Abstract
Background: Gastric cancer is increasingly recognized in Zambia. Although nutritional factors contribute to gastric cancer risk, their effect in Zambia is unknown.
Objective: The objective was to investigate the association between intake of dietary antioxidants, urinary 8-iso prostaglandin F2α (8-iso PGF2α) as a marker of oxidative stress, and gastric cancer.
Design: This was a case-control study at the University Teaching Hospital in Zambia. Gastric cancer cases were compared with age- and sex-matched controls. Urine 8-iso PGF2α was measured primarily by ELISA, and by gas chromatography–mass spectrometry in a subset, expressed as a ratio to creatinine. Blood was collected for Helicobacter pylori, HIV serology, gastrin-17, and pepsinogen 1 and 2 concentrations. Clinical and dietary data were collected by using questionnaires. Food items were broadly classified into 7 major categories (fruit, vegetables, fish, meat, insects, cereals, and starches).
Results: Fifty cases with gastric cancer (mean age: 61 y; n = 31 males) and 90 controls (mean age: 54 y; n = 41 males) were enrolled. Median urinary 8-iso PGF2α excretion was higher in cases (0.014; IQR: 0.008–0.021) than in controls (0.011; IQR: 0.006–0.018; P = 0.039). On univariate analysis, habitual fruit intake was lower in cases than in controls during the dry season (P = 0.02). On multivariate analysis, smoking (OR: 7.22; IQR: 1.38–37.9) and gastric atrophy (OR: 2.43; IQR: 1.12–5.13) were independently associated with cancer, and higher fruit intake was protective (OR: 0.44; IQR: 0.20–0.95). Isoprostane excretion was inversely correlated with total fruit intake (ρ = −0.23; n = 140; P = 0.006).
Conclusion: Urinary 8-iso PGF2α excretion was associated with the risk of gastric cancer, as were smoking and gastric atrophy, but increased fruit intake conferred protection. This trial was registered at www.pactr.org as ISRCTN52971746.
INTRODUCTION
Gastric cancer is the fourth most common type of cancer and the second most frequent cause of cancer death worldwide (1–3). The WHO predicts an increase in cancer rates by >50% over the next 20 y (1). Hypothesized risk factors for increasing rates include inflammatory, infectious, and environmental factors (3–6). Environmental factors particularly related to diet have been implicated in gastric carcinogenesis through a direct (nitrosamines in smoked foods) or indirect mechanism (altering cellular dynamics of gastric mucosa) (7). Nitrosamine ingestion in smoked and pickled foods has been proposed as an explanation for the high incidence of gastric cancer in Japan. In Zambia, the epidemiology of gastric cancer is largely unexplored, but a recent audit of endoscopy and pathology records suggested that the incidence has shifted to younger adults (8). Furthermore, the contribution of Helicobacter pylori, which has a seroprevalence of 81%, remains unknown in the Zambian gastric cancer population (9). We postulated that dietary factors might be associated with gastric cancer risk in Zambian adults. We examined gastric cancer risk in relation to diet, antioxidant status, and established risk factors in a case-control study that accounted for H. pylori status.
The Zambian diet predominantly consists of a high intake of maize starch as a hot paste (nshima) served with vegetables, with fish, and less frequently with meat. The diet is not rich in smoked food, but does vary seasonally following the availability of fruit, vegetables, and insects. Oxidative stress potentially plays a key role in the development of cancer (10–12). Oxidative stress consists of the presence of increased free radicals triggering DNA damage or lipid peroxidation, which in turn predispose to cancer formation (10, 13, 14). Until recently, there has been a lack of reliable markers to measure levels of oxidative stress, although indexes of dietary antioxidant intake (vitamin E, vitamin C, and selenium) have been used. Urinary isoprostanes were recently shown to be reliable markers for in vivo oxidative stress (13). Isoprostanes are prostaglandin-like substances produced in vivo by free radical–induced peroxidation of arachidonic acid (13, 14). In this study, we looked for evidence that a low intake of fruit and vegetables predisposes to gastric cancer through the oxidative stress pathway and that this would be mediated through impaired antioxidant status as evidenced by abnormal urinary isoprostane excretion.
SUBJECTS AND METHODS
The study was a prospective case-control study carried out at the University Teaching Hospital (UTH), Lusaka, Zambia—a national referral hospital based in the capital city that evaluates patients from all over the country. The study was approved by the Biomedical Research Ethics Committee of the University of Zambia School of Medicine, Lusaka, Zambia; the Siteman Cancer Center Protocol Review Monitoring Committee; and the Human Research Protection Office (Institutional Review Board) at Washington University School of Medicine, St Louis, MO.
