios (RR). Equivalent analyses for a similar time period were performed for stage distribution and 5-year relative survival.
Results
Age-specific incidence rates were higher for men, for all life-time periods. However, the magnitude of the male predominance was age-dependent. The RR and RD did not remain constant over time: they increased gradually with age, peaked at 70–74 years, and declined thereafter. The distribution of stage at diagnosis was similar between men and women, but women seemed to have better survival, until the age of 64 years for colon cancer and 74 years for rectal cancer.
Conclusions
There seem to be significant age-related sex differences in the incidence of colorectal cancer, and maybe also in its prognosis.
Keywords: colorectal cancer, incidence, SEER, male to female ratio, estrogen
Background
Colorectal cancer (CRC) is one of the most common malignancies in both men and women worldwide. Despite improvements in prevention in recent years, the reported global incidence is still about one million per year. CRC accounts for more than 500,000 deaths annually, making it the third leading cause of cancer-related mortality [1]. The 5-year survival proportion is 64% in Western countries [2]. Most affected patients are elderly, giving rise to the assumption that the disease is slow-developing. Therefore, the identification of its main etiological factors could help clinicians reduce the health burden of the disease by more effective screening and treatment strategies.
There is cumulative evidence of sex-related differences in the incidence, anatomic site distribution, and chemoresponsiveness of CRC. Incidence rates are lower in women than men, with a particularly striking discrepancy between pre-menopausal women and age-matched men [3,4]. Women have a greater susceptibility to right-sided tumors (proximal and transverse segments of the colon) than to left-sided tumors (distal colon and rectum) [2], and white men having the greatest risk for distal CRC [5]. Women are more likely than men to respond to 5-fluorouracil (5-FU)-based chemotherapy [6], perhaps owing to the lower cellular level of thymidilate synthase, the main target of the drug, in tumors in women [7]. On the molecular level, sex differences have been found in the “mutator phenotype” which causes genetic instability in the form of deletion and insertion mutations in simple repetitive DNA sequences at microsatellite loci. Microsatellite instability (MSI) occurs in 10% to 20% of cases of sporadic CRC and is more characteristic of women than men.
Based on the observation that most of the reported sex-related differences in clinical and molecular parameters of CRC were also age-related, the aim of the present study was to investigate the effect of potential sex-age interactions on the development and progression of the disease. Specifically, we sought to determine if the overall male predominance is constant over the lifetime of men and women or is subject to sex-age interactions. If a similar pattern affects the stage distribution at diagnosis and patient outcome.
Material and Methods
Data source
All data presented and analyzed were obtained from the Surveillance, Epidemiology and End Results (SEER) website of the United States (US) National Cancer Institute (www.seer.cancer.gov), which provides information on cancer statistics [8]. We used the sex- and age-specific statistical data on incidence rates, stage distribution, and survival of CRC in the white (race) population from 17 geographic areas in the US (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New-Mexico, Seattle, Utah, Atlanta, San Jose-Monterey, Los Angeles, Alaska Native Registry, Rural Georgia, California excluding SF/SJM/LA, Kentucky, Louisiana, and New Jersey). We limited the calculation to whites for two main reasons. First, the white population represents the majority of US residents and US patients with CRC (82% of all CRC cases in 2002–2006) and may be representative of the general western white population. Second, limiting our study sample eliminated the comparison complexity of possible endogenous or exogenous race-related factors [9].
Analyses were performed according to anatomic cancer subsites (colon, rectum) using incidence and survival rates per 100,000 individuals (men or women). The age-adjusted incidence rates (for the 2000 US standard population) and age-specific incidence rates covered the period of 2002–2006; the age-specific rates were categorized in 5-year age groups, from 45–49 years to 80–84 years. We excluded the <45-year-group because of small rate counts for the combined years and the >85-year group because of the possibility of confounders complicating sex differences. Stage distribution percentage was based on the latest statistical data available for the years 2000–2006. Categories for stage at diagnosis were coded according to the SEER Summary Stage 2000 [10]: malignant microscopically confirmed tumors were defined as localized (invasive tumors confined to the colon or rectum), regional (tumors that invaded surrounding tissues, organs, lymph nodes), distant (metastasized tumors), and unstaged. Sex- and age-specific incidence rates were stratified by stage according to the percentage distribution. Survival rates were based on the latest statistical data available for the years 1999–2005.
Calculations
To gain a better understanding of the observed disparities, measures of sex differences were based on both absolute variables (incidence rate difference, RD) and relative variables (incidence rate ratio, RR). Rates ratios were calculated as follows: (A) male-to-female RR for age-specific incidence rates, including age-specific incidence rates that were stratified according to stage distribution, because the male-to-female RR value is >1; (B) female-to-male RR for survival, because most female-to-male RR values are >1.
Results
Incidence Rates
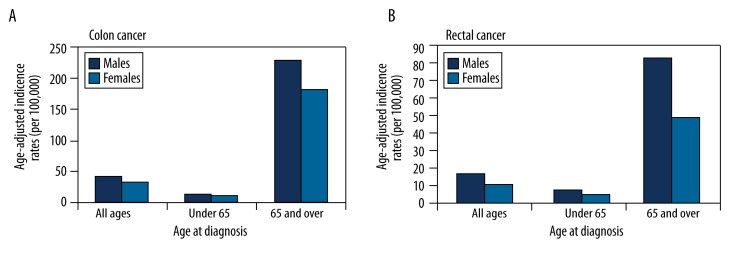
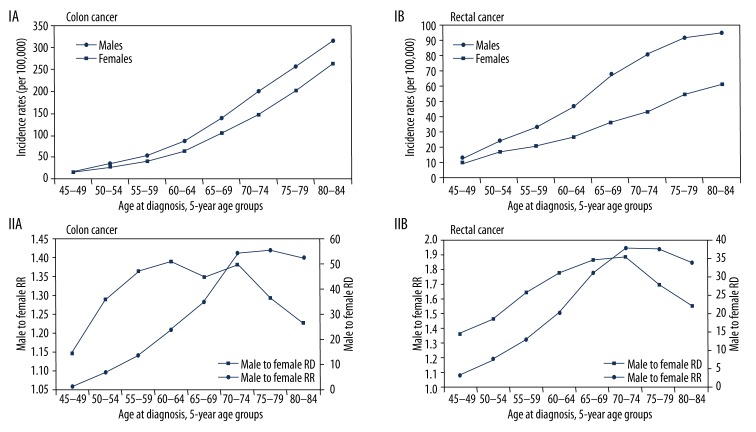
The reported number of diagnosed cases in the 17 SEER areas for whites (both sexes) in 2002–2006 was 105,256 for colon cancer and 40,692 for rectal (standard errors 0.11 and 0.07, respectively). The median age at diagnosis of CRC for all races (2002–2006) was 71 years; approximately 65% of patients were aged 65 years or more. Figure 1 compares the age-adjusted SEER incidence rates of colon or rectal cancers by sex and by age at diagnosis. Although the majority of cases were diagnosed at age 65 years or more, the magnitude of the male predominance was age-dependent; that is, it was more pronounced at age 65 years or more than at age less than 65 years; the male-to-female ratio for incidence was 1.70 in the older population and 1.51 in the younger population. Further subdividing the population into 5-year age groups (Figure 2) revealed that the age-specific colon and rectal cancer incidence rates rose overall with increasing age for both sexes, but within each age group, the rates were always lower in women than in men. The male predominance was stronger for rectal than for colon cancer (Figure 2, IA, IB). Using the relative and absolute measures (Figure 2, IIA, IIB), we found that for each subtype of cancer, the RR and RD did not remain constant over time; rather, they gradually increased with age and peaked at 70–74 years. Interestingly, in the 75+ years age group, the RR decreased whereas the RD was almost stable. For colon cancer, the RR increased from ages 45–49 years (1.11) to 70–74 years (1.37), meaning that rates were 11% higher for men than for women at ages 45–49 years, and this difference increased to 37% at ages 70–74 years. For rectal cancer, the age-related difference in male-to-female incidence rates was even greater: the RR increased from 1.36 at 45–49 years (36% higher in men) to 1.88 at 70–74 years (88% higher in men). The absolute measure (RD) showed that at age 70–74 years, there were 54 more cases of invasive colon cancer and 38 more cases of rectal cancer per 100,000 men than per 100,000 women.