Patients and recruitment
Patients (≥18 y of age) presenting to the endoscopy unit at UTH with upper gastrointestinal symptoms for esophagogastroduodenoscopy were considered for recruitment if they were willing to provide informed consent. Cases were defined as patients with gastric adenocarcinoma confirmed by histopathology. The only exclusion criterion was current radiation or chemotherapy, but no patients were excluded on this basis. Controls were defined as patients with upper gastrointestinal symptoms without any endoscopic evidence of upper gastrointestinal pathology. Controls were recruited sequentially within 1 mo of cases (to ensure equal representation by season of recruitment), matched by sex and within comparable age groups, as follows: 18–30, 31–45, 46–60, or >60 y. Although the protocol was designed for 2 controls per case of gastric cancer, this could not be achieved in the oldest age group because of the unavailability of older-age control males in the study population.
Data collection
Informed consent was obtained from all patients (cases and controls) at the time of study enrollment. A questionnaire was used to collect demographic data [age, sex, occupation, and socioeconomic status indicators such as low household income (<K1,000,000/mo; exchange rate $1 = K5000) and education achievement]. A medical history (including family and social history, smoking, and alcohol consumption) was obtained, and a physical examination was performed by the same investigator for uniformity in data collection. Height and weight were recorded as part of the physical examination, and BMI was calculated according to the standard formula, ie, weight (in kg)/height (in m) squared (2). Midupper arm circumference was recorded as a measure of nutritional status.
A detailed food-frequency questionnaire was developed a priori to include foods available in Zambia and was used exclusively for this study. In this questionnaire, food items were broadly classified into 7 major categories (fruit, vegetables, fish, meat, insects, cereals and starches, and other). A total of 51 food items were assessed. Participants were asked to report their habitual (ie, premorbid, for cases) frequency of consumption of each food category, segregated as never, once per month, once per week, 2–3 times/wk, 4–6 times/wk, daily, 2–3 times/d, 4–6 times/d, and 7 times/d.
An esophagogastroduodenoscopy was performed to determine gastric cancer status. When identified, samples were obtained for histopathology. Gastric adenocarcinoma was further divided by using the Lauren classification into diffuse or intestinal type (15). Controls underwent biopsy sampling to exclude mucosal processes; none had evidence of dysplasia or adenocarcinoma on histopathologic examination.
Urine samples were collected to measure concentrations of isoprostanes. Butylated hydroxytoluene preservative stock solution was prepared with 50 mg/mL methanol and stored in a refrigerator at 4°C. Fasting spot urine samples were collected from all subjects; aliquots of 0.9 mL were added to 0.1 mL butylated hydroxytoluene in a cryopreservation tube and then stored at −80°C. The seroprevalence of HIV in Zambia is 16% (15, 16) and that of Helicobacter pylori, a known gastric carcinogen (7), is 81% (9); therefore, serum was collected to assess seropositivity for these infections. In addition, serum pepsinogen 1 and 2 and gastrin-17 concentrations were measured as markers of gastric atrophy (see Laboratory assays).
Laboratory assays
Urinary isoprostane and creatinine concentrations were measured by using Isoprostane and Creatinine Microplate Assays (Oxford Biomedical) according to the manufacturer's instructions. A subset (28%) of duplicates of the urine aliquots was transported to St Louis for gas chromatography–mass spectrometry quantitation of total (free + esterified) urinary 8-iso prostaglandin-F2α (8-iso-PGF2α). Briefly, 4 ng deuterated internal standard [8-iso PGF2α-d4 (8-iso PGF2α-3,3,4,4-d4) Cayman Chemical Co] (17) was added to each urine sample (400 μL). After alkaline hydrolysis, urine 8-isoprostanes were isolated by using an immunoaffinity resin (Cayman Chemical Co), which was chemically derivatized to form their pentafluorobenzyl ester-trimethylsilyl ether, and analyzed by gas chromatography–negative chemical ionization mass spectrometry by using selective ion monitoring at m/z 569 and 573. The 569/573 signal intensity area ratios were calculated, compared with the same signals generated with a concentration standard curve, and used to quantify the amount of total 8-iso PGF2α in urine.
H. pylori serology, pepsinogen 1 and 2, and gastrin-17 assays were performed by using Biohit Gastro Panel ELISA kits (Biohit); for CagA, ELISA kits were obtained from Genesis Diagnostics and used according to the manufacturer's instructions. The presence of HIV infection was determined by the virology laboratory of the UTH by using Determine (Alere) for screening and Unigold test strips (Trinity Biotech) for confirmation.