Figure 1.
Age-adjusted SEER incidence rates (per 100,000), 2002–2006 (17 areas), white, by sex.
Figure 2.
(I) Age-specific SEER incidence rates (per 100,000), 2002–2006 (17 areas), white, by sex. (II) Male-to-female incidence rate ratio (RR) and incidence rate difference (RD).
Stage distribution
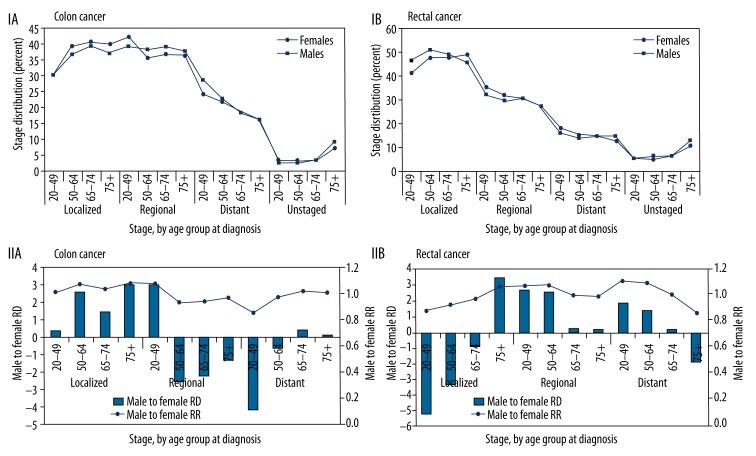
Figure 3 shows the distribution of cases of colon and rectal cancer separately by stage at diagnosis (SEER Summary Stage 2000) for men and women. The majority of cancers were localized or regional at diagnosis. After stratifying incidence rates according to stage distribution for all ages and within each age group (20–49, 50–64, 65–74, 75+ years), rates remained similar in men and women. This observation was true also for colon and rectal tumors separately (Figure 3, IA, IB). On further analysis using the male-to-female RR and RD (Figure 3, IIA, IIB), we noted that the pattern of sex- and age-related differences shown for incidence was not maintained when stratifying by cancer stage. Within the age groups 50–64, 65–74, and 75+ years, a similar incidence rate was shown for male and female cases of colon or rectal cancers in the localized or regional stage, yielding a male-to-female RR of ~1.0. However, for distant-stage colon cancer, the male-to-female RR was 0.85 for the 20–49-year age group, meaning that in this age group colon cancer was diagnosed at the distant stage in 18% more women than men. The male-to-female RR in distant-stage colon cancer rose gradually with an increase in age, achieving equality (RR=1) in the 75+ age group (Figure 3, IIA). For distant-stage rectal cancer, a reverse pattern was noted: there was a 12% male predominance at ages 20–49 years which gradually declined, and by age 75+ years, there was a 14% female predominance (Figure 3, IIB).
Figure 3.
(I) Stage distribution (SEER summary stage), 2000–2006 (17 areas), white, at age at diagnosis by sex. (II) Male-to-female stage-stratified incidence rate ratio (RR) and stage-stratified incidence rate difference (RD).
Survival rates
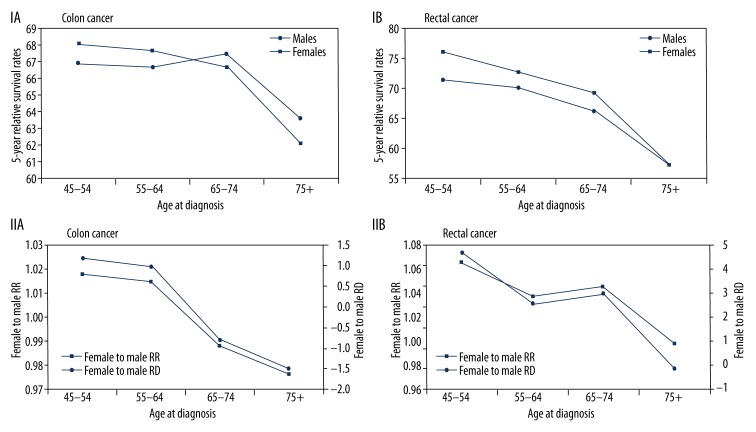
The 5-year relative survival rates by age at diagnosis (45–54, 55–64, 65–74, and 75+ years) and by sex showed different patterns for colon cancer and rectal cancer. For colon cancer, women had a slight survival advantage over men until age 64 years; thereafter, men had a slight advantage (Figure 4, IA, IIA). For rectal cancer, women maintained their advantage to age 74 years, and thereafter, survival was similar for men and women (Figure 4, IB, IIB).