Statistical analysis
Isoprostane and food-frequency data did not follow a Gaussian distribution, so continuous variables are presented as medians and IQRs. Food-frequency data were analyzed per patient, and the total daily intake of portions of each food were computed for each case or control. Only total consumption in each group was analyzed (except for the “other” food category in which items were analyzed individually) to minimize the number of statistical tests required. Seasonality was analyzed by dividing the month of recruitment into 3 seasons: cold (May–August), hot (September–November), and rainy (December–April). A pepsinogen 1:2 ratio was regarded as low (indicative of atrophy of the body/fundus) if ≤3.0, and gastrin-17 was classified as low if <1.0 pmol/L. All analyses were performed by using Stata 10.1 (Stata Corp). Associations between risk factors and cancer are presented as ORs with 95% CIs, and were determined by using Fisher's exact test. To compare continuous variables in cases and controls, the Kruskal-Wallis test was used. For multivariate analysis, continuous variables were dichotomized around the median, and a backward stepwise strategy was used to derive a final unconditional logistic regression model. In all instances, a P value <0.05 was required for statistical significance.
RESULTS
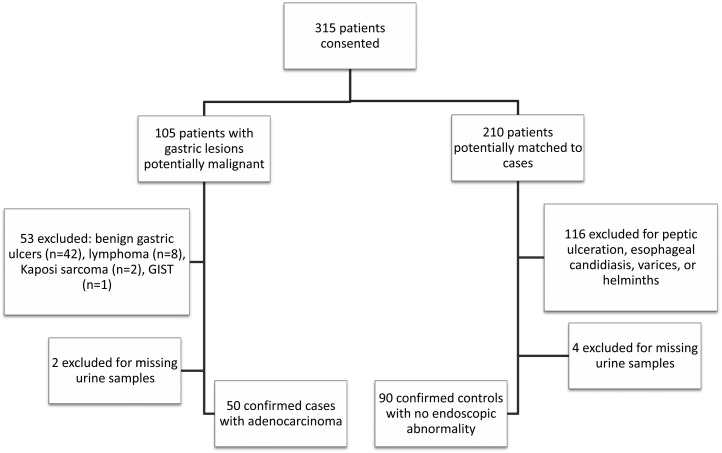
Between November 2010 and January 2012, a total of 315 patients gave consent for inclusion in the study. Of the 105 patients whose endoscopy showed ulcers or potentially malignant lesions, 50 were confirmed by histology and were included as cases; 65 were not, and thus were excluded. Of the 210 potential controls identified as matches for age and sex, 120 were ineligible because of upper gastrointestinal conditions (Figure 1). Men outnumbered women among cases in a ratio of 1.7:1, and the mean age was 61 y (Table 1). Midupper arm circumference was lower in cases than in controls, but other demographic and clinical characteristics, including BMI, did not differ between cases and controls (Table 1). Of the 50 cases of gastric cancer, 30 (60%) were located in the antrum, 17 (34%) in the gastric body, and 10 (20%) in the cardia, with some extending across more than one site. When segregated by the Lauren classification, 39 (78%) were intestinal type adenocarcinoma, 7 (14%) were diffuse, and 2 (4%) were mixed; 2 were not classified. H. pylori serology was positive in 76% of gastric cancer cases, which was comparable with that in controls (87%; P = 0.10). Thirty-one cases and 26 controls were CagA+ (OR: 1.08; 95% CI: 0.5, 2.5; P = 1.00). In total, 4 cases and 7 controls were seropositive for HIV (OR: 1.03; 95% CI: 0.2, 4.3; P = 1.00).
FIGURE 1.
Flow diagram of enrollment of cases and controls. GIST, gastrointestinal stromal tumor.
TABLE 1.
Baseline characteristics1
| Variable | Case (n = 50) | Control (n = 90) | P |
| Age group [n (%)] | |||
| <30 y | 2 (4) | 4 (4) | |
| 30–45 y | 8 (16) | 26 (29) | |
| 46–60 y | 10 (20) | 26 (29) | |
| >60 y | 30 (60) | 34 (38) | 0.08 |
| Male sex [n (%)] | 31 (62) | 42 (47) | 0.11 |
| Education [n (%)] | |||
| None | 8 (16) | 11 (12) | |
| Primary | 18 (36) | 33 (37) | |
| Secondary | 18 (36) | 25 (28) | |
| Tertiary | 5 (10) | 21 (23) | 0.24 |
| Income [n (%)] | |||
| Low | 12 (24) | 22 (24) | |
| High | 14 (28) | 45 (50) | |
| Irregular/unsure | 23 (46) | 22 (24) | 0.02 |
| Smoking status [n (%)] | |||
| Never | 38 (76) | 85 (94) | |
| Ever | 11 (22) | 5 (6) | 0.002 |
| Alcohol intake [n (%)] | |||
| Never | 37 (74) | 77 (85) | |
| Ever | 13 (26) | 14 (15) | 0.11 |
| BMI (kg/m2) | 21.4 (18.2–28.1)2 | 24.0 (21.2–29.0) | 0.08 |
| HIV seropositive (n) | 4 | 7 | 1.00 |
| MUAC (cm) | 25.6 (22.0–31.0) | 28.3 (25.5–32.8) | 0.004 |
| Concurrent medication [n (%)] | |||
| None | 24 (48) | 40 (44) | 0.73 |
| Proton pump inhibitors | 6 (12) | 19 (21) | 0.25 |
| H2 antagonists | 1 (2) | 7 (8) | 0.26 |
| Antihypertensives | 0 | 15 (17) | 0.001 |
| NSAIDs | 4 (8) | 7 (8) | 1.00 |
1The Kruskal-Wallis test was used to compare continuous variables in cases and controls. MUAC, midupper arm circumference; NSAID, nonsteroidal antiinflammatory drug.
2Median; IQR in parentheses (all such values).
Urinary isoprostane excretion
After adjustment for creatinine, median (IQR) urinary 8-iso PGF2α excretion (urinary isoprostane:creatinine ratio) was higher in patients with gastric cancer (median: 0.014; IQR: 0.008–0.021) than in controls (median: 0.011; IQR: 0.006–0.018; P = 0.039). In a subset of samples (14 cases and 24 controls), isoprostane was measured by both ELISA and gas chromatography–mass spectrometry; a significant correlation was found between the 2 methods (Spearman's ρ = 0.35; P = 0.03).
The urinary isoprostane:creatinine ratio was compared in cases and controls within groups with known risk factors for gastric cancer. However, this ratio did not differ significantly in smokers (current or past) or with alcohol consumption (current or past). Urinary isoprostane excretion was higher (median: 0.013, IQR: 0.009–0.024) in 57 study patients with a low pepsinogen 1:2 ratio (including cases and controls) than in 83 with a normal ratio (median: 0.010; IQR: 0.005–0.170; P = 0.009). When compared between cases and controls, neither low nor high gastrin-17 was associated with isoprostane in a univariate analysis (data not shown).
Food-frequency questionnaire estimation of dietary intake
As expected in the Zambian population, all respondents reported consumption of maize, onions, and tomatoes on a daily basis; therefore, these were not compared between cases and controls. In a univariate analysis, ingestion of foods within the 7 different food groups did not differ between cases and controls (Table 2).
TABLE 2.
Food frequency per day1
| Median (IQR) | |||
| Food item | Cases | Controls | P1 |
| Fruit | 0.06 (0.02 dry season; 0.18 wet season) | ||
| Banana | 0.03 (0.03–0.14) | 0.14 (0.03–0.35) | |
| Apple | 0 (0–0.03) | 0.03 (0.03–0.14) | |
| Avocado | 0.03 (0–0.03) | 0.03 (0–0.14) | |
| Papaya | 0.03 (0–0.03) | 0.03 (0–0.03) | |
| Watermelon | 0 (0–0.03) | 0.03 (0–0.03) | |
| Guava | 0 (0–0.03) | 0 (0–0.03) | |
| Exotic (grape, strawberry, mulberry, peach) | 0 (0–0) | 0 (0–0) | |
| Indigenous (masuku, mpundu, masau) | 0 (0–0) | 0 (0–0) | |
| Citrus (orange, tangerine, lemon) | 0.03 (0–0.14) | 0.03 (0–0.14) | |
| Vegetables | 0.23 | ||
| Bean leaves | 0.03 (0–0.03) | 0.03 (0–0.03) | |
| Cassava leaves | 0.03 (0–0.03) | 0.03 (0–0.03) | |
| Green peppers | 0 (0–0.03) | 0.015 (0–0.14) | |