Figure 4.
(I) 5-year relative survival rates, 1999–2005 (17 areas), white, at age at diagnosis by sex. (II) Male to female survival rate ratio (RR) and survival rate difference (RD).
Discussion
Earlier studies reported sex related differences in the incidence, presentation and clinical outcome of CRC patients. The aim of the present study was to evaluate these differences and, more importantly, to determine if they are constant over time or age-dependent. We found that differences in incidence, incidence rates by stage, and survival between male and female CRC patients are subject to substantial age-dependent changes. As previously reported, men had a higher incidence of CRC for all ages, but our analysis suggested that this excess of risk in men has a clear peak: it increases up to the mid-eighth decade of life and decreases steadily thereafter, with a greater effect of age in rectal than in colon cancer. Data on disease stage at diagnosis showed only minor sex differences by age, and those were limited to the distant stage, with opposing trends for colon and rectal cancer. We found that as men age, they are more likely than women to be diagnosed with metastatic colon cancer, but less likely than women to be diagnosed with metastatic rectal cancer. Survival analysis demonstrated only a slight overall 5-year survival advantage for women over men, until age 64 years for colon cancer and age 74 years for rectal cancer. Overall, women harbored a protective effect against both the development of CRC and CRC-related mortality until the seventh to eighth decade of life, with no clear effect of sex-age interactions on the extent of disease at presentation.
Previous data from the US showed that men have a higher incidence of CRC, in all age groups and races and for all colonic subsites, and that this sex disparity increases with age and is greatest in the ≥65-year-old population [2,11,12]. The present study sought to determine if there is a specific pattern to the sex differences over time by breaking the ≥65-year age group into several smaller-range groups. Applying the relative (RR) and absolute (RD) male-to-female rate differences, we found what we believe is a previously unrecognized pattern of sex-related difference by age for CRC occurrence. Similar to others [11,12], we noted that while the age-specific incidence rates for both male and female colon or rectal cancer increase with age, the rates in males grow in larger numbers. However, the sex disparity increases with an increase in age only to age 74 years and decreases thereafter, indicating that the disparity in magnitude is age-dependent. This pattern was true for both colon and rectal cancer separately, yet with a clearer male predominance for rectal cancer. According to these results, men are at greater risk than women of developing CRC, and this excess of risk increases up to the mid-eighth decade of life and then decreases steadily.
Women have been described as having a significantly better survival than men for many cancer types, including CRC [13,14]. However, similar to the age-dependent sex-related differences in the incidence of the disease, we also found that the better CRC outcome of women is also age-dependent: for colon cancer, women had a slight 5-year survival advantage which reversed at age 65 years, and for rectal cancer, the higher survival rates of women decreased over time to equality at age 74 years. To the best of our knowledge, this is the first report on the effect of the sex-age interaction on the outcome of CRC in the US. Data from Europe suggest a similar pattern: Micheli et al. [13] reported that women’s survival advantage for combined CRC increases with age to 64 years and declines thereafter. The survival advantage of female patients with CRC cannot be explained by a sex-related imbalance of disease stage at presentation, as the stage distribution was grossly similar between the sexes. These findings are consistent with most previous reports [15].
The reason for the apparent age-dependent protective effect against both the development and progression of CRC remains unknown. The effect for CRC occurrence is present until the eighth decade of life, and for CRC mortality, to the seventh (colon) or eighth (rectum) decades. The fact that it is a combined effect suggests a causative underlying endogenous factor that either poses a risk to men or is protective in women. This assumption is supported by the parallel findings of a wide geographic range in CRC incidence and mortality rates, even among ethnic populations living in the same regions, and a common worldwide trend of higher incidence and mortality rates in men [10,15,16] and age-dependent survival advantage for women [10,13,15].