| Sweet potatoes | 0.03 (0–0.14) | 0.03 (0.03–0.14) | |
| Garlic | 0 (0–0) | 0 (0–0.03) | |
| Baby marrow | 0 (0–0) | 0 (0–0) | |
| Rape | 0.7 (0.30–1.0) | 0.7 (0.14–1.0) | |
| Cabbage | 0.35 (0.03–0.07) | 0.35 (0.14–0.7) | |
| Pumpkin leaves | 0.35 (0.03–0.4) | 0.35 (0.03–0.7) | |
| Okra | 0.14 (0–0.35) | 0.03 (0–0.35) | |
| Mushroom | 0 (0–0.03) | 0.03 (0–0.03) | |
| Impwa | 0.03 (0–0.6) | 0.03 (0–0.14) | |
| Wondwe | 0.03 (0–0.14) | 0.03 (0–0.14) | |
| Kanuka | 0 (0–0.03) | 0 (0–0) | |
| Peas | 0 (0–0.35) | 0 (0–0) | |
| Green beans | 0 (0–0.03) | 0.03 (0–0.03) | |
| Carrots | 0 (0–0.03) | 0.03 (0–0.03) | |
| Spinach | 0 (0–0.14) | 0.03 (0–0.14) | |
| Eggplant | 0 (0–0.008) | 0 (0–0.03) | |
| Fish | |||
| Kapenta | 0.03 (0–0.14) | 0.03 (0–0.14) | 0.31 |
| Buka Buka | 0 (0–0.03) | 0 (0–0.03) | |
| Tilapia | 0.015 (0–0.03) | 0 (0–0.03) | |
| Insects | 0.35 | ||
| Grasshoppers | 0 (0–0) | 0 (0–0) | |
| Inswa | 0 (0–0.03) | 0 (0–0) | |
| Caterpillars | 0 (0–0.03) | 0 (0–0.03) | |
| Animal origin | 0.41 | ||
| Meat | 0.14 (0.03–0.35) | 0.14 (0.03–0.35) | |
| Pork | 0 (0–0.03) | 0 (0–0.03) | |
| Chicken | 0.14 (0.03–0.35) | 0.35 (0.14–0.35) | |
| Starch | 0.56 | ||
| Irish potatoes | 0.03 (0–0.14) | 0.03 (0–0.14) | |
| Cassava | 0.03 (0–0.03) | 0.03 (0–0.03) | |
| Rice | 0.245 (0.03–0.7) | 0.35 (0.03–0.35) | |
| Bread | 1 (0.19–1.0) | 1 (0.35–1.0) | |
| Miscellaneous | |||
| Chibuku | 0 (0–0) | 0 (0–0) | 0.0005 |
| Sugarcane | 0.03 (0–0.03) | 0.03 (0–0.03) | 0.83 |
| Peanuts | 0.14 (0.03–0.7) | 0.35 (0.03–0.7) | 0.4 |
| Chikanda | 0 (0–0.03) | 0.03 (0–0.03) | 0.41 |
| Milk | 0.35 (0.14–1.0) | 0.35 (0.14–1.0) | 0.86 |
1P values refer to the results of the Kruskal-Wallis test. Only 8 cases and 1 control reported regular intake of chibuku (traditional beer), so medians are low; however, there is a significant difference that is consistent with the results of dichotomized intakes (yes or no), where P = 0.001 (OR: 16.9; 95% CI: 2.1, 760). Because the numbers of participants who reported regular intake were low, the biological relevance of this statistically significant association is uncertain.
Associations between food intake and isoprostane excretion were analyzed. Isoprostane excretion inversely correlated with total fruit intake (r = −0.23; n = 140: P = 0.006); this was most marked in nonsmokers (ρ = −0.24; n = 127; P = 0.007). Isoprostane excretion was also inversely correlated with fish intake, but only in controls (ρ = −0.39; n = 50; P = 0.006). Intakes of other food groups did not correlate with isoprostane excretion. No association was found between fruit intake or isoprostane excretion in relation to smoking or alcohol consumption, past or current (data not shown).
Risk factors for gastric cancer
To further evaluate the contribution of dietary intakes and isoprostane excretion to known risk factors for gastric cancer (current smoking status, current alcohol consumption, H. pylori seropositivity, income <K1,000,000/mo, a low serum pepsinogen 1:2 ratio, and low serum gastrin-17), isoprostane excretion and fruit intake were dichotomized with a median split. Multivariate regression models were designed that included the risk factors described above, isoprostane excretion, and fruit intake, with gastric cancer as the outcome variable. High fruit intake and high urinary isoprostane excretion were each independent predictors of gastric cancer (Table 3), but predictability was lost when both entities were included in the same analysis, which suggested that these are codependent variables. Neither isoprostane excretion nor any of the main dietary food groups was associated with gastric atrophy in the cases or controls, whether measured by a low pepsinogen 1:2 ratio or low gastrin-17 (data not shown).
TABLE 3.
Logistic regression models of the association of risk factors with gastric cancer
| Risk factor | OR (95% CI) | P |
| Model 1 (n = 140) | ||
| Upper half of isoprostane excretion | 2.15 (1.01, 4.59) | 0.046 |
| Current smoker | 6.88 (1.34, 35.2) | 0.021 |
| Low pepsinogen 1:2 ratio | 2.29 (1.07, 4.91) | 0.032 |
| Model 2 (n = 140) | ||
| Upper half of fruit intake | 0.44 (0.20, 0.95) | 0.038 |
| Current smoker | 7.22 (1.38, 37.9) | 0.019 |
| Low pepsinogen 1:2 ratio | 2.43 (1.12, 5.13) | 0.024 |
Seasonality
Isoprostane excretion was not seasonal, although fruit and vegetable intakes were (P = 0.03 and P = 0.02, respectively). The median intake of fruit was 0.81 (IQR: 0.5–1.8) items per day in cases and 1.4 (0.6–2.1) in controls (P = 0.06). This difference was significantly different for dry season intakes (P = 0.02) between cases (0.6; 0.2–1.2) and controls (1.1, 0.5–1.8) but not for rainy season intakes (P = 0.18) between cases (1.2; IQR: 0.6–2.2) and controls (1.6; 0.7–2.5). Analysis by season did not show a relation between other foodstuffs and cancer. The relation between isoprostane excretion and cancer was affected by seasonality in multivariate analysis. When dichotomized and used in a univariate analysis, the upper half of isoprostane excretion was associated with cancer (OR: 2.43; 95% CI: 1.2, 5.3; P = 0.02), but only during the rainy season (OR: 4.1; 95% CI: 1.3, 13.6; P = 0.01), and the association was not significant during the cold or hot seasons.
DISCUSSION
In this study designed to examine the relation between dietary intakes, oxidative stress quantified by urinary isoprostane concentration, and gastric cancer, we report that urinary isoprostane is a potential marker for oxidative stress in gastric cancer, and higher fruit intake is protective against gastric cancer. Isoprostane excretion may change after the onset of cancer; however, taken together with the relation between isoprostane excretion and fruit intake, our data suggest that antioxidant status is an important contributor to carcinogenesis. There was no suggestion that isoprostane excretion determines gastric atrophy, from which we inferred that any influence is on the final steps in the Correa pathway. The Correa pathway explains the stages of gastric carcinogenesis as progression from chronic gastritis, atrophy, intestinal metaplasia, dysplasia, and ultimately cancer (5).
Gastric cancer has a multifactorial etiopathogenesis, believed to result from interaction between host, environmental (including dietary), and microbial factors. Relations between fruit and vegetable intakes and gastric cancer have been extensively studied (7), and type of diet, mode of preparation, and frequency of consumption may also influence cancer development. High salt intakes have been implicated in some studies, in the form of burnt fish (Iceland) or preserved meats, fish, or pickled vegetables (Japan, China) (18). A recent multicentric, multinational European study analyzed 477,312 subjects and found that fresh fruit and citrus fruit consumption may protect smokers from developing gastric cancer (19). There is a conspicuous paucity of data of this sort from Africa, primarily from underreporting because of a lack of diagnostic resources and limited manpower (4, 8). This has led to suggestions that the reported incidence is lower in Africa than in industrialized countries. However, according to the International Agency for Research on Cancer (IARC-GloboCan 2008), gastric cancer is the ninth leading cause of cancer mortality in Zambia (2). Therefore, recognizing risk factors for the development of gastric cancer specific to Zambia may lead to targeting preventive measures such as lifestyle modifications (limiting salt intake), nutritional intervention (increasing fruit intake), and treatment or prevention of H. pylori infection.
The role of oxidative stress in contributing to carcinogenesis has been widely investigated in several cancers. The inference from these investigations is that antioxidants may have a protective role. Measurement of antioxidant status, however, is not straightforward. Several measures have been used, including blood concentrations of antioxidant micronutrients such as vitamins A, C, or E; selenium or zinc; and oxidation products of nucleosides, but no single measure is without limitations. Urinary isoprostanes have been used as a marker of oxidative stress and cancer risk with other cancers, such as lung cancer (20) and prostate cancer (21), which suggests that this test may have value in other cancers. To our knowledge, there are no published studies evaluating gastric cancer and urinary isoprostane as a marker of oxidative stress. The consistency between fruit intake and isoprostane excretion in our data encourages us to believe that isoprostane excretion is contributing important information in gastric cancers. In a cross-sectional study of 285 adolescents (ages 13–17 y), Holt et al (22) showed that urinary isoprostane was inversely correlated with intake of total fruit and vegetables, which is consistent with our findings.