A reasonable candidate host factor is female sex hormone status, particularly estrogen serum levels. First, circulating levels of the main estrogenic compound, 17β-estradiol (E2), are largely higher in women than in men, and they are age-dependent. During the child-bearing years, from adolescence to the fifth decade, women are exposed to relatively high levels of endogenous E2; however, after menopause, levels drop to close to those of men. By contrast, in men, E2 levels remain low and constant, and decline only marginally with advanced age. Second, the concept that estrogen exerts an effect on the gastrointestinal tract (GIT) is not new. Estrogen has already been implicated in upper GIT cancers, such as esophageal and gastric cancers, which, like CRC, are characterized by a higher incidence and mortality rates among men, with a peak in male predominance at a certain age [17,18].Also in his research Wang and friends demonstrated that people with high incidence of esophageal cancer have low levels of estrogen compared to heathly subjects [19] This finding was supported by experimental studies showing that estrogen regulates growth, cell differentiation, and cell function in the GIT [20]. The possible protective role of estrogen in CRC has been suggested in recent years by several lines of epidemiologic, clinical and experimental evidence [20,21]. For example, use of hormone replacement therapy was found to reduce the risk of CRC [22,23]. Liang et al. [24] reported that the risk of second primary CRC was lower in premenopausal women, but after age 55 years, incidence rates increased much more rapidly in women than in men. The authors linked these findings to the corresponding decline in estrogen levels with increasing age in women.
We hypothesize that in women, circulating levels of E2 may exert a cumulative protective effect against the CRC carcinogenic process for up to 20–25 years after menopause. Such a long period may represent the time needed for a normal mucosal cell to undergo tumorigenic transformation and reach diagnostic size. As to the age-dependent survival advantage of female patients, it is possible that CRC developing in high-E2 environments may have a less aggressive biology, among other differences, than CRC developing in low-E2 environments.
Diet is one of the main underlying environmental etiological factors in CRC. Jacobs et al. [25] reviewed sex differences in diet-related factors in CRC. Although some of the factors seemed to have slightly stronger effects in men than women, the most pronounced evidence of a sex-specific effect was observed for obesity. Obesity was reported to be a major risk factor for CRC in men and to a lesser extent in women [26]. However, this observation may have been influenced by the differential effects of menopausal status in women, as obesity increased the risk of CRC in the presence of estrogen [27,28]. Estrogen’s impact on the incidence and behavior of CRC may also interact with basic molecular process in CRC. For example, accumulating data suggest that estrogen may exert its protective effects via interference with MSI status and gene hypermethylation [29]. Thus, estrogen-related factors may interplay with other endogenous, environmental and behavioral risk factors for CRC through multiple pathways to yield differential effects between the sexes and among women according to menopausal status.
Conclusions
In summary, this study demonstrated significant age-related sex differences in the incidence of CRC, and to a lesser extent, in its progression. Expanding studies like ours to other races in the US and to other nations seems warranted in order to confirm our data and to provide insight on the underlying mechanisms of this phenomenon. The findings may have important implications for developing effective approaches to the screening, early detection, and perhaps treatment of CRC.
Acknowledgement
There was no funding for this study.