Because the availability of different fruit in Zambia varies seasonally, we anticipated that seasonality would be an important factor to take into account. The study design ensured that cases and controls were, as far as possible, recruited within the same season. Respondents were asked separately about their usual intakes of fruit, vegetables, and insects during the dry and rainy seasons, the 2 seasons in which availability was most likely to affect intake. As expected, fruit and vegetable intakes were seasonal in both cases and controls, but isoprostane excretion was not. Despite the fact that seasonality did not influence isoprostane excretion, it did influence the relation between isoprostane excretion and cancer, which was mainly apparent during the rainy season. The relation between fruit intake and cancer was prominently observed for fruit intake in the dry season. This may suggest that adults who maintain a high fruit intake during the dry season (when fruit is less available) are protected from gastric cancer, in contrast with those whose intake falls off in the dry season. We also observed an effect of season of recruitment, which is not easily explained: the relation between fruit intake and cancer was much stronger for those cases and controls recruited in the cold season (OR: 0.08; 95% CI: 0.01, 0.5; P = 0.003), even though the food-frequency questionnaire was designed to reflect habitual intakes before the onset of symptoms attributable to cancer and so should have been unaffected by season of recruitment. It is possible that the season of recruitment generated a recall bias. This likely reflects longitudinal trends beyond the realm of analysis of a case-control study; a cohort study design will be needed to further dissect out seasonal influences on carcinogenesis.
One of the strengths of our study was the detailed characterization of dietary intake in the Zambian population. The questionnaire used for dietary data collection was developed a priori because no such questionnaire was in existence. With this questionnaire, there was remarkable consistency in the differences between cases and controls, both for fruit and vegetable intake, and there was a statistically significant correlation between fruit intake and isoprostane excretion. This suggests that the questionnaire generated for this study is robust, although it has not been formally validated against observed intakes. Furthermore, this template can potentially be adapted for other populations, both in Africa and elsewhere.
A case-control study design is subject to certain limitations. We could not ascertain whether poor antioxidant status in patients with gastric cancer is a cause or a consequence of cancer. In addition, information about cancer stage was unavailable for most patients because patient financial limitations precluded testing for staging or because patients died before oncology referral. Furthermore, because the antioxidant content of some Zambian foods is unknown, categorization of food groups (eg, fruit, vegetables) is rather crude. Difficulty with dietary recall, especially related to portion sizes, may have had an effect on estimation of exposure. Because cancer incidence is dependent on age and sex, we attempted to match cases with controls by these factors, but this proved difficult because the number of men older than 60 y of age with normal endoscopic results did not match the recruitment of gastric cancer cases. Therefore, unconditional analysis was favored over conditional logistic regression (23), because of concerns about small study numbers, large numbers of independent variables, and the broad age bands used for matching cases with controls. However, we checked each model to determine whether sex and age contributed significantly to the association. For completeness, we also ran conditional logistic regression models and obtained results in general agreement with the models shown in Table 3. Our findings were therefore consistent across the different analyses that were performed, which improved confidence in our results and conclusions despite these limitations.
In conclusion, we report that fruit intake and antioxidant status alter gastric cancer risk in Zambian patients and that fruit intake is a protective factor against gastric neoplasia, even in a population in whom diet is dominated by vegetable rather than animal products. To our knowledge this is the first formal study of diet and antioxidant status as risk factors for gastric cancer in Africa. Further research is needed to characterize dietary intakes and the antioxidant properties of foods consumed in Africa, where until now this subject has received little attention.
Acknowledgments
The authors’ responsibilities were as follows—PK: had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; AWA, VK, CPG, DCR, KY, CA-S, and PK: designed the study; AWA, VK, MM-L, KY, RC, ES, SM, and PK: conducted the research (acquisition of data); AWA, VK, MM-L, CPG, DCR, VM, KY, RC, GC, CA-S, and PK: provided essential material to conduct the research; PK: performed the statistical analysis; AWA, VK, MM-L, CPC, DR, GC, CA-S, VM, KY, SM, and PK: analyzed the data; AWA, VK, MM-L, CPG, DR, GC, CA-S, VM, KY, ES, SM, and PK: wrote the manuscript and had primary responsibility for the final manuscript. AWA was a Fogarty International Clinical Research Fellow supported by the Fogarty International Center–NIH. None of the authors declared a conflict of interest.
REFERENCES
- 1.Stewart BW, Kleihues P, eds. World cancer report. Lyon, France: IARC Press, 2003.; [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, cancer incidence and mortality worldwide: IARC CancerBase no. 10. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr (cited 25 April 2012);
- 3.Plummer M, Franceschi S, Muñoz N. Epidemiology of gastric cancer. IARC Sci Publ 2004;7(157):311–26 [PubMed] [Google Scholar]
- 4.Parkin DM, Ferlay M, Hamdi-Cherif F, Sitas JO, Thomas H, Wabinga SL. Cancer in Africa. Epidemiology and prevention. Lyon, France: International Agency for Research on Cancer, 2003;153. Available from: http://www.iarc.fr/en/publications/pdfs-online/epi/index.php.
- 5.Correa P. A human model of gastric carcinogenesis. Cancer Res 1988;48:3554–60. [PubMed] [Google Scholar]
- 6.Wabinga H. Helicobacter pylori and hisopathological changes of gastric mucosa in Uganda population with varying prevalence of cancer. Afr Health Sci 2005;5:234–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asombang AW, Kelly P. Gastric cancer in Africa: what do we know about incidence and risk factors? Trans R Soc Trop Med Hyg 2012;106:69–74. [DOI] [PubMed] [Google Scholar]
- 8.Kelly P, Katema M, Amadi B, Zimba L, Aparicio S, Mudenda V, Baboo KS, Zulu I. Gastrointestinal pathology in the University Teaching Hospital, Lusaka, Zambia: review of endoscopic and pathology records. Trans R Soc Trop Med Hyg 2008;102:194–9. [DOI] [PubMed] [Google Scholar]
- 9.Fernando N, Holton J, Zulu I, Vaira D, Mwaba P, Kelly P. Helicobacter pylori infection in an urban African population. J Clin Microbiol 2001;39:1323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diplock AT. Antioxidants and disease prevention. Mol Aspects Med 1994;15:293–376. [DOI] [PubMed] [Google Scholar]
- 11.Nair U, Barstch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med 2007;43:1109–20. [DOI] [PubMed] [Google Scholar]
- 12.Patel BP, Rawal U, Dave T, Rawal R, Shukla S, Shah P, Patel P. Lipid peroxidation, total antioxidant status and total thiol levels predict overall survival in patients with oral squamous cell carcinoma. Integr Cancer Ther 2007;6:365–72. [DOI] [PubMed] [Google Scholar]
- 13.Montuschi P, Barnes P, Roberts J. Isoprostanes: markers and mediators of oxidative stress. FASEB J 2004;18:1791–800. [DOI] [PubMed] [Google Scholar]
- 14.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997;82:291–5. [DOI] [PubMed] [Google Scholar]
- 15.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- 16.Grabbe K, Stephenson R, Vwalika B, Chomba E, Karita E, Kayitenkore K, Tichacek A, Allen S. Knowledge, use, and concerns about contraceptive methods among sero-discordant couples in Rwanda and Zambia. J Womens Health (Larchmt) 2009;18:1449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milne GL, Yin H, Brooks JD, Sanchez S, Jackson Roberts L II. , Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol 2007;433:113–26. [DOI] [PubMed] [Google Scholar]
- 18.Binici DN, Koca T, Dursun H. Dietary habits, demographical, and socio-economical risk factors of the newly diagnosed gastric cancers in the eastern Anatolia region of Turkey: an endemic upper gastrointestinal cancer region. Dig Dis Sci 2009;54:2629–33. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez CA, Lujan-Barroso L, de Mesquita HB, Jenab M, Duell EJ, Agudo A, Tjonneland A, Boutron-Ruault MC, Clavel-Chapelon F, Touillaud M, et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a re-analysis of the European prospective investigation into cancer and nutrition (EPIC-EURGAST) study after a longer follow-up. Int J Cancer 2012;131(12):2910–9. [DOI] [PubMed] [Google Scholar]
- 20.Epplein M, Franke AA, Cooney RV, Morris JS, Wilkens LR, Goodman MT, Murphy SP, Henderson BE, Kolonel LN, Le Marchand L. Association of plasma micronutrient levels and urinary isoprostane with risk of lung cancer: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 2009;18:1962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barocas DA, Motley S, Cookson MS, Chang SS, Penson DF, Dai Q, Milne G, Roberts LJ, 2nd, Morrow J, Concepcion RS, et al. Oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. J Urol 2011;185:2102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt EM, Steffen LM, Moran A, Basu S, Steinberger J, Ross JA, Hong CP, Sinaiko AR. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc 2009;109:414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland S, Schwartzbaum JA, Finkle WD. Problems due to small samples and sparse data in conditional logistic regression analysis. Am J Epidemiol 2000;151:531–9. [DOI] [PubMed] [Google Scholar]