Footnotes
Source of support: Self financing
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.DeCosse JJ, Ngoi SS, Jascobson JS, Cennerazzo WJ. Gender and colorectal cancer. Eur J Cancer Prev. 1993;2:105–15. doi: 10.1097/00008469-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433–42. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson RL, Dollear T, Freels S, Persky V. The relation of age, race, and gender to the subsite location of colorectal carcinoma. Cancer. 1997;80:193–97. doi: 10.1002/(sici)1097-0142(19970715)80:2<193::aid-cncr4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Elsaleh H, Joseph D, Grieu F, et al. Association of tumor site, and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–50. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 7.Wong NA, Brett L, Stewart M, et al. Nuclear thymidilate synthase expression, p53 expression and 5-FU response in colorectal carcinoma. Br J Cancer. 2001;5:1937–43. doi: 10.1054/bjoc.2001.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horner MJ, Ries LAG, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2006, based on November 2008 SEER data submission, posted to the SEER web site, 2009. [Google Scholar]
- 9.Dominik DA, Waterbor J, Hughes T, et al. African-American and Caucasian disparities in colorectal cancer mortality and survival by data source: An epidemiologic review. Cancer Biomark. 2007;3:301–13. doi: 10.3233/cbm-2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson-Thompson J, Ahmed F, German R, et al. Descriptive epidemiology of colorectal cancer in the United States, 1998–2001. Cancer. 2006;107(Suppl 5):1103–11. doi: 10.1002/cncr.22007. [DOI] [PubMed] [Google Scholar]
- 11.Rim SH, Seeff L, Ahmed F, et al. Colorectal cancer incidence in the United States, 1999–2004. Cancer. 2009;115:1967–76. doi: 10.1002/cncr.24216. [DOI] [PubMed] [Google Scholar]
- 12.Brenner H, Hoffmeister M, Arndt V, Haug U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer. 2007;96:828–31. doi: 10.1038/sj.bjc.6603628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micheli A, Ciampichini R, Oberaigner W, et al. The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur J Cancer. 2009;45:1017–27. doi: 10.1016/j.ejca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Bossard N, Velten M, Remontet L, et al. Survival of cancer patients in France: The population-based study from the association of the French Cancer Registries study (FRANCIM) Eur J Cancer. 2007;43:149–60. doi: 10.1016/j.ejca.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Koo JH, Jalaludin B, Wong SKC, et al. Improved survival in young women with colorectal cancer. Am J Gastroenterol. 2008;103:1488–95. doi: 10.1111/j.1572-0241.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- 16.Umar A, Greenwald P. Alarming colorectal cancer incidence trends: A case for early detection and prevention. Cancer Epidemiol Biomarkers Prev. 2009;18:1672–73. doi: 10.1158/1055-9965.EPI-09-0320. [DOI] [PubMed] [Google Scholar]
- 17.Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in female results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213–19. doi: 10.1007/s101200200037. [DOI] [PubMed] [Google Scholar]
- 18.Derakhshan MH, Liptrot S, Paul J, et al. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58:16–23. doi: 10.1136/gut.2008.161331. [DOI] [PubMed] [Google Scholar]
- 19.Wang QM, Yuan L, Qi YJ, et al. Estrogen analogues: Promising target for prevention and treatment of esophageal squamous cell carcinoma in high risk areas. Med Sci Monit. 2010;16(7):HY19–22. [PubMed] [Google Scholar]
- 20.Singh S, Langman MJ. Oestrogen and colonic epithelial cell growth. Gut. 1995;37:737–79. doi: 10.1136/gut.37.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennelly R, Kavanagh DO, Hogan AM, Winter DC. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 2008;9:385–91. doi: 10.1016/S1470-2045(08)70100-1. [DOI] [PubMed] [Google Scholar]
- 22.Grodstein F, Newcomb PA, Stampler MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med. 1999;106:574–82. doi: 10.1016/s0002-9343(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 23.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Women,s Health Initiative Investigators: Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 24.Liang W. Age, sex and the risk of grade-specific second primary colorectal cancer: evidence for the protective effect of female hormone. Eur J Cancer. 2007;43:1856–61. doi: 10.1016/j.ejca.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs ET, Thompson PA, Martinez ME. Diet, gender, and colorectal neoplasia. J Clin Gastroenterol. 2007;41:731–46. doi: 10.1097/MCG.0b013e3180338e56. [DOI] [PubMed] [Google Scholar]
- 26.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–65. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 27.Slattery ML, Ballard-Barbash R, Edwards S, et al. Body mass index and colon cancer: an evaluation of the modifying effects of estrogen (United States) Cancer Causes Control. 2003;14:75–84. doi: 10.1023/a:1022545017867. [DOI] [PubMed] [Google Scholar]
- 28.Reeves GK, Pirie K, Beral V, et al. Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo JH, Leong RWL. Sex differences in epidemiological, clinical and pathological characteristics of colorectal cancer. J Gastroenterol Hepatol. 2010;25:33–42. doi: 10.1111/j.1440-1746.2009.05992.x. [DOI] [PubMed] [Google Scholar